Abstract
Proliferation and activation of microglial cells is a neuropathological characteristic of brain injury and neurodegeneration, including Alzheimer's disease. Microglia act as the first and main form of immune defense in the nervous system. While the primary function of microglia is to survey and maintain the cellular environment optimal for neurons in the brain parenchyma by actively scavenging the brain for damaged brain cells and foreign proteins or particles, sustained activation of microglia may result in high production of proinflammatory mediators that disturb normal brain functions and even cause neuronal injury. Glycogen synthase kinase-3β has been recently identified as a major regulator of immune system and mediates inflammatory responses in microglia. Glycogen synthase kinase-3β has been extensively investigated in connection to tau and amyloid β toxicity, whereas reports on the role of this enzyme in neuroinflammation in Alzheimer's disease are negligible. Here we review and discuss the role of glycogen synthase-3β in immune cells in the context of Alzheimer's disease pathology.
1. Inflammation in Alzheimer's Disease
In addition to progressive loss of neurons and accumulation of intra- and extracellular protein deposits, chronic inflammation is a major pathological hallmark of Alzheimer's disease (AD) [1, 2]. Neuroinflammation in AD is characterized by the existence of inflammatory mediator cells surrounding the β-amyloid (Aβ) plaques and sites of neuronal injury [3–5]. Even though microglia, the main immune cells of the brain, have been extensively studied in AD, the exact role of inflammation in the disease pathogenesis remains elusive [3–10]. There is substantial evidence that microglia and the monocytic cells derived from the blood or bone marrow at least initially protect neurons from neurotoxic accumulation of Aβ and even release neurotrophic factors and extracellular proteases which may support neuronal survival and regeneration [5–11]. On the other hand, extensive and long-term release of proinflammatory mediators and reactive oxygen or nitrogen species (ROS and RNS) by the inflammatory cells is thought to accelerate neurodegeneration and disturb cognitive functions [3–10].
The primary inflammatory cells in the central nervous system are microglia, constituting around 10% of all cells in the brain. They represent the innate immune system and form the first line of defense against invading pathogens in the brain [12–14]. Microglia serve as sensors for disturbed brain tissue homeostasis as they accumulate and proliferate locally in response to neuronal injury or penetration of foreign material in the brain [13, 14]. In AD, such activation can result from extracellular deposition of Aβ, neuronal injury caused by Aβ or tau toxicity [5–15], to some extent from ischemic or traumatic brain injury, and may be contributed even by local or systemic infection [16, 17]. In addition to microglia, astrocytes, pericytes, endothelial cells and neurons are thought to play a role in inflammatory responses relevant to AD [18]. However, most of the data on the impact of inflammation in AD originate from studies with microglia.
2. Glycogen Synthase Kinase 3-β in the Nervous System
Glycogen synthase kinase 3 (GSK-3) is a multifunctional serine/threonine kinase present in all eukaryotes. There are two highly homologous isoforms of GSK-3, GSK-3α and GSK-3β, that are usually equivalent in actions. In addition, there is an alternatively spliced GSK-3β variant that encodes GSK-3β2, which has a 13-residue insert in the kinase domain [19–25] and is expressed exclusively in the nervous system [19–25]. GSK-3 shows partial constitutive activity and is known to phosphorylate more than 50 different substrates. The most important mechanism for regulation of GSK-3β activity is inhibitory phosphorylation of Ser9 by protein kinase A (PKA) protein kinase B (PKB)/Akt and protein kinase C (KPC). Other kinases may phosphorylate the regulatory Ser9 as well. Activation of GSK-3β is enhanced when also the regulatory Tyr216 is phosphorylated [19–25].
In the brain, GSK-3β is known to be involved in neurogenesis, neuronal migration, neuronal polarization, and axon growth and guidance. GSK3β2 shows the highest expression in the nervous system during development and seems to have a special role in neuronal morphogenesis [25–32]. GSK-3β affects axon growth by controlling microtubule dynamics through phosphorylation of microtubule-associated proteins (MAPs) such as Tau, MAP-1B and adenomatous polyposis coli [25–32]. Importantly, GSK-3β plays a key role in neuropathology of AD, schizophrenia, autism and Parkinson's disease (PD). Also, the polymorphisms in GSK3β interact with the microtubule-associated protein Tau (MAPT) haplotypes to increase the risk for idiopathic PD and AD [32–35].
There is substantial evidence that activation of GSK-3β contributes to tau pathology, Aβ synthesis, and apoptotic neuronal death and it is thus not surprising that GSK-3β has been recognized as a potential therapeutic target in AD [35–37]. However, GSK-3β is a well-known regulator of innate and adaptive immune responses and plays a key role also in pathways of microglial activation relevant to AD [19, 20, 38–41]. Considering that neuroinflammation is a characteristic of AD brain pathology, the role of GSK-3β in glial cells is of great interest. The therapeutically interesting role of GSK-3β in regulating inflammation in AD is emphasized by the fact that various forms of Aβ promote microglial activation and release of proinflammatory mediators and ROS/RNS. In addition, in vitro studies suggest that microglial activation may in turn induce accumulation of tau in neurites though microglial ROS production [15].
3. GSK-3β and Migration of Microglia
Migration of blood and bone marrow-derived monocytic cells as well as endogenous microglia to the sites of brain injury and abnormal proteins, such as Aβ, is a necessary step before production of proinflammatory mediators or neurotoxins and attempts of phagocytosis [10, 11, 13, 42–48]. GSK-3β has been reported to be a key kinase regulating migration of various cell types, such as different stem cells and other cells related to development, cancer cells, endothelial cells, and blood-derived inflammatory cells [39, 49–52]. Similarly, GSK-3β has been shown to promote microglial migration both in vitro and in situ [39]. When random and directed migration of BV2 cells were studied using transwell migration and scratch assays, respectively, GSK inhibitors were found to inhibit both types of microglial migration by far more than 50% [39]. The same authors also demonstrated that GSK-3β mediates migration of endogenous mouse microglia in response to slice injury of hippocampus [39]. It is possible that GSK-3β promotes migration/mobility of microglia at least partially by triggering upregulation of CD11b, the αMβ2 integrin and complement receptor, which are needed for adhesion and migration of leukocytes, including microglia [23]. Studies on the role of GSK3 in migration of other cell types support the conclusion that GSK-3β controls multiple pathways involved in migration [28, 30, 51, 53].
4. GSK-3β and Microglial Inflammatory Cytokines, Chemokines and Reactive Oxygen/Nitrogen Species
The production of proinflammatory molecules is a crucial feature of cells needed for innate immune response and the most widely investigated function of microglia in neuroinflammation coupled to AD. Microglia are able to secrete a variety of cytokines and chemokines upon activation by Aβ. These include interleukin 1 (IL-1), interleukin-6 (IL-6), interleukin-8 (IL-8), tumor necrosis factor alpha (TNF-α), as well as chemokines such as macrophage inflammatory protein-1 (MIP-1) and monocyte chemotactic protein-1 (MCP-1) [54]. These secretory products have been postulated to contribute to neuronal death seen in AD brain. In general, cytokines function by regulating the intensity and duration of the immune response [55, 56]. Thus, IL-1 can induce IL-6 production and stimulate synthesis and release of nitric oxide by triggering inducible nitric oxide synthase (iNOS) [57]. This neuroinflammatory stimulation of microglia is further characterized by activation of the complement cascade and induction of the prostanoid generating enzyme cyclooxygenase-2 (COX-2) [57–60]. In addition to this general proinflammatory role, Aβ-induced release of cytokines may promote further Aβ production in microglia. Certain cytokines, such as IL-1, can interact with the amyloid precursor protein (APP) processing pathway resulting in increased cleavage of Aβ [61]. In turn, fibrillar Aβ has been reported to increase neurotoxic secretory products, proinflammatory cytokines and RNS/ROS [5–7]. Eventually, these interactions between cytokines and APP processing establish a self-propagating cycle of neuronal injury [62, 63]. Indeed, several lines of evidence suggest that continuous cytokine production and inflammation-driven cascades cause further activation and recruitment of microglia and can exacerbate disease progression or even sensitize to AD pathology [8, 9]. This continuous reactive microgliosis has been described as the cycle of neuronal death: as in AD brain the cause (Aβ) of microglial activation is not effectively cleared, microglia may enhance their secretion of inflammatory mediators and thus promote toxicity to nearby neurons. However, the causal relationship between microglial activation, cytokine production, Aβ accumulation and neuronal death has not been completely resolved [14].
It is important to note that microglia have also a potential beneficial role in neuroinflammation when another general category of cytokines are released. These antiinflammatory cytokines include IL-1 receptor antagonist (IL-1Ra), IL-4, IL-10, and tumor growth factor beta (TGF-β) [64–68]. IL-4 and TGF-β have a potential to reduce the expression and activity of CD40 and class II MHC [69]. TGFβ has also been reported to reduce Aβ burden in the brain parenchyma in a transgenic mouse model of AD [64]. On the other hand, IL-4 can counteract AD pathology by selectively inducing the clearance of oligomeric Aβ by primary microglia [64, 65]. Similarly, IL-10 can reduce proinflammatory state of microglia by inhibiting the synthesis of cytokines TNFα, IL-1, IL-6, IL-12, granulocyte-macrophage colony stimulating factor (GM-CSF) and chemokines MIP-2, MCP-1 and RANTES [66, 67, 70].
5. Signaling of GSK-3β in Inflammation
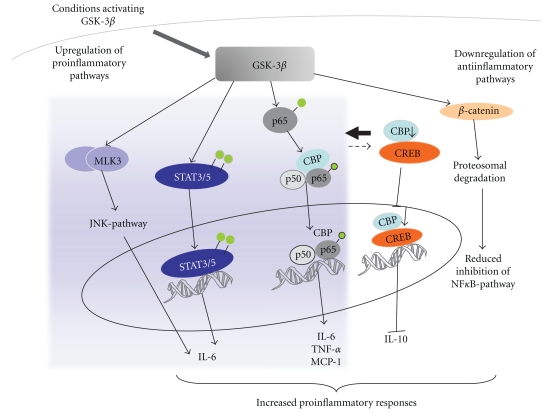
GSK-3β is a major regulator of the balance between the above-mentioned proinflammatory and antiinflammatory mediators in immune cells, including microglia [38, 39]. This regulation is manifested by multiple pathways and include interactions with nuclear factor κB (NF-κB) and mixed lineage kinase 3 (MLK3)/c-Jun N-terminal kinase (JNK) signaling pathways [38, 39, 49] (Figure 1).
Figure 1.
GSK-3β regulates the production of microglial inflammatory molecules. GSK-3β activation has been shown to increase the production of proinflammatory mediators via multiple mechanisms. GSK-3β is able to activate JNK-, STAT3/5- and NF-κB pathways leading to increased cytokine and chemokine production. GSK-3β is able to increase the binding of coactivator CREB-binding protein (CBP) to p65 thus enhancing NF-κB mediated transcription. Since CREB competes for CBP binding, activation of GSK-3β shifts the balance in favor of NF-κB pathway and CREB-mediated induction of IL-10 is reduced. Moreover, GSK-3β activation leads to proteosomal degradation of beta-catenin, thus resulting in reduced inhibition of NF-κB-activation. All these events lead to enhanced production of proinflammatory molecules.
NF-κB is a dimer protein complex that controls the DNA transcription. In resting microglia, the NF-κB dimers are sequestered in the cytoplasm by inhibitors of κB (IκBs) [71]. Activation of the NF-κB is triggered by the signals that result in degradation of IκB, thereby freeing the NF-κB complex to enter the nucleus and interact with the DNA binding sites of NF-κB [72, 73]. Activation of NF-κB mediates expression of several proinflammatory cytokines and iNOS [74]. Once activated, NF-κB transcriptional activity is further regulated by inducible posttranslational modifications, including phosphorylation and acetylation [49, 75–80]. In certain conditions, GSK-3β may regulate NF-κB activation by phosphorylation of p65 subunit of NF-κB upon TNFα treatment, whereas in cultured microglia LPS treatment induces NF-κB activation by increasing acetylation of p65 at lysine 310 through GSK-3β [49, 75–80]. In fact, several studies support the idea that such acetylation of p65 is required for the full transcriptional activity of NF-κB and that GSK-3β increases the p65 binding of the coactivator CREB-binding protein (CBP), which has acetylase activity. CBP is present in limited amounts and also binds and acetylates transcription factor CREB. Thus, these two transcription factors, the p65 subunit of NF-κB and CREB, compete for CBP and activation of GSK-3β pathway shifts the balance in favor of NF-κB [20, 49, 75–80]. The GSK-3β-mediated increase in NF-κB activity results in expression of proinflammatory cytokines and chemokines, such as TNFα, IL-6 and MCP-1. Simultaneously, the expression of IL-10 is reduced, partially because of the reduced DNA binding activity of CREB and also AP1, which are the main transcription factors contributing to IL-10 expression. The eventual proinflammatory effect of GSK-3β signaling is mediated by reduced IL-10 expression, which leads to further increased synthesis of various cytokines and chemokines [20, 49, 75–80].
β-catenin is a transcriptional coactivator of WNT signaling and a direct target of GSK-3β phosphorylation. β-catenin regulates cell proliferation and inhibits NF-κB [81, 82]. Upon GSK-3β phosphorylation, β-catenin enters the proteasomal degradation pathway resulting in reduced inhibition of NF-κB and thereby increased NF-κB-mediated inflammatory responses [81, 82]. β-catenin expression is increased in microglia of transgenic AD mice and Wnt signaling has been reported to play a role in impaired cognitive functions in transgenic AD mouse models [83, 84].
Activation of IL-6 receptors executes proinflammatory response through activation of STAT3 transcription factor, leading to increased expression of proinflammatory molecules, including IL-6 itself. GSK3β selectively promotes STAT3 and STAT5 activation and thereby IL6-induced proinflammatory responses [38, 49, 85].
Finally, certain proinflammatory responses, such as the LPS-induced activation of microglia involve JNK pathway that is regulated by MLK3. GSK-3β phosphorylation may be needed for proper function and dimerization of MLK3, which eventually leads to increased activity of JNK pathway and TNF-α synthesis [49, 86].
Even though there is substantial evidence for proinflammatory role of GSK-3β in several cell types, including microglial cell lines and primary rodent microglia, there are also studies demonstrating an opposite role for GSK-3β. The contradictory results most likely reflect the dependence on the type of cell, stimulus and experimental conditions as the targets of GSK-3β phosphorylation are numerous and of interacting signaling pathways [87–89].
6. GSK-3β and Phagocytosis
Phagocytosis is a main function of microglia. In vitro microglia have the capacity to phagocytose Aβ, but several studies have failed to show actual Aβ-laden vesicles in microglial cells in animal models of AD or in AD [4, 11, 64]. At least the capacity of successful phagocytosis by microglia is very limited in AD brain and not sufficient to prevent the formation of Aβ plaques [4, 11, 64]. However, modulation of microglial activity may enable microglia to effectively phagocytose Aβ as evidenced by activation of microglia for example by Aβ opsonisation [90, 91]. In models of AD, the pathway resulting in Aβ phagocytosis is initiated when Aβ binds a complex of microglial surface receptors consisting of the α6β1 integrin, CD36, CD47, and the class A scavenger receptor (SRA) [10]. In addition, Toll-like receptors (TLRs) which function as dimers and are often coupled to CD14 coreceptors, functionally interact with other partners of the microglial Aβ binding receptor complex [92–96] and execute phagocytosis associated with increased ROS. In turn, engagement of this receptor complex activates tyrosine kinase-based signaling cascades [10, 97, 98] resulting in beneficial phagocytosis but also in production of reactive oxygen species (ROS) and secretion of cytokines [99, 100].
The role of TLR2 and TLR4 in Aβ phagocytosis and AD is emphasized by numerous studies. The expression of TLR2 and TLR4 receptors are upregulated in both AD brains and in related transgenic mouse models of AD. Also, the microglia associated with Aβ plaques show increased levels of mRNA coding for TLR2, -4, -5, -7, and -9 [101]. In addition, AD mice deficient in TLR4 show increased brain Aβ burden. Stimulation of microglial cells with TLR2 and TLR4 ligands boosts indirect clearance of Aβin vitro [102]. Moreover, induction of monocyte recruitment in response to foreign particles, including Aβ, may require activation of TLR-based signaling pathway. Gene delivery of TLR2-lentivirus into the bone marrow cells can rescue the cognitive decline of TLR2 deficient AD mice [103]. Upon Aβ stimulation, monocytes from normal subjects upregulate TLRs, whereas monocytes from AD patients may fail to do so [104]. Also, the level of TLR4 in monocytic cells of AD patients may be lower compared to levels of TLR4 in the same cell population of healthy controls. Finally, bisdemethoxycurcumin, an antiinflammatory compound, improves the defective clearance of Aβ and the transcription and translation of TLR2-4 in monocytic cells of AD patients [104]. These studies point to the importance of TLR signaling in the phagocytic activity of blood-derived monocytic cells in AD.
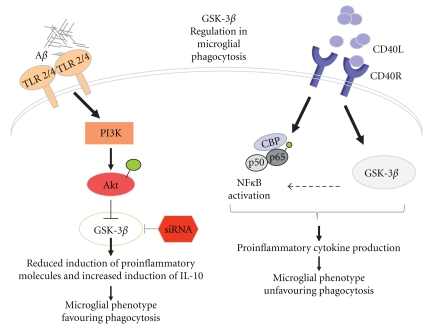
Signaling of several TLRs, including TLR2, TLR4, TLR5, and TLR9 is regulated by GSK-3β in human monocytes and is coupled to production of cytokines [39, 41, 105] (Figure 2). Stimulation of TLR receptors activates phosphatidylinositol 3-OH kinase (PI(3)K) pathway activated Akt leading to phosphorylation and inhibition of GSK-3β. As a result, the cells produce less proinflammatory molecules but upregulates production of antiinflammatory cytokines, such as IL-10 [105].
Figure 2.
GSK-3β in the regulation of microglial phagocytosis. Microglial phagocytosis has been shown to be enhanced through activation of TLR2/4 pathway. Binding of Aβ to TLR2/4 may result in activation of PI3K signalling eventually leading to inhibition of GSK-3β activation. This in turn shifts the cellular balance towards increase in the production of antiinflammatory cytokines favouring phagocytic microglial phenotype. On the other hand, CD40R-CD40L interaction results in both NF-κB and GSK-3β activation thus increasing proinflammatory cytokine production. This may shift the phenotype of microglial cells being less capable of clearing Aβ.
Another pathway relevant for Aβ clearance is triggered by activation of CD40R, a transmembrane receptor of the TNF gene superfamily that is expressed on a variety of cells, such as monocytes, B-cells, antigen presenting cells, endothelial, smooth muscle cells, fibroblasts, and microglia [50]. CD40L is an immunoregulatory molecule that is expressed by activated T-cells, for example. By preventing the CD40-CD40L interaction in AD transgenic mice [106, 107] the Aβ burden is reduced. Aβ stimulation in the presence of CD40-CD40L interaction has been demonstrated to cause diminished microglial phagocytosis and a shift in balance towards an adaptive, antigen-presenting state [108]. It is conceivable that CD40R is activated in microglial cells in AD. The interaction between CD40 and CD40L enhances the expression of cytokines, chemokines, matrix metalloproteinases, growth factors, and adhesion molecules, mainly through the stimulation of NF-κB and also by GSK-3β, which has a role in CD40-mediated response and polarization of naïve CD4+ T cells to Th2 cells [50, 51].
7. Concluding Remarks
Inflammation and especially microglial activation is a contributory factor in neurodegeneration, including AD. Without question, GSK-3β is a central mediator molecule of harmful inflammatory mechanisms relevant to AD. Several studies convincingly link the role of tau and Aβ to increased activity of GSK-3β in the brain [109–114]. Indeed, both human and rodent model studies on AD indicate that inhibition of GSK-3β can be expected to be beneficial in AD [115–120]. Even though some small molecules inhibiting GSK-3β reduce memory/learning deficits and also inflammation in transgenic mouse models of AD [115–120], the link between GSK-3β and harmful inflammation in AD has not been much explored. There are hardly any investigations on the Aβ or tau-related harmful inflammation through mechanisms involving GSK-3β. Based on the overall literature on inflammation, microglia, and AD, we hypothesize that GSK-3β is a potential therapeutic target uniting Aβ deposition, tau aggregation, and inflammation, which represent all the key components of AD pathology.
Acknowledgments
The authors thank The Academy of Finland and The Sigrid Juselius Foundation for their support.
References
- 1.Hardy JA, Higgins GA. Alzheimer’s disease: the amyloid cascade hypothesis. Science. 1992;256(5054):184–185. doi: 10.1126/science.1566067. [DOI] [PubMed] [Google Scholar]
- 2.Crouch PJ, Harding SME, White AR, Camakaris J, Bush AI, Masters CL. Mechanisms of Aβ mediated neurodegeneration in Alzheimer’s disease. International Journal of Biochemistry and Cell Biology. 2008;40(2):181–198. doi: 10.1016/j.biocel.2007.07.013. [DOI] [PubMed] [Google Scholar]
- 3.Michelucci A, Heurtaux T, Grandbarbe L, Morga E, Heuschling P. Characterization of the microglial phenotype under specific pro-inflammatory and anti-inflammatory conditions: effects of oligomeric and fibrillar amyloid-β. Journal of Neuroimmunology. 2009;210(1-2):3–12. doi: 10.1016/j.jneuroim.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 4.Heneka MT, O’Banion MK, Terwel D, Kummer MP. Neuroinflammatory processes in Alzheimer’s disease. Journal of Neural Transmission. 2010;117(8):919–947. doi: 10.1007/s00702-010-0438-z. [DOI] [PubMed] [Google Scholar]
- 5.Eikelenboom P, Van Gool WA. Neuroinflammatory perspectives on the two faces of Al’zheimer’s disease. Journal of Neural Transmission. 2004;111(3):281–294. doi: 10.1007/s00702-003-0055-1. [DOI] [PubMed] [Google Scholar]
- 6.Eikelenboom P, Zhan SS, Van Gool WA, Allsop D. Inflammatory mechanisms in Alzheimer’s disease. Trends in Pharmacological Sciences. 1994;15(12):447–450. doi: 10.1016/0165-6147(94)90057-4. [DOI] [PubMed] [Google Scholar]
- 7.McGeer PL, McGeer EG. The inflammatory response system of brain: implications for therapy of Alzheimer and other neurodegenerative diseases. Brain Research Reviews. 1995;21(2):195–218. doi: 10.1016/0165-0173(95)00011-9. [DOI] [PubMed] [Google Scholar]
- 8.Akiyama H, Barger S, Barnum S, et al. Inflammation and Alzheimer’s disease. Neurobiology of Aging. 2000;21(3):383–421. doi: 10.1016/s0197-4580(00)00124-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McGeer PL, McGeer EG. Inflammation, autotoxicity and Alzheimer disease. Neurobiology of Aging. 2001;22(6):799–809. doi: 10.1016/s0197-4580(01)00289-5. [DOI] [PubMed] [Google Scholar]
- 10.Bamberger ME, Harris ME, McDonald DR, Husemann J, Landreth GE. A cell surface receptor complex for fibrillar β-amyloid mediates microglial activation. Journal of Neuroscience. 2003;23(7):2665–2674. doi: 10.1523/JNEUROSCI.23-07-02665.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Malm TM, Koistinaho M, Pärepalo M, et al. Bone-marrow-derived cells contribute to the recruitment of microglial cells in response to β-amyloid deposition in APP/PS1 double transgenic Alzheimer mice. Neurobiology of Disease. 2005;18(1):134–142. doi: 10.1016/j.nbd.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 12.Ransohoff RM. Chemokines and chemokine receptors: standing at the crossroads of immunobiology and neurobiology. Immunity. 2009;31(5):711–721. doi: 10.1016/j.immuni.2009.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hanisch UK, Kettenmann H. Microglia: active sensor and versatile effector cells in the normal and pathologic brain. Nature Neuroscience. 2007;10(11):1387–1394. doi: 10.1038/nn1997. [DOI] [PubMed] [Google Scholar]
- 14.Hanisch UK. Microglia as a source and target of cytokines. GLIA. 2002;40(2):140–155. doi: 10.1002/glia.10161. [DOI] [PubMed] [Google Scholar]
- 15.Gorlovoy P, Larionov S, Pham TTH, Neumann H. Accumulation of tau induced in neurites by microglial proinflammatory mediators. FASEB Journal. 2009;23(8):2502–2513. doi: 10.1096/fj.08-123877. [DOI] [PubMed] [Google Scholar]
- 16.Koistinaho M, Koistinaho J. Role of p38 and p44/42 mitogen-activated protein kinases in microglia. GLIA. 2002;40(2):175–183. doi: 10.1002/glia.10151. [DOI] [PubMed] [Google Scholar]
- 17.Koistinaho M, Koistinaho J. Interactions between Alzheimer’s disease and cerebral ischemia - Focus on inflammation. Brain Research Reviews. 2005;48(2):240–250. doi: 10.1016/j.brainresrev.2004.12.014. [DOI] [PubMed] [Google Scholar]
- 18.Lue LF, Yan SD, Stern DM, Walker DG. Preventing activation of receptor for advanced glycation endproducts in Alzheimer’s disease. Current Drug Targets. 2005;4(3):249–266. doi: 10.2174/1568007054038210. [DOI] [PubMed] [Google Scholar]
- 19.Beurel E, Jope RS. Glycogen synthase kinase-3 regulates inflammatory tolerance in astrocytes. Neuroscience. 2010;69(3):1063–1070. doi: 10.1016/j.neuroscience.2010.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Beurel E, Michalek SM, Jope RS. Innate and adaptive immune responses regulated by glycogen synthase kinase-3 (GSK3) Trends in Immunology. 2010;31(1):24–31. doi: 10.1016/j.it.2009.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Woodgett JR. Molecular cloning and expression of glycogen synthase kinase-3/factor A. EMBO Journal. 1990;9(8):2431–2438. doi: 10.1002/j.1460-2075.1990.tb07419.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mukai F, Ishiguro K, Sano Y, Fujita SC. Aternative splicing isoform of tau protein kinase I/glycogen synthase kinase 3β. Journal of Neurochemistry. 2002;81(5):1073–1083. doi: 10.1046/j.1471-4159.2002.00918.x. [DOI] [PubMed] [Google Scholar]
- 23.Solovjov DA, Pluskota E, Plow EF. Distinct roles for the α and β subunits in the functions of integrin αβ. Journal of Biological Chemistry. 2005;280(2):1336–1345. doi: 10.1074/jbc.M406968200. [DOI] [PubMed] [Google Scholar]
- 24.Schaffer B, Wiedau-Pazos M, Geschwind DH. Gene structure and alternative splicing of glycogen synthase kinase 3 beta (GSK-3β) in neural and non-neural tissues. Gene. 2003;302(1-2):73–81. doi: 10.1016/s0378-1119(02)01092-2. [DOI] [PubMed] [Google Scholar]
- 25.Wood-Kaczmar A, Kraus M, Ishiguro K, Philpott KL, Gordon-Weeks PR. An alternatively spliced form of glycogen synthase kinase-3β is targeted to growing neurites and growth cones. Molecular and Cellular Neuroscience. 2009;42(3):184–194. doi: 10.1016/j.mcn.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 26.Hur E-M, Zhou F-Q. GSK3 signalling in neural development. Nature Reviews Neuroscience. 2010;11(8):539–551. doi: 10.1038/nrn2870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Castaño Z, Gordon-Weeks PR, Kypta RM. The neuron-specific isoform of glycogen synthase kinase-3β is required for axon growth. Journal of Neurochemistry. 2010;113(1):117–130. doi: 10.1111/j.1471-4159.2010.06581.x. [DOI] [PubMed] [Google Scholar]
- 28.Owen R, Gordon-Weeks PR. Inhibition of glycogen synthase kinase 3β in sensory neurons in culture alters filopodia dynamics and microtubule distribution in growth cones. Molecular and Cellular Neuroscience. 2003;23(4):626–637. doi: 10.1016/s1044-7431(03)00095-2. [DOI] [PubMed] [Google Scholar]
- 29.Yoshimura T, Kawano Y, Arimura N, Kawabata S, Kikuchi A, Kaibuchi K. GSK-3β regulates phosphorylation of CRMP-2 and neuronal polarity. Cell. 2005;120(1):137–149. doi: 10.1016/j.cell.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 30.Farooqui R, Zhu S, Fenteany G. Glycogen synthase kinase-3 acts upstream of ADP-ribosylation factor 6 and Rac1 to regulate epithelial cell migration. Experimental Cell Research. 2006;312(9):1514–1525. doi: 10.1016/j.yexcr.2006.01.018. [DOI] [PubMed] [Google Scholar]
- 31.Kim WY, Wang X, Wu Y, et al. GSK-3 is a master regulator of neural progenitor homeostasis. Nature Neuroscience. 2009;12(11):1390–1397. doi: 10.1038/nn.2408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bultje RS, Castaneda-Castellanos DR, Jan LY, Jan YN, Kriegstein AR, Shi SH. Mammalian par3 regulates progenitor cell asymmetric division via notch signaling in the developing neocortex. Neuron. 2009;63(2):189–202. doi: 10.1016/j.neuron.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kwok JBJ, Hallupp M, Loy CT, et al. GSK3B polymorphisms alter transcription and splicing in Parkinson’s disease. Annals of Neurology. 2005;58(6):829–839. doi: 10.1002/ana.20691. [DOI] [PubMed] [Google Scholar]
- 34.Kwok JBJ, Loy CT, Hamilton G, et al. Glycogen synthase kinase-3β and tau genes interact in Alzheimer’s disease. Annals of Neurology. 2008;64(4):446–454. doi: 10.1002/ana.21476. [DOI] [PubMed] [Google Scholar]
- 35.Shin J, Yu S-B, Yu UY, Jo SA, Ahn J-H. Swedish mutation within amyloid precursor protein modulates global gene expression towards the pathogenesis of Alzheimer's disease. BMB Reports. 2010;43(10):704–709. doi: 10.5483/BMBRep.2010.43.10.704. [DOI] [PubMed] [Google Scholar]
- 36.Avila J, Wandosell F, Hernández F. Role of glycogen synthase kinase-3 in Alzheimer’s disease pathogenesis and glycogen synthase kinase-3 inhibitors. Expert Review of Neurotherapeutics. 2010;10(5):703–710. doi: 10.1586/ern.10.40. [DOI] [PubMed] [Google Scholar]
- 37.Mao Y, Ge X, Frank CL, et al. Disrupted in schizophrenia 1 regulates neuronal progenitor proliferation via modulation of GSK3β/β-Catenin Signaling. Cell. 2009;136(6):1017–1031. doi: 10.1016/j.cell.2008.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Beurel E, Jope RS. Lipopolysaccharide-induced interleukin-6 production is controlled by glycogen synthase kinase-3 and STAT3 in the brain. Journal of Neuroinflammation. 2009;6, article 9 doi: 10.1186/1742-2094-6-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yuskaitis CJ, Jope RS. Glycogen synthase kinase-3 regulates microglial migration, inflammation, and inflammation-induced neurotoxicity. Cellular Signalling. 2009;21(2):264–273. doi: 10.1016/j.cellsig.2008.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ding Y, Qiao A, Fan GH. Indirubin-3’-monoxime rescues spatial memory deficits and attenuates β-amyloid-associated neuropathology in a mouse model of Alzheimer’s disease. Neurobiology of Disease. 2010;39(2):156–168. doi: 10.1016/j.nbd.2010.03.022. [DOI] [PubMed] [Google Scholar]
- 41.Huang WC, Lin YS, Wang CY, et al. Glycogen synthase kinase-3 negatively regulates anti-inflammatory interleukin-10 for lipopolysaccharide-induced iNOS/NO biosynthesis and RANTES production in microglial cells. Immunology. 2009;128(1, supplement):e275–e286. doi: 10.1111/j.1365-2567.2008.02959.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Malm T, Koistinaho M, Muona A, Magga J, Koistinaho J. The role and therapeutic potential of monocytic cells in Alzheimer’s disease. GLIA. 2010;58(8):889–900. doi: 10.1002/glia.20973. [DOI] [PubMed] [Google Scholar]
- 43.Choi J, Zheng Q, Katz HE, Guilarte TR. Silica-based nanoparticle uptake and cellular response by primary microglia. Environmental Health Perspectives. 2010;118(5):589–595. doi: 10.1289/ehp.0901534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Reed-Geaghan EG, Savage JC, Hise AG, Landreth GE. CD14 and toll-like receptors 2 and 4 are required for fibrillar Aβ-stimulated microglial activation. Journal of Neuroscience. 2009;29(38):11982–11992. doi: 10.1523/JNEUROSCI.3158-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Koronyo-Hamaoui M, Ko MK, Koronyo Y, et al. Attenuation of AD-like neuropathology by harnessing peripheral immune cells: local elevation of IL-10 and MMP-9. Journal of Neurochemistry. 2009;111(6):1409–1424. doi: 10.1111/j.1471-4159.2009.06402.x. [DOI] [PubMed] [Google Scholar]
- 46.Yamamoto M, Kiyota T, Walsh SM, Liu J, Kipnis J, Ikezu T. Cytokine-mediated inhibition of fibrillar amyloid-β peptide degradation by human mononuclear phagocytes. Journal of Immunology. 2008;181(6):3877–3886. doi: 10.4049/jimmunol.181.6.3877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Teixeira AL, Reis HJ, Coelho FM, et al. All-or-nothing type biphasic cytokine production of human lymphocytes after exposure to Alzheimer's β-Amyloid peptide. Biological Psychiatry. 2008;64(10):891–895. doi: 10.1016/j.biopsych.2008.07.019. [DOI] [PubMed] [Google Scholar]
- 48.Reale M, Iarlori C, Feliciani C, Gambi D. Peripheral chemokine receptors, their ligands, cytokines and Alzheimer’s disease. Journal of Alzheimer’s Disease. 2008;14(2):147–159. doi: 10.3233/jad-2008-14203. [DOI] [PubMed] [Google Scholar]
- 49.Wang M-J, Huang H-Y, Chen W-F, Chang H-F, Kuo J-S. Glycogen synthase kinase-3β inactivation inhibits tumor necrosis factor-α production in microglia by modulating nuclear factor κB and MLK3/JNK signaling cascades. Journal of Neuroinflammation. 2010;31(1):p. 99. doi: 10.1186/1742-2094-7-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chatzigeorgiou A, Lyberi M, Chatzilymperis G, Nezos A, Kamper E. CD40/CD40L signaling and its implication in health and disease. BioFactors. 2009;35(6):474–483. doi: 10.1002/biof.62. [DOI] [PubMed] [Google Scholar]
- 51.Ono T, Yanagawa Y, Iwabuchi K, Nonomura K, Onoé K. Glycogen synthase kinase 3 activity during development of bone marrow-derived dendritic cells (DCs) essential for the DC function to induce T helper 2 polarization. Immunology. 2007;122(2):189–198. doi: 10.1111/j.1365-2567.2007.02627.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li Q, Michaud M, Canosa S, Kuo A, Madri JA. GSK-3β: a signaling pathway node modulating neural stem cell and endothelial cell interactions. doi: 10.1007/s10456-011-9201-9. Angiogenesis, 2011, In press. [DOI] [PubMed] [Google Scholar]
- 53.Neumann H, Linnartz B, Wang Y. Microglial immunoreceptor tyrosine-based activation and inhibition motif signaling in neuroinflammation. International Journal of Alzheimer's Disease. 2010;2010:7 pages. doi: 10.4061/2010/587463. Article ID 587463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rogers J, Lue LF. Microglial chemotaxis, activation, and phagocytosis of amyloid β-peptide as linked phenomena in Alzheimer’s disease. Neurochemistry International. 2001;39(5-6):333–340. doi: 10.1016/s0197-0186(01)00040-7. [DOI] [PubMed] [Google Scholar]
- 55.Tuppo EE, Arias HR. The role of inflammation in Alzheimer’s disease. International Journal of Biochemistry and Cell Biology. 2005;37(2):289–305. doi: 10.1016/j.biocel.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 56.Heneka MT, O’Banion MK. Inflammatory processes in Alzheimer’s disease. Journal of Neuroimmunology. 2007;184(1-2):69–91. doi: 10.1016/j.jneuroim.2006.11.017. [DOI] [PubMed] [Google Scholar]
- 57.Abbas N, Bednar I, Mix E, et al. Up-regulation of the inflammatory cytokines IFN-γ and IL-12 and down-regulation of IL-4 in cerebral cortex regions of APP transgenic mice. Journal of Neuroimmunology. 2002;126(1-2):50–57. doi: 10.1016/s0165-5728(02)00050-4. [DOI] [PubMed] [Google Scholar]
- 58.Bezzi P, Domercq M, Brambilla L, et al. CXCR4-activated astrocyte glutamate release via TNFa: amplification by microglia triggers neurotoxicity. Nature Neuroscience. 2001;4(7):702–710. doi: 10.1038/89490. [DOI] [PubMed] [Google Scholar]
- 59.Bezzi P, Domercq M, Vesce S, Volterra A. Neuron-astrocyte cross-talk during synaptic transmission: physiological and neuropathological implications. Progress in Brain Research. 2001;132:255–265. doi: 10.1016/S0079-6123(01)32081-2. [DOI] [PubMed] [Google Scholar]
- 60.Brown GC, Bal-Price A. Inflammatory neurodegeneration mediated by nitric oxide, glutamate, and mitochondria. Molecular Neurobiology. 2003;27(3):325–355. doi: 10.1385/MN:27:3:325. [DOI] [PubMed] [Google Scholar]
- 61.Blasko I, Marx F, Steiner E, Hartmann T, Grubeck-Loebenstein B. TNFα plus IFNγ induce the production of alzheimer β-amyloid peptides and decrease the secretion of APPs. FASEB Journal. 1999;13(1):63–68. doi: 10.1096/fasebj.13.1.63. [DOI] [PubMed] [Google Scholar]
- 62.Griffin WST. IL-1 and the cytokine cycle in Alzheimer’s disease. Journal of Neurochemistry. 2000;74:p. S52. [Google Scholar]
- 63.Mrak RE, Griffin WST. Interleukin-1, neuroinflammation, and Alzheimer’s disease. Neurobiology of Aging. 2001;22(6):903–908. doi: 10.1016/s0197-4580(01)00287-1. [DOI] [PubMed] [Google Scholar]
- 64.Wyss-Coray T, Lin C, Yan F, et al. TGF-β1 promotes microglial amyloid-β clearance and reduces plaque burden in transgenic mice. Nature Medicine. 2001;7(5):612–618. doi: 10.1038/87945. [DOI] [PubMed] [Google Scholar]
- 65.Kiyota T, Okuyama S, Swan RJ, Jacobsen MT, Gendelman HE, Ikezu T. CNS expression of anti-inflammatory cytokine interleukin-4 attenuates Alzheimer's disease-like pathogenesis in APP+PS1 bigenic mice. FASEB Journal. 2010;24(8):3093–3102. doi: 10.1096/fj.10-155317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hu S, Chao CC, Ehrlich LC, et al. Inhibition of microglial cell RANTES production by IL-10 and TGF-β. Journal of Leukocyte Biology. 1999;65(6):815–821. doi: 10.1002/jlb.65.6.815. [DOI] [PubMed] [Google Scholar]
- 67.Moore KW, De Waal Malefyt R, Coffman RL, O’Garra A. Interleukin-10 and the interleukin-10 receptor. Annual Review of Immunology. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- 68.Shimizu E, Kawahara K, Kajizono M, Sawada M, Nakayama H. IL-4-induced selective clearance of oligomeric β-amyloid peptide(1–42) by rat primary type 2 microglia. Journal of Immunology. 2008;181(9):6503–6513. doi: 10.4049/jimmunol.181.9.6503. [DOI] [PubMed] [Google Scholar]
- 69.Benveniste EN, Nguyen VT, O’Keefe GM. Immunological aspects of microglia: relevance to Alzheimer’s disease. Neurochemistry International. 2001;39(5-6):381–391. doi: 10.1016/s0197-0186(01)00045-6. [DOI] [PubMed] [Google Scholar]
- 70.Hao J, Wang ZD, Yang YX. Study on β-amyloid peptide 1–42 induced chemokine rantes in U251 cell. Journal of Sichuan University. 2009;40(6):1011–1014. [PubMed] [Google Scholar]
- 71.Didonato JA, Hayakawa M, Rothwarf DM, Zandi E, Karin M. A cytokine-responsive IκB kinase that activates the transcription factor NF-κB. Nature. 1997;388(6642):548–554. doi: 10.1038/41493. [DOI] [PubMed] [Google Scholar]
- 72.Karin M, Ben-Neriah Y. Phosphorylation meets ubiquitination: the control of NF-[kappa]B activity. Annual Review of Immunology. 2000;18:621–663. doi: 10.1146/annurev.immunol.18.1.621. [DOI] [PubMed] [Google Scholar]
- 73.Hanada T, Yoshimura A. Regulation of cytokine signaling and inflammation. Cytokine and Growth Factor Reviews. 2002;13(4-5):413–421. doi: 10.1016/s1359-6101(02)00026-6. [DOI] [PubMed] [Google Scholar]
- 74.Morris KR, Lutz RD, Choi HS, Kamitani T, Chmura K, Chan ED. Role of the NF-κB signaling pathway and κB cis-regulatory elements on the IRF-1 and iNOS promoter regions in mycobacterial lipoarabinomannan induction of nitric oxide. Infection and Immunity. 2003;71(3):1442–1452. doi: 10.1128/IAI.71.3.1442-1452.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Vermeulen L, De Wilde G, Notebaert S, Vanden Berghe W, Haegeman G. Regulation of the transcriptional activity of the nuclear factor-κB p65 subunit. Biochemical Pharmacology. 2002;64(5-6):963–970. doi: 10.1016/s0006-2952(02)01161-9. [DOI] [PubMed] [Google Scholar]
- 76.Hayden MS, Ghosh S. Signaling to NF-κB. Genes and Development. 2004;18(18):2195–2224. doi: 10.1101/gad.1228704. [DOI] [PubMed] [Google Scholar]
- 77.Schmitz ML, Mattioli I, Buss H, Kracht M. NF-κB: a multifaceted transcription factor regulated at several levels. ChemBioChem. 2004;5(10):1348–1358. doi: 10.1002/cbic.200400144. [DOI] [PubMed] [Google Scholar]
- 78.Gong R, Rifai A, Ge Y, Chen S, Dworkin LD. Hepatocyte growth factor suppresses proinflammatory NFκB activation through GSK3β inactivation in renal tubular epithelial cells. Journal of Biological Chemistry. 2008;283(12):7401–7410. doi: 10.1074/jbc.M710396200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chen LF, Mu Y, Greene WC. Acetylation of RelA at discrete sites regulates distinct nuclear functions of NF-κB. EMBO Journal. 2002;21(23):6539–6548. doi: 10.1093/emboj/cdf660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kiernan R, Brès V, Ng RWM, et al. Post-activation turn-off of NF-κB-dependent transcription is regulated by acetylation of p65. Journal of Biological Chemistry. 2003;278(4):2758–2766. doi: 10.1074/jbc.M209572200. [DOI] [PubMed] [Google Scholar]
- 81.Coyle-Rink J, Del Valle L, Sweet T, Khalili K, Amini S. Developmental expression of Wnt signaling factors in mouse brain. Cancer Biology & Therapy. 2002;1(6):640–645. doi: 10.4161/cbt.313. [DOI] [PubMed] [Google Scholar]
- 82.Duan Y, Liao AP, Kuppireddi S, Ye Z, Ciancio MJ, Sun J. β-Catenin activity negatively regulates bacteria-induced inflammation. Laboratory Investigation. 2007;87(6):613–624. doi: 10.1038/labinvest.3700545. [DOI] [PubMed] [Google Scholar]
- 83.Li HL, Wang HH, Liu SJ, et al. Phosphorylation of tau antagonizes apoptosis by stabilizing beta-catenin, a mechanism involved in Alzheimer's neurodegeneration. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(9):3591–3596. doi: 10.1073/pnas.0609303104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Halleskog C, Mulder J, Dahlström J, et al. WNT signaling in activated microglia is proinflammatory. GLIA. 2011;59(1):119–131. doi: 10.1002/glia.21081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Takada Y, Fang X, Jamaluddin MS, Boyd DD, Aggarwal BB. Genetic deletion of glycogen synthase kinase-3β abrogates activation of IκBα kinase, JNK, Akt, and p44/p42 MAPK but potentiates apoptosis induced by tumor necrosis factor. Journal of Biological Chemistry. 2004;279(38):39541–39554. doi: 10.1074/jbc.M403449200. [DOI] [PubMed] [Google Scholar]
- 86.Leung IWL, Lassam N. Dimerization via tandem leucine zippers is essential for the activation of the mitogen-activated protein kinase kinase kinase, MLK-3. Journal of Biological Chemistry. 1998;273(49):32408–32415. doi: 10.1074/jbc.273.49.32408. [DOI] [PubMed] [Google Scholar]
- 87.Shen F, Zhao MW, He B, Yang JJ, Pei F, Wang YZ. The changes and significance of interleukin-17 in rat models of chronic obstructive pulmonary disease and asthma. Zhonghua Jie He He Hu Xi Za Zhi. 2004;27(10):654–658. [PubMed] [Google Scholar]
- 88.Shen E, Fan J, Peng T. Glycogen synthase kinase-3beta suppresses tumor necrosis factor-alpha expression in cardiomyocytes during lipopolysaccharide stimulation. Journal of Cellular Biochemistry. 2008;104(1):329–338. doi: 10.1002/jcb.21629. [DOI] [PubMed] [Google Scholar]
- 89.Vines A, Cahoon S, Goldberg I, Saxena U, Pillarisetti S. Novel anti-inflammatory role for glycogen synthase kinase-3β in the inhibition of tumor necrosis factor-α- and interleukin-1β-induced inflammatory gene expression. Journal of Biological Chemistry. 2006;281(25):16985–16990. doi: 10.1074/jbc.M602446200. [DOI] [PubMed] [Google Scholar]
- 90.Bard F, Cannon C, Barbour R, et al. Peripherally administered antibodies against amyloid beta-peptide enter the central nervous system and reduce pathology in a mouse model of Alzheimer disease. Nature Medicine. 2000;6(8):916–919. doi: 10.1038/78682. [DOI] [PubMed] [Google Scholar]
- 91.Schenk D, Barbour R, Dunn W, et al. Immunization with amyloid-β attenuates Alzheimer disease-like pathology in the PDAPP mouse. Nature. 1999;400(6740):173–177. doi: 10.1038/22124. [DOI] [PubMed] [Google Scholar]
- 92.Reed-Geaghan EG, Savage JC, Hise AG, Landreth GE. CD14 and toll-like receptors 2 and 4 are required for fibrillar Aβ-stimulated microglial activation. Journal of Neuroscience. 2009;29(38):11982–11992. doi: 10.1523/JNEUROSCI.3158-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kielian T. Toll-like receptors in central nervous system glial inflammation and homeostasis. Journal of Neuroscience Research. 2006;83(5):711–730. doi: 10.1002/jnr.20767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Pfeiffer A, Böttcher A, Orsó E, et al. Lipopolysaccharide and ceramide docking to CD14 provokes ligand-specific receptor clustering in rafts. European Journal of Immunology. 2001;31(11):3153–3164. doi: 10.1002/1521-4141(200111)31:11<3153::aid-immu3153>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 95.Triantafilou M, Gamper FGJ, Haston RM, et al. Membrane sorting of toll-like receptor (TLR)-2/6 and TLR2/1 heterodimers at the cell surface determines heterotypic associations with CD36 and intracellular targeting. Journal of Biological Chemistry. 2006;281(41):31002–31011. doi: 10.1074/jbc.M602794200. [DOI] [PubMed] [Google Scholar]
- 96.Triantafilou M, Morath S, Mackie A, Hartung T, Triantafilou K. Lateral diffusion of Toll-like receptors reveals that they are transiently confined within lipid rafts on the plasma membrane. Journal of Cell Science. 2004;117(17):4007–4014. doi: 10.1242/jcs.01270. [DOI] [PubMed] [Google Scholar]
- 97.McDonald DR, Brunden KR, Landreth GE. Amyloid fibrils activate tyrosine kinase-dependent signaling and superoxide production in microglia. Journal of Neuroscience. 1997;17(7):2284–2294. doi: 10.1523/JNEUROSCI.17-07-02284.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Combs CK, Johnson DE, Cannady SB, Lehman TM, Landreth GE. Identification of microglial signal transduction pathways mediating a neurotoxic response to amyloidogenic fragments of β-amyloid and prion proteins. Journal of Neuroscience. 1999;19(3):928–939. doi: 10.1523/JNEUROSCI.19-03-00928.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Koenigsknecht J, Landreth G. Microglial phagocytosis of fibrillar β-amyloid through a β integrin-dependent mechanism. Journal of Neuroscience. 2004;24(44):9838–9846. doi: 10.1523/JNEUROSCI.2557-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wilkinson B, Koenigsknecht-Talboo J, Grommes C, Lee CY, Landreth G. Fibrillar beta-amyloid-stimulated intracellular signaling cascades require Vav for induction of respiratory burst and phagocytosis in monocytes and microglia. The Journal of Biological Chemistry. 2006;281(30):20842–20850. doi: 10.1074/jbc.M600627200. [DOI] [PubMed] [Google Scholar]
- 101.Frank S, Copanaki E, Burbach GJ, Müller UC, Deller T. Differential regulation of toll-like receptor mRNAs in amyloid plaque-associated brain tissue of aged APP23 transgenic mice. Neuroscience Letters. 2009;453(1):41–44. doi: 10.1016/j.neulet.2009.01.075. [DOI] [PubMed] [Google Scholar]
- 102.Tahara K, Kim HD, Jin JJ, Maxwell JA, Li L, Fukuchi K. Role of toll-like receptor signalling in Abeta uptake and clearance. Brain. 2006;129(11):3006–3019. doi: 10.1093/brain/awl249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Richard KL, Filali M, Préfontaine P, Rivest S. Toll-like receptor 2 acts as a natural innate immune receptor to clear amyloid β and delay the cognitive decline in a mouse model of Alzheimer’s disease. Journal of Neuroscience. 2008;28(22):5784–5793. doi: 10.1523/JNEUROSCI.1146-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Fiala M, Liu PT, Espinosa-Jeffrey A, et al. Innate immunity and transcription of MGAT-III and Toll-like receptors in Alzheimer’s disease patients are improved by bisdemethoxycurcumin. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(31):12849–12854. doi: 10.1073/pnas.0701267104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Martin M, Rehani K, Jope RS, Michalek SM. Toll-like receptor—mediated cytokine production is differentially regulated by glycogen synthase kinase 3. Nature Immunology. 2005;6(8):777–784. doi: 10.1038/ni1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Tan J, Town T, Crawford F, et al. Role of CD40 ligand in amyloidosis in transgenic Alzheimer's mice. Nature Neuroscience. 2002;5(12):1288–1293. doi: 10.1038/nn968. [DOI] [PubMed] [Google Scholar]
- 107.Laporte V, Lombard Y, Levy-Benezra R, Tranchant C, Poindron P, Warter J-M. Uptake of Aβ 1–40- and Aβ 1–42-coated yeast by microglial cells: a role for LRP. Journal of Leukocyte Biology. 2004;76(2):451–461. doi: 10.1189/jlb.1203620. [DOI] [PubMed] [Google Scholar]
- 108.Town T, Nikolic V, Tan J. The microglial “activation” continuum: from innate to adaptive responses. Journal of Neuroinflammation. 2005;2, article 24 doi: 10.1186/1742-2094-2-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Terwel D, Muyllaert D, Dewachter I, et al. Amyloid activates GSK-3β to aggravate neuronal tauopathy in bigenic mice. American Journal of Pathology. 2008;172(3):786–798. doi: 10.2353/ajpath.2008.070904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Muyllaert D, Kremer A, Jaworski T, et al. Glycogen synthase kinase-3β, or a link between amyloid and tau pathology? Genes, Brain and Behavior. 2008;7(supplement 1):57–66. doi: 10.1111/j.1601-183X.2007.00376.x. [DOI] [PubMed] [Google Scholar]
- 111.Li HL, Wang HH, Liu SJ, et al. Phosphorylation of tau antagonizes apoptosis by stabilizing beta-catenin, a mechanism involved in Alzheimer's neurodegeneration. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(9):3591–3596. doi: 10.1073/pnas.0609303104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Leroy K, Yilmaz Z, Brion JP. Increased level of active GSK-3β in Alzheimer’s disease and accumulation in argyrophilic grains and in neurones at different stages of neurofibrillary degeneration. Neuropathology and Applied Neurobiology. 2007;33(1):43–55. doi: 10.1111/j.1365-2990.2006.00795.x. [DOI] [PubMed] [Google Scholar]
- 113.Takashima A. GSK-3 is essential in the pathogenesis of Alzheimer's disease. Journal of Alzheimer's Disease. 2006;9(supplement 3):309–317. doi: 10.3233/jad-2006-9s335. [DOI] [PubMed] [Google Scholar]
- 114.Mateo I, Infante J, Llorca J, Rodríguez E, Berciano J, Combarros O. Association between glycogen synthase kinase-3β genetic polymorphism and late-onset Alzheimer's disease. Dementia and Geriatric Cognitive Disorders. 2006;21(4):228–232. doi: 10.1159/000091044. [DOI] [PubMed] [Google Scholar]
- 115.Bustanji Y, Taha MO, Almasri IM, Al-Ghussein MAS, Mohammad MK, Alkhatib HS. Inhibition of glycogen synthase kinase by curcumin: Investigation by simulated molecular docking and subsequent in vitro/in vivo evaluation. Journal of Enzyme Inhibition and Medicinal Chemistry. 2009;24(3):771–778. doi: 10.1080/14756360802364377. [DOI] [PubMed] [Google Scholar]
- 116.Lim GP, Chu T, Yang F, Beech W, Frautschy SA, Cole GM. The curry spice curcumin reduces oxidative damage and amyloid pathology in an Alzheimer transgenic mouse. Journal of Neuroscience. 2001;21(21):8370–8377. doi: 10.1523/JNEUROSCI.21-21-08370.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lim GP, Yang F, Chu T, et al. Ibuprofen suppresses plaque pathology and inflammation in a mouse model for Alzheimer's disease. Journal of Neuroscience. 2000;20(15):5709–5714. doi: 10.1523/JNEUROSCI.20-15-05709.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Greenspan EJ, Madigan JP, Boardman LA, Rosenberg DW. Ibuprofen inhibits activation of nuclear β-catenin in human colon adenomas and induces the phosphorylation of GSK-3β. Cancer Prevention Research. 2011;4(1):161–171. doi: 10.1158/1940-6207.CAPR-10-0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Malm TM, Iivonen H, Goldsteins G, et al. Pyrrolidine dithiocarbamate activates Akt and improves spatial learning in APP/PS1 mice without affecting β-amyloid burden. Journal of Neuroscience. 2007;27(14):3712–3721. doi: 10.1523/JNEUROSCI.0059-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Nurmi A, Goldsteins G, Närväinen J, et al. Antioxidant pyrrolidine dithiocarbamate activates Akt-GSK signaling and is neuroprotective in neonatal hypoxia-ischemia. Free Radical Biology and Medicine. 2006;40(10):1776–1784. doi: 10.1016/j.freeradbiomed.2006.01.011. [DOI] [PubMed] [Google Scholar]