Abstract
We report the development of a chitosan modified carbon fiber microelectrode for in vivo detection of serotonin. We find that chitosan has the ability to reject physiological levels of ascorbic acid interferences and facilitate selective and sensitive detection of in vivo levels of serotonin, a common catecholamine neurotransmitter. Presence of chitosan on the microelectrode surface was investigated using scanning electron microscopy (SEM) and cyclic voltammetry (CV). The electrode was characterized using differential pulse voltammetry (DPV). A detection limit of 1.6 nM serotonin with a sensitivity of 5.12 nA/µM, a linear range from 2 to 100 nM and a reproducibility of 6.5 % for n=6 electrodes were obtained. Chitosan modified microelectrodes selectively measure serotonin in presence of physiological levels of ascorbic acid. In vivo measurements were performed to measure concentration of serotonin in the live embryonic zebrafish intestine. The sensor quantifies in vivo intestinal levels of serotonin while successfully rejecting ascorbic acid interferences. We demonstrate that chitosan can be used as an effective coating to reject ascorbic acid interferences at carbon fiber microelectrodes, as an alternative to Nafion, and that chitosan modified microelectrodes are reliable tools for in vivo monitoring of changes in neurotransmitter levels.
Keywords: carbon fiber microelectrode, chitosan, ascorbic acid, interferences, in vivo detection of neurotransmitters, zebrafish, intestine
1. INTRODUCTION
In vivo measurements with carbon fiber microelectrodes (CFMEs) and fast electrochemical methods, like fast-scan cyclic voltammetry [1], amperometry [2] and differential pulse voltammetry (DPV) [3] are commonly used in analytical and neuroscience research to detect physiological levels of neurotransmitters [4, 5]. Electrochemical sensors are small, relatively simple to fabricate, easily implantable and can provide real-time measurements in biological environments with excellent spatial and temporal resolution [6]. The main challenge is to achieve adequate selectivity and sensitivity in the complex biological environment, where other coexisting species can interfere. The major interfering compound that can be present at high micromolar concentrations in physiological conditions is ascorbic acid, an easily oxidizable species at low applied potentials. Typically, the electrochemical response of ascorbic acid oxidation on bare electrodes interferes with that of common neurotransmitters like dopamine and serotonin, causing overlapping signals and limiting selectivity. This makes it difficult to discriminate an individual analyte. Therefore, selective and sensitive determination of neurotransmitters in the presence of ascorbic acid is of great physiological importance.
To achieve selectivity, CFMEs are usually coated with polymeric films to restrict access of interferences to the electrode surface. Most of these sensors use Nafion, a fluorosulfonated polyanion as electrode coating. Nafion excludes ascorbic acid interferences through electrostatic repulsion [7, 8]. In other works, ascorbic acid interferences were eliminated using overoxidized polypyrrole [9], carbon nanotubes [10] or mixed composite films of Nafion and carbon nanotubes [11]. Here we report that chitosan, a well-known biopolymer, has a sieving function against physiological levels of ascorbic acid and can be used as a selective coating in the fabrication of implantable CFMEs. Chitosan is an attractive surface coating for a variety of reasons. It is widely available, possesses excellent film-forming ability, high mechanical strength and remarkable adherence to surfaces. In addition, it is chemically inert, has low toxicity and is biocompatible, which makes it ideal for use in implantable devices. Chitosan has been used as surface coating in a variety of applications. In electrochemical sensors, it has been mainly applied to macroelectrodes in conjunction with enzymes, as an enzyme immobilization matrix, and as a linker, in composite forms with carbon nanotubes and nanoparticles. We have recently showed that chitosan acts as a protective layer enhancing the stability of a NO sensor [12] and that it can be successfully used for the immobilization of tyrosinase in the design of an enzymatic dopamine biosensor [2]. While chitosan has been applied in electrochemical sensors before, its sieving properties and its use as a selective coating for CFMEs have not been reported. In addition, the operation of chitosan modified carbon fiber microelectrodes in implantable conditions has not been established.
The goal of this study is to demonstrate that chitosan is an efficient membrane for rejecting ascorbic acid interferences, thereby enhancing selectivity of implantable CFMEs for in vivo detection of neurotransmitters. The role of chitosan in the sensing layer and the rejection capacity was systematically studied in relation to the analytical performance of the corresponding CFMEs microelectrodes. Selectivity and sensitivity of the microelectrode were studied for serotonin, as a model neurotransmitter, in presence of ascorbic acid using DPV as the detection technique. The sensor was tested both in standard solutions and in vivo, measuring physiological levels of serotonin in live embryonic zebrafish. Serotonin (5-Hydroxytryptamine or 5-HT) is an important neurotransmitter in both the central nervous system (CNS) and enteric nervous system (ENS).
Zebrafish is a widely used biological model to study organ development, metabolism and toxicity due to their anatomic and physiological similarities with vertebrates including humans [13]. In a previous study using zebrafish embryos, serotonin was analyzed electrochemically using DPV with Nafion-carbon nanotube modified CFMEs [3]. Here, we show that chitosan is an effective and more stable coating on CFMEs for the selective in vivo detection of intestinal serotonin concentrations. These studies demonstrate that chitosan-modified electrochemical CFMEs microsensors can be used as a reliable tool for monitoring neurological processes and neurodevelopmental mechanisms.
2. EXPERIMENTAL
2.1. Reagents
Chitosan (from shrimp shells; practical grade), L-ascorbic acid, 2-propanol (99.5%; ACS grade), potassium phosphate (monobasic) were purchased from Sigma-Aldrich. Sodium phosphate (dibasic) and potassium ferricyanide were obtained from Fisher Scientific. Serotonin hydrochloride (99%) was purchased from Acros Organics. Methanol (100%), acetic acid (glacial) and potassium nitrate were obtained from J. T. Baker. Carbon wire and Dumont #5 forceps were purchased from WPI (World Precision Instruments Inc.). Silver conductive epoxy was obtained from MG Chemicals. Non-conductive epoxy was purchased from Devcon. Distilled, deionized water (Millipore, Direct-Q System) with a resistivity of 18.2Ω.cm was used for preparation of solutions.
2.2. Instrumentation
Differential pulse voltammetry and cyclic voltammetric experiments were performed using a CH Instrument electrochemical analyzer potentiostat (CH Instruments Inc.). All optimization experiments were carried out using a conventional electrochemical cell with the chitosan modified CFME as the working electrode, a Ag/AgCl/3 M NaCl (BAS MF-2052, RE-5B) as reference electrode and a platinum wire as counter electrode. All potentials were referred to the Ag/AgCl reference electrode. The morphology of the electrode surface after deposition of the chitosan layer was assessed with a JEOL JSM-7400F, high resolution field emission scanning electron microscope.
2.3. Preparation of Carbon Fiber Microelectrodes
Single fiber carbon microelectrodes (~5 µm diameter) were fabricated from carbon fibers from WPI. A single carbon fiber was glued to a copper wire with a conductive silver epoxy paste and aspirated into a pulled glass capillary tube. The upper end of the capillary tube was sealed with a non-conductive epoxy resin and cured at 100°C for 10 min. The capillary tube with the connecting wire was pulled using a Narishige PP-83 microelectrode puller. The length of the fiber extending from glass was fixed to ~0.5 mm from the glass seal using a scalpel. Microelectrodes were immersed in isopropanol to clean carbon fibers for 10 min and air dried. Before modification, the microelectrodes were electrochemically treated by repeated oxidation in 0.1 M PBS (phosphate buffer saline, pH 7.0) in the potential range from −0.4 to 1.4 V at 500 V/s. Modification with chitosan was performed using a dip-coating procedure. Preparation of the 1% chitosan solution was performed as described elsewhere [14]. The carbon fiber microelectrodes were dipped for 15 s in chitosan solution. The microelectrodes were air-dry for at least 30 min before testing. All values are reported as the mean ± standard deviation for at least three replicate measurements. In vivo measurements are reported as the mean ± standard deviation for measurements with at least three embryos. The limit of detection was determined according to the 3 Sb/m criteria where m is the slope of the linear calibration plot and Sb is the relative standard deviation of the electrochemical signal of the blank.
2.4. Procedures for in vitro and in vivo testing of serotonin
DPV was used for all in vivo and in vitro measurements in a potential range of 0 to 0.6 V. Measurements were performed at a scan increment of 4.0 mV. The pulse amplitude, width and period were 50 mV, 50 ms and 200 ms respectively. A background DPV obtained in absence of serotonin was subtracted from each voltammogram obtained in presence of serotonin. In vitro experiments were performed in 0.1 M PBS, pH 7.4. The microelectrodes were electrochemically cleaned in between each measurement by cyclic voltammetry in the range from −0.4 to 1.4 V at 50 V/s in 0.1 M PBS, pH 7.4. To study ascorbic acid interferences, 200 µM AA was injected into the electrochemical cell after recording the background voltammogram.
All in vivo experiments were performed in live zebrafish embryos to record the normal physiological concentrations of serotonin. Pigmented five day postfertilization (dpf) zebrafish larvae were used for electrochemical measurements. Electrochemical measurements were completed in E3 medium which is an electrolyte solution containing 5 mM NaCl, 0.17 mM KCl, 0.33 mM MgSO4, 0.33 mM CaCl2. AB wild type fish were used for all procedures [15]. Fish maintenance and matings were performed as previously described [16]. To perform in vivo electrochemical experiments, embryos were immobilized on agarose pad. Microsensors were inserted into the middle segment of the intestine using a micromanipulator. All voltammograms are shown after background subtraction.
3. RESULTS and DISCUSSION
3.1. Preparation and characterization of the chitosan layer
To evaluate the presence and effect of chitosan on the surface of the CFME, we first characterized the electrochemical response of the electrode with and without chitosan using cyclic voltammetry. Chitosan has strong adhesion properties and it is quickly attached onto the surface of the carbon fiber. Figure 1 shows the electrochemical characteristics of the bare and chitosan modified CFME in 10 mM potassium ferricyanide solution prepared in 1 M KNO3 in the potential range of −0.4 to + 0.8 V vs. Ag/AgCl reference electrode at a scan rate of 100 mV/s. Both the oxidation and reduction current decreased after the treatment of the electrode with chitosan, indicating that a less-conductive layer is deposited onto the electrode surface.
Figure 1.
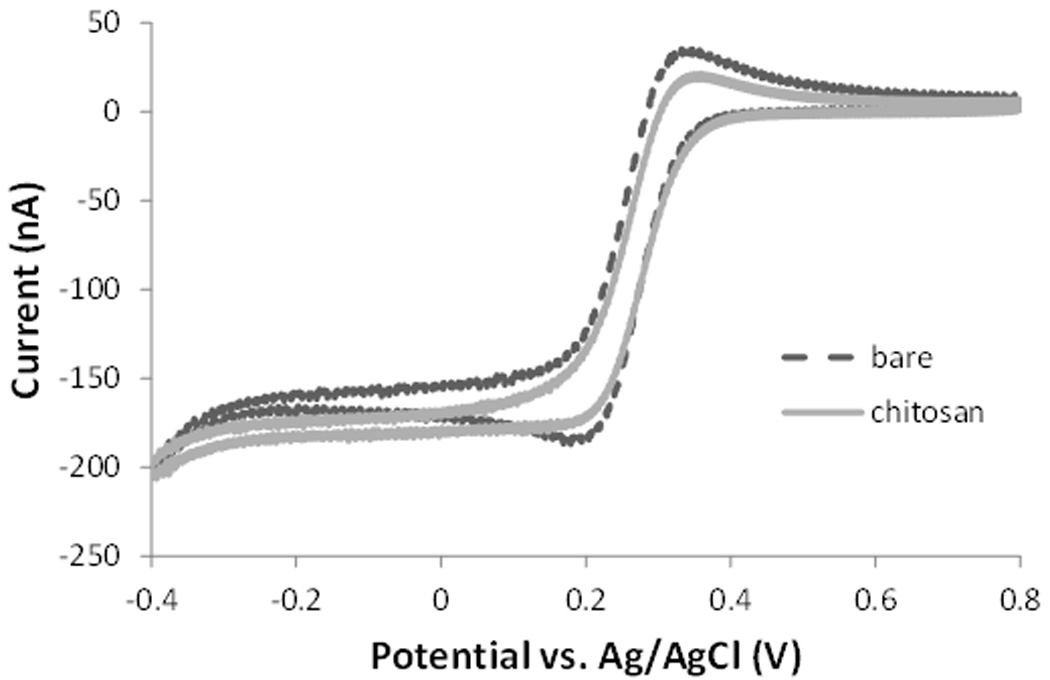
Comparative cyclic voltammograms of the bare and chitosan-modified CFME in 10 mM K3[Fe(CN)6] in 0.1M KNO3.
The presence of the chitosan layer onto the electrode surface was further confirmed by scanning electron microscopy (SEM). Figure 2 shows scanning electron micrographs of the bare (Figure 2A) and chitosan treated electrode (Figure 2B). The SEM images of the chitosan modified electrode show uniform coverage along the fiber with a much smoother surface as compared to the bare electrode (Figure 2B). The white deposit visible onto the tip of the electrode (Figure 2C) indicates the presence of chitosan. In an aqueous environment such as the one of the electrochemical measurements, chitosan swallows, creating a tridimensional porous network, further improving the quality of the coating over the entire surface of the carbon fiber. Thus, we speculate that in the measurement environment the electrode coverage is further enhanced. The SEM images, in addition to the voltammetric characterization of the electrode in potassium ferricyanide demonstrate formation of a chitosan layer on the surface of the carbon fiber.
Figure 2.

Scanning electron micrograph images of bare (A) and chitosan modified (B and C) carbon fiber microelectrode. (C) shows the chitosan layer onto the tip of the fiber. The scale bar is 100 nm. All images are shown at 100 000 × magnification.
3.2. Selectivity of the chitosan film
Figure 3 shows cyclic voltammetric responses at bare and chitosan coated carbon fiber electrodes in the presence of 200 µM ascorbic acid and 20 µM serotonin. The bare electrode shows a distinct voltammetric response corresponding to the oxidation of ascorbic acid with a maximum current at a potential of 0.32 V. For serotonin, a well-defined peak was recorded at 0.34 V, strongly overlapping with that of ascorbic acid. This indicates that serotonin cannot be successfully discriminated in mixtures with ascorbic acid. Under the same experimental conditions but with the chitosan modified CFME the peak corresponding to the oxidation of ascorbic acid is no longer produced. We explain this phenomenon by an electrostatic repulsion of the negatively charged ascorbic acid at the chitosan coated electrode, suggesting that chitosan has a sieving function against ascorbic acid. Chitosan has reactive amino functional groups on its polysaccharide backbone with a pKa value of ~6.5 [17]. The pKa value of ascorbic acid is 4.2. Therefore at a physiological pH, chitosan can efficiently reject ascorbic acid. At the same time, there is a favorable interaction between serotonin and chitosan, increasing the sensitivity of the coated electrode. At physiological pH, the amino groups of serotonin are protonated and serotonin is positively charged [18]. Therefore, serotonin can be electrostatically attracted and accumulated onto the chitosan coating of the CFME, increasing sensitivity. For example, the intensity of the oxidation signal for 20 µM serotonin at the chitosan coated electrode was 17 nA, which was increased as compared to that at the bare electrode (10.5 nA). The increased signal can be explained by a pre-concentration of serotonin at the electrode surface.
Figure 3.
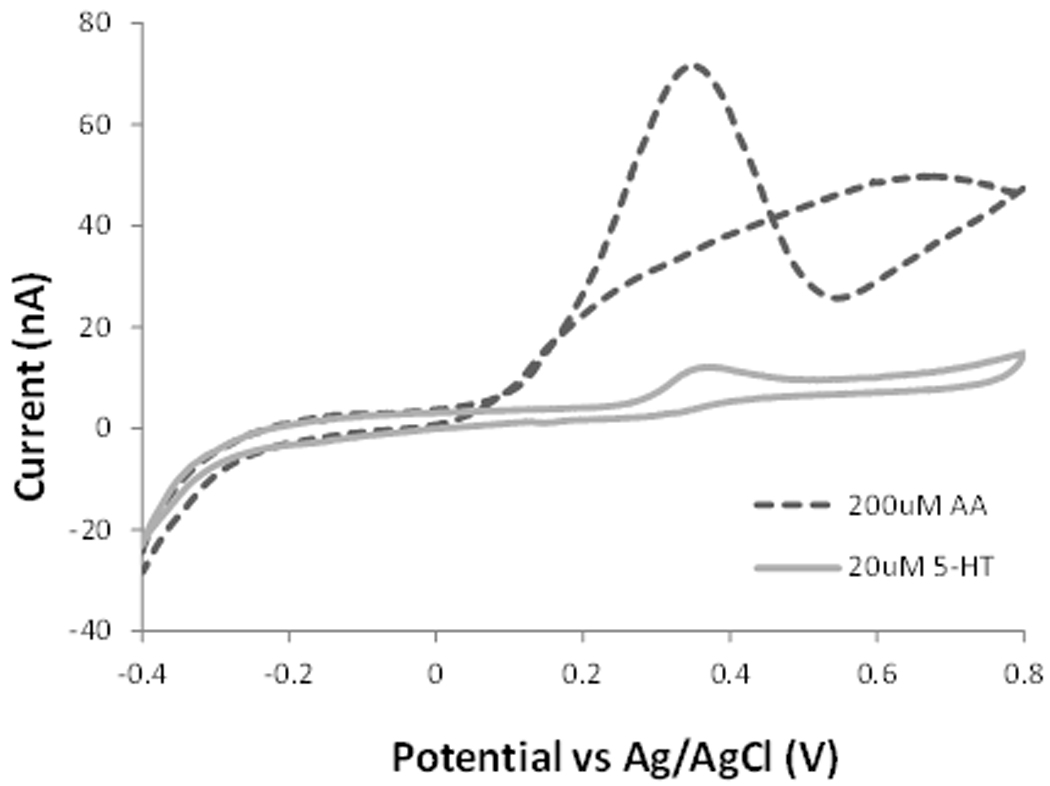
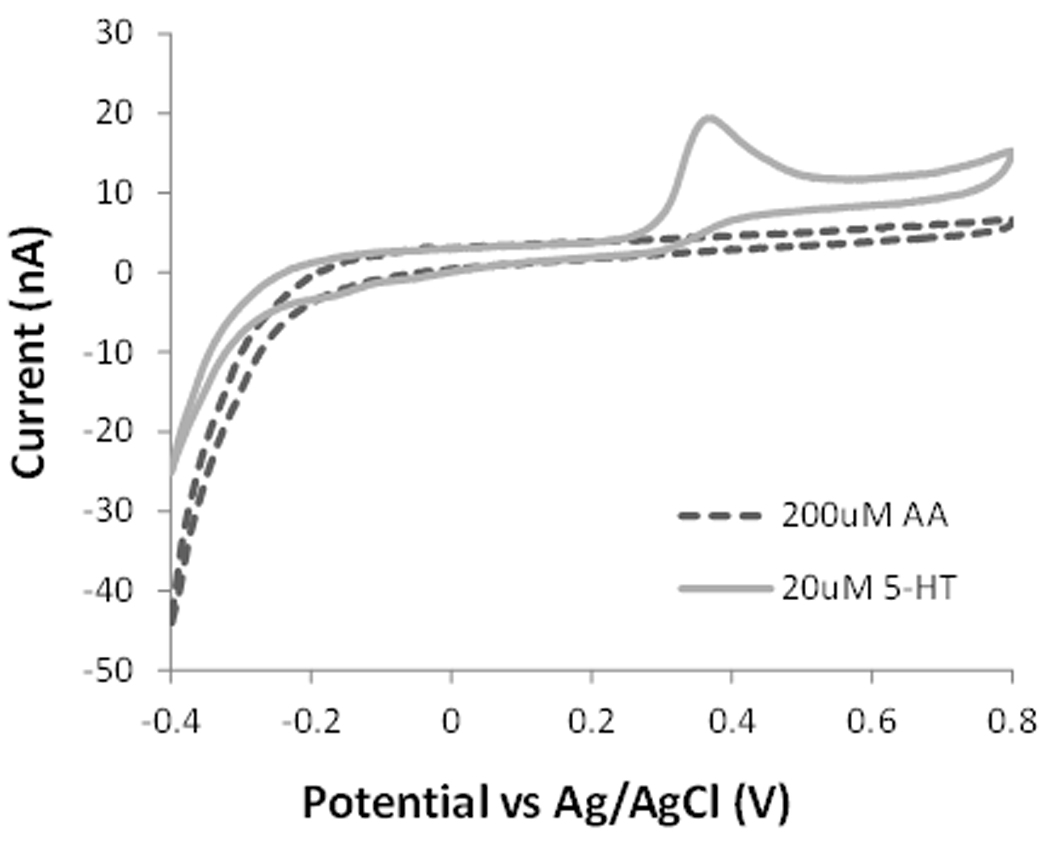
Comparison of cyclic voltammograms of 200 µM ascorbic acid and 20 µM serotonin (5-HT) at bare (A) and chitosan modified (B) CFMEs in 0.1 M PBS solution at pH 7.4.
3.3. Electrochemical determination of 5-HT and analytical characterization
Electrochemical determination of serotonin in the presence of ascorbic acid and analytical characterization of the chitosan modified CFME were performed using DPV. A potential window from 0 to + 0.6 V was chosen for this analysis. Experiments were performed in mixtures of serotonin and ascorbic acid with both the bare and chitosan coated electrode. Figure 4A shows the DPV voltammograms at the bare electrode of 200 µM ascorbic acid in the presence and absence of 10 nM serotonin. The broad peak at ~ 0.12 V corresponds to the oxidation of ascorbic acid. In the presence of both ascorbic acid and serotonin, this peak increases concomitant with the appearance of a small shoulder at 0.33 V. With increasing concentrations of serotonin, the peak at 0.33 V increases (results not shown). The peak corresponding to the oxidation of ascorbic acid completely disappears at the chitosan modified electrode (Figure 4B). It is clear from this result that chitosan prevents access of ascorbic acid to the electrode surface and eliminates ascorbic acid interference. The sieving function is efficient for up to 500 µM ascorbic acid, which is higher than physiological concentrations. Even in the presence of high concentrations of ascorbic acid, the ascorbic acid signal is still lower than the serotonin signal. Moreover, modification of the carbon fiber with chitosan also improves the background current, increasing the sensitivity of the measurements. These results indicate that ascorbic acid is effectively discriminated by chitosan, allowing selective and sensitive determination of serotonin. Thus, measurements of serotonin in the presence of physiological levels of ascorbic acid at the chitosan modified CFME are possible. The procedure can also be used for the detection of other neurotransmitters such as dopamine.
Figure 4.

Differential pulse voltammogram of the bare (A) and chitosan modified CFMEs (B) for 200 µM AA (light gray line) and a mixture of 200 µM AA and 10 nM serotonin (solid black line). The inset in (A) shows the response of the bare electrode to 10 nM serotonin. (C) displays the differential pulse voltammograms of various concentrations of serotonin at the chitosan modified microelectrode after background subtraction and (D) shows the corresponding linear range of the sensor. The supporting electrolyte solution was 0.1 M PBS at pH 7.4.
Figures 4C and D show a typical DPV and the corresponding calibration curve obtained with increasing concentrations of serotonin at the chitosan modified CFME. The detection limit of the microelectrode was 1.6 nM with a sensitivity of 5.12 nA/µm. The linear range spanned from 2 to 100 nM after background subtraction (Figure 4D). The reproducibility of the chitosan microsensor was evaluated by preparing a series of electrodes and performing experiments in identical conditions using the same concentration of serotonin. A current value of 0.1851 nA was recorded for injection of 20 nM serotonin with a standard deviation of 0.0121 for n = 6 randomly-prepared sensors, corresponding to a coefficient of variation of 6.5 %. The sensitivity of the sensor, when taking into account the electrode area, compares favorably or even better to that of other microelectrodes fabricated with alternative coatings reported in the literature. The analytical characteristics of the sensor in comparison with literature data are summarized in Table 1. The chitosan coated CFME has a lower detection limit and higher sensitivity for serotonin than CFMEs with overoxidized polypyrrole [19] and boron doped electrodes [20]. The higher sensitivity of the carbon nanotubes/Nafion coated CFME (85.65 nA/µM) [3] is due to the higher surface area of the electrode (30 µm vs 5 µm). A higher sensitivity was reported for a CFME coated with Nafion and a polymeric Ni-tetrasulfonated phtalocyanine. It should be noted that most of those electrodes were not implanted or tested in an in vivo system.
Table 1.
Analytical characteristics of the chitosan modified microsensor and comparison with the literature data.
| Detection Method |
Diameter Size (µm) |
Linear Range (mol/L) |
Sensitivity/ | LOD (nM) |
Rejection of interferences |
Ref. | |
|---|---|---|---|---|---|---|---|
| DNA-PPyox/CFME | DPV | 6 | 1×10−8 – 1×10−6 | - | 7 | AA up to 2×10−2M | [20] |
| BDD-Pt | DPV | 40 | 2×10−6 – 10 ×10−6 | 19.4 pA/µM | 1050 | - | [19] |
| CFME | DPV | 30 | 7.2 pA/µM | 3080 | - | [19] | |
| Poly[Ni(TSPc)]/Nafion/CFME | SWV | 8 | 5×10−9 – 9×10−8 | 36 nA/µM | 3.8 | 5-HIAA up to 31 mg/L DOPAC up to 11 mg/L Rejects AA | [23] |
| GNP/LC/GCE | SWV | 3000 | 6×10−8 – 6×10−6 | - | 20 | AA up to 6×10−4M | [24] |
| BDD-Si | Amperometry / Flow injection | Geometric area of 0.64 cm2 | 1×10−8 – 5×10−5 | 25 nA/µM | 20 | - | [25] |
| CNTs/Nafion/CFME | DPV | 30 | 5×10−9 – 2×10−7 | 83.65 nA/µM | 1 | - | [3] |
| Chit/CFME | DPV |
5 Geometric area of 0.079 mm2 |
2×10−9 – 1×10−7 | 5.1243 nA/µM | 1.6 | AA up to 5×10−4M | This work |
PPyox: overoxidized polypyrrole; BDD: boron doped diamond; Poly[Ni(TSPc)]: polymeric tetrasulfonated phtalocyanines; GNP: gold nanoparticles; LC: L-cysteine; AA: ascorbic acid; DOPAC: 3,4-dihydroxyphenylaceticacid; 5-HIAA: 5-Hydroxyindole-3-acetic acid, GCE: glassy carbon electrode; CNTs: carbon nanotubes; SWV: square wave voltammetry; DPV: differential pulse voltammetry.
To evaluate the stability and reusability of the sensor, we studied the ability of the sensor to respond reproducibly to successive exposures to serotonin. It is well known that the products of serotonin oxidation strongly adsorb onto carbon fiber microelectrodes, quickly and irreversibly passivating the carbon surface [7]. Figure 5 shows the operational stability of the sensor for 5 consecutive measurements of 20 nM serotonin in controlled conditions obtained with the same electrode. Results indicate that after the first measurement of serotonin, the bare electrode loses 15% sensitivity. After the fourth measurement the decrease is 50 %. On the other hand, the chitosan coated electrode shows no decrease in the signal after 4 consecutive measurements (without electrochemical reconditioning in between runs). If the electrode is electrochemically reconditioned, the chitosan coated microelectrode could be reused for more than 10 consecutive assays in vitro in controlled conditions. This demonstrates that chitosan provide protection against fouling. In the presence of chitosan, the chitosan layer acts as a protective barrier against direct adsorption of the oxidation products onto the carbon fiber, thus reducing the extent of fouling. All measurements were performed in the physiological pH range.
Figure 5.
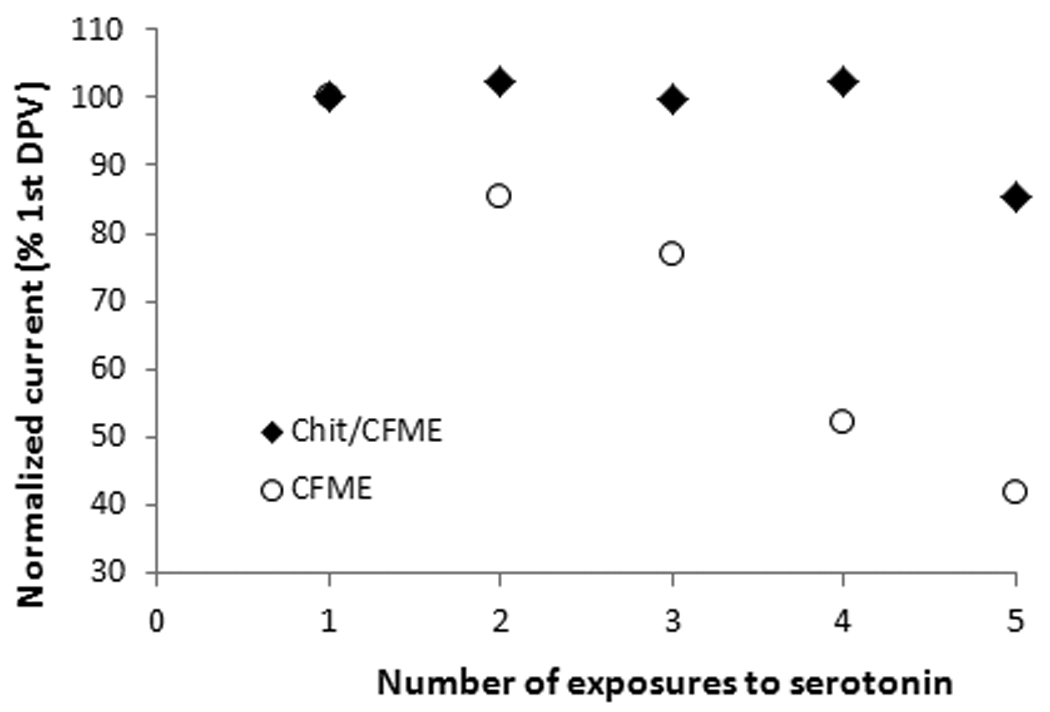
Operational stability of the bare (CFME) and chitosan coated carbon fiber microelectrode (Chit/CFME) in controlled conditions to injections of 20 nM serotonin with washing the cell in between measurements (without electrochemical reconditioning of the electrode in between runs).
The effect of interferences and specificity of the chitosan coated vs the bare electrode was tested with ascorbic acid (200 µM) and uric acid (200 µM) and with several catecholamines including epinephrine, norepinephrine, DOPAC (dihydroxyphenylacetic acid) and the main metabolite of serotonin, 5-HIAA (5-Hydroxyindoleacetic acid), all tested at a concentration of 1 µM. Figure 6 shows electrode selectivity and comparison of electrode responses to these species. For the same concentration range, chitosan has a sieving function against another negatively charged interfering compound, uric acid (Figure 6B) but this is less effective than that for ascorbic acid. When testing the different catecholamines (Figure 6A) results show that the chitosan coating enhances specificity for serotonin. The modified electrode has decreased response for anions like DOPAC and 5-HIAA. For example, the response to 1 µM 5-HIAA of the bare electrode is reduced from 0.135 nA to 0.018 nA for the modified electrode. The chitosan coated microelectrode is nearly 70 times more sensitive to serotonin than to 5-HIAA. For the same concentration range tested, a high response and little difference between the bare and modified electrodes were observed for norepinephrine.
Figure 6.
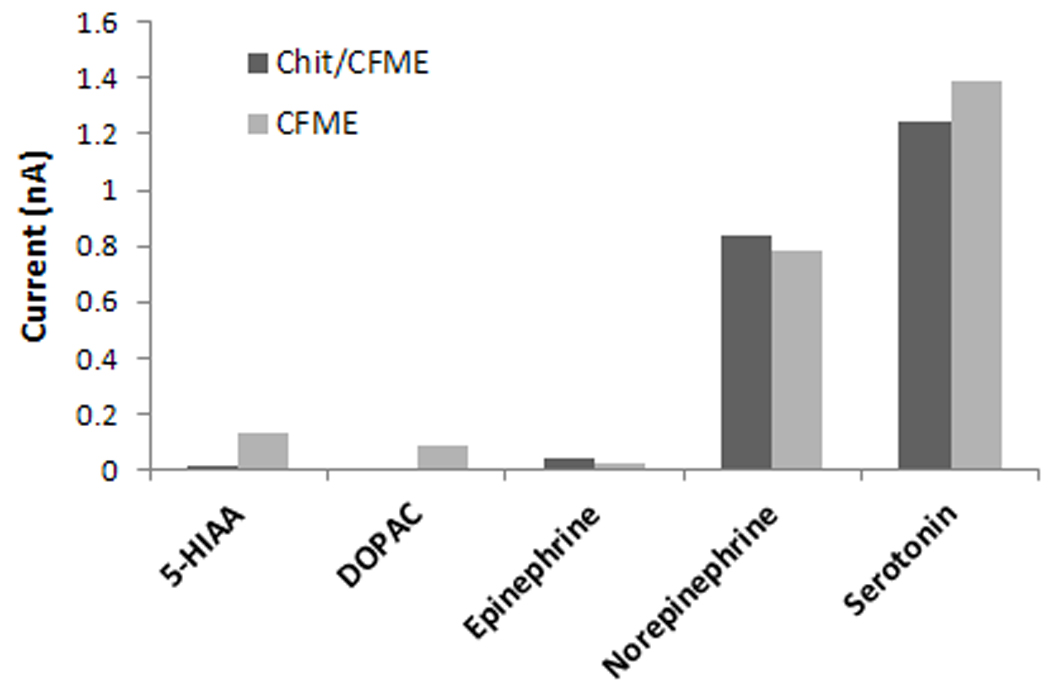
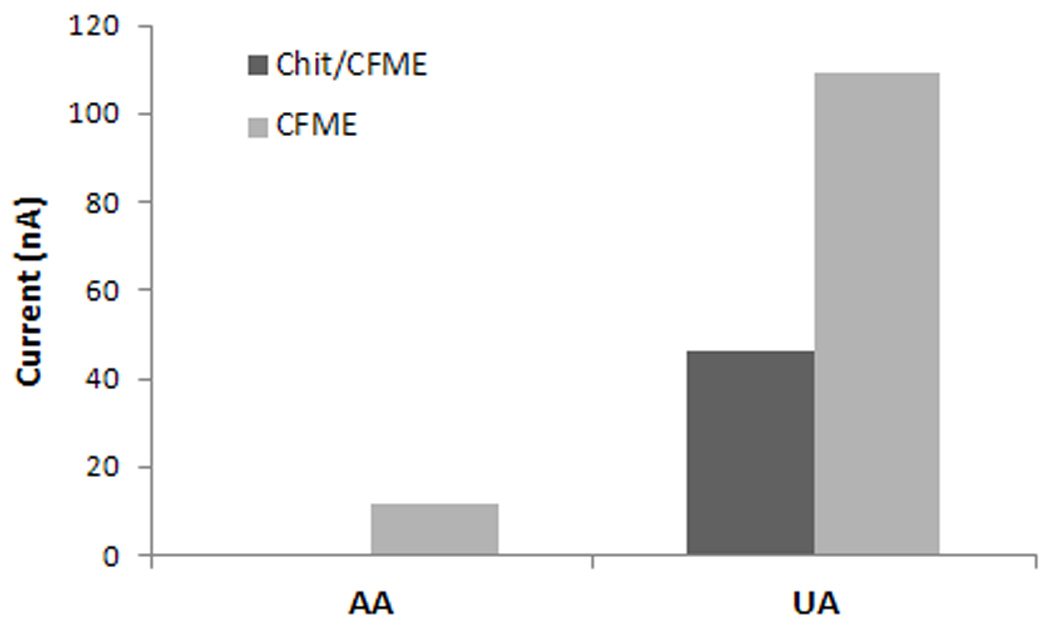
Detection selectivity and comparison of the responses of bare (CFME) vs chitosan coated microelectrode (Chit/CFME) to A: epinephrine, norepinephrine, DOPAC (dihydroxyphenylacetic acid) and 5-HIAA (5-Hydroxyindoleacetic acid), each at 1 µM concentration, and B: ascorbic acid and uric acid (each at 200 µM).
To demonstrate the functionality of the chitosan modified CFME for direct in vivo measurements, we used zebrafish embryos as a model organism by performing electrochemical measurements within the intestine, which contains high levels of 5-HT.
3.4. In vivo measurements with the chitosan functionalized electrode
In vivo serotonin detection was performed with an electrode implanted in the middle segment of the intestine of zebrafish embryos. Enteric neurons and enterochromaffin cells (EC) within the digestive system produce approximately 95% of the serotonin in the vertebrate body [21]. Serotonin plays a role in gastrointestinal motility, secretion, and food absorption [22]. Previously, we measured zebrafish intestinal serotonin concentrations using implanted Nafion-modified CFMEs and demonstrated the validity of the electrochemical signal, confirming identification of serotonin in vivo [3]. Here, we demonstrate that implanted chitosan modified CFMEs provide selective determination of serotonin. Figure 7 shows the DPV results recorded in vivo with the bare and chitosan modified CFME. A very broad peak with a maximum of 0.25 V was recorded with the bare CFME. This peak corresponds to overlapping electrochemical signals arising from oxidation of both ascorbic acid and serotonin, which coexist in the intestine. Therefore, the serotonin oxidation peak cannot be observed and effectively discriminated from that of ascorbic acid. On the other hand, in the voltammogram recorded with the chitosan coated CFME, a well-defined peak with a maximum at ~ 0.35 V corresponding to serotonin was obtained. This current corresponds to a concentration of 30.8(±3.4) nM serotonin in physiological conditions, as calculated from the calibration curve of the microsensor. A previous study on zebrafish embryos using CNTs/Nafion/CFMEs and DPV reported physiological intestinal levels of serotonin of 29.9 (±1.13) nM which is close to that found in this study with chitosan functionalized electrodes [3]. The CNTs/Nafion/CFMEs used previously were fabricated from multiple carbon fibers and had a diameter of 30 µm (vs a single fiber of 5 µm in this study). We were however unable to obtain uniform and reproducible deposition of Nafion onto a single carbon fiber; in many cases the Nafion layer was missing and the electrode recorded high electrochemical signals corresponding to the oxidation of ascorbic acid, just like the bare electrode, overlapping that of serotonin (Figure 7 for the bare electrode). The chitosan coating on the other hand provided consistent and reproducible results in vivo (less than 6% variability between electrodes), by using a simple dip-coating procedure. The in vivo DPV data with the chitosan coated microelectrode shows a single well-defined peak corresponding to serotonin while the Nafion coated electrode recorded a second unidentified peak at 570 mV. This second peak was not present at the chitosan modified electrode. It is possible that chitosan is effectively removing this electrochemically active compound from the electrode surface, further enhancing selectivity against serotonin.
Figure 7.
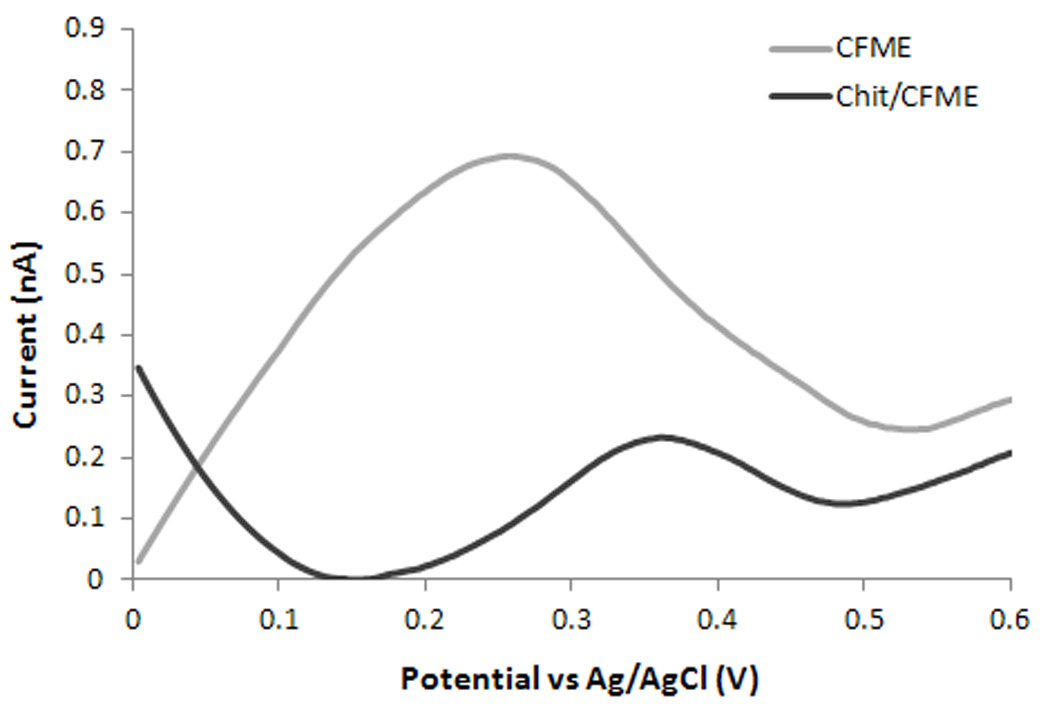
Differential pulse voltammogram recorded in vivo with the implanted bare (CFME) and chitosan coated microelectrode (Chit/CFME). The supporting electrolyte was E3 medium.
The chitosan film used as a selective coating was firmly attached onto the electrode surface, while conserving the same analytical performance. The film was stable and resistant to multiple implantations. The modified electrode can be reused for up to four sequential in vivo measurements with brief electrochemical reconditioning in between runs. The average serotonin current recorded in vivo for four consecutive readings/implantations with the same electrode was 0.162 (±0.004) nA. After the fourth implantation, the current response increased considerably to 0.65 nA and the DPV signal was very broad. We attribute these changes to build-up tissue and pre-concentration of serotonin onto the electrode surface, which cannot be removed by electrochemical treatment.
4. CONCLUSION
We fabricated a chitosan based carbon fiber microelectrode and demonstrated that chitosan can be used as a selective coating in implanted microelectrodes for rejecting ascorbic acid interferences. Results indicate enhanced selectivity and sensitivity for the detection of the neurotransmitter serotonin compared with the bare electrode, with no interference from physiological levels of ascorbic acid. The microsensor measures low nanomolar concentrations of serotonin, with a detection limit of 1.6 nM, a sensitivity of 5.12 nA/µM, a linear range of 2 – 100 nM and a reproducibility of 6.5 % for n=6 electrodes.
In vivo results demonstrate that the chitosan modified electrode measures serotonin produced in the zebrafish intestine with high spatial and temporal resolution. The electrochemical signal recorded in vivo with the implanted chitosan modified microelectrode corresponded to a serotonin concentration of 30.8(±3.4) nM in normal physiological conditions. The chitosan membrane was very stable allowing implantation and multiple measurements with the same electrode. The response of the microelectrode to zebrafish intestinal serotonin indicates that the sensor operates effectively in an in vivo environment. The inherent biocompatibility and remarkable adherence makes chitosan an excellent coating for use in implantable sensors to selectively detect and monitor levels of in vivo neurotransmitters. This method can also be used for construction of implantable microelectrodes to detect other neurotransmitters.
Acknowledgements
This work was supported by grants NSF-0954919 to SA and NIH 1R15DK089474-01 to KW.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Makos MA, et al. In Vivo Electrochemical Measurements of Exogenously Applied Dopamine in Drosophila melanogaster. Analytical Chemistry. 2009;81(5):1848–1854. doi: 10.1021/ac802297b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Njagi J, et al. Amperometric Detection of Dopamine in Vivo with an Enzyme Based Carbon Fiber Microbiosensor. Analytical Chemistry. 2010;82(3):989–996. doi: 10.1021/ac9022605. [DOI] [PubMed] [Google Scholar]
- 3.Njagi J, et al. Electrochemical Quantification of Serotonin in the Live Embryonic Zebrafish Intestine. Analytical Chemistry. 2010;82(5):1822–1830. doi: 10.1021/ac902465v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Venton BJ, Troyer KP, Wightman RM. Response Times of Carbon Fiber Microelectrodes to Dynamic Changes in Catecholamine Concentration. Analytical Chemistry. 2001;74(3):539–546. doi: 10.1021/ac010819a. [DOI] [PubMed] [Google Scholar]
- 5.Venton BJ, Wightman RM. Psychoanalytical Electrochemistry: Dopamine and Behavior. Analytical Chemistry. 2003;75(19):414 A–421 A. [Google Scholar]
- 6.Peters JL, et al. Ultrastructure at carbon fiber microelectrode implantation sites after acute voltammetric measurements in the striatum of anesthetized rats. Journal of Neuroscience Methods. 2004;137(1):9–23. doi: 10.1016/j.jneumeth.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 7.Hashemi P, et al. Voltammetric Detection of 5-Hydroxytryptamine Release in the Rat Brain. Analytical Chemistry. 2009;81(22):9462–9471. doi: 10.1021/ac9018846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brown FO, Lowry JP. Microelectrochemical sensors for in vivo brain analysis: an investigation of procedures for modifying Pt electrodes using Nafion (R) Analyst. 2003;128(6):700–705. doi: 10.1039/b300266g. [DOI] [PubMed] [Google Scholar]
- 9.Pihel K, Walker QD, Wightman RM. Overoxidized polypyrrole-coated carbon fiber microelectrodes for dopamine measurements with fast-scan cyclic voltammetry. Analytical Chemistry. 1996;68(13):2084–2089. doi: 10.1021/ac960153y. [DOI] [PubMed] [Google Scholar]
- 10.Viry L, et al. Discrimination of dopamine and ascorbic acid using carbon nanotube fiber microelectrodes. Physical Chemistry Chemical Physics. 2010;12(34):9993–9995. doi: 10.1039/c0cp00367k. [DOI] [PubMed] [Google Scholar]
- 11.Swamy BEK, Venton BJ. Carbon nanotube-modified microelectrodes for simultaneous detection of dopamine and serotoninin vivo. Analyst. 2007;132(9):876–884. doi: 10.1039/b705552h. [DOI] [PubMed] [Google Scholar]
- 12.Njagi J, et al. A sensitive electrochemical sensor based on chitosan and electropolymerized Meldola blue for monitoring NO in brain slices. Sensors and Actuators B-Chemical. 2010;143(2):673–680. [Google Scholar]
- 13.Wallace KN, Pack M. Unique and conserved aspects of gut development in zebrafish. Developmental Biology. 2003;255(1):12–29. doi: 10.1016/s0012-1606(02)00034-9. [DOI] [PubMed] [Google Scholar]
- 14.Njagi J, Ispas C, Andreescu S. Mixed Ceria-Based Metal Oxides Biosensor for Operation in Oxygen Restrictive Environments. Analytical Chemistry. 2008;80(19):7266–7274. doi: 10.1021/ac800808a. [DOI] [PubMed] [Google Scholar]
- 15.Westerfield M. The zebrafish book : a guide for the laboratory use of zebrafish (Brachydanio rerio) Eugene, OR: M. Westerfield; 1993. 1 v. (unpaged). [Google Scholar]
- 16.Wallace KN, et al. Intestinal growth and differentiation in zebrafish. Mechanisms of Development. 2005;122(2):157–173. doi: 10.1016/j.mod.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 17.Fernandes SC, et al. Development of a biomimetic chitosan film-coated gold electrode for determination of dopamine in the presence of ascorbic acid and uric acid. Electrochimica Acta. 2010;55(23):7152–7157. [Google Scholar]
- 18.Pratuangdejkul J, et al. Conformational dependence of serotonin theoretical pKa prediction. Chemical Physics Letters. 2006;420(4–6):538–544. [Google Scholar]
- 19.Patel BA. Continuous amperometric detection of co-released serotonin and melatonin from the mucosa in the ileum. Analyst. 2008;133(4):516–524. doi: 10.1039/b717034c. [DOI] [PubMed] [Google Scholar]
- 20.Jiang X, Lin X. Overoxidized polypyrrole film directed DNA immobilization for construction of electrochemical micro-biosensors and simultaneous determination of serotonin and dopamine. Analytica Chimica Acta. 2005;537(1–2):145–151. [Google Scholar]
- 21.Olden T, et al. Differentiation of the zebrafish enteric nervous system and intestinal smooth muscle. genesis. 2008;46(9):484–498. doi: 10.1002/dvg.20429. [DOI] [PubMed] [Google Scholar]
- 22.Sikander A, Rana SV, Prasad KK. Role of serotonin in gastrointestinal motility and irritable bowel syndrome. Clinica Chimica Acta. 2009;403(1–2):47–55. doi: 10.1016/j.cca.2009.01.028. [DOI] [PubMed] [Google Scholar]
- 23.de Irazu S, et al. Multimembrane carbon fiber microelectrodes for amperometric determination of serotonin in human urine. Analyst. 2001;126(4):495–500. doi: 10.1039/b009703i. [DOI] [PubMed] [Google Scholar]
- 24.Wei X, et al. Selective detection of neurotransmitter serotonin by a gold nanoparticle-modified glassy carbon electrode. Analyst. 2010;135(9):2286–2290. doi: 10.1039/c0an00256a. [DOI] [PubMed] [Google Scholar]
- 25.Sarada BV, et al. Electrochemical Oxidation of Histamine and Serotonin at Highly Boron-Doped Diamond Electrodes. Analytical Chemistry. 2000;72(7):1632–1638. doi: 10.1021/ac9908748. [DOI] [PubMed] [Google Scholar]


