Abstract
D-galactose induced neurotoxicity is well known model for studying aging and related oxidative damage and memory impairment. Aging is a biological process, characterized by the gradual loss of physiological functions by unknown mechanism. Centella asiatica, Indian pennywort has been documented in the treatment of various neurological disorders including aging. Therefore, present study has been conducted in order to explore the possible role of Centella asiatica against D-galactose induced cognitive impairment, oxidative and mitochondrial dysfunction in mice. Chronic administration of D-galactose (100 mg/kg s.c.) for a period of six weeks significantly impaired cognitive task (both in both Morris water maze and elevated plus maze) and oxidative defense (Increased lipid peroxidation, nitrite concentration and decreased activity of superoxide dismutase, catalase and non-protein thiols) and impaired mitochondrial complex (I, II and III) enzymes activities as compared to sham group. Six weeks Centella asiatica (150 and 300 mg/kg, p.o) treatment significantly improved behavioral alterations, oxidative damage and mitochondrial enzyme complex activities as compared to contro l (D-galactose). Centella asiatica also attenuated enhanced acetylcholine esterase enzyme level in D-galactose senescence mice. Present study highlights the protective effect of Centella asiatica against D-galactose induced behavioral, biochemical and mitochondrial dysfunction in mice.
1. Introduction
Aging is a slow and gradual biological process, associated with various morphological and biochemical changes in biological system [1]. Aging diminishes homeostasis and increases vulnerability to cognitive dysfunctions in addition to physical, mental, or social activities in human beings. Aging also accompanied by changes in membrane fatty acid composition including decrease levels of polyunsaturated fatty acid (PUFAs) such as arachidonic acid (AA). A correlation between the concentration of AA and long-term potentiation suggests that oxidation depletion of AA may be related to cognitive deficits in animal. It is now well known that many age-related behavioral changes in motor and cognitive performance occur even in the absence of specific, age-related, neurodegenerative diseases such as Alzheimer disease or Parkinson disease [2]. The behavioral dysfunctions have been proposed to be associated with a decreased mitochondrial electron transfer complex activity with aging [3]. Some factors implicated in aging include chronological risk factors, telomere shortening, advanced glycation endproducts, and free radicals damage. Brain senescence plays an important role in cognitive dysfunction which is commonly associated with many neurodegenerative disorders. D-galactose is a normal reducing sugar in body. Galactose is normally metabolized by D-galactokinase and galactose-1-phosphate uridyltransferase in animals, but over-supply of D-galactose results in abnormality of metabolism [4]. D-galactose converts into galactitol, which is not metabolized by above enzymes but accumulate in the cell, leading to osmotic stress and production of reactive oxygen species [5]. Researchers have a strong opinion that D-galactose reacts with the free amines of amino acids in proteins and peptides to form advanced glycation end products (AGE), which in turn causes activation of receptor for advanced glycation end products (RAGE). This sequence of events results an oxidative stress and cellular damage [6–8]. However, our earlier study had reported that D-galactose toxicity is associated with mitochondria dysfunction in mice [9]. The implication of mitochondria both as producers and as targets of ROS has been the basis for the mitochondrial theory of aging [10]. The theory postulates that random alterations of mitochondrial DNA (mtDNA) in somatic cells are responsible for the energetic decline accompanying senescence. It has also been proposed that accumulation of somatic mutations of mtDNA, induced by exposure to ROS, leads to errors in the mtDNA-encoded polypeptides [11]. These errors are stochastic and randomly transmitted during mitochondrial and cell division. The consequence of these alterations, which affect exclusively the mitochondrial complexes involved in energy conservation, would be defective electron transfer and oxidative phosphorylation. Respiratory chain defects may be associated with increased ROS production, thus establishing a vicious circle [12].
Centella asiatica (CA) L. Urban (syn. Hydrocotyle asiatica L.) belonging to family Apiaceae (Umbelliferae) is a psychoactive medicinal plant, being used from centuries in Ayurvedic system of medicine as a medhya rasayna [13]. It has been reported to possess various biological activities such as stimulatory-nervine tonic, rejuvenant, sedative, anxiolytic, and intelligence promoting property [14]. Asiaticoside, active constitute of Centella asiatica has been reported as a dementia-treating agent and cognitive enhancer [15]. Asiaticoside has been found to have therapeutic value against β-amyloid neurotoxicity [16]. Previous reports also demonstrated that Centella asiatica leaf extract involved in the morphology of hippocampal CA3 and amygdal neuronal dendritic arborization in neonatal rats [17, 18]. Beside experiments it has been shown to improve learning and memory in mice during early postnatal developmental period [19]. CA has been reported to contain more than 70 constituents such as caffeic acid derivative, flavonoid, triterpenoids and in particular quercetin and kaempferol, catechin, rutin, sterols, and lipid, some of which have been proved to have a potent antioxidants [20]. Despite the beneficial effects of CA, its therapeutic effect against mitochondrial dysfunction and free radical mediated toxicity has not been well understood so far.
Therefore, the present study has been designed to explore the possible role of Centella asiatica against D-galactose-induced cognitive dysfunction, oxidative damage, and mitochondrial dysfunction in senescence mice.
2. Materials and Methods
2.1. Animals
Male Laca mice (25–30 g), 2-3 months old, (Central Animal House, Panjab University, Chandigarh) were used. Animals were acclimatized to the laboratory conditions room temperature prior to the experiment. Animals were kept under standard condition of 12 hour light/dark cycle with food and water ad libitum in plastic cages with soft bedding. Experiment was carried out between 9.00 and 17.00 h. The protocol was approved by the Institutional Animal Ethics Committee and was carried out in accordance with the Indian National Science Academy Guidelines for the use and care of animals.
2.2. Drugs and Treatment Schedule
D-galactose (CDH, India) solution and standardized aqueous extract of Centella asiatica (CA) (Dabur Research Foundation, Ghaziabad, India) was used. D-galactose was dissolved in distilled water for subcutaneous (s.c.) administration. CA extract suspended in 0.25% w/v sodium carboxy-methyl-cellulose and administered orally in a dose of 1 mL/100 g body weight. Animals were randomized into five groups, each consists of 12 animals.
Naïve group received 0.5% sodium carboxymethyl cellulose.
D-galactose-treated group received 100 mg/kg of D-galactose administered subcutaneously.
CA (300 mg/kg, p.o.) was administered to the per se groups.
CA (150 mg/kg, p.o.) were administered to D-galactose-treated mice.
CA (300 mg/kg, p.o.) were administered to D-galactose-treated mice.
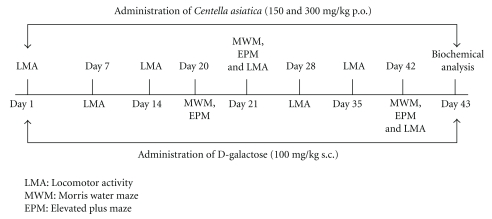
The doses of CA and D-galactose were selected based on our report in the literature [9, 21, 22]. The study was carried out for a period of 42 days (6 weeks) as in Figure 1.
Figure 1.
Drug treatment was given as per the flow diagram.
2.3. Behavioral Assessments
2.3.1. Assessment of Cognitive Performance
(a) Morris Water Maze Task —
The acquisition and retention of memory was evaluated by using Morris water maze [21]. Morris water maze consisted of large circular pool (150 cm in diameter, 45 cm in height, filled to a depth of 30 cm with water at 28 ± 1°C). Pool was divided into four equal quadrants with the help of two threads, fixed at right angle to each other. The pool was placed in illuminated light room among the several colored clues. These external clues remained unchanged throughout the experimental period and used as reference memory. A circular platform (4.5 cm diameter) was placed in one quadrant of the pool, 1 cm above the water level during the acquisition phase. A similar platform was placed 1 cm below the water level for retention phase. The position of the platform was not changed in any quadrant during assessment of both phases. Each animal was subjected to four consecutive trials with gap of 5 min. The mouse was gently placed in the water of the pool between quadrants, facing the wall of the pool with drop location, change for each trial, and allowed 120 s to locate the platform. Then, it was allowed to stay on the platform for the following 20 s. If animal failed to reach the platform within 120 s, same was guided to reach the platform.
Maze Acquisition Phase (Training) —
Animals received a training session consisting of 4 trials on day 20. Starting position was different in all the four trials. The time taken by the mouse to reach the visual platform was taken as the initial acquisition latency (IAL). At the end of each trial, mice were returned to their respective home cages.
Maze Retention Phase (Testing for Retention of the Learned Task) —
Following 24 hour (day 21) and 21 days (day 42) after IAL, mouse was released randomly at one of the edges facing the wall of the pool to assess for memory retention. Time taken by mice to find the hidden platform on day 21 and 42 following start of D-galactose administration was recorded, termed as first retention latency (1st RL) and second retention latency (2nd RL), respectively.
(b) Elevated Plus Maze Paradigm —
The elevated plus maze consists of two opposite white open arms (16 × 5 cm), crossed with two closed walls (16 × 5 cm) with 12 cm high walls. The arms were connected with a central square of dimensions 5 × 5 cm. The entire maze was placed 25 cm high above the ground. Acquisition of memory was tested on day 20. A mouse was placed individually at one end of the open arm facing away from the central square. The time taken by the animal to move from the open arm to the closed arm was recorded as the initial transfer latency (ITL). Animals were allowed to explore the maze for 10 s after recording ITL. If animal did not enter the enclosed arm within 90 s, same was guided to the enclosed arm and ITL was recorded as 90 s. Retention of memory was assessed by placing the mouse in an open arm on day 21 and day 42 of the ITL, termed as the first retention transfer latency (1st RTL) and second retention transfer latency (2nd RTL), respectively [23].
2.3.2. Assessment of Gross Behavioral Activity
Gross behavioral activity was observed at weekly intervals. Each animal was placed in a square (30 cm) closed arena equipped with infrared light sensitive photocells using digital actophotometer. The animal was observed for a period of 5 min and expressed as counts/5 min. The apparatus was placed in a darkened, light and sound attenuated and ventilated test room [21].
2.4. Mitochondrial Complex Estimation
Isolation of Mice Brain Mitochondria —
The whole brain (excluding cerebellum) was used for mitochondrial isolation. Mice brain mitochondria were isolated by differential centrifugation [24]. The mice brain is homogenized in 10 mL of homogenizing buffer containing 225 mM mannitol, 75 mM sucrose, 5 mM HEPES, 1 mM EGTA, and 1 mg/mL BSA, pH 7.4. The homogenate is brought to 30 mL with the same buffer and centrifuged at 2000 g for 3 min at 4°C. The pellet is discarded, and the supernatant is divided into 2 tubes and centrifuged at 12000 g for 10 min. The pellet containing the mixture of synaptosomes and mitochondria is suspended in 10 mL of homogenization buffer containing 0.02% digitonin to lyse the synaptosomes followed by centrifugation at 12000 g for 10 min to pellet down both extrasynaptosomal and intrasynaptosomal mitochondria. The mitochondrial pellet is washed twice in the same buffer without EGTA, BSA, or digitonin.
2.4.1. Complex-I (NADH Dehydrogenase Activity)
COMPLEX-I was measured spectrophotometrically by the method of King and Howard [25]. The method involves catalytic oxidation of NADH to NAD+ with subsequent reduction of cytochrome C. The reaction mixture contained 0.2 M glycyl glycine buffer pH 8.5, 6 mM NADH in 2 mM glycyl glycine buffer and 10.5 mM cytochrome C. The reaction was initiated by addition of requisite amount of solubilized mitochondrial sample and followed absorbance change at 550 nm for 2 min.
2.4.2. Complex-II (Succinate Dehydrogenase (SDH) Activity)
SDH was measured spectrophotometrically according to King [26]. The method involves oxidation of succinate by an artificial electron acceptor, potassium ferricyanide. The reaction mixture contained 0.2 M phosphate buffer pH 7.8, 1% BSA, 0.6 M succinic acid, and 0.03 M potassium ferricyanide. The reaction was initiated by the addition of mitochondrial sample, and absorbance change was followed at 420 nm for 2 min.
2.4.3. COMPLEX-III (MTT Ability)
The MTT assay is based on the reduction of (3-(4,5-dimethylthiazol-2-yl)-2, 5-diphenyl-H-tetrazolium bromide (MTT) by hydrogenase activity in functionally intact mitochondria. The MTT reduction rate was used to assess the activity of the mitochondrial respiratory chain in isolated mitochondria by the method of Liu et al. [27]. Briefly, 100 μL mitochondrial samples were incubated with 10 μL MTT for 3 hours at 37°C. The blue formazan crystals were solubilized with dimethylsulfoxide and measured by an ELISA reader at 580 nm filter.
2.5. Biochemical Assessment
Biochemical tests were conducted 24 hrs after last behavioral test. The animals were sacrificed by decapitation. Brains were removed and rinsed with ice-cold isotonic saline. Brains were then homogenized with ice-cold 0.1 mmol/L phosphate buffer (pH 7.4). The homogenates (10% w/v) were then centrifuged at 10,000 g for 15 min and the supernatant so formed was used for the biochemical estimations.
2.5.1. Measurement of Lipid Peroxidation
The extent of lipid peroxidation in the brain was determined quantitatively by performing the method as described by0020Wills [28]. The amount of malondialdehyde (MDA) was measured by reaction with thiobarbituric acid at 532 nm using Perkin Elmer Lambda 20 spectrophotometer.
2.5.2. Estimation of Nitrite
The accumulation of nitrite in the supernatant, an indicator of the production of nitric oxide, was determined by a colorimetric assay with Greiss reagent (0.1% N-(1-napththyl) ethylene diamine dihydrochloride, 1% sulphanilamide and 5% phosphoric acid.) [29]. The absorbance was measured at 540 nm using Perkin Elmer Lambda 20 spectrophotometer. The concentration of nitrite in the supernatant was determined from sodium nitrite standard curve.
2.5.3. Estimation of Non Protein Thiols (NP-SH)
NP-SH was estimated according to the method described by Ellman [30]. A 1 mL supernatant was precipitated with 1 mL of 4% sulphosalicylic acid and cold digested for 1 hour at 4°C. The samples were then centrifuged at 1,200 g for 15 min at 4°C. To 1 mL of the supernatant obtained, 2.7 mL of phosphate buffer (0.1 mmol/L, pH 8) and 0.2 mL of 5, 5′ dithio-bis (2-nitrobenzoic acid) was added. The yellow color developed was measured at 412 nm using Perkin Elmer Lambda 20 spectrophotometer.
2.5.4. Superoxide Dismutase Activity
Superoxide dismutase (SOD) activity was assayed by the method of Kono [31]. The assay system consists of EDTA 0.1 mM, sodium carbonate 50 mM, and 96 mM of nitro blue tetrazolium (NBT). In the cuvette, 2 mL of the above mixture, 0.05 mL of hydroxylamine, and 0.05 mL of the supernatant was added and auto-oxidation of hydroxylamine was measured for 2 min at 30 s interval by measuring absorbance at 560 nm using Perkin Elmer Lambda 20 spectrophotometer.
2.5.5. Catalase Activity
Catalase activity was assessed by the method of Luck [32], wherein the breakdown of H2O2 is measured. Briefly, assay mixture consists of 3 mL of H2O2 phosphate buffer and 0.05 mL of the supernatant of the tissue homogenate. The change in absorbance was recorded for 2 min at 30 s interval at 240 nm using Perkin Elmer Lambda 20 spectrophotometer.
2.5.6. Glutathione-S-Transferase Activity
The activity of glutathione-s-transferase was assayed by the method of Habig and Jakoby [33]. Briefly, the assay mixture consisted of 2.7 mL of phosphate buffer, 0.1 mL of reduced glutathione, 0.1 mL of 1-chloro-2, 4-dinitrobenzene (CDNB) as substrate and 0.1 mL of supernatant. The increase in the absorbance was recorded at 340 nm for 5 min at 1 min interval using Perkin Elmer Lambda 20 spectrophotometer.
2.5.7. Estimation of Acetyl Cholinesterase (AChE) Activity
AchE is a marker of loss of cholinergic neurons in the forebrain. The AchE activity was assessed by Ellman method [34]. The assay mixture contained 0.05 mL of supernatant, 3 mL of sodium phosphate buffer (pH 8), 0.1 mL of acetylthiocholine iodide, and 0.1 mL of DTNB (Ellman reagent). The change in absorbance was measured for 2 min at 30 s interval at 412 nm using Perkin Elmer Lambda 20 spectrophotometer.
2.5.8. Protein Estimation
The protein content was estimated by Biuret method [35] using bovine serum albumin as a standard.
2.6. Statistical Analysis
Values are expressed as mean ± S.E.M. The behavioral assessment data were analyzed by a repeated measures two-way analysis of variance (ANOVA) with drug-treated groups as between and sessions as the within-subjects factors. The biochemical estimations were separately analyzed by one-way ANOVA. Post hoc comparisons between groups were made using Tukey's test. P < .05 was considered significant.
3. Results
3.1. Centella Asiatica (CA) Improved on Behavioral Alteration in D-Galactose-Treated Mice
(I) Morris Water Maze —
D-galactose-treated mice significantly delayed acquisition latency to reach the visual platform as compared to naïve group, indicating memory deficits. CA (150 & 300 mg/kg) treatment significantly improved memory performance (shortened mean acquisition latency) on day 19 and 20 (P < .05) in D-galactose-treated group. Following training, visual platform was kept 1 cm below water level. D-galactose treatment significantly delayed mean acquisition latency and retention latencies (1st and 2nd on days 21 and 42, resp.) to escape onto the hidden platform as compared to naïve group. These results suggest that D-galactose caused significant cognitive impairment. Further, chronic CA treatment (150 & 300 mg/kg) significantly improved memory performance (increased memory retention) on 1st and 2nd RL on days 21 and 42, respectively, as compared to D-galactose-treated mice. However, CA (300 mg/kg) per se treatment did not influence any acquisition and retention latencies as compared to naïve animals (Table 1).
Table 1.
Effect of Centella asiatica (CA; 150 and 300 mg/kg, p.o.) on memory performance in Morris water maze in D-galactose-treated mice.
| Treatment (mg/Kg) | Day 20 (IAL) | Day 21 (1st RL) | Day 42 (2nd RL) |
|---|---|---|---|
| Naïve | 48.1 ± 2.9 | 17.3 ± 3.2 | 12.3 ± 1.4 |
| D-gal (100) | 49.8 ± 3.0 | 58.0 ± 2.2a | 55.1 ± 1.7a |
| CA (300) | 48.6 ± 2.6 | 12.5 ± 2.5 | 9.5 ± 2.4 |
| CA (150) + D-gal (100) | 46.5 ± 2.56 | 43.0 ± 2.7b | 40.6 ± 2.6b |
| CA (300) + D-gal (100) | 46.3 ± 2.7 | 28.5 ± 2.5b,c | 25.6 ± 2.4b,c |
The initial acquisition latencies (IAL) on day 20 and retention latencies on days 21 (1st RL) and 42 (2nd RL) following D-gal concurrent treatment were observed. Values are mean ± S.E.M. aP < .05 as compared to naive group; bP < .05 as compared to D-gal-treated group; cP < .05 as compared to CA (150) + D-gal group (repeated measures two-way ANOVA followed by Tukey's test for multiple comparisons).
(II) Elevated Plus Maze —
In the present study, D-galactose-treated group significantly increased ITL as compared to naïve group on day 20. Further, D-galactose-treated group showed a significant delayed 1st RTL and 2nd RTL on days 21 and 42, respectively, as compared to naïve group, demonstrating that chronic D-galactose-induced marked memory impairment. Chronic CA (150 & 300 mg/kg) treatment significantly shortened acquisition latency on day 20 as well as mean retention transfer latencies to enter close arm on days 21 and 42 as compared to control (D-galactose-treated group) (P < .05). However, CA (150 mg/kg) per se treatment did not show any significant alteration in both acquisition as well as retention as compared to naïve group (Table 2).
Table 2.
Effect of Centella asiatica (CA; 150 and 300 mg/kg, p.o.) on memory performance in elevated plus maze paradigm in D-galactose-treated mice.
| Treatment (mg/ Kg) | Day 20 (ITL) | Day 21 (1st RTL) | Day 42 (2nd RTL) |
|---|---|---|---|
| Naïve | 77.5 ± 2.4 | 15.0 ± 2.7 | 10.0 ± 2.5 |
| D-gal (100) | 77.6 ± 2.3 | 87.8 ± 2.6a | 84.3 ± 2.3a |
| CA (300) | 79.8 ± 2.6 | 13.5 ± 2.4 | 11.0 ± 2.2 |
| CA (150) + D-gal (100) | 78.3 ± 2.7 | 50.6 ± 2.6b | 47.0 ± 2.5b |
| CA (300) + D-gal (100) | 79.0 ± 2.6 | 40.8 ± 2.6b,c | 38.0 ± 2.3b,c |
The initial acquisition latencies (IAL) on day 20 and retention latencies on days 21 (1st RL) and 42 (2nd RL) following AlCl3 concurrent treatment were observed. Values are mean ± S.E.M. aP < .05 as compared to naive group; bP < .05 as compared to D-gal-treated group; cP < .05 as compared to CA (150) + D-gal group (repeated measures two-way ANOVA followed by Tukey's test for multiple comparisons).
3.2. Centella asiatica (CA) on Locomotor Activity in D-Galactose Treated Mice
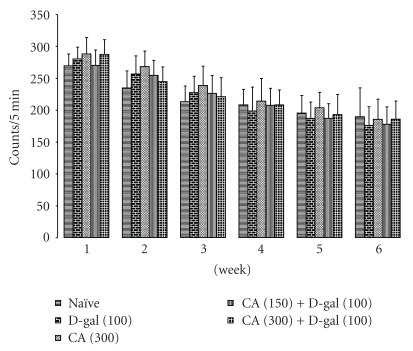
D-galactose-treatment did not influence significantly the locomotor activity as compared to naïve animals. Chronic administration of CA (300 mg/kg) per se treatment as well as CA (150 and 300 mg/kg) treatment in D-galactose-treated mice did not cause any alteration in the locomotor activity as compared to control (D-galactose) (Figure 2).
Figure 2.
Effect of Centella asiatica (CA; 150 and 300 mg/kg, p.o.) on locomotor activity in D-galactose-treated mice. Values are mean ± S.E.M. Data was analyzed by two-way ANOVA.
3.3. Antioxidant Effect of Centella asiatica (CA) in D-Galactose-Treated Mice
Chronic administration of D-galactose significantly raised MDA and nitrite concentration, and caused depletion of NP-SH, glutathione-S-tranferase, superoxide dismutase, and catalase level as compared to naïve mice (P < .05). However, chronic CA (150 and 300 mg/kg, p.o.) treatment significantly attenuated the oxidative damage as indicated by reduced MDA, nitrite concentration, and restoration of NP-SH, glutathione-S-transferase, superoxide dismutase, and catalase level as compared to control group (D-gal-treated group) levels. Further, CA (300 mg/kg, p.o.) per se treatment did not produce any significant effect on oxidative stress parameter as compared to naïve mice (Table 3).
Table 3.
Effect of Centella asiatica (CA; 150 and 300 mg/kg, p.o.) on D-gal-induced oxidative stress parameters in rat brain.
| Treatment (mg/kg) | MDA levels nmol MDA/mg of protein (% of control) | Nitrite levels μmol/mg of protein (% of control) | Non protein Thiol nmol/mg of protein (% of control) | Catalase μmol of hydrogen peroxide decomposed/min/mg of protein (% of control) | Superoxide dismutase Units/mg of protein (% of control) | Glutathione-S-transferase nmol of CDNB conjugated/min/mg of protein (% of control) |
|---|---|---|---|---|---|---|
| Naïve | 0.1768 ± 0.04 (100) | 216.8 ± 30.06 (100) | 0.0653 ± 0.005 (100) | 0.709 ± 0.04 (100) | 48.252 ± 3.19 (100) | 97.9 ± 5 (100) |
| D-gal (100) | 0.5965 ± 0.05a (338.63) | 605.83 ± 28.32a (279.44) | 0.0256 ± 0.003a (39.203) | 0.147 ± 0.018a (20.73) | 13.873 ± 2.11a (28.75) | 37.8 ± 5.3a (38.61) |
| CA (300) | 0.172 ± 0.03 (97.82) | 218 ± 26.7 (100.55) | 0.0648 ± 0.004 (99.23) | 0.703 ± 0.03 (99.15) | 47.22 ± 3.111 (97.86) | 99.9 ± 5.2 (102.04) |
| CA (150) + D-gal (100) | 0.486 ± 0.03b (247.88) | 456 ± 16.2b (210.33) | 0.041 ± 0.001b (62.78) | 0.309 ± 0.023b (43.58) | 21.93 ± 2.049b (45.44) | 47.67 ± 2.3b (48.69) |
| CA (300) + D-gal (100) | 0.279 ± 0.04b,c (157.8) | 353.83 ± 18.6b,c (163.2) | 0.051 ± 0.001b,c (78.1) | 0.427 ± 0.018b,c (60.22) | 35.872 ± 2.05b,c (74.34) | 64.6 ± 1.8b,c (65.98) |
Values are mean ± S.E.M. aP < .05 as compared to naive group; bP < .05 as compared to D-gal-treated group; cP < .05 as compared to CA (150) group+ D-gal group (repeated measures one-way ANOVA followed by Tukey's test for multiple comparisons).
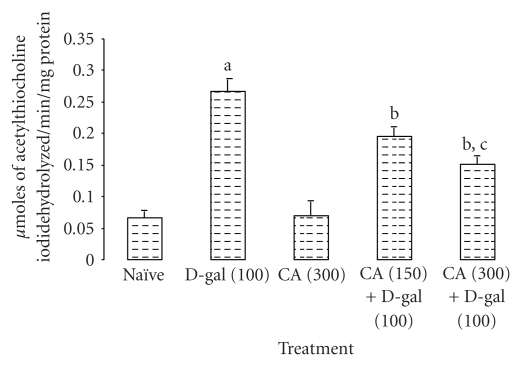
3.4. Reversal of Increased Brain Acetyl Cholinesterase Levels by Centella asiatica (CA) in D-Galactose-Treated Mice
Chronic administration D-galactose caused a significant increase in acetyl cholinesterase activity as compared to naïve mice (P < .05). However, chronic CA (150 and 300 mg/kg, p.o.) treatment significantly decreased acetylcholinesterase activity as compared to D-galactose-treated mice. Further, there was no alteration in acetyl cholinesterase activity in CA (300 mg/kg, p.o.) per se treatment as compared to naïve mice (Figure 3).
Figure 3.
Effect of Centella asiatica (CA; 150 and 300 mg/kg, p.o.) on acetylcholinesterase activity in D-galactose-treated mice. Values are mean ± S.E.M. aP < .05 as compared to naïve group; bP < .05 as compared to D-gal-treated group; cP < .05 as compared to CA (150) + D-gal group; (repeated measures one-way ANOVA followed by Tukey's test for multiple comparisons).
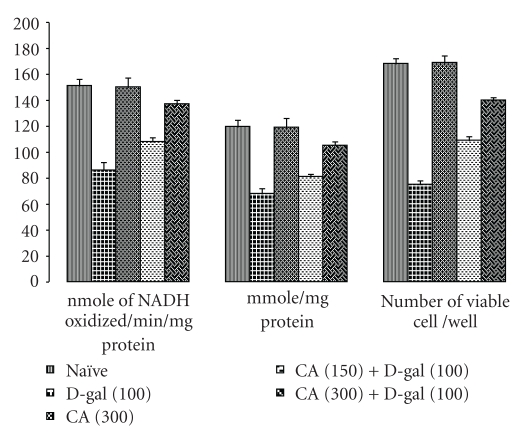
3.5. Centella asiatica (CA) Improved on Mitochondrial Enzyme Alteration in D-Galactose-Treated Mice
Chronic administration of D-galactose caused marked mitochondrial enzyme complex dysfunction and significantly decreased NADH dehydrogenase, succinate dehydrogenase, and MTT ability as compared to naïve mice (P < .05). Chronic CA (150 and 300 mg/kg, p.o.) administration to D-galactose-treated mice significantly prevented the mitochondrial dysfunction. Further, there were no alteration in the brain mitochondrial levels of NADH dehydrogenase, succinate dehydrogenase, and MTT ability in CA (150 and 300 mg/kg, p.o.) per se treatment as compared to naïve mice (Figure 4).
Figure 4.
Effect of Centella asiatica (CA; 150 and 300 mg/kg, p.o.) on NADH Dehydrogenase activity, succinate dehydrogenase activity, and MTT ability in D-galactose-treated mice. Values are mean ± S.E.M. aP < .05 as compared to naïve group; bP < .05 as compared to D-gal treated group; cP < .05 as compared to CA (150) + D-gal group (repeated measures one-way ANOVA followed by Tukey's test for multiple comparisons).
4. Discussion
The present study demonstrates that Centella asiatica extract prevents memory deficits against D-galactose-induced senescence in mice.
D-galactose plays a prime role in the pathogenesis of aging. Various hypotheses have been put forward to explain the mechanism of action of D-galactose in aging including glycometabolism block, formation of advanced glycation end product (AGE), and free radical injury with the evidence of increase levels of malondialdehyde, lipofuscin, decrease in SOD, glutathione peroxidase activity, and decrease in NP-SH level [36–38]. D-galactose administration mimics some characters of cognitive dysfunction and oxidative damage; therefore, it is gradually accepted by people and used in age-related disorders like AD. In our study, D-galactose senescence mice spent a longer time in finding the hidden platform during the retrieval trial in the Morris water maze test which indicates impairment of memory. Observation has been further strengthened by EPM test in which D-galactose showed more latency time to enter into closed arm. In our previous report, we found that D-galactose produced memory impairment in mice for 6 weeks [9, 21]. Earlier, it has been reported that rodents injected with D-galactose for 6–10 weeks shows progressive deterioration of learning and memory capacity and increases production of free radicals in the brain [6]. In our study, chronic administration of D-galactose resulted in a marked oxidative stress as indicated by increasing lipid peroxidation, nitrite concentration, and depletion of NP-SH levels, catalase, superoxide dismutase, and glutathione-s-transferase activity, suggesting oxidative damage. Afterwards, growing evidence revealed there was learning and memory impairment and neuropathological changes like oxidative damage occurred in the brain of rodents treated with D-galactose [39, 40]. The cholinergic system has been implicated in the age-related disorders like AD. Chronic administration of D-galactose showed marked increase of actetylcholine estrase (AChE) enzyme, one of the specific cholinergic markers in aging mice. Recently, Zhong et al. reported that AChE activity, which is responsible for degredation of acetyl choline in synaptic cleft increased significantly in D-galactose-treated mice [41]. Additionally, CA leaf extract has been reported to improve spatial learning performance and enhance memory retention in neonatal rats during growth spurt period, but also found efficient in enhancing hippocampal CA3 neuronaldendritic arborization in rats [17, 18].
It has been shown that aging is associated with a marked decline in mitochondrial function, characterized by a decrease in oxidative phosphorylation and ATP synthesis, an increase in mtDNA (mitochondrial deoxyribonucleic acid) mutations, an increase in abnormal mitochondrial cristae structures, and a marked rise in free radical production, all of which may predispose to age-related disorders [42–44]. It is well known that energy production, realized by oxidative phosphorylation activity, occurs in mitochondria and is catalysed by membrane bound protein complexes, namely NADH-ubiquinol oxidoreductase (Complex I), succinate-ubiquinol oxidoreductase (Complex II), and ubiquinol cytochrome c oxidoreductase (Complex III). Mitochondrial oxidative damage is based on the fact that electron leakage from electron transport chain (ETC), mainly through complex I and complex III and the amount of O2∙− increases dramatically if these complexes are inhibited [45]. The results of the present study indicate that chronic administration of D-galactose results in mitochondrial dysfunction as indicated by decrease in the NADH dehydrogenase, succinate dehydrogenase activity and MTT ability.
Chronic administration of extract of CA was found to improve not only the memory impairment but also reduced oxidative damage induced by chronic D-galactose administration. A previous study showed that different accessions of Centella asiatica exhibited a high antioxidative activity that was comparable to that exhibited by both α-tocopherol and BHT [46]. In our study, CA significantly protected the elevated MDA level and nitric oxide of D-galactose aging mice. Glutathione is an endogenous antioxidant present in the reduced form within the cells. It has been shown to react with free radicals and prevent generation of hydroxyl free radicals. In the present study, CA restored the NP-SH and glutathione-s-transferase level and activity of SOD, catalase in brain of D-galactose-treated mice indicate that CA extract participates in the elimination of free radicals induced by D-galactose. These findings promoted to us to examine that CA would render protection of mitochondrial dysfunction induced by D-galactose in mice. Current evidence suggests that mitochondria are a major source of intracellular ROS and are particularly vulnerable to oxidative stress and its potential connection with mitochondrial enzyme alteration, have been implicated in brain aging. Our result showed CA extract markedly upregulated NADH dehydrogenase, succinate dehydrogenase activity, and MTT ability in D-galactose-induced senescence mice. There is another interesting finding that chronic treatment with CA extract reversed the AChE activity of D-galactose-induced aging mice which reflects that this compound may improve dysfunction of the cholinergic system long term exposed to oxidative stress. The present study highlights that CA attenuates D-galactose-induced behavioral, biochemical, and mitochondrial dysfunction by involving antioxidant and mitochondrial pathways in senescence mice. However, further cellular studies are required to understand the effect of CA on oxidative stress and mitochondrial pathways and the interaction in different experimental system.
Acknowledgment
Authors gratefully acknowledge the financial support of the Indian Council of Medical Research (ICMR), New Delhi for carrying out this work.
References
- 1.Troen BR. The biology of aging. Mount Sinai Journal of Medicine. 2003;70(1):3–22. [PubMed] [Google Scholar]
- 2.Crivello NA, Rosenberg IH, Dallal GE, Bielinski D, Joseph JA. Age-related changes in neutral sphingomyelin-specific phospholipase C activity in striatum, hippocampus, and frontal cortex: implication for sensitivity to stress and inflammation. Neurochemistry International. 2005;47(8):573–579. doi: 10.1016/j.neuint.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 3.Navarro A, Sánchez Del Pino MJ, Gómez C, Peralta JL, Boveris A. Behavioral dysfunction, brain oxidative stress, and impaired mitochondrial electron transfer in aging mice. American Journal of Physiology. 2002;282(4):R985–R992. doi: 10.1152/ajpregu.00537.2001. [DOI] [PubMed] [Google Scholar]
- 4.Kaplan LA, Pesce AJ. Clinical Chemistry. 3rd edition. St. Louis, Mo, USA: Mosby; 1996. [Google Scholar]
- 5.Hsieh HM, Wu WM, Hu ML. Soy isoflavones attenuate oxidative stress and improve parameters related to aging and Alzheimer’s disease in C57BL/6J mice treated with d-galactose. Food and Chemical Toxicology. 2009;47(3):625–632. doi: 10.1016/j.fct.2008.12.026. [DOI] [PubMed] [Google Scholar]
- 6.Song XU, Bao M, Li D, Li YM. Advanced glycation in D-galactose induced mouse aging model. Mechanisms of Ageing and Development. 1999;108(3):239–251. doi: 10.1016/s0047-6374(99)00022-6. [DOI] [PubMed] [Google Scholar]
- 7.Baynes JW. The role of AGEs in aging: causation or correlation. Experimental Gerontology. 2001;36(9):1527–1537. doi: 10.1016/s0531-5565(01)00138-3. [DOI] [PubMed] [Google Scholar]
- 8.Takeuchi M, Yamagishi SI. Possible involvement of advanced glycation end-products (AGEs) in the pathogenesis of Alzheimer’s disease. Current Pharmaceutical Design. 2008;14(10):973–978. doi: 10.2174/138161208784139693. [DOI] [PubMed] [Google Scholar]
- 9.Kumar A, Dogra S, Prakash A. Effect of carvedilol on behavioral, mitochondrial dysfunction, and oxidative damage against d-galactose induced senescence in mice. Naunyn-Schmiedeberg’s Archives of Pharmacology. 2009;380(5):431–441. doi: 10.1007/s00210-009-0442-8. [DOI] [PubMed] [Google Scholar]
- 10.Linnane AW, Marzuki S, Ozawa T, Tanaka M. Mitochondrial DNA mutations as an important contributor to ageing and degenerative diseases. The Lancet. 1989;1(8639):642–645. doi: 10.1016/s0140-6736(89)92145-4. [DOI] [PubMed] [Google Scholar]
- 11.Harman D. Aging: a theory based on free radical and radiation chemistry. Journal of gerontology. 1956;11(3):298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- 12.Ozawa T. Genetic and functional changes in mitochondria associated with aging. Physiological Reviews. 1997;77(2):425–464. doi: 10.1152/physrev.1997.77.2.425. [DOI] [PubMed] [Google Scholar]
- 13.Sharma PV. Dravyaguna Vignana. 13th edition. New Delhi, India: Chaukhamba Publications Vishwa Bharati Academy; 1999. [Google Scholar]
- 14.Kumar MHV, Gupta YK. Effect of different extracts of Centella asiatica on cognition and markers of oxidative stress in rats. Journal of Ethnopharmacology. 2002;79(2):253–260. doi: 10.1016/s0378-8741(01)00394-4. [DOI] [PubMed] [Google Scholar]
- 15.De Souza ND, Shah V, Desai PD, Inamdar PK, D’Sa A, Ammonamanchi R. 2,3,23-trihydroxy-urs-12-ene and its derivatives, processes for their preparation and their use. European Patent., 383 171 A2, 1990.
- 16.Mook-Jung I, Shin JIE, Yun HS, et al. Protective effects of asiaticoside derivatives against beta-amyloid neurotoxicity. Journal of Neuroscience Research. 1999;58(3):417–425. [PubMed] [Google Scholar]
- 17.Mohandas Rao KG, Muddanna Rao S, Gurumadhva Rao S. Centella asiatica (L.) leaf extract treatment during the growth spurt period enhances hippocampal CA3 neuronal dendritic arborization in rats. Evidence-Based Complementary and Alternative Medicine. 2006;3(3):349–357. doi: 10.1093/ecam/nel024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mohandas Rao KG, Muddanna Rao S, Gurumadhva Rao S. Enhancement of amygdaloid neuronal dendritic arborization by fresh leaf juice of Centella asiatica (Linn) during growth spurt period in rats. Evidence-Based Complementary and Alternative Medicine. 2009;6(2):203–210. doi: 10.1093/ecam/nem079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rao SB, Chetana M, Uma DP. Centella asiatica treatment during postnatal period enhances learning and memory in mice. Physiology and Behavior. 2005;86(4):449–457. doi: 10.1016/j.physbeh.2005.07.019. [DOI] [PubMed] [Google Scholar]
- 20.Hussin M, Abdul-Hamid A, Mohamad S, Saari N, Ismail M, Bejo MH. Protective effect of Centella asiatica extract and powder on oxidative stress in rats. Food Chemistry. 2007;100(2):535–541. [Google Scholar]
- 21.Kumar A, Prakash A, Dogra S. Naringin alleviates cognitive impairment, mitochondrial dysfunction and oxidative stress induced by d-galactose in mice. Food and Chemical Toxicology. 2010;48(2):626–632. doi: 10.1016/j.fct.2009.11.043. [DOI] [PubMed] [Google Scholar]
- 22.Kumar A, Dogra S, Prakash A. Neuroprotective effects of Centella asiatica against intracerebroventricular colchicine—induced cognitive impairment and oxidative stress. International Journal of Alzheimer's Disease. 2009;2009:8 pages. doi: 10.4061/2009/972178. Article ID 972178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sharma AC, Kulkarni SK. Evaluation of learning and memory mechanisms employing elevated plus-maze in rats and mice. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 1992;16(1):117–125. doi: 10.1016/0278-5846(92)90014-6. [DOI] [PubMed] [Google Scholar]
- 24.Berman B, Hastings G. Dopamine oxidation alters mitochondrial respiration and induces permeability transition in brain mitochondria: implications for Parkinson's disease. Journal of Neurochemistry. 1997;63:1185–1195. doi: 10.1046/j.1471-4159.1999.0731127.x. [DOI] [PubMed] [Google Scholar]
- 25.King TE. Preparations and properties of soluble NADH dehydrogenases from cardiac muscle. Methods in Enzymology. 1967;10:275–294. [Google Scholar]
- 26.King TE. Preparation of succinate dehydrogenase and reconstitution of succinate oxidase. Methods in Enzymology. 1967;10:322–331. [Google Scholar]
- 27.Liu Y, Peterson DA, Kimura H, Schubert D. Mechanism of cellular 3-(4,5-dimethylthiazol-2-yl)-2,5- diphenyltetrazolium bromide (MTT) reduction. Journal of Neurochemistry. 1997;69(2):581–593. doi: 10.1046/j.1471-4159.1997.69020581.x. [DOI] [PubMed] [Google Scholar]
- 28.Wills ED. Mechanisms of lipid peroxide formation in animal tissues. Biochemical Journal. 1966;99(3):667–676. doi: 10.1042/bj0990667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Green LC, Wagner DA, Glogowski J. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Analytical Biochemistry. 1982;193:265–275. doi: 10.1016/0003-2697(82)90118-x. [DOI] [PubMed] [Google Scholar]
- 30.Ellman GL. Tissue sulfhydryl groups. Archives of Biochemistry and Biophysics. 1959;82(1):48670–48677. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- 31.Kono Y. Generation of superoxide radical during autoxidation of hydroxylamine and an assay for superoxide dismutase. Archives of Biochemistry and Biophysics. 1978;186(1):189–195. doi: 10.1016/0003-9861(78)90479-4. [DOI] [PubMed] [Google Scholar]
- 32.Luck H. Catalase. In: Bergmeyer HU, editor. Methods of Enzymatic Analysis. New York, NY, USA: Academic Press; 1971. pp. 885–893. [Google Scholar]
- 33.Habig WH, Jakoby WB. Assays for differentiation of glutathione S-transferases. Methods in Enzymology. 1981;77:398–405. doi: 10.1016/s0076-6879(81)77053-8. [DOI] [PubMed] [Google Scholar]
- 34.Ellman GL, Courtney KD, Andres V, Featherstone RM. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochemical Pharmacology. 1961;7(2):88–95. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- 35.Gornall AG, Bardawill CT, David MM. Determination of serum proteins by means of Biuret reaction. The Journal of Biological Chemistry. 1949;177:751–766. [PubMed] [Google Scholar]
- 36.Cui XU, Zuo P, Zhang Q, et al. Chronic systemic D-galactose exposure induces memory loss, neurodegeneration, and oxidative damage in mice: protective effects of R-α-lipoic acid. Journal of Neuroscience Research. 2006;84(3):647–654. doi: 10.1002/jnr.20899. [DOI] [PubMed] [Google Scholar]
- 37.Ho SC, Liu JH, Wu RY. Establishment of the mimetic aging effect in mice caused by D-galactose. Biogerontology. 2003;4(1):15–18. doi: 10.1023/a:1022417102206. [DOI] [PubMed] [Google Scholar]
- 38.Shen YUX, Xu SY, Wei W, et al. Melatonin reduces memory changes and neural oxidative damage in mice treated with D-galactose. Journal of Pineal Research. 2002;32(3):173–178. doi: 10.1034/j.1600-079x.2002.1o850.x. [DOI] [PubMed] [Google Scholar]
- 39.Ya-Zhen S, Gong M, Xiao-Xia Z, Bao-Yuan W, Su-Ting L. Improving effects of SSF on memory deficits and pathological changes of neural and immunological systems in senescent mice. Acta Pharmacologica Sinica. 2001;22(12):1078–1083. [PubMed] [Google Scholar]
- 40.Wei H, Li L, Song Q, Ai H, Chu J, Li W. Behavioural study of the D-galactose induced aging model in C57BL/6J mice. Behavioural Brain Research. 2005;157(2):245–251. doi: 10.1016/j.bbr.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 41.Zhong SZ, Ge QH, Qu R, Li Q, Ma SP. Paeonol attenuates neurotoxicity and ameliorates cognitive impairment induced by d-galactose in ICR mice. Journal of the Neurological Sciences. 2009;277(1-2):58–64. doi: 10.1016/j.jns.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 42.Pallotti F, Chen XI, Bonilla E, Schon EA. Evidence that specific mtDNA point mutations may not accumulate in skeletal muscle during normal human aging. American Journal of Human Genetics. 1996;59(3):591–602. [PMC free article] [PubMed] [Google Scholar]
- 43.Cortopassi G, Wang E. Modelling the effects of age-related mtDNA mutation accumulation; Complex I deficiency, superoxide and cell death. Biochimica et Biophysica Acta. 1995;1271(1):171–176. doi: 10.1016/0925-4439(95)00025-y. [DOI] [PubMed] [Google Scholar]
- 44.Long J, Wang X, Gao H, et al. D-Galactose toxicity in mice is associated with mitochondrial dysfunction: protecting effects of mitochondrial nutrient R-alpha-lipoic acid. Biogerontology. 2007;8(3):373–381. doi: 10.1007/s10522-007-9081-y. [DOI] [PubMed] [Google Scholar]
- 45.Turrens JF. Superoxide production by the mitochondrial respiratory chain. Bioscience Reports. 1997;17(1):3–8. doi: 10.1023/a:1027374931887. [DOI] [PubMed] [Google Scholar]
- 46.Zainol MK, Abdul-Hamid A, Yusof S, Muse R. Anti-oxidative activity and total polyphenolic compounds of leaf, root and petiole of four accessions of Centella asiatica (L.) urban. Food Chemistry. 2003;81:575–581. [Google Scholar]