Abstract
Despite recent advances in perforator flap reconstruction, there can be significant variability in vessel size and location. Although pre-operative evaluation may provide valuable information, real-time intraoperative methods have the potential to provide the greatest benefit. Our laboratory has developed the Fluorescence-Assisted Resection and Exploration (FLARE™) near-infrared (NIR) fluorescence imaging system for visualizing, intraoperatively, details of the underlying vasculature. The FLARE™ system uses indocyanine green, a safe and reliable NIR fluorophore already FDA-approved for other indications. The system has been optimized in large animal models for the identification of perforator size, location, and perfusion, and has also been translated to the clinic for use during breast reconstruction after mastectomy. In this paper, we review our pre-clinical and clinical data, as well as literature describing the use of similar NIR fluorescence imaging systems in plastic and reconstructive surgery.
Keywords: Intraoperative imaging, near-infrared fluorescence angiography, microsurgery, perforator flap, indocyanine green
Introduction
The use of perforator flaps in reconstructive surgery has increased over the past decade. The ability to isolate flaps without muscle has provided a significant advantage, while simultaneously minimizing morbidity. Despite these advances, the key element in designing a perforator flap remains vessel identification and selection. As the perforating vessels demonstrate a high degree of variability, various imaging modalities have been used to identify vessels and increase reliability. The use of the handheld Doppler, Duplex ultrasound, computed tomography (CT), and magnetic resonance imaging (MRI) all require a static pre-operative evaluation correlated to observations made during surgery. Ideally, an intraoperative imaging system would provide the most information and utility in real-time flap design.
Our laboratory has previously described the FLARE™ (Fluorescence-Assisted Resection and Exploration) near-infrared (NIR) fluorescence imaging system and its use for angiography.1-6 This technology uses NIR light, which can penetrate several millimeters into living tissue, to assess sub-surface structures, such as the vasculature.7 The agent used for NIR fluorescence angiography is indocyanine green, which is already FDA-approved for other indications. The concept is similar to intravenous (IV) fluorescein, which is used to assess perfusion in reconstructive surgery. However, NIR light provides orders of magnitude higher sensitivity than visible light, i.e., light from fluorescein's green fluorescence. The FLARE™ imaging system is also capable of displaying surgical anatomy, NIR fluorescence, and a pseudo-colored merge of the two images simultaneously, in real-time, and at high resolution. Using these features, digital images can be replayed and reviewed to determine optimal vessel choice during plastic and reconstructive surgery.
In this review, we describe our experience with NIR fluorescence imaging in large animal model systems and during pilot clinical trials. We also review the existing plastic surgery literature describing similar fluorescent imaging systems that are in early stages of clinical use.
Methods
NIR Fluorescence Imaging System
The FLARE™ imaging system (Figure 1) has been previously described by our laboratory.1,2,8-10 The light source and optics are attached to an articulated arm, which permits their positioning anywhere over the surgical field. Working distance from the bottom of the imaging head to the patient is 18”. The entire arm and cart are covered by a sterile drape.2 The electronics, computer, and monitors are housed on a portable cart. White (400 - 650 nm) light and NIR fluorescence excitation (745 - 779 nm) light are generated by light emitting diodes (LEDs) over a 15-cm diameter area.2 Color video of the surgical field and NIR fluorescence images are obtained simultaneously via custom-designed optics and software.10 A 6-pedal footswitch permits hands-free operation.11 Images can be acquired and displayed as a snapshot or a cine loop (i.e., movie). Color video (i.e., surgical anatomy) and NIR fluorescence images can be displayed separately and merged. Merged images are formed by selecting an unnatural pseudo-color, from a palette of 256 possible colors, converting the grayscale NIR image to this pseudo-color, and overlaying it on top of the color video image. All images are refreshed on the monitor at rates up to 15 times per second (15 Hz).
Figure 1. The FLARE™ (Fluorescence-Assisted Resection and Exploration) Near-Infrared Fluorescence Imaging System.
The articulated arm has a reach of 50” laterally and 70” vertically, which permits positioning of the imaging head anywhere over the surgical field. The footswitch and satellite monitor are positioned according to surgeon preference.
Animals
Animals were studied under the supervision of an approved institutional protocol. Female 35-kg Yorkshire pigs (E. M. Parsons and Sons, Hadley, MA) were used during our study. The pigs were induced with 4.4 mg/kg intramuscular Telazol®, intubated, and maintained with 2% isoflurane (Baxter Healthcare Corp., Deerfield, Illinois). Physiologic parameters were monitored during all experiments.
Perforator Flap Design with NIR Fluorescence Angiography
Animals were injected with ICG as a rapid IV bolus. The given doses ranged from 0.01 to 5.0 mg (mean: 2.49 mg) per injection. Images were acquired at 67-msec exposure every 500 msec for the first 1 min, every 1 sec for the next 1 min, and every minute from 3 to 10 min post-injection. Flaps were designed to encompass the selected perforators; there were two anatomic sites evaluated, the deep superior epigastric artery (DSEA) perforator flap and submental artery perforator flap. Flap perfusion was evaluated with subsequent ICG injections. Multiple injections were given at a minimal time interval of 10 min to reduce the dose stacking effect of ICG.6
Pilot clinical study
The clinical trial was approved by the Institutional Review Board (IRB) of the Beth Israel Deaconess Medical Center and was performed in accordance with the ethical standards of the Helsinki Declaration of 1975. The IRB deemed the FLARE™ imaging system a “non-significant risk” device. All patients gave informed consent and were anonymized. Clinical trial participants were women undergoing breast reconstruction after mastectomy. The patient shown was injected intravenously with 2 mg ICG for each condition imaged. NIR fluorescence angiography was performed using FLARE™ and its LED-based light source,2 as described in detail previously, and with the following system settings: 40,000 lux of white (400-650 nm) light, 14 mW/cm2 of 760 nm NIR fluorescence excitation light, and 67 msec camera acquisition times.
Results
Initial application of NIR fluorescence imaging for flap design
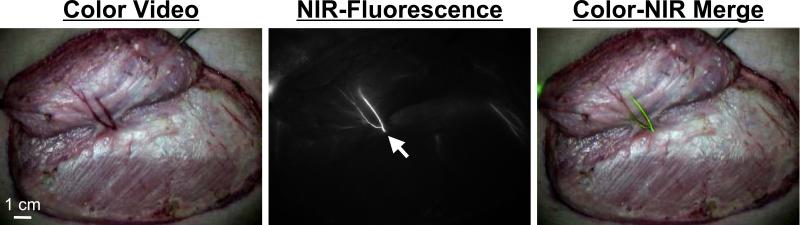
Our initial study looked at the feasibility of intraoperative NIR imaging of the perforating vessels.6 We evaluated 20 abdominal DSEA perforator flaps in 20 pigs using the FLARE™ imaging system. A single ICG injection allowed for localization of the perforating vessels for flap design. Fluorescent activity appeared at the perforating vessel within 20 sec after the injection, then quickly spread into the flap (Figure 2). At 30 to 60 sec post-injection, a venous pattern can be identified as fluorescence was gradually eliminated within 10 to 20 min down to the pre-injection level. After flap elevation, subsequent injections allowed for assessment of flap perfusion. In addition, the perforating vessels could be evaluated on the undersurface of the flap (Figure 3).
Figure 2. Real-Time Quantitative Assessment of Flaps using NIR Fluorescence Angiography.
Pre-operative assessment of an abdominal perforator flap. Shown are the color video (left) image, NIR fluorescence (middle) image, and a pseudo-colored (lime green) merge of the two (right). Images acquired at 8 sec post-injection of ICG. White arrow identifies the origin of a perforating vessel. N: nipple.
Figure 3. Assessment of Vessel Patency.
After flap creation, the vessels on the undersurface of the flap (arrow) can also be evaluated. Shown are the color video image (left), NIR fluorescence image (middle), and a pseudo-colored (lime green) merge of the two (right). Images were acquired 10 sec post-injection of ICG.
Validation of NIR technology for perforator mapping using x-ray fluoroscopy
Our next study looked at confirmation of perforator location with x-ray fluoroscopy.3 We evaluated 8 DSEA perforator flaps in 8 pigs using the FLARE™ imaging system. A flap was then designed by identifying the perforating vessels as well as the source vessel (DSEA). The source vessel was cannulated and a bolus of iodine contrast injected (Renografin®-60, Bracco Diagnostics, Princeton, NJ). Images were recorded by C-arm fluoroscopy (Series 9400 X-ray Imaging System, GE Healthcare, Princeton, NJ). In the 8 flaps evaluated, there was 100% correlation of the number of perforators identified between FLARE™, x-ray angiography, and anatomic dissection.
Quantitative assessment of flap perfusion in perforator flaps
The next study evaluated patterns of perfusion in perforator flaps.5 We isolated the deep superior epigastric vessels and placed occlusive clamps on the artery and vein prior to injection of ICG. Epinephrine was used as a local irrigant to induce vessel spasm in select flaps. When considering our entire experience with the FLARE™ system, which included 345 injections in 39 flaps, we were able to identify, consistently, patterns of vascular compromise. These included arterial spasm, epinephrine induced spasm, total arterial occlusion, and total venous occlusion. From these observations, we identified two indices for arterial and venous compromise, the Tmax Ratio (TR) and Drainage Ratio (DR), respectively. These indices were able to provide a high degree of sensitivity and specificity (TR, 75%, 100%, respectively; DR 100%, 98.9%, respectively).
Assessment of alternate models - the submental perforator flap
We next looked at an alternate model for perforator flap assessment, the submental flap model in the pig.4 In this study, we used the FLARE™ system to perform quantitative perfusion assessment at multiple points in time. The submental perforator flap was designed in a total of 18 pigs separated into three groups: 1 perforator preserved (n = 6), 2 perforators preserved (n = 6), and 3 perforators preserved (n = 6). We evaluated the time to maximum perfusion, venous drainage (DR), and percent of total flap perfused. After initial flap creation, flaps with multiple perforators had an improved time to maximum perfusion and venous drainage compared to a single perforator flap. However, at 6 hrs, all three groups eventually reached nearly 100% perfusion demonstrating that a single perforator flap can redistribute flow by 6 hrs after flap creation.
We also looked at the long-term survival of submental perforator flaps in relation to perfusion at 72 hrs after surgery (Matsui et al., manuscript in preparation). In this study, we evaluated the importance of perforator size (dominant, non-dominant) in relation to location (central, peripheral). At 72 hrs, a central, dominant perforator had significantly improved perfusion indices compared to a peripheral, non-dominant perforator. This may have clinical relevance in predicting areas that may result in fat necrosis long term.
Clinical translation of the technology
After two years of refinement of the FLARE™ system in large animal model systems, we were able to proceed with successful clinical translation.10 In an ongoing pilot study, we have used FLARE™ with patients undergoing deep inferior epigastric perforator flap breast reconstruction. The feasibility of clinical use was assessed, and optimal dosing of ICG was determined. We were able to capture perfusion within hemi-abdominal flaps prior to flap elevation, after flap elevation with isolation on selected perforators, and after microsurgical transfer (Figure 4).
Figure 4. Pilot Clinical Study.
Evaluation of perforators in a deep inferior epigastric perforator flap breast reconstruction prior to vessel dissection (first row, right abdomen; second row, left abdomen). Evaluation of perfusion after isolation of flap on selected perforating vessels of the left abdomen, but prior to transfer (third row). Evaluation of flap perfusion after successful transfer and microsurgical anastomosis (fourth row).
Discussion
The use of an intraoperative NIR fluorescence imaging system as an adjunct for perforator flap design has been used extensively in our laboratory. Our studies demonstrate that use of the FLARE™ imaging system is both simple and effective during large animal surgery6 and during clinical use.10 Through a set of systematic studies, we were able to establish:
Feasibility in abdominal and submental perforator flaps in a pig model.6
Correlation between NIR imaging, x-ray angiography, and surgical exploration.3
Quantitative assessment of perfusion and vascular compromise.5
Assessment of submental flap physiology and perfusion (number of perforators and vessel dominance).4
Feasibility in clinical translation in deep inferior epigastric perforator flap breast reconstruction (Lee et al., manuscript in preparation).
The early animal studies in the literature that used similar technology examined the combination of ICG and NIR fluorescence to assess intraoperative and post-operative global flap perfusion.12-14 Studies dating back to 1994 assessed axial pattern skin flap perfusion in rats and established the pharmacokinetic profile of ICG and its advantages over fluorescein.12,13 Recent studies have examined at random pattern skin flaps in rats and used ICG fluoroscopy to predict flap necrosis while defining thresholds for perfusion.14
Early clinical studies examined perfusion in pedicled flaps.15,16 These descriptive studies showed excellent correlation with clinical findings. Still et al. (1999) used fluorescent imaging in 21 flaps and found no compromise of circulation in 16 flaps. Clinically diminished perfusion in three flaps was seen with fluorescence imaging. Finally, there was one flap that appeared well clinically, showed poor perfusion by imaging, and eventually developed complete flap necrosis. Holm et al. (2002) initially evaluated 15 pedicled skin flaps.16 A one-time injection of ICG was obtained and the surgeon was blinded to the results. The flap was evaluated at one week and the results of the ICG video angiography was compared with excellent correlation. Intraoperative filling defects were always associated with delayed wound healing in this series. In their next report, Holm et al. used NIR imaging for evaluating microsurgery in 20 flaps.17 A one-time assessment was performed after flap transfer and the surgeon was blinded to the results. There were one partial and one total flap loss and both complications were detected by ICG imaging.
Mothes et al. (2004) used ICG video angiography to actively assess tissue perfusion in microsurgery in 25 patients.18 They noted that the post-operative management was altered in 47.2% of cases based on images obtained. In their nine flap series, Krishnan et al. assessed post-operative venous congestion using ICG video imaging.19,20 These flaps included random, pedicled, and free flaps, and their initial observation was that ICG NIR imaging was too sensitive for venous congestion.
The most recent clinical studies examined abdominal flap perfusion using ICG and dynamic laser fluorescence videoangiography.21-23 Yamaguchi et al. (2004) evaluated pedicled transverse rectus abdominis myocutaneous (TRAM) flap perfusion in 10 flaps. They reported consistent and reliable perfusion of the ipsilateral abdominal flap, however, variability of the distal extent of perfusion on the contralateral side. Holm et al. (2006) looked for zones of perfusion in 15 deep inferior epigastric perforator (DIEP) flaps and found an axial pattern of perfusion on the ipsilateral half of the abdomen compared to a random pattern and variable blood supply on the contralateral half. In 2008, the superficial inferior epigastric artery (SIEA) flap was mapped out in 25 patients and the vascular territory did not cross the midline in 64%. The authors specifically recommended intraoperative perfusion measurements in SIEA flaps as they found perfusion to be highly variable. A recent report on ICG angiography described an initial experience in 10 microsurgical abdominal flap breast reconstructions with a commercially available system (Novadaq Technologies Inc., Toronto, Canada).24 In this series, ICG angiography was able to identify 4 cases with perfusion abnormalities requiring operative intervention.
The imaging systems used in these earlier papers were limited, with most utilizing a handheld camcorder device. Such handheld systems provide limited resolution, are subjective, and pose difficulties for real-time quantitation. The Novadaq device requires laser excitation and displays only a single grayscale image of NIR fluorescence. The FLARE™ imaging system permits simultaneous acquisition of color video (i.e., surgical anatomy) and NIR fluorescence angiography, and permits real-time acquisition and quantitative analysis of data.3,5,6 The FLARE™ system also utilizes LEDs instead of lasers.2
Regardless of the imaging system employed, there are obvious limitations to this technology. As with all early technology, the clinical benefits of decreased complications or operative times are still being evaluated. Although data acquisition and display can be performed in real-time, NIR fluorescence imaging will necessarily increase operating room time by approximately 2 min per ICG injection. Field-of-view, presently 15 cm for the FLARE™ imaging system, may not be adequate for the assessment of large flaps. Finally, one must be careful in re-positioning the imaging optics during repeat ICG injections to ensure proper quantitation. However, these limitations are offset by the benefit of being able to assess tissue perfusion before, during and after flap creation and transfer.
Conclusion
Intraoperative evaluation of perforator flaps is possible with a real-time, NIR fluorescence imaging system. Early studies in our laboratory with the FLARE™ imaging system show promise in identifying perforators and for assessing tissue perfusion. In addition, perforating vessels can be visualized on the undersurface of the flap, and assessment of a microsurgical anastomosis can also be performed. Clinical studies are underway to assess potential outcome benefits.
Acknowledgements
We thank Barbara L. Clough for editing and Lorissa A. Moffitt and Eugenia Trabucchi for administrative assistance. This work was funded by the National Institutes of Health grants R01-EB-005805 and R01-CA-115296 to JVF.
Abbreviations
- CT
computed tomography
- DR
Drainage Ratio
- DSEA
deep superior epigastric artery
- DIEP
deep inferior epigastric perforator
- FLARE™
Fluorescence-Assisted Resection and Exploration
- FDA
Food and Drug Administration
- ICG
indocyanine green
- IV
intravenous
- LEDs
light emitting diodes
- MRI
magnetic resonance imaging
- NIR
near-infrared
- SIEA
superficial inferior epigastric artery
- TR
Tmax Ratio
- TRAM
transverse rectus abdominis myocutaneous
Footnotes
Financial Disclosure
All intellectual property for the FLARE™ imaging system is owned by the Beth Israel Deaconess Medical Center, a teaching hospital of Harvard Medical School. As the inventor of the technology, Dr. Frangioni may someday receive royalties if the system is commercialized.
References
- 1.De Grand AM, Frangioni JV. An operational near-infrared fluorescence imaging system prototype for large animal surgery. Technol Cancer Res Treat. 2003;2:553–562. doi: 10.1177/153303460300200607. [DOI] [PubMed] [Google Scholar]
- 2.Gioux S, Kianzad V, Ciocan R, et al. High power, computer-controlled, LED-based light sources for fluorescence imaging and image-guided surgery. Mol Imaging. 2009;8:156–165. [PMC free article] [PubMed] [Google Scholar]
- 3.Matsui A, Lee BT, Winer JH, Kianzad V, Frangioni JV. Image-guided perforator flap design using invisible near-infrared light and validation with x-ray angiography. Ann Plast Surg. 2009;63:327–330. doi: 10.1097/SAP.0b013e318193493d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Matsui A, Lee BT, Winer JH, Laurence RG, Frangioni JV. Submental perforator flap design with a near-infrared fluorescence imaging system: the relation between number of perforators, flap perfusion, and venous drainage. Plastic Recon Surg. 2009 doi: 10.1097/PRS.0b013e3181b5a44c. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Matsui A, Lee BT, Winer JH, Laurence RG, Frangioni JV. Quantitative assessment of perfusion and vascular compromise in perforator flaps using a near-infrared fluorescence-guided imaging system. Plast Reconstr Surg. 2009;124:451–460. doi: 10.1097/PRS.0b013e3181adcf7d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Matsui A, Lee BT, Winer JH, et al. Real-time intraoperative near-infrared fluorescence angiography for perforator identification and flap design. Plast Reconstr Surg. 2009;123:125e–127e. doi: 10.1097/PRS.0b013e31819a3617. [DOI] [PubMed] [Google Scholar]
- 7.Frangioni JV. In vivo near-infrared fluorescence imaging. Curr Opin Chem Biol. 2003;7:626–634. doi: 10.1016/j.cbpa.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 8.Nakayama A, del Monte F, Hajjar RJ, Frangioni JV. Functional near-infrared fluorescence imaging for cardiac surgery and targeted gene therapy. Mol Imaging. 2002;1:365–377. doi: 10.1162/15353500200221333. [DOI] [PubMed] [Google Scholar]
- 9.Tanaka E, Choi HS, Fujii H, Bawendi MG, Frangioni JV. Image-guided oncologic surgery using invisible light: completed pre-clinical development for sentinel lymph node mapping. Ann Surg Oncol. 2006;13:1671–1681. doi: 10.1245/s10434-006-9194-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Troyan SL, Kianzad V, Gibbs-Strauss SL, et al. The FLARE(TM) Intraoperative Near-Infrared Fluorescence Imaging System: A First-in-Human Clinical Trial in Breast Cancer Sentinel Lymph Node Mapping. Ann Surg Oncol. 2009 doi: 10.1245/s10434-009-0594-2. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gioux S, De Grand AM, Lee DS, et al. Improved optical sub-systems for intraoperative near-infrared fluorescence imaging. SPIE Proceedings. 2005;6009:39–48. [Google Scholar]
- 12.Rubben A, Eren S, Krein R, et al. Infrared videoangiofluorography of the skin with indocyanine green--rat random cutaneous flap model and results in man. Microvasc Res. 1994;47:240–251. doi: 10.1006/mvre.1994.1018. [DOI] [PubMed] [Google Scholar]
- 13.Eren S, Rubben A, Krein R, Larkin G, Hettich R. Assessment of microcirculation of an axial skin flap using indocyanine green fluorescence angiography. Plast Reconstr Surg. 1995;96:1636–1649. doi: 10.1097/00006534-199512000-00018. [DOI] [PubMed] [Google Scholar]
- 14.Giunta RE, Holzbach T, Taskov C, et al. Prediction of flap necrosis with laser induced indocyanine green fluorescence in a rat model. Br J Plast Surg. 2005;58:695–701. doi: 10.1016/j.bjps.2005.02.018. [DOI] [PubMed] [Google Scholar]
- 15.Still J, Law E, Dawson J, et al. Evaluation of the circulation of reconstructive flaps using laser-induced fluorescence of indocyanine green. Ann Plast Surg. 1999;42:266–274. doi: 10.1097/00000637-199903000-00007. [DOI] [PubMed] [Google Scholar]
- 16.Holm C, Mayr M, Hofter E, et al. Intraoperative evaluation of skin-flap viability using laser-induced fluorescence of indocyanine green. Br J Plast Surg. 2002;55:635–644. doi: 10.1054/bjps.2002.3969. [DOI] [PubMed] [Google Scholar]
- 17.Holm C, Tegeler J, Mayr M, et al. Monitoring free flaps using laser-induced fluorescence of indocyanine green: a preliminary experience. Microsurgery. 2002;22:278–287. doi: 10.1002/micr.10052. [DOI] [PubMed] [Google Scholar]
- 18.Mothes H, Donicke T, Friedel R, et al. Indocyanine-green fluorescence video angiography used clinically to evaluate tissue perfusion in microsurgery. J Trauma. 2004;57:1018–1024. doi: 10.1097/01.ta.0000123041.47008.70. [DOI] [PubMed] [Google Scholar]
- 19.Krishnan KG, Schackert G, Steinmeier R. The role of near-infrared angiography in the assessment of post-operative venous congestion in random pattern, pedicled island and free flaps. Br J Plast Surg. 2005;58:330–338. doi: 10.1016/j.bjps.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 20.Krishnan KG, Schackert G, Steinmeier R. Near-infrared angiography and prediction of postoperative complications in various types of integumentary flaps. Plast Reconstr Surg. 2004;114:1361–1362. doi: 10.1097/01.prs.0000141630.66941.ca. [DOI] [PubMed] [Google Scholar]
- 21.Holm C, Mayr M, Hofter E, Ninkovic M. Perfusion zones of the DIEP flap revisited: a clinical study. Plast Reconstr Surg. 2006;117:37–43. doi: 10.1097/01.prs.0000185867.84172.c0. [DOI] [PubMed] [Google Scholar]
- 22.Yamaguchi S, De Lorenzi F, Petit JY, et al. The “perfusion map” of the unipedicled TRAM flap to reduce postoperative partial necrosis. Ann Plast Surg. 2004;53:205–209. doi: 10.1097/01.sap.0000116284.51679.ea. [DOI] [PubMed] [Google Scholar]
- 23.Holm C, Mayr M, Hofter E, Raab N, Ninkovic M. Interindividual variability of the SIEA Angiosome: effects on operative strategies in breast reconstruction. Plast Reconstr Surg. 2008;122:1612–1620. doi: 10.1097/PRS.0b013e31818a9a3f. [DOI] [PubMed] [Google Scholar]
- 24.Newman MI, Samson MC. The application of laser-assisted indocyanine green fluorescent dye angiography in microsurgical breast reconstruction. J Reconstr Microsurg. 2009;25:21–26. doi: 10.1055/s-0028-1090617. [DOI] [PubMed] [Google Scholar]