Abstract
Objective
To characterize the course of cognitive decline during the prodromal phase of Alzheimer's disease.
Design
Longitudinal cohort study with up to 16 years of observation.
Participants
Older persons from two projects underwent annual clinical evaluations that included cognitive function testing and clinical classification of mild cognitive impairment, dementia, and Alzheimer's disease. At baseline, there were 2,071 individuals without dementia and 1,511 without cognitive impairment.
Main Outcome Measures
Change in previously established composite measures of global cognition and specific cognitive domains assessed in mixed-effects models that allow rate of decline to shift at specific points.
Results
During follow-up, 462 persons developed Alzheimer's disease (20 with dementia solely due to another condition were excluded). Five to six years before the diagnosis, rate of global cognitive decline sharply accelerated by more than 15-fold. The acceleration in decline occurred slightly earlier for semantic memory (76 months before diagnosis) and working memory (75 months) than other cognitive functions. Mild cognitive impairment was also preceded by years of cognitive decline which began earlier (80 months before diagnosis) and proceeded more rapidly (annual loss of 0.102 unit) in the amnestic than nonamnestic (62 months, 0.072 unit) subtype.
Conclusion
Dementia due to Alzheimer's disease is preceded by about five to six years of accelerated decline in multiple cognitive functions. By contrast, little decline is evident in persons not developing Alzheimer's disease.
Dementia due to Alzheimer's disease (AD), by definition, is preceded by a minimum of six months of cognitive decline. With recognition of the importance of developing strategies to delay the onset of AD symptoms [1,2], scientific interest in the prodromal cognitive changes has increased. There have been relatively few prospective longitudinal studies of this prodromal phase of the disease [3-9], however, and estimates of its duration range from about 1 year [6] to more than 10 years [5]. Several factors are probably contributing to this variability. One issue is that most studies have had less than 100 subjects with incident disease [3,7-9]. In addition, prodromal changes may occur earlier in some cognitive domains than others [6,8]. Studies also likely differ in where the cutpoint between normality and dementia is placed.
Here we examine change in cognitive function during the prodromal phase of AD and before the onset of mild cognitive impairment (MCI), a common precursor to dementia in AD. Data are from two longitudinal cohort studies, the Religious Orders Study [10] and the Rush Memory and Aging Project [11]. For up to 16 years, more than 2,000 participants underwent annual clinical evaluations that included detailed testing of cognitive function and clinical classification of MCI, dementia, and AD. In mixed-effects models that allowed rate of cognitive change to shift, we characterized change in global and specific measures of cognition in the years preceding and following dementia onset in AD and conducted similar analyses of cognitive change before and after incidence of MCI.
Methods
Participants
Subjects are from two ongoing longitudinal studies, the Religious Orders Study which began in 1994 and the Rush Memory and Aging Project which began in 1997. Both studies involve annual clinical evaluation and brain donation at death and were approved by the Institutional Review Board of Rush University Medical Center.
At the time of these analyses, 2, 323 individuals had completed the baseline evaluations in these studies and been found to be without dementia. Of these, 62 died before follow-up and 40 had been in the study less than one year. This left 2,221 individuals eligible for follow-up and 2,071 (93.2%) had follow-up data. The first set of analyses are based on this group. They completed a mean of 7.4 annual evaluations (SD=3.8). At baseline, they had a mean age of 77.4 (SD=7.7), a mean of 16.2 years of education (SD=3.8), and a mean score of 28.2 (SD=1.9) on the Mini-Mental State Examination; 72.0% were woman and 87.9% were white and non-Hispanic. A second set of analyses focused on a subset of this group: 1,511 persons without MCI or dementia at baseline. They completed a mean of 7.8 annual evaluations (SD=3.9) and had a mean baseline age of 76.3 (SD=7.4) and mean of 16.4 years of education (SD=3.8); 72.8% were women and 90.7% were white and non-Hispanic.
Clinical Evaluation
At baseline and annually thereafter, subjects in each study underwent a uniform structured clinical evaluation that included a medical history, complete neurological examination, and cognitive performance testing. Based on these data and in person evaluation of the subject, an experienced clinician diagnosed dementia and AD using the criteria of the joint working group of the National Institute of Neurologic and Communicative Disorders and Stroke and the Alzheimer's Disease and Related Disorders Association [12]. The criteria require a history of cognitive decline and impairment in at least two cognitive domains, one of which must be memory for a diagnosis of AD. As previously described [13], impairment in 5 cognitive functions (orientation, attention, memory, language, visuospatial ability) was determined in a 2-step process. First, an algorithm rated impairment in each area of function based on educationally adjusted cutoff scores on 11 individual tests [14]. Second, based on all test data and information on education, sensorimotor problems, and effort, a neuropsychologist agreed or disagreed with each rating, and supplied a new rating in the event of disagreement. Persons who had cognitive impairment but did not meet criteria for dementia were classified as MCI and divided into amnestic (i.e. with episodic memory impaired) and nonamnestic subtypes, as previously described [15]. Individuals meeting these criteria for MCI have been found to have intermediate levels of mortality [14,16], cognitive decline [14,17,18], and plaques, tangles, and cerebral infarction [19] relative to persons without cognitive impairment and dementia. All clinical classification was done blinded to previously collected data.
Assessment of Cognitive Function
Nineteen cognitive performance tests were administered in each study. The Mini-Mental State Examination was used for descriptive purposes and Complex Ideational Material was used only in clinical classification. Analyses were based on the remaining 17 tests. There were 7 measures of episodic memory: Word List Memory, Recall, and Recognition plus immediate and delayed recall of the East Boston story and story A from Logical Memory; 3 measures of semantic memory: verbal fluency, a 15-item form of the Boston Naming Test, and a measure of reading recognition; 3 measures of working memory: Digit Span Forward, Digit Span Backward, and Digit Ordering; 2 measures of perceptual speed: Number Comparison and Symbol Digit Modalities Test; and 2 measures of visuospatial ability: short forms of Judgment of Line Orientation and Standard Progressive Matrices. The primary outcome was a composite measure of global cognition using all 17 individual measures. Raw scores on each of the 17 measures were converted to z scores using the baseline mean and SD of all subjects, and z scores were then averaged to yield the composite measure of global cognition. Based in part on previous factor analyses of these cognitive measures in these [20-22] and other [23,24] cohorts, individual cognitive measures were grouped into specific cognitive domains. Within each domain, individual z scores were averaged to yield composite scores that were used in secondary analyses: episodic memory (based on 7 individual measures), semantic memory (3 measures), working memory (3 measures), perceptual speed (2 measures), and visuospatial ability (2 measures). Further information on the individual tests and the derivation of the composite scores is contained in previous publications [20-22].
Data Analysis
We used mixed-effects regression models [25] to characterize paths of change in cognitive function. The models include change points that allowed rate of cognitive change to shift at some time before AD (or MCI) was diagnosed and again at the time of diagnosis. To identify the point before diagnosis when rate of cognitive change shifted, we constructed a series of models with change points ranging from a few months to several years before diagnosis and chose the analysis with the highest log likelihood value, indicating the best model fit. To take advantage of all cognitive data, a composite measure of global cognition was the primary outcome. For AD, we conducted additional analyses excluding those with MCI at baseline and using composite measures of specific cognitive domain as outcomes. For MCI, the core model was followed by separate analyses of MCI subtypes. All analyses controlled for age, sex, and education. Programming was done in SAS [25].
Results
Cognitive Decline in the Prodromal Phase of AD
The 2,071 persons without dementia at study enrollment had up to 16 years of annual follow-up (mean=7.0, SD=4.0). During this period, 462 persons developed incident AD. For these analyses, we excluded the 20 people who developed other forms of dementia. Those who developed AD were older than those who did not (81.3 years versus 76.3, t [845] =14.1, p <.001), but the groups were similar in education (16.4 years versus 16.2, t [2,049] = 1.4, p=.165) and sex distribution (72.7% women versus 71.9%, χ2 [1] =0.1, p=.737).
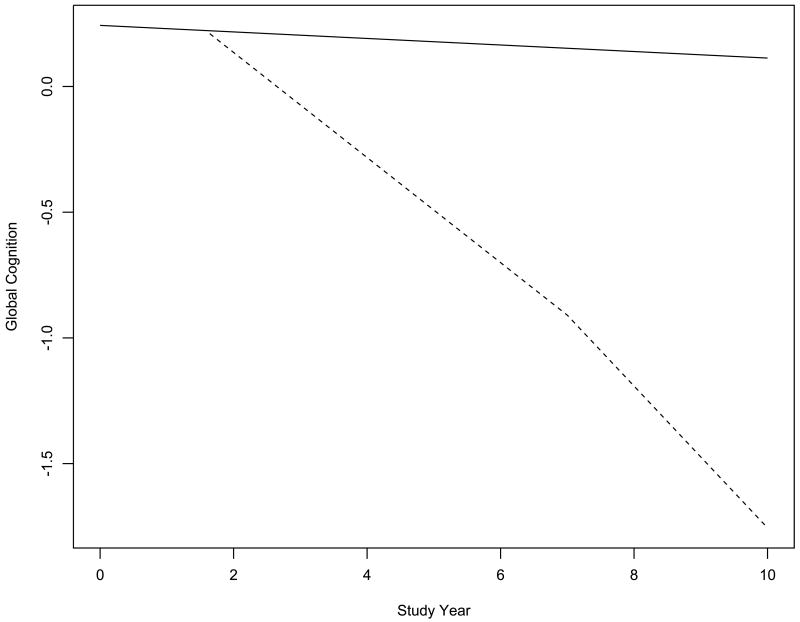
We used mixed-effects models to characterize change in cognitive function before and after AD was clinically diagnosed. To determine if rate of decline accelerated at some point before the diagnosis, we fit a series of models that allowed for a shift in rate of cognitive change during periods ranging from 6 to 120 months before the diagnosis plus a second shift at the time of diagnosis. We used a composite measure of global cognition in initial analyses. At baseline, scores ranged from -1.910 to 1.466 (mean=0.094, SD=0.529), with higher values indicating better function. The best fitting model set the change point at 65 months before the diagnosis. In this model (Table 1), the global cognitive score declined a mean of 0.013-unit per year before the change point and 0.209-unit per year thereafter, a more than 15-fold increase. After AD was diagnosed, there was a further increase of about one third in annual rate of global cognitive decline. Figure 1, which is based on this analysis, shows the predicted 10-year paths of change in global cognition for two typical participants. Little cognitive decline is evident in the person who remained dementia free (solid line). In the person who developed AD (dashed line), the diagnosis in study year 7 was preceded by more than 5 years of accelerated global cognitive decline.
Table 1.
Change in Cognitive Function Before and After Dementia Onset in Alzheimer's Disease*
WB: from rerun.requst12.using_estimate.txt
| Cognitive Outcome | Model Term | Estimate | St.Error | P |
|---|---|---|---|---|
| Global Cognition | Time preceding prodrome | -0.013 | 0.001 | <.001 |
| Time during prodrome | -0.209 | 0.005 | <.001 | |
| Time after dementia onset | -0.282 | 0.014 | <.001 | |
| Episodic Memory | Time preceding prodrome | 0.0003 | 0.002 | .862 |
| Time during prodrome | -0.251 | 0.007 | <.001 | |
| Time after dementia onset | -0.283 | 0.016 | <.001 | |
| Perceptual Speed | Time preceding prodrome | -0.044 | 0.002 | <.001 |
| Time during prodrome | -0.227 | 0.007 | <.001 | |
| Time after dementia onset | -0.265 | 0.014 | <.001 | |
| Working Memory | Time preceding prodrome | -0.020 | 0.001 | <.001 |
| Time during prodrome | -0.120 | 0.005 | <.001 | |
| Time after dementia onset | -0.217 | 0.014 | <.001 | |
| Semantic Memory | Time preceding prodrome | -0.013 | 0.001 | <.001 |
| Time during prodrome | -0.157 | 0.006 | <.001 | |
| Time after dementia onset | -0.281 | 0.018 | <.001 | |
| Visuospatial ability | Time preceding prodrome | -0.013 | 0.001 | <.001 |
| Time during prodrome | -0.181 | 0.007 | <.001 | |
| Time after dementia onset | -0.283 | 0.018 | <.001 |
From separate mixed-effects models adjusted for age, sex, education. Each estimate is the mean annual rate of cognitive change predicted by the model.
Figure 1.
Global Cognitive Decline Before and After Dementia Onset in Alzheimer's Disease. Predicted 10-year paths of decline in global cognition for typical participants who remained dementia free (solid line) or developed AD in study year 7 (dashed line), adjusted for age, sex, and education.
At baseline, 552 subjects had MCI. To assess how this subgroup affected results, we excluded them and repeated the analyses in the remaining 1,499, of whom 217 developed AD. The best fitting change point was at 54 months before AD was diagnosed. Global cognition declined a mean of 0.016 unit per year before this point (SE = 0.001, p<.001). Rate of annual decline increased more than 15-fold to 0.262 unit (SE =0.008, p<.001) during the 54 month prodromal period and to 0.314 unit (SE=0.021, p=<.001) after AD was diagnosed.
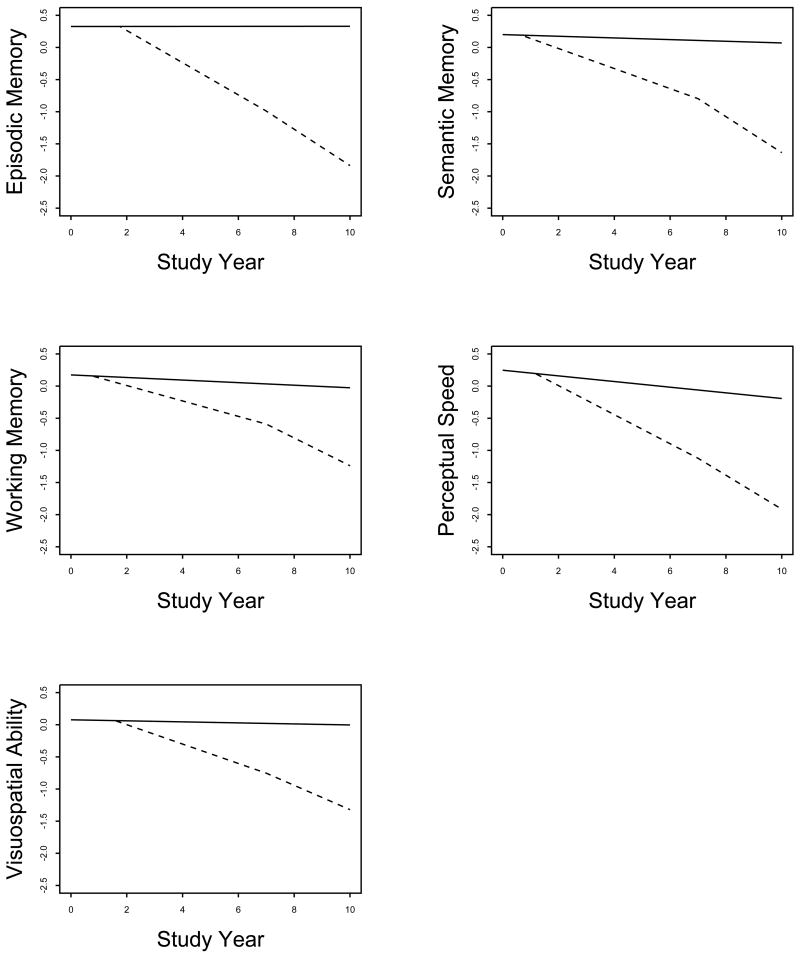
To see if results varied across cognitive domains, we conducted separate analyses using composite measures of specific cognitive functions (Table 1). As shown in Figure 2, the prodromal period began about 63 months before dementia onset for episodic memory, 76 months for semantic memory, 75 months for working memory, 70 months for perceptual speed, and 65 months for visuospatial ability. Prior to the prodromal period, episodic memory was stable and there was very gradual decline in the other domains. Decline in all functions increased sharply during the prodromal period with a further less marked acceleration after AD was diagnosed.
Figure 2.
Decline in Specific Cognitive Functions Before and After Dementia Onset in Alzheimer's Disease. Predicted 10-year paths of cognitive decline for typical participants who remained dementia free (solid line) or developed AD in study year 7 (dashed line), adjusted for age, sex, and education.
Cognitive Decline in the Prodromal Phase of Mild Cognitive Impairment
To further examine prodromal AD, we assessed change in cognitive function during the development of MCI, widely recognized as a precursor to AD. Of 1,511 persons without cognitive impairment at study enrollment, 742 developed cognitive impairment on follow-up. Those who developed MCI were older (78.1 years versus 74.6, t [1,509]=9.4, p<.001) and slightly more educated (16.6 years versus 16.2, t [1,509]=1.9, p=.05) than those who did not, with a comparable sex distribution (71.0% women versus 74.5, χ2 [1] = 2.3, p=.128). There was an approximately 4.5-year period of gradual global cognitive decline prior to MCI onset (as shown by the term for time during prodrome in Table 2), with a further doubling in the rate of decline after MCI was diagnosed.
Table 2.
Change in Global Cognitive Function Before and After Onset of Mild Cognitive Impairment *
WB: from rerun.requst12.using_estimate.txt
| MCI Type | Model Term | Estimate | St.Error | P |
|---|---|---|---|---|
| Any Type | Time preceding prodrome | 0.002 | 0.001 | .106 |
| Time during prodrome | -0.067 | 0.003 | <.001 | |
| Time after MCI onset | -0.134 | 0.008 | <.001 | |
| Amnestic MCI | Time preceding prodrome | -0.008 | 0.001 | <.001 |
| Time during prodrome | -0.102 | 0.004 | <.001 | |
| Time after MCI onset | -0.188 | 0.011 | <.001 | |
| Nonamnestic MCI | Time preceding prodrome | -0.027 | 0.003 | <.001 |
| Time during prodrome | -0.072 | 0.004 | <.001 | |
| Time after MCI onset | -0.108 | 0.009 | <.001 |
From separate mixed-effects models adjusted for age, sex, and education. Each estimate is the mean annual rate of global cognitive change predicted by the model. MCI indicates mild cognitive impairment.
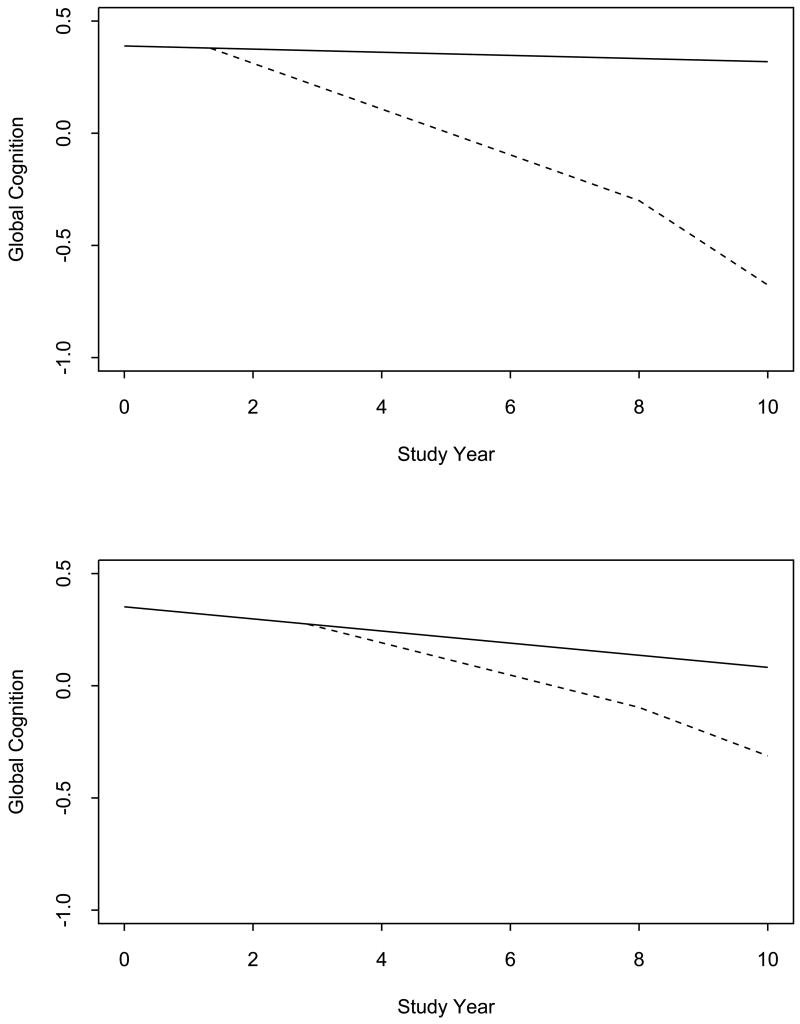
Because memory impairment in MCI has been associated with increased risk of dementia and AD [27,28], we conducted separate analyses for amnestic (observed in 487) and nonamnestic (observed in 442) MCI to examine their developmental histories (Table 2, Figure 2). A mean of 80 months before the diagnosis of amnestic MCI (upper panel of Figure 2, dotted line) annual rate of global cognitive decline increased more than tenfold with a further increase of about 80% following the diagnosis. By contrast, in nonamnestic MCI (lower panel of Figure 2, dotted line) the prodromal period began later (62 months before diagnosis) and the acceleration in global cognitive decline was less marked, with an approximately 2.5-fold increase during the prodromal period and a further 50% increase following the diagnosis.
Comment
In a group of more than 2,000 old people followed annually for up to 16 years, we assessed change in cognitive function during the prodromal phases of AD and its precursor, MCI. Rate of cognitive decline increased sharply about 5 to 6 years before dementia was diagnosed and showed a modest increase approximately of 4 to 6 years before MCI was diagnosed. The results indicate that dementia in AD is preceded by many years of progressively accelerating cognitive decline.
We are aware of only one previous study of cognitive change preceding development of MCI and results were comparable to the present study, with accelerated cognitive decline 3 to 4 years before the MCI diagnosis [9]. In addition, we found that the prodromal period for amnestic MCI began 1 to 2 years earlier than the nonamnestic MCI prodrome and was characterized by more rapid cognitive decline. These findings are consistent with neuroimaging [29] and neuropathologic [19] data suggesting that many people with amnestic MCI actually have mild AD.
As noted above, estimates of the temporal course of cognitive decline prior to dementia onset in AD have varied widely. The 5 to 6 years of cognitive decline preceding dementia observed in the present study is close to the midpoint of comparable studies [3-8]. Several factors are probably contributing to this variability. One suggested by the present analyses is the extent to which symptomatic individuals are included. When we excluded people with MCI at baseline, the prodromal period was briefer and the acceleration of cognitive decline was more marked. This may be because individuals with more mild disease take longer to transition from no cognitive impairment to dementia and so are less likely to be identified with observation periods of less than 2 or 3 decades. Another factor contributing to variability in findings is study differences in the effective cutpoint for dementia. Thus, in a diagnostic system that regards MCI as mild AD [30], the cognitive prodromal period is relatively brief [6]. By contrast, in the study with the longest estimate of the prodromal period [5], the mean Mini-Mental State Examination score in the pre-AD group declined below 20 before the diagnosis was made, a relatively low dementia cutpoint.
There is also evidence that prodromal cognitive decline in AD varies across cognitive domains. We found that prodromal decline in semantic memory and working memory preceded decline in other cognitive domains, consistent with findings from the PAQUID study [5]. However, the differences between domains in this study were not large and other studies suggest that prodromal decline in AD begins in other domains, including episodic memory [8] and visuospatial ability [6]. Thus, cognition is clearly globally affected in the prodromal phase of AD, and some of the variability between cognitive outcomes probably reflects metric factors including retest effects [31] rather than the cognitive domain being assessed. On the other hand, prodromal cognitive decline in amnestic MCI began earlier and progressed more rapidly than in nonamnestic MCI, in line with neuropathologic [32,33] and neuroimaging [34,35] data suggesting that medial temporal lobe structures that support episodic memory are among the first brain regions affected by the disease.
These data show that by the time individuals meet clinical criteria for a diagnosis of AD, they have already experienced many years of accelerating cognitive decline. This has important public health implications because it is generally assumed that treatments for AD will be more effective if introduced before this prodromal period begins and cognitive systems are manifestly dysfunctional. Further, an effective early treatment could compress the cognitive morbidity of AD [36] whereas effective treatment after dementia onset might prolong it, underscoring the need for biologic and behavioral markers to aid in early diagnosis.
Little cognitive decline was evident in people who did not develop MCI or AD. This observation is consistent with prior research [5,6,37,38] and suggests that cognitive decline may not be an inevitable consequence of old age.
Strengths and limitations of this study should be noted. Clinical classification of MCI, dementia, and AD was based on a uniform clinical evaluation and widely accepted criteria applied by experienced clinicians, thereby minimizing diagnostic error. The large cohort, high rate of follow-up participation, and availability of previously established psychometrically sound measures of cognition allowed us to capture subtle nonlinear changes in function. The principal limitation is that subjects are selected and so the generalizability of the findings will need to be established.
Figure 3.
Global Cognitive Decline Before and After Onset of Amnestic (upper panel) or Nonamnestic (lower panel) Mild Cognitive Impairment. Predicted 10-year paths of decline in global cognition for typical participants whose cognition remained intact (solid line) or became impaired in study year 8 (dashed line), adjusted for age, sex, and education.
Acknowledgments
The authors thank the many Illinois residents who have participated in the Rush Memory and Aging Project and the many Catholic nuns, priests, and brothers who have participated in the Religious Orders Study; Traci Colvin, MPH, Julie Bach, MSW, George Hoganson, and Tracy Faulkner for coordinating the studies; Woojeong Bang, MS, for statistical programming; John Gibbons, MS, and Greg Klein for data management; and Priya Patel for preparing the manuscript.
Supported by National Institute on Aging Grants R01 AG17917, R01 AG024871, R01 AG15819, and P30 AG10161, and the Illinois Department of Public Health. The sponsors had no role in the design or conduct of the study; in the collection management, analysis, or interpretation of the data; or in the preparation, review, or approval of the manuscript.
References
- 1.Brookmeyer R, Gray S, Kawas C. Projections of Alzheimer's disease in the United States and the public health impact of delaying disease onset. Am J Pub Health. 1998;88:1337–1342. doi: 10.2105/ajph.88.9.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sloane PD, Zimmerman S, Suchindran C, Reed P, Wang L, Boustani M, Sudha S. The public health impact of Alzheimer's disease, 2000-2050: potential implication of treatment advances. Ann Rev Pub Health. 2002;23:213–231. doi: 10.1146/annurev.publhealth.23.100901.140525. [DOI] [PubMed] [Google Scholar]
- 3.Hall CB, Lipton RB, Sliwinski M, Stewart WF. A change point model for estimating the onset of cognitive decline in preclinical Alzheimer's disease. Stat Med. 2000;19:1555–1566. doi: 10.1002/(sici)1097-0258(20000615/30)19:11/12<1555::aid-sim445>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 4.Amieva H, Jacqmin-Gadda H, Orgogozo JM, et al. The 9 year cognitive decline before dementia of Alzheimer type: a prospective population-based study. Brain. 2005;128:1093–1101. doi: 10.1093/brain/awh451. [DOI] [PubMed] [Google Scholar]
- 5.Amieva H, LeGaff M, Millet X, et al. Prodromal Alzheimer's disease: successive emergence of clinical symptoms. Ann Neurol. 2008;64:492–498. doi: 10.1002/ana.21509. [DOI] [PubMed] [Google Scholar]
- 6.Johnson DK, Storandt M, Morris JC, Galvin JE. Longitudinal study of the transition from healthy aging to Alzheimer disease. Arch Neurol. 2009;66:1254–1259. doi: 10.1001/archneurol.2009.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Small BJ, Backman L. Longitudinal trajectories of cognitive change in preclinical Alzheimer's disease: a growth mixture modeling analysis. Cortex. 2007;43:826–834. doi: 10.1016/s0010-9452(08)70682-8. [DOI] [PubMed] [Google Scholar]
- 8.Grober E, Hall CB, Lipton RB, Zonderman AB, Resnick SM, Kawas C. Memory impairment, executive dysfunction, and intellectual decline in preclinical Alzheimer's disease. J Int Neuropsychol Soc. 2008;14:266–278. doi: 10.1017/S1355617708080302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Howieson DB, Carlson NE, Moore MM, Wasserman D, Abendroth CD, Payne-Murphy J, Kaye JA. Trajectory of mild cognitive impairment onset. J Int Neuropsychol Soc. 2008;14:192–198. doi: 10.1017/S1355617708080375. [DOI] [PubMed] [Google Scholar]
- 10.Wilson RS, Bienias JL, Evans DA, Bennett DA. Religious Orders Study: Overview and change in cognitive and motor speed. Aging Neuropsychol Cogn. 2004;11:280–303. [Google Scholar]
- 11.Bennett DA, Schneider JA, Buchman AS, Mendes de Leon C, Bienias JL, Wilson RS. The Rush Memory and Aging Project: study design and baseline characteristics of the study cohort. Neuroepidemiol. 2005;25:163–175. doi: 10.1159/000087446. [DOI] [PubMed] [Google Scholar]
- 12.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan E. Clinical diagnosis of Alzheimer's disease: report of the NINCDS/ADRDA work group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 13.Bennett DA, Schneider JA, Aggarwal NT, Arvanitakis Z, Shah RC, Kelly JF, Fox JH, Cochran EJ, Arends D, Treinkman AD, Wilson RS. Decision rules guiding the clinical diagnosis of Alzheimer's disease in two community-based cohort studies compared to standard practice in a clinic-based cohort study. Neuroepidemiol. 2006;27:169–176. doi: 10.1159/000096129. [DOI] [PubMed] [Google Scholar]
- 14.Bennett DA, Wilson RS, Schneider JA, Evans DA, Beckett LA, et al. Natural history of mild cognitive impairment in older persons. Neurology. 2002;59:198–205. doi: 10.1212/wnl.59.2.198. [DOI] [PubMed] [Google Scholar]
- 15.Wilson RS, Schneider JA, Arnold SE, Tang Y, Boyle PA, Bennett DA. Olfactory identification and incidence of mild cognitive impairment in older age. Arch Gen Psychiatry. 2007;64:802–808. doi: 10.1001/archpsyc.64.7.802. [DOI] [PubMed] [Google Scholar]
- 16.Wilson RS, Aggarwal NT, Barnes LL, Bienias JL, Mendes de Leon CF, Evans DA. Biracial population study of mortality in mild cognitive impairment and AD. Arch Neurol. doi: 10.1001/archneurol.2009.80. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boyle PA, Wilson RS, Aggarwal NT, Tang Y, Bennett DA. Mild cognitive impairment: risk of Alzheimer's disease and rate of cognitive decline. Neurology. 2006;67:441–445. doi: 10.1212/01.wnl.0000228244.10416.20. [DOI] [PubMed] [Google Scholar]
- 18.Wilson RS, Aggarwal NT, Barnes LL, Mendes de Leon CF, Hebert LE, Evans DA. Cognitive decline in incident Alzheimer disease in a community population. Neurology. 2010;74:951–955. doi: 10.1212/WNL.0b013e3181d64786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bennett DA, Schneider JA, Wilson RS, Bienias JL, Arnold SE. Mild cognitive impairment is related to Alzheimer's disease pathology and cerebral infarctions. Neurology. 2005;64:834–841. doi: 10.1212/01.WNL.0000152982.47274.9E. [DOI] [PubMed] [Google Scholar]
- 20.Wilson RS, Beckett LA, Barnes LL, Schneider JA, Bach J, Evans DA, Bennett DA. Individual differences in rates of change in cognitive abilities of older persons. Psychol Aging. 2002;17:179–193. [PubMed] [Google Scholar]
- 21.Wilson RS, Barnes LL, Bennett DA. Assessment of lifetime participation in cognitively stimulating activities. J Clin Exp Neuropsychol. 2003;25:634–42. doi: 10.1076/jcen.25.5.634.14572. [DOI] [PubMed] [Google Scholar]
- 22.Wilson RS, Barnes LL, Krueger KR, Hoganson G, Bienias JL, Bennett DA. Early and late life cognitive activity and cognitive systems in old age. J Int Neuropsychol Soc. 2005;11:400–407. [PubMed] [Google Scholar]
- 23.Krueger KR, Wilson RS, Bennett DA, Aggarwal NT. A battery of tests for assessing cognitive function in older latino persons. Alzheimer Dis Assoc Disord. 2009;23:384–388. doi: 10.1097/WAD.0b013e31819e0bfc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aggarwal NT, Wilson RS, Bienias JL, et al. The association of magnetic resonance imaging measures with cognitive function in a biracial population sample. Arch Neurol. 2010;67:475–482. doi: 10.1001/archneurol.2010.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Laird NM, Ware J. Random-effects models for longitudinal data. Biometrics. 1982;38:963–974. [PubMed] [Google Scholar]
- 26.SAS Institute Inc. SAS.STAT User's Guide, Version 8. Cary, NC: SAS Institute Inc; 2000. [Google Scholar]
- 27.Aggarwal NT, Wilson RS, Beck TL, Bienias JL, Bennett DA. Mild cognitive impairment in different functional domains and incident Alzheimer's disease. J Neurol Neurosurg Psychiatry. 2005;76:1479–1484. doi: 10.1136/jnnp.2004.053561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yaffe K, Petersen RC, Lindquist K, Kramer J, Miller B. Subtype of mild cognitive impairment and progression to dementia and death. Dement Geriatr Cogn Disord. 2006;22:312–319. doi: 10.1159/000095427. [DOI] [PubMed] [Google Scholar]
- 29.He J, Farias S, Martinez O, Reed B, Mungas D, DeCarli C. Differences in brain volume, hippocampal volume, cerebrovascular risk factors, and apolipoprotein E4 among mild cognitive impairment subtypes. Arch Neurol. 2009;66:1393–1399. doi: 10.1001/archneurol.2009.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Morris JC. Mild cognitive impairment is early-stage Alzheimer's disease. Arch Neurol. 2006;63:15–16. doi: 10.1001/archneur.63.1.15. [DOI] [PubMed] [Google Scholar]
- 31.Wilson RS, Li Y, Bienias JL, Bennett DA. Cognitive decline in old age: separating retest effects from the effects of growing older. Psychol Aging. 2006;21:774–789. doi: 10.1037/0882-7974.21.4.774. [DOI] [PubMed] [Google Scholar]
- 32.Hyman BT, Van Hoesen GW, Kromer C, Damasio A. Alzheimer's disease: cell-specific pathology isolates the hippocampal formation. Science. 1984;225:1168–1170. doi: 10.1126/science.6474172. [DOI] [PubMed] [Google Scholar]
- 33.Braak H, Braak E. Neuropathological staging of Alzheimer-related changes. Acta Neuropathol. 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- 34.Killiany RJ, Hyman BT, Gomez-Isla T, Moss MB, Kikinis R, Jolesz F, Tanzi R, Jones K, Albert MS. MRI measures of entorhinal cortex vs hippocampus in preclinical AD. Neurology. 2002;58:1188–1196. doi: 10.1212/wnl.58.8.1188. [DOI] [PubMed] [Google Scholar]
- 35.Kesslak JP, Nalcioglu O, Cotman CW. Quantification of magnetic resonance scans for hippocampal and parahippocampal atrophy in Alzheimer's disease. Neurology. 1991;41:51–54. doi: 10.1212/wnl.41.1.51. [DOI] [PubMed] [Google Scholar]
- 36.Wilson RS, Barnes LL, Aggarwal NT, et al. Cognitive activity and the cognitive morbidity of Alzheimer's disease. Neurology. doi: 10.1212/WNL.0b013e3181f25b5e. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rubin EH, Storandt M, Miller JP, et al. A prospective study of cognitive function and the onset of dementia in cognitively healthy elders. Arch Neurol. 1998;55:395–401. doi: 10.1001/archneur.55.3.395. [DOI] [PubMed] [Google Scholar]
- 38.Wilson RS, Beckett LA, Bennett DA, Albert MS, Evans DA. Change in cognitive function in older persons from a community population: relation to age and Alzheimer's disease. Arch Neurol. 1999;56:1274–1279. doi: 10.1001/archneur.56.10.1274. [DOI] [PubMed] [Google Scholar]