Abstract
Aversive events are typically more debilitating when they occur unpredictably than predictably. Studies in humans and animals indicate that predictable and unpredictable aversive events can induce phasic and sustained fear, respectively. Research in rodents suggests that anatomically related but distinct neural circuits may mediate phasic and sustained fear. We explored this issue in humans by examining threat predictability in three virtual reality contexts, one in which electric shocks were predictably signaled by a cue, a second in which shocks occurred unpredictably but never paired with a cue, and a third in which no shocks were delivered. Evidence of threat-induced phasic and sustained fear was presented using fear ratings and skin conductance. Utilizing recent advances in functional magnetic resonance imaging (fMRI), we were able to conduct whole-brain fMRI at relatively high spatial resolution and still have enough sensitivity to detect transient and sustained signal changes in the basal forebrain. We found that both predictable and unpredictable threat evoked transient activity in the dorsal amygdala, but that only unpredictable threat produced sustained activity in a forebrain region corresponding to the bed nucleus of the stria terminalis complex. Consistent with animal models hypothesizing a role for the cortex in generating sustained fear, sustained signal increases to unpredictable threat were also found in anterior insula and a frontoparietal cortical network associated with hypervigilance. In addition, unpredictable threat led to transient activity in the ventral amygdala–hippocampal area and pregenual anterior cingulate cortex, as well as transient activation and subsequent deactivation of subgenual anterior cingulate cortex, limbic structures that have been implicated in the regulation of emotional behavior and stress responses. In line with basic findings in rodents, these results provide evidence that phasic and sustained fear in humans may manifest similar signs of distress, but appear to be associated with different patterns of neural activity in the human basal forebrain.
Keywords: Fear, Anxiety, Dorsal amygdala, BNST, Functional imaging, fMRI
Introduction
In humans and animals, unpredictable aversive events produce debilitating behavioral, cognitive, and somatic effects similar to those found in anxiety and mood disorders (Grillon et al., 2004; Mineka and Kihlstrom, 1978). These effects are usually not found when the same aversive events or threats are predictable (Grillon et al., 2004; Mineka and Hendersen, 1985), suggesting that unpredictable threats are generally more harmful. Studies in humans and rats indicate that predictable threats typically induce phasic fear, a short-lasting apprehension concerning imminent threat, and that unpredictable threats generally induce sustained fear, a longer-lasting apprehension elicited by potential or temporally uncertain threat (Davis et al., 2010b). Rodent data suggest that highly related but partially distinct functional neuroanatomical networks may underlie phasic and sustained fear (Davis et al., 2010b). It is currently unclear whether similar mechanisms support the expression of phasic and sustained fear in humans. The present study investigated this issue using a threat predictability procedure adapted for high-resolution fMRI and virtual reality presentation.
Predictable and unpredictable threats induce similar signs of fear but elicit distinct behavioral and neural responses. In humans and animals, a brief, discrete cue that predictably signals an imminent threat evokes a rapid apprehensive state (phasic fear) that diminishes quickly once the threat is terminated, and triggers active defensive responses such as attack, escape, and physiological reactivity (Fanselow, 1994; Grillon, 2008). In contrast, a diffuse cue that signals a temporally unpredictable threat (e.g., a hazardous environment) elicits a longer-lasting anxiety-like state (sustained fear) associated with passive defensive behaviors such as hypervigilance, avoidance, and quiescence (Blanchard and Blanchard, 2008; Grillon, 2008). Rodent data suggest that phasic fear responses rely on the central nucleus of the amygdala (CeA), which in humans is located in the dorsal part of the amygdala (Amunts et al., 2005), whereas sustained fear responses are dependent on the bed nucleus of the stria terminalis (BNST), which is located in the ventromedial basal forebrain and receives input from the CeA and the more ventrally located basolateral amygdala complex (BLC) (Walker and Davis, 2008). Together with neuronal groups alongside the stria terminalis (supracapsular BNST) and those located beneath the globus pallidus (sublenticular BNST), the CeA and BNST form a neuronal continuum known as the extended amygdala (Alheid and Heimer, 1988; Heimer et al., 1999). Because the CeA and BNST project to the same neural mediators of fear symptoms, each of these components of the extended amygdala are capable of generating defensive responses (Davis and Whalen, 2001).
Consistent with animal research centrally implicating the amygdala in cued fear conditioning and extinction, neuroimaging studies in humans have likewise implicated the amygdala in fear acquisition and extinction (Buchel et al., 1998; LaBar et al., 1998; Milad et al., 2007; Phelps et al., 2004). To identify the neural mechanisms that underlie fear expression, however, it may be more advantageous to examine instructed fear in humans rather than fear conditioning (Davis et al., 2010b). In studies of instructed fear, subjects are verbally informed beforehand of the likelihood of experiencing an aversive event when encountering a stimulus, rather than having to learn this probability through direct experience. For example, Phelps et al. (2001) instructed individuals that they were at risk of receiving an electric shock when they encountered a briefly presented cue of one color (threat cue) but not another color (safe cue). Though no shocks were ever administered, the threat cue evoked enhanced arousal and fear as indexed by skin conductance, and relative to the safe cue, produced a rapid and short-lasting activation in left dorsal amygdala. In a similar study but one using [O-15]H20 positron emission tomography and the delivery of occasional shocks to enhance procedural credibility, Hasler et al. (2007) found that a visual threat cue increased cerebral blood flow in the left amygdala relative to a comparable safe cue. Studies of instructed fear that have examined neural activity underlying both anticipation of and responses to briefly presented aversive pictures have also revealed dorsal amygdala activation (Mackiewicz et al., 2006; Nitschke et al., 2006), provided that the aversive events are predictably signaled (Sarinopoulos et al., 2010). These previous findings support the hypothesis that a neural circuit within the dorsal amygdala may mediate phasic fear in humans.
While many neuroimaging studies in humans have investigated the role of the amygdala in phasic fear, few have examined the specific role of the BNST in sustained fear (Straube et al., 2007). However, Somerville et al. (2010) recently examined the role of the BNST in anxious-related vigilance. In the present study, we investigated whether the BNST was associated with a sustained state of anxiety. Based on the above pre-clinical research in animals and previous neuroimaging studies, we hypothesized that in humans a cue signaling imminent shock would elicit phasic fear responses and transient activity in the dorsal amygdala, whereas the threat of temporally unpredictable shock would produce sustained fear responses and sustained activity in the BNST. Based in part on anterograde and retrograde tract-tracing studies showing that anterior insular cortex projects heavily to the extended amygdala (McDonald et al., 1999), animal models of sustained fear posit that cortical inputs from the insula may also underlie sustained fear, possibly mediating cognitive components of anxious apprehension (Davis et al., 2010b). Therefore, we further hypothesized that the threat of unpredictable shock would generate sustained activity in anterior insula, an area that also has strong projections to medial prefrontal cortical regions associated with the regulation of emotions and visceral reactions (Price and Drevets, 2010).
We tested these predictions using a well-validated instructed threat procedure (Davis et al., 2010b; Grillon et al., 2004), and neuroimaging methods that assured good signal detection of transient cue- and context-related responses, as well as sustained context-related responses in the basal forebrain. Because of partial volume effects from cerebrospinal fluid (CSF) in the cerebral ventricles, it is methodologically challenging to use BOLD fMRI contrast to examine relatively small structures in the basal forebrain. Standard fMRI voxel volumes (e.g., 3.8 × 3.8 × 4.0 mm3) are typically not small enough to decrease partial volume effects nor do they provide the spatial resolution required to detect fMRI signal changes from small brain regions. Imaging at a higher spatial resolution can decrease partial volume effects but can come at the cost of a reduced MRI signal-to-noise ratio (SNR) (Edelstein et al., 1986). Therefore, to boost SNR, we employed a multi-element surface coil array and conducted parallel imaging fMRI (Bodurka et al., 2004). This allowed us to conduct whole-brain fMRI at roughly 4 times higher spatial resolution (1.7 × 1.7 × 3.5 mm3) and still have enough sensitivity to detect BOLD signal changes (Bodurka et al., 2007). Moreover, combining parallel imaging and voxel volume reduction provides the added benefit of reducing susceptibility-related signal dropout from different tissue interfaces (Bellgowan et al., 2006).
Materials and methods
Subjects
Eighteen healthy volunteers (10 males, mean age = 24.7 years, SD = 3.7 years) participated in the study and gave written informed consent approved by the NIMH Human Investigation Review Board. Inclusion criteria included 1) no past or current psychiatric disorders as per Structured Clinical Interview for DSM-IV; 2) no medical condition that interfered with the study objectives; and 3) no use of illicit drugs or psychoactive medications as per urine screen. All subjects in the study exhibited minimal head motion during imaging (mean= 2.1 mm, SD=1.6). Besides the 18 subjects included in the study, 4 were excluded because of excessive shock-related head motion (n=1), failure of the shock delivery system (n=2), and scanner malfunction (n=1).
Experimental procedures
Virtual reality stimuli
The software application (VR Worlds, Psychology Software Tools, Pittsburgh, PA) consisted of several virtual reality (VR) environments, three of which served as contexts in the present study (a restaurant, casino, and bank; Fig. 1B). In addition, a static VR image of the outdoors, a backyard scene extracted from a fourth VR environment, was presented during each inter-trial interval (ITI) to signal a rest period between context presentations. Recordings of each context were made by first having the same person navigate each VR environment from a first person perspective. The recordings were then divided into different scenarios of each context. Each scenario lasted 40-s and was arranged into one of six runs as described below under Instructed threat procedure. During each scenario, subjects virtually entered the restaurant, casino, or bank at one of several locations, and continuously toured the context until transitioning to rest. Because the scenarios were pre-recorded, and passively viewed on a projector screen during scanning, subjects were unable to control navigation in any context. Since entry into each context occurred at several locations within the VR environment, it is unlikely that the onset of a context would be associated with any specific cue within a context. Rather, participants were required to form a mental representation of each context. Context onset occurred over 1 s during which the context emerged from a black background that separated the rest period from the following context presentation. This transition at context onset prevented unwanted orienting responses. Previous research in our laboratory has shown that similarly constructed VR stimuli are effective for studying context manipulations in the MRI environment (Alvarez et al., 2008). Presentation and timing of VR stimuli were achieved using the VR Worlds video editor and PSYLAB (Contact Precision Instruments, London, Great Britain) on Dell laptops running Windows XP (Dell Computer Corp., Round Rock, TX).
Fig. 1.
Experimental paradigm. (A) In each of six runs (one shown), subjects were presented with three different virtual reality (VR) contexts: no-shock (N), predictable shock (P), and unpredictable shock (U). During each 40-s context presentation, a 3-s tone (250 Hz) was presented 1–3 times, followed by a 40-s rest period. In the P context, shocks were delivered as the tone terminated making it a cue for shock; tones had no signal value in the U and N contexts. In the U context, shocks were delivered at any time, making it a signal for temporally unpredictable shock. In the N context, no shock was ever administered, making it a clear signal of safety. (B) Snapshots of the VR contexts. The restaurant was always the no-shock context, and the casino and bank served as predictable and unpredictable contexts counterbalanced across subjects.
Instructed threat procedure
A novel VR paradigm was used to study predictable and unpredictable threat with fMRI (Fig. 1A). For half the subjects, the virtual casino and bank served as predictable and unpredictable shock contexts, respectively. Context assignment was reversed for the remaining subjects so as to counterbalance across subjects which environment was associated with predictable and unpredictable shock; the virtual restaurant was always the no-shock context. In the predictable context (Pcxt), electric shocks were consistently delivered at the offset of a 3-s auditory tone (250 Hz), which served as a predictable threat cue; the tone was meaningless in the other contexts. Shocks were consistently administered in an un-signaled or semi-random manner in the unpredictable context (Ucxt), and no shocks were delivered during the no-shock context (Ncxt).
Each context was presented for 40-s with an ITI of equal duration. There were 6 runs, each followed by a brief rest. In each run, each context was presented once for a total of 6 Ncxt, 6 Pcxt, and 6 Ucxt. The order of context presentation was such that across runs each context appeared an equal number of times as the first, second, and third context presentation. During each context presentation, an auditory cue (250 Hz tone) was presented 1–3 times for a total of 13 no-shock cues (Ncue), 13 predictable cues (Pcue), and 13 unpredictable cues (Ucue). In Pcue, 7 of the 13 cues were reinforced with shock; one Pcxt presentation had two cues reinforced with shock. In Ucxt, the shock was delivered, on average, 17.8 s following the onset of the unpredictable context (range 6–28 s) for a total of 7 shocks; one Ucxt presentation was associated with two un-signaled shocks. There were an equal number of shocks in Pcxt and Ucxt but shocks were administered during the cue in Pcxt only. Therefore, the absence of cues in Pcxt may have signaled relative safety; however, unlike Ncxt, Pcxt was not a safe context in an absolute sense because Pcxt was associated with cue-related shock, much like the context in background contextual conditioning studies (Calandreau et al., 2005). In contrast, there were no safe periods at all in Ucxt, which signaled temporally uncertain threat. Although shocks were never delivered during a cue in Ucxt, it is unlikely that unpredictable cues served as signals of safety since subjects were instructed that a shock could occur at any time during the unpredictable context.
Participants were instructed that they would hear a tone (250 Hz, 3-s duration) in three virtual environments, and that in one (Pcxt; e.g., the casino) they were at risk of receiving a shock only when they heard a tone, and that in another (Ucxt; e.g., the bank) they could receive a shock at any time, but that in the third environment (Ncxt; the restaurant) they would never receive a shock. They were also instructed to rest while viewing the outdoor scene (i.e., during ITI), which they were to fixate until an indoor environment appeared. To ensure that subjects understood the threat procedure, they were exposed to the VR contexts and tone prior to the study, and were required to paraphrase the shock and no-shock contingencies. Retrospective fear ratings obtained immediately following scanning were used to confirm understanding of the threat instructions. This differs from fear conditioning procedures in which contingencies are learned rather than instructed. Therefore, rather than reflecting fear learning per se, this paradigm was expected to enhance fear expression during the predictable cue (phasic fear) and unpredictable context (sustained fear) relative to the no-shock cue and no-shock context, respectively.
Subjective fear and skin conductance assessment
To index contextually induced sustained fear (or anxiety) and cue-induced phasic fear, subjective fear ratings were obtained immediately following the last run using a 0–10 scale of fearfulness (0, not at all; 5, moderately; 10, extremely). Subjects were asked to retrospectively rate their overall levels of fear during the entire 40-s presentation of the restaurant, casino, and bank, in the absence of the auditory cue, as well as during the 3-s cue itself. In addition, throughout each run left volar skin conductance was recorded on the sole of the left foot according to published recommendations (Prokasy and Ebel, 1967). Stimulation and recording were controlled by a commercial system (Contact Precision Instruments, London, Great Britain). Electric shocks up to 5 mA and 200 ms duration served as threat stimuli. The shocks were produced by a constant current stimulator and were administered on the right foot. Shock level was determined individually during a pre-scan test involving the administration of up to three sample shocks. Subjects rated the shock level used in the study as moderately painful (mean=3.4; SD=0.4) based on a 1–5 scale (1, not at all; 3, moderately; 5, extremely).
Skin conductance responses (SCRs) were scored as the largest response initiated 1–5 s after cue or context onset. Additional assessment of tonic skin conductance level was not possible because of the density of cues and shocks that were delivered in each context. Nonetheless, previous research in our laboratory indicates that SCRs to context onset serve as reasonable indices of contextually induced anxiety when elicited by virtual spatial contexts (Alvarez et al., 2008). SCRs were determined by subtracting the skin conductance level at the onset of the SCR from the peak skin conductance level. SCRs to cue and context onset that were contaminated by a shock, or an early cue in the case of a context, were omitted from analyses, assuring that the SCR data were not affected by nuisance factors. A log transformation (log [1 + SCR]) was performed to normalize the distribution, and the magnitudes were range corrected (SCR/SCRmax) for each subject to attain statistical normality and to reduce error variance (Lykken and Venables, 1971).
MRI data acquisition
All subjects were scanned on a 3 T General Electric Signa HDx MRI scanner (60 cm bore, whole body gradient inset with gradient amplitude of 40 mT/m, and slew rate 150 T/m/s, whole body transmit/receive RF coil) equipped with an 8-channel receive-only surface coil brain array. For anatomical reference and alignment purposes high-resolution whole-brain anatomical images were acquired for each subject using a T1-weighted Magnetization Prepared Rapid Gradient Echo (MPRAGE) imaging sequence (axial prescription, number slices per slab 126, slice thickness=1.0 mm, image matrix 256 × 256, square FOV=24 cm). Functional data were acquired using a single-shot, gradient-echo echo-planar imaging (EPI) sequence (matrix size: 128×128, FOV: 220 mm, in-plane resolution: 1.7×1.7 mm2, axial plane: 32 slices, slice thickness: 3.5 mm with no slice gap, TR: 2000 ms, TE: 23 ms, flip angle: 90°, number of volumes: 124). Whole-brain coverage was achieved in all subjects and included coverage of the basal forebrain structures on which we focused (i.e., the BNST complex and dorsal amygdala).
Image preprocessing and realignment
Functional data were processed and analyzed using the AFNI software package (Cox, 1996). To achieve accurate coregistration of functional and anatomical data, especially in the midline where internal brain structures such as the BNST and the CSF-filled ventricles are located, a weighted local Pearson coefficient (LPC) cost functional was used to align anatomical to EPI datasets for each subject (Saad et al., 2009). The advantage of this LPC-based alignment process for imaging ventromedial basal forebrain structures is that it computes the transformations necessary for aligning anatomical and EPI datasets with minimal interpolation, and compared to generic cost functionals, provides superior spatial alignment between T2*- and T1-weighted images in brain structures near the ventricles (Saad et al., 2009). The quality of coregistration between EPI and anatomical datasets was visually verified for each subject. After performing slice-timing correction and volume registration, the first 4 volumes were discarded to allow for T1 equilibrium effects. The EPI data were then scaled by the mean of the time series at each voxel. To optimize our ability to detect fMRI signal changes from small brain structures and to avoid any reduction in spatial resolution, we did not spatially filter or smooth the EPI data. The anatomical data were transformed into Talairach–Tournoux standard space (Talairach and Tournoux, 1988) by manually aligning anterior commissure–posterior commissure and inferior-superior axes, and scaling to Talairach–Tournoux atlas brain size. Landmarks were placed on the high-resolution MPRAGE anatomical dataset of each subject, and the same transformation was applied to each subject's EPI data prior to individual subject analysis. The data from the six runs were concatenated before individual subject analysis.
Data analysis
Subjective fear and skin conductance
To assess changes in subjective fear and SCRs across conditions, Stimulus Type (Context, Cue) × Condition (No-shock, Predictable, Unpredictable) repeated-measures ANOVAs were performed for both sets of data. Follow-up tests involved paired t-tests between the predictable cue and other cue conditions, the unpredictable context and other context conditions, and the predictable cue and predictable context. Note that to eliminate any influence of shock on SCR analyses of cue data, analyses were based only on cue trials in which no shock was present. Because previous work measuring startle reactivity and subjective fear during inter-trial intervals (i.e., contexts) have shown that instructed threat of shock produces a linear increase in fear from the no-shock to the predictable shock to the unpredictable shock context (Grillon et al., 2004), presumably reflecting a gradual increase in contextually induced sustained anxiety, we predicted that in the present study subjective fear ratings and SCRs to Ncxt, Pcxt, and Ucxt would likewise show a linear trend. To assess this hypothesis without stimulus type differences introducing a confound, trend analysis was performed on context data using a one-factor (Context) ANOVA. The significance level was set at 0.05 for all statistical tests and all tests were two-tailed. Greenhouse–Geisser corrections were used for statistical effects involving more than two levels.
Imaging
Data were analyzed using AFNI-implemented multiple regression. Transient responses to cues (Ncue, Pcue, and Ucue) were modeled with 3 basis functions: a gamma variate function with derivatives for time and dispersion, with the magnitude of responses assessed using only the gamma variate function. Transient responses to the onset of each context (Nons, Pons, Uons) were modeled in the same manner as cues, whereas sustained activity for each context (Ncxt, Pcxt, Ucxt) was modeled as the convolution of a gamma and box-car function spanning the entire context; therefore, sustained activity reflected the magnitude of the response throughout the entire context duration. To minimize transient noise associated with context offset and the recovery of the BOLD response, context offsets were modeled identically to cues and context onsets and were treated as regressors of no interest. Regressors of no interest also included 6 head movement parameters, baseline drift, and the response to shocks. The latter was modeled with 3 basis functions as was done for other transient signals. Drifting effects, or low frequency confounds, were modeled with a separate quadratic polynomial for each run. Analysis of transient responses to cues was restricted to trials in which no shock was present to ensure that transient responses to predictable cues were not influenced by the presence of shock during 7 of the 13 trials. Comparisons between cues were based on equal numbers of trials (6 Ncue, 6 Pcue, and 6 Ucue) by including only the no-shock and unpredictable cues that occurred in closest proximity to each predictable cue.
Individual subject analyses were performed using 3dREMLfit, a regression program in AFNI that estimates the serial correlation structure of the noise with an ARMA(1, 1) model, and uses the subsequent temporal correlation matrix to estimate beta parameters using the generalized least squares (GLSQ) method. Following individual analyses, whole-brain group analysis was performed using 3dMEMA (http://www.afni.nimh.nih.gov/sscc/gangc/HBMabstract2010.pdf), a program that incorporates individual beta precision estimates into group effects using a mixed-effects meta-analytic approach. This approach is robust against the violation of within-subject variability assumptions and some types of outliers, and provides more accurate statistical testing at the group level.
To examine the hypothesis that the extended amygdala is differentially implicated in predictable threat (Pcue>Ncue) and unpredictable threat (Uons>Nons, Ucxt>Ncxt), subject-specific β coefficients from each condition and the corresponding standard errors were entered into paired-sample testing as appropriate. Because of the small size of the extended amygdala, it would be too conservative to assess our a priori hypothesis concerning this region by correcting for multiple tests across the whole brain; therefore, statistical conclusions concerning the extended amygdala were based on small volume correction (SVC) for multiple tests (Worsley et al., 1996). To implement SVC at the P<0.05 level, we used AlphaSim, an AFNI program that uses Monte Carlo simulations to estimate the minimum cluster size threshold corresponding to a specific voxel-wise probability and volume threshold. AlphaSim takes into account any intrinsic spatial correlation in the data. Consequently, the residual time series was obtained for each subject and used to calculate the average spatial correlation in the data across subjects (FWHMx=2.67, FWHMy=2.71, FWHMz=3.73; in mm). Statistical maps of the extended amygdala were based on a voxel-wise threshold of P<0.05. Volume correction was calculated based on the total volume of bilateral dorsal amygdala and BNST regions of interest (ROIs). A detailed description of how anatomical ROIs were determined is provided below ROI analyses.
Statistical maps of brain regions outside the extended amygdala were also corrected for multiple tests at the P<0.05 level using AlphaSim. However, for brain regions other than the extended amygdala, the voxel-wise threshold was P<0.005 and volume correction was based on a whole-brain, grey matter mask created by means of the segmentation tool FAST in FSL. The above correction procedures resulted in a minimum cluster size threshold of 40 mm3 for the extended amygdala, and a minimum cluster size threshold of 120 mm3 for all other brain regions. Both specialized and standard brain atlases were used to verify anatomical localization of brain activations (Sakamoto et al., 1999; Mai et al., 2007; Duvernoy, 1999).
Time course data
To depict the time course of transient activity from extended amygdala regions associated with predictable threat (Pcue>Ncue) and unpredictable threat (Uons>Nons), mean subject-specific β values were extracted from individual hemodynamic response curves from the peaks of group activation clusters, then averaged across subjects at each time point (2–14 s after stimulus onset), and plotted for visualization purposes. Sustained activity for each context was modeled with a single basis function providing a straightforward and strong test of sustained activity; however, this modeling approach does not allow for the reconstruction of the hemodynamic response function (HRF) associated with each context. Therefore, strictly to depict the time course of sustained activity from extended amygdala and related regions (e.g., anterior insula), individual analyses were performed as before except modeling each context as the sum of 9 cubic spline basis functions covering 4–36 s post-stimulus onset and omitting the transient response to context onset. From the resulting hemodynamic response curves, mean subject-specific β values were extracted per individual from the peak of the group activation cluster associated with sustained activity to unpredictable threat in the original analysis. These betas were then averaged across subjects at each time point (4–36 s after stimulus onset) and plotted for visualization purposes only. Deconvolution analysis was performed using the AFNI program 3dDeconvolve. Although the time course data derived from 3dDeconvolve are based on beta parameters estimated using the ordinary least squares (OLSQ) method rather than the GLSQ method used by 3dREMLfit, the beta values are comparable because of the statistical property of unbiasedness in the OLSQ method. Therefore, the sustained time course data provide an accurate depiction of the nature of sustained activity in the extended amygdala and related brain regions.
ROI analyses
To further assess the role of the extended amygdala in responses to predictable and unpredictable threat, ancillary analyses were performed on anatomical ROIs of the dorsal amygdala and BNST complex. The dorsal amygdala ROI was derived from bilateral cytoarchitectonic probability maps of the centromedial amygdala (Amunts et al., 2005). Note that the centromedial amygdala map encompasses both the central nucleus and the medial nucleus of the amygdala. Because the BNST complex is currently not included in any available cytoarchitectonic probability map, ROIs of the BNST were anatomically defined according to atlases of the human brain (Mai et al., 2007) and basal forebrain (Sakamoto et al., 1999).
In human and monkey (Martin et al., 1991), the BNST is comprised of several divisions including a dorsally located supracapsular division that courses alongside the stria terminalis and merges rostrally with the lateral BNST (Shammah-Lagnado et al., 2000), the division of the BNST that has been previously implicated in anxiety-like responses in rodents (Walker and Davis, 2008) and humans (Straube et al., 2007; Somerville et al., 2010). The anterior extent of the BNST was defined as the region that merges with the posterior nucleus accumbens ventrolaterally, the caudate nucleus dorsolaterally, and the ventro-lateral septum medially. More posteriorly, the BNST was defined as bounded by the preoptic and hypothalamic area ventrally, the caudate nucleus dorsally, the internal capsule laterally and the lateral ventricle and fornix medially (see Fig. S1 in the Supplementary Information).
Because some boundaries of the BNST cannot be easily identified on high-resolution anatomical images (e.g., the border with the posterior nucleus accumbens), the above guidelines for anatomical demarcation were supplemented by additional rules based on anatomical markers that are clearly visible on MRI images such as the presence of the anterior commissure, the upper extent of the external globus pallidus, and the proximity of the supracapsular or dorsal BNST to the internal capsule and lateral ventricle. Furthermore, there was no attempt to include the sublenticular portion of the BNST as this region is known to be interrupted by fiber bundles and to intermingle with other structures such as the basal nucleus of Meynert (Heimer et al., 2008). Thus, the BNST ROIs in this study comprised two easily distinguishable components bilaterally, an anterior BNST region (approximately 127 mm3) and a dorsal BNST region (approximately 84 mm3). Compared to previously published divisions of the human BNST (Martin et al., 1991), our anterior BNST ROI primarily encompassed anterior, lateral, and medial divisions of the BNST complex (omitting the ventral/sublenticular divisions), and our dorsal BNST ROI corresponded roughly to the supracapsular division (see Fig. S2). Because the supracapsular BNST is anatomically related to the lateral BNST (Shammah-Lagnado et al., 2000), and is known to be highly developed in humans (Strenge et al., 1977), ROI analyses were conducted for both anterior and dorsal regions of the BNST. Anatomical delineation of the BNST was performed using a standardized protocol by a trained research assistant blind to the subjects' identity and adherence to the protocol was assessed by the first author.
The data for ROI analyses were obtained by extracting mean subject-specific β values from anatomical ROIs of dorsal amygdala, anterior BNST, and dorsal BNST, for each condition. To assess whether brain activity in the BNST complex showed a linear increase from Ncxt to Pcxt to Ucxt, paralleling that predicted for SCRs and fear ratings, subject-specific β values corresponding to each context were extracted from anterior and dorsal BNST ROIs, and then entered into a one-factor (Context) ANOVA and subjected to linear trend analysis.
Results
Fear ratings (ANX) and skin conductance responses (SCRs) to contexts and cues differed depending on shock predictability [Stimulus Type × Condition interaction, ANX: F(1.6, 27.5)=38.13, P<0.001; Fig. 2A; SCR: F(1.6, 28.5)= 17.74, P<0.001; Fig. 2B]. Paired t-tests revealed that the predictable cue (Pcue) [ANX: mean (SEM) 7.3 (0.3); SCR: 0.45 (0.06)] was more anxiogenic than the predictable context (Pcxt) [ANX: 4.3 (0.5); t17=6.0, P<0.001; SCR: 0.26 (0.04); t17=5.2, P<0.001] as well as the no-shock cue (Ncue) [ANX: 1.3 (0.3); t17=13.3, P<0.001; SCR: 0.13 (0.03); t17=7.5, P<0.001], and unpredictable cue (Ucue) [ANX: 5.2 (0.5); t17=4.0, P<0.001; SCR: 0.23 (0.04); t17=4.9, P<0.001], and that the unpredictable context (Ucxt) [ANX: 7.3 (0.3); SCR: 0.33 (0.06)] was more anxiogenic than the no-shock context (Ncxt) [ANX: 0.7 (0.2); t17=18.5, P<0.001; SCR: 0.14 (0.03); t17=4.8, P<0.001] and predictable context (Pcxt) [ANX: 4.3 (0.5); t17=7.7, P<0.001; SCR: 0.26 (0.04); t17=1.8, P=0.09]. There was also a significant linear trend from Ncxt to Pcxt to Ucxt [ANX: F(1,17)=343.2, P<0.001; SCR: F(1,17)=22.9, P<0.001] indicating that contextually induced sustained anxiety increased linearly across conditions.
Fig. 2.
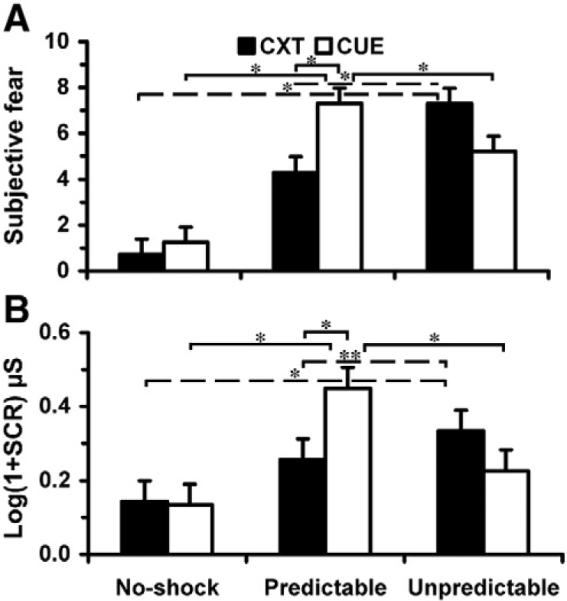
Behavioral data. (A) Subjective fear ratings and (B) SCRs to context (CXT) onset by Stimulus Type (CXT, CUE) and Condition (No-shock, Predictable, Unpredictable). *P<0.001; **P=0.09. Error bars are 95% confidence intervals.
Although the SCR difference between Ucxt and Pcxt was marginally significant compared to the ANX difference, probably because SCRs to context onset are less sensitive indicators of sustained fear states, these results support the view that aversive events produce more sustained anxiety when they occur unpredictably than predictably. These results also confirm that under predictable threat, the cue elicited a phasic apprehensive state that was greater than that elicited by other cues, and greater than the predictable context in the absence of a cue.
To examine transient brain activity associated with predictable threat, we contrasted Pcue with Ncue. Predictable threat evoked phasic activity in the dorsal amygdala (Table 1 and Fig. 3A), roughly in the vicinity of the CeA. No other brain regions were statistically significant after correcting for multiple tests. As a quality control check, therefore, we examined brain activity associated with the predictable cue relative to baseline to determine whether there was activity in the rostral dorsomedial prefrontal/dorsal anterior cingulate (dmPFC/dACC), an area that is consistently activated in instructed fear and classical fear conditioning studies (Mechias et al., 2010). Relative to baseline, the predictable cue elicited transient activity in dmPFC/dACC among other regions (Fig. 4A, Table S1). We also contrasted Pcue with Ucue to assess whether phasic activity in dorsal amygdala was greater to Pcue despite Ucue occurring in an unpredictable shock context. This contrast revealed greater brain activity to Pcue than Ucue in a region of the dorsal amygdala overlapping the dorsal amygdala activation in Pcue>Ncue (Table 1). We also found that Pcue elicited less activity relative to Ucue in the posterior cingulate and precuneus (Table 2).
Table 1.
Transient and sustained activity to predictable and unpredictable threat in extended amygdala.
| Contrast | fMRI signal | Region | x | y | z | t value | Volume (μl) |
|---|---|---|---|---|---|---|---|
| Pcue>Ncue | Transient | L dorsal amygdala | −22 | −5 | −8 | 3.32 | 81 |
| Pcue>Ucue | Transient | L dorsal amygdala | −22 | −5 | −8 | 2.28 | 40 |
| Uons>Nons | Transient | R anterior BNST | 9 | 0 | 10 | 4.88 | 40 |
| L anterior BNST | −9 | 2 | 10 | 3.69 | 40 | ||
| Uons>Pons | Transient | R anterior BNST | 9 | 0 | 10 | 2.23 | 40 |
| L anterior BNST | −9 | 2 | 10 | 2.32 | 40 | ||
| Pons>Nons | Transient | R anterior BNST | 9 | 0 | 10 | 4.39 | 40 |
| L anterior BNST | −6 | 0 | 6 | 2.90 | 40 | ||
| Ucxt>Ncxt | Sustained | L dorsal BNST | −10 | −2 | 16 | 2.13 | 40 |
| Ucxt>Pcxt | Sustained | – | – | – | – | – | – |
| Pcxt>Ncxt | Sustained | – | – | – | – | – | – |
Note. Peak coordinates are listed in Talairach space. t values are based on random effects, paired t-tests thresholded at the voxel-wise level of P<0.05 and cluster size corrected for multiple tests at P<0.05 using small volume correction. Volume indicates cluster size of ventral basal forebrain activity based on single voxel dimensions of 1.7 mm × 1.7 mm × 3.5 mm and a single voxel volume of 10.1 μl.
L, left; R, right; BNST, vicinity of bed nucleus of the stria terminalis complex.
Fig. 3.
Threat-induced transient and sustained brain activity in the extended amygdala. Predictable threat evoked transient activity in the (A) dorsal amygdala (AMYG) (peak at −22 −5 −8), whereas the onset of unpredictable threat elicited transient activity in the (B) bed nucleus of the stria terminalis (BNST) complex (peak at −9 2 10, left; 9 0 10, right). In addition, unpredictable threat produced sustained, increased activity in the vicinity of the (C) supracapsular division of the BNST (peak at −10 −2 16). Brain activity appears on a group structural image aligned to functional data. Adjoining each image is a plot depicting peristimulus time courses of the hemodynamic response. Error bars reflect SE.
Fig. 4.
Additional brain regions showing transient and sustained brain activity to predictable and unpredictable threat. (A) Compared to baseline, the predictable threat cue evoked transient activity in dorsomedial prefrontal/dorsal anterior cingulate (dmPFC/dACC), medial orbital (morb), insula/inferior frontal (INS/ifg), and inferior parietal (ipar) regions. (B) The onset of unpredictable threat was associated with transient activity in pregenual anterior cingulate cortex (pACC), dorsal anterior cingulate cortex (dACC), subgenual anterior cingulate cortex (sACC), medial caudate (CdM) and middle temporal (mtp) regions. (C) In addition, unpredictable threat produced sustained, increased activity in frontopolar (fp), cingulate/superior frontal (sf), anterior insula (aINS), insula (INS), middle frontal (mfr), right and left inferior frontal (ifg), and precentral (prc) regions, as well as sustained decreased activity in sACC. (D) Peristimulus time course plots of the hemodynamic response for selected brain regions in C and Table 2: L anterior insula (−32 21 7); R anterior insula (34 24 10); R inferior frontal (48 14 3); R inferior parietal (56 −46 38); R subgenual ACC (14 34 −11); L medial orbital (−5 46 −15). Error bars reflect SE. L=left; R=right.
Table 2.
Other brain regions showing transient and sustained activity to predictable/unpredictable threat.
| Contrast | Brain region | x | y | z | t value | Volume (μl) |
|---|---|---|---|---|---|---|
| Pcue>Ucue (transient) | L posterior cingulate (deactivation) | −3 | −54 | 24 | 5.02 | 160 |
| L precuneus (deactivation) | −12 | −63 | 24 | 3.80 | 120 | |
| Uons>Nons (transient) | R subgenual anterior cingulate | 10 | 34 | −15 | 13.65 | 400 |
| L medial caudate | −7 | 16 | 6 | 6.64 | 160 | |
| L medial orbital | −8 | 46 | −8 | 3.90 | 120 | |
| L subgenual anterior cingulate | −8 | 24 | −4 | 5.99 | 120 | |
| R pregenual anterior cingulate | 3 | 34 | 3 | 10.12 | 120 | |
| R middle temporal | 60 | −42 | 6 | 3.04 | 120 | |
| R dorsal anterior cingulate | 3 | 14 | 31 | 5.23 | 120 | |
| R ventral amygdala/hippocampus* | 24 | −2 | −17 | 11.45 | 360 | |
| Uons>Pons (transient) | R thalamus | 14 | −13 | 17 | 4.27 | 180 |
| R mid-cingulate | 3 | −13 | 34 | 4.27 | 180 | |
| R pregenual anterior cingulate | 3 | 34 | 3 | 7.61 | 120 | |
| Pons>Nons (transient) | R subgenual anterior cingulate (ACC) | 14 | 34 | −11 | 9.13 | 120 |
| R dorsal anterior cingulate | 3 | 14 | 24 | 4.83 | 120 | |
| Ucxt>Ncxt (sustained) | R subgenual ACC (deactivation) | 14 | 34 | −11 | 1.20 | 620 |
| R superior frontal | 22 | 58 | −4 | 0.89 | 520 | |
| R anterior insula | 34 | 24 | 10 | 0.66 | 400 | |
| R inferior frontal, pars orbitalis | 52 | 34 | −1 | 0.89 | 360 | |
| R inferior frontal (frontal operculum) | 48 | 14 | 3 | 1.08 | 360 | |
| L anterior insula | −32 | 21 | 7 | 0.57 | 360 | |
| L superior frontal | −26 | 60 | −1 | 1.21 | 320 | |
| L middle frontal | −26 | 53 | 21 | 0.50 | 320 | |
| L middle frontal | −35 | 34 | 38 | 0.64 | 280 | |
| R middle frontal | 31 | 56 | 14 | 0.55 | 240 | |
| R medial orbital (deactivation) | 7 | 28 | −15 | 3.89 | 220 | |
| L middle frontal | −29 | 53 | 3 | 0.63 | 200 | |
| L mid-cingulate | −2 | 7 | 42 | 0.65 | 200 | |
| R inferior parietal | 51 | −52 | 42 | 0.49 | 200 | |
| R middle frontal | 37 | 46 | −1 | 0.60 | 180 | |
| L inferior frontal/precentral | −53 | 4 | 10 | 0.57 | 180 | |
| L medial orbital (deactivation) | −5 | 46 | −15 | 1.75 | 160 | |
| R medial orbital (deactivation) | 3 | 41 | −8 | 0.88 | 160 | |
| R frontopolar | 17 | 65 | −4 | 1.04 | 160 | |
| R superior frontal | 31 | 55 | −1 | 0.63 | 160 | |
| R insula | 37 | 17 | 3 | 0.55 | 160 | |
| R inferior frontal, pars triangularis | 48 | 41 | 7 | 0.45 | 160 | |
| L cingulate/superior frontal | −2 | 21 | 28 | 0.61 | 160 | |
| R inferior parietal | 56 | −46 | 38 | 0.43 | 140 | |
| Ucxt>Pcxt (sustained) | R subgenual ACC (deactivation)* | 0 | 31 | −11 | 2.69 | 1180 |
| Pcxt>Ncxt (sustained) | R superior frontal | 26 | 58 | 3 | 0.70 | 360 |
| R middle frontal | 31 | 41 | 24 | 0.36 | 240 | |
| L middle frontal | −26 | 51 | 21 | 0.42 | 220 | |
| R cerebellum (deactivation) | 7 | −46 | −22 | 0.36 | 160 | |
| L medial orbital (deactivation) | −3 | 50 | −15 | 1.57 | 120 |
Note. Thresholded at P<0.005 unless asterisked, and whole-brain corrected
indicates P<0.05.
To assess transient and sustained brain activity elicited by unpredictable threat, we separately contrasted the onset and entire duration of the unpredictable shock and no-shock contexts. Unpredictable threat transiently increased activity in the anterior portion of the BNST complex (Table 1, Fig. 3B) in the approximate vicinity of the lateral BNST. In addition, we found increased transient activity in subgenual, pregenual, and dorsal regions of the anterior cingulate, the medial caudate, and medial orbital and middle temporal cortices (Table 2 and Fig. 4B). Transient activation of the ventral amygdala was present but not statistically significant after correction. When whole-brain correction was performed using a more lenient voxel-wise threshold (P<0.05), however, a large cluster (360 μl) in the ventral amygdala and anterior hippocampus (24 −2 −17, peak; 26 −4 −15, center-of-mass) showed significant transient activation to unpredictable threat.
In addition, we contrasted Uons with Pons to examine whether transient activity in the BNST was greater for a context associated with unpredictable threat than predictable threat. As in the contrast with the no-shock context, the unpredictable context transiently increased activity in the BNST complex relative to the predictable context (Table 1). Compared to the predictable context, the unpredictable context induced enhanced transient activity in the thalamus, mid-cingulate, and pregenual anterior cingulate cortex as well (Table 2). To determine whether transient activation of the BNST was unique to the unpredictable context, or also present in response to the predictable context, Pons was contrasted with Nons. As was found in Uons>Nons and Uons>Pons, the predictable context transiently increased activity bilaterally in the anterior part of the BNST complex compared to the no-shock context (Table 1). The peak coordinates of the right BNST (9 0 10) activation were identical to those associated with the onset of unpredictable threat, but the peak coordinates of the left BNST (−6 0 6) activation were slightly more posteromedial and ventral compared to those associated with unpredictable threat onset (−9 2 10 ). Relative to the no-shock context, the predictable context also increased transient activity in subgenual and dorsal anterior cingulate cortex (Table 2).
Importantly, unpredictable threat elicited sustained increased activity in the dorsal portion of the BNST complex (Table 1, Fig. 3C) in the approximate vicinity of the supracapsular BNST. We also found sustained increased activity in anterior insula, frontal (superior, inferior, middle, frontopolar) and inferior parietal cortical regions, and the mid-cingulate (Table 2, Fig. 4C and D). Following a transient increase in brain activity, unpredictable threat also induced a sustained decrease in activity in subgenual anterior cingulate and medial orbital cortex (Table 2, Fig. 4C and D).
To assess whether sustained activation of the BNST was unique to unpredictable threat, or also associated with the predictable context, we contrasted Ucxt with Pcxt and Pcxt with Ncxt. We found no significant differences in sustained activity in the extended amygdala between unpredictable and predictable contexts, or predictable and no-shock contexts (Table 1). However, when whole-brain correction was performed using a voxel-wise threshold of P<0.05, the unpredictable context showed a sustained deactivation in subgenual anterior cingulate cortex (sACC) relative to the predictable context (Table 2), indicating that after a transient increase in activity in sACC in both threat contexts, there was a sustained decrease in activity in sACC in the unpredictable context compared to the predictable context (Fig. S3). No other significant signal changes were detected in this comparison. The predictable context also generated sustained increased activity in superior and middle frontal cortex, and sustained decreased activity in the cerebellum and medial orbital cortex relative to the no-shock context (Table 2).
To examine the role of the extended amygdala in predictable and unpredictable threat in greater detail, ancillary anatomical ROI analyses were performed on dorsal amygdala and BNST ROIs. Previous studies of instructed fear using simple threat cues (e.g., colored squares) have associated phasic fear responses with a left hemisphere bias (Phelps et al., 2001; Funayama et al., 2001; Hasler et al., 2007). Nevertheless, ROI analysis of the dorsal amygdala failed to detect significantly greater transient activity to Pcue than Ncue when comparing mean subject-specific β values for each condition extracted from bilateral dorsal amygdala (t17=0.55, P=0.59). Follow-up analyses of left and right dorsal amygdala ROIs yielded a similar result (left, t17=0.23, P=0.82; right, t17=0.71, P=0.49).
Because animal models of sustained fear hypothesize that a part of the CeA different from that involved in phasic fear may be transiently involved in initiating sustained fear (Davis et al., 2010b), we tested for a transient fear response to unpredictable threat in bilateral dorsal amygdala. Voxel-wise analyses revealed no activation in dorsal amygdala to unpredictable threat, but our analysis based on ROI data showed that transient activity in dorsal amygdala tended to be greater to the unpredictable context onset than the onset of the no-shock context (t17=1.95, P=0.067). Follow-up analyses indicated that increased transient activity in response to unpredictable threat was significant in the right dorsal amygdala but not the left (Fig. 5A; left, t17=0.58; P=0.57; right, t17=2.57; P=0.02).
Fig. 5.
ROI and linear trend analyses. (A) ROI data showing significant transient activity to unpredictable threat onset (Uons-Nons) in right dorsal amygdala (AMYG) but not left dorsal amygdala. (B) ROI data from the anterior portion of the bed nucleus of the stria terminalis complex (BNST) showing a linear trend in transient brain activity from the no-shock to predictable shock to unpredictable shock context. (C) ROI data from the dorsal portion of the BNST showing a linear trend in sustained brain activity from the no-shock to predictable shock to unpredictable shock context. Error bars indicate SEs.
Overall, analyses of the ROI data from bilateral anterior BNST replicated the pattern of results found in the voxel-wise analyses. Whereas a comparison between Uons and Nons revealed increased transient activity in anterior BNST (t17=4.41, P<0.001), a comparison between Ucxt and Ncxt showed no significant difference in sustained activity in anterior BNST (t17=1.42, P=0.18). In addition, Uons compared to Pons showed that transient activity in anterior BNST was greater to the unpredictable context than the predictable context (t17=2.27, P<0.05), and Pons compared to Nons showed that transient activation of anterior BNST tended to be greater to the predictable context than the no-shock context (t17=1.93, P<0.07).
We also extracted mean subject-specific β values reflecting sustained activity from bilateral dorsal BNST. Consistent with the voxel-wise analyses, there was a trend for Ucxt to show greater sustained activity in dorsal BNST relative to Ncxt (t17=1.82, P=0.08). Follow-up analyses indicated that the trend for increased sustained activity in response to unpredictable threat was present in both hemispheres in dorsal BNST, but relatively stronger in the left hemisphere (left, t17=2.05; P=0.056; right, t17=1.31; P=0.21). It is noteworthy that one subject's beta value for Ucxt met the Hampel identifier criteria for being an outlier (Davies and Gather, 1993), in this case, an unusually small value. With this value temporarily removed from the analysis, the difference in sustained activity between Ucxt and Ncxt was significant in bilateral dorsal BNST (t16=2.27, P=0.037) and left dorsal BNST (left, t16=2.89, P=0.011; right, t16=1.44, P=0.17). As in the voxel-wise analyses, no significant differences were found in sustained activity in dorsal BNST between unpredictable and predictable contexts (Ucxt>Pcxt, t17=1.13, P=0.28), or predictable and no-shock contexts (Pcxt>Ncxt, t17=0.75, P=0.47).
Data in rodents and humans suggest that sustained states of apprehension, including contextually induced anxiety, may stem in part from neural activity in the BNST (Davis et al., 2010b). If this potential brain mechanism extends to humans, BNST activity should track contextually induced sustained anxiety, that is, be greatest to the unpredictable context, least to the no-shock context, and intermediate to the predictable context. In support of this hypothesis, trend analyses performed on ROI data from the BNST complex revealed a significant linear trend from Nons to Pons to Uons in bilateral anterior BNST (Fig. 5B) (F(1, 17)=19.44, P<0.001; left, F(1, 17)=14.45, P=0.0014; right, F(1, 17)=5.80, P=0.027), and a trend for such an effect from Ncxt to Pcxt to Ucxt in bilateral dorsal BNST (Fig. 5C) (F(1, 17)=3.32, P=0.086; left, F(1, 17)=4.21, P=0.055; right, F(1, 17)=1.72, P=0.206). Note that with the previously indentified “outlier” removed from the analysis, the linear trend in sustained activity across contexts was significant in bilateral dorsal BNST (F(1, 16)=5.17, P=0.037) and left dorsal BNST (left; F(1, 16)=8.37, P=0.010; right, F(1, 16)=2.07, P=0.169).
Discussion
The present study compared neural processing of phasic and sustained fear in humans as induced by predictable and unpredictable threat, respectively. Behaviorally, predictable and unpredictable threat evoked pronounced emotional reactions as indicated by subjective fear ratings and skin conductance. In addition, aversive events invoked greater sustained anxiety when they occurred unpredictably than predictably. Using high-resolution fMRI, we found that predictable and unpredictable threat produced different patterns of neural activity in a forebrain region consistent with the extended amygdala, which encompasses the dorsal amygdala and BNST complex (Heimer et al., 2008). Concurring with animal models of phasic and sustained fear (Davis et al., 2010b), both predictable and unpredictable threat increased transient activity in the dorsal amygdala, but only unpredictable threat yielded significant sustained activity in the BNST complex. Beyond the amygdala, predictable threat evoked transient activity in rostral dorsomedial prefrontal and dorsal anterior cingulate cortex. In support of a role for the cortex in sustained fear, unpredictable threat produced sustained activity in anterior insula, mid-cingulate, and frontoparietal cortical networks. In addition, unpredictable threat transiently activated the ventral amygdala-anterior hippocampus area, the anterior portion of the BNST complex, and induced dynamic signal changes in a medial prefrontal network (pregenual, subgenual, and dorsal anterior cingulate cortex, medial caudate, and medial orbital cortex) associated with mood disorders and anxiety dysfunction (Price and Drevets, 2010; Hasler et al., 2004).
Predictable and unpredictable threat are associated with different patterns of brain activation
In the present study, transient neural activity in the dorsal amygdala to a predictable threat cue likely reflected processes in the CeA that lead to a rapid phasic fear response. In support of this interpretation, phasic fear responses and transient activity in dorsal amygdala were greater to the predictable cue than the no-shock cue and unpredictable cue. Because subjects in the present study were forewarned of the threat associated with each cue, subjects encountering a predictable cue would be expected to engage in conscious appraisal of imminent threat. Conscious appraisal processes have been hypothesized to involve a neural circuit including the dmPFC/dACC (Mechias et al., 2010). The present finding that a predictable threat cue evoked transient activation of dmPFC/dACC supports this hypothesis. Neural activity in the posterior cingulate and precuneus were attenuated by the predictable cue compared to the unpredictable cue. The posterior cingulate and precuneus are part of a cortical midline network associated with self-referential processing in the spatial domain (Northoff et al., 2006), suggesting that imminent threats likely evoke greater phasic fear than temporally unpredictable threats, but the latter may give rise to greater vigilance.
We suggest that transient activity in the dorsal amygdala to the onset of an unpredictable threat context may reflect processes in the CeA that lead to the initiation of sustained fear. In support of this view, a ROI analysis revealed that transient activity to unpredictable threat context onset was enhanced in the right dorsal amygdala. This result is consistent with studies in rodents, primates, and humans that demonstrate a role for the CeA in mediating behavioral and physiological responses evoked by conditioned and unconditioned stimuli including contexts (Davis and Whalen, 2001; Kalin et al., 2004; LeDoux, 2000).
The conclusion that dorsal amygdala activation in the present study may reflect involvement of the CeA is supported by previous neuroimaging findings (Whalen et al., 2001). In addition, the peak coordinates of the amygdala activation to predictable threat (−22 −5 −8) are compatible with dorsal amygdala designations in previous fMRI studies (Davis et al., 2010a). However, the relatively small size of the CeA, the close proximity of surrounding nuclei (e.g., basal nucleus of Meynert), and the spatial resolution of fMRI make it extremely difficult to localize the specific neuronal source underlying any dorsal amygdala activation. Hence, any conclusions regarding fMRI signals in the CeA must remain tentative. This point is underscored by the inability to replicate the predictable threat effect in the ROI analysis of the dorsal amygdala. This null finding is perplexing considering that ROIs typically increase the power to detect an effect. It may be that using a ROI that included the entire centromedial amygdala contributed to poor statistical power. It is worth noting however, that detection of amygdala activation in instructed fear studies has been somewhat unreliable (Mechias et al., 2010).
We suggest that transient neural activity in the ventral amygdala to unpredictable threat reflected processes in the BLC that lead to activation of the extended amygdala. This conjecture is supported by research indicating that the BLC is well positioned to receive incoming sensory information concerning threats in the environment, and to convey this information to output areas including the CeA and BNST (Amaral et al., 1992; Dong et al., 2001). Transient activation in ventral amygdala/anterior hippocampus to unpredictable threat context onset provides further evidence that the amygdala (Alvarez et al., 2008; Davis et al., 2010b) and hippocampus (Marschner et al., 2008) play time-limited roles during sustained aversive contexts. It is also consistent with previous studies showing a rapid reduction in amygdala activation over time in fear conditioning (Buchel et al., 1998; LaBar et al., 1998) and threat of shock (Phelps et al., 2001). The absence of activity in the ventral amygdala to the predictable threat cue (tone) is not clear, but it may be that a more complex stimulus (e.g., a context) elicits a more robust signal in the BLC (Yaniv et al., 2004).
In both the voxel-wise and ROI results of the present study, we observed transient activity to the onset of unpredictable threat in the anterior part of the BNST complex in the approximate vicinity of the lateral BNST. Straube et al. (2007) reported increased activity in a similar region of the BNST in phobics relative to controls during the anticipation of phobia-related pictures. Because enhanced activity in the BNST occurred during an anticipatory period of 10–28 s, Straube et al. (2007) interpreted their finding as evidence for BNST involvement in anticipatory anxiety. In line with this interpretation, in the present study transient activity in the BNST complex was greater to the onset of the unpredictable context than the predictable context. Transient activity in the BNST was also greater to the onset of the predictable context than the no-shock context, as would be expected considering the association between the predictable context and cue-related shock. A comparable area of the BNST has been associated with hypervigilant threat monitoring (Somerville et al., 2010).
The present voxel-wise and ROI results indicate that sustained activity in the BNST was restricted to the dorsal part of the BNST complex, roughly corresponding to the supracapsular BNST (Heimer et al., 1999). The supracapsular BNST is a small basal forebrain structure that is relatively well developed in humans (Strenge et al., 1977). The supracapsular and lateral BNST share a similar cellular and neurochemical makeup, and the supracapsular BNST merges into and shares afferent and efferent pathways with the lateral BNST (Shammah-Lagnado et al., 2000); therefore, it is possible that these continuous components of the extended amygdala function together to bring about sustained anxiety. This proposal is compatible with a recently described neural model of sustained fear (Davis et al., 2010b). According to this model, cortical and subcortical input converge on the basolateral amygdala and lateral CeA, which in turn project to the lateral BNST and trigger a cascade of neurochemical release, resulting in a relatively long-lasting apprehensive state. Supporting the notion that the supracapsular and lateral BNST might function together to generate sustained anxiety, the basolateral amygdala and lateral CeA provide extensive inputs to both the supracapsular and lateral BNST (Shammah-Lagnado et al., 2000). The supracapsular BNST also receives strong input from dysgranular anterior insula (Shammah-Lagnado et al., 2000), a cortical region that projects strongly to the basolateral amygdala, lateral CeA, and lateral BNST (McDonald et al., 1999).
It has been suggested that anterior insula might mediate cognitive aspects of worry (Walker and Davis, 2008). On the basis of its connections to the extended amygdala and brainstem (McDonald et al., 1999; Pascoe and Kapp, 1987), it may be more parsimonious to posit that sustained neural activity in the anterior insula to unpredictable threat likely reflects modulation of visceral arousal during apprehension. Previous neuroimaging findings indicating that contextual fear conditioning elicits sustained activity in anterior insula are consistent with this interpretation (Alvarez et al., 2008).
The finding that unpredictable threat induced sustained activity of frontoparietal areas involved in sustained attention (Pardo et al., 1991), is consistent with previous neuroimaging studies linking unpredictable threat to hypervigilance (Carlsson et al., 2006; Hasler et al., 2007). Subgenual anterior cingulate cortex (sACC) is central to the medial prefrontal network that has been associated with mood and anxiety dysregulation, and has been implicated in the regulation of visceral responses during stress and anxiety (Price and Drevets, 2010). This may account for the dynamic pattern of activation observed in sACC in response to unpredictable threat. Neural activity in sACC showed a strong transient increase to the onset of unpredictable threat followed by a sustained decreased response. Clinical studies indicate that metabolism in sACC is elevated in the depressed phase relative to the remitted phase of the same patients with major depressive disorder (Drevets et al., 2008). This suggests that a sustained decrease that follows a transient increase in sACC activity might reflect changes in the regulation of visceral reactions during threat exposure.
In the present study, unpredictable threat contexts compared to predictable threat contexts elicited greater transient activity in pregenual anterior cingulate cortex (pACC), a cingulate subregion that differs from sACC in its cytological and transmitter receptor architecture (Palomero-Gallagher, et al., 2008). Whereas both predictable and unpredictable threat contexts evoked transient activity in sACC, thereafter unpredictable threat contexts were associated with a greater sustained decrease in sACC activity. Although the medial prefrontal network was not the focus of this study, the activations observed in this network are consistent with previous imaging studies implicating medial prefrontal cortex in distal threat (Mobbs et al., 2009), and suggest that contextually induced sustained anxiety may involve complex oscillations in neural activity in key prefrontal regions such as pACC and sACC.
Summary and caveats
Data in rodents and humans (Davis et al., 2010b) support the hypothesis that the amygdala and BNST form the core of a neural system underlying phasic and sustained states of apprehension. The present study provides further support for this hypothesis. We propose that cues that predictably signal imminent threat evoke a short-duration fear response mediated by transient activity in the dorsal amygdala. Conscious appraisal of predictable threat cues may activate threat evaluation processes in dmPFC/dACC. Contexts that signal a longer duration threat initially elicit transient activity in the ventral amygdala/hippocampus and dorsal amygdala, with the former structures possibly being responsible for activation of the latter. As the duration of threat persists, transient activation of the dorsal amygdala gives way to activation of the BNST complex to help maintain anxiety. In addition, we suggest that long-duration states of anxiety engender hypervigilance mediated by frontoparietal networks, and modulation of visceral function via mechanisms within anterior insula, pACC and sACC.
The model outlined above predicts that increases in sustained anxiety should be paralleled by increases in BNST activation. Supporting this prediction, in the present study anatomical ROI analyses revealed a linear trend in transient and sustained activity in the BNST complex, paralleling a linear trend found in contextually induced sustained anxiety as indexed by subjective fear and skin conductance. The observation that unpredictable threat produced significantly sustained activity in a brain region corresponding to the BNST, and not the dorsal amygdala, is consistent with the hypothesis that sustained fear may depend on different neural mechanisms in the extended amygdala than phasic fear (Davis et al., 2010b). It is important to interpret this finding cautiously, however, because the presence of a significant signal change to unpredictable threat, and the absence of such a change to predictable threat, does not demonstrate a significant difference between the two threat conditions. Because such contrasts are problematic for fMRI studies manipulating threat predictability using cue and context stimuli differing in stimulus duration, it will be important to develop imaging methods that allow for direct comparisons between predictable and unpredictable threat. In addition, future studies should consider incorporating measures of phasic and sustained fear beyond subjective ratings and electrodermal responses, which are limited in their ability to index anxiety online, and can reflect emotional as well as non-emotional factors (Boucsein, 1992).
Notwithstanding the limitations of this investigation, the present study took advantage of recent advances in fMRI data acquisition and processing, allowing us to conduct whole-brain fMRI at about 4 times higher spatial resolution than typical fMRI studies, yet still have sufficient sensitivity to detect small signal changes in the basal forebrain. To sum up, the present study suggests that predictable and unpredictable threats can induce phasic and sustained fear in humans, and that these apprehensive states elicit distinct patterns of activation in the basal forebrain including, potentially, the extended amygdala. It is likely that increased knowledge of the extended amygdala and its interaction with the rest of the basal forebrain will advance our understanding of normal and pathological anxiety.
Supplementary Material
Acknowledgments
This study was supported by the Intramural Research Program of the National Institute of Mental Health.
Footnotes
Supplementary materials related to this article can be found online at doi:10.1016/j.neuroimage.2010.11.057.
References
- Alheid GF, Heimer L. New perspectives in basal forebrain organization of special relevance for neuropsychiatric disorders: the striatopallidal, amygdaloid, and corticopetal components of substantia innominata. Neuroscience. 1988;27:1–39. doi: 10.1016/0306-4522(88)90217-5. [DOI] [PubMed] [Google Scholar]
- Alvarez RP, Biggs A, Chen G, Pine DS, Grillon C. Contextual fear conditioning in humans: cortical-hippocampal and amygdala contributions. J. Neurosci. 2008;28:6211–6219. doi: 10.1523/JNEUROSCI.1246-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaral DG, Price JL, Pitkanen A, Carmichael ST. Anatomical organization of the primate amygdaloid complex. In: Aggleton JP, editor. The amygdala: neurobiological aspects of emotion, memory, and mental dysfunction. Wiley-Liss; New York: 1992. [Google Scholar]
- Amunts K, Kedo O, Kindler M, Pieperhoff P, Mohlberg H, Shah NJ, Habel U, Schneider F, Zilles K. Cytoarchitectonic mapping of the human amygdala, hippocampal region and entorhinal cortex: intersubject variability and probability maps. Anat. Embryol. (Berl) 2005;210:343–352. doi: 10.1007/s00429-005-0025-5. [DOI] [PubMed] [Google Scholar]
- Bellgowan PS, Bandettini PA, van Gelderen P, Martin A, Bodurka J. Improved BOLD detection in the medial temporal region using parallel imaging and voxel volume reduction. Neuroimage. 2006;29:1244–1251. doi: 10.1016/j.neuroimage.2005.08.042. [DOI] [PubMed] [Google Scholar]
- Blanchard D, Blanchard R. Defensive behaviors, fear, and anxiety. In: Blanchard RJ, Blanchard DC, Griebel G, Nutt D, editors. Handbook of anxiety and fear. Academic Press; Amsterdam, The Netherlands: 2008. [Google Scholar]
- Bodurka J, Ledden PJ, van Gelderen P, Chu R, De Zwart JA, Morris D, Duyn JH. Scalable multichannel MRI data acquisition system. Magn. Reson. Med. 2004;51:165–171. doi: 10.1002/mrm.10693. [DOI] [PubMed] [Google Scholar]
- Bodurka J, Ye F, Petridou N, Murphy K, Bandettini PA. Mapping the MRI voxel volume in which thermal noise matches physiological noise–implications for fMRI. Neuroimage. 2007;34:542–549. doi: 10.1016/j.neuroimage.2006.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boucsein W. Electrodermal activity. Plenum; New York: 1992. [Google Scholar]
- Buchel C, Morris J, Dolan RJ, Friston KJ. Brain systems mediating aversive conditioning: an event-related fMRI study. Neuron. 1998;20:947–957. doi: 10.1016/s0896-6273(00)80476-6. [DOI] [PubMed] [Google Scholar]
- Calandreau L, Desmedt A, Decorte L, Jaffard R. A different recruitment of the lateral and basolateral amygdala promotes contextual or elemental conditioned association in Pavlovian fear conditioning. Learn. Mem. 2005;12:383–388. doi: 10.1101/lm.92305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsson K, Andersson J, Petrovic P, Petersson KM, Ohman A, Ingvar M. Predictability modulates the affective and sensory-discriminative neural processing of pain. Neuroimage. 2006;32:1804–1814. doi: 10.1016/j.neuroimage.2006.05.027. [DOI] [PubMed] [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput. Biomed. Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Davies L, Gather U. The identification of multiple outliers (with discussion) J. Am. Stat. Assoc. 1993;88:782–792. [Google Scholar]
- Davis M, Whalen PJ. The amygdala: vigilance and emotion. Mol. Psychiatry. 2001;6:13–34. doi: 10.1038/sj.mp.4000812. [DOI] [PubMed] [Google Scholar]
- Davis FC, Johnstone T, Mazzulla EC, Oler JA, Whalen PJ. Regional response differences across the human amygdaloid complex during social conditioning. Cereb. Cortex. 2010a;20(3):612–621. doi: 10.1093/cercor/bhp126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M, Walker DL, Miles L, Grillon C. Phasic vs sustained fear in rats and humans: role of the extended amygdala in fear vs anxiety. Neuropsychopharmacology. 2010b;35(1):105–135. doi: 10.1038/npp.2009.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong HW, Petrovich GD, Swanson LW. Topography of projections from amygdala to bed nuclei of the stria terminalis. Brain Res. Brain Res. Rev. 2001;38:192–246. doi: 10.1016/s0165-0173(01)00079-0. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Savitz J, Trimble M. The subgenual anterior cingulate cortex in mood disorders. CNS Spectr. 2008;13:663–681. doi: 10.1017/s1092852900013754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duvernoy HM. The human brain: surface, three-dimensional sectional anatomy with mri, and blood supply. Springer-Verlag; New York: 1999. [Google Scholar]
- Edelstein WA, Glover GH, Hardy CJ, Redington RW. The intrinsic signal-to-noise ratio in NMR imaging. Magn. Reson. Med. 1986;3:604–618. doi: 10.1002/mrm.1910030413. [DOI] [PubMed] [Google Scholar]
- Fanselow Neural organization of the defensive behavior system responsible for fear. Psychon. Bull. Rev. 1994;1:429–438. doi: 10.3758/BF03210947. [DOI] [PubMed] [Google Scholar]
- Funayama ES, Grillon C, Davis M, Phelps EA. A double dissociation in the affective modulation of startle in humans: effects of unilateral temporal lobectomy. J. Cogn. Neurosci. 2001;13:721–729. doi: 10.1162/08989290152541395. [DOI] [PubMed] [Google Scholar]
- Grillon C. Models and mechanisms of anxiety: evidence from startle studies. Psychopharmacology (Berl) 2008;199:421–437. doi: 10.1007/s00213-007-1019-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillon C, Baas JP, Lissek S, Smith K, Milstein J. Anxious responses to predictable and unpredictable aversive events. Behav. Neurosci. 2004;118:916–924. doi: 10.1037/0735-7044.118.5.916. [DOI] [PubMed] [Google Scholar]
- Hasler G, Drevets WC, Manji HK, Charney DS. Discovering endophenotypes for major depression. Neuropsychopharmacology. 2004;29:1765–1781. doi: 10.1038/sj.npp.1300506. [DOI] [PubMed] [Google Scholar]
- Hasler G, Fromm S, Alvarez RP, Luckenbaugh DA, Drevets WC, Grillon C. Cerebral blood flow in immediate and sustained anxiety. J. Neurosci. 2007;27:6313–6319. doi: 10.1523/JNEUROSCI.5369-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heimer L, Alheid GF, Pearson J, Sakamoto N, Shinoda K, Marksteiner J, Switzer RC., III . The human basal forebrain. Part II. In: Bloom Fe BA, Hokfelt T, editors. Handbook of chemical neuroanatomy. Elsevier; Amsterdam: 1999. [Google Scholar]
- Heimer L, van Hoesen GW, Trimble M, Zahm DS. Anatomy of neuropsychiatry: the new anatomy of the basal forebrain and its implications for neuropsychiatric illness. Elsevier; Amsterdam: 2008. [Google Scholar]
- Kalin NH, Shelton SE, Davidson RJ. The role of the central nucleus of the amygdala in mediating fear and anxiety in the primate. J. Neurosci. 2004;24:5506–5515. doi: 10.1523/JNEUROSCI.0292-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labar KS, Gatenby JC, Gore JC, Ledoux JE, Phelps EA. Human amygdala activation during conditioned fear acquisition and extinction: a mixed-trial fMRI study. Neuron. 1998;20:937–945. doi: 10.1016/s0896-6273(00)80475-4. [DOI] [PubMed] [Google Scholar]
- Ledoux JE. Emotion circuits in the brain. Annu. Rev. Neurosci. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- Lykken DT, Venables PH. Direct measurement of the skin conductance: a proposal for standardization. Psychophysiology. 1971;8:656–672. doi: 10.1111/j.1469-8986.1971.tb00501.x. [DOI] [PubMed] [Google Scholar]
- Mackiewicz KL, Sarinopoulos I, Cleven KL, Nitschke JB. The effect of anticipation and the specificity of sex differences for amygdala and hippocampus function in emotional memory. Proc. Natl Acad. Sci. USA. 2006;103:14200–14205. doi: 10.1073/pnas.0601648103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mai J, Paxinos G, Voss T. Atlas of the human brain. Academic Press; New York: 2007. [Google Scholar]
- Marschner A, Kalisch R, Vervliet B, Vansteenwegen D, Buchel C. Dissociable roles for the hippocampus and the amygdala in human cued versus context fear conditioning. J. Neurosci. 2008;28:9030–9036. doi: 10.1523/JNEUROSCI.1651-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin LJ, Powers RE, Dellovade TL, Price DL. The bed nucleus–amygdala continuum in human and monkey. J. Comp. Neurol. 1991;309:445–485. doi: 10.1002/cne.903090404. [DOI] [PubMed] [Google Scholar]
- McDonald AJ, Shammah-Lagnado SJ, Shi C, Davis M. Cortical afferents to the extended amygdala. Ann. NY Acad. Sci. 1999;877:309–338. doi: 10.1111/j.1749-6632.1999.tb09275.x. [DOI] [PubMed] [Google Scholar]
- Mechias ML, Etkin A, Kalisch R. A meta-analysis of instructed fear studies: implications for conscious appraisal of threat. Neuroimage. 2010;49:1760–1768. doi: 10.1016/j.neuroimage.2009.09.040. [DOI] [PubMed] [Google Scholar]
- Milad MR, Wright CI, Orr SP, Pitman RK, Quirk GJ, Rauch SL. Recall of fear extinction in humans activates the ventromedial prefrontal cortex and hippocampus in concert. Biol. Psychiatry. 2007;62:446–454. doi: 10.1016/j.biopsych.2006.10.011. [DOI] [PubMed] [Google Scholar]
- Mineka S, Hendersen RW. Controllability and predictability in acquired motivation. Annu. Rev. Psychol. 1985;36:495–529. doi: 10.1146/annurev.ps.36.020185.002431. [DOI] [PubMed] [Google Scholar]
- Mineka S, Kihlstrom JF. Unpredictable and uncontrollable events: a new perspective on experimental neurosis. J. Abnorm. Psychol. 1978;87:256–271. doi: 10.1037//0021-843x.87.2.256. [DOI] [PubMed] [Google Scholar]
- Mobbs D, Marchant JL, Hassabis D, Seymour B, Tan G, Gray M, Petrovic P, Dolan RJ, Frith CD. From threat to fear: the neural organization of defensive fear systems in humans. J. Neurosci. 2009;29:12236–12243. doi: 10.1523/JNEUROSCI.2378-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitschke JB, Sarinopoulos I, Mackiewicz KL, Schaefer HS, Davidson RJ. Functional neuroanatomy of aversion and its anticipation. Neuroimage. 2006;29:106–116. doi: 10.1016/j.neuroimage.2005.06.068. [DOI] [PubMed] [Google Scholar]
- Northoff G, Heinzel A, de Greck M, Bermpohl F, Dobrowolny H, Panksepp J. Self-referential processing in our brain–a meta-analysis of imaging studies on the self. Neuroimage. 2006;31:440–457. doi: 10.1016/j.neuroimage.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Palomero-Gallagher N, Mohlberg H, Zilles K, Vogt B. Cytology and receptor architecture of human anterior cingulate cortex. J. Comp. Neurol. 2008;508:906–926. doi: 10.1002/cne.21684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardo JV, Fox PT, Raichle ME. Localization of a human system for sustained attention by positron emission tomography. Nature. 1991;349:61–64. doi: 10.1038/349061a0. [DOI] [PubMed] [Google Scholar]
- Pascoe JP, Kapp BS. Responses of amygdaloid central nucleus neurons to stimulation of the insular cortex in awake rabbits. Neuroscience. 1987;21:471–485. doi: 10.1016/0306-4522(87)90136-9. [DOI] [PubMed] [Google Scholar]
- Phelps EA, O'Connor KJ, Gatenby JC, Gore JC, Grillon C, Davis M. Activation of the left amygdala to a cognitive representation of fear. Nat. Neurosci. 2001;4:437–441. doi: 10.1038/86110. [DOI] [PubMed] [Google Scholar]
- Phelps EA, Delgado MR, Nearing KI, Ledoux JE. Extinction learning in humans: role of the amygdala and vmPFC. Neuron. 2004;43:897–905. doi: 10.1016/j.neuron.2004.08.042. [DOI] [PubMed] [Google Scholar]
- Price JL, Drevets WC. Neurocircuitry of mood disorders. Neuropsychopharmacology. 2010;35:192–216. doi: 10.1038/npp.2009.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prokasy WF, Ebel HC. Three components of the classically conditioned GSR in human subjects. J. Exp. Psychol. 1967;73:247–256. [Google Scholar]
- Saad ZS, Glen DR, Chen G, Beauchamp MS, Desai R, Cox RW. A new method for improving functional-to-structural MRI alignment using local Pearson correlation. Neuroimage. 2009;44:839–848. doi: 10.1016/j.neuroimage.2008.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto N, Pearson J, Shinoda K, Alheid GF. The human basal forebrain. Part 1. An overview. In: Bloom Fe BA, Hokfelt T, editors. Handbook of chemical neuroanatomy. Elsevier; Amsterdam: 1999. [Google Scholar]
- Sarinopoulos I, Grupe DW, Mackiewicz KL, Herrington JD, LOR M, Steege EE, NITSCHKE JB. Uncertainty during anticipation modulates neural responses to aversion in human insula and amygdala. Cereb. Cortex. 2010;20:929–940. doi: 10.1093/cercor/bhp155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shammah-Lagnado SJ, Beltramino CA, McDonald AJ, Miselis RR, Yang M, de Olmos J, Heimer L, Alheid GF. Supracapsular bed nucleus of the stria terminalis contains central and medial extended amygdala elements: evidence from anterograde and retrograde tracing experiments in the rat. J. Comp. Neurol. 2000;422:533–555. doi: 10.1002/1096-9861(20000710)422:4<533::aid-cne5>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Somerville L, Whalen P, Kelley W. Human bed nucleus of the stria terminalis indexes hypervigilant threat. Biol. Psychiatry. 2010 Sep 1;68(5):416–424. doi: 10.1016/j.biopsych.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straube T, Mentzel HJ, Miltner WH. Waiting for spiders: brain activation during anticipatory anxiety in spider phobics. Neuroimage. 2007;37:1427–1436. doi: 10.1016/j.neuroimage.2007.06.023. [DOI] [PubMed] [Google Scholar]
- Strenge H, Braak E, Braak H. Nucleus striae terminalis in the brain of adult man. Pigment architectionical study. Z. Mikrosk.-Anat. Forsch. 1977;91:105–118. [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain. Thieme Medical Publishers; New York: 1988. [Google Scholar]
- Walker DL, Davis M. Role of the extended amygdala in short-duration versus sustained fear: a tribute to Dr. Lennart Heimer. Brain Struct. Funct. 2008;213:29–42. doi: 10.1007/s00429-008-0183-3. [DOI] [PubMed] [Google Scholar]
- Whalen PJ, Shin LM, McInerney SC, Fischer H, Wright CI, Rauch SL. A functional MRI study of human amygdala responses to facial expressions of fear versus anger. Emotion. 2001;1:70–83. doi: 10.1037/1528-3542.1.1.70. [DOI] [PubMed] [Google Scholar]
- Worsley KJ, Marrett S, Neelin P, Vandal AC, Friston KJ, Evans AC. A unified statistical approach for determining significant signal images of cerebral activation. Hum. Brain Mapp. 1996;4:58–73. doi: 10.1002/(SICI)1097-0193(1996)4:1<58::AID-HBM4>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Yaniv D, Desmedt A, Jaffard R, Richter-Levin G. The amygdala and appraisal processes: stimulus and response complexity as an organizing factor. Brain Res. Brain Res. Rev. 2004;44:179–186. doi: 10.1016/j.brainresrev.2003.08.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.






