Abstract
As life expectancy continues to rise, in the future there will be an increasing number of older people prone to falling. Accordingly, there is an urgent need for comprehensive testing of older individuals to collect data and to identify possible risk factors for falling. Here we use a low-cost force platform to rapidly assess deficits in balance under various conditions. We tested 21 healthy older adults and 24 young adults during static stance, unidirectional and rotational displacement of their centre of pressure (COP). We found an age-related increase in postural sway during quiet standing and a reduction of maximal COP displacement in unidirectional and rotational displacement tests. Our data show that even low-cost computerized assessment tools allow for the comprehensive testing of balance performance in older subjects.
1. Introduction
1.1. Balance and Aging
Several mechanisms have been proposed to explain the changes in balance during aging. Since aging affects almost all physiological processes [1], reduction in postural stability can be explained by various factors, such as a loss of receptor cells in the vestibular organ [2], impaired sensory perception, a decline in muscle strength, and increased reaction times [3]. Especially proprioception, which refers to the sensing where the body is located in space [4], is known to be a critical source of sensory feedback for the preservation of balance during upright standing in the elderly (for review see [5]). Besides physiological alterations, impaired balance at high age can also be explained by a general age-related reduction in physical activities, which in turn may further reduce the ability of living a physically active life [6]. Balance disorders represent a growing public health concern due to the association with falls and fall-related injuries. Thereby falls often mark the beginning of a loss of independence and are the leading cause of injury-related institutionalizations in older adults [3, 7]. Consequently, the documentation and understanding of age-related changes in balance performance is of utmost importance [7].
1.2. Methods for Assessing Standing Balance
Clinical scales, in addition to tests of stance and reach, are often used to rate balance performance. Functional assessments of this type have the advantage that they can be quickly applied and rarely require expensive equipment [8] but they often lack a more detailed rating of impairment [9]. By contrast, posturography is used to measure and quantify postural sway [9, 10] by assessing COP deflections [11]. Force platforms are the gold standard for posture assessment as they exceed many other techniques concerning reliability [12] and simplicity of their design [9]. Recently Clark and coworkers compared COP-data obtained with a Wii Balance Board with a laboratory-grade force platform and reported a high test-retest reliability of the Wii Board for COP assessment in young subjects [13]. We here investigated if age-related changes in standing balance can be revealed by using a Wii Balance Board in aged participants. To this aim, we compared the performance of young and older subjects during quiet standing and controlled displacement of their COP.
2. Methods
2.1. Subjects
The target group was comprised of 21 older subjects aged 60–94 years (69.0 ± 7.7 years; 8 male), and the control group was comprised of 24 adults aged 20–32 years (25.2 ± 3.4 years; 9 male). At the time of testing, no subject reported any orthopedic or neurological conditions (according to self-report, medical history and independence in activities of daily living (Everyday competence questionnaire (ECQ-score >9) [14])) that could interfere with testing. Moreover, subjects underwent the Mini Mental Status Examination (MMSE-score >27) [15] to test for demetia. We collected individual height (m) and weight (kg) for calculation of body-mass-indices (BMI). The average BMI of young subjects was 21.42 ± 2.62 kg/m2 and 26.35 ± 4.2 kg/m2 for elderly subjects. All subjects gave their written informed consent. The study was approved by the local Ethics Committee of the Ruhr-University Bochum.
2.2. Force Platform and Data Analyses
We used the Wii Balance Board (Nintendo, Kyoto, Japan) as a measuring device and the WiiUse library [16] to enable communication. According to a recent study, the Wii Balance Board was shown to exhibit high concurrent validity for COP assessment with a laboratory-grade force platform (intraclass correlation coefficients = 0.77–0.89) [13]. As raw sensor data (RSD) are linearly correlated with the weight of the subject [13], RSD changes reflect changes in the COP-position. We therefore used RSD to quantify subjects' balance performance. Data from all four pressure-sensors were recorded with a sampling rate of 50 Hz and were written to a file for offline analysis. For obtaining the COP, the RSD of the four sensors were transformed to xy-coordinates of a standard Cartesian coordinate system. For this purpose, for each individual the offsets of the RSD from the middle of the coordinate system were recorded prior to the various subtests in a separate session. Therefore, the subjects were required to stand still for 30 seconds with eyes open looking at a fixed reference point and arms at the sides of the body. For calibration, the individual COP-offset (difference between actual COP coordinates and central point (i.e., the centre of the platform)) was calculated and subtracted from all values acquired in every subsequent measurement. In every subsequent subtest, percentage changes of the COP-position were determined by calculating deviations from the normalized initial RSD (% RSD).
2.3. Subtests for the Assessment of Balance
Subjects were unshod and instructed to stand quietly on the platform. The distance between the feet was 30 cm. The subjects had to perform 8 subtests, with subtest 1–8 lasting 30 s each.
Subtests 1–3 were used to detect and quantify displacements of the COP, when visual information was prevented or posture had to be altered in a test-specific way. In subtest 1, (looking at a fixed reference point), subjects were instructed to stand still with eyes open. Both arms reached out to the front (angle of 90° to the body). In subtest 2, subjects had to stand still with arms at the sides of the body and eyes closed. Subtest 3 was the same as subtest 1, but with eyes closed. Subtests 4–7 were used to quantify the subjects' ability to shift their COP without losing balance. Thereby, the subjects were asked to keep their body rigid, maintain the full plantar surface of the feet in contact with the platform, and lean over towards specified directions (subtest 4: forward right; subtest 5: forward left; subtest 6: backwards right, subtest 7: backwards left). Subtest 8 was used to quantify the subjects' ability to continuously change posture by circular movement in clockwise direction, trying to reach maximal displacement of their COP.
In subtests 4–7, subjects' ability to shift their COP forward as far as possible without taking a step forward or falling was tested. These subtests provide information about the ability to deflect the centre of gravity (COG) towards the edge of the base of support. The tests resemble the so-called Functional reach test, where subjects do not lean over, but actually reach forward [17]. The Functional reach test has been shown to offer high inter-rater reliability and good predictive validity of subjects at risk of falls [18].
For subtests 1–7, we calculated the average xy-coordinates of COP and the appending scatter, that is, standard deviations of COP positions in anterior-posterior and medio-lateral directions.
To analyze performance in subtest 8, we calculated the mean COP coordinate for each sector of the coordinate system. Subsequently, we calculated the Euclidian distance between the origin of the coordinate system and these COP coordinates. The four resulting Euclidian distances were averaged and used as radius for the calculation of the circumference of COP rotation.
2.4. Statistical Analyses
Results are presented as mean COP coordinates ± SD (“scatter”) with coordinates describing balance performance and scatter describing postural sway. Statistical evaluation was carried out using Student's t-test and repeated measures analysis of variance (rmANOVA) with within-subject-factor DIRECTION and between-subject-factor GROUP to calculate differences in anterior-posterior and medio-lateral displacements of COP (DIRECTION) of young and older subjects (GROUP) as well as interactions of both factors. A P value of <.05 was considered significant.
Correlation analyses were carried out by means of Pearson-correlations of postural performance (COP positions, COP scatter) and individual age of elderly subjects. Within the group of young subjects, no age-correlations were calculated because of the narrow age-range.
3. Results
3.1. Quiet Standing (Subtests 1–3)
In subtests 1 and 3, we tested the impact of different postural positions on standing stability. Generally, displacements in anterior-posterior direction were larger than in medio-lateral direction. These circumstances were found in the data of young subjects and were even more pronounced in the data of older subjects. Absolute COP displacements revealed no significant between-group differences in medio-lateral direction, but in anterior-posterior direction for data of subtests 2 and 3 with older subjects showing larger displacements. For young and older subjects, the scatter in all three subtests was larger in anterior-posterior directions than in medio-lateral directions. Average COP scatter was higher in older subjects in medio-lateral direction for subtest 1 and 3 and anterior-posterior direction for subtest 3. See Table 1 for details. Within the group of older subjects, correlation analyses of individual age and postural performance revealed no significant results for subtests 1, 2, and 3 (r ≤ 0.358; P ≥ .111) indicating a lack of dependence of posturographic performance on individual age.
Table 1.
Posturographic performance of young and older subjects as determined in subtests 1–7. Data are given as average percent shift of raw sensor data (% RSD) for x- and y-coordinates (Mean ± SD). Repeated measures ANOVA (rmANOVA) and t-tests were used to calculate direction-specific differences (D), group-specific differences (G), and according interactions (DG).
| Subtest | x | y | rmANOVA | Scatter | Scatter | rmANOVA | |
|---|---|---|---|---|---|---|---|
| D: DIRECTION | (Medio-lateral) | (Anterior-posterior) | D: DIRECTION | ||||
| DG: DIRECTION ∗ GROUP | DG: DIRECTION ∗ GROUP | ||||||
| (1) Arms reached out | Young | −0.38 ± 1.92 | 3.03 ± 7.61 | D: F(1,43) = 16.269; P ≤ .001* | 0.59±0.28 | 2.12 ± 0.66 | D: F(1,43) = 332.804; P ≤ .001* |
| Old | −0.22 ± 2.08 | 6.06 ± 6.38 | 0.72 ± 0.21 | 2.31 ± 0.48 | |||
| t-test (P) | .396 | .079 | DG: F(1,43) = 1.437; P = .237 | .049* | .134 | DG: F(1,43) = 0.160; P = .691 | |
| (2) Eyes closed | Young | 0.0002 ± 0.00 | 0.0007 ± 0.00 | D: F(1,43) = 4.001; P = .052 | 0.73 ± 0.37 | 2.71 ± 0.92 | D: F(1,43) = 316.592; P ≤ .001* |
| Old | 0.0001 ± 0.00 | 0.0027 ± 0.00 | 0.88 ± 0.51 | 3.23 ± 1.22 | |||
| t-test (P) | .281 | ≤.001* | DG: F(1,43) = 10.048; P = .003* | .127 | .054 | DG: F(1,43) = 2.339; P = .134 | |
| (3) Arms reached out, eyes closed | Young | 0.00 ± 1.90 | 3.61 ± 5.23 | D: F(1,43) = 41.743; P ≤ .001* | 0.66 ± 0.23 | 2.64 ± 0.90 | D: F(1,43) = 373.838; P ≤ .001* |
| Old | 0.14 ± 2.15 | 7.80 ± 5.38 | 0.97 ± 0.46 | 3.47 ± 0.94 | |||
| t-test (P) | .410 | .006* | DG: F(1,43) = 5.386; P = .025* | .002* | .002* | DG: F(1,43) = 4.833; P = .033* | |
| (4) Displacement to upper right | Young | 24.91 ± 9.31 | 21.86 ± 12.06 | D: F(1,43) = 3.231; P = .079 | 1.38 ± 0.68 | 2.79 ± 0.82 | D: F(1,43) = 160.176; P ≤ .001* |
| Old | 20.76 ± 10.82 | 14.64 ± 13.79 | 1.63 ± 0.69 | 3.37 ± 0.97 | |||
| t-test (P) | .087 | .034* | DG: F(1,43) = 0.361; P = .551 | .114 | .018* | DG: F(1,43) = 1.720; P = .197 | |
| (5) Displacement to upper left | Young | −28.83 ± 7.14 | 30.91 ± 8.85 | D: F(1,43) = 341.238; P ≤ .001* | 1.94 ± 0.81 | 3.39 ± 1.77 | D: F(1,43) = 41.173; P ≤ .001* |
| Old | −24.48 ± 12.00 | 16.75 ± 14.62 | 1.96 ± 0.52 | 3.49 ± 1.08 | |||
| t-test (P) | .071 | ≤.001* | DG: F(1,43) = 11.457; P = .002* | .467 | .408 | DG: F(1,43) = 0.035; P = .853 | |
| (6) Displacement to lower right | Young | 22.48 ± 7.02 | −19.32 ± 9.96 | D: F(1,43) = 250.449; P ≤ .001* | 1.46 ± 0.63 | 3.11 ± 1.02 | D: F(1,43) = 141.001; P ≤ .001* |
| Old | 12.07 ± 10.63 | −10.01 ± 9.94 | 1.86 ± 0.77 | 3.78 ± 1.39 | |||
| t-test (P) | ≤.001* | .002* | DG: F(1,43) = 23.880; P ≤ .001* | .031* | .036* | DG: F(1,43) = 0.783; P = .381 | |
| (7) Displacement to lower left | Young | −20.47 ± 8.05 | −15.86 ± 9.43 | D: F(1,43) = 5.339; P = .026* | 2.14 ± 1.11 | 3.22 ± 1.31 | D: F(1,43) = 120.483; P ≤ .001* |
| Old | −15.92 ± 9.51 | −9.47 ± 10.34 | 1.95 ± 1.29 | 3.83 ± 1.84 | |||
| t-test (P) | .045* | .018* | DG: F(1,43) = 0.147; P = .703 | .303 | .102 | DG: F(1,43) = 8.656; P = .005* | |
3.2. Controlled Displacement of COP (Subtests 4–7)
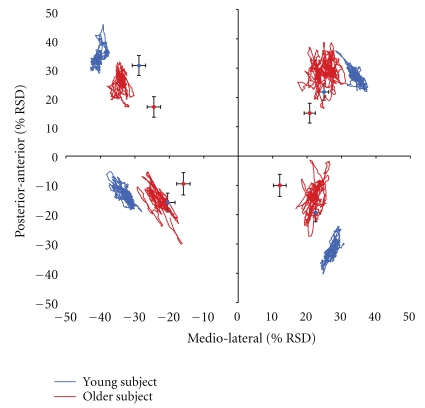
In general, there was a higher COP displacement in young subjects compared to older subjects, except for x-coordinates of subtests 4 and 5, showing an age-related reduction in the ability to shift the COP to the edge of the base of support. In subjects of both groups, we found significantly higher COP displacements in medio-lateral directions than in anterior-posterior directions, although the effect was more distinct in older subjects. Analysis of scatter revealed higher values in anterior-posterior direction than in medio-lateral direction for all subjects. Scatter in medio-lateral direction was higher for older subjects in subtest 6. In anterior-posterior direction, older subjects showed increased scatter subtests 4 and 6. Data of subtests 4–7 and the according statistics are summarized in Table 1 and depicted in Figure 1. Within the data of the group of older subjects we found significant correlations between age and postural performance. Within the group of older subjects, the ability to shift the COP in subtests 4, 5, 6, and 7 to extreme medio-lateral positions was particularly reduced in subjects of the upper age-range, while participants of the lower age-range showed less impairment (r ≥ −0.448; P ≤ .042). Furthermore, the ability to shift the COP to extreme anterior-posterior positions was reduced in subtest 5 (r = −0.510; P = .018). There were no significant correlations between age and COP scatter in any direction (r ≤ 0.288; P ≥ .205).
Figure 1.
Single-subject data of a young subject (32 years) and an older subject (70 years) depicted as statokinesigram (blue and red lines) for subtest 4 (shifting the COP to the upper right), subtest 5 (shifting the COP to the upper left), subtest 6 (shifting the COP to the lower right), and subtest 7 (shifting the COP to the lower left). Group data for young (N = 24) and older subjects (N = 21) are displayed by blue and red diamonds in the respective sectors of the diagram. COP deviations are given in percent changes of raw sensor data (RSD) in relation to origin of the coordinate system. Standard deviations of the average COP-positions (black bars) are given for medio-lateral and anterior-posterior directions.
3.3. Rotational Displacement of COP
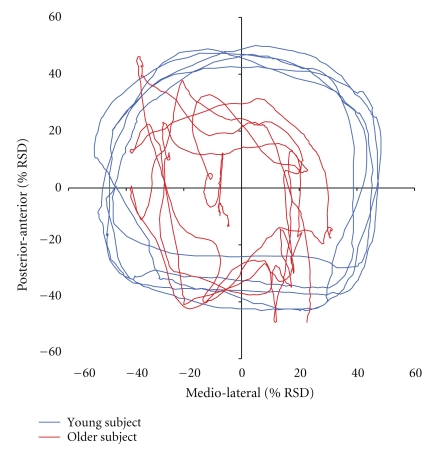
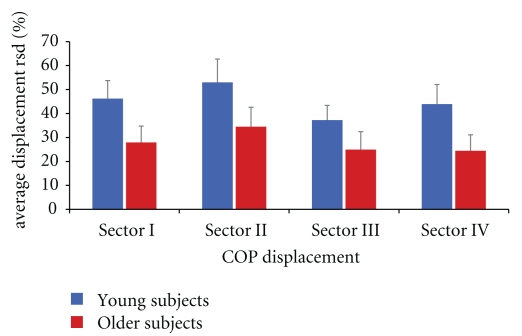
In subtest 8, subjects had to shift their COP continuously by repeated clockwise rotation (Figure 2). Therefore, COP coordinates were transformed to radii and averaged for each sector of the coordinate system. Young subjects reached wider displacements of their COP in all sectors (I(upper right): 46.20 ± 7.53%-RSD; II(upper left): 52.97 ± 9.74%-RSD; III(lower left): 37.26 ± 6.12%-RSD; IV(lower right): 43.93 ± 8.18%-RSD) compared to older subjects (I(upper right): 27.90 ± 6.86%-RSD; II(upper left): 34.49 ± 8.12%-RSD; III(lower left): 24.94 ± 7.49%-RSD; IV(lower right): 24.41 ± 6.67%-RSD) (t-test, P ≤ .001) (Figure 3). The average radius of all sectors per subject was used to calculate the circumference of COP displacement for young (283.32 ± 36.22 RSD) and older subjects (175.51 ± 61.04 RSD). Accordingly, young subjects showed significantly larger rotations of their COP as compared to older subjects (t-test, P ≤ .001). Analyses of data obtained within the group of aged subjects showed a significant correlation between individual age and the ability to displace the COP in sectors I, II, and IV (r ≥ −0.447; P ≤ 0.042), where the oldest participants showed the largest degradation in performance, indicating the existence of a gradient within the group of elderly participants.
Figure 2.
Single-subject data of a young subject (32 years; blue line) and an older subject (74 years; red line) depicted as statokinesigram for subtest 8. COP deviations are given in percent changes of raw sensor data (% RSD). Subjects were asked to perform clockwise rotational movements and thereby try to displace their COP maximally without losing balance.
Figure 3.
Bar charts indicate group data of young (blue bars) and older subjects (red bars) as assessed in subtest 8 (rotational displacement of COP). Error bars depict standard deviation. Displacement of COP was higher for young subjects as compared to older subjects for every sector (t-test, P ≤ .001).
4. Discussion
In the present study, we used the Wii Balance Board to investigate age-related changes in balance by comparing the performance of healthy young subjects and older adults during static stance, unidirectional and rotational displacement of their centre of pressure (COP). Our data show that even low-cost computerized assessment tools allow for the detection of task-specific age-related degradation in balance performance in a group of older adults.
We observed postural sway, that is, scatter of COP, in all subjects. COP scatter was significantly higher in anterior-posterior direction than in medio-lateral direction. This observation appears reasonable as the position of the feet (30 cm apart) assures a more stable position in medio-lateral rather than anterior-posterior direction. In anterior-posterior direction, the base of support is limited allowing larger displacements and higher scatter of the COP. This directionality has been observed in other studies employing force platform measurements as well [19].
When the subjects were asked to change posture by reaching out their arms, older subjects showed larger displacements of their COP in anterior direction, as compared to young subjects. Older participants might have developed a reduced sensitivity for the detection of posture-dependent changes of their COP. Alternatively, the threshold for the release of equilibrium reactions might be raised - resulting in greater deviations of COP shifts before a compensatory action is taken. During the quiet standing tests with eyes closed (subtests 2 and 3), the older subjects significantly shifted their COP in the anterior direction. This finding is in line with previous data obtained with conventional force platforms [19]. We assume that the displacement towards the anterior base of support increased the subjects' margin of safety. This assumption is in line with reports where the impact of proprioceptive input for postural control in older subjects was investigated during measurements of COP displacement [20]. The authors found that in older subjects the sensitivity for proprioceptive information shifted from the trunk to the ankles, resulting in an even higher sensibility of the ankle muscles than that observed in young subjects. These data were taken as an argument that proprioceptive information coming from the ankle muscles is especially important for balance control in older individuals as it compensates for the age-related loss of vestibular, visual and pressoreceptive information [20]. Accordingly, the large COP shifts of older subjects recorded in subtests 1–3 can be interpreted as compensatory action to enhance afferent proprioceptive information flow from the ankles to allow efficient postural control.
In subtest 4–7, we investigated age-related changes in balance while standing at the limits of stability [7]. For the individual, forward and backward leaning performance is of particular interest as being a crucial component of the individual limit of stability, typically decreasing with advanced age and thereby possibly leading to an increased risk of falling in older adults [21, 22]. This notion is supported by the observation that postural sway increased dramatically when the position of the subjects came close to the limit of stability [23]. The data of our tests are in accordance with such findings, as older subjects showed reduced abilities to lean to anterior and posterior directions. Although older subjects attained a smaller displacement of their COP as compared to young subjects, the postural sway was in the same range or only little higher than in young subjects. This finding supports the assumption that healthy older subjects are aware of their reduced balance abilities and adjust their range of leaning accordingly.
To find out whether there is a progressive increase of age-related degradation within the group of older participants, we performed a correlation analysis, confirming that especially participants of very high age show an impairment of balance in more demanding tasks. For nondemanding subtests 1, 2, and 3, we found no significant correlations between individual age and postural performance. However, subtest 4–7 characterized by increased complexity showed significant correlations between age and postural performance, suggesting a progressive increase of age-related degradation within the group of elderly participants. Our results are in accordance with studies on hand motor performance and increasing age, suggesting an “age-threshold” up to which increasing task-complexity can by compensated by the individual [24]. Once exceeded, this threshold is associated with a steady age-related impairment. In subtest 8, we found significant group-differences in performance for every sector of the rotation movement and the overall circumference of COP rotation. Concomitantly, there were significant correlations between older subjects' age and the according performance in subtest 8, suggesting again a close relation between an age-related impairment of balance and the complexity of the task.
5. Conclusions
Low-cost force platforms can be used for the assessment of balance performance in older subjects, thereby bridging the gap between sophisticated laboratory test equipments and low-level functional tests [13]. Low-cost force platforms might turn to be out a simple but efficient way to assure data acquisition in short time on a routine basis. A particularly interesting option could be the combination of low-cost force platforms and established functional tests.
Acknowledgments
The work was supported by the Deutsche Forschungsgemeinschaft (Di 334/19-1, HRD; Te 315/4-1, MT), the Medical Faculty of the Ruhr-University Bochum (FoRUM F637-08, TK), a scholarship from the Research Department of Neuroscience of the Ruhr-University Bochum to TK, and a scholarship from the Allgemeiner Deutscher Tanzlehrerverband (ADTV) to Jan-Christoph Kattenstroth. Both authors T. Kalisch and J.-C. Kattenstroth contributed equally to this work.
References
- 1.Young A. Ageing and physiological functions. Philosophical Transactions of the Royal Society B. 1997;352(1363):1837–1843. doi: 10.1098/rstb.1997.0169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Young MS, Triggs WJ, Bowers D, Greer M, Friedman WA. Stereotactic pallidotomy lengthens the transcranial magnetic cortical stimulation silent period in Parkinson’s disease. Neurology. 1997;49(5):1278–1283. doi: 10.1212/wnl.49.5.1278. [DOI] [PubMed] [Google Scholar]
- 3.Matsumura BA, Ambrose AF. Balance in the elderly. Clinics in Geriatric Medicine. 2006;22(2):395–412. doi: 10.1016/j.cger.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 4.Gandevia SC, Refshauge KM, Collins DF. Proprioception: peripheral inputs and perceptual interactions. Advances in Experimental Medicine and Biology. 2002;508:61–68. doi: 10.1007/978-1-4615-0713-0_8. [DOI] [PubMed] [Google Scholar]
- 5.Goble DJ, Coxon JP, Wenderoth N, Van Impe A, Swinnen SP. Proprioceptive sensibility in the elderly: degeneration, functional consequences and plastic-adaptive processes. Neuroscience and Biobehavioral Reviews. 2009;33(3):271–278. doi: 10.1016/j.neubiorev.2008.08.012. [DOI] [PubMed] [Google Scholar]
- 6.Perrin PP, Gauchard GC, Perrot C, Jeandel C. Effects of physical and sporting activities on balance control in elderly people. British Journal of Sports Medicine. 1999;33(2):121–126. doi: 10.1136/bjsm.33.2.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sturnieks DL, St George R, Lord SR. Balance disorders in the elderly. Neurophysiologie Clinique. 2008;38(6):467–478. doi: 10.1016/j.neucli.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 8.Berg K, Norman KE. Functional assessment of balance and gait. Clinics in Geriatric Medicine. 1996;12(4):705–723. [PubMed] [Google Scholar]
- 9.Browne J, O’Hare N. Review of the different methods for assessing standing balance. Physiotherapy. 2001;87(9):489–495. [Google Scholar]
- 10.Yelnik A, Bonan I. Clinical tools for assessing balance disorders. Neurophysiologie Clinique. 2008;38(6):439–445. doi: 10.1016/j.neucli.2008.09.008. [DOI] [PubMed] [Google Scholar]
- 11.Winter DA. Human balance and posture control during standing and walking. Gait and Posture. 1995;3(4):193–214. [Google Scholar]
- 12.Benvenuti F, Mecacci R, Gineprari I, et al. Kinematic characteristics of standing disequilibrium: reliability and validity of a posturographic protocol. Archives of Physical Medicine and Rehabilitation. 1999;80(3):278–287. doi: 10.1016/s0003-9993(99)90138-7. [DOI] [PubMed] [Google Scholar]
- 13.Clark RA, Bryant AL, Pua Y, McCrory P, Bennell K, Hunt M. Validity and reliability of the Nintendo Wii Balance Board for assessment of standing balance. Gait and Posture. 2010;31(3):307–310. doi: 10.1016/j.gaitpost.2009.11.012. [DOI] [PubMed] [Google Scholar]
- 14.Kalisch T, et al. Questionnaire-based evaluation of everyday competence in older adults. Clinical Interventions in Aging. 2011;6:37–46. doi: 10.2147/CIA.S15433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Folstein MF, Folstein SE, McHugh PR. ’Mini mental state’. A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 16.Laforest M. Wiiuse. 2010, http://www.wiiuse.net/docs/index.htm.
- 17.Duncan PW, Weiner DK, Chandler J, Studenski S. Functional reach: a new clinical measure of balance. Journals of Gerontology. 1990;45(6):M192–M197. doi: 10.1093/geronj/45.6.m192. [DOI] [PubMed] [Google Scholar]
- 18.Duncan PW, Studenski S, Chandler J, Prescott B. Functional reach: predictive validity in a sample of elderly male veterans. Journals of Gerontology. 1992;47(3):M93–M98. doi: 10.1093/geronj/47.3.m93. [DOI] [PubMed] [Google Scholar]
- 19.Era P, Sainio P, Koskinen S, Haavisto P, Vaara M, Aromaa A. Postural balance in a random sample of 7,979 subjects aged 30 years and over. Gerontology. 2006;52(4):204–213. doi: 10.1159/000093652. [DOI] [PubMed] [Google Scholar]
- 20.Brumagne S, Cordo P, Verschueren S. Proprioceptive weighting changes in persons with low back pain and elderly persons during upright standing. Neuroscience Letters. 2004;366(1):63–66. doi: 10.1016/j.neulet.2004.05.013. [DOI] [PubMed] [Google Scholar]
- 21.Melzer I, Benjuya N, Kaplanski J. Postural stability in the elderly: a comparison between fallers and non-fallers. Age and Ageing. 2004;33(6):602–607. doi: 10.1093/ageing/afh218. [DOI] [PubMed] [Google Scholar]
- 22.Melzer I, Benjuya N, Kaplanski J, Alexander N. Association between ankle muscle strength and limit of stability in older adults. Age and Ageing. 2009;38(1):119–123. doi: 10.1093/ageing/afn249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hasselkus BR, Shambes GM. Aging and postural sway in women. Journals of Gerontology. 1975;30(6):661–667. doi: 10.1093/geronj/30.6.661. [DOI] [PubMed] [Google Scholar]
- 24.Smith CD, Umberger GH, Manning EL, et al. Critical decline in fine motor hand movements in human aging. Neurology. 1999;53(7):1458–1461. doi: 10.1212/wnl.53.7.1458. [DOI] [PubMed] [Google Scholar]