Abstract
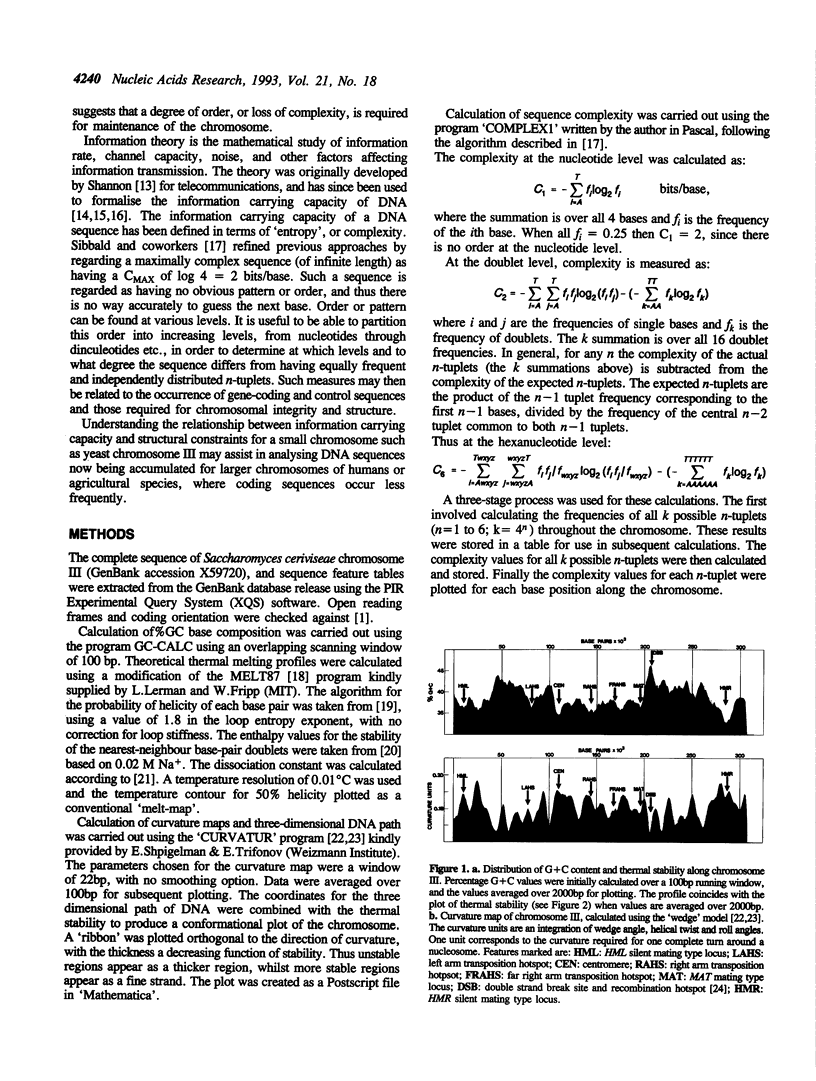
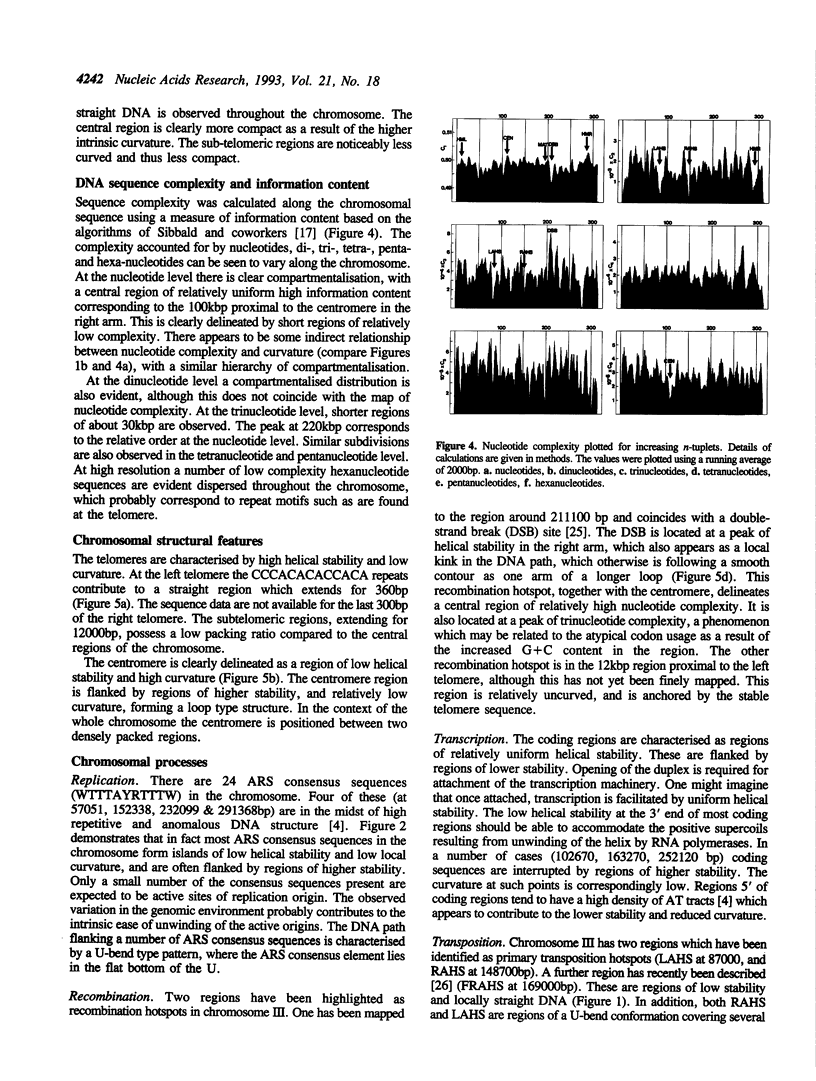
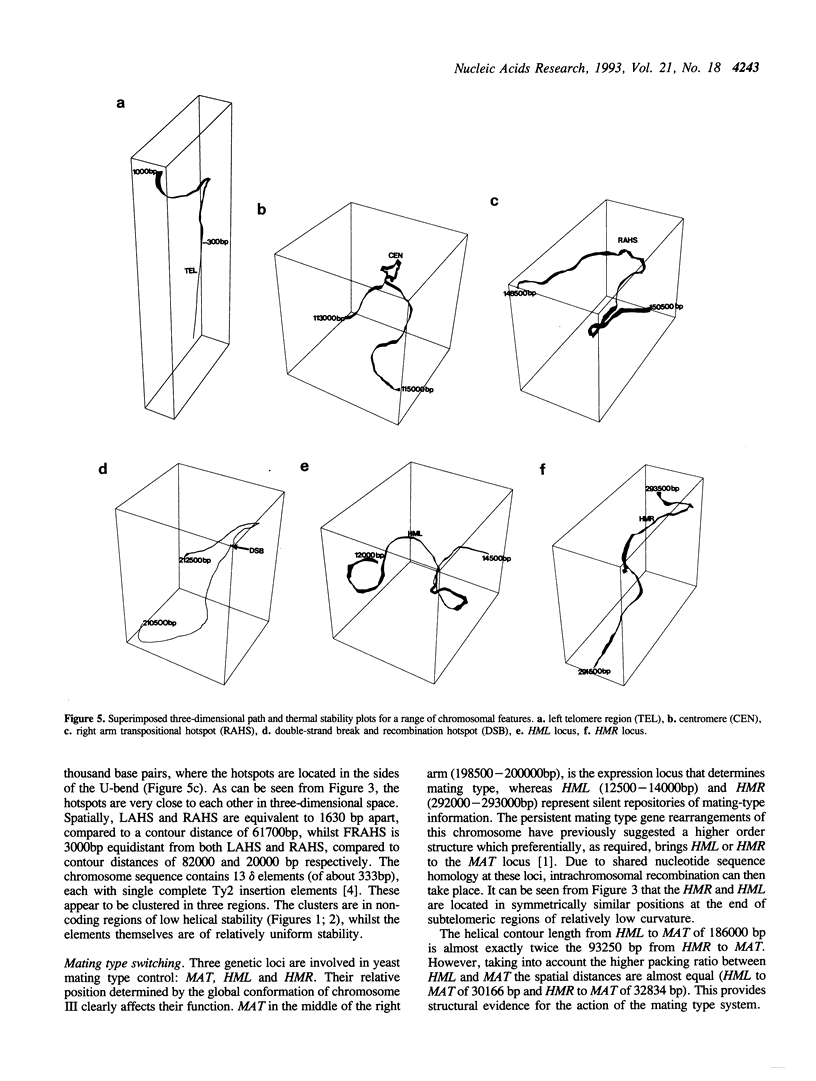
The complete sequence of yeast chromosome III provides a model for studies relating DNA sequence and structure at different levels of organisation in eukaryotic chromosomes. DNA helical stability, intrinsic curvature and sequence complexity have been calculated for the complete chromosome. These features are compartmentalised at different levels of organisation. Compartmentalisation of thermal stability is observed from the level delineating coding/non-coding sequences, to higher levels of organisation which correspond to regions varying in G + C content. The three-dimensional path reveals a symmetrical structure for the chromosome, with a densely packed central region and more diffuse and linear subtelomeric regions. This interspersion of regions of high and low curvature is reflected at lower levels of organisation. Complexity of n-tuplets (n = 1 to 6) also reveals compartmentalisation of the chromosome at different levels of organisation, in many cases corresponding to the structural features. DNA stability, conformation and complexity delineate telomeres, centromere, autonomous replication sequences (ARS), transposition hotspots, recombination hotspots and the mating-type loci.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnold J., Cuticchia A. J., Newsome D. A., Jennings W. W., 3rd, Ivarie R. Mono- through hexanucleotide composition of the sense strand of yeast DNA: a Markov chain analysis. Nucleic Acids Res. 1988 Jul 25;16(14B):7145–7158. doi: 10.1093/nar/16.14.7145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benight A. S., Wartell R. M. Influence of base-pair changes and cooperativity parameters on the melting curves of short DNAs. Biopolymers. 1983 May;22(5):1409–1425. doi: 10.1002/bip.360220512. [DOI] [PubMed] [Google Scholar]
- Bernardi G., Bernardi G. Compositional constraints and genome evolution. J Mol Evol. 1986;24(1-2):1–11. doi: 10.1007/BF02099946. [DOI] [PubMed] [Google Scholar]
- Bolshoy A., McNamara P., Harrington R. E., Trifonov E. N. Curved DNA without A-A: experimental estimation of all 16 DNA wedge angles. Proc Natl Acad Sci U S A. 1991 Mar 15;88(6):2312–2316. doi: 10.1073/pnas.88.6.2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bork P., Ouzounis C., Sander C., Scharf M., Schneider R., Sonnhammer E. Comprehensive sequence analysis of the 182 predicted open reading frames of yeast chromosome III. Protein Sci. 1992 Dec;1(12):1677–1690. doi: 10.1002/pro.5560011216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breslauer K. J., Frank R., Blöcker H., Marky L. A. Predicting DNA duplex stability from the base sequence. Proc Natl Acad Sci U S A. 1986 Jun;83(11):3746–3750. doi: 10.1073/pnas.83.11.3746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dershowitz A., Newlon C. S. The effect on chromosome stability of deleting replication origins. Mol Cell Biol. 1993 Jan;13(1):391–398. doi: 10.1128/mcb.13.1.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fangman W. L., Brewer B. J. A question of time: replication origins of eukaryotic chromosomes. Cell. 1992 Oct 30;71(3):363–366. doi: 10.1016/0092-8674(92)90505-7. [DOI] [PubMed] [Google Scholar]
- Ferguson B. M., Fangman W. L. A position effect on the time of replication origin activation in yeast. Cell. 1992 Jan 24;68(2):333–339. doi: 10.1016/0092-8674(92)90474-q. [DOI] [PubMed] [Google Scholar]
- Fixman M., Freire J. J. Theory of DNA melting curves. Biopolymers. 1977 Dec;16(12):2693–2704. doi: 10.1002/bip.1977.360161209. [DOI] [PubMed] [Google Scholar]
- Goldway M., Arbel T., Simchen G. Meiotic nondisjunction and recombination of chromosome III and homologous fragments in Saccharomyces cerevisiae. Genetics. 1993 Feb;133(2):149–158. doi: 10.1093/genetics/133.2.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldway M., Sherman A., Zenvirth D., Arbel T., Simchen G. A short chromosomal region with major roles in yeast chromosome III meiotic disjunction, recombination and double strand breaks. Genetics. 1993 Feb;133(2):159–169. doi: 10.1093/genetics/133.2.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotoh O. Prediction of melting profiles and local helix stability for sequenced DNA. Adv Biophys. 1983;16:1–52. doi: 10.1016/0065-227x(83)90007-2. [DOI] [PubMed] [Google Scholar]
- Karlin S., Blaisdell B. E., Sapolsky R. J., Cardon L., Burge C. Assessments of DNA inhomogeneities in yeast chromosome III. Nucleic Acids Res. 1993 Feb 11;21(3):703–711. doi: 10.1093/nar/21.3.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauc G., Ilić I., Heffer-Lauc M. Entropies of coding and noncoding sequences of DNA and proteins. Biophys Chem. 1992 Jan;42(1):7–11. doi: 10.1016/0301-4622(92)80002-m. [DOI] [PubMed] [Google Scholar]
- Lerman L. S., Silverstein K. Computational simulation of DNA melting and its application to denaturing gradient gel electrophoresis. Methods Enzymol. 1987;155:482–501. doi: 10.1016/0076-6879(87)55032-7. [DOI] [PubMed] [Google Scholar]
- Matassi G., Montero L. M., Salinas J., Bernardi G. The isochore organization and the compositional distribution of homologous coding sequences in the nuclear genome of plants. Nucleic Acids Res. 1989 Jul 11;17(13):5273–5290. doi: 10.1093/nar/17.13.5273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarroll R. M., Fangman W. L. Time of replication of yeast centromeres and telomeres. Cell. 1988 Aug 12;54(4):505–513. doi: 10.1016/0092-8674(88)90072-4. [DOI] [PubMed] [Google Scholar]
- Natale D. A., Schubert A. E., Kowalski D. DNA helical stability accounts for mutational defects in a yeast replication origin. Proc Natl Acad Sci U S A. 1992 Apr 1;89(7):2654–2658. doi: 10.1073/pnas.89.7.2654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natale D. A., Umek R. M., Kowalski D. Ease of DNA unwinding is a conserved property of yeast replication origins. Nucleic Acids Res. 1993 Feb 11;21(3):555–560. doi: 10.1093/nar/21.3.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver S. G., van der Aart Q. J., Agostoni-Carbone M. L., Aigle M., Alberghina L., Alexandraki D., Antoine G., Anwar R., Ballesta J. P., Benit P. The complete DNA sequence of yeast chromosome III. Nature. 1992 May 7;357(6373):38–46. doi: 10.1038/357038a0. [DOI] [PubMed] [Google Scholar]
- Riggs A. D., Pfeifer G. P. X-chromosome inactivation and cell memory. Trends Genet. 1992 May;8(5):169–174. doi: 10.1016/0168-9525(92)90219-t. [DOI] [PubMed] [Google Scholar]
- Sharp P. M., Lloyd A. T. Regional base composition variation along yeast chromosome III: evolution of chromosome primary structure. Nucleic Acids Res. 1993 Jan 25;21(2):179–183. doi: 10.1093/nar/21.2.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenkin P. S., Erman B., Mastrandrea L. D. Information-theoretical entropy as a measure of sequence variability. Proteins. 1991;11(4):297–313. doi: 10.1002/prot.340110408. [DOI] [PubMed] [Google Scholar]
- Sibbald P. R., Banerjee S., Maze J. Calculating higher order DNA sequence information measures. J Theor Biol. 1989 Feb 22;136(4):475–483. doi: 10.1016/s0022-5193(89)80159-6. [DOI] [PubMed] [Google Scholar]
- Takahashi M. A fractal model of chromosomes and chromosomal DNA replication. J Theor Biol. 1989 Nov 8;141(1):117–136. doi: 10.1016/s0022-5193(89)80012-8. [DOI] [PubMed] [Google Scholar]
- Turnell W. G., Travers A. A. Algorithms for prediction of histone octamer binding sites. Methods Enzymol. 1992;212:387–399. doi: 10.1016/0076-6879(92)12025-l. [DOI] [PubMed] [Google Scholar]
- Ulanovsky L. E., Trifonov E. N. Estimation of wedge components in curved DNA. Nature. 1987 Apr 16;326(6114):720–722. doi: 10.1038/326720a0. [DOI] [PubMed] [Google Scholar]
- Wada A., Suyama A. Local stability of DNA and RNA secondary structure and its relation to biological functions. Prog Biophys Mol Biol. 1986;47(2):113–157. doi: 10.1016/0079-6107(86)90012-x. [DOI] [PubMed] [Google Scholar]
- Wada A., Suyama A. Variation of double-helix stability along DNA molecular thread and its biological implications: homostabilizing propensity of gene double-helix. Prog Clin Biol Res. 1985;172A:37–46. [PubMed] [Google Scholar]



