Abstract
Surface grafting of cyclic olefins with low strain energies, including cyclopentene (CP), 1,4-cyclohexadiene (CHD), cycloheptene (CHP), cis-cyclooctene (CO), cis,cis-1,5-cyclooctadiene (COD), 1,3,5,7-cyclooctatetraene (COT), cyclododecene (CD), and trans,trans,cis-1,5,9-cyclododecatriene (CDT), were explored using ring-opening metathesis polymerization in the vapor phase. These monomers do not polymerize when SiROMP is carried out in solution due to pronounced chain transfer on surfaces where chains are in close proximities. In the vapor phase, however, chain transfer is suppressed at the solid-vapor interfaces, which permits the polymerization of most of these monomers. A minimal required strain energy of 2.2 kcal/mol was determined in this study, which is significantly lower than the estimated 13.3 kcal/mol for SiROMP carried out in solution, indicating that the enhancement in monomer polymerizability is significant using the vapor phase approach. A series of polyalkenamers with controlled fraction of unsaturation from 8% to 50% along the polymer backbone were grafted to solid substrates. It was observed that the logarithm of largest grafted layer thickness obtained before the removal of chain transfer products – which correlates with the extent of polymerization – scales with monomer strain energy. This confirms that the release of ring strain is the thermodynamic driving force for SiROMP. It was also found that although chain transfer is suppressed in the vapor phase, it is important in monomer/polymer systems where the fraction of unsaturated bonds is high. In these cases, grafted polymer thickness is dominated by chain transfer, rather than by monomer strain energy. A quantitative relationship is established for estimating graft thickness of a particular monomer using its strain energy and fraction of unsaturated bonds in the monomer.
Introduction
Ring-opening metathesis polymerization (ROMP) has become one of the most powerful synthetic strategies for the preparation of linear polymers.1 These polymers have found a wide range of applications because functional groups can be conveniently incorporated into the macromolecular backbone through the use of functionalized cyclic olefins or the subsequent chemical transformation of the conserved olefins.2 A great deal of the success of ROMP can be credited to the development of well-defined catalysts. In particular, ruthenium complexes are useful due to their thermal stability, high metathesis activity and most notably, their excellent tolerance to many polar functionalities, e.g. alcohols, esters, halides, nitriles.3 Advances in the ruthenium-based catalysis have allowed access to novel polymeric materials, utilizing a range of unsubstituted as well as functionalized monomers in high yields under mild conditions.4–7
ROMP is thermodynamically favored for cyclic olefins with sufficient strain energy.8 The extent of polymerization depends on the amount of the ring strain released, which compensates for the loss in entropy upon polymer growth. Monomers with five-, six- and seven-membered rings and those with strain energies below a critical value of 5 kcal/mol are reported to not polymerize readily.8 In these situations, the equilibrium position can be shifted toward ring-opened products by using high monomer concentrations and operating at reaction temperatures considerably below the ceiling temperature of the system.9 These strategies, in addition to using highly reactive initiators, have been employed to polymerize cyclohexene with a strain energy of 2.5 kcal/mol.10
We are interested in the covalent attachment of new polymers onto substrates using surface-initiated ROMP (SiROMP). In general, surface-initiated polymerization or the “grafting from” method ensures surface attachment of polymers and offers control of polymer film thickness and graft density.11 In a typical surface-initiated polymerization, an initiator is first tethered onto a functionalized substrate followed by the addition of monomers resulting in the growth of polymer chains. The straightforward process and rapid kinetics render SiROMP an attractive tool to tune surface properties of a number of substrates including Au,12–17 SiO2,18–21 Si,22 and carbon.23 While olefin metathesis is established for synthesis of polyalkenamers in the bulk using a wide variety of cyclic olefins, previous studies on surface functionalization using ROMP have been limited to norbornene (Nbn) and its derivatives. Polynorbornene films were grafted under relatively mild conditions, producing polymer films from nano- to micrometer thick depending on the catalyst type, polymerization temperature and time, and monomer concentration. The growing number of studies on SiROMP of Nbn is likely due to its superior reactivity stemming from its high strain energy (27.2 kcal/mol) and the ease of derivatization of the monomer.
In our recent reports, we demonstrated the feasibility of SiROMP of cis,cis-1,5-cyclooctadiene (COD), a monomer with a lower ring strain (13.26 kcal/mol).24,25 We showed that carrying out SiROMP of COD in the vapor phase is a viable approach in grafting polybutadiene (PBd) on Si/SiO2 substrates, yielding ~ 10 nm thick films after 30 min of polymerization time at room temperature. Commonly, ROMP of low-strain cyclic olefins is complicated by competing chain transfer reactions. This involves the reaction between active catalysts at propagating chain ends with olefins along polymer backbones, either intra- or intermolecularly, resulting in high polydispersity of graft chains and low graft thickness.1 In comparison to the conventional approach carried out in solution, vapor phase SiROMP reduces the extent of chain transfer by lowering chain mobility at the solid-vapor interfaces. Vapor phase SiROMP was previously described by Zauscher et al. in the preparation of nanopatterned polymer brushes on silicon substrates using 5-ethylidene-2-norbornene as the monomer.26 Xu and co-workers have also shown the polymerization of Nbn and cyclooctatetraene (COT) in the vapor phase, albeit using physisorbed ruthenium initiators.27–29
In this report, vapor phase SiROMP of a series of monomers with lower strain energies than Nbn (Figure 1) was surveyed; polyolefins with controlled fraction of unsaturation (from 8% to 50%) along the polymer backbone were prepared from these monomers on solid substrates (Scheme 1). Factors affecting thermodynamics and kinetics of SiROMP were elucidated and a thermodynamic limit of this approach was estimated.
Figure 1.
Strain energies of cyclic olefins.
Scheme 1.
The preparation of polymers from low strain cyclic olefins.
Experimental
General
Silicon wafers were obtained from International Wafer Service (100 orientation, P/B doped, resistivity 1–10 Ωcm, thickness 450–575 μm). 5-Hexenyldimethylchlorosilane (95%, Gelest) was transferred to a custom-built Schlenk flask and stored under nitrogen. Grubbs 2nd generation catalyst, 1,3-bis-(2,4,6-trimethylphenyl)-2-imidazolidinylidene-dichloro(phenylmethylene)-(tricyclohexylphosphine)ruthenium, 9-borabicyclo[3.3.1]nonane (9-BBN) dimer (crystalline, 98%), ethyl vinyl ether, cyclopentene (CP), 1,4-cyclohexadiene (CHD), cycloheptene (CHP), cis-cyclooctene (CO), redistilled 1,5-cyclooctadiene (COD), 1,3,5,7-cyclooctatetraene (COT), cyclododecene (CD), and trans,trans,cis-1,5,9-cyclododecatriene (CDT) were obtained from Sigma-Aldrich. Hydrogen peroxide (30%) and concentrated sulfuric acid were received from Fisher. All reagents were used as received unless specified otherwise. Dichloromethane (HPLC grade, Pharmco-Aaper) was dried via a solvent purification system (Pure Solv, Innovative Technology, Inc.), and deoxygenated by purging with nitrogen for one hour. House-purified water (reverse osmosis) was further purified using a Millipore Milli-Q system that involves reverse osmosis, ion-exchange, and filtration steps (18.2 MΩ/cm).
Instrumentation
Thickness measurements were carried out using an LSE Stokes Ellipsometer. The light source is a He-Ne laser and the angle of incidence is 70° (from the normal). Thickness was calculated using the following parameters: air, no = 1; silicon oxide, silane- and polymer-derived layers, no = 1.46; silicon substrate, ns = 3.85 and ks = −0.02. A Veeco Dektak Stylus profilometer was used for measuring polymer thicknesses that were greater than 1 μm. Samples were scratched using a thin blade followed by scanning a diamond tipped stylus across sample surfaces. Thickness measurements were performed on multiple samples from at least two sets of experiments; three to five measurements were carried out in different areas of each sample. The standard deviation in reported thickness is typically less than 10%. Contact angles were measured using a Rame-Hart telescope goniometer with a Gilmont syringe and a 24-gauge flat-tipped needle; Milli-Q water was used as the probe fluid. Dynamic advancing (θA) and receding (θR) angles were recorded while the probe fluid was added to and withdrawn from the drop, respectively. Reported contact angle values were averages of three to five measurements on each sample; individual contact angle data were within ± 2° of the averages. X-ray photoelectron spectra (XPS) were recorded with a Physical Electronics Quantum 2000 ESCA Microprobe with Al Kα excitation. Spectra were obtained at two take-off angles, 15° and 75° (between the plane of the surface and the entrance lens of the detector optics). Reported XPS atomic compositions were averages of at least three experimental data points with standard deviations less than 5%. Atomic force microscopy images were taken using a Veeco Metrology Dimension 3100 scanning force microscope with a silicon tip operated in tapping mode (scan rate of 0.1 Hz).
Immobilization of a Silane Monolayer
Silicon wafers were cut into 1.3 × 1.5 cm pieces and cleaned by submerging in a freshly prepared piranha solution (H2SO4:H2O2 = 7:3 by volume) for 1 h. Caution: Piranha solution reacts violently with organic matter. The wafers were then rinsed thoroughly with water and dried in an oven at 110 °C for 30 min. Upon cooling, clean wafers were immediately placed in a custom-designed glass holder (slotted glass cylinder), which was transferred to a clean Schlenk flask. 0.5 mL of 5-hexenyldimethylchlorosilane was introduced to the Schlenk flask with no direct contact between the reagent and the samples. Vapor phase silanization was carried out at 70 °C for 16 h in the sealed flask. The wafers were then rinsed individually with toluene (2x), ethanol, and water, dried under reduced pressure for 1 h, and transferred to a nitrogen-filled glove box (Innovative Technology, Inc). Silanized silicon wafers were used within 24 h of preparation.
Attachment of Initiators and SiROMP in the Vapor Phase
In the glove box, 1.5 mL of the monomer of interest and 9-BBN (5 wt%) were stirred overnight in a closed 250 mL wide-mouthed jar. Silane-functionalized silicon wafers were immersed in a solution containing 1.0 mM Grubbs second generation catalyst in dichloromethane for 20 min in the glove box. The substrates were thoroughly rinsed in dichloromethane before being placed on a stainless steel wire mesh in the jar; there was no contact between the substrates and the monomer mixture. The jar was sealed for a desired amount of polymerization time at room temperature. Some of the substrates (unrinsed samples) were set aside for characterization. The remainder of the samples (rinsed) were placed in 10 mL of dichloromethane containing 1 mL of ethyl vinyl ether for 5 min before being individually rinsed with dichloromethane (3x) in the glove box. Sample exposure to the ambient environment was kept at minimum before and during characterization.
Results and Discussion
The general procedure for vapor phase SiROMP of low strain monomers was similar to the one used for COD24,25 and is outlined in Scheme 2. We recently reported the acute sensitivity of tethered unsaturated polyolefins to oxidative degradation during solvent rinse under ambient conditions.25 Hence, all the preparative steps described herein were carried out under inert atmosphere. In addition, it was necessary to mix COD with a small amount of 9-BBN prior to polymerization to remove 4-vinylcyclohexene impurity from COD and to afford grafted PBd films with appreciable thicknesses.30 Similarly, it was found that thicker polymer films were obtained when other monomers were mixed with 9-BBN. Presumably 9-BBN reacts with isomers containing acyclic double bonds to render them nonvolatile and unable to participate in vapor phase polymerization.
Scheme 2.
SiROMP of COD with data on thickness increase (T) and advancing and receding water contact angles (CA=θA/θR) of the samples after each step.
In the subsequent discussion of SiROMP, graft thickness is often referred to. Graft thickness is naturally a function of both graft density and molecular weight of graft polymers. Unfortunately, neither could be reliably determined in our system. When a low surface-area substrate is used, there is an insufficient amount of polymer for molecular weight determination. Furthermore, polymer molecular weight is not an accurate indicator for the extent of reaction when molecular weight distribution is wide resulting from pronounced intermolecular chain transfer reactions among chains in close proximities on surfaces. Lastly, the differences in graft density among various monomers are not expected to be large considering that the same initiation method was used. Graft thickness, therefore, is a reasonable approximation of the extent of reaction in this study.
SiROMP was carried out for all the cyclic monomers. Figure 2 shows graft thicknesses (on a logarithmic scale in order to spread out data points) before and after solvent rinse at different polymerization times. Data for CHD and COT are not included due to the negligible and very low graft thicknesses obtained, respectively. 30 min was chosen as the shortest polymerization time examined since it requires time for monomer vapor pressure to reach equilibrium in closed reaction vessels; graft thicknesses at shorter reaction times were irreproducible. This is an inherent limitation of the vapor phase approach. Little additional growth in graft thicknesses after 1200 min was observed, presumably due to the loss of catalyst activity and/or because reaction equilibria were reached.
Figure 2.
SiROMP kinetics of various monomers: filled symbols represent graft thicknesses of unrinsed samples and open symbols represent graft thicknesses of rinsed samples.
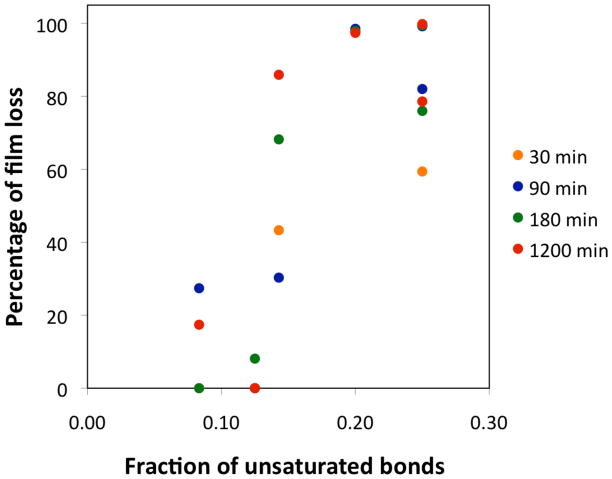
A quick glance at the pairs of filled and open symbols reveals that the differences in graft thicknesses before and after solvent rinse vary significantly depending on the monomer type. Solvent rinse removes extractable monomers, oligomers, and polymers, which are products of inter- and intramolecular chain transfer reactions. Polymerization of CO resulted in a graft thickness of ~ 2 μm and there was negligible thickness change upon solvent extraction. On the other hand, grafted PBd from SiROMP of COD had an initial thickness of ~5 μm and only ~ 10 nm thick film remained upon removal of extractable species. This indicates that SiROMP in the vapor phase does reduce chain mobility and the extent of chain transfer to allow the polymerization of low strain cyclic monomers, however, chain transfer still occurs extensively in some cases. The difference between unrinsed and rinsed film thicknesses can be used as a measure for the extent of chain transfer during polymerization. When percentages of film loss upon solvent extraction are plotted against fractions of unsaturated bonds in monomers/polymers (χu.b.) at all polymerization times examined (30, 90, 180, and 1200 min), a positive correlation is obtained as shown in Figure 3. This indicates that the extent of chain transfer is statistically correlated to the density of unsaturated bonds along the polymer backbone, which is an intrinsic property of the monomer/polymer system under consideration.
Figure 3.
Percentage of film loss upon solvent rinse as a function of fraction of unsaturated bonds in monomers at different polymerization times.
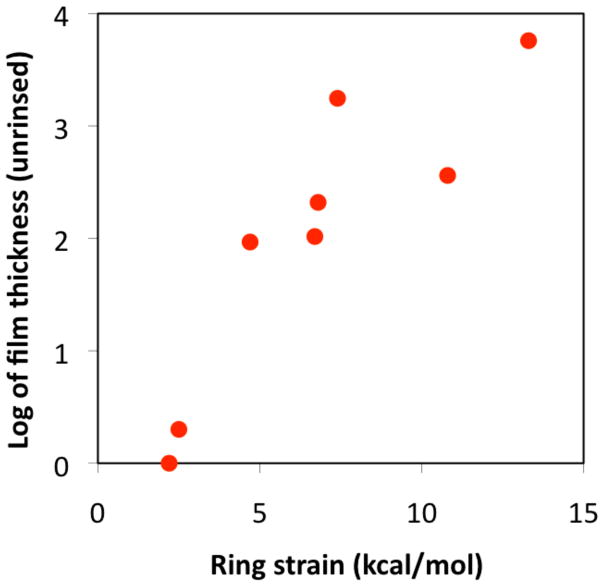
If the thermodynamic driving force of SiROMP is the release of monomer ring strain, the polymerizability and the extent of polymerization of a monomer should be affected by its strain energy. Figure 4 shows a near linear relationship between the logarithm of graft thickness (before solvent rinse) at the longest reaction time and monomer strain energy. Thickness before solvent extraction was used because it correlates to the extent of polymerization without taking into account the reduction of polymer molecular weight and the overall graft thickness due to chain transfer. The positive correlation shown in Figure 4 confirms that the driving force of SiROMP is the release of monomer strain energy (SE). In addition, a critical ring strain (SE0) of ~ 2.2 kcal/mol corresponding to that of CHD is predicted, below which cyclic olefins are not polymerizable using vapor phase SiROMP. A non-zero value of ring strain is necessary to offset the entropy loss upon polymerization. This value is much lower than the critical value of 5 kcal/mol predicted by Ivin and Grubbs for ROMP in solution/bulk.1,8 Since COD does not graft from solid-solution interfaces in our laboratory and it has not been reported in the literature, its strain energy of 13.26 kcal/mol is a good estimate for the critical ring strain of SiROMP in solution. It is thus apparent that the polymerizability of low strain cyclic olefin using the vapor phase approach is increased significantly. All polymerizations were carried out at room temperature in this study.Lower reaction temperatures should lower the unfavorable contribution from entropy thus further increasing the polymerizability of cyclic olefins.
Figure 4.
Logarithm of graft film thicknesses (in nm) of unrinsed samples as a function of monomer strain energies.
Other factors affecting thermodynamics of SiROMP include monomer vapor pressure (v.p.), rigidity of polymer, and solubility of monomer in polymer thin film. Their effects are difficult to quantify but are included in equation 1 for predicting the graft thickness (after the removal of extractable species) of a given monomer using the vapor phase SiROMP approach.
| (1) |
where k1 and k2 are proportionality constants. Given the linearity of Figures 3 and 4, strain energy (first term) and chain transfer (second term) are expected to have the predominant effects on the overall graft thickness. The open symbols in Figure 2 represent thicknesses of grafted polymer chains, ranging from 3 nm (CP) to ~2 μm (CO). It is remarkable that COD and CDT result in PBd films with similar graft thickness of ~ 10 nm. They have different ring strains but the same fraction of unsaturated bonds. This indicates that although ring strain determines the extent of polymerization, the extent of chain transfer ultimately controls the amount of grafted chains remaining on surfaces.
The kinetics aspects of SiROMP in the vapor phase were also examined. Patton and McCarthy reported that the rate of polymerization is affected by the reactivity of the propagating carbenes in the tungsten hexachloride/tetramethyltin-catalyzed ROMP of cyclic olefins in solution and argued that monomer ring strain does not affect ROMP kinetics.31,32 In an attempt to compare polymerization rates of various monomers, graft thicknesses of unrinsed samples after the shortest reaction time of 30 min were analyzed. The observed rate order in our SiROMP study is COD > CO > CP ~ CD > CDT > CHP, which is very close to the expected order of CO > CP > CD ~ COD ~ CDT > CHP when the reactivity of the propagating carbenes is considered as the determining factor,32 with the exception of COD. Because COD has the highest strain energy among the monomers examined, it is not surprising that COD yielded the thickest polymer film as shown in Figure 2 and as discussed earlier. The data obtained at the shortest reaction time of 30 min are perhaps already influenced by the thermodynamics of the polymerization. Furthermore, ROMP is first order with respect to monomer concentration, which varies with the nature of monomer. Considering that reliable data at early reaction times were not available and that monomer concentration varies for each monomer system, the observed order in this study is in good agreement with the order predicted by early studies.
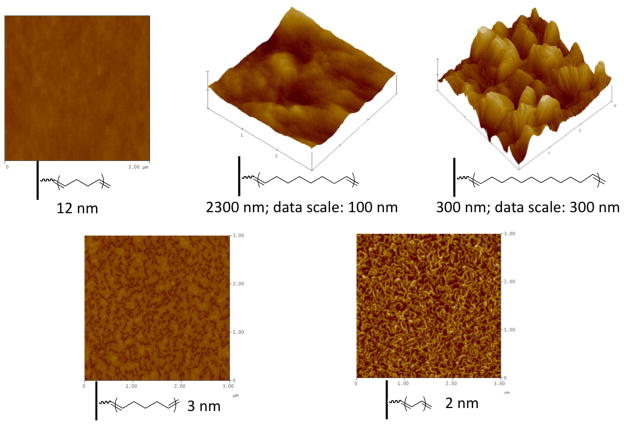
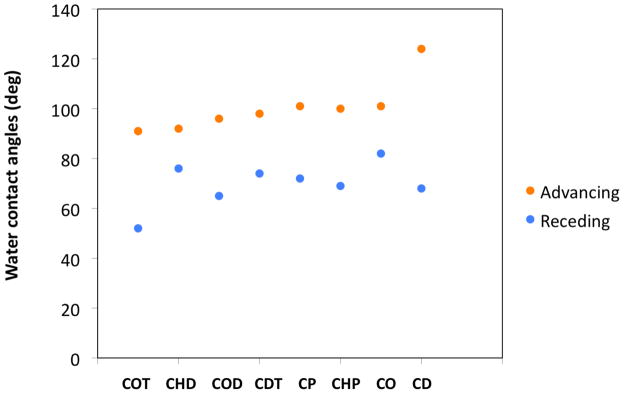
Surface topographies of some representative polymer films are shown in Figure 5. As previously reported for PBd, graft polymers appear patchy and discontinuous initially and become continuous and smooth during the course of polymerization.25 PolyCDT and polyCHP showed similar surface topographies as PBd. Toward the later stage of polymerization, thick polymer films are generally continuous nevertheless with varying roughness. Among the three continuous films shown (top images), PBd is smooth, polyCO is rougher, and polyCD is the roughest. XPS analyses indicated that all of the samples with continuous polymer coverage contained 1–3% of oxygen with the remainder of the detected composition being carbon at 15° take-off angle (outermost ~10 Å) and a negligible amount of oxygen at 75° take-off angle (outermost ~40 Å). The presence of measurable and comparable amounts of oxygen on polymer surfaces is most likely due to the autooxidation of unsaturated hydrocarbons. Oxidation, however, is limited to the outermost surfaces. The drastic difference in surface topography of these polymer films thus cannot be caused by surface oxidation alone. The only difference among these polymer samples is the length of the more stiff and hydrophobic –(CH2)n– moieties. Presumably, oxidation exacerbates the difference between the saturated and (oxidized) unsaturated domains, which provides an additional driving force for phase segregation. When the graft thickness is low (bottom images), the polymer domains formed at the early stage of polymerization do not completely coalesce to form a continuous film. PolyCP patches and polyCOT (or polyacetylene) fibrous structures are shown as examples here. Water contact angles on polymer-grafted surfaces follow certain trends as well. Advancing contact angles increase as the fraction of unsaturated bonds decreases since methylene groups are more hydrophobic than unsaturated groups. Hysteresis – the difference between advancing and receding contact angles – is large when surfaces are rough, such as in the cases of polyCOT and polyCD.
Figure 5.
AFM images (size: 3 μm × 3 μm; data scale: 20 nm unless specified otherwise) of some representative grafted polycycloolefins.
Conclusions
We have demonstrated that carrying out surface-initiated ring-opening metathesis polymerization in the vapor phase allows cyclic olefins with low strain energies to be polymerized from solid substrates. The release of ring strain is the thermodynamic driving force for SiROMP and a critical strain energy of 2.2 kcal/mol is required to render the polymerization in the vapor phase thermodynamically feasible. The vapor phase approach drastically enhances monomer polymerizability because the extent of chain transfer is reduced at the solid-vapor interfaces compared to that at the solid-liquid interfaces in the conventional method. Chain transfer is nevertheless still present in SiROMP due to close proximities of polymer chains on surfaces. In fact, chain transfer is more dominant than ring strain in determining grafted-polymer thickness for monomer/polymer systems where the fraction of unsaturated bonds is high. Kinetic considerations indicate that the rate of SiROMP is affected by the reactivity of propagating carbenes. The versatile reactivity of olefins suggests rich opportunities for further derivatizations of these grafted polymers.
Figure 6.
Water contact angles of surfaces with grafted polycycloolefins.
Acknowledgments
Financial support was provided by the National Institutes of Health (2R15EB139-2), the National Science Foundation (DMR -1005324), and the Camille & Henry Dreyfus Foundation. The use of the NSF-MRSEC central facilities at the University of Massachusetts is also acknowledged.
References
- 1.Bielawski CW, Grubbs RH. Prog Polym Sci. 2007;32:1–29. [Google Scholar]
- 2.Leitgeb A, Wappel J, Slugovc C. Polymer. 2010;51:2927–2946. and references cited therein. [Google Scholar]
- 3.Trnka TM, Grubbs RH. Acc Chem Res. 2001;34:18–29. doi: 10.1021/ar000114f. [DOI] [PubMed] [Google Scholar]
- 4.Hejl A, Scherman OA, Grubbs RH. Macromolecules. 2005;38:7214–7218. [Google Scholar]
- 5.Bielawski CW, Grubbs RH. Angew Chem, Int Ed. 2000;39:2903–2906. doi: 10.1002/1521-3773(20000818)39:16<2903::aid-anie2903>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 6.Lehman SE, Wagener KB, Akvan S. J Polym Sci Part A: Polym Chem. 2005;43:6134–6145. [Google Scholar]
- 7.Lehman SE, Wagener KB. Organometallics. 2005;24:1477–1482. [Google Scholar]
- 8.Ivin KJ, Mol JC. Olefin metathesis and metathesis polymerization. Academic Press; San Diego: 1997. [Google Scholar]
- 9.Odian GG. Principles of polymerization. 4. Wiley-Interscience; Hoboken, N.J: 2004. [Google Scholar]
- 10.Patton PA, Lillya CP, McCarthy TJ. Macromolecules. 1986;19:1266–1268. [Google Scholar]
- 11.Ayres N. Polym Chem. 2010;1:769–777. [Google Scholar]
- 12.Rutenberg IM, Scherman OA, Grubbs RH, Jiang W, Garfunkel E, Bao Z. J Am Chem Soc. 2004;126:4062–4063. doi: 10.1021/ja035773c. [DOI] [PubMed] [Google Scholar]
- 13.Weck M, Jackiw JJ, Rossi RR, Weiss PS, Grubbs RH. J Am Chem Soc. 1999;121:4088–4089. [Google Scholar]
- 14.Kong B, Lee JK, Choi IS. Langmuir. 2007;23:6761–6765. doi: 10.1021/la700568j. [DOI] [PubMed] [Google Scholar]
- 15.Berron BJ, Graybill EP, Jennings GK. Langmuir. 2007;23:11651–11655. doi: 10.1021/la7017902. [DOI] [PubMed] [Google Scholar]
- 16.Berron BJ, Payne PA, Jennings GK. Ind Eng Chem Res. 2008;47:7707–7714. [Google Scholar]
- 17.Faulkner CJ, Fischer RE, Jennings GK. Macromolecules. 2010;43:1203–1209. [Google Scholar]
- 18.Jeon NL, Choi IS, Whitesides GM, Kim NY, Laibinis PE, Harada Y, Finnie KR, Girolami GS, Nuzzo RG. Appl Phys Lett. 1999;75:4201–4203. [Google Scholar]
- 19.Harris RF, Ricci MJ, Farrer RA, Praino J, Miller SJ, Saleh BEA, Teich MC, Fourkas JT. Adv Mater. 2005;17:39–42. [Google Scholar]
- 20.Harada Y, Girolami GS, Nuzzo RG. Langmuir. 2003;19:5104–5114. [Google Scholar]
- 21.Jordi MA, Seery TAP. J Am Chem Soc. 2005;127:4416–4422. doi: 10.1021/ja044456i. [DOI] [PubMed] [Google Scholar]
- 22.Juang A, Scherman OA, Grubbs RH, Lewis NS. Langmuir. 2001;17:1321–1323. [Google Scholar]
- 23.Faulkner CJ, Payne PA, Jennings GK. J Coll Interf Sci. 2010;351:248–253. doi: 10.1016/j.jcis.2010.07.044. [DOI] [PubMed] [Google Scholar]
- 24.Feng JX, Stoddart SS, Weerakoon KA, Chen W. Langmuir. 2007;23:1004–1006. doi: 10.1021/la0630110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lerum MFZ, Chen W. Langmuir. 2009;25:11250–11254. doi: 10.1021/la902340z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee WK, Caster KC, Kim J, Zauscher S. Small. 2006;2:848–853. doi: 10.1002/smll.200500470. [DOI] [PubMed] [Google Scholar]
- 27.Gu HW, Fu D, Weng LT, Xie J, Xu B. Adv Funct Mater. 2004;14:492–500. [Google Scholar]
- 28.Gu HW, Zheng RK, Zhang XX, Xu B. Adv Mater. 2004;16:1356–1359. [Google Scholar]
- 29.Fu DG, Weng LT, Du BY, Tsui OKC, Xu B. Adv Mater. 2002;14:339–343. [Google Scholar]
- 30.Ji SX, Hoye TR, Macosko CW. Macromolecules. 2004;37:5485–5489. [Google Scholar]
- 31.Patton PA, McCarthy TJ. Macromolecules. 1984;17:2939–2940. [Google Scholar]
- 32.Patton PA, McCarthy TJ. Macromolecules. 1987;20:778–782. [Google Scholar]