Abstract
Human neuropeptide Y (NPY) is an important biologics that regulates multitude of physiological functions and could be amenable to therapeutic manipulations in certain disease states. However, rapid (minutes) enzymatic degradation and inactivation of NPY precludes its development as a drug. Accordingly, we determined whether self-association of NPY with biocompatible and biodegradable sterically stabilized phospholipid micelles (SSM) improves its stability and bioactivity. We found that in saline NPY spontaneously aggregates whereas in the presence of SSM it self-associates with the micelles as monomers. Three NPY molecules self-associate with one SSM at saturation. This process stabilizes the peptide in α-helix conformation, abrogates its degradation by dipeptidyl peptidase-4 and potentiates NPY-induced inhibition of cAMP elaboration in SK-N-MC cells. Collectively, these data indicate that self-association of NPY with SSM stabilizes and protects the peptide in active monomeric conformation, thereby amplifying its bioactivity in vitro. We propose further development of NPY in SSM as a novel, long-acting nanomedicine.
Keywords: human neuropeptide Y (NPY) medicine, sterically stabilized micelles (SSM), stability, dipeptydyl peptidase-4, cAMP
Background
Human neuropeptide Y (NPY), a ubiquitous 36-amino acid amphipathic neuropeptide, regulates a range of physiological functions, including food intake, anxiety, cognition, pain and cardiovascular homeostasis.1–5 In addition, the peptide has been shown to modulate oxidative fuel selection6, bone homeostasis, intestinal fluid secretion, inflammatory responses and cancer growth.6–10 These responses are mediated, in part, through activation of NPY/ Y(1) receptor subtype in target organs and tissues.11–14 Overexpression of this receptor in malignant neoplasms was reported, particularly in breast cancer.10, 15 Importantly, stimulation of NPY/Y(1) receptor pathway inhibits growth of Ewing sarcoma in vivo and several cultured cancer cell lines.16, 17
Taken together, these data suggest that NPY could be developed as a drug for the disease conditions characterized by dysregulation of NPY-dependent metabolic pathway(s). However, clinical development of NPY is hampered by its short half-life (minutes) in vivo due to rapid enzymatic degradation predominantly by dipeptidyl peptidase-4 (DPP-4).18, 19 Formation of folded/unfolded peptide monomers, dimers and aggregates in aqueous environment also impedes development of the peptide formulation.20–22
To circumvent NPY instability, we adopted our innovative strategy where native amphipathic peptide drug candidates, such as NPY, self-associate with long-circulating, sterically stabilized, biocompatible and biodegradable phospholipid micelles (SSM).23–25 These micelles (graphical abstract schema) are composed of the PEGylated phospholipid molecules, 1,2-distearoyl-sn-glycero-3-phosphatidylethanolamine-N- [methoxy(poly-ethyleneglycol)-2000] (DSPE-PEG2000) that self-assemble in aqueous media above its critical micelle concentration (CMC) of 0.5–1 μM.26 Given their very low CMC, SSM are relatively stable upon intravenous administration and dilution in the bloodstream.
Additional advantages of SSM are ease of preparation and ability to be lyophilized without any lyo- or cryoprotectants to provide an acceptable shelf-life23. In our previous studies, using another amphiphilic peptide, vasoactive intestinal peptide (VIP) we found that the peptide bioactivity was prolonged from minutes to hours after systemic administration to laboratory animals.24, 27 This phenomenon was related, in part, to a change in molecular conformation of VIP from degradation-prone random coil in aqueous environment to potentially proteolysis-resistant α-helix in SSM. In vivo circulation time was also prolonged by the stealth property due to the outer polyethylene glycol (PEG) corona of the micellar carrier. In addition, the α-helical conformation of VIP was previously shown to be the preferred conformation for ligand-receptor interactions in target cells.28, 29
However, not all peptide drugs intrinsically possess equal potential to form a successful nanomedicine using SSM and such ability should be tested experimentally for each peptide. Accordingly, the purpose of this study was to determine whether NPY self-associates with SSM, and if so, does this interaction stabilize the peptide and amplifies its bioactivity in vitro.
Methods
Materials
1,2-Distearoyl-sn-glycero-3-phosphatidylethanolamine-N- [methoxy(polyethyleneglycol)-2000] sodium salt (DSPE-PEG2000) was purchased from Northern Lipids Inc. (Vancouver, BC, Canada). Human neuropeptide Y (NPY) was obtained from American Peptide Company (Sunnyvale, CA); sterile saline (0.9% NaCl injection USP) from Baxter Healthcare Corporation (Deerfield, IL), and human dipeptidyl-peptidase 4 from ProSpec Tany TechnoGene (Rehovot, Israel). SK-N-MC cells (HTB-10) were from American Type Culture Collection (Manassas, VA). All other reagents were acquired from Fisher Scientific (Itasca, IL) or Sigma-Aldrich (St. Louis, MO).
Sample preparation
Method to prepare novel NPY-SSM formulation was based on our previous experience with peptide-SSM association.23 Briefly, weighed amount of DSPE-PEG2000 was combined with appropriate volume of saline and vortexed for 2 min (Thermolyne Maxi Mix II). The dispersion was equilibrated at 25 °C for 1 hour in dark. This resulted in the formation of sterically stabilized micelles (SSM) at concentrations above lipid critical micelle concentration. Thereafter, measured volume of NPY stock solution in saline was added to SSM dispersion. Resulted NPY-SSM dispersion was further incubated for 2 h at 25 °C in dark. All samples were prepared fresh and diluted with saline according to the experiment.
Particle size analysis
The aggregation behavior of NPY in saline was assessed in presence and absence of DSPE-PEG2000 by dynamic light scattering (DLS) using Agilent 7030 NICOMP DLS (Agilent Technologies, Santa Clara, CA). SSM, NPY and NPY-SSM in saline at 5 mM lipid and 20 μM peptide concentrations were evaluated. The mean hydrodynamic particle diameter dℏ was computed from the diffusion coefficient using Stokes-Einstein equation. Measurements were carried out at 23 °C with autoadjusted light scattering intensity of 300 KHz and fixed detector angel of 90°. Dispersing medium parameters of 0.933 cP for viscosity and 1.33 for refractive index were used. As a minimum of three dℏ values were averaged for each reported experimental result from the analysis of the autocorrelation function accumulated over at least 15 min.
Fluorescence emission spectroscopy
The maximal self-association number of NPY molecules per SSM was determined by fluorescence spectroscopy as previously described in our laboratory.23
Samples were prepared with varying DSPE-PEG2000: NPY molar ratios ranging from 2:1 to 100:1. Peptide concentration was kept constant at 5 μM while lipid concentration was increased from 0.01 to 0.5 mM. The peak fluorescence emission intensity (Emmax) of tyrosine residues of NPY was recorded on SLM Aminco 8000 Spectrofluorimeter (SLM Instruments, Rochester, NY) at excitation wavelength 275 nm. The respective Emmax was plotted against corresponding lipid:peptide molar ratio with an assistance of SigmaPlot® (Systat Software Inc., San Jose, CA) and integrated into curve fitting function of the software. The lipid: NPY molar ratio at saturation was determined as the lowest lipid: NPY molar ratio at which Emmax of NPY was not significantly different from the intensity measured at plateau (in the presence of excessive lipid). Given that approximately 90 lipid monomers form one SSM30 the maximal number of NPY molecules that could associate with each micelle was calculated by the following equation:
Where NNPY is the number of NPY molecules associated with one SSM and x is the number of lipid molecules at the saturation.
Circular dichroism spectroscopy
The peptide secondary structure in NPY-SSM or NPY in saline (20 μM peptide, 5mM lipid) was determined by circular dichroism (CD) as previously described in our laboratory.28, 31 CD spectra for NPY were recorded on Jasco J-710 spectropolarimeter (Jasco Inc., Easton, MD) calibrated with D-10-camphorsulfonic acid in range 190–260nm at room temperature in 1mm path length fused quartz cuvette. The resulted spectra were corrected for corresponding SSM or saline scans and smoothed according to manufacturer's instructions by the Savitzky-Golay algorithm.
Mass spectrometry analysis of NPY and its DPP-4 cleaved products in presence and absence of SSM
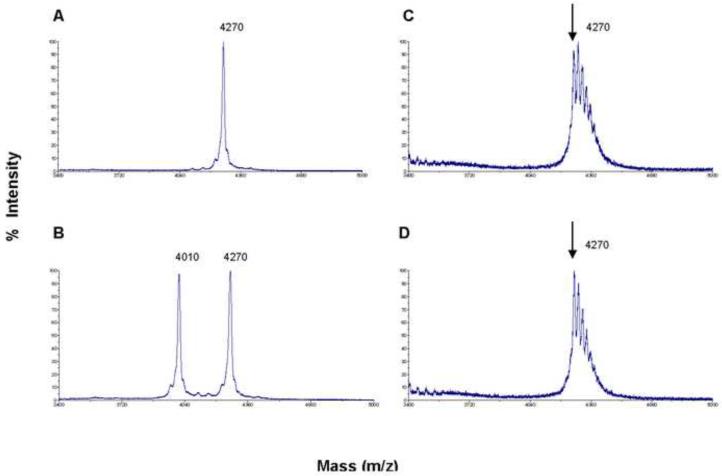
The purpose of this study was to assess the stability of NPY in self-association with SSM to dipeptidyl peptidase-4 (DPP-4) degradation in comparison to the peptide in saline. DPP-4 is a major blood and tissue associated enzyme involved in regulation of Y(1) to non-Y(1) receptor signaling through NPY truncation at N-terminal penultimate proline.18, 22, 32
Stock solutions of NPY in saline and NPY-SSM were prepared as outlined above. Measured volume of stock solutions were added to 1 nM DPP-4 in phosphate buffered saline (pH 7.4) to obtain the final concentrations of 10 μM for NPY and 300 μM for the lipid. The resulting samples were incubated for 1 h at 37 °C followed by analysis with matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS).33, 34 Samples were mixed with the matrix solution (10 mg α-cyano-4-hydroxycinnamic acid in 1 ml aqueous solution of 50 % acetonitrile containing 0.1 % trifluoroacetic acid, v/v) at 1:1 ratio. Aliquots of the mixtures (1.3 μl) were spotted onto a MALDI-TOF target and analyzed using Voyager-DE PRO Mass Spectrometer (Applied Biosystems, Foster City, CA) equipped with a 337 nm pulsed nitrogen laser. Peptide mass was measured using positive-ion linear mode over the range m/z 1000–6500.
Bioactivity of NPY-SSM in vitro
The purpose of this experiment was to determine whether NPY in association with SSM retained its biological activity to diminish cAMP accumulation in cultured human SK-N-MC brain neuroepithelioma cells.35, 36 The selected cell line endogenously expresses neuropeptide Y(1) receptors that upon binding to NPY inhibit intracellular cAMP production.35, 37
Stock solutions of NPY-SSM, NPY in saline and their respective controls were prepared as described above with the corresponding NPY and lipid concentrations of 1 μM and 100 μM followed by dilution with saline for further cell culture studies. The method used in this experiment has been previously described in literature.35 Briefly, cells were seeded in 24-well plates at density 2 × 105 cells per well and cultured in EMEM growth medium supplemented with 10% fetal bovine serum for 24 h at 37 °C in humidified, 5% CO2 atmosphere. On the day of experiment, growth medium was aspirated and replaced with serum-free medium containing 5 mM theophylline, a non-specific phosphodiesterase inhibitor, and incubated for 1 h at 37 °C in humidified, 5% CO2 atmosphere. After that, 20 μl of 5 mM DSPE-PEG2000 solution were introduced to the wells to be treated with SSM containing solutions to maintain the lipid concentration above CMC. The corresponding amount of saline was added into all other wells to keep up matching well volume. Immediately hereafter, equal volumes of NPY in saline, NPYSSM or their respective controls were added into designated wells to achieve NPY concentration ranging from 0.01–10 nM. Following 3 min incubation, forskolin was added into all wells (100 μl; final concentration, 10 μM) to induce cAMP production. Thereafter, cells were incubated for additional hour at 37 °C in humidified, 5% CO2 atmosphere. At the conclusion of the incubation period, cells were lysed and cAMP content in cell lysates was determined by enzyme-linked immunosorbent assay (ELISA) according to manufacturer's instructions (cAMP Biotrak EIA Kit, GE Healthcare, Piscataway, NJ). Data were normalized to total protein content measured by BCA (bicinchoninic acid) protein assay (Pierce, Rockford, IL).
Data and statistical analyses
Data are expressed as mean ± SD. Statistical analysis was performed using analysis of variance (ANOVA) followed by Tukey's test. A value of p<0.05 was considered statistically significant.
Results
Particle size analysis
The mean particle size and particle distribution of blank SSM, NPY-SSM and NPY in saline were analyzed by dynamic light scattering (Figure 1). Monomodal particle distributions as evaluated by volume- and intensity-weighted Nicomp analysis were observed in all tested samples; number-weighted analysis revealed similar particle allocation (data not shown). Neuropeptide Y (20 μM) in saline formed large soluble aggregates with mean hydrodynamic diameter of 557±100 nm (Figure 1A; volume-weighted averaged value of 3 separate experiments). By contrast, mean hydrodynamic diameter of NPY (20 μM) in SSM (5 mM DSPE-PEG2000) was 14±3 nm (Figure 1B), which was not significantly different from blank SSM 15±3 nm (Figure 1C) (n=3/group; p>0.05).
Figure 1.
Representative particle size distribution by intensity- and volume-weighted DLS NICOMP analysis after 2 h incubation of blank (A) SSM (100% of the particles form a single peak with average diameter 14 ± 3 nm by volume-weighted analysis), (B) neuropeptide Y (NPY) in saline (average hydrodynamic diameter of the aggregates ranged 557 ± 100 nm in a single peak), and (C) NPY-SSM (monomodal distribution was observed with mean particle diameter not significantly different from SSM alone, 15 ± 3 nm volume-weighted).
Fluorescence emission spectroscopy
Intrinsic fluorescence of NPY tyrosine residues at constant peptide concentration (5 μM) enhanced with increasing concentration of DSPE-PEG2000 until reaching a plateau at lipid:petide molar ratio of 29:1 and beyond (Figure 2). Based on the information that one SSM is formed from ~90 DSPE-PEG2000 monomers30 the maximal number of NPY molecules that could self-associate with one SSM was determined to be three.
Figure 2.
Peak fluorescence intensity of NPY at varying lipid: NPY molar ratios (each point n=3). The arrow (↑) indicates lipid: NPY saturation molar ratio of 29:1.
Circular dichroism spectroscopy
The CD spectra of NPY in SSM and saline were recorded at 20 μm peptide concentration in far-UV range (Figure 3). Notable amount of α-helix for the peptide in saline was detected. Association of NPY with SSM lead to further slight increase in NPY α-helicity, observed by deepening of negative bands at 208 and 222 nm which are characteristics of α-helical peptides. This finding is supported by previously reported NPY potential for enhanced α-helicity in nonpolar environment38, implying that NPY interaction with SSM stabilizes the peptide in its physiologically active helical form.
Figure 3.
Representative circular dichroism spectra of neuropeptide Y (NPY; 20 μM) in saline (Sal-NPY) and in SSM (NPY-SSM; 5 mM DSPE-PEG2000).
Mass spectrometry analysis of NPY-SSM in the absence and presence of DPP-4
We found that one hour incubation of NPY(1–36) (molecular mass of 4270 Da) with DPP-4 aminopeptidase at 37 °C in PBS (pH 7.4) generated a considerable amount of secondary product with molecular mass around 4000 Da, indicating production of truncated NPY(3–36) form equivalent in intensity to the parent peptide (Figure 4B). In contrast, such secondary peak was not detectable upon incubation NPY-SSM samples with DPP-4 (Figure 4D), similar to NPY and NPY-SSM samples without the enzyme (Figure 4 A, C) at the same experimental conditions. Splitting of the peptide peak observed for the NPY-SSM mass spectra in the presence and absence of enzyme (Figure 4 C, D) was most probably accounted to variation of the PEG and/or lipid chain length in DSPE-PEG2000 lipid of SSM.
Figure 4.
Representative MALDI-TOF mass spectra of (A) NPY (10 μM) in saline (Sal-NPY), (B) Sal-NPY + DPP-4 (1 nM), (C) NPY-SSM (300 μM DSPE-PEG2000) (D) and NPY-SSM + DPP-4. Samples were incubated at 37 °C for 1 h in PBS (pH 7.4) prior the analysis.
Bioactivity of neuropeptide Y in SSM in vitro
Effects of NPY in saline and SSM on cAMP accumulation in human SK-N-MC brain neuroepithelioma cells are depicted in Figure 5. Cyclic AMP content decreased significantly in a concentration-dependent manner in the presence of NPY in SSM and NPY in saline. However, a greater inhibition of cAMP production was observed for the cells treated with NPY-SSM compared to NPY in saline at the same peptide concentration. Cyclic AMP concentration reduced significantly to 50.2±2.7 % compared to forskolin-stimulated saline-treated cells (positive control; 100 %) in the presence of 0.1 nM NPY-SSM but only to 86.5±14.7 % in the presence of 0.1 nM NPY in saline (Figure 5; n=3/group; p<0.05). Likewise, cAMP concentration decreased significantly to 44.7±2.9 % in the presence of 1.0 nM NPY in SSM but only to 59.6±6.8 % at the same NPY concentration in saline (Figure 5; each group n=3; p<0.05). Conversely, cellular cAMP level decreased to similar concentration in the presence of 10 nM NPY-SSM and NPY in saline (30.5±3.5 % and 41.6± 6.9 %, respectively; Fig. 5; n=3/group; p>0.05). The effect of NPY in micelles at 0.1 nM was similar to the effect of NPY in saline at 10 nM 50.2±2.7 % and 41.6± 6.9 %, respectively (Figure 5; each group, n=3; p>0.05), despite 100 times lower peptide content in SSM. SK-N-MC cell incubation with blank SSM, corresponding to the highest lipid concentration of tested NPY-SSM samples, resulted in no significant change of cAMP level in comparison to saline treated control (Figure 5, inset).
Figure 5.
Cyclic AMP level in forskolin-stimulated SK-N-MC neuroepithelioma cells treated with NPY in saline (Sal-NPY) or NPY-SSM at various peptide concentrations (data normalized against positive control). Each group, n=3 experiments; *p<0.05 in comparison to forskolin-stimulated saline treated cells (NPY 0 nM; positive control); +p<0.05 in comparison to NPY in saline at the same peptide concentration. Inset figure represents cAMP level in SK-N-MC cells incubated with saline, blank SSM (vehicle control) or forskolin (positive control); n=3/group.
Discussion
Human neuropeptide Y (NPY) is a neurotransmitter and hormonal peptide that is undergoing intensive investigation for the treatment of cachexia, certain psychiatric disorders and neuropathic pain.1, 3, 4, 39, 40 Delivery of NPY directly to peripheral tissues possess an additional interest and can be beneficial when peptide action in central nervous system (CNS) is not required. Anti-proliferative and proapoptotic10 effects of NPY mediated through Y(1) receptor activation can be exploited in treatment of solid tumors overexpressing this receptor subtype. Moreover, targeting of peripheral energy utilization6 as supplementary treatment of anorexia and downregulation of T-helper 1 hypersensitivity41 in acute stages of inflammation with NPY are therapeutically beneficial. Besides direct therapeutic application, labeled NPY analogs could serve as a diagnostic tool in imaging. This approach was recently tested in humans to image breast cancer and metastasis with technetium 99 tagged NPY.42 We anticipate that labeled NPY in SSM should be more effective in imaging due to enhanced accumulation at a tumor site through passive and active targeting.
However, fast proteolytic degradation of NPY upon administration is a major barrier for its successful clinical application.2 The new findings of this study showed that NPY, which underwent spontaneous self-association with SSM, was protected from enzymatic degradation and its bioactivity was significantly amplified.
Self-association of monomeric NPY with SSM abrogated formation of peptide aggregates in aqueous environment (saline) as shown by particle size analysis (Figure 1). We postulate that intermolecular hydrophobic interactions between NPY molecules at the dimer/tetramer interface are superseded by more energetically-favorable interactions of NPY molecules with PEGylated phospholipid micelles. This was further evidenced by increased fluorescence emission of NPY in the presence of SSM (Figure 2). The enhanced fluorescence intensity was likely due to reduced fluorescence quenching between aggregated NPY molecules.21 To this end, we found that up to three NPY molecules self-associate with each micelle freely, without interaction with each other.
Furthermore, our circular dichroism study showed that the association of NPY with biocompatible and biodegradable sterically stabilized phospholipid micelles in aqueous environment resulted in stabilization of α-helical peptide confirmation (Figure 3). This was in agreement with the results of another research group on NPY conformational changes with a model membrane system (dodecylphosphocholine micelle). This study revealed a raise in NPY α-helicity, particularly in its C-terminal segment upon interaction with the dodecylphosphocholine micelles.11 Given that the C-terminal of NPY is critical for binding to its targets, this conformational change favors peptide-receptor interaction.11, 20, 43, 44 Increased α-helicity of NPY in SSM is likely contributed, in part, by the adoption of helical conformation at the C-terminal pentapeptide. Moreover, NPY most likely reside within the PEG palisade of PEGylated micellar system, rather than binding to the phospholipid headgroup surface as claimed with dodecylphosphocholine micelles free of PEG corona.11 Therefore, the PEG layer of SSM should provide an additional steric barrier against physical aggregation and enzymatic degradation of NPY, which would result in further increase in the stability of NPY.
This notion is further supported by our findings that self-association of NPY with SSM abrogated enzymatic peptide cleavage induced by DPP-4 (Figure 4) and potentiated NPY bioactivity as indicated by enhanced suppression of cAMP accumulation in forskolin stimulated SK-N-MC cells (Figure 5). These cells express neuropeptide Y(1) receptors that upon activation reduce intracellular cAMP accumulation which underlies many of the NPY-induced central and peripheral responses.6, 11–13, 15, 37 Since SK-N-MC cells have been reported to exogenously express DPP-4,45 enhanced bioactivity of NPY-SSM in these cells could be related, in part, to NPY molecule protection within SSM. However, we believe that such protection is “reversible” and do not interfere with receptor/ligand interaction. This, in turn, enables more intact full length NPY molecules in favorable α-helix conformation to interact with their receptors thereby amplifying peptide bioactivity. Moreover, the peptide will be presumably protected by SSM from the enzymatic cleavage not only in vicinity of their cell targets but also in circulation prolonging its half-life. The possibility of interaction of NPY in SSM with Y(2), Y(4) and Y(5) receptors can not be ruled out. Clearly, additional studies are needed to evaluate this fact.
The results of this study represent the first step in our overall goal to safely and effectively deliver human NPY by the intravenous or intranasal routes. The former path is more applicable for peripheral peptide delivery since our nanomicellar technology most likely will not allow blood brain barrier crossing. From another hand, feasibility of intranasal delivery of peptide drugs has been shown for various nanoparticulate carriers, including liposomes and PEGylated polymeric nanoparticles46–48 and should be applicable for NPY-SSM as well. Moreover, NPY is currently being tested by intranasal route on humans in a new clinical trial,49 but not as a nanoparticulate formulation.
In conclusion, we found that self-association of NPY with sterically stabilized phospholipid micelles amplifies its bioactivity in vitro. This may be related, in part, to decreased aggregation and increased stability of NPY molecules by the carrier. This, in turn, obviates the need to chemically modify NPY molecule to improve its stability thereby circumventing possible immunogenicity and adverse events.24, 28, 50 Phospholipid micelles used in this study are composed of PEGylated lipid, which is a component of U.S. FDA approved product Doxil®. The final product, NPY-SSM dispersion is easy to prepare, scale up and freeze-dry for long-term storage. Therefore, we propose further development of NPY in SSM as a novel, long-acting nanomedicine for conditions with peptide deficiency and where NPY/receptor Y(1) pathway mediation is needed.
Acknowledgement
The authors thank Ernest Gemeinhart for his technical assistance in cell culture experiments.
This study was supported, in part, by NIH grants AG024026, CA121797; Parenteral Drug Association Pre-Doctoral Fellowship and VA Merit Review grant. This investigation was conducted in a facility constructed with support from Research Facilities Improvement Program Grant Number CO6RR15482 from the National Center for Research Resources, NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no conflict of interest.
References
- 1.Eaton K, Sallee FR, Sah R. Relevance of neuropeptide Y (NPY) in psychiatry. Curr Top Med Chem. 2007;7:1645–59. doi: 10.2174/156802607782341037. [DOI] [PubMed] [Google Scholar]
- 2.Harro J. CCK and NPY as anti-anxiety treatment targets: Promises, pitfalls, and strategies. Amino Acids. 2006;31:215–30. doi: 10.1007/s00726-006-0334-x. [DOI] [PubMed] [Google Scholar]
- 3.Laviano A, Inui A, Meguid MM, Molfino A, Conte C, Rossi Fanelli F. NPY and brain monoamines in the pathogenesis of cancer anorexia. Nutrition. 2008;24:802–5. doi: 10.1016/j.nut.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 4.Morley JE, Farr SA. Cachexia and neuropeptide Y. Nutrition. 2008;24:815–9. doi: 10.1016/j.nut.2008.06.020. [DOI] [PubMed] [Google Scholar]
- 5.Benarroch EE. Neuropeptide Y: Its multiple effects in the CNS and potential clinical significance. Neurology. 2009;72:1016–20. doi: 10.1212/01.wnl.0000345258.18071.54. [DOI] [PubMed] [Google Scholar]
- 6.Zhang L, Macia L, Turner N, Enriquez RF, Riepler SJ, Nguyen AD, et al. Peripheral neuropeptide Y Y1 receptors regulate lipid oxidation and fat accretion. Int J Obes (Lond) 2010;34:357–73. doi: 10.1038/ijo.2009.232. [DOI] [PubMed] [Google Scholar]
- 7.Lee NJ, Herzog H. NPY regulation of bone remodelling. Neuropeptides. 2009;43:457–63. doi: 10.1016/j.npep.2009.08.006. [DOI] [PubMed] [Google Scholar]
- 8.Moriya R, Shirakura T, Hirose H, Kanno T, Suzuki J, Kanatani A. NPY Y2 receptor agonist PYY(3–36) inhibits diarrhea by reducing intestinal fluid secretion and slowing colonic transit in mice. Peptides. 2010;31:671–5. doi: 10.1016/j.peptides.2009.11.005. [DOI] [PubMed] [Google Scholar]
- 9.Prod'homme T, Weber MS, Steinman L, Zamvil SS. A neuropeptide in immune-mediated inflammation, Y? Trends Immunol. 2006;27:164–7. doi: 10.1016/j.it.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 10.Korner M, Reubi JC. NPY receptors in human cancer: A review of current knowledge. Peptides. 2007;28:419–25. doi: 10.1016/j.peptides.2006.08.037. [DOI] [PubMed] [Google Scholar]
- 11.Bader R, Zerbe O. Are hormones from the neuropeptide Y family recognized by their receptors from the membrane-bound state? Chembiochem. 2005;6:1520–34. doi: 10.1002/cbic.200400439. [DOI] [PubMed] [Google Scholar]
- 12.Berglund MM, Hipskind PA, Gehlert DR. Recent developments in our understanding of the physiological role of PP-fold peptide receptor subtypes. Exp Biol Med (Maywood) 2003;228:217–44. doi: 10.1177/153537020322800301. [DOI] [PubMed] [Google Scholar]
- 13.Redrobe JP, Dumont Y, Fournier A, Quirion R. The neuropeptide Y (NPY) Y1 receptor subtype mediates NPY-induced antidepressant-like activity in the mouse forced swimming test. Neuropsychopharmacology. 2002;26:615–24. doi: 10.1016/S0893-133X(01)00403-1. [DOI] [PubMed] [Google Scholar]
- 14.Baldock PA, Lee NJ, Driessler F, Lin S, Allison S, Stehrer B, et al. Neuropeptide Y knockout mice reveal a central role of NPY in the coordination of bone mass to body weight. PLoS One. 2009;4:e8415. doi: 10.1371/journal.pone.0008415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reubi JC, Gugger M, Waser B, Schaer JC. Y(1)-mediated effect of neuropeptide Y in cancer: Breast carcinomas as targets. Cancer Res. 2001;61:4636–41. [PubMed] [Google Scholar]
- 16.Kitlinska J, Abe K, Kuo L, Pons J, Yu M, Li L, et al. Differential effects of neuropeptide Y on the growth and vascularization of neural crest-derived tumors. Cancer Res. 2005;65:1719–28. doi: 10.1158/0008-5472.CAN-04-2192. [DOI] [PubMed] [Google Scholar]
- 17.Ruscica M, Dozio E, Motta M, Magni P. Role of neuropeptide Y and its receptors in the progression of endocrine-related cancer. Peptides. 2007;28:426–34. doi: 10.1016/j.peptides.2006.08.045. [DOI] [PubMed] [Google Scholar]
- 18.Abid K, Rochat B, Lassahn PG, Stocklin R, Michalet S, Brakch N, et al. Kinetic study of neuropeptide Y (NPY) proteolysis in blood and identification of NPY3–35: A new peptide generated by plasma kallikrein. J Biol Chem. 2009;284:24715–24. doi: 10.1074/jbc.M109.035253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sun LS, Du F, Schechter WS, Quaegebeur JM, Vulliemoz Y. Plasma neuropeptide Y and catecholamines in pediatric patients undergoing cardiac operations. J Thorac Cardiovasc Surg. 1997;113:278–84. doi: 10.1016/S0022-5223(97)70324-6. [DOI] [PubMed] [Google Scholar]
- 20.Bader R, Bettio A, Beck-Sickinger AG, Zerbe O. Structure and dynamics of micelle-bound neuropeptide Y: Comparison with unligated NPY and implications for receptor selection. J Mol Biol. 2001;305:307–29. doi: 10.1006/jmbi.2000.4264. [DOI] [PubMed] [Google Scholar]
- 21.Dyck M, Losche M. Interaction of the neurotransmitter, neuropeptide Y, with phospholipid membranes: Film balance and fluorescence microscopy studies. J Phys Chem B. 2006;110:22143–51. doi: 10.1021/jp056697y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Frerker N, Wagner L, Wolf R, Heiser U, Hoffmann T, Rahfeld JU, et al. Neuropeptide Y (NPY) cleaving enzymes: Structural and functional homologues of dipeptidyl peptidase 4. Peptides. 2007;28:257–68. doi: 10.1016/j.peptides.2006.09.027. [DOI] [PubMed] [Google Scholar]
- 23.Lim SB, Rubinstein I, Onyuksel H. Freeze drying of peptide drugs self-associated with long-circulating, biocompatible and biodegradable sterically stabilized phospholipid nanomicelles. Int J Pharm. 2008;356:345–50. doi: 10.1016/j.ijpharm.2008.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Onyuksel H, Ikezaki H, Patel M, Gao XP, Rubinstein I. A novel formulation of VIP in sterically stabilized micelles amplifies vasodilation in vivo. Pharm Res. 1999;16:155–60. doi: 10.1023/a:1018847501985. [DOI] [PubMed] [Google Scholar]
- 25.Lim SB, Rubinstein I, Sadikot RT, Artwohl JE, Onyuksel H. A novel peptide nanomedicine against acute lung injury: GLP-1 in phospholipid micelles. Pharm Res. 2010 doi: 10.1007/s11095-010-0322-4. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ashok B, Arleth L, Hjelm RP, Rubinstein I, Onyuksel H. In vitro characterization of PEGylated phospholipid micelles for improved drug solubilization: Effects of PEG chain length and PC incorporation. J Pharm Sci. 2004;93:2476–87. doi: 10.1002/jps.20150. [DOI] [PubMed] [Google Scholar]
- 27.Sethi V, Onyuksel H, Rubinstein I. Enhanced circulation half-life and reduced clearance of vasoactive intestinal peptide (VIP) loaded in sterically stabilized micelles (SSM) in mice with collagen-induced arthritis (CIA) AAPS Pharm Sci. 2003;5:M1045. [Google Scholar]
- 28.Rubinstein I, Patel M, Ikezaki H, Dagar S, Onyuksel H. Conformation and vasoreactivity of VIP in phospholipids: Effects of calmodulin. Peptides. 1999;20:1497–501. doi: 10.1016/s0196-9781(99)00161-8. [DOI] [PubMed] [Google Scholar]
- 29.Gololobov G, Noda Y, Sherman S, Rubinstein I, Baranowska-Kortylewicz J, Paul S. Stabilization of vasoactive intestinal peptide by lipids. J Pharmacol Exp Ther. 1998;285:753–8. [PubMed] [Google Scholar]
- 30.Arleth L, Ashok B, Onyuksel H, Thiyagarajan P, Jacob J, Hjelm RP. Detailed structure of hairy mixed micelles formed by phosphatidylcholine and PEGylated phospholipids in aqueous media. Langmuir. 2005;21:3279–90. doi: 10.1021/la047588y. [DOI] [PubMed] [Google Scholar]
- 31.Lim Y, Lee M. Nanostructures of β-sheet peptides: Steps towards bioactive functional materials. Journal of Materials Chemistry. 2008;18:723–7. [Google Scholar]
- 32.Mentlein R, Dahms P, Grandt D, Kruger R. Proteolytic processing of neuropeptide Y and peptide YY by dipeptidyl peptidase IV. Regul Pept. 1993;49:133–44. doi: 10.1016/0167-0115(93)90435-b. [DOI] [PubMed] [Google Scholar]
- 33.Lee BS, Krishnanchettiar S, Lateef SS, Gupta S. Analyses of the in vitro non-enzymatic glycation of peptides/proteins by matrix-assisted laser desorption/ionization mass spectrometry. International Journal of Mass Spectrometry. 2007;260:67–74. [Google Scholar]
- 34.Lee BS, Krishnanchettiar S, Lateef SS, Gupta S. Characterization of oligosaccharide moieties of glycopeptides by microwave-assisted partial acid hydrolysis and mass spectrometry. Rapid Commun Mass Spectrom. 2005;19:1545–50. doi: 10.1002/rcm.1956. [DOI] [PubMed] [Google Scholar]
- 35.Sheriff S, F Qureshy A, T Chance W, Kasckow JW, Balasubramaniam A. Predominant role by CaM kinase in NPY Y(1) receptor signaling: Involvement of CREB. Peptides. 2002;23:87–96. doi: 10.1016/s0196-9781(01)00583-6. corrected. [DOI] [PubMed] [Google Scholar]
- 36.Zhang H, Pandey SC. Effects of PKA modulation on the expression of neuropeptide Y in rat amygdaloid structures during ethanol withdrawal. Peptides. 2003;24:1397–402. doi: 10.1016/j.peptides.2003.08.008. [DOI] [PubMed] [Google Scholar]
- 37.Gordon EA, Kohout TA, Fishman PH. Characterization of functional neuropeptide Y receptors in a human neuroblastoma cell line. J Neurochem. 1990;55:506–13. doi: 10.1111/j.1471-4159.1990.tb04164.x. [DOI] [PubMed] [Google Scholar]
- 38.Nordmann A, Blommers MJ, Fretz H, Arvinte T, Drake AF. Aspects of the molecular structure and dynamics of neuropeptide Y. Eur J Biochem. 1999;261:216–26. doi: 10.1046/j.1432-1327.1999.00263.x. [DOI] [PubMed] [Google Scholar]
- 39.Thiele TE, Badia-Elder NE. A role for neuropeptide Y in alcohol intake control: Evidence from human and animal research. Physiol Behav. 2003;79:95–101. doi: 10.1016/s0031-9384(03)00109-4. [DOI] [PubMed] [Google Scholar]
- 40.Jung SJ, Chang JW, Won R, Cha MH, Nam TS, Lee HJ, et al. Modulation of neuropathic pain by galanin and neuropeptide Y at the level of the medulla in rats. Int J Neurosci. 2009;119:1941–55. doi: 10.1080/00207450903263661. [DOI] [PubMed] [Google Scholar]
- 41.Bedoui S, Miyake S, Lin Y, Miyamoto K, Oki S, Kawamura N, et al. Neuropeptide Y (NPY) suppresses experimental autoimmune encephalomyelitis: NPY1 receptor-specific inhibition of autoreactive Th1 responses in vivo. J Immunol. 2003;171:3451–8. doi: 10.4049/jimmunol.171.7.3451. [DOI] [PubMed] [Google Scholar]
- 42.Khan IU, Zwanziger D, Bohme I, Javed M, Naseer H, Hyder SW, et al. Breast-cancer diagnosis by neuropeptide Y analogues: From synthesis to clinical application. Angew Chem Int Ed Engl. 2010;49:1155–8. doi: 10.1002/anie.200905008. [DOI] [PubMed] [Google Scholar]
- 43.Bettio A, Beck-Sickinger AG. Biophysical methods to study ligand-receptor interactions of neuropeptide Y. Biopolymers. 2001;60:420–37. doi: 10.1002/1097-0282(2001)60:6<420::AID-BIP10183>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 44.Lerch M, Mayrhofer M, Zerbe O. Structural similarities of micelle-bound peptide YY (PYY) and neuropeptide Y (NPY) are related to their affinity profiles at the Y receptors. J Mol Biol. 2004;339:1153–68. doi: 10.1016/j.jmb.2004.04.032. [DOI] [PubMed] [Google Scholar]
- 45.Arscott WT, LaBauve AE, May V, Wesley UV. Suppression of neuroblastoma growth by dipeptidyl peptidase IV: Relevance of chemokine regulation and caspase activation. Oncogene. 2009;28:479–91. doi: 10.1038/onc.2008.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Arumugam K, Subramanian GS, Mallayasamy SR, Averineni RK, Reddy MS, Udupa N. A study of rivastigmine liposomes for delivery into the brain through intranasal route. Acta Pharm. 2008;58:287–97. doi: 10.2478/v10007-008-0014-3. [DOI] [PubMed] [Google Scholar]
- 47.Tin-Tin-Win-Shwe, Mitsushima D, Yamamoto S, Fukushima A, Funabashi T, Kobayashi T, et al. Changes in neurotransmitter levels and proinflammatory cytokine mRNA expressions in the mice olfactory bulb following nanoparticle exposure. Toxicol Appl Pharmacol. 2008;226:192–8. doi: 10.1016/j.taap.2007.09.009. [DOI] [PubMed] [Google Scholar]
- 48.Gao X, Tao W, Lu W, Zhang Q, Zhang Y, Jiang X, et al. Lectin-conjugated PEG-PLA nanoparticles: Preparation and brain delivery after intranasal administration. Biomaterials. 2006;27:3482–90. doi: 10.1016/j.biomaterials.2006.01.038. [DOI] [PubMed] [Google Scholar]
- 49.Intranasal administration of neuropeptide Y in healthy male volunteers (NPY); NCT00748956. 2010 January 20; 2010. Available from: http://clinicaltrials.gov/ct2/show/NCT00748956?term=npy&rank=1.
- 50.Krishnadas A, Onyuksel H, Rubinstein I. Interactions of VIP, secretin and PACAP(1–38) with phospholipids: A biological paradox revisited. Curr Pharm Des. 2003;9:1005–12. doi: 10.2174/1381612033455206. [DOI] [PubMed] [Google Scholar]