Abstract
We present an approach to design spiking silicon neurons based on dynamical systems theory. Dynamical systems theory aids in choosing the appropriate level of abstraction, prescribing a neuron model with the desired dynamics while maintaining simplicity. Further, we provide a procedure to transform the prescribed equations into subthreshold current-mode circuits. We present a circuit design example, a positive-feedback integrate-and-fire neuron, fabricated in 0.25 μm CMOS. We analyze and characterize the circuit, and demonstrate that it can be configured to exhibit desired behaviors, including spike-frequency adaptation and two forms of bursting.
Index Terms: Neuromorphic engineering, silicon neuron, dynamical systems, bifurcation analysis, bursting
I. Silicon Neurons
Neuromorphic engineering aims to reproduce the spike-based computation of the brain by morphing its anatomy and physiology into custom silicon chips, which simulate neuronal networks in real-time (i.e., emulate). The basic unit of these chips is the silicon neuron, designed using analog circuits for spike generation and digital ones for spike communication. Engineers have built many silicon neuron chips, ranging in complexity from simple current-to-spike frequency generators to multicompartment, multichannel models, and ranging in density from a single neuromorphic neuron to arrays of ten thousand neurons [1, 2, 3, 4, 5, 6, 7]. Systems of silicon neurons have realized numerous computations, such as visual orientation maps, echolocation, and winner-take-all selection [1, 8, 9, 10].
A fundamental choice in designing silicon neurons is selecting the appropriate level of abstraction, with the field segregated into two design styles, a top-down approach and a bottom-up approach. The top-down approach aims to copy neurobiology, building every possible detail into silicon neurons. In this manner, designers aim to ensure that they include all of neurobiology’s computing power. This approach comes with a high price; engineers are unable to build large arrays of such complex neurons. Further, even small arrays are difficult to use, suffering from large variations among neurons, pushing them off the precipice into the Valley of Death.1 On the other hand, the bottom up approach aims to build minimal neuron models, exploiting the inherent features of a technology. Engineers are able to build dense arrays of simple neurons, often expressing little variation, compared to complex models; however, these simple models often fail to reflect the properties of neurobiology. Unable to behave like their biological counterparts, these models fail to compute like them as well.
To avoid the precipice leading into the Valley of Death, we aim to design silicon neurons that realize the simplest complete model for intended tasks, keeping in mind that once a chip is fabricated we can tune neuron properties (e.g., thresholds, time constants, etc.) but cannot modify structure (i.e., we cannot add missing components). Models should be complex enough to express the same qualitative behavior and neurocomputational properties as their biological counterparts, in contrast to simple integrate-and-fire models; but they must accomplish this goal without superfluous detail and complexity, in contrast to biophysically detailed models [15, 16]. Recent work suggests that simple phenomenological models can not only reproduce the qualitative properties of biological neurons, but also their precise spike times [14]. The tool we use to select the appropriate level of abstraction between integrate-and-fire and biophysical models is dynamical systems theory (DST).
DST prescribes how a system’s states evolve, focusing on qualitative transitions among dynamical regimes as inputs and other parameters vary. DST achieves this function by finding equilibrium points, analyzing stability, and determining bifurcations [17]. For example, from the DST perspective, a bifurcation is responsible for a neuron’s transition from resting to spiking, and can aid us in deducing a neuron’s computational properties as they are linked to the type of bifurcation(s) a neuron undergoes. Experimental and computational neuroscientists have used DST to study the behavior of both individual neurons [18, 19] and neural networks [20], providing insight into how neural systems move among behavioral regimes (for a review see [21]). Engineers have used DST to analyze silicon models, elucidating their behavior [22, 23] and showing similarities between silicon and neurobiology [5]. In addition, one group has used DST to as a tool to transform a neuron with one type of dynamics into another type with distinct qualitative behavior [24].
In this paper, we use DST to design a neuron with simple but rich dynamics realized compactly in silicon. Until now, no formal procedure existed to choose the appropriate level of abstraction for a silicon neuron, and once the level of abstraction is selected no systematic process existed to design the neuron circuit. These challenges are particularly daunting in designing neurons for large-scale models of the brain, as they must optimize silicon area while maintaining flexibility. Following the philosophy that the simplest capable neuron results in the least variance, we limit our scope to single-compartment neurons with two degrees of freedom:
| (1) |
Such models have been studied from computational [25, 26] and dynamical [21] perspectives, and are sufficient to produce all the (practical) bifurcations between resting and spiking. However, we do provide one example with three variables.
In Section II, we introduce the concept of dynamical systems design, focusing on neuron bifurcations and their influence on behavior. In Section III, we describe the design process, focusing on how we use DST to create a positive-feedback integrate-and-fire neuron. In addition, we describe how we synthesize the neuron using log-domain circuits. In Section IV, we analyze and characterize the neuron, focusing on the neuron’s frequency-current relationship and showing the match between the theoretical behavior and the silicon neuron’s behavior. In Sections V through VII, we reapply the design procedure, enabling the neuron to exhibit bursting and spike-frequency adaptation by augmenting it with model ion-channel populations, and we verify its behavior in silicon. In Section VIII, we discuss the implications of our work, focusing on neuron variation and the future of silicon neuron design.
II. Dynamical System Theory
To design our silicon neuron, we employ DST to achieve the desired neuron properties while maintaining simplicity—dynamical system design (DSD). First, we determine the appropriate bifurcation(s) for the neuron to transition from resting to spiking. Second, we select whether the neuron should support bistability, monostability, or both. Third, we include additional properties that are important for a given neuron’s application, or that relate to a property we wish to study (e.g., spike-frequency adaptation). In general, a neuron’s required degree of complexity would depend strongly on the application(s) for which it is intended; however application-driven specifications are outside the scope of this paper.
Though biological and silicon neurons realize their transitions from resting to spiking in a myriad of ways, from a dynamical systems perspective, they all transition via two bifurcation types: saddle node or Androv–Hopf [21]. A saddle-node bifurcation occurs when two equilibrium points, one stable and one unstable, collide and annihilate each other. An Androv–Hopf bifurcation occurs when a stable equilibrium point transforms into an unstable one. In both cases, the elimination of the stable point causes the neuron’s potential to increase, eliciting a spike. These two bifurcation types result in different behaviors: Neurons expressing a saddle-node bifurcation integrate; excitatory input promotes spiking and inhibitory input represses spiking [27]. In contrast, neurons expressing a Androv–Hopf bifurcation resonate; excitatory or inhibitory input promotes or represses depending on timing [28].
In addition to the bifurcation type, we must choose whether the neuron expresses bistability. Each bifurcation type comes in two varieties, depending on whether a stable (spiking) limit cycle exists before the bifurcation occurs or only after. If the stable limit cycle exists before the bifurcation, the neuron is bistable since both resting and spiking are simultaneously stable. In addition, this bistability results in hysteresis: If the input current increases enough to cause spiking, the neuron will continue to spike even after it decreases below the bifurcation point. On the other hand, for a monostable neuron, the same input may cause a single spike, rather than continuous spiking.
The four combinations of bifurcation types and stability yield different neuron membrane (black) behaviors when driven with an increasing ramp of current (gray), followed by a decreasing one (Table I). Neurons expressing a monostable saddle-node bifurcation integrate, exhibiting a continuous current-frequency curve. They spike at a near-zero frequency when the current surpasses a threshold. As the current drops below this threshold, they cease spiking. Neurons expressing a bistable saddle-node bifurcation also integrate, but exhibit a discontinous current-frequency curve. They spike at a nonzero frequency when the current surpasses a threshold. As the current drops below the original threshold, they continue spiking, until it decreases further to a new threshold. Neurons expressing a monostable Androv–Hopf bifurcation resonate, expressing increasing subthreshold oscillations until spikes reach their full amplitude. As the current drops, their spikes cease and the oscillation’s amplitudes decrease. Neurons expressing a bistable Androv–Hopf bifurcation resonate, but express only low-amplitude subthreshold oscillations until spikes jump to their full amplitude. As the current drops below the original threshold, they continue spiking, until it decreases further to a new threshold.
Table I.
Resting-to-Spiking & Spiking-to-Resting Bifurcations
 |
Using the DST strategy, we can design a neuron that integrates, resonates, or does one or the other, depending on its settings. Such reconfigurability can be realized by adding an inhibitory or excitatory state variable that opposes the membrane potential’s change, activating or inactivating as the membrane potential increases [28]. Depending on the variable’s speed and sensitivity the neuron could integrate, resonate, or switch between types [29]. In this paper, we focus on the saddle-node bifurcation, designing a neuron that integrates rather than one that resonates.
III. Neuron Design
In this section, we present a method to design and to implement a silicon neuron, using DSD. We show how to move from the dimensionless ordinary differential equation (ODE) that describes the desired bifurcation to one in terms of currents. Finally, we show how this ODE can be implemented in circuits with logarithmically related voltages (V ∝ log(I)).
A. Saddle-Node Bifurcation
The saddle-node bifurcation is simpler than the Androv–Hopf one, requiring only a single state variable (plus a reset) to realize spiking. We choose this simpler type because we are not exploring subthreshold oscillations or other effects of resonance with this chip.
The canonical saddle-node bifurcation uses quadratic positive feedback [17, 21]:
| (2) |
where r and x are the neuron’s dimensionless input current and membrane potential, respectively. τ is its membrane time constant, rth = 1/2 is its threshold input, and xi = 1 is the inflection point, above which x accelerates (i.e., spiking occurs). The −x term corresponds to a leak conductance, a current proportional to membrane potential. We use the slightly more complex cubic system:
| (3) |
with xi = 1 and rth = 2/3. Using cubic positive feedback has two advantages over quadratic feedback: its circuit implementation is more flexible and its spike acceleration is sharper, better approximating biological spike-generating sodium currents [16, 30].
The saddle-node bifurcation occurs as r increases, moving from resting to spiking. For small r, Equation 3 has two equilibrium points, one stable (near r) and one unstable (higher): Starting at x = 0, the system will settle at the stable equilibrium (Fig. 1). As r increases, the stable and unstable equilibrium points increase and decrease, respectively. At r = rth, the two equilibrium points collide, annihilating each other—a saddle-node bifurcation. Once this bifurcation occurs, x increases without bound, until it reaches xMAX (≫ 1), at which point a spike is declared and the neuron reset.
Fig. 1.
Measured silicon neuron membrane traces (x, normalized) rise like a resistor-capacitor circuit, with a positive feedback spike for several step-input current levels (r=0.48, 0.57, 0.69, 0.82, 0.98, and 1.2). Below a threshold input, the neuron reaches a steady state at which the input and positive feedback are insufficient to overpower the leak. Above threshold, larger inputs enable the positive feedback to overcome the leak more quickly, resulting in a shorter time to spike. Inset The neuron’s phase plot shows ẋ versus x (black), fit with a cubic (gray). The neuron has two fixed points (circles) when its input is small, r < 2/3, one stable (filled) and one unstable (open). As r increases above 2/3, the fixed points merge and destroy each other, undergoing a saddle-node bifurcation, the transition from resting to spiking. Phase plot traces filtered by a 5 ms halfwidth gaussian.
To visualize the dynamics of the spike, we use a DST workhorse, the phase plot (see [17, 21]). Phase plots show the relationships among states and dynamics of the system. For our one-dimensional neuron, we plot ẋ versus x, revealing how the neuron’s membrane changes depending on its current state (Fig. 1 inset).
B. Circuit Synthesis
To implement the neuron circuit, we compute the polynomial expressions by using currents to represent our variables of interest. In the subthreshold regime, a (metal-oxide semiconductor) transistor’s (gate–source) voltage is logarithmically related to its (drain–source) current. Therefore, currents can be multiplied simply by adding voltages. Furthermore, since we store the circuit’s state on capacitors, as voltage, we need to compute the derivative of the logarithm.
The process to convert the neuron ODE into a circuit requires three steps. First, we transform the ODE into a current-mode description by replacing each dimensionless variable with a ratio of currents. Second, based on a current’s time derivative, we compute the capacitor voltage’s time derivative (CV̇ = ΣIi(t)). Third, we design a subcircuit to implement each current term, Ii(t).
First, we transform Equation 3 by replacing each dimensionless variable with a ratio of currents, , where IC is constant, yielding:
| (4) |
To find a description in terms of a capacitor’s voltage, we find the relation between İx and V̇x, where Vx is the gate voltage (referenced below the positive voltage supply, VDD) of the transistor whose current is Ix. Since we tune the PMOS transistors to be in the subthreshold regime:
| (5) |
where I0 and κ are transistor parameters, and Ut is the thermal voltage. Substituting Equation 5 into Equation 4, we find:
| (6) |
where (see [31]), and we define .2
To realize the circuit we require three currents to drive the capacitor (Fig. 2): one current decreases the capacitor voltage (towards VDD) and two increase it (towards GND). The first current term (IL) corresponds to x’s decay towards zero; ML accomplishes this decay.
Fig. 2.
The neuron circuit comprises a capacitor Cx, driven by three currents: an input (Mr), a leak (ML), and positive feedback (MF1–5). When the input and positive feedback currents drive Vx low enough, REQ transitions from low to high, signaling a spike.
The second term corresponds to r, realized by driving Vx towards GND. It equals the product of the input (Ir) and leak currents (IL), divided by the membrane current (Ix). We generate this current using the translinear principle for subthreshold transistors [32, 33]. In short, we set Mr’s gate to VL + Vr to generate ILIr. Connecting its source and bulk to Vx realizes division by Ix, yielding .
The third term corresponds to the cubic (positive) feedback, x3/3, driving Vx towards GND. It equals the membrane current squared divided by a constant current (Ipth). Again, we use the translinear principle, mirroring Ix (MF2,3) to a differential pair (MF4,5) and shorting one side’s gate to the mirror, which realizes the square. The bias voltage on the other side of the pair sets Ipth, which sets the current–voltage range over which the neuron operates. Alternatively, we could implement quadratic feedback by removing MF4,5, but we would have less control, as our operating range would be set by transistor sizing alone.
The base circuit requires eight transistors and one capacitor (Fig. 2). When the input and positive feedback currents drive Vx low enough, Mx and MF1 behave like an inverter [34], generating a digital request, REQ, which transitions from low to high, signaling a spike. After a neuron spikes, it is reset.
The neuron’s reset drives x to xRES by driving Vx towards VDD. In the simplest implementation, the reset briefly sets VL = 0; ML overcomes the positive feedback and resets Vx. The duration of the reset pulse (tr) sets xRES. A long reset pulse, sets Vx = VDD, corresponding to xRES = 0; a shorter reset pulse, sets Vx < VDD, corresponding to xRES > 0. In practice, we do not use ML, but use another circuit that provides better control over tr (see Fig. 7).
Fig. 7.
To implement adapting and bursting the neuron circuit is augmented with two ion-channel population circuits, realizing gk (Mk) and rca (Mca), and a reset circuit (Mres).
Biasing the neuron circuit requires us to set each current such that ratios of currents realize the dimensionless variables in Equation 3. From Equation 6, we see that we only need to set three currents: IL, Ipth, and Ir. First, we set IL to obtain the desired τ. Next, we set Ipth to obtain the desired threshold, which defines the sub-spike-threshold range (Vpth = 0.8 V for all data in this paper). In general, as Vpth increases the neuron uses greater power, but the signals sent off-chip are easier to measure. Finally, we set Ir to achieve a specified r, using .
The Neuron chip was fabricated through MOSIS in 1P5M 0.25 μm CMOS process, consuming 10 mm2 of area. It has a 16 by 16 array of microcircuits. Each microcircuit contains four neurons (77 by 14 μm each) and one interneuron (not used here). Each neuron has 21 STDP circuits (not used here). The Neuron Chip uses the address-event representation (AER) to transmit spikes off chip and to receive spike input [35, 36] in addition to an analog scanner that allows us to observe the state of one neuron at a time. The chip consumes on the order of 1–5 mW with all neurons and interneurons spiking at average rates up to 40 Hz.3 All data figures in this work are recorded from a single neuron on this chip. For a complete description of the Neuron chip, circuit layout, and test board, see [31].
IV. Dynamical Systems Analysis
In this section, we analyze and characterize the neuron’s frequency-current relation. First, we use Equation 3 to obtain both exact and approximate solutions to the neuron’s period as a function of input current in the monostable regime. Then, we use these results to fit measured data from a silicon neuron. Next, we show that we can configure the neuron in the bistable regime in which it expresses spike-frequency hysteresis.
A. Frequency-Current Relation
As r surpasses rth, the neuron moves from the resting state to the spiking state (reset to xres = 0). Once the neuron enters the spiking state, its frequency increases as r increases above rth. We view this relationship in terms of the neuron’s phase plot (Fig. 1 inset). Increasing r drives the phase plot up, increasing ẋ for all x values. If ẋ lies just above zero, x increases slowly near the inflection point, resulting in a long period, T, and a low spike frequency, f. Increasing ẋ decreases the time x spends near its inflection, increasing the spike frequency.
We calculate T by summing the time intervals the neuron takes to increase from x to x + Δx for constant values of r and xres, equivalent to integrating from Equation 3 with respect to x from xres to ∞:
| (7) |
where pi is the ith root of the neuron’s characteristic polynomial: p3/3 − p + r. Though analytically tractable this exact solution is sufficiently complicated to preclude us from gaining much insight into neuron behavior, so we choose not to evaluate it further.
To gain intuition, we simplify Equation 7 in the regime where r ≫ x. We ignore x and replace r with r − rth, which preserves the bifurcation point. Also, to simplify the integral, we set xres = 0, yielding:
| (8) |
We see that for large r, f(r, 0) = 1/T(r, 0) increases as r2/3.
To characterize the neuron’s frequency-current relationship, we drove the neuron by stepping r from zero to a range of values (input currents) and measured the neuron’s time to spike, the inverse of which corresponds to f.4 We step r up by stepping Vr down towards GND (transistor Mr’s gate bias Vr + VL; see Fig. 2), and measure f over a range of Vr values.
We fit f versus r to Equation 7 (Fig. 3). We found that f was within 5% of the predicted value for all r values (τ = 27.1 ms). We calibrated Ir’s dependence on Vr (Ir = I0eκVr/Ut) using a property of the monostable saddle-node bifurcation: The neuron spikes when and near the bifurcation [21]. Therefore, we find the smallest five values of Ir that result in the neuron spiking and fit the frequency squared to find r’s value as a function of Ir (Fig. 3 inset). This procedure yielded I0 and the value of Ir that corresponds to .5
Fig. 3.
The silicon neuron responds sublinearly to current above a threshold (dots). See text for fit equation. Inset f2 is fit (gray line) versus the input to find and normalize to the threshold r = 2/3.
B. Frequency Bistability
We verified that our saddle-node-bifurcation neuron is capable of expressing bistability (Fig. 4). We put the neuron in the bistable regime by setting xres ≈ 1.7. We drove the neuron with a below-threshold input current, r = 0.36. The neuron did not spike; its membrane settled to a constant level. Next, we increased r to 0.70, causing the neuron to spike repeatedly at 36 Hz. Then, we returned the input to the previous level 0.36, but because the neuron’s membrane potential x was never pulled below it’s unstable point (about 1.57 for r = 0.36), it continued spiking at a reduced rate of 22 Hz.
Fig. 4.
The neuron expresses frequency bistability when reset to 1.7. Initially, when the input is low, r = 0.36, the neuron is silent, but when it increases to 0.70 then, drops back to 0.36, the neuron spikes at 22 Hz. Inset The neuron trajectory (black) follows the fits for r = 0.36, 0.70 (gray). When it is reset above the unstable point (open circle) for r = 0.36, the neuron spikes; otherwise it sits at the stable point (filled circle). Phase plot traces filtered by a 5 ms halfwidth gaussian.
V. Square-Wave Bursting
In this and the following two sections, we apply the dynamical systems design approach to design neurons capable of bursting and adaptation by adding model voltage-gated ion channels. We chose types of bursting and adaptation that are commonly found in computational models as well as observed in biology [21]. We implement these dynamics with channels that increment their activity only during a spike (high-threshold variety) and decay thereafter, and limit ourselves to adding at most one excitatory and one inhibitory channel population to the neuron.
To augment the neuron, we follow the DSD procedure introduced in Sections II and III: We determine the bifurcation(s) and equation(s) that implement the desired dynamics and implements them in circuits. In the case of reconfiguring the neuron, we modify and augment Equation 3, rather than starting from a canonical equation.
Square-wave bursting (SWB) is characterized by a silent phase with low membrane potential followed by a high-frequency spiking phase with high membrane potential (i.e., xres > 1) (Fig. 5). During the high phase, sodium positive feedback remains active, causing repetitive spiking (Fig. 5 inset). Spiking ends when an inhibitory potassium conductance grows strong enough to overcome the positive feedback, which pulls the membrane low, removing the positive feedback and terminating the burst until the potassium conductance decays. We use DSD to endow our neuron with the appropriate bifurcations to realize SWB.
Fig. 5.
In square-wave bursting, as gk decays the burst begins, x spikes rapidly until gk overcomes the positive feedback. Inset The neuron rests at its stable point (closed circle), which becomes destabilized when the ẋ versus x curve rises as gk decays, starting a burst (arrows). During the burst, the ẋ versus x curve drops as gk rises, eventually pushing the unstable point (open circle) above xres, which terminates the burst. Phase plot and gk traces filtered by a 5 ms halfwidth gaussian.
A. Design
SWB requires two bifurcations, one from resting to spiking and one from spiking to resting. For its resting-to-spiking bifurcation, SWB employs a bistable saddle, setting its reset above the saddle’s unstable equilibrium (as in Section IV-B). For its spiking-to-resting bifurcation, SWB employs a homoclinic bifurcation, moving its unstable point up to intersect the (spiking) limit cycle; an inhibitory conductance (gk), which increments after each spike, achieves this. When the unstable point crosses the limit cycle, spiking ceases and x drops to the stable point. In a dynamical-systems context SWB is referred to as fold-homoclinc bursting [37]; a bistable saddle is also known as a fold bifurcation.
gk is situated in parallel with the neuron’s leak, augmenting Equation 3:
| (9) |
As gk increases, the bifurcation point, , increases, reducing r − rth and hence ẋ. We model gk after the M-type potassium (MK) channel [38]. An MK channel population has a slow decay and high activation threshold. Its high threshold ensures that the MK population is only activated during spikes and decays during interspike intervals. Its slow decay enables MK channels to integrate across spikes, increasing their conductance in proportion to spike frequency.
We model gk as a first-order ODE with a spike response described by:
| (10) |
where pk(t) equals one for a brief duration (tk) after the neuron spikes, resulting in an increase in gk by Δgk = gmaxtm/τk (for gk ≪ gmax).
B. Circuit
Once we know the dynamics and equations we want to implement, we need to generate circuit implementations for the channel population (Equation 10) and connect it to the neuron circuit (Equation 9). We use the same approach presented in Section III-B: We replace each variable with a ratio of currents ( , where Ik = I0eκVk/Ut) and use the translinear principle to construct appropriate subcircuits. The resulting circuit equations are:
| (11) |
where ILk determines gk’s decay constant: . gk’s first-order dynamics are realized in the same manner as the neuron circuit with the addition of a series transistor to gate the input by pk(t), which is provided by a pulse-extender circuit (Fig. 6). SWB is realized by placing the pulse extender and low-pass filter in a feedback loop around the positive-feedback neuron (Fig. 7). To bias gk, we set τk with ILk, and we set gmax with Ikmax relative to IL. For example, if gmax is 10, we set Ikmax to 10 times IL.
Fig. 6.
The ion-channel population circuit consists of two modules, one implements the pulse-like spike response, p(t) (Mp1,2 and Cp), and the other implements the first-order dynamics, Vch (Mch1–3 and Cch). Vch generates the output current, Ich, when it is applied to the gate of an output transistor Mout.
C. Analysis and Characterization
To understand the mechanics of SWB, we recall the bistable neuron without an MK conductance. If xRES > 1, the sodium overpowers the leak conductance, and therefore even if we lower r spiking continues. The high reset realizes bistability in the spike rate: For r near the bifurcation, the neuron can be active or silent. Similarly, for gk near the bifurcation, the neuron can be active or silent.
To characterize SWB, we set xRES = 2.35 and drove the neuron with r = 0.9, observing bursting with three spikes per burst. We calculate the values to which gk must decay and grow to initiate and terminate the burst, respectively. To initiate the burst, gk must decay enough such that , which yields . To terminate the burst, gk must grow enough such that xRES equals x’s unstable equilibrium. We find this equilibrium by setting ẋ = 0 (Equation 9) and solving for gk given x = xRES, which yields . To analyze SWB, we again use a phase plot.
The SWB neuron’s phase plot considers the relationship between x and gk (Fig. 8). For gk below 0.22, x has no (positive) equilibria points (it has only a stable spiking limit cycle). For gk above 0.22, x has two equilibria points, one stable and one unstable (as well as the stable spiking limit cycle). However for gk greater than 1.22, the unstable equilibrium enters the spiking limit cycle, destabilizing it. When its limit cycle becomes unstable the neuron can still spike if it is kicked into the limit cycle’s region of phase space, but only once, then the neuron is reset and descends to the stable equilibrium.
Fig. 8.
In square-wave bursting, x and gk follow a trajectory (black) in which x moves towards either a stable equilibrium or a spiking limit cycle. Initially for low gk (below 0.22), x enters the spiking limit cycle (light gray shading). As gk increases (between 0.22 and 1.22), x remains in its spiking limit cycle, even though a stable point and an unstable point have appeared (solid and dashed lines). Once gk exceeds 1.22, the limit cycle becomes unstable (dark gray shading); x drops to its only stable point, until gk decays below 0.22, repeating the cycle. Traces filtered by a 5 ms halfwidth gaussian; dots are 5 ms apart.
From this phase-space perspective, we see that for small gk, after a silent phase, x enters the spiking limit cycle. As gk increases, the spiking limit cycle remains stable despite the creation of the two other equilibria; so in the absence of perturbation, the neuron continues spiking. Only when gk exceeds 1.22 does the limit cycle become unstable, resulting in one last spike, ending the burst. During the silent phase, as gk decays, x follows its calculated (not fit) stable equilibrium.
VI. Spike-Frequency Adaptation
Spike-frequency adaptation (SFA) reduces a neuron’s sensitivity to sustained excitatory input, lowering its spike frequency over time (Fig. 9). For example, if an adapting neuron receives a potent input current step, it will fire several rapid spikes, but each subsequent period will be longer than the one before, until the neuron reaches a steady-state frequency. Like SWB, SFA is realized by a potassium conductance that builds up from one spike to another. Unlike SWB, however, the neuron is reset below its unstable point, eliminating bistability.
Fig. 9.
In spike-frequency adaptation, as gk (dark gray) increases x’s (light gray) spike frequency decreases. Inset gk decreases frequency by decreasing ẋ (black) towards zero, slowing spiking. Fits (gray) are a guide for the eye. Phase plot and gk traces filtered by 10 and 5 ms halfwidth gaussians, respectively.
A. Design
We realize SFA by pushing the neuron progressively closer to the saddle-node bifurcation responsible for its transition from resting to spiking. For a fixed input current r, the bifurcation occurs at . As gk approaches this value, ẋ decreases, lowering the neuron’s frequency (Fig. 9 inset).
B. Circuit
SFA’s circuit is identical to the one used in SWB (Fig. 7), described by Equation 11. The one difference is that we set the reset circuit to deliver a longer reset pulse, resulting in xres = 0, below the unstable point.
C. Analysis and Characterization
Once the circuit is implemented, we analyze and characterize its behavior, focusing on SFA’s response to steps to a range of r values. We drove the adapting neuron with five r values (1.0, 2.0, 3.7, 6.7, and 11.8) and observed f and gk. We consider f instead of x, since gk and f directly depend on each other, whereas x influences gk only through f. For each r value, we allow the neuron’s frequency to reach steady state.
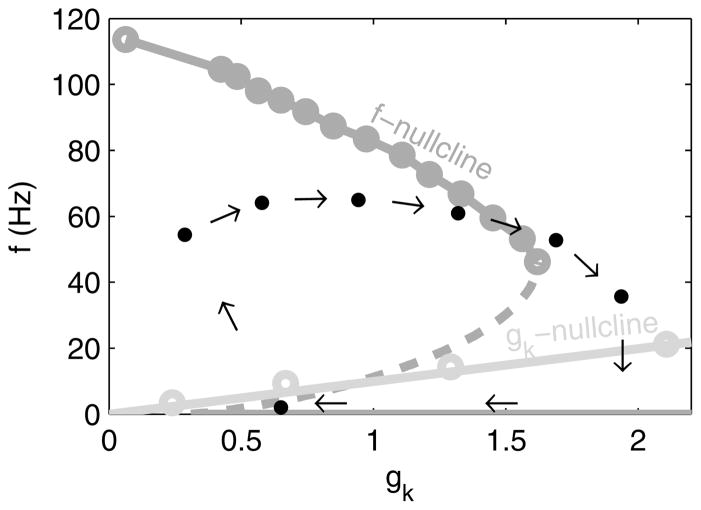
To visualize the dynamics, we again use a phase plot, showing the f-gk trajectory (Fig. 10). In addition, we plot a nullcline for each variable. The f-nullcline is the curve where f is in equilibrium for a given value of . Similarly, the gk-nullcline is the curve where gk is in equilibrium for a given value of . Nullclines provide insight into the dynamics of the system; from any given point on the phase plot, f and gk always move along their respective axes towards their respective nullclines. Eventually, f and gk arrive at the intersection of their nullclines, an equilibrium point. This equilibrium is stable: If we perturb the system, it returns.
Fig. 10.
In spike-frequency adaptation, f starts high but decreases as gk increases (arrows), following the f-nullcline (light gray) until it crosses the gk nullcline, reaching a stable equilibrium. Each dot corresponds to one interspike interval’s instantaneous f and mean gk. gk values filtered by a 5 ms halfwidth gaussian.
To find the neuron’s f-nullcline, we calculate T in the same manner as Section IV, by integrating from Equation 9 with respect to x, from xres(= 0) to ∞:
| (12) |
where pi is the ith root of the neuron’s characteristic polynomial: p3/3 − p(1 + gk) + r. f(r, xres, gk) = 1/T corresponds to the f nullclines (Fig. 10). These nullclines assume gk is constant; because we set gk to be slow (τk = 190 ms, fitted), it is approximately constant over a neuron’s interspike interval.
To find the neuron’s gk-nullcline with respect to f, we calculate gk’s steady-state conductance, gk∞(f) by equating its decay over one period to its growth, Δgk:
| (13) |
which is a good approximation (within 8%) for f τk > 1 (Fig. 10). This linear relationship enables us to obtain Δgk(= 0.60) from the gk-nullcline’s slope.
VII. Parabolic Bursting
Parabolic bursting (PB) is characterized by a quiescent phase followed by a bursting phase in which the spike frequency rises and falls like a parabola (Fig. 11). After the quiescent phase, each interspike interval becomes shorter until a peak frequency is reached, after which each interspike interval becomes longer until the burst terminates, resulting in another quiescent phase. This smooth frequency increase and decrease contrasts with SWB’s abrupt frequency changes, which arise from bistability in the membrane potential. Reseting its membrane potential to zero after a spike eliminates this source of bistability and calls for a different mechanism, which directly produces bistability in spike frequency. The membrane potential’s bifurcations remain monostable—also known as circle bifurcations—hence PB is referred to as circle-circle bursting [37].
Fig. 11.
In parabolic bursting, as gk (dark gray) decays the burst begins, x (light) spikes faster then slower until the burst ends. Inset gk shifts the conceptual f(rca) curve. For medium gk (middle curve), two stable points coexist (filled circles), one at a high frequency and one at a low, with an unstable point in between (open circle). For low gk (top curve), the low stable point collides with the unstable point, leaving only the high stable point, initiating spiking. For high gk (bottom curve), the high stable point collides with the unstable point, leaving only the low stable point, terminating spiking. gk filtered by a 5 ms halfwidth gaussian.
A. Design
We realize frequency bistability by introducing positive feedback in the form of an excitatory current, rca, which models a calcium channel (CC) population:
| (14) |
Each time the neuron spikes, rca increments, but decays slowly, increasing the drive to the neuron and making it spike more frequently. This linear frequency-to-rca curve (see below) intersects the neuron’s compressive current-to-frequency curve in three places, producing two stable points separated by an unstable one (Fig. 11 Inset). This situation pertains to intermediate gk values that shift the neuron’s current-to-frequency curve to the right a moderate amount. For lower gk values, the lower stable point disappears (transition from resting to spiking); for higher gk values, the higher stable point disappears (transition from spiking to resting).
As the CC population has a high threshold, only activating during spikes, we model rca in the same manner as gk:
| (15) |
where pca(t) equals one for a period (tca) after the neuron spikes, resulting in an increase in rca by Δrca = rmaxtca/τca (for rca ≪ rmax). We tune CC to have a decay slow enough to enable it to integrate across spikes. In that case, Equation 13 applies, yielding a linear frequency relationship rca∞ = fτcaΔrca for rca’s steady-state value. Note that this design assumes that both rca and gk approach values proportional to f, requiring both to decay on a time scale slower than the membrane and spike dynamics. We measured τk = 190 ms and τca = 53 ms, compared to a typical intraburst spike interval of 17ms.
B. Circuit
Except for the addition of ion-channel population circuit for , PB’s circuit (Fig. 7) is identical to SFA’s:
| (16) |
where and gk’s equation is omitted (See Equation 11). Notice that Ica and Ir are in parallel. We realize pca(t) by putting a transistor in series with the input transistor ( ), turned on for period tca after each spike (Fig. 7). To bias rca, we set τca with ILca, and we set Irmax in the same manner we set Ir in Section III-B.
C. Analysis and Characterization
To understand PB, we again employ a two-dimensional phase plot with two nullclines, for f and gk (Fig. 12).6 Whereas the gk nullcline is identical to the one for SFA, the f nullcline has three branches, which trace the loci of f’s three fixed points as gk varies (Fig. 11 Inset). As gk increases, the upper stable point drops (in frequency), the lower stable point remains at zero, and the unstable point rises. These three branches coexist over an intermediate range of gk values (bistable region) whose lower and upper limits correspond to the values of gk at which resting-to-spiking and spiking-to-resting transitions occur, respectively. We determine the lower limit the same way we did for SWB’s transition to spiking, yielding for r = 1.0. We determine the upper limit by finding when the two curves in Fig. 11 Inset are tangential, which does not yield a closed-form solution.
Fig. 12.
In parabolic bursting, f and gk follow a trajectory (black) in which both move towards stable portions of their respective nullclines (dark and light gray). Each dot corresponds to one interspike interval’s instantaneous f and mean gk. gk values filtered by a 5 ms halfwidth gaussian.
Although we also cannot obtain the f-nullcline analytically, we can characterize it experimentally by varying Δgk. Reducing Δgk increases the gk-nullcline’s slope, causing it to intersect the f-nullcline’s upper stable branch, and the neuron to fire at the corresponding frequency. Thus, we can trace this branch by sweeping Δgk until the neuron starts bursting. We estimate the middle branch by extrapolating between this point and the calculated lower limit. The resulting nullcline is consistent with the measured bursting limit cycle (Fig. 12).
Once we have obtained the nullcline, we track a burst through its phase space (Fig. 12). A burst initiates when gk decays below its lower limit, causing f to move towards its nullcline’s upper branch. This trajectory crosses the gk nullcline, causing gk to move to the right. Eventually, this upward and rightward trajectory crosses the f nullcline. The burst terminates when gk exceeds its upper limit, causing f to move towards its nullcline’s lower branch. The trajectory crosses the gk nullcline once again, causing gk to move to the left, initiating a new burst.
VIII. Discussion
We presented DSD, an approach to design silicon neurons at the appropriate level of abstraction, epitomized by simple but complete. This approach uses dynamical systems theory to develop equations that describe model neurons. Our work offers a solution to selecting an appropriate level of abstraction, a fundamental challenge in neuromorphic design.
DSD strikes a balance between biologically-detailed top-down and technologically-simple bottom-up approaches, taking strengths from each one. DSD allows the designer to match biological neurons’ dynamics, thereby reproducing much of their computing power. Further, it allows the designer to reproduce these dynamics while maintaining circuit simplicity and density, keeping the system away from the Valley of Death.
We provided a method to transform DSD-derived equations into subthreshold log-domain (current-mode) circuits, an efficient implementation technique. We demonstrated this technique by designing compact neuron circuits that realize regular spiking, square-ware bursting, spike-frequency adaptation, and parabolic bursting. The 1,024 excitatory neurons on the chip presented in this work express a spike-frequency coefficient of variation (CV) between 18 and 25 percent, depending on their settings, whereas we have previously designed neurons with similar behaviors but with greater transistors counts expressing variations close to 100 percent [11], validating the simple but complete axiom.
DSD will remain a viable design strategy as technology advances due to its flexibility. Agnostic to current, voltage, or any physical quantity, DSD simply prescribes the behavior of the system. Such flexibility is crucial to enable the use of whatever circuit medium is available to scale artificial neurons towards the integration density and energy efficiency of neurobiology.
Appendix A.
Transistor Sizes and Capacitor Values
| Transistor | Width/Length (μm/μm) | Transistor | Width/Length (μm/μm) |
|---|---|---|---|
| ML, p2, ch3, k | 0.7/4.7 | Mp1 | 1.0/1.6 |
| Mr, ca | 1.4/2.0 | Mch1 | 1.2/4.7 |
| Mx, out | 1.4/3.2 | Mch2 | 1.7/2.5 |
| MF1–3, F5 | 0.6/2.4 | Mres | 0.6/1.4 |
| MF4 | 0.6/2.2 | ||
| Capacitor | Value (fF) | Capacitor | Value (fF) |
| Cx | 900 | Cp | 60 |
| Cch | 840 | ||
Acknowledgments
The authors would like to thank the students of the 2007 and 2008 BioE 332 classes for their questions and enthusiasm, which helped to clarify much of this work.
The NIH Director’s Pioneer Award provided funding (DPI-OD000965).
Biographies

John V. Arthur received the B.S.E. degree (summa cum laude) in electrical engineering from Arizona State University, Tempe, and the Ph.D. degree in bioengineering from the University of Pennsylvania, Philadelphia, in 2000 and 2006, respectively.
He was a Post-Doctoral Scholar in bioengineering at Stanford University, Stanford, CA, where he worked on the SyNAPSE project. In addition, he was the lead analog and neuron designer for the Neurogrid project, which aims to build a desktop supercomputer for neuroscientists, allowing them to model complex cortical systems of up to 106 neurons at a fraction of the cost of a general purpose supercomputer.
He is currently a research staff member at IBM Almaden Research Center, San Jose, CA. His research interests include dynamical systems design, neuromorphic learning systems, silicon olfactory recognition, generation of neural rhythms, and asynchronous interchip communication.

Kwabena A. Boahen Kwabena A. Boahen is an Associate Professor in the Bioengineering Department at Stanford University. He is a bioengineer who is using silicon integrated circuits to emulate the way neurons compute, linking the seemingly disparate fields of electronics and computer science with neurobiology and medicine. His contributions to the field of neuromorphic engineering include a silicon retina that could be used to give the blind sight and a self-organizing chip that emulates the way the developing brain wires itself up. His scholarship is widely recognized, with over seventy publications to his name, including a cover story in the May 2005 issue of Scientific American. He has received several distinguished honors, including a Fellowship from the Packard Foundation (1999), a CAREER award from the National Science Foundation (2001), a Young Investigator Award from the Office of Naval Research (2002), and the National Institutes of Health Directors Pioneer Award (2006). Professor Boahens BS and MSE degrees are in Electrical and Computer Engineering, from the Johns Hopkins University, Baltimore MD (both earned in 1989), where he made Tau Beta Kappa. His PhD degree is in Computation and Neural Systems, from the California Institute of Technology, Pasadena CA (1997), where he held a Sloan Fellowship for Theoretical Neurobiology. From 1997 to 2005, he was on the faculty of the University of Pennsylvania, Philadelphia PA, where he held the first Skirkanich Term Junior Chair.
Footnotes
The Valley of Death is a conceptural region of neuron complexity where neurons are too complex to be well matched and too simple to auto-compensate for variation, resulting in poor system performance [11, 12]. Eventually, complexity (∝ transistor count) increases to the degree that neurons can compensate for their variations, as occurs in neurobiology, rescuing system performance [13].
The last term of Equation 6 is implemented as , which approximates for Ix ≪ Ipth. This assumption is violated during the period when Ipth < Ix < Iinv, the current level where REQ transitions from low to high, signaling a spike. This period is negligible for large Ipth (Vpth ≥ 0.5 V).
In simulation, when spiking at 100 Hz, a single neuron consumes 50–1000 nW depending on its biases.
Alternatively, we could have measured the neuron’s interspike intervals; however, then we would have to account for the effects of the reset–refractory period.
We measured κ = 0.69 in a separate experiment.
It is important to note that f’s dynamics reflect rca’s, since the latter changes much more slowly.
Personal use of this material is permitted. However, permission to use this material for any other purposes must be obtained from the IEEE by sending an email to pubs-permissions@ieee.org.
Contributor Information
John V. Arthur, Email: arthurjo@us.ibm.com.
Kwabena Boahen, Email: boahen@stanford.edu.
References
- 1.Mead CA. Analog VLSI and Neural Systems. Reading, MA: Addison-Wesley; 1989. [Google Scholar]
- 2.Indiveri G, Chicca E, Douglas R. A VLSI array of low-power spiking neurons and bistable synapses with spike-timing dependent plasticity. IEEE Transactions on Neural Networks. 2006;17(1):211–221. doi: 10.1109/TNN.2005.860850. [DOI] [PubMed] [Google Scholar]
- 3.Folowosele F, Harrison A, et al. A switched capacitor implementation of the generalized linear integrate-and-fire neuron. International Symposium on Circuits and Systems, ISCAS’09; IEEE Press; 2009. pp. 24–27. [Google Scholar]
- 4.Mahowald M, Douglas R. A silicon neuron. Nature. 1991;354:515–518. doi: 10.1038/354515a0. [DOI] [PubMed] [Google Scholar]
- 5.Simoni MF, Cymbalyuk GS, Sorensen ME, Calabrese RL, DeWeerth SP. A multiconductance silicon neuron with biologically matched dynamics. IEEE Transactions on Biomedical Engineering. 2004;51(2):342–354. doi: 10.1109/TBME.2003.820390. [DOI] [PubMed] [Google Scholar]
- 6.Hynna KM, Boahen K. Thermodynamically-equivalent silicon models of ion channels. Neural Computation. 2007;19(2):327–350. doi: 10.1162/neco.2007.19.2.327. [DOI] [PubMed] [Google Scholar]
- 7.Toumazou C, Georgiou J, Drakakis EM. Current-mode analogue circuit representation of Hodgkin and Huxley neuron equations. Electronics Letters. 1998;34(14):1376–1377. [Google Scholar]
- 8.Merolla P, Boahen K. A Recurrent Model of Orientation Maps with Simple and Complex Cells. In: Thrun S, Saul L, Schölkopf B, editors. Advances in Neural Information Processing Systems. Vol. 16. Cambridge, MA: MIT Press; 2004. pp. 995–1002. [Google Scholar]
- 9.Shi RZ, Horiuchi TK. A Neuromorphic VLSI model of bat interaural level difference processing for azimuthal echolocation. IEEE Transactions on Circuits and Systems I. 2007;54:74–88. [Google Scholar]
- 10.Oster M, Wang Y, Douglas R, Liu SC. Quantification of a spike-based winner-take-all VLSI network. IEEE Transactions on Circuits and Systems I. 2008;55:3160–3169. [Google Scholar]
- 11.Arthur JV, Boahen K. Recurrently connected silicon neurons with active dendrites for one-shot learning. International Joint Conference on Neural Networks, IJCNN’04; IEEE Press; 2004. pp. 1699–1704. [Google Scholar]
- 12.Douglas R, Mahowald M, Mead C. Neuromorphic analogue VLSI. Annu Rev Neurosci. 1995;18:255–281. doi: 10.1146/annurev.ne.18.030195.001351. [DOI] [PubMed] [Google Scholar]
- 13.Renart A, Song P, Wang XJ. Robust spatial working memory through homeostatic synaptic scaling in heterogeneous cortical networks. Neuron. 2003;38:473–485. doi: 10.1016/s0896-6273(03)00255-1. [DOI] [PubMed] [Google Scholar]
- 14.Gerstner W, Naud R. How good are neuron models? Science. 2009;326(5951):379–380. doi: 10.1126/science.1181936. [DOI] [PubMed] [Google Scholar]
- 15.Lapicque L. Recherches quantitatives sur lexcitation electrique des nerfs traitee comme une polarization. J Physiol Pathol Gen. 1907;9:620–635. [Google Scholar]
- 16.Hodgkin AL, Huxley AF. A quantitative description of membrane current and its application to conduction and excitation in nerve. Journal of Physiololgy. 1952;117(4):500544. doi: 10.1113/jphysiol.1952.sp004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Strogatz SH, Herbert DE. Nonlinear Dynamics and Chaos. Reading, MA: Addison-Wesley; 1994. [Google Scholar]
- 18.Manor Y, Rinzel J, Segev I, Yarom Y. Low-amplitude oscillations in the inferior olive: a model based on electrical coupling of neurons with heterogeneous channel densities. Journal of Neurophysiology. 1997;77:2736–2752. doi: 10.1152/jn.1997.77.5.2736. [DOI] [PubMed] [Google Scholar]
- 19.Tateno T, Harsch A, Robinson HPC. Threshold firing frequency-current relationships of neurons in rat somatosensory cortex: type 1 and type 2 dynamics. Journal of Neurophysiology. 2004;92:2283–2294. doi: 10.1152/jn.00109.2004. [DOI] [PubMed] [Google Scholar]
- 20.Stopfer M, Jayaraman V, Laurent G. Intensity versus identity coding in an olfactory system. Neuron. 2003;39:991–1004. doi: 10.1016/j.neuron.2003.08.011. [DOI] [PubMed] [Google Scholar]
- 21.Izhikevich E. Dynamical Systems in Neuroscience: The Geometry of Excitability and Bursting. Cambridge, MA: The MIT press; 2007. [Google Scholar]
- 22.Basu A, Petre C, Hasler P. Bifurcations in a silicon neuron. International Symposium on Circuits and Systems, ISCAS’08; IEEE Press; 2008. pp. 428–431. [Google Scholar]
- 23.Kohno T, Aihara K. A MOSFET-based model of a class 2 nerve membrane. IEEE Transactions on Neural Networks. 2005;16(3):754–773. doi: 10.1109/TNN.2005.844855. [DOI] [PubMed] [Google Scholar]
- 24.Basu A, Hasler P. Nullcline-based design of a silicon neuron. IEEE Transactions on Circuits and Systems I. doi: 10.1109/TBCAS.2010.2051224. in press. [DOI] [PubMed] [Google Scholar]
- 25.Mihalas S, Niebur E. A generalized linear integrate-and-fire neural model produces diverse spiking behaviors. Neural Computation. 2009;21(1):704–718. doi: 10.1162/neco.2008.12-07-680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Izhikevich EM. Which model to use for cortical spiking neurons? IEEE Transactions on Neural Networks. 2004;15(5):1063–1070. doi: 10.1109/TNN.2004.832719. [DOI] [PubMed] [Google Scholar]
- 27.Rinzel J, Ermentrout GB. Analysis of neural excitability and oscillations. In: Koch C, Segev I, editors. Methods in Neuronal Modeling. Cambridge, MA: MIT Press; 1989. [Google Scholar]
- 28.Izhikevich EM. Resonate-and-fire neurons. Neural Networks. 2001;14:883–894. doi: 10.1016/s0893-6080(01)00078-8. [DOI] [PubMed] [Google Scholar]
- 29.Touboul J, Brette R. Dynamics and bifurcations of the adaptive exponential integrate-and-fire model. Biological Cybernetics. 2008;99(4–5):319–334. doi: 10.1007/s00422-008-0267-4. [DOI] [PubMed] [Google Scholar]
- 30.Brette R, Gerstner W. Adaptive exponential integrate-and-fire model as an effective description of neuronal activity. Journal of Neurophysiology. 2005;94:3637–3642. doi: 10.1152/jn.00686.2005. [DOI] [PubMed] [Google Scholar]
- 31.Arthur JV, Boahen KA. Synchrony in silicon: the gamma rhythm. IEEE Transactions on Neural Networks. 2007;18(6):1815–1825. doi: 10.1109/TNN.2007.900238. [DOI] [PubMed] [Google Scholar]
- 32.Gilbert B. Translinear circuits: a proposed classification. Electronics Letters. 1975;11(1):14–16. [Google Scholar]
- 33.Himmelbauer W, Andreou AG. Log-domain circuits in subthresh-old MOS. Proceedings of the 40th Midwest Symposium on Circuits and Systems; 1997; IEEE Press; 1997. pp. 26–30. [Google Scholar]
- 34.Culurciello E, Etienne-Cummings R, Boahen K. A Biomorphic Digital Image Sensor. Journal of Solid State Circuits. 2003;38:281–294. [Google Scholar]
- 35.Boahen K. A burst-mode word-serial address-event link-I: transmitter design. IEEE Transactions on Circuits and Systems I. 2004;51:1269–1280. [Google Scholar]
- 36.Boahen K. A burst-mode word-serial address-event link-II: receiver design. IEEE Transactions on Circuits and Systems I. 2004;51:1281–1291. [Google Scholar]
- 37.Rinzel J, Ermentrout GB. Analysis of neural excitability and oscillations. In: Koch C, Segev I, editors. Methods in Neuronal Modeling. The MIT Press; Cambridge, MA: 1989. [Google Scholar]
- 38.Adams PR, Brown DA, Constanti A. M-currents and other potassium currents in bullfrog sympathetic neurones. Journal of Physiology. 330:537–572. doi: 10.1113/jphysiol.1982.sp014357. [DOI] [PMC free article] [PubMed] [Google Scholar]