Abstract
Exposure to severe stressors increases the risk for psychiatric disorders in vulnerable individuals, but can lead to positive outcomes for others. However, it remains unknown how severe stress affects neural functioning in humans and what factors mediate individual differences in the neural sequelae of stress. The amygdala is a key brain region involved in threat detection and fear regulation, and previous animal studies have suggested that stress sensitizes amygdala responsivity and reduces its regulation by the prefrontal cortex. In this study, we used a prospective design to investigate the consequences of severe stress in soldiers before and after deployment to a combat zone. We found that combat stress increased amygdala and insula reactivity to biologically salient stimuli across the group of combat-exposed individuals. In contrast, its influence on amygdala coupling with the insula and dorsal anterior cingulate cortex was dependent on perceived threat, rather than actual exposure, suggesting that threat appraisal affects interoceptive awareness and amygdala regulation. Our results demonstrate that combat stress has sustained consequences on neural responsivity, and suggest a key role for the appraisal of threat on an amygdala-centered neural network in the aftermath of severe stress.
Keywords: ACC, amygdala, combat, fMRI, PTSD, stress
Introduction
Highly stressful experiences, such as natural disaster, terrorism, assault or military combat, are significant events in the lives of people. Such stressful life events have large impact on the exposed individual, but its influence on subsequent psychological well-being is highly variable between individuals. Whereas traumatic experiences lead to positive changes for some,1, 2 they increase the risk for post-traumatic stress disorder (PTSD) and depression for others,3, 4 which suggests that the influence of stress is highly variable between individuals. However, the neural consequence of severe stress on neural functioning in humans remains unknown. Patients with PTSD show exaggerated amygdala responses and deficient prefrontal cortex function, in particular in the anterior cingulate cortex and ventromedial prefrontal cortex.5, 6, 7, 8 The amygdala and prefrontal cortex are key brain regions involved in threat detection and fear regulation.9, 10, 11 These neural alterations in PTSD presumably result from a combination of stress exposure and stress vulnerability.12, 13, 14 Nevertheless, animal studies suggest that severe stress has lasting influences on neural functioning.15, 16 Specifically, recent animal studies have shown that prolonged severe stress sensitizes amygdala responsivity and reduces its regulation by the prefrontal cortex.17, 18 Furthermore, acute stress exposure increases amygdala reactivity in humans.19 To investigate whether prolonged severe stress also affects amygdala functioning in humans, even in the absence of acute stress, we used a prospective study design. We assessed amygdala functioning in soldiers before and after deployment to a combat zone, which is typically associated with severe stress exposure. In addition, we included a group of soldiers who were never deployed to control for repeated testing and unspecific time effects.
Materials and methods
Participants
The combat stress group consisted of 33 healthy soldiers who were deployed for 4 months to Afghanistan as part of the North Atlantic Treaty Organization International Security Assistance Force peacekeeping operation. We investigated them before and on average 1.5 (s.d.=0.8) months after their first military deployment. They were recruited from a larger prospective study on the development of stress-related disorders following military deployment in the Dutch armed forces. Their duties included combat patrols, clearing or searching homes and buildings, participation in demining operations and transportation across enemy territory. They were exposed to typical war-zone stressors, such as enemy fire, armed combat and seeing seriously injured fellow soldiers and civilians (including women and children). The control group consisted of 26 soldiers who were recruited from training bases and army divisions currently not involved in combat missions. One soldier of the control group scored above a clinical threshold for PTSD symptoms on a self-report questionnaire (see below) at baseline, and one soldier of the combat group scored above the threshold after deployment. Both participants were therefore excluded from the analyses. The groups did not differ significantly in sex ratio, age and intelligence quotient (see Table 1). Furthermore, we investigated both groups at the same test-retest interval (mean±s.d.; 6.9±1.6 months; t(55)=0.5, P=0.65). Because the number of females in the study was small, we also conducted all analyses with males only, which did not alter the pattern of results (data not shown). The study was in accordance with the declaration of Helsinki and institutional guidelines of the local ethics committee (CMO Regio Arnhem-Nijmegen, The Netherlands), and all participants provided written informed consent after written and oral description of the study.
Table 1. Demographic and questionnaire data (mean±s.d.).
|
Combat stress group (n=32) |
Control group (n=25) |
Statistics |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Baseline |
Follow-up |
Baseline |
Follow-up |
Baselinea | Group × timeb | |||||
| Mean | s.d. | Mean | s.d. | Mean | s.d. | Mean | s.d. | P | P | |
| Age | 24.3 | 8.0 | 23.9 | 6.7 | 0.82 | |||||
| IQ | 89.4 | 10.6 | 92.1 | 13.3 | 0.40 | |||||
| Sex (M/F) | 31/1 | 23/2 | 0.41c | |||||||
| Questionnaires | ||||||||||
| PTSD symptoms | 27.6 | 4.6 | 27.6 | 5.9 | 26.5 | 3.8 | 26.8 | 4.6 | 0.35 | 0.77 |
| Positive affect | 32.0 | 5.5 | 33.1 | 5.6 | 32.6 | 5.0 | 31.4 | 6.7 | 0.64 | 0.10 |
| Negative affect | 12.9 | 4.8 | 11.6 | 2.3 | 11.5 | 2.6 | 11.2 | 2.0 | 0.21 | 0.26 |
| State anxiety | 31.6 | 7.6 | 30.7 | 6.7 | 30.2 | 6.8 | 28.2 | 5.3 | 0.54 | 0.50 |
Abbreviations: F, female; IQ, intelligence quotient; M, male; PTSD, post-traumatic stress disorder.
Two-sample t-test
Group × time analysis of variance.
Pearson's χ2.
Questionnaires
To evaluate the influence of severe stress exposure on PTSD symptoms, mood and anxiety, we used three questionnaires. The short version of a previously validated Dutch questionnaire, the self-rating inventory for PTSD, was used to assess self-reported PTSD symptoms. We excluded individuals with possible PTSD from all analyses, as defined by the cutoff score of 52.20 The Dutch version of the Positive and Negative Affect Schedule was used to assess positive and negative mood,21 and Dutch state version of the State Trait Anxiety Inventory was administered to assess state anxiety.22 To quantify individual differences in stress exposure, we measured combat exposure and perceived threat during deployment using the Combat experiences and Perceived threat scales of the Deployment Risk and Resilience Inventory.23 The scores on these scales were not significantly correlated (r=0.10, P=0.58), suggesting that the amount of perceived threat during the time of the military deployment was not directly related to the number of actual combat experiences.
Behavioral task
The experimental paradigm that was performed during functional magnetic resonance imaging scanning consisted of a blocked design, including an emotion condition with angry and fearful face stimuli (http://www.macbrain.org) and a visuomotor control condition with scrambled ellipse stimuli.24, 25, 26 Two emotion blocks were interleaved with three control blocks, and each 30 s block consisted of six 5 s trials. Each trial consisted of three simultaneously presented stimuli, with the cue stimulus presented above the target and distractor. In the emotion condition, an angry or fearful face was presented on top as cue, and participants had to indicate by an appropriate button press which of the bottom two faces (one angry and one fearful) matched the cue in emotional expression. In the control condition, a horizontally or vertically oriented ellipse was presented as cue above two ellipses (one vertical and one horizontal), and participants had to select the identically oriented ellipse.
Functional magnetic resonance image acquisition
Magnetic resonance data were acquired with a 1.5T Siemens (Erlangen, Germany) Avanto magnetic resonance scanner, equipped with a standard head coil. T2*-weighted bold images were acquired using echo planner imaging with an echo time of 35 ms to reduce signal dropout in the medial temporal lobes. Each image volume consisted of 32 axial slices (3.5 mm, 0.35 mm slice gap, repetition time (TR)=2.340 s, 64 × 64 matrix, field of view=212 mm, flip angle (FA)=90°). In addition, a high-resolution T1-weighted structural magnetic resonance image with optimized gray/white matter contrast was acquired (three-dimensional magnetization-prepared rapid acquisition with gradient echo, 1 × 1 × 1 mm3 voxels, TR=2.730 s, echo time (TE)=2.95 ms, inversion time (TI)=1000 ms, field of view=256 mm, FA=7).
Functional magnetic resonance data analysis
Image analysis was performed with SPM5 (http://www.fil.ion.ucl.ac.uk/spm). The first five echo planner imaging volumes were discarded and the remaining images were realigned to the first volume. Images were then co-registered to the anatomical scan, corrected for differences in slice acquisition time, spatially normalized to the Montreal Neurological Institute (MNI) T1 template, resampled into 2 × 2 × 2 mm3 voxels, and spatially smoothed (8 mm full width at half maximum). Statistical analysis was performed within the framework of the general linear model.27 To assess the influence of combat stress on neural responsivity, the two experimental conditions were modeled as box-car regressors convolved with the canonical hemodynamic response function of SPM5. In addition, the realignment parameters were included to model potential movement artefacts, as well as a constant. Furthermore, a high-pass filter (cutoff 1/128 Hz) was included, and temporal autocorrelation was modelled with an AR(1) process. Contrast images subtracting the visuomotor control condition from the emotion condition were obtained, and analyzed in subsequent random effects models.
Functional connectivity
To assess the influence of severe stress on the amygdala network, we performed an additional functional connectivity (psychophysiological interaction) analysis using SPM5. The time course of amygdala activity was obtained for each scanning session. The first eigenvariate of a sphere with 5 mm radius around the peak group × time interaction in the right amygdala (24,−4 and −18) was extracted and entered as an additional regressor to the original functional magnetic resonance imaging model, as well as the interaction between the experimental conditions and amygdala time course.28 Psychophysiological interaction (time course × condition) images were obtained, and analyzed in subsequent random effects models.
Voxel-based morphometry
To assess the influence of severe stress on regional gray matter volume, we performed voxel-based morphometry using SPM5 with standard procedures29 and default parameters of the VBM5 toolbox (http://dbm.neuro.uni-jena.de/vbm.html).30 Analyses were performed on gray matter segmentations, which were multiplied by the nonlinear components derived from the normalization matrix in order to preserve local gray matter volumes (that is, the modulated images) and spatially smoothed (12 mm full width at half maximum).
Statistical testing
Mixed model analysis of variances with the factors group and time were used to test whether changes in brain structure and function over time were different between the combat stress and control groups. To test whether stress-induced changes were related to individual differences in combat exposure and perceived threat, additional correlation analyses were performed. Statistical tests were family-wise error rate corrected (P<0.05) for multiple comparisons across the entire brain, or for the search volume for regions of interest using a small volume correction.31 The search volumes for the amygdala, insula and anterior cingulate cortex (Brodmann areas 24 and 32) were anatomically defined using the WFU Pickatlas toolbox implemented in SPM5.32
Results
Questionnaires
We observed no significant differences in self-reported PTSD symptoms, state anxiety, positive affect and negative affect scores at baseline, nor different changes in these variables between the combat stress group and control group (see Table 1), indicating that changes in neural activity did not reflect increases in symptomatology. In addition, we measured combat exposure and perceived threat during deployment to quantify the individual differences in stress exposure. The average score for combat exposure (mean±s.d.; 5.0±2.5) was higher than that of a previously reported reference population of Gulf War veterans (4.0±3.2),23 confirming that the combat group was exposed to typical combat zone stressors such as armed combat, combat patrols, and exposure to enemy fire, as well as modern warfare with a risk of exposure to improvised explosive devises. To explore whether there was a delayed onset of PTSD symptoms, we contacted the deployed individuals again 6 months after their deployment, but observed no significant changes in PTSD symptoms over time in those individuals that completed all three questionnaires (n=16; F(2,14)=1.3, P=0.31).
Behavioral performance
Across both groups and measurements, task accuracy was higher in the emotion than control condition (Z=−2.0, P=0.04; median±interquartile range; emotion condition: 100.0±4.2%, control condition: 97.2±5.6%) but reaction times were slower (F(1,55)=171.1, P<0.001; mean±s.d.; emotion condition: 1863±346 ms, control condition: 1141±391 ms). However, no significant changes in accuracy (emotion condition: U=362.0, Z=−0.73, P=0.47; control condition: U=387.0,Z=−0.22, P=0.83) or reaction times (F<1) over time between the combat stress and control groups were observed, which suggests that the imaging results are unlikely explained by differences in behavioral performance.
Neural responsivity
To verify that the task indeed activated the amygdala, we compared the emotion condition with the control condition across both groups and measurements. As expected, the task increased activity in the amygdala, as well as in the insula, dorsal anterior cingulate cortex (dACC), occipitotemporal cortex, hippocampus, orbitofrontal cortex, inferior and middle frontal gyri, thalamus, precuneus, angular gyrus and cerebellum (all Pcor<0.05). These activation patterns were not significantly different between groups at baseline (Pcor>0.05), suggesting that neural functioning was comparable before stress exposure.
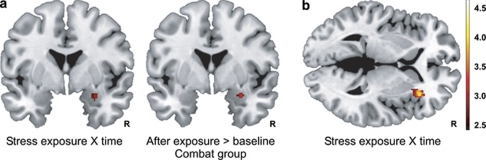
Next, we compared the change in amygdala reactivity over time between groups. The increase in amygdala reactivity was significantly larger in the combat stress group than the control group (peak Montreal Neurological Institute coordinate (x, y and z); (24, −4 and −18); Z=3.1, Pcor=0.037). Subsequent tests showed that combat stress exposure increased amygdala reactivity (30, 0 and−18; Z=3.1, Pcor=0.044; see Figure 1a), whereas no significant change in activity was observed in the control group (Pcor>0.05). A similar group × time interaction was also observed in the anterior insula (32, 24 and 2; Z=3.8, Pcor=0.037; see Figure 1b), which is a brain region involved in interoceptive awareness and thought to signal internal body states.33, 34 Subsequent tests showed that combat stress exposure also increased insula reactivity (P=0.002), whereas insula reactivity decreased with repeated testing in the control group (P<0.001), but these additional tests did not remain significant after correction for multiple comparisons. These results demonstrate that severe and long-term stress exposure sensitized amygdala and insula reactivity to biologically salient stimuli in humans. Next, to evaluate whether these activity changes over time were related to individual differences in stress exposure, we performed additional correlation analyses. However, we observed no significant correlations between activity changes and combat experiences or perceived threat (Pcor>0.05).
Figure 1.
Severe stress exposure increases amygdala and insula reactivity to biologically salient stimuli. (a) Combat exposure increased amygdala reactivity in military soldiers, whereas no significant change in amygdala reactivity was observed in soldiers that were never deployed. (b) Combat exposure also increased insula reactivity in soldiers relative to response habituation over time in the control group. The figures show statistical parametric maps illustrating the significant effects (P<0.05, corrected) at P<0.005, uncorrected.
Amygdala connectivity
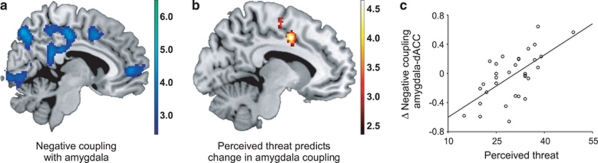
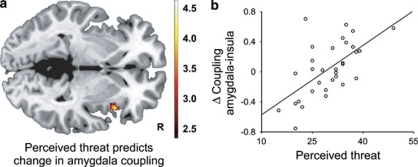
To investigate whether the influence of severe stress on amygdala reactivity also affected the neural network that is centered around the amygdala,11, 35 we performed a functional connectivity analysis. Across groups and investigations, the amygdala was negatively coupled to cingulate cortex areas (see Figure 2a) including the dorsal anterior cingulate cortex (dACC; (6, 8 and 44), Z=4.1, Pcor=0.017), pregenual ACC (6, 48 and 2; Z=3.8, Pcor=0.040, middle cingulate cortex; 4, −20 and 42; Z=4.3, Pcor=0.006) and posterior cingulate cortex (−2,−36 and 26), Z=5.9, Pcor<0.001). These connectivity patterns were not significantly different between groups at baseline (Pcor>0.05), suggesting that amygdala coupling was comparable before stress exposure. Changes in functional connectivity over time were not significantly different between the groups (Pcor>0.05), but were related to the amount of perceived threat in stress-exposed individuals. Importantly, the strength of amygdala coupling with the dACC was positively related to perceived threat (−10, 0 and 42; Z=4.5, Pcor=0.003; see Figures 2b and c). Thus, negative coupling of the amygdala with the dACC was enhanced in individuals that perceived little threat, but reduced in individuals that perceived much threat. In addition, perceived threat also enhanced amygdala coupling with the insula (42, 16 and −2; Z=3.8, Pcor=0.039; see Figure 3). In contrast, no significant correlations between actual combat exposure and functional connectivity changes were observed (Pcor>0.05). Moreover, the associations with perceived threat remained significant after correcting for combat exposure, suggesting that perceived threat rather than actual exposure influenced amygdala coupling.
Figure 2.
Individual differences in perceived threat during military deployment affects functional coupling of the amygdala with the dorsal anterior cingulate cortex (dACC). (a) The dACC and other midline structures were in general negatively coupled to the amygdala when analyzed across stress exposure groups and testing sessions. (b) The change in amygdala coupling with the dACC after stress exposure was positively correlated to perceived threat during military deployment. (c) The scatter plot illustrates the correlation in panel b at the peak voxel. Panels a and b show statistical parametric maps illustrating the significant effects (P<0.05, corrected) at P<0.005, uncorrected.
Figure 3.
Individual differences in perceived threat during military deployment affects functional coupling of the amygdala with the insula. (a) The change in amygdala coupling with the insula after stress exposure was positively correlated to perceived threat during military deployment. The statistical parametric map illustrates the significant effect (P<0.05, corrected) at P<0.005, uncorrected. (b) The scatter plot illustrates the correlation in panel a at the peak voxel.
Brain structure
To investigate whether the stress-induced changes in neural activity and connectivity were related to changes in brain structure, we compared regional brain volumes using voxel-based morphometry. We observed no significant volume differences at baseline, no significant changes over time between the combat stress group and control group, and no significant correlations between combat experiences or perceived threat and regional volume changes (Pcor>0.05).
Discussion
To study the neural consequences of severe stress exposure, we investigated soldiers before and after deployment to a combat zone. Our results demonstrate that combat stress increases amygdala and insula reactivity to biologically salient stimuli. These neural changes were not observed in the control group and occurred in the absence of self-reported changes in post-traumatic stress symptoms. This suggests that sustained enhanced reactivity to biologically salient stimuli is a common adaptive response to prolonged environmental threat. The amygdala is a crucial brain region for the detection of threat and the enhancement of vigilance, and both the amygdala and insula are part of a larger salience network.9, 36 Therefore these adaptations may be beneficial to maintain sustained vigilance in continuously dangerous situations, such as the combat zone the soldiers were deployed to. But as heightened amygdala and insula reactivity is thought to increase the risk for mood and anxiety disorders,37, 38 these alterations may not be adaptive for safe environments. Although our second follow-up assessment suggests that the combat group did not develop PTSD symptoms within half a year after deployment, it remains possible that stress symptoms do evolve later on. Furthermore, the amygdala and insula sensitization may also increase the vulnerability to future stressors.
To investigate whether the influence of severe stress on amygdala reactivity also affected the neural network that is centered around the amygdala, we performed an additional functional connectivity analysis. Our results show that the influence of severe stress on amygdala coupling with the dACC and insula was dependent on individual differences in perceived threat. Individuals that perceived little threat during deployment showed enhanced negative amygdala–dACC coupling, whereas individuals that perceived much threat showed reduced coupling. Given that the dACC is thought to regulate amygdala activity,10, 11 this finding suggests that the perception of threat during severe stress exposure alters amygdala regulation thereafter. In addition, perceived threat also enhanced amygdala–insula coupling. The insula is involved in interoceptive awareness and is thought to signal internal body states,33, 34 which suggests that this may reflect increased bodily awareness in those individuals that perceive the most threat.
The influence of perceived threat rather than actual combat exposure on the amygdala network is in line with the appraisal theory, which posits that the cognitive appraisal of threat rather than the actual environmental stressor determines the impact of stress exposure.39, 40 Thus, our results suggest that cognitive appraisal also has a critical role in determining the impact of severe stress on the amygdala network. Interestingly, perceived threat also seems a better predictor for PTSD symptoms than actual combat exposure.23 Even though previous studies have demonstrated a close relation between combat experiences and the prevalence of PTSD,4 path analyses suggest that the influences of combat exposure on PTSD symptoms is mediated by its influence on perceived threat.41
The divergent influences of severe stress on the amygdala network may help to explain why some individuals are vulnerable to stress, whereas others are stress resilient. Our results show opposite effects on amygdala–dACC and amygdala–insula coupling depending on how the stressful experience is perceived. In turn, this may lead to opposite effects on amygdala regulation and interoceptive awareness, with better emotion regulation in those individuals that perceive little threat but worse emotion regulation in those that experience much threat. These individual differences may explain in part why we did not observe consistent changes in symptomatology across the group of combat-exposed individuals, even though their amygdala and insula reactivity had increased, and suggest a neural mechanism by which traumatic experiences could lead to highly variable outcomes.1, 2, 3, 4
The stress-induced changes in the amygdala network did not lead to consistent changes in mood and anxiety as assessed here. We have measured the changes in neural reactivity during affective stimulation. But in addition to increased reactivity to biologically salient stimuli, combat exposure may also have altered the manner in which the brain restores after stress exposure. For example, the release of cortisol after stress exposure normalizes amygdala reactivity,42 and adaptive changes in this process may prevent the development of symptoms. Interestingly, patients with PTSD have abnormalities in the hypothalamic–pituitary–adrenal axis,43 and soldiers that develop PTSD symptoms after combat exposure have a high preexisting number of glucocorticoid receptors.44 This suggests that PTSD symptoms could result from unsuccessful normalization after exposure to consecutive stressors.45, 46 Therefore, we propose that whereas the stress-induced changes in neural reactivity appear a common adaptive response to prolonged environmental threat, maladaptive normalization of neural hyperactivity may lead to the development of stress symptoms, which could be addressed in future studies.
The neural sequelae of severe stress exposure were remarkably similar to the neural alterations in PTSD, which include amygdala and insula hyperactivity and dACC hypoactivity.8, 47 This is in line with the idea that PTSD reflects the upper end of a stress response continuum.48 However, the neural basis of PTSD presumably results from a combination of stress exposure and stress vulnerability.12, 49 Previous studies have shown that high amygdala reactivity to masked stimuli (which prevents conscious awareness) and dACC metabolism before stress exposure increase the risk for subsequent stress symptoms.13, 14 Our results now suggest that sustained increases in amygdala and insula reactivity to biologically salient stimuli reflect a consequence of stress exposure, which may be additive to preexisting vulnerability factors. In contrast, the individual differences in altered amygdala coupling suggest that the impact of stress on the amygdala network is a consequence of the interaction between stress vulnerability and stress exposure.
The similarity between the neural consequences of stress exposure and PTSD pathophysiology has important implications for studies investigating the neural basis of PTSD. Previous neuroimaging studies with PTSD patients have included a control group of trauma-exposed individuals that did not develop PTSD, to control for trauma exposure (for example, see Rauch et al.5). Our results underscore the importance for this procedure by demonstrating that trauma exposure by itself also influences brain functioning. Comparing PTSD patients to non-trauma-exposed individuals may result in misattribution of group differences to PTSD pathophysiology.
One of the 33 soldiers (3%) scored above the clinical threshold for PTSD symptoms on a self-report questionnaire after their 4-month deployment to Afghanistan. This is comparable with PTSD prevalence rates for Dutch and United Kingdom soldiers that have been deployed to Iraq or Afghanistan,50, 51 but lower than the estimate for United States soldiers.4 Differences in these prevalence rates may have various causes, including differences in study populations, level of combat exposure, and screening methods.52 Although our sample seems representative for the military population, it may not be representative for the general population. For example, the process of self-selection to serve in the military may lead to a relatively resilient population. To address this issue, future studies may investigate the consequences of severe and traumatic stress in non-military samples.
In conclusion, our results demonstrate that severe stress exposure sensitizes amygdala and insula reactivity. In addition, individual differences in threat perception predicted divergent influences on the amygdala network, which may explain why some individuals are vulnerable to stress, whereas others are stress resilient. Long-term follow-up studies are required to determine whether these stress-induced neural changes indeed represent resilience or vulnerability factors.
Acknowledgments
We thank A Beekman and M Groenewald for their assistance with data collection and P Gaalman for his technical support. This work was supported by a grant (918.66.613) from the Netherlands Organization for Scientific Research (NWO) and the Dutch Ministry of Defense. The sponsors had no role in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
The authors declare no conflict of interest.
References
- Helgeson VS, Reynolds KA, Tomich PL. A meta-analytic review of benefit finding and growth. J Consult Clin Psychol. 2006;74:797–816. doi: 10.1037/0022-006X.74.5.797. [DOI] [PubMed] [Google Scholar]
- Linley PA, Joseph S. Positive change following trauma and adversity: a review. J Trauma Stress. 2004;17:11–21. doi: 10.1023/B:JOTS.0000014671.27856.7e. [DOI] [PubMed] [Google Scholar]
- Galea S, Ahern J, Resnick H, Kilpatrick D, Bucuvalas M, Gold J, et al. Psychological sequelae of the September 11 terrorist attacks in New York City. N Engl J Med. 2002;346:982–987. doi: 10.1056/NEJMsa013404. [DOI] [PubMed] [Google Scholar]
- Hoge CW, Castro CA, Messer SC, McGurk D, Cotting DI, Koffman RL. Combat duty in Iraq and Afghanistan, mental health problems, and barriers to care. N Engl J Med. 2004;351:13–22. doi: 10.1056/NEJMoa040603. [DOI] [PubMed] [Google Scholar]
- Rauch SL, Whalen PJ, Shin LM, McInerney SC, Macklin ML, Lasko NB, et al. Exaggerated amygdala response to masked facial stimuli in posttraumatic stress disorder: a functional MRI study. Biol Psychiatry. 2000;47:769–776. doi: 10.1016/s0006-3223(00)00828-3. [DOI] [PubMed] [Google Scholar]
- Shin LM, Whalen PJ, Pitman RK, Bush G, Macklin ML, Lasko NB, et al. An fMRI study of anterior cingulate function in posttraumatic stress disorder. Biol Psychiatry. 2001;50:932–942. doi: 10.1016/s0006-3223(01)01215-x. [DOI] [PubMed] [Google Scholar]
- Shin LM, Wright CI, Cannistraro PA, Wedig MM, McMullin K, Martis B, et al. A functional magnetic resonance imaging study of amygdala and medial prefrontal cortex responses to overtly presented fearful faces in posttraumatic stress disorder. Arch Gen Psychiatry. 2005;62:273–281. doi: 10.1001/archpsyc.62.3.273. [DOI] [PubMed] [Google Scholar]
- Etkin A, Wager TD. Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. Am J Psychiatry. 2007;164:1476–1488. doi: 10.1176/appi.ajp.2007.07030504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M, Whalen PJ. The amygdala: vigilance and emotion. Mol Psychiatry. 2001;6:13–34. doi: 10.1038/sj.mp.4000812. [DOI] [PubMed] [Google Scholar]
- Quirk GJ, Beer JS. Prefrontal involvement in the regulation of emotion: convergence of rat and human studies. Curr Opin Neurobiol. 2006;16:723–727. doi: 10.1016/j.conb.2006.07.004. [DOI] [PubMed] [Google Scholar]
- Stein JL, Wiedholz LM, Bassett DS, Weinberger DR, Zink CF, Mattay VS, et al. A validated network of effective amygdala connectivity. Neuroimage. 2007;36:736–745. doi: 10.1016/j.neuroimage.2007.03.022. [DOI] [PubMed] [Google Scholar]
- True WR, Rice J, Eisen SA, Heath AC, Goldberg J, Lyons MJ, et al. A twin study of genetic and environmental contributions to liability for posttraumatic stress symptoms. Arch Gen Psychiatry. 1993;50:257–264. doi: 10.1001/archpsyc.1993.01820160019002. [DOI] [PubMed] [Google Scholar]
- Admon R, Lubin G, Stern O, Rosenberg K, Sela L, Ben-Ami H, et al. Human vulnerability to stress depends on amygdala's predisposition and hippocampal plasticity. Proc Natl Acad Sci USA. 2009;106:14120–14125. doi: 10.1073/pnas.0903183106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin LM, Lasko NB, Macklin ML, Karpf RD, Milad MR, Orr SP, et al. Resting metabolic activity in the cingulate cortex and vulnerability to posttraumatic stress disorder. Arch Gen Psychiatry. 2009;66:1099–1107. doi: 10.1001/archgenpsychiatry.2009.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlides C, Nivon LG, McEwen BS. Effects of chronic stress on hippocampal long-term potentiation. Hippocampus. 2002;12:245–257. doi: 10.1002/hipo.1116. [DOI] [PubMed] [Google Scholar]
- Alfarez DN, Joëls M, Krugers HJ. Chronic unpredictable stress impairs long-term potentiation in rat hippocampal CA1 area and dentate gyrus in vitro. Eur J Neurosci. 2003;17:1928–1934. doi: 10.1046/j.1460-9568.2003.02622.x. [DOI] [PubMed] [Google Scholar]
- Correll CM, Rosenkranz JA, Grace AA. Chronic cold stress alters prefrontal cortical modulation of amygdala neuronal activity in rats. Biol Psychiatry. 2005;58:382–391. doi: 10.1016/j.biopsych.2005.04.009. [DOI] [PubMed] [Google Scholar]
- Rosenkranz JA, Venheim ER, Padival M. Chronic stress causes amygdala hyperexcitability in rodents. Biol Psychiatry. 2010;67:1128–1136. doi: 10.1016/j.biopsych.2010.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Marle HJ, Hermans EJ, Qin S, Fernández G. From specificity to sensitivity: how acute stress affects amygdala processing of biologically salient stimuli. Biol Psychiatry. 2009;66:649–655. doi: 10.1016/j.biopsych.2009.05.014. [DOI] [PubMed] [Google Scholar]
- Hovens JE, van der Ploeg HM, Bramsen I, Klaarenbeek MT, Schreuder JN, Rivero VV. The development of the self-rating inventory for posttraumatic stress disorder. Acta Psychiatr Scand. 1994;90:172–183. doi: 10.1111/j.1600-0447.1994.tb01574.x. [DOI] [PubMed] [Google Scholar]
- Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS scales. J Pers Soc Psychol. 1988;54:1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- Spielberger CD, Gorsuch RL, Lushene RE. STAI Manual for the State Trait Anxiety Inventory. Consulting Psychologists Press: Palo Alto; 1970. [Google Scholar]
- King LA, King DW, Vogt DS, Knight J, Samper RE. Deployment risk and resilience inventory: a collection of measures for studying deployment-related experiences of military personnel and veterans. Mil Psychol. 2006;18:89–120. [Google Scholar]
- Hariri AR, Mattay VS, Tessitore A, Kolachana B, Fera F, Goldman D, et al. Serotonin transporter genetic variation and the response of the human amygdala. Science. 2002;297:400–403. doi: 10.1126/science.1071829. [DOI] [PubMed] [Google Scholar]
- van Wingen GA, van Broekhoven F, Verkes RJ, Petersson KM, Bäckström T, Buitelaar JK, et al. Progesterone selectively increases amygdala reactivity in women. Mol Psychiatry. 2008;13:325–333. doi: 10.1038/sj.mp.4002030. [DOI] [PubMed] [Google Scholar]
- van Wingen GA, Zylicz SA, Pieters S, Mattern C, Verkes RJ, Buitelaar JK, et al. Testosterone increases amygdala reactivity in middle-aged women to a young adulthood level. Neuropsychopharmacology. 2009;34:539–547. doi: 10.1038/npp.2008.2. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Holmes AP, Worsley KJ, Poline JB, Frith CD, Frackowiak RSJ. Statistical parametric maps in functional imaging: a general linear approach. Hum Brain Mapp. 1995;2:189–210. [Google Scholar]
- Friston KJ, Buechel C, Fink GR, Morris J, Rolls E, Dolan RJ. Psychophysiological and modulatory interactions in neuroimaging. Neuroimage. 1997;6:218–229. doi: 10.1006/nimg.1997.0291. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Unified segmentation. Neuroimage. 2005;26:839–851. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Luders E, Toga AW, Lepore N, Gaser C. The underlying anatomical correlates of long-term meditation: larger hippocampal and frontal volumes of gray matter. Neuroimage. 2009;45:672–678. doi: 10.1016/j.neuroimage.2008.12.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worsley KJ, Marrett S, Neelin P, Vandal AC, Friston KJ, Evans AC. A unified statistical approach for determining significant signals in images of cerebral activation. Hum Brain Mapp. 1996;4:58–73. doi: 10.1002/(SICI)1097-0193(1996)4:1<58::AID-HBM4>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19:1233–1239. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- Craig AD. How do you feel? Interoception: the sense of the physiological condition of the body. Nat Rev Neurosci. 2002;3:655–666. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Stein MB. An insular view of anxiety. Biol Psychiatry. 2006;60:383–387. doi: 10.1016/j.biopsych.2006.03.042. [DOI] [PubMed] [Google Scholar]
- Ghashghaei HT, Hilgetag CC, Barbas H. Sequence of information processing for emotions based on the anatomic dialogue between prefrontal cortex and amygdala. Neuroimage. 2007;34:905–923. doi: 10.1016/j.neuroimage.2006.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, et al. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 2007;27:2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein MB, Simmons AN, Feinstein JS, Paulus MP. Increased amygdala and insula activation during emotion processing in anxiety-prone subjects. Am J Psychiatry. 2007;164:318–327. doi: 10.1176/ajp.2007.164.2.318. [DOI] [PubMed] [Google Scholar]
- Wolfensberger SP, Veltman DJ, Hoogendijk WJ, Boomsma DI, de Geus EJ. Amygdala responses to emotional faces in twins discordant or concordant for the risk for anxiety and depression. Neuroimage. 2008;41:544–552. doi: 10.1016/j.neuroimage.2008.01.053. [DOI] [PubMed] [Google Scholar]
- Lazarus RS, Folkman S. Stress, Appraisal, and Coping. Springer Publishing Company: New York; 1984. [Google Scholar]
- Solomon Z, Mikulincer M, Benbenishty R. Locus of control and combat-related post-traumatic stress disorder: the intervening role of battle intensity, threat appraisal and coping. Br J Clin Psychol. 1989;28:131–144. doi: 10.1111/j.2044-8260.1989.tb00823.x. [DOI] [PubMed] [Google Scholar]
- King DW, King LA, Gudanowski DM, Vreven DL. Alternative representations of war zone stressors: relationships to posttraumatic stress disorder in male and female Vietnam veterans. J Abnorm Psychol. 1995;104:184–195. doi: 10.1037//0021-843x.104.1.184. [DOI] [PubMed] [Google Scholar]
- Henckens MJAG, van Wingen GA, Joëls M, Fernández G. Time-dependent effects of corticosteroids on human amygdala processing. J Neurosci. 2010;30:12725–12732. doi: 10.1523/JNEUROSCI.3112-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yehuda R, LeDoux J. Response variation following trauma: a translational neuroscience approach to understanding PTSD. Neuron. 2007;56:19–32. doi: 10.1016/j.neuron.2007.09.006. [DOI] [PubMed] [Google Scholar]
- van ZuidenM, Geuze E, Willemen HLDM, Vermetten E, Maas M, Heijnen CJ, et al. Pre-existing high glucocorticoid receptor number predicts development of posttraumatic stress symptoms after military deployment. Am J Psychiatry. 2011;168:89–96. doi: 10.1176/appi.ajp.2010.10050706. [DOI] [PubMed] [Google Scholar]
- de Kloet ER, Joëls M, Holsboer F. Stress and the brain: from adaptation to disease. Nat Rev Neurosci. 2005;6:463–475. doi: 10.1038/nrn1683. [DOI] [PubMed] [Google Scholar]
- Karst H, Berger S, Erdmann G, Schutz G, Joels M. Metaplasticity of amygdalar responses to the stress hormone corticosterone. Proc Natl Acad Sci USA. 2010;107:14449–14454. doi: 10.1073/pnas.0914381107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauch SL, Shin LM, Phelps EA. Neurocircuitry models of posttraumatic stress disorder and extinction: human neuroimaging research-past, present, and future. Biol Psychiatry. 2006;60:376–382. doi: 10.1016/j.biopsych.2006.06.004. [DOI] [PubMed] [Google Scholar]
- Ruscio AM, Ruscio J, Keane TM. The latent structure of posttraumatic stress disorder: a taxometric investigation of reactions to extreme stress. J Abnorm Psychol. 2002;111:290–301. [PubMed] [Google Scholar]
- Gilbertson MW, Shenton ME, Ciszewski A, Kasai K, Lasko NB, Orr SP, et al. Smaller hippocampal volume predicts pathologic vulnerability to psychological trauma. Nat Neurosci. 2002;5:1242–1247. doi: 10.1038/nn958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotopf M, Hull L, Fear NT, Browne T, Horn O, Iversen A, et al. The health of UK military personnel who deployed to the 2003 Iraq war: a cohort study. Lancet. 2006;367:1731–1741. doi: 10.1016/S0140-6736(06)68662-5. [DOI] [PubMed] [Google Scholar]
- Engelhard IM, van den Hout MA, Weerts J, Arntz A, Hox JJ, McNally RJ. Deployment-related stress and trauma in Dutch soldiers returning from Iraq. Prospective study. Br J Psychiatry. 2007;191:140–145. doi: 10.1192/bjp.bp.106.034884. [DOI] [PubMed] [Google Scholar]
- Sundin J, Fear NT, Iversen A, Rona RJ, Wessely S. PTSD after deployment to Iraq: conflicting rates, conflicting claims. Psychol Med. 2010;40:367–382. doi: 10.1017/S0033291709990791. [DOI] [PubMed] [Google Scholar]