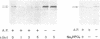Abstract
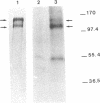
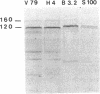
Xfin is a Xenopus zinc finger protein which is expressed in the cytoplasm of the oocyte and throughout embryogenesis, as well as in the cytoplasm of some specific and highly differentiated cell types (De Lucchini et al., Mech. Dev. 36, 31-40, 1991). In this paper we present a characterization of some structural features of the protein and of its nucleic acid binding properties. We found that Xfin is a phosphoprotein, is present in the soluble fraction of the cytoplasm, and is actively phosphorylated in cytosolic extracts. Several putative phosphorylation sites are present in the cDNA-derived protein sequence, mostly located at specific positions within the Zn-fingers. In an in vitro assay a fusion protein containing part of the finger region of Xfin exhibits specific binding to a poly (G) RNA homopolymer, while it does not bind DNA. The RNA binding activity of the protein is significantly enhanced by phosphorylation. A putative Xfin homolog, which appears to be evolutionarily conserved with regard to size, cytoplasmic expression and antigenic specificity, is present in representatives of five Vertebrate classes. Taken together, these results may suggest that, by virtue of its RNA binding activity modulated through phosphorylation, Xfin could serve some evolutionarily conserved function in post-transcriptional regulation processes.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bellefroid E. J., Poncelet D. A., Lecocq P. J., Revelant O., Martial J. A. The evolutionarily conserved Krüppel-associated box domain defines a subfamily of eukaryotic multifingered proteins. Proc Natl Acad Sci U S A. 1991 May 1;88(9):3608–3612. doi: 10.1073/pnas.88.9.3608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellini M., Lacroix J. C., Gall J. G. A putative zinc-binding protein on lampbrush chromosome loops. EMBO J. 1993 Jan;12(1):107–114. doi: 10.1002/j.1460-2075.1993.tb05636.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavrier P., Zerial M., Lemaire P., Almendral J., Bravo R., Charnay P. A gene encoding a protein with zinc fingers is activated during G0/G1 transition in cultured cells. EMBO J. 1988 Jan;7(1):29–35. doi: 10.1002/j.1460-2075.1988.tb02780.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darby M. K., Joho K. E. Differential binding of zinc fingers from Xenopus TFIIIA and p43 to 5S RNA and the 5S RNA gene. Mol Cell Biol. 1992 Jul;12(7):3155–3164. doi: 10.1128/mcb.12.7.3155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Lucchini S., Rijli F. M., Ciliberto G., Barsacchi G. A Xenopus multifinger protein, Xfin, is expressed in specialized cell types and is localized in the cytoplasm. Mech Dev. 1991 Dec;36(1-2):31–40. doi: 10.1016/0925-4773(91)90069-i. [DOI] [PubMed] [Google Scholar]
- Deschatrette J., Weiss M. C. Characterization of differentiated and dedifferentiated clones from a rat hepatoma. Biochimie. 1974;56(11-12):1603–1611. doi: 10.1016/s0300-9084(75)80286-0. [DOI] [PubMed] [Google Scholar]
- Dignam J. D., Lebovitz R. M., Roeder R. G. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983 Mar 11;11(5):1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox C. A., Sheets M. D., Wahle E., Wickens M. Polyadenylation of maternal mRNA during oocyte maturation: poly(A) addition in vitro requires a regulated RNA binding activity and a poly(A) polymerase. EMBO J. 1992 Dec;11(13):5021–5032. doi: 10.1002/j.1460-2075.1992.tb05609.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guddat U., Bakken A. H., Pieler T. Protein-mediated nuclear export of RNA: 5S rRNA containing small RNPs in xenopus oocytes. Cell. 1990 Feb 23;60(4):619–628. doi: 10.1016/0092-8674(90)90665-2. [DOI] [PubMed] [Google Scholar]
- Honda B. M., Roeder R. G. Association of a 5S gene transcription factor with 5S RNA and altered levels of the factor during cell differentiation. Cell. 1980 Nov;22(1 Pt 1):119–126. doi: 10.1016/0092-8674(80)90160-9. [DOI] [PubMed] [Google Scholar]
- Jackson S. P., MacDonald J. J., Lees-Miller S., Tjian R. GC box binding induces phosphorylation of Sp1 by a DNA-dependent protein kinase. Cell. 1990 Oct 5;63(1):155–165. doi: 10.1016/0092-8674(90)90296-q. [DOI] [PubMed] [Google Scholar]
- Jacobs G. H. Determination of the base recognition positions of zinc fingers from sequence analysis. EMBO J. 1992 Dec;11(12):4507–4517. doi: 10.1002/j.1460-2075.1992.tb05552.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joho K. E., Darby M. K., Crawford E. T., Brown D. D. A finger protein structurally similar to TFIIIA that binds exclusively to 5S RNA in Xenopus. Cell. 1990 Apr 20;61(2):293–300. doi: 10.1016/0092-8674(90)90809-s. [DOI] [PubMed] [Google Scholar]
- Kamata T., Kung H. F. Modulation of maturation and ribosomal protein S6 phosphorylation in Xenopus oocytes by microinjection of oncogenic ras protein and protein kinase C. Mol Cell Biol. 1990 Mar;10(3):880–886. doi: 10.1128/mcb.10.3.880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp B. E., Pearson R. B. Protein kinase recognition sequence motifs. Trends Biochem Sci. 1990 Sep;15(9):342–346. doi: 10.1016/0968-0004(90)90073-k. [DOI] [PubMed] [Google Scholar]
- Kennelly P. J., Krebs E. G. Consensus sequences as substrate specificity determinants for protein kinases and protein phosphatases. J Biol Chem. 1991 Aug 25;266(24):15555–15558. [PubMed] [Google Scholar]
- Knöchel W., Pöting A., Köster M., el Baradi T., Nietfeld W., Bouwmeester T., Pieler T. Evolutionary conserved modules associated with zinc fingers in Xenopus laevis. Proc Natl Acad Sci U S A. 1989 Aug;86(16):6097–6100. doi: 10.1073/pnas.86.16.6097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köster M., Kühn U., Bouwmeester T., Nietfeld W., el-Baradi T., Knöchel W., Pieler T. Structure, expression and in vitro functional characterization of a novel RNA binding zinc finger protein from Xenopus. EMBO J. 1991 Oct;10(10):3087–3093. doi: 10.1002/j.1460-2075.1991.tb07861.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köster M., Pieler T., Pöting A., Knöchel W. The finger motif defines a multigene family represented in the maternal mRNA of Xenopus laevis oocytes. EMBO J. 1988 Jun;7(6):1735–1741. doi: 10.1002/j.1460-2075.1988.tb03002.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller J., McLachlan A. D., Klug A. Repetitive zinc-binding domains in the protein transcription factor IIIA from Xenopus oocytes. EMBO J. 1985 Jun;4(6):1609–1614. doi: 10.1002/j.1460-2075.1985.tb03825.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray M. T., Krohne G., Franke W. W. Different forms of soluble cytoplasmic mRNA binding proteins and particles in Xenopus laevis oocytes and embryos. J Cell Biol. 1991 Jan;112(1):1–11. doi: 10.1083/jcb.112.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray M. T., Schiller D. L., Franke W. W. Sequence analysis of cytoplasmic mRNA-binding proteins of Xenopus oocytes identifies a family of RNA-binding proteins. Proc Natl Acad Sci U S A. 1992 Jan 1;89(1):11–15. doi: 10.1073/pnas.89.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nietfeld W., Mentzel H., Pieler T. The Xenopus laevis poly(A) binding protein is composed of multiple functionally independent RNA binding domains. EMBO J. 1990 Nov;9(11):3699–3705. doi: 10.1002/j.1460-2075.1990.tb07582.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ollo R., Maniatis T. Drosophila Krüppel gene product produced in a baculovirus expression system is a nuclear phosphoprotein that binds to DNA. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5700–5704. doi: 10.1073/pnas.84.16.5700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otte A. P., Kramer I. M., Mannesse M., Lambrechts C., Durston A. J. Characterization of protein kinase C in early Xenopus embryogenesis. Development. 1990 Oct;110(2):461–470. doi: 10.1242/dev.110.2.461. [DOI] [PubMed] [Google Scholar]
- Payre F., Vincent A. Genomic targets of the serendipity beta and delta zinc finger proteins and their respective DNA recognition sites. EMBO J. 1991 Sep;10(9):2533–2541. doi: 10.1002/j.1460-2075.1991.tb07793.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham H. R., Brown D. D. A specific transcription factor that can bind either the 5S RNA gene or 5S RNA. Proc Natl Acad Sci U S A. 1980 Jul;77(7):4170–4174. doi: 10.1073/pnas.77.7.4170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picard B., Wegnez M. Isolation of a 7S particle from Xenopus laevis oocytes: a 5S RNA-protein complex. Proc Natl Acad Sci U S A. 1979 Jan;76(1):241–245. doi: 10.1073/pnas.76.1.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rijli F. M., De Lucchini S., Ciliberto G., Barsacchi G. A Zn-finger protein, Xfin, is expressed during cone differentiation in the retina of the frog Xenopus laevis. Int J Dev Biol. 1993 Jun;37(2):311–317. [PubMed] [Google Scholar]
- Rosati M., Marino M., Franzè A., Tramontano A., Grimaldi G. Members of the zinc finger protein gene family sharing a conserved N-terminal module. Nucleic Acids Res. 1991 Oct 25;19(20):5661–5667. doi: 10.1093/nar/19.20.5661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz i Altaba A., Perry-O'Keefe H., Melton D. A. Xfin: an embryonic gene encoding a multifingered protein in Xenopus. EMBO J. 1987 Oct;6(10):3065–3070. doi: 10.1002/j.1460-2075.1987.tb02613.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seiler-Tuyns A., Mérillat A. M., Haefliger D. N., Wahli W. The human estrogen receptor can regulate exogenous but not endogenous vitellogenin gene promoters in a Xenopus cell line. Nucleic Acids Res. 1988 Sep 12;16(17):8291–8305. doi: 10.1093/nar/16.17.8291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith L. D. The induction of oocyte maturation: transmembrane signaling events and regulation of the cell cycle. Development. 1989 Dec;107(4):685–699. doi: 10.1242/dev.107.4.685. [DOI] [PubMed] [Google Scholar]
- Spandidos D. A., Wilkie N. M. Malignant transformation of early passage rodent cells by a single mutated human oncogene. Nature. 1984 Aug 9;310(5977):469–475. doi: 10.1038/310469a0. [DOI] [PubMed] [Google Scholar]
- Stillman D. J., Bankier A. T., Seddon A., Groenhout E. G., Nasmyth K. A. Characterization of a transcription factor involved in mother cell specific transcription of the yeast HO gene. EMBO J. 1988 Feb;7(2):485–494. doi: 10.1002/j.1460-2075.1988.tb02836.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theunissen O., Rudt F., Guddat U., Mentzel H., Pieler T. RNA and DNA binding zinc fingers in Xenopus TFIIIA. Cell. 1992 Nov 13;71(4):679–690. doi: 10.1016/0092-8674(92)90601-8. [DOI] [PubMed] [Google Scholar]
- Wilkinson D. G., Bhatt S., Chavrier P., Bravo R., Charnay P. Segment-specific expression of a zinc-finger gene in the developing nervous system of the mouse. Nature. 1989 Feb 2;337(6206):461–464. doi: 10.1038/337461a0. [DOI] [PubMed] [Google Scholar]
- Xu H. P., Rajavashisth T., Grewal N., Jung V., Riggs M., Rodgers L., Wigler M. A gene encoding a protein with seven zinc finger domains acts on the sexual differentiation pathways of Schizosaccharomyces pombe. Mol Biol Cell. 1992 Jul;3(7):721–734. doi: 10.1091/mbc.3.7.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- el-Baradi T., Bouwmeester T., Giltay R., Pieler T. The maternal store of zinc finger protein encoding mRNAs in fully grown Xenopus oocytes is not required for early embryogenesis. EMBO J. 1991 Jun;10(6):1407–1413. doi: 10.1002/j.1460-2075.1991.tb07661.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- el-Baradi T., Pieler T. Zinc finger proteins: what we know and what we would like to know. Mech Dev. 1991 Nov;35(3):155–169. doi: 10.1016/0925-4773(91)90015-x. [DOI] [PubMed] [Google Scholar]