Summary
Proteasomes play a fundamental role in the processing of intracellular antigens into peptides that bind to MHC class I molecules for presentation to CD8 T cells. Three IFNγ-inducible catalytic proteasome (immuno)subunits as well as the IFNγ-inducible proteasome activator PA28 dramatically accelerate the generation of a subset of MHC class I-presented antigenic peptides. To determine whether these IFNγ-inducible proteasome components play a compounded role in antigen processing, we generated mice lacking both PA28 and the immunosubunits β5i/LMP7 and β2i/MECL-1. Analyses of MHC class I cell surface levels ex vivo demonstrated that PA28-deficiency reduced the production of MHC class I-binding peptides both in cells with and without immunosubunits, in the last cells on top of an already diminished production of MHC ligands in the absence of immunoproteasomes. In contrast, the immunosubunits but not PA28 appeared to be of critical importance for the induction of CD8 T cell responses to multiple dominant Influenza and Listeria-derived epitopes. Taken together, our data demonstrate that PA28 and the proteasome immunosubunits use fundamentally different mechanisms to enhance the supply of MHC class I-binding peptides. However, only the immunosubunit-imposed effects on proteolytic epitope processing appear to have substantial effects on the fine-specificity of pathogen-specific CD8 T cell responses.
Keywords: Antigen processing, immunoproteasome, CD8 T cell, PA28, MHC class I
Introduction
Cells continuously turn-over their intracellular proteins, leaving short peptides of which a small percentage is translocated into the ER and binds to MHC class I molecules for display on the cell surface. Here, they can be recognized by CD8 T cells, allowing these cells to react to intracellular pathogens. Previous studies have shown that the kinetics and efficiency of epitope processing from pathogen-derived antigens play an important role both in CD8 T cell activation [1] [2][3][4] and their ability to recognize infected cells [5][6][7]. The production of most MHC class I-presented peptides starts with the proteolysis of mature proteins or defective ribosomal products by the proteasome, an abundant cellular protease. Proteasomes consist of a catalytic core particle (20S) and one or more regulatory complexes. In most cells, the enzymatic activity of the 20S particle is exerted by three constitutively expressed subunits, β1, β2 and β5 [8]. In contrast, lymphoid cells and cells exposed to cytokines such as type I interferons [9], TNFα [10] and IFNγ [11][12] express three facultative, homologous subunits, induced (i) β1i/LMP2, β5i/LMP7 and β2i/MECL-1, that replace the constitutive subunits in newly assembled so called immunoproteasome complexes. Cytokine-exposure further upregulates the expression of the proteasome regulator PA28αβ (PA28) [13], which is found at enhanced quantities also in professional APC (pAPC) [14][15][16], as well as that of many other proteins involved in antigen processing.
Both immunosubunit and PA28 expression are IFNγ-inducible, suggesting that these components of the proteasome system play an important role in immune recognition of infected host cells. A variety of studies have shown that incorporation of the immunosubunits β1i/LMP2, β5i/LMP7 and β2i/MECL-1 changes the 20S’ cleavage preferences and enhances the proteasome-mediated generation of a significant number of antigenic peptides [17]. This is most clear in cells lacking β5i/LMP7, where a defect in peptide supply leads to reduced MHC class I cell surface levels [18]. The immunosubunit-induced changes of proteasome-mediated epitope production have clear effects on the fine specificity of CD8 T cell responses to intracellular pathogens. Thus, mice lacking β1i/LMP2, β5i/LMP7 and/or β2i/MECL-1 fail to mount CD8 T cell responses to epitopes that are inefficiently generated by the constitutive proteasome subunits, probably because epitopes that are presented at relatively late time points after infection fail to prime CD8 T cell responses [1]. Nevertheless, the absence of immunosubunits does not impair the responses against other epitopes that are made by constitutive proteasomes and mice lacking either β1i/LMP2, β5i/LMP7 or β2i/MECL-1 or both β5i/LMP7 and β2i/MECL-1, despite partially impaired CD8 T cell responses, are capable of resolving infections with intracellular pathogens [19][20]. Thus, the inducible proteasome subunits do not seem to play an essential role in immune protection. Instead, a recent study suggested that immunoproteasomes may serve primarily to remove protein aggregates, accumulating due to interferon-induced oxidative stress [21].
The effects of PA28 on antigen processing and CD8 T cell responses are less clear. The PA28 α and β subunits form a heptamer [22] that attaches to the 20S particle and activates this in vitro, leading to an enhanced turn over of short peptide substrates and an increase in double cleavages in polypeptide substrates [23][24]. In vitro studies, in which polypeptide substrates were digested with 20S proteasome/PA28 complexes, PA28αβ-gene-deficient cells or cells transfected with PA28α and or PA28β encoding vectors were used, indicated that PA28 enhanced MHC class I presentation of several antigenic peptides [17][25], but that the effects of PA28 were confined to specific MHC class I alleles [26]. Mice lacking PA28 did not show clear defects in CD8 T cell responses to Influenza virus or viral resistance [25], however, were not analyzed for CD8 T cell responses or resistance to any other pathogen. Notably, peritoneal macrophages of these mice failed to upregulate MHC class I cell surface expression following IFNγ treatment [27]. Such defects in MHC class I upregulation were not observed in mouse embryonal cells of these gene-deficient mice [28]. Taken together, these studies suggest that the effects of PA28 on antigen processing and immune reponses are rather mild.
Although cytokine-exposed cells in infected tissues as well as professional antigen presenting cells usually express both the immunosubunits and PA28, the effects of PA28 or immunosubunits on MHC class I antigen processing and CD8 T cell responses have been studied separately so far. Thus, it is unknown whether these two types of IFNγ-inducible proteasome components have synergistic, additive or perhaps largely overlapping roles in antigen processing. To dissect the effects of PA28 on peptide production by immuno- versus constitutive proteasomes, we quantified 20S/PA28-mediated peptide liberation from a model substrate in vitro, and crossed PA28 gene-deficient [25] with β5i/LMP7+β2i/MECL-1 gene-deficient mice [29] and quantified proteasome-mediated production of MHC class I ligands and CD8 T cell responses in the different proteasome component-deficient strains.
Results
PA28 and immunosubunits have synergistic effects on 20S-mediated liberation of selected peptide products in vitro
Previous studies have shown that PA28 enhances the proteasome-mediated production of different antigenic peptides [6][30][31], but neither directly compared the effects of PA28 versus immunosubunits on 20S-mediated fragmentation of substrates, nor determined the effects of PA28 on the cleavage profile of constitutive versus immunoproteasomes. To test how PA28 modulated the activity of constitutive versus immunoproteasomes, we digested a synthetic polypeptide derived from the Hepatitis B Virus core antigen (HBV cAg) (Fig. 1A) with purified constitutive and immunoproteasome complexes, in the presence or absence of PA28. Peptide products were analyzed by RP-HPLC and on-line mass spectrometry. As shown previously, cleavage of the HBV polypeptide by constitutive proteasomes liberated the peptides Ser141-Val149 and Ser141-Arg152 [32]. Even higher quantities of the same two fragments and, in addition, the fragments Ser141-150 and Ser141-Arg151 [32] were found in the digests of immunoproteasomes. Remarkably, the presence of PA28 in the digests dramatically enhanced the efficiency of liberation of the 141-149 fragment by both constitutive and immunoproteasomes, and of 141-150 by immunoproteasomes (Fig. 1B) (note the difference in time scale between upper and lower panel). The presence of PA28, however, did not enhance the generation of fragments 141-151 and 141-152 by either constitutive or immunoproteasomes (Fig. 1B). Thus, PA28 enhanced the generation of a subset of 20S double cleavage products only and failed to confer new cleavage specificities to the 20S complex, which was most evident from the finding that PA28 enhanced the liberation of 141-150 by immunoproteasomes but did not enable constitutive proteasomes to generate this fragment. Notably, as the set of peptides produced by immunoproteasomes seems more diverse (Fig. 1) and better suited for MHC class I binding [18][33] than that produced by constitutive proteasomes, it is possible that PA28 further enhances the immunoproteasome-mediated generation of MHC class I ligands.
Figure 1. Kinetic analysis of the effects of PA28 and immunosubunits on generation of HBV cAg 131-162 cleavage products.
(A) Amino acid sequence of the HBV cAg131-162 polypeptide substrate. Arrows indicate dominant cleavage sites of constitutive or immunoproteasomes (32). (B) HBV cAg131-162 was incubated with constitutive or immunoproteasomes of T2 or T2 + β1i/LMP2+β5i/LMP7 cells, in the absence or presence of a four-molar excess of PA28, purified from induced MEC-PA28 cells [6], as indicated. Digestion products were separated by RP-HPLC and identified by mass spectrometry. Accumulation of different peptide products over time of digestion is shown. HBV cAg131-162 incubated with PA28 without proteasomes was not degraded.
Generation of a gene-deficient mouse strain lacking PA28 and immunosubunit expression
To be able to determine the respective and potentially compounded roles of the IFNγ inducible catalytic subunits and PA28 in MHC class I antigen processing, β5i/LMP7+β2i/MECL-1 double-gene deficient mice [29] were crossed with PA28αβ-double gene-deficient mice [25]. Because 20S complexes containing β1i/LMP2 assemble inefficiently in the absence of β5i/LMP7 [34], lymphoid - and cytokine-exposed cells of β5i/LMP7+β2i/MECL-1-deficient mice contain predominantly constitutive proteasomes. Homozygous mice lacking the genes coding for PA28α, PA28β, β5i/LMP7 and β2i/MECL-1 were identified by PCR (data not shown) and deficiency of these proteasome components was confirmed by immunoblot analyses (Fig. 2). The PA28+β5i/LMP7+β2i/MECL-1 gene-deficient mice did not show any phenotypic characteristics or abnormalities in growth or fertility.
Figure 2. Absence of PA28 and immunosubunit expression in gene-deficient mouse strains.
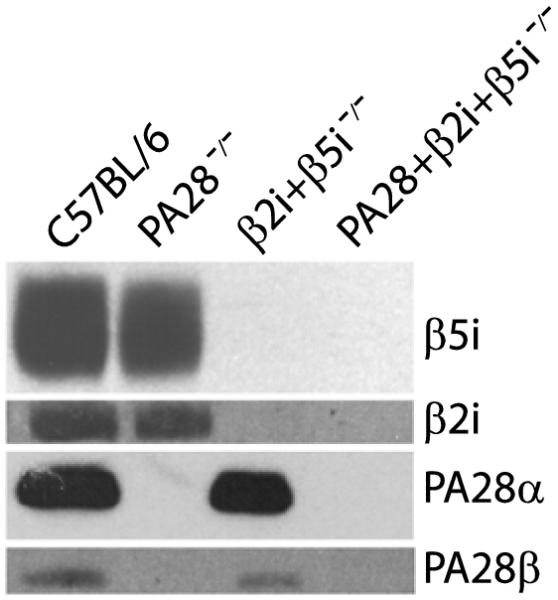
Splenocytes of indicated mouse strains were extracted, the lysates were separated by SDS/PAGE, and β5i/LMP7, β2i/MECL-1, PA28α and PA28β were detected by Western blotting.
PA28-deficiency does not alter the ratios of CD4:CD8 T cell subsets
Previous studies have shown that, compared to wt B6 mice, mice lacking the immunosubunit β2i /MECL-1 display an enhanced relative ratio of CD4 to CD8 T cells in the spleen [29][35][36]. Comparing the cellular composition of the spleens of the different gene-deficient mouse strains (Fig. 3A), we found reduced frequencies of CD8 T cells in β5i/LMP7+β2i/MECL-1-deficient compared to wt mice, consistent with these previous studies. Also PA28-/- mice showed slightly diminished frequencies of CD8 T cells in comparison to wt B6 mice, but no clear differences were discernible between β5i/LMP7+β2i/MECL-1-/- and PA28+β5i/LMP7+β2i/MECL-1-/- mice (Fig. 3A). Frequencies of splenic CD4 T cells were similar in wt, PA28-/- and PA28+β5i/LMP7+β2i/MECL-1-/- mice, whereas in β5i/LMP7+β2i/MECL-1-/- mice the numbers of CD4 T cells were slightly decreased (not shown). Expressed as ratios, we detected increased CD4:CD8 ratios in β5i/LMP7+β2i/MECL-1-/- and PA28+β5i/LMP7+β2i/MECL-1-/- mice in comparison to wt or PA28-deficient mice, both in the spleen (Fig. 3B) and lymph nodes (not shown), whereas CD4:CD8 T cell ratios in the thymus were similar in all groups (Fig. 3B), consistent with Caudill et al. [29]. Thus, the increased CD4:CD8 T cell ratios in mice deficient for the immunosubunits (and PA28) are only found in the secondary lymphoid organs. They probably are caused by effects of β2i/MECL-1 on homeostatic expansion [35], and are not affected by the presence or absence of PA28.
Figure 3. Effects of PA28 and immunosubunits on CD4:CD8 T cell ratios in lymphoid tissues of uninfected mice.
(A) CD8 T cells were detected in the spleens of B6 wt, PA28-/-, β5i/LMP7+β2i/MECL-1-/- and PA28+β5i/LMP7+β2i/MECL-1-/- mice by staining with anti-CD8α mAb and FACS analysis. Frequencies of CD8 T cells as percentage of total lymphocytes are shown (means plus SEM, n=3). (B) CD4:CD8 T cell ratios in spleen and thymus of B6 wt, PA28-/-, β5i/LMP7+β2i/MECL-1-/- and PA28+β5i/LMP7+β2i/MECL-1-/- mice. Data are representative for two independent experiments (means plus SEM; n=3).
Both PA28 and immunosubunits play an important but additive role in the generation of MHC class I ligands
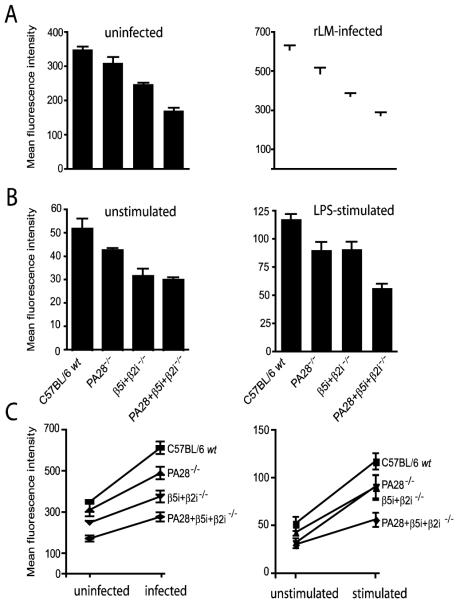
H2b cells that lack PA28 or β5i/LMP7 expression display diminished amounts of cell surface MHC class I molecules, indicating that both these proteasome components play an important role in the production of MHC class I ligands. [18][26][29][35][33][37]. As PA28 increases the production of only a subset of the peptides that are liberated by proteasomes (Fig. 1) and immunosubunit incorporation changes the proteasomal cleavage profile to support the production of MHC class I ligands [18][29][35], it is possible that PA28 further enhances the positive effects of the immunosubunits on MHC class I antigen processing. To address this possibility, we compared the MHC class I cell surface levels on splenocytes of wt and the different proteasome component-deficient mice. As expected, we found that defective immunosubunit expression resulted in reduced cell surface expression of MHC class I H2-Kb and Db molecules on splenocytes, such as B cells (Fig. 4A) and T cells (not shown), and also on BM DC (not shown). The absence of PA28 led to a reduction of MHC class I cell surface expression on immunoproteasome-positive splenic B cells, for H2-Kb to 14,3 % of that on wt cells and, surprisingly, reduced the already diminished MHC class I cell surface expression on β5i/LMP7+β2i/MECL-1-/- cells even further (Fig. 4), with 18,8 % (Table 1). These similar reductions in MHC class I cell surface levels for PA28-deficient cells with and without immunoproteasomes argue against a compounded role of PA28 and immunosubunits in MHC class I antigen processing. Thus, PA28 enhances the generation of MHC class I-binding peptides by constitutive and immunoproteasomes equally.
Figure 4. Role of immunosubunits and PA28 in the production of MHC class I ligands.
(A) Splenocytes of uninfected mice and mice, infected 8 days earlier with rLM, were stained with anti-B220 and a conformation-dependent anti-H-2Kb mAb. (B) Unstimulated and LPS-stimulated splenocytes of uninfected mice were stained with anti-CD19 and anti-H-2Kb mAbs. MFI of H-2Kb staining on B cells are shown (means plus SEM; n=3). (C) Upregulation of MHC class I H-2Kb cell surface levels on B220+ splenocytes of rLM-infected mice compared to uninfected mice (left panel) or on LPS-stimulated compared to unstimulated B220+ splenocytes (right panel). MFI are shown (means plus SD; n=2-4).
Table 1.
Reduced MHC class I expression on lymphoid cells lacking PA28.
| wt | PA28-/- | β5i+β2i -/- | PA28+β5i+β2i -/- | |
|---|---|---|---|---|
| Kb (unstim) | 100 | 85,7a | 100 | 81,2 |
| Kb (LPS) | 100 | 74,4 | 100 | 72,0 |
| Kb (inf) | 100 | 80 | 100 | 74,5 |
| Db (unstim) | 100 | 94,1 | 100 | 88,6 |
| Db (LPS) | 100 | 78,4 | 100 | 69,8 |
| Db (inf) | 100 | 91,6 | 100 | 82,5 |
Relative amounts of MHC class I H2-Kb or Db molecules on PA28-deficient or PA28+β5i/LMP7+β2iMECL-1-deficient unstimulated (unstim) or LPS-stimulated B cells of uninfected or infected (inf) mice, expressed as percentage of H2-Kb or Db expression on similarly treated B cells of wt or β5i/LMP7+β2iMECL-1-deficient mice. MHC class I expression was measured by immunofluorescence staining and FACS analysis. Data represent mean values of 2-4 experiments with 3-5 mice per group.
Listeria monocytogenes, administered i.v., infects the mouse spleen and liver, leading to the recruitment of immune effector cells and cytokine secretion in these tissues. This, in turn, enhances the expression of the components of the MHC class I antigen processing pathway [38] [39] and therewith the demand for MHC class I ligands. To determine whether a potentially compounded role of PA28 and immunosubunits in antigenic peptide production is detectable under such conditions, we analyzed MHC class I cell surface expression on splenic B cells of the different gene-deficient mice, 8 days after i.v. infection with recombinant Listeria. Indeed, the mean fluorescence channels detected for MHC class I (Kb) on splenocytes of rLM-infected mice were consistently higher than these on cells of uninfected mice, measured in the same experiment (Fig. 4A). Upregulation of MHC class I expression was detected for all mouse groups. Thus, despite the lack of expression of immunosubunits and/or PA28, cells are able to upregulate MHC class I cell surface expression in response to external stimuli, although wt B cells upregulated MHC class I markedly more efficiently than either PA28-/-, β5i/LMP7+β2i/MECL-1-/- or PA28+β5i/LMP7+β2i/MECL-1-deficient cells (Fig. 4C). Consistent with the observations on splenocytes of uninfected mice, PA28-deficiency diminished MHC class I cell surface expression on both immunoproteasome positive and -deficient cells, again to approximately similar extents (Fig. 4A and Table 1). To further verify these findings, MHC class I expression on splenocytes stimulated with LPS in vitro was compared to that on unstimulated B cells, analyzed ex vivo. As shown in Figs. 4B and 4C, LPS-treatment led to a similar upregulation of MHC class I Kb and Db (not shown) cell surface expression as cytokine exposure, with the highest levels reached for cells of wt mice and the lowest upregulation observed on cells lacking both the immunosubunits and PA28. Again, PA28-deficiency reduced the relative quantities of H2Kb and Db molecules on immunoproteasome-positive and immunoproteasome-deficient cells similarly (Fig. 4B, Table 1). The cell surface levels of MHC class II and CD19 molecules were similar for gene-deficient and wt cells (not shown), indicating that PA28 does not interfere with cell surface trafficking of glycoproteins in general. Furthermore, H2-Kb levels on immunosubunit and PA28-deficient LPS blasts were restored upon overnight incubation with synthetic ova257-264 peptide (not shown), indicating that overall generation of MHC class I molecules was not impaired, but that the reduced expression of Kb (and Db) in mice deficient for one or more of the inducible components of the proteasome must be attributed to defects in the supply of peptides that bind MHC class I with high affinity. Taken together, these data show that both the immunosubunits and PA28 play an important role in proteasome-mediated production of high affine MHC class I ligands and that PA28 potentiates the production of MHC class I ligands by immunoproteasomes and constitutive proteasomes similarly. Moreover, the effects of PA28 and the immunosubunits are additive, indicating that they use different, largely non-overlapping mechanisms to enhance proteasome-mediated generation of MHC class I ligands.
PA28-deficiency has no dramatic effects on pathogen-specific CD8 T cell responses
While the proteasome immunosubunits are known to shape the immunodominance hierarchy of pathogen-specific CD8 T cell responses [1][4][40][41], the effects of PA28 on the specificity of CD8 T cell responses are less clear. To determine whether PA28-deficiency diminishes the ability of wt or β5i/LMP7+β2i/MECL-1-deficient mice to mount T cell responses, control B6, PA28-/-, β5i/LMP7+β2i/MECL-1-/- and PA28+β5i/LMP7+β2i/MECL-1-/- mice were infected with a recombinant Listeria monocytogenes strain secreting the p60E1 model antigen (rLM-E1) [1], with rLM-ova [42] or with Influenza virus strain HKx31. Quantification of CD8 T cell responses to rLM epitopes in the spleen, 8 days after infection, showed that rLM-E1-infected mice lacking β5i/LMP7 and β2i/MECL-1 failed to respond to the immunoproteasome dependent epitope E1B192-200 (Fig. 5A), consistent with our previous data [1]. In contrast, mice that lacked PA28 but expressed the immunosubunits mounted clearly detectable E1B192-200-specific responses (Fig. 5A) that did not significantly differ in magnitude from the responses detected in wt mice (Mann Whitney, two tailed, 95% C.I). Thus, PA28 does not play a critical role in processing of the immunoproteasome-dependent epitope E1B192-200. After rLM-ova-infection, all mice responded vigorously to the immunoproteasome-independent ova257-264 epitope (Fig. 5C), with insignificant difference in frequencies of ova-specific CD8 T cells between PA28-deficient and PA28-positive mouse groups. Thus, PA28 is not required for the activation of ova-specific CD8 T cells. In these experiments, frequencies of CD4 T cells responding to the listeriolysin O–derived epitope LLO189-201 were similar in all mouse groups, indicating all mice had been infected and responses to this CD4 T cell epitope were not affected by the absence or presence of immunosubunits and/or PA28 (Fig. 5A, S1 and not shown).
Figure 5. Effects of PA28 and immunosubunits on T cell responses to rLM- and Influenza-derived antigens.
B6 wt, PA28-/-, β5i/LMP7+β2i/MECL-1-/- and PA28+β5i/LMP7+β2i/MECL-1-/- mice were infected with 5×103 rLm-E1 (A), 5×104 U Influenza/HKx31 (B) or 1×105 rLM-ova (C) or immunized i.v. with PA224-233-pulsed BM DC (D). (A, C) Eight days after rLM-infection, frequencies of E1B192-200- or ova257-264 -specific CD8 T cells or LLO189-201-specific CD4 T cells were determined in the spleen by staining for CD8 or CD4 and intracellular IFNγ. B, Percentages of NP366-374 and PA224-233 specific CD8 T cells in the BAL, seven days after Influenza infection. D, Percentages of PA224-233-specific CD8 T cells in the spleen, seven days after injection of peptide-pulsed DC. Background responses measured in the absence of stimulating peptides were subtracted. Data are representative for 2-4 experiments. Means plus SEM (A-C) or values for individual mice (D) are shown.
Responses to the Influenza HKx31-derived NP366-374 and PA224-233 epitopes were quantified 7 days after infection, in the bronchioalveolar lavage (BAL) (Fig. 5B). While all mice responded to NP366-374, we failed to detect PA224-233–specific CD8 T cell responses in mice deficient for β5i/LMP7+β2i/MECL-1, as expected [40]. The absence of a PA224-233–specific response could not be explained by defects in the T cell repertoire, as β5i/LMP7+β2i/MECL-1-deficient mice showed PA224-233–specific CD8 T cell responses comparable to wt mice that were immunized with GM-CSF expanded BM-derived DCs loaded with synthetic PA224-233 peptide (Fig. 5D). PA28-/- mice showed a robust response to PA224-233 comparable to the response in wt B6 mice.
Taken together, we find that in contrast to the immunosubunits, PA28 has no dramatic qualitative effects on the size of CD8 T cell responses mounted against several dominant Influenza- and rLM-derived epitopes.
Discussion
Stimulation of cells with pro-inflammatory cytokines or IFNγ upregulates the expression of the proteasome immunosubunits and of PA28. While the immunosubunits are known to play an important role in epitope processing and to shape the immunodominance hierarchy of pathogen-specific CD8 T cell responses, the impact of PA28 on immune defense still remains enigmatic. We here demonstrate that both the proteasome immunosubunits and PA28 fulfill a central function in the production of MHC class I ligands in vivo. Importantly, we find that spleen-derived lymphoid cells of mice that are gene-deficient for PA28 display lower MHC class I expression levels than wt mice, and observe a similar reduction in MHC class I levels on cells of PA28+β5i/LMP7+β2i/MECL-1-deficient mice, in comparison to mice gene deficient for the immunosubunits only (Fig. 4, Table 1). These differences in MHC class I cell surface levels become even more apparent after infection of the different mouse strains with rLM, a pathogen that targets the spleen (Fig. 4) and induces the secretion of Th1 cytokines, or after stimulation of B cells from uninfected wt or proteasome component-deficient mice with LPS, in cell culture (Fig. 4). Thus, PA28 plays an important role in the production of MHC class I binding peptides in lymphoid cells. Thereby, in quantitative terms, PA28 is the second most important component of the proteasome system that contributes to the production of MHC class I ligands, surpassed only by β5i/LMP7, whose absence is responsible for the reduction in class I levels observed in mice that lack β5i/LMP7+β2i/MECL-1 [29][35]. Although β2i/MECL-1 (and β1i/LMP2) play an important role in the processing of a variety of epitopes [35][36][43], their effects are not substantial enough to influence overall MHC class I expression [35]. Thus, both the proteasome immunosubunits and PA28 should be considered as integral components of the MHC class I antigen processing machinery. They play an important role in the production of high-affinity peptides required to maintain the basal MHC class I cell surface levels on lymphoid cells, as well as in providing additional ligands to stabilize newly synthesized MHC class I molecules following cytokine exposure.
The finding that PA28-deficiency leads to reduced MHC class I cell surface levels both on immunoproteasome-positive and -negative cells, on which MHC expression is diminished already due to the absence of immunosubunits (Fig. 4), indicates that the effects of the immunosubunits and PA28 are additive. Thus, these proteasome components enhance the production of different MHC class I ligands. This is consistent with the different working mechanisms of PA28 and the catalytic immunosubunits. PA28 binds to the 20S complex, leading to coordinated double cleavages and apparently thereby an enhanced generation of MHC class I ligands [23], whereas the immunosubunits change the 20S cleavage site preferences. In particular the replacement of the constitutive subunit β5 by β5i/LMP7 leads to enhanced cleavage at the C-terminus of hydrophobic residues, which are critical anchors for binding to MHC class I molecules [33]. In addition, enhanced proteolytic activity in the presence of immunoproteasomes may lead to enhanced antigenic peptide generation [21], although our previous studies failed to show any differences in kinetics of presentation of two immunoproteasome-independent epitopes between rLM-infected β5i/LMP7+β2i/MECL-1-deficient and -sufficient DC in vitro [1].
Remarkably, deficiency of PA28+β5i/LMP7+β2i/MECl-1 reduces MHC class I expression only to approximately 53 % on unstimulated cells compared to wt and these levels can still be upregulated by exposure to LPS or cytokines. Thus, IFNγ-induced expression of other components of the antigen processing pathway such as of β1i/LMP2 (which however poorly assembles into 20S complexes in the absence of β5i/LMP7 [45]) or of aminopeptidases may lead to the production of additional peptides that stabilize the newly synthesized MHC class I molecules on stimulated PA28+β5i/LMP7+β2i/MECL-1-deficient cells. Alternatively, the increase in numbers of cell surface MHC class I /peptide complexes may result from enhanced synthesis of MHC class I heavy chains and thus a renewed balance between gain and loss of MHC class I molecules, complexed with low affinity peptides, from the cell surface. In support of this, addition of synthetic ova257-264 to LPS blast cultures restores the H2-Kb levels on PA28+β5i/LMP7+β2i/MECL-1-deficient cells to nearly wt levels (not shown), indicating that MHC class I molecules traffick to the cell surface also in cells that lack these proteasome components.
Importantly, in contrast to the profound effects of immunosubunits on the generation of specific epitopes and the fine-specificity of pathogen-specific CD8 T cell responses, the contributions of PA28 to peptide presentation had little impact on the sizes of the responses to Influenza- and rLM-derived epitopes. Taken together, all of four dominant CD8 T cell epitopes tested triggered robust responses in PA28-deficient mice. Previous studies have shown that PA28 enhances 20S-mediated processing of the ovalbumin-derived ova257-264 epitope [27][46]. However, our data do not show any significant differences in responses to this epitope in PA28-deficient compared to PA28-positive mice (Fig. 5). Thus, reduced ova257-264 presentation in the absence of PA28 has no or only minor effects on CD8 T cell activation. This contrasts to the dramatic reduction in size of CD8 T cell responses in immunoproteasome-deficient mice to immunoproteasome-dependent epitopes, of which two (E1B192-200 and PA224-233) were included in this study.
It has been suggested that the hsp90 chaperone provides an alternative pathway for epitope supply to MHC class I molecules in cells that lack PA28 expression, and also in PA28-positive lymphoid cell lines unless stimulated with IFNγ [27]. More recent studies however demonstrated that hsp90 binds proteasome-generated protein fragments to protect these from further degradation, and makes such fragments available for MHC class I loading [47][48]. Thus, hsp90 acts downstream of the proteasome, which was confirmed also for PA28-deficient cells [28]. Also our finding that MHC class I expression levels are reduced on PA28-deficient compared to wt spleen cells implies that any compensatory effects of hsp90 on the peptide pool available for MHC class I binding in the absence of PA28 are partial at most, and is consistent with the notion that the hsp90 pathway does not produce new MHC class I ligands but enhances the presentation of peptides that are produced by the proteasome.
Taken together, we here show that both PA28 and the immunosubunits play an important and additive role in proteasome-mediated processing of intracellular proteins into peptides that bind to MHC class I molecules. The role of PA28 in the production of class I ligands is much greater than expected, based on previous studies [25]. We showed previously that the early kinetics of MHC class I antigen processing following infection are strongly influenced by the immunosubunits and determine the immunogenicity of individual antigenic peptides [1]. Like the immunosubunits, also PA28 enhances MHC class I presentation of specific epitopes (like ova257-264) at early time points after infection. Nevertheless, consistent with previous findings [25], our analyses of rLM and Influenza-specific CD8 T cell responses indicate that the effects of PA28 on antigen processing do not have any dramatic effects on the size and fine specificity of CD8 T cell responses to these pathogens. Remarkably, a recent study showed that, like immunoproteasomes, also PA28 is involved in degradation of oxidized proteins. Therefore, these components of the proteasome system may play a role also beyond antigen processing and immune recognition, in cellular adaptation to oxidative stress [21][48].
Materials and methods
Cells
MEC-PA28 and T2 cells were cultured as described [6][32]. LPS blasts were expanded by stimulation of splenocytes with 30 μg/ml LPS in RPMI with 10% FCS, glutamax, antibiotics and 30 μM β-mercaptoethanol.
Mice
C57BL/6 (B6) mice were purchased from Charles River. B6 β5i/LMP7+β2i/MECL-1 gene-deficient [29] and PA28αβ gene-deficient mice [25] were maintained by in-house breeding. PA28+β5i/LMP7+β2i/MECL-1 gene-deficient mice were generated by crossing PA28-/- with β5i/LMP7+β2i/MECL-1-/- mice. All animal experiments were performed with age-matched mice and approved by the Committee of Animal Experiments of the University of Utrecht.
Infection and immunization of mice
Infections with rLM and immunizations with peptide-pulsed DC were performed as described [1]. For infection with Influenza strain HKx31, mice were anesthetized with isofluorane and then infected i.n. with 5×104 hemagglutination units in 30 μl PBS.
Western blot analysis
Presence of PA28, β5i/LMP7 or β2i/MECL-1 was analyzed by Western blotting as described [6][35].
Antibodies and flow cytometry
MAbs used in this study included fluorochrome- or biotin-conjugated anti-mouse H2-Kb (clone AF6-88.5) and anti-mouse H-2Db (clone 28-14-8), fluorochrome-conjugated anti-mouse CD8α (clone 53-6.7), anti-mouse CD19 (clone MB19-1), anti-mouse CD4 (clone RM 4-5), anti-mouse IFNγ (clone XMG1.2) and anti-mouse B220. Immunofluorescence staining and FACS-analyses of specific cell subsets were performed as described [35].
Intracellular cytokine staining
Mice were sacrificed at the time points specified in the figure legends and spleens or BAL collected. Percentages of E1B192-200-, NP366-374 - or PA224-233 -specific CD8 T cells and LLO189-201-specific CD4 T cells were determined by intracellular cytokine staining and FACS analysis as described [1].
Peptides
The synthetic polypeptide AYRPPNAPILSTLPETTVVRRRGRSPRRRTPS (HBV subtype ayw, cAg amino acids 131–162) was synthesized by Dr. P. Henklein of the Charité, Humboldt University, Berlin.
In vitro digestion assays
PA28 was purified from 2 × 109 MEC-PA28 cells [6], grown in the absence of tetracycline, as described [30]. Constitutive and immuno-20S proteasomes were purified from 6–8×108 T2 and T2 LMP2+7 cells, respectively, as described [32]. The purity of the preparates was checked by Coomassie-stained SDS-PAGE and was >90%.
20 μg of HBV 32-mer polypeptide, 3 μg of purified proteasomes and 1.5 μg of purified PA28 were incubated in 300 μl assay buffer (20 mM Hepes/KOH, pH 7.8, 1 mM dithiothreitol) at 37°C for the time periods specified in the figure legends. Digestions in the absence of PA28 were performed in assay buffer containing 20 mM Hepes/KOH, pH 7.8, 2mM MgAc2, and 1 mM DTT. Samples of the digestion reactions were frozen at −20°C until analysis by RP-HPLC and mass spectrometry, as described [30].
Supplementary Material
Acknowledgements
We thank Dr. J. Monaco of the University of Cincinnati, OH, for the kind gift of the β5i/LMP7+β2i/MECL-1 gene-deficient mice. This work was supported by the Netherlands Organization for Scientific Research (MEERVOUD program) and NIH grant AI064576 (to A.J.A.M.S.).
Abbreviations
- BAL
bronchoalveolar lavage
- HBV cAg
hepatitis B virus core antigen
- LMP
low-molecular-mass polypeptide
- MECL-1
multicatalytic endopeptidase complex-like-1
- PA28
proteasome activator 28 kDalton
- rLM
recombinant Listeria monocytogenes
Footnotes
Conflict of interest None
References
- 1.Deol P, Zaiss DM, Monaco JJ, Sijts AJ. Rates of processing determine the immunogenicity of immunoproteasome-generated epitopes. J. Immunol. 2007;178:7557–7562. doi: 10.4049/jimmunol.178.12.7557. [DOI] [PubMed] [Google Scholar]
- 2.Probst HC, Tschannen K, Gallimore A, Martinic M, Basler M, Dumrese T, Jones E, van den Broek MF. Immunodominance of an antiviral cytotoxic T cell response is shaped by the kinetics of viral protein expression. J. Immunol. 2003;171:5415–5422. doi: 10.4049/jimmunol.171.10.5415. [DOI] [PubMed] [Google Scholar]
- 3.Deng Y, Yewdell JW, Eisenlohr LC, Bennink JR. MHC affinity, peptide liberation, T cell repertoire, and immunodominance all contribute to the paucity of MHC class I-restricted peptides recognized by antiviral CTL. J. Immunol. 1997;158:1507–1515. [PubMed] [Google Scholar]
- 4.Chen W, Norbury CC, Cho Y, Yewdell JW, Bennink JR. Immunoproteasomes shape immunodominance hierarchies of antiviral CD8(+) T cells at the levels of T cell repertoire and presentation of viral antigens. J. Exp. Med. 2001;193:1319–1326. doi: 10.1084/jem.193.11.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sijts AJ, Standera S, Toes RE, Ruppert T, Beekman NJ, van Veelen PA, Ossendorp FA, et al. MHC class I antigen processing of an adenovirus CTL epitope is linked to the levels of immunoproteasomes in infected cells. J. Immunol. 2000;164:4500–4506. doi: 10.4049/jimmunol.164.9.4500. [DOI] [PubMed] [Google Scholar]
- 6.van Hall T, Sijts A, Camps M, Offringa R, Melief C, Kloetzel PM, Ossendorp F. Differential influence on cytotoxic T lymphocyte epitope presentation by controlled expression of either proteasome immunosubunits or PA28. J. Exp. Med. 2000;192:483–494. doi: 10.1084/jem.192.4.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schwarz K, van Den Broek M, Kostka S, Kraft R, Soza A, Schmidtke G, Kloetzel PM, Groettrup M. Overexpression of the proteasome subunits LMP2, LMP7, and MECL-1, but not PA28 alpha/beta, enhances the presentation of an immunodominant lymphocytic choriomeningitis virus T cell epitope. J. Immunol. 2000;165:768–778. doi: 10.4049/jimmunol.165.2.768. [DOI] [PubMed] [Google Scholar]
- 8.Coux O, Tanaka K, Goldberg AL. Structure and functions of the 20S and 26S proteasomes. Annu. Rev. Biochem. 1996;65:801–847. doi: 10.1146/annurev.bi.65.070196.004101. [DOI] [PubMed] [Google Scholar]
- 9.Shin EC, Seifert U, Kato T, Rice CM, Feinstone SM, Kloetzel PM, Rehermann B. Virus-induced type I IFN stimulates generation of immunoproteasomes at the site of infection. J. Clin. Invest. 2006;116:3006–3014. doi: 10.1172/JCI29832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hallermalm K, Seki K, Wei C, Castelli C, Rivoltini L, Kiessling R, Levitskaya J. Tumor necrosis factor-alpha induces coordinated changes in major histocompatibility class I presentation pathway, resulting in increased stability of class I complexes at the cell surface. Blood. 2001;98:1108–1115. doi: 10.1182/blood.v98.4.1108. [DOI] [PubMed] [Google Scholar]
- 11.Nandi D, Jiang H, Monaco JJ. Identification of MECL-1 (LMP-10) as the third IFN-gamma-inducible proteasome subunit. J. Immunol. 1996;156:2361–2364. [PubMed] [Google Scholar]
- 12.Boes B, Hengel H, Ruppert T, Multhaup G, Koszinowski UH, Kloetzel PM. Interferon gamma stimulation modulates the proteolytic activity and cleavage site preference of 20S mouse proteasomes. J. Exp. Med. 1994;179:901–909. doi: 10.1084/jem.179.3.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Realini C, Dubiel W, Pratt G, Ferrell K, Rechsteiner M. Molecular cloning and expression of a gamma-interferon-inducible activator of the multicatalytic protease. J. Biol. Chem. 1994;269:20727–20732. [PubMed] [Google Scholar]
- 14.Ossendorp F, Fu N, Camps M, Granucci F, Gobin SJ, van den Elsen PJ, Schuurhuis D, et al. Differential expression regulation of the alpha and beta subunits of the PA28 proteasome activator in mature dendritic cells. J. Immunol. 2005;174:7815–7822. doi: 10.4049/jimmunol.174.12.7815. [DOI] [PubMed] [Google Scholar]
- 15.Soza A, Knuehl C, Groettrup M, Henklein P, Tanaka K, Kloetzel PM. Expression and subcellular localization of mouse 20S proteasome activator complex PA28. FEBS Lett. 1997;413:27–34. doi: 10.1016/s0014-5793(97)00864-8. [DOI] [PubMed] [Google Scholar]
- 16.Macagno A, Gilliet M, Sallusto F, Lanzavecchia A, Nestle FO, Groettrup M. Dendritic cells up-regulate immunoproteasomes and the proteasome regulator PA28 during maturation. Eur. J. Immunol. 1999;29:4037–4042. doi: 10.1002/(SICI)1521-4141(199912)29:12<4037::AID-IMMU4037>3.0.CO;2-T. 2-T. [DOI] [PubMed] [Google Scholar]
- 17.Sijts A, Sun Y, Janek K, Kral S, Paschen A, Schadendorf D, Kloetzel PM. The role of the proteasome activator PA28 in MHC class I antigen processing. Mol. Immunol. 2002;39:165–169. doi: 10.1016/s0161-5890(02)00099-8. [DOI] [PubMed] [Google Scholar]
- 18.Fehling HJ, Swat W, Laplace C, Kuhn R, Rajewsky K, Muller U, von Boehmer H. MHC class I expression in mice lacking the proteasome subunit LMP-7. Science. 1994;265:1234–1237. doi: 10.1126/science.8066463. [DOI] [PubMed] [Google Scholar]
- 19.Sijts A, Zaiss D, Kloetzel PM. The role of the ubiquitin-proteasome pathway in MHC class I antigen processing: Implications for vaccine design. Curr. Mol. Med. 2001;1:665–676. doi: 10.2174/1566524013363230. [DOI] [PubMed] [Google Scholar]
- 20.Nussbaum AK, Rodriguez-Carreno MP, Benning N, Botten J, Whitton JL. Immunoproteasome-deficient mice mount largely normal CD8+ T cell responses to lymphocytic choriomeningitis virus infection and DNA vaccination. J. Immunol. 2005;175:1153–1160. doi: 10.4049/jimmunol.175.2.1153. [DOI] [PubMed] [Google Scholar]
- 21.Seifert U, Bialy LP, Ebstein F, Bech-Otschir D, Voigt A, Schroter F, Prozorovski T, et al. Immunoproteasomes preserve protein homeostasis upon interferon-induced oxidative stress. Cell. 2010;142:613–624. doi: 10.1016/j.cell.2010.07.036. [DOI] [PubMed] [Google Scholar]
- 22.Zhang Z, Krutchinsky A, Endicott S, Realini C, Rechsteiner M, Standing KG. Proteasome activator 11S REG or PA28: Recombinant REG alpha/REG beta hetero-oligomers are heptamers. Biochemistry. 1999;38:5651–5658. doi: 10.1021/bi990056+. [DOI] [PubMed] [Google Scholar]
- 23.Dick TP, Ruppert T, Groettrup M, Kloetzel PM, Kuehn L, Koszinowski UH, Stevanovic, et al. Coordinated dual cleavages induced by the proteasome regulator PA28 lead to dominant MHC ligands. Cell. 1996;86:253–262. doi: 10.1016/s0092-8674(00)80097-5. [DOI] [PubMed] [Google Scholar]
- 24.Shimbara N, Nakajima H, Tanahashi N, Ogawa K, Niwa S, Uenaka A, Nakayama E, Tanaka K. Double-cleavage production of the CTL epitope by proteasomes and PA28: Role of the flanking region. Genes Cells. 1997;2:785–800. doi: 10.1046/j.1365-2443.1997.1610359.x. [DOI] [PubMed] [Google Scholar]
- 25.Murata S, Udono H, Tanahashi N, Hamada N, Watanabe K, Adachi K, Yamano T, et al. Immunoproteasome assembly and antigen presentation in mice lacking both PA28alpha and PA28beta. EMBO J. 2001;20:5898–5907. doi: 10.1093/emboj/20.21.5898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yamano T, Sugahara H, Mizukami S, Murata S, Chiba T, Tanaka K, Yui K, Udono H. Allele-selective effect of PA28 in MHC class I antigen processing. J. Immunol. 2008;181:1655–1664. doi: 10.4049/jimmunol.181.3.1655. [DOI] [PubMed] [Google Scholar]
- 27.Yamano T, Murata S, Shimbara N, Tanaka N, Chiba T, Tanaka K, Yui K, Udono H. Two distinct pathways mediated by PA28 and hsp90 in major histocompatibility complex class I antigen processing. J. Exp. Med. 2002;196:185–196. doi: 10.1084/jem.20011922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gonciarz-Swiatek M, Rechsteiner M. Proteasomes and antigen presentation: Evidence that a KEKE motif does not promote presentation of the class I epitope SIINFEKL. Mol. Immunol. 2006;43:1993–2001. doi: 10.1016/j.molimm.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 29.Caudill CM, Jayarapu K, Elenich L, Monaco JJ, Colbert RA, Griffin TA. T cells lacking immunoproteasome subunits MECL-1 and LMP7 hyperproliferate in response to polyclonal mitogens. J. Immunol. 2006;176:4075–4082. doi: 10.4049/jimmunol.176.7.4075. [DOI] [PubMed] [Google Scholar]
- 30.Sun Y, Sijts AJ, Song M, Janek K, Nussbaum AK, Kral S, Schirle M, et al. Expression of the proteasome activator PA28 rescues the presentation of a cytotoxic T lymphocyte epitope on melanoma cells. Cancer Res. 2002;62:2875–2882. [PubMed] [Google Scholar]
- 31.Kuckelkorn U, Ferreira EA, Drung I, Liewer U, Kloetzel PM, Theobald M. The effect of the interferon-gamma-inducible processing machinery on the generation of a naturally tumor-associated human cytotoxic T lymphocyte epitope within a wild-type and mutant p53 sequence context. Eur. J. Immunol. 2002;32:1368–1375. doi: 10.1002/1521-4141(200205)32:5<1368::AID-IMMU1368>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 32.Sijts AJ, Ruppert T, Rehermann B, Schmidt M, Koszinowski U, Kloetzel PM. Efficient generation of a hepatitis B virus cytotoxic T lymphocyte epitope requires the structural features of immunoproteasomes. J. Exp. Med. 2000;191:503–514. doi: 10.1084/jem.191.3.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Toes RE, Nussbaum AK, Degermann S, Schirle M, Emmerich NP, Kraft M, Laplace C, et al. Discrete cleavage motifs of constitutive and immunoproteasomes revealed by quantitative analysis of cleavage products. J. Exp. Med. 2001;194:1–12. doi: 10.1084/jem.194.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.De M, Jayarapu K, Elenich L, Monaco JJ, Colbert RA, Griffin TA. Beta 2 subunit propeptides influence cooperative proteasome assembly. J. Biol. Chem. 2003;278:6153–6159. doi: 10.1074/jbc.M209292200. [DOI] [PubMed] [Google Scholar]
- 35.Zaiss DM, de Graaf N, Sijts AJ. The proteasome immunosubunit multicatalytic endopeptidase complex-like 1 is a T-cell-intrinsic factor influencing homeostatic expansion. Infect. Immun. 2008;76:1207–1213. doi: 10.1128/IAI.01134-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Basler M, Moebius J, Elenich L, Groettrup M, Monaco JJ. An altered T cell repertoire in MECL-1-deficient mice. J. Immunol. 2006;176:6665–6672. doi: 10.4049/jimmunol.176.11.6665. [DOI] [PubMed] [Google Scholar]
- 37.Preckel T, Fung-Leung WP, Cai Z, Vitiello A, Salter-Cid L, Winqvist O, Wolfe TG, et al. Impaired immunoproteasome assembly and immune responses in PA28-/- mice. Science. 1999;286:2162–2165. doi: 10.1126/science.286.5447.2162. [DOI] [PubMed] [Google Scholar]
- 38.Strehl B, Joeris T, Rieger M, Visekruna A, Textoris-Taube K, Kaufmann SH, Kloetzel PM, et al. Immunoproteasomes are essential for clearance of listeria monocytogenes in nonlymphoid tissues but not for induction of bacteria-specific CD8+ T cells. J. Immunol. 2006;177:6238–6244. doi: 10.4049/jimmunol.177.9.6238. [DOI] [PubMed] [Google Scholar]
- 39.Khan S, van den Broek M, Schwarz K, de Giuli R, Diener PA, Groettrup M. Immunoproteasomes largely replace constitutive proteasomes during an antiviral and antibacterial immune response in the liver. J. Immunol. 2001;167:6859–6868. doi: 10.4049/jimmunol.167.12.6859. [DOI] [PubMed] [Google Scholar]
- 40.Pang KC, Sanders MT, Monaco JJ, Doherty PC, Turner SJ, Chen W. Immunoproteasome subunit deficiencies impact differentially on two immunodominant influenza virus-specific CD8+ T cell responses. J. Immunol. 2006;177:7680–7688. doi: 10.4049/jimmunol.177.11.7680. [DOI] [PubMed] [Google Scholar]
- 41.Basler M, Youhnovski N, Van Den Broek M, Przybylski M, Groettrup M. Immunoproteasomes down-regulate presentation of a subdominant T cell epitope from lymphocytic choriomeningitis virus. J. Immunol. 2004;173:3925–3934. doi: 10.4049/jimmunol.173.6.3925. [DOI] [PubMed] [Google Scholar]
- 42.Foulds KE, Zenewicz LA, Shedlock DJ, Jiang J, Troy AE, Shen H. Cutting edge: CD4 and CD8 T cells are intrinsically different in their proliferative responses. J. Immunol. 2002;168:1528–1532. doi: 10.4049/jimmunol.168.4.1528. [DOI] [PubMed] [Google Scholar]
- 43.Van Kaer L, Ashton-Rickardt PG, Eichelberger M, Gaczynska M, Nagashima K, Rock KL, Goldberg AL, et al. Altered peptidase and viral-specific T cell response in LMP2 mutant mice. Immunity. 1994;1:533–541. doi: 10.1016/1074-7613(94)90043-4. [DOI] [PubMed] [Google Scholar]
- 44.Griffin TA, Nandi D, Cruz M, Fehling HJ, Kaer LV, Monaco JJ, Colbert RA. Immunoproteasome assembly: Cooperative incorporation of interferon gamma (IFN-gamma)-inducible subunits. J. Exp. Med. 1998;187:97–104. doi: 10.1084/jem.187.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Niedermann G, Grimm R, Geier E, Maurer M, Realini C, Gartmann C, Soll J, et al. Potential immunocompetence of proteolytic fragments produced by proteasomes before evolution of the vertebrate immune system. J. Exp. Med. 1997;186:209–220. doi: 10.1084/jem.186.2.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kunisawa J, Shastri N. Hsp90alpha chaperones large C-terminally extended proteolytic intermediates in the MHC class I antigen processing pathway. Immunity. 2006;24:523–534. doi: 10.1016/j.immuni.2006.03.015. [DOI] [PubMed] [Google Scholar]
- 47.Srivastava PK, Udono H, Blachere NE, Li Z. Heat shock proteins transfer peptides during antigen processing and CTL priming. Immunogenetics. 1994;39:93–98. doi: 10.1007/BF00188611. [DOI] [PubMed] [Google Scholar]
- 48.Pickering AM, Koop AL, Teoh CY, Ermak G, Grune T, Davies KJA. The immunoproteasome, the 20S proteasome and PA28αβ proteasome regulator are oxidative-stress-adaptive proteolytic complexes. Biochem. J. doi: 10.1042/BJ20100878. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.