Abstract
Heterogeneity in the composition and in the polydispersity of heparin has motivated the development of homogeneous heparin mimics, and peptides of appropriate sequence and chemical function have therefore recently emerged as potential replacements for heparin in selected applications. Here, we report the assessment of the binding affinities of multiple sulfated peptides (SPs) for a set of heparin-binding peptides (HBPs) and for vascular endothelial growth factor isoform 165 (VEGF165); these binding partners have application in the selective immobilization of proteins and in hydrogel formation through non-covalent interactions. Sulfated peptides were produced via solid-phase methods, and their affinity for the HBPs and VEGF165 was assessed via affinity liquid chromatography (ALC), surface plasmon resonance (SPR), and in selected cases, isothermal titration calorimetry (ITC). The shortest peptide, SPa, showed the highest affinity binding of HBPs and VEGF165 in both ALC and SPR measurements, with slight exceptions. Of the investigated HBPs, a peptide based on the heparin-binding domain of human platelet factor 4 showed greatest binding affinities toward all of the SPs, consistent with its stronger binding to heparin. The affinity between SPa and PF4ZIP was indicated via SPR (KD = 5.27 μM) and confirmed via ITC (KD = 8.09 μM). The binding by SPa of both VEGF and HBPs suggests its use as a binding partner to multiple species, and the use of these interactions in assembly of materials. Given that the peptide sequences can be varied to control binding affinity and selectivity, opportunities are also suggested for the production of a wider array of matrices with selective binding and release properties useful for biomaterials applications.
Keywords: Sulfated peptide, Heparin mimic, Heparin-binding peptides, Affinity chromatography, Growth factor delivery, Hydrogel
1. Introduction
Growth factor delivery has been a topic of increased recent research interest owing to both increasing interests in controlled and targeted drug delivery as well as the expansion of recombinant DNA techniques that have facilitated growth factor production [9,45,59]. Heparin, a highly sulfated glycosaminoglycan, has been used to mediate the local delivery of growth factors from various matrices such as microcapsules [14], fibers [33,34], grafts [29,64], and hydrogels [50,53,54,66–69], since it is known to bind and stabilize many growth factors and potentiate their activity. In particular, heparin has been shown to protect growth factors from inactivation [22,45], increase their affinity to receptors [49], improve the loading efficiency of growth factor into delivery vehicles [34], and sustain the release of growth factors over extended periods of time [33,34]. The chemical diversity, versatility, and heterogeneity of heparin (H), and the related heparan sulfate (HS) that is found in tissue, permits the binding of over 50 growth factors [34], as well as mediation by H/HS of many important biological processes including thrombosis, cell adhesion, lipid metabolism, enzyme regulation, and cytokine action [1,3,27,35,51].
We have reported previously the non-covalent assembly of hydrogels via interaction between heparin and heparin-binding peptides or growth factors, as well as the delivery of growth factors from these matrices via passive or receptor-mediated matrix erosion (Scheme 1) [66–69]. Four-arm star poly(ethylene glycol) (PEG) modified with heparin has been shown to form hydrogels when mixed with star PEGs modified with heparin-binding peptides, as illustrated in the left path in Scheme 1; the peptides used are derived from heparin-interacting protein, antithrombin III, or platelet factor 4 (HIP [36–39], ATIII [61], and PF4ZIP [5,69], respectively). Such modified polymers form hydrogels through non-covalent (primarily electrostatic) interactions, and the hydrogels can be loaded with therapeutic growth factors. The mechanical strength of the hydrogels can be controlled by choosing different heparin-binding peptides and polymer compositions [66,69], and the distribution of the charged heparin throughout the matrix may also facilitate homogeneous incorporation of growth factors into the delivery vehicle [34]. The lack of toxic cross-linking agents in the gels [53], coupled with the potential for their non-invasive administration (e.g., via injection) should permit their use in multiple applications.
Scheme 1.
Non-covalently assembled hydrogels for growth factor delivery. PEG stands for poly(ethylene glycol); LMWH for low molecular weight heparin; HBP for heparin-binding peptide; GF for growth factor.
In addition, dimeric growth factors, such as vascular endothelial growth factor (VEGF), can be directly employed as cross-links in the assembly of these hydrogel networks, since they provide two cross-linking points per molecule (right path in Scheme 1) [68]. Upon administration at a target site, the growth factor may be removed from the gel via binding with its receptors, and subsequently through receptor-mediated endocytosis [43], which would cause erosion due to the loss of physical cross-linking points and would theoretically permit elimination of the polymer matrix should PEG molecules of appropriate molecular weight be used [10,30,46,47,55]. Such a delivery system could be flexibly applied for numerous targets, depending on the desired growth factor employed in the matrix; in theory, multiple growth factors could also be used in a single matrix to permit delivery and erosion on multiple timescales that depend on the affinity of the growth factor for heparin. In addition, the delivery of multiple proteins would be expanded if additional affinity ‘tags’ could be identified with various affinities for heparin-binding proteins of relevance in biomedical applications; such identification would be facilitated by the use of homogeneous molecules with compositions that could be readily and controllably varied.
The inherent heterogeneity of heparin makes its controlled use in the above applications challenging [67]. The number of saccharides in heparin, the sulfation pattern, and the exact site of conjugation of polymers, can vary substantially from sample to sample, which has motivated the identification of heparin surrogates that are homogeneous and straightforward to synthesize. Among the many kinds of heparin mimics previously reported, including anionic polymers and small molecules [13,20,21,25,28,63], a sulfated tyrosine containing tetrapeptide reported by Maynard and Hubbell [42], which binds specifically to VEGF165, attracted our attention owing to its potential use in growth factor delivery and VEGF-mediated hydrogel assembly [68]. The use of sulfated tyrosine as the sulfated species in these molecules may offer advantages with respect to biological activity, as such post-translational modifications occur naturally [31]; indeed, it has been suggested that the electrostatic interaction mediated by sulfated tyrosine is one of the important factors in the antithrombotic action of heparin cofactor II (HCII) [24], the hirudin–thrombin interaction [2,41,57], and interaction between chemokine receptor CCR5 and HIV (human immunodeficiency virus) gp120 [17]. In addition, the ability to tailor sequences via solid-phase methods will facilitate identification of additional peptides of desired homogeneity for binding with specific heparin-binding partners. Taken together with our studies summarized above, these reports suggest that sulfated peptides of select and defined sequences could be useful in the production of materials with targeted mechanical properties and protein delivery profiles.
In this study, a series of sulfated peptides, including the previously reported sulfated tetrapeptide [42], was synthesized. The binding affinities between the sulfated peptides and heparin-binding peptides or VEGF165 were measured via affinity liquid chromatography (ALC) and surface plasmon resonance (SPR) techniques. The results were compared with the binding affinities of heparin. The importance of the amino acid sequence of the sulfated peptides in the binding event was indicated by the differences in affinities among the various binding pairs, despite the similarity of sequence and charge of the sulfated peptides. Isothermal titration calorimetry (ITC) experiments confirmed the dissociation constant of the SP-HBP pair of highest binding affinity. These results suggest the potential use of the SPs for the immobilization of multiple proteins with different affinities in biomaterials and delivery systems where heterogeneous heparin has been conventionally used.
2. Experimental
2.1. Materials
Fmoc-Tyr(OH)-OH, 2-(1H-benzotriazole-1-yl)-1,1,3,3-tetramethyluronium hexaflurophosphate (HBTU), 2-chlorotrityl chloride resin, Rink-amide MBHA resin and all other peptide synthesis reagents were purchased from Novabiochem® (Merck Biosciences AG, Switzerland), except for piperidine (Aldrich, Allentown, PA), 4-methylmorpholine (Aldrich, Milwaukee, WI), and N,N-dimethylformamide (DMF, Fisher Scientific Inc., Newark, DE). The sulfur trioxide-pyridine complex and N-hydroxysuccinimide (NHS) were purchased from Aldrich (Milwaukee, WI). Triisopropylsilane (TIS) and 1,1,1,3,3,3-hexafluoro-isopropanol (HFIP) were purchased from Aldrich (Allentown, PA). The tetrabutylammonium hydrogen sulfate was purchased from Sigma (St. Louis, MO). The 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride) (EDC) was purchased from Pierce (Rockford, IL). Other chemicals were purchased from Sigma–Aldrich unless otherwise noted. All chemicals were used as received. A pRSET-VEGF165 plasmid was a generous gift from Dr. Andreas H. Zisch (Department of Obstetrics and Gynecology, University Hospital Zurich, Switzerland). The recombinant VEGF165 protein was expressed and isolated from Escherichia coli according to the reported protocol [68,70,71], and purified via heparin–agarose affinity chromatography. All HPLC experiments were conducted on a Waters Delta 600 HPLC system (Waters Co., Milford, MA), with various choices of columns and eluent conditions. All nuclear magnetic resonance (NMR) spectra were acquired on a DRX400 (Bruker BioSpin Co., Billerica, MA) under standard quantitative conditions.
2.2. Synthesis and characterization of the sulfated peptides
Fmoc-Tyr(SO3·NnBu4)-OH was synthesized as previously described [62]. Fmoc-Try(OH)-OH was sulfated via treatment with sulfur trioxide–pyridine complex in DMF. The counterion of the sulfate was exchanged to tetrabutylammonium in order to improve the acid-stability [6,65] and the solubility of the amino acid. The degree of sulfation of the amino acid was determined via 1H NMR (400 MHz, in MeOD containing an internal reference TMS, 512 scans): Fmoc-Tyr(OH)-OH, 1H NMR (400 MHz, MeOD, 512 scans): δ = 6.69 (2H, tyrosyl meta, d), 7.04 (2H, tyrosyl ortho, d), 7.29 (2H, Fmoc, m), 7.39 (2H, Fmoc, t), 7.59 (2H, Fmoc, d), and 7.78 ppm (2H, Fmoc, d); Fmoc-Tyr(OSO3)-OH, 1H NMR (400 MHz, MeOD, 512 scans): δ = 7.20 (4H, tyrosyl aromatic, m), 7.31 (2H, Fmoc, m), 7.39 (2H, Fmoc, t), 7.59 (2H, Fmoc, d), and 7.78 ppm (2H, Fmoc, d). The purity of the sulfated tyrosine was confirmed via reverse phase HPLC on a Waters DeltaPak® C18 column. The eluent was subjected to a linear gradient from 95:5 to 30:70 of 0.1 M ammonium acetate aqueous/acetonitrile over 35 min at 5 ml/min, and the absorbance was observed at 214 nm.
The sulfated tyrosine containing peptides were synthesized via standard solid-phase peptide synthesis methods on the PS3™ automated peptide synthesizer (Protein Technologies Inc., Tucson, AZ), using 2-chlorotrityl chloride resin as a polymer support and HBTU as a coupling reagent. The synthesized peptides were cleaved from the resin by treating the resin with HFIP/DCM (1:4, v/v) at room temperature for 1 h [4,62]. The solution was precipitated in cold ether to obtain a white solid. The side-chain protecting groups of the peptides were removed in pre-cooled TFA/TIS/water mixture (95:2.5:2.5, v/v/v) in an ice bath for 1 h. The solution was precipitated into cold ether to give a white solid. The sulfated peptides were purified via anion-exchange chromatography on an ÄKTA™ explorer system equipped with a HiTrap DEAE FF 5 ml column (GE Healthcare Bio-Sciences Corp.: formerly Amersham Biosciences, Piscataway, NJ). The peptides were eluted with a linear gradient of sodium chloride concentration from 0 to 2 M in 5 mM sodium phosphate (pH 7.4), over 20 min at 5 ml/ min, and the absorbance was observed at 215 nm. Fractions with the highest affinity (requiring the highest concentration of salt for elution) were collected and lyophilized. The collected peptides were then purified in a final step via RP-HPLC with elution via a linear gradient from 100:0 to 30:70 of water/acetonitrile over 35 min; this was conducted to confirm the purity of the sulfated peptide and to remove the salts from the previous purification step. Obtained peptides were characterized via amino acid analysis (Molecular Analysis Facility, University of Iowa), and showed purities of greater than 95%. The degree of sulfation was confirmed via NMR; 1H NMR (400 MHz, D2O, 512 scans): δ = 7.20 ppm (8H, sulfated tyrosyl aromatic, m); the peptides showed essentially 100% sulfation after purification. Reliable mass spectral analysis of intact, sulfated peptides was impossible owing to the lability of the sulfate groups under the required experimental conditions.
2.3. Synthesis of heparin-binding peptides
Two of the three heparin binding peptides (HBP) investigated, i.e. ATIII (NH2-KAFAKLAARLYRKA-CONH2) [61,66] and HIP (NH2-RPKAKAKAKAKDQTK-CONH2) [36–39,66,67], were synthesized via a standard Fmoc solid-phase peptide techniques as previously described [8,66,67]. Each peptide was purified via preparative HPLC on a Waters Symmetry® C18 column, under a linear gradient from 95:5 to 5:95 of water/acetonitrile (containing 0.1% of trifluoroacetic acid) at 5 ml/min. The molecular weights of each peptide were verified via matrix-assisted laser desorption/ionisation–time of flight mass spectrometry (MALDI–TOFMS, OnmiFlex-NT, Bruker Daltonics Inc., Billerica, MA), m/z = 1608.33 [(M + H)+, calcd 1607.00] for the ATIII peptide, and m/z = 1667.44 [(M + H)+, calcd 1668.02] for the HIP peptide. The purities of peptides were re-confirmed via HPLC. The ATIII was 99% pure as determined via separation on Waters Analytical Symmetry® C18 column under a linear gradient of 95:5 to 5:95 of water/acetonitrile (containing 0.1% trifluoroacetic acid) over 50 min. HIP was 99% pure as determined via separation on a Waters Symmetry® C18 column under a linear gradient from 100:0 to 0:100 of water/acetonitrile (containing 0.1% trifluoroacetic acid) over 50 min. The PF4ZIP peptide (NH2-RMKQLEDKVKKLLKKNYHLENEVARLKKLVG-CONH2) [5,69] was a generous gift from Le Zhang and was of similar purity.
2.4. Affinity liquid chromatography
A heparin-mimetic column was produced via immobilization of the sulfated peptides on a solid support. The sulfated peptides were immobilized on an NHS-preactivated cross-linked agarose column (GE Healthcare Bio-Sciences Corp.: formerly Amersham Biosciences, Piscataway, NJ) via amine coupling of the single amine at the N-terminus of the peptide. The sulfated peptides were dissolved in the binding buffer at a concentration ca. 15 mg/1 ml (0.2 M NaHCO3, 0.5 M NaCl, pH 8.3), and then mixed with the NHS-activated resin. The reaction was conducted for 40 min at room temperature with constant exposure to peptide solution. Any excess active groups were deactivated and any non-specifically bound ligands were removed by washing the columns with 0.5 M ethanolamine/0.5 M NaCl (pH 10) followed by 0.1 M sodium acetate/0.5 M NaCl (pH 6.5), with a total of five wash cycles. The modified columns were used in binding assays on the ÄKTA™ Explorer system (GE Healthcare Bio-Sciences Corp.: formerly Amersham Bio-sciences, Piscataway, NJ).
The heparin-binding peptides or VEGF165 were injected onto each column and unbound molecules were removed by washing the column with 5 mM sodium phosphate buffer (pH 7.4) over five column volumes. The elution of peptides was detected via UV, at an absorbance of 215 nm. The bound peptides were eluted with a linear gradient of sodium chloride at concentrations ranging from 0 to 2 M in 5 mM sodium phosphate solution (pH 7.4). The relative binding affinities between each sulfated peptide and heparin-binding peptide/protein are estimated from the concentration of NaCl (M) required to elute the heparin-binding peptide from the column. The experiments were done in at least triplicate and the average NaCl concentrations reported. The binding affinity between heparin and VEGF165 was measured on a heparin sepharose column (HiTrap Heparin HP, 1 ml). The VEGF165 was injected and eluted under a linear gradient of 0–1 M NaCl in 5 ml sodium phosphate (pH 7.4) at 1 ml/min for 20 min, with UV detection at 215 nm.
Control experiments to probe the role of non-specific electrostatic interactions in the binding of the HBPs to the modified columns were conducted on non-peptide functionalized columns equipped with carboxylate groups. The NHS-preactivated agarose column was hydrolyzed to give free carboxyl end-groups by treating with 20 column-volumes of water, 20 column-volumes of 5 mM sodium phosphate solution (pH 7.4), and then 20 column-volumes of 2 M sodium chloride solution (in 5 mM sodium phosphate, pH 7.4). The HBPs and VEGF165 were loaded and eluted from this column via the same protocols as described above.
2.5. Surface plasmon resonance
The association and dissociation between sulfated peptides and HBPs were monitored via SPR, in which the refractive index change and resulting surface plasmon angle change, due to binding at a modified gold surface, are measured. Corresponding dissociation constants (KD) were obtained via non-linear regression fitting to a Langmuir binding model, as described in a previous report [66,69]. All experiments were conducted on the Biacore® 3000 with running buffer HBS-EP (Biacore Inc., Piscataway, NJ). The sulfated peptides were immobilized on a carboxymethylated dextran CM5 sensor chip (Biacore Inc., Piscataway, NJ) through an amine coupling reaction. The surface of the sensor chip was activated by injecting a mixture of 0.15 M NHS and 0.6 M EDC for 2 min at 5 μl/min. The prepared sulfated peptide solution (ca.12mg/ 0.1 ml) in HBS-EP running buffer (100 mM HEPES, 150 mM NaCl, 3 mM EDTA, pH 7.4 containing 0.005% (v/v) P-20 surfactant) was then injected for 10 min at 5 μl/min. The unreacted NHS groups were inactivated via injection of 1 M ethanolamine. Permanent increases in the resonance units of the chips confirmed immobilization (122.7 RU for SPa, 58.4 RU for SPb, and 45.4 RU for SPc). A reference flow cell was treated with ethanolamine as a ligand instead of sulfated peptide. For binding studies, a series of concentrations of each heparin-binding peptide (0, 5, 10, 25, 50, 100, and 250 μM in HBS-EP buffer) or of VEGF (0, 0.85, 1.7, 3.4, 6.79, 13.6, and 27.2 μM in HBS-EP buffer) were injected over the sulfated peptide-modified chips. After a 15 min dissociation phase, the sensor chip surface was regenerated for the next peptide sample injection, via treatment with two 1 min pulse injections of regenerating buffer (2 M NaCl in HBS-EP, pH 7.4) at 100 μl/min. The resonance units of the baseline returned to the initial value after the regeneration step, confirming the removal of all bound analytes. The SPR measurement of the binding affinities of HBPs to a heparin-modified sensor chip has been described in a previous report [66,69].
2.6. Isothermal titration calorimetry
All ITC experiments were conducted on a MicroCal VP-ITC calorimeter (MicroCal, LLC, Northampton, MA). A solution of the PF4ZIP peptide (0.2 mM in 5 mM sodium phosphate buffer, pH 7.4, 1.34 ml) was placed in the sample cell. After baseline stabilization, a total of 125 aliquots of 10 μl SPa solution (2.146 mM in 5 mM sodium phosphate buffer, pH 7.4) were injected, with a time interval of 600 s and stirring at 260 rpm. The reference cell power and temperature were set at 10 μcal/s and 25 °C, respectively. Control experiments were conducted to measure the heats of dilution, via titration of buffer solution into buffer solution, buffer solution into PF4ZIP solution, and SPa solution into buffer. The data from these control experiments were subtracted from the appropriate sets of data prior to the analysis. The raw data was fit to various binding models using Origin® 7.0 software; the best fit was assessed on the basis of a minimized χ2 value. The thermodynamic parameters were obtained from the standard Gibbs free energy equation, ΔG = ΔH TΔS = –RT ln Ka as described elsewhere [11,12], to determine KD values and the number of binding sites.
3. Results
3.1. Sequences and synthesis of sulfated peptides
Despite the utility of heparinized polymers in the production of hydrogels in our previous reports [66–69], variation in chemical modification and hydrogel mechanical properties resulted from the intrinsic heterogeneity of the heparin employed. The goal of this study was to investigate the potential feasibility, through assessments of binding affinities, of employing heparin mimetic sulfated peptides in the non-covalent assembly of hydrogels via interaction with heparin-binding peptides. The sequences of studied sulfated peptides are shown in Table 1, with the regions that mediate binding indicated in bold. The SPa peptide was chosen as a first target given its previous identification, via a combinatorial library method, as a binding partner for VEGF165 [42]. The terminal glycine residues were added to improve accessibility to the peptide in solid-phase binding assays. In these sequences, serine, aspartic acid, and sulfated tyrosine are chosen as mimics of the hydroxyl, carboxyl, and sulfate functional groups in heparin, as reported by Maynard and Hubbell [42]; these functional groups are purported to be the most important in the binding activities of the glycosaminoglycan.
Table 1.
Sequences of sulfated peptides
| Peptides | Sequencesa | MWb |
|---|---|---|
| SPac | NH2-GGGG-SYSO3DYSO3 GGGG-OH | 1163.0 |
| SPb | NH2-GGGG-SYSO3DYSO3 SYSO3DYSO3 G-OH | 1680.3 |
| SPc | NH2-GGGG-YSO3YSO3GGYSO3DYSO3 G-OH | 1505.4 |
The sequences in bold font are the purported active binding sites.
The molecular weights are calculated values. Experimental estimates of mass were impossible due to substantial desulfation of the peptides under all mass spectra conditions investigated.
The sequence in bold was previously reported to bind toVEGF165 [42].
For comparison, SPb was synthesized to investigate the effect of the size of the peptide containing the same pattern of functional groups as SPa. It has been reported that the minimum required size of heparin for binding to ATIII and HIP is a pentasaccharide, while four tetrasaccharides are required for high affinity binding to PF4. The heparin disaccharide has on average one to two O-sulfates, which suggests an average minimum of two to four O-sulfates to mediate binding to the peptides. Therefore, sulfated peptides of different sizes (and therefore different numbers of O-sulfates) might show modulated binding affinities to the peptides. The sequence of SPc was chosen to probe the effect of variation in the positioning and number of the sulfate groups in binding to these different targets. The fact that SPc might have some binding affinity for heparin-binding proteins and peptides was suggested from the screening of a combinatorial library (Maynard, personal communication). Studies were therefore conducted to determine if this sequence had any increased affinity for the heparin-binding peptides of interest in hydrogel studies.
The synthesis of sulfated Tyr-containing peptides has not been widely employed due to the intrinsic acid-lability of O-sulfates during synthesis. In these studies, sulfated, Fmoc-protected Tyr was first synthesized via previously reported protocols [62] and employed in standard Fmoc-based solid-phase peptide synthesis strategies employing the highly acid-labile, 2-chlorotrityl chloride resin, which permitted cleavage of the peptide from the resin with mild acid treatment and minimal desulfation. The cleavage was conducted under mild acidic conditions, and the side chains were deprotected in cold TFA solution (0 °C) in order to minimize desulfation. A two-step purification via anionic-exchange chromatography followed by RP-HPLC resulted in a highly pure and homogeneous peptide. The final degree of sulfation of the peptides was confirmed via 1H NMR. Fig. 1 shows the comparison of 1H NMR spectra of non-sulfated Fmoc-Tyr-OH, sulfated Fmoc-Tyr(SO3)-OH and the sulfated SPa peptide. The aromatic ortho and meta proton shifts of the non-sulfated Fmoc-Tyr-OH were distinctly separated at 7.04 and 6.69 ppm, respectively (Fig. 1a). After sulfation, the ortho and meta protons shift and overlap as a multiplet near 7.20 ppm (Fig. 1b). The degree of sulfation of the peptide can therefore be estimated from the integration of the aromatic protons of the sulfated portion and comparison to the total number of aromatic protons. As shown in the figure, the spectrum of SPa (Fig. 1c) shows a high degree of sulfation with a negligible extent of desulfation. High degrees of sulfation of SPb and SPc were confirmed in the same manner. The data for all three peptides reproducibly indicated essentially 100% sulfation for the peptides produced and purified via these protocols (supporting information).
Fig. 1.
The 1H NMR (400 MHz) spectra of non-sulfated and sulfated tyrosines, and the sulfated tyrosine-containing peptide. (a) Fmoc-Tyr(OH)-OH: δ = 6.69 (2H, tyrosyl meta, d), 7.04 (2H, tyrosyl ortho, d). (b) Fmoc-Tyr(OSO3)-OH: δ = 7.20 (4H, sulfated tyrosyl aromatic, m). (c) SPa peptide: δ = 7.20 ppm (8H, sulfated tyrosyl aromatic, m).
3.2. Binding studies via affinity liquid chromatography
Binding affinities between sulfated peptides and select heparin binding peptides, as well as VEGF165, were measured via ALC. In our studies designed to probe the binding of these HBPs to the sulfated peptides, the sulfated peptides were immobilized on an NHS-preactivated column. The unbound sulfated peptide molecules were removed and the non-reacted NHS groups were deactivated by washing the column with ethanolamine solution and sodium acetate buffers alternately, as described in Section 2. Heparin-binding peptides (or VEGF165) were then applied and allowed to bind to the sulfated column. The bound HBPs (or VEGF165) were eluted under a linear concentration gradient of NaCl in a phosphate buffer.
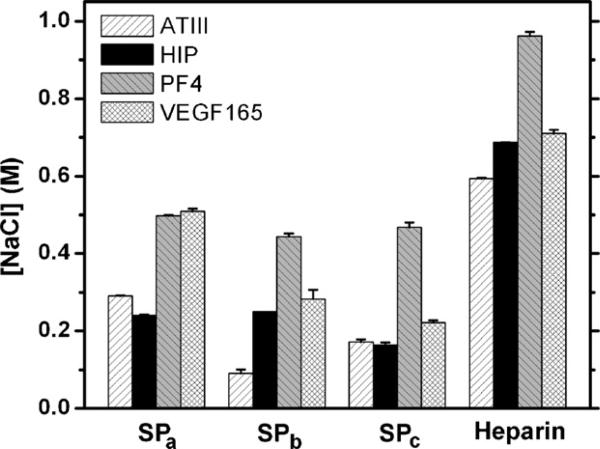
The concentration of NaCl required to elute bound HBPs or VEGF165 was measured; the results are shown in Fig. 2. The SPa-modified column required 0.290 ± 0.002, 0.240 ± 0.002, 0.498 ± 0.002, and 0.509 ± 0.007 M NaCl for the elution ATIII, HIP, PF4ZIP, and VEGF165, respectively. The SPb-modified column required 0.090 ± 0.011, 0.25 ± 0, 0.444 ± 0.008, and 0.283 ± 0.024 M for the elution of ATIII, HIP, PF4ZIP, and VEGF165, while the SPc-modified column required 0.172 ± 0.006, 0.163 ± 0.007, 0.468 ± 0.012, and 0.222 ± 0.006 M of NaCl. The NaCl concentrations required to elute the peptides or VEGF165 from an unmodified column were also measured; the hydrolyzed column carries carboxylic acid moieties that can interact with the positively charged peptides and protein via electrostatic interactions. The results of these control experiments indicated that only 0.156 ± 0.001, 0.136 ± 0.003, 0.082 ± 0.007, and 0.148 ± 0.011 M NaCl was required for elution of the ATIII, HIP, PF4ZIP, and VEGF, confirming that while some non-specific electrostatic interaction may occur during the ALC measurement, this electrostatic attraction is not the primary source of the measured binding affinity of the HBPs and VEGF for the SP-modified columns.
Fig. 2.
The affinity chromatography of sulfated peptides/heparin and heparin-binding peptides/VEGF165. The NaCl concentration required to elute the bound analytes from sulfated peptidyl or heparin columns was recorded as an indicator of binding affinity. The error bars were derived from standard deviations of the average of three or more samples, except for VEGF165. The chromatography data of heparin column and ATIII, HIP, and PF4 were taken from previous reports [66,69].
3.3. Binding studies via surface plasmon resonance
The binding kinetics and affinities between sulfated peptides and heparin binding peptides/VEGF165 were also assessed via surface plasmon resonance (SPR) [23,52]. In our study, each sulfated peptide was directly immobilized on the sensor chip via amine coupling, and changes in the refractive index accompanied by the binding of analytes (i.e. HBPs and VEGF165) were recorded. Prior to the concentration measurement, appropriate control experiments were conducted to confirm that there were no mass transfer limitations at the flow rates used in this study and that there was no non-specific binding during injection of analyte. The binding between the surface of the reference cell and analytes, which was insignificant compared to the binding of the analytes to the sulfated peptide-modified surface, was subtracted from the data in order to exclude the contribution of any non-specific electrostatic interactions.
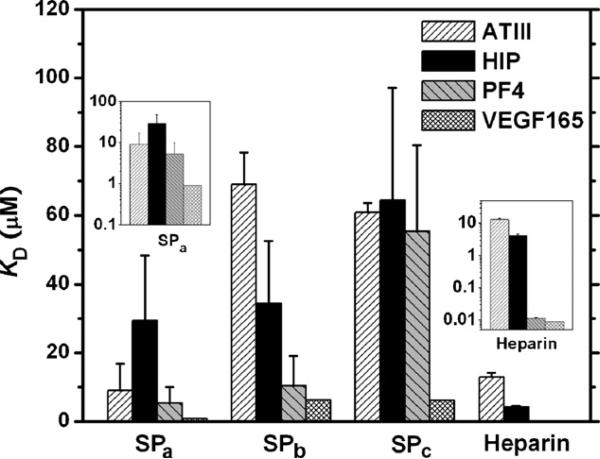
A series of binding sensorgrams was obtained upon injection of various concentrations of HBPs to each sulfated-peptide-modified chip. The equilibrium dissociation constants, KD, were estimated via a non-linear regression fitting to a Langmuir binding model, as previously described [69]; the results are shown graphically in Fig. 3. The SPa showed KD values of 9.09 ± 7.71, 29.4 ± 19.0, 5.27 ± 4.72 and 0.907 ± 0.118 μM for ATIII, HIP, PF4ZIP, and VEGF165, respectively. The SPb showed KD values of 69.0 ± 9.4, 34.4 ± 18.3, 10.4 ± 8.72, 6.29 ± 1.32 μM for ATIII, HIP, PF4ZIP, and VEGF165, while the SPc showed KD values of 60.8 ± 2.8, 64.4 ± 32.7, 55.3 ± 25.2, and 6.10 ± 1.10 μM. The kon and koff values for these measurements are given in supporting information.
Fig. 3.
The measured dissociation constants of sulfated peptides/heparin and their binding partners measured via SPR. A series of concentrations of ATIII, HIP, PF4, and VEGF165 analytes were injected to the sulfated peptides/heparin immobilized sensor chip. The binding responses with various concentrations of analytes were fit to a Langmuir model in order to give the binding constants. The insets show the KD of SPa and heparin in log-scale. The error bars are derived from the standard deviations of multiple samples. The data of heparin binding with ATIII, HIP, and PF4 were taken from previous reports [66,69].
3.4. Isothermal titration calorimetry
The SPa and PF4ZIP interaction was shown to be of high affinity in both ALC and SPR experiments. Therefore, these binding partners would be appropriate first choices in the generation of homogeneous assembling polymers, as described in Scheme 1, and would provide useful comparisons to previously reported hydrogels assembled via interactions of LMWH and PF4ZIP, mainly in assessing the role of homogeneity in hydrogel assembly and properties. Therefore, ITC experiments were carried out in order to characterize the binding of SPa and PF4ZIP in greater detail, and under the solution conditions in which assembly would be conducted. The ITC experiment provides a direct measurement of the heat associated with binding of two molecules in solution, and provides assessments of both the enthalpic and entropic contributions, as well as the stoichiometry, of the binding event [11,12].
In this study, the PF4ZIP solution was titrated with SPa solution and the evolved heat was detected. Heats of dilutions of PF4ZIP solution and SPa solution were subtracted from the data prior to analysis. The reverse titration data, i.e. PF4ZIP titration into SPa solution, suggested that the PF4ZIP peptide exists in different association states at the different concentrations in the ITC experiment, consistent with its expected behavior (data not shown). Therefore, when small aliquots of high concentration of PF4ZIP peptide were titrated into the SPa solution, the heat of dissociation of the PF4ZIP oligomer dominated the overall enthalpy change. Thus, the experiments were conducted in the order described for determination of the thermodynamic parameters in the binding event.
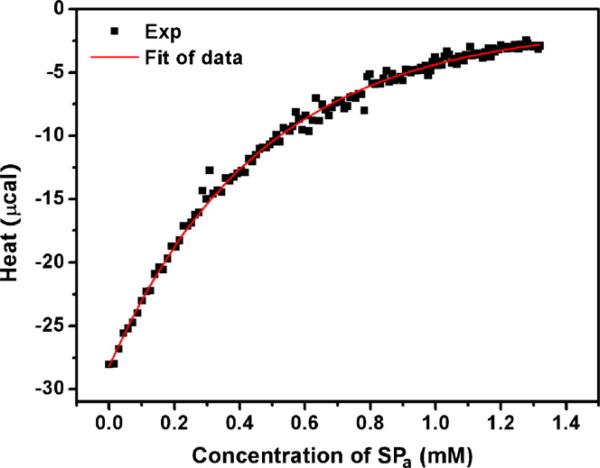
The molar enthalpy of binding, after subtracting the heats of dilution, is shown in Fig. 4. The fit to the data, obtained with a sequential three-site binding model (chosen on the basis of minimal χ2 values) is shown in Fig. 4 as a solid line. The KD1 = 8.09 μM, ΔH1 = –1320 cal/mol, and ΔS1 = 18.9 cal/mol-K; KD2 = 113 μM, ΔH2 = –1339 cal/mol, and ΔS2 = 13.6 cal/mol-K; KD3 = 5515 μM, ΔH3 = –20690 cal/mol, and ΔS3 = –59.0 cal/mol-K, indicating that the first and second binding events are tight and the third sequential binding event is of very low affinity.
Fig. 4.
Binding isotherm of SPa interacting with PF4ZIP. The total heat of each injection was integrated and plotted (■). The solid line was the fit of the experimental data which was used to calculate KD, ΔH, and ΔS.
4. Discussion
4.1. Choice of peptide sequences
Delivery of growth factors and other proteins, utilizing the interaction between polysaccharides (i.e. heparin) and proteins/polypeptides has recently emerged in the field of drug delivery and tissue engineering [14,29,33,34,50,53,54,64,66–69]. Previously we have reported assembly of non-covalently cross-linked hydrogel systems, via interactions between heparin and heparin-binding peptides, for the delivery of growth factors [66–69]. The intrinsic heterogeneity of heparin, however, resulted in the variations in chemical modification and mechanical properties in such systems. In this study, the feasibility of employment of heparin mimetic sulfated peptides in these types of hydrogels was therefore investigated.
The heparin-binding peptides investigated here were selected because of their successful implementation, via interaction with heparin, in the formation of the previously reported non-covalently assembled networks. Their similar binding to sulfated peptides would permit alternative modes of assembly, and perhaps increased elastic moduli of the resulting materials, owing to increased homogeneity and increased number of binding sites within the network. The ATIII peptide employed in these studies contains the high-affinity heparin-binding domain from the plasma glycoprotein antithrombin III; binding of ATIII with heparin is essential for the anticoagulative properties of ATIII. The HIP peptide is derived from the heparin interacting protein (HIP) [36–39], which has been employed because of HIPs heparin affinity and because HIP and bFGF are reported to have distinct binding sites on heparin; a hydrogel produced via the interactions of HIP and heparin would therefore retain binding sites for bFGF. The PF4ZIP peptide has also been incorporated in growth factor delivery networks in our previous investigations [69], owing to its higher heparin-binding affinity than that of the ATIII and HIP peptides. It is designed to mimic two pairs of lysine residues in the heparin-binding domain of human platelet factor 4, via the use of a GCN4-based coiled-coil motif [5].
The three sulfated peptides studied in this report contain YSO3-X-YSO3 sequences that had been previously indicated as a common binding motif in the sulfated peptides screened by Maynard and Hubbell [42]; peptides in which the X residue was aspartic acid (D) were indicated to show greatest affinities and this sequence was therefore employed in the three peptides here. The differences in the general trend of affinities observed in our studies, despite the presence of the YSO3-D-YSO3 in all three peptides, may result from differences in functional group presentation, as well as from conformational entropy considerations upon binding. In the fully extended structures (trans conformation of peptide bonds) expected for these short peptides, the sulfated residues at the n and n + 2 positions are presented on one face of the chain, which likely improves their accessibility to the positively charged heparin-binding domains and may also mediate their presentation at distances that approximate and better mimic the presentation of sulfate groups on heparin [44]. These considerations may account for the previously observed frequency of this sequence in sulfated peptides of higher affinity for VEGF165.
4.2. Comparisons of affinities between SPs and HBPs
The interactions between the SPs and HBPs were characterized via ALC and SPR. A high KD value from SPR experiments corresponds to low binding affinity, which is indicated by the low concentration of salt required for elution in ALC. The results show the same general trends in affinity in both ALC and SPR, although the KD determined for the binding between ATIII and SPa appears unusually low. In the binding of SPb and SPc, ATIII, HIP, and PF4ZIP showed the same trend in ALC and SPR. In addition, the HBPs generally show similar trends in affinity to the sulfated peptides as the trends in their affinities toward heparin (previously reported [66,69] and included in Figs. 2 and 3). However, the VEGF binding affinity was not consistent between ALC and SPR measurements. The VEGF had the highest affinity among four analytes in SPR while PF4ZIP had the highest in ALC. This discrepancy between SPR and ALC may be due to the fact that the PF4 is known to bind to an oligosaccharide of 16 saccharides while VEGF is thought to bind to a pentasaccharide sequence. Thus, the measured affinity of PF4ZIP may be lower in SPR measurements because of a lower peptide surface density in the SPR experiment versus the ALC experiment. The overall slight differences in binding trends between the SPR and ALC data may also result from the differences in the experimental assessments of binding affinity by the two experiments, as the SPR measures the kinetics of the association and dissociation events versus the bulk assessment of equilibrium binding of the ALC experiment. Slight differences in the sulfated surfaces in the two sets of measurements may also contribute to differences in measured binding.
The kinetics data from the SPR experiment are provided in supporting information. Although there was no single overriding trend, the data suggested that measured changes in affinity resulted mainly from changes in kon, although changes in koff also contributed in select cases. The concentration of immobilized peptides on the surface may affect the kon and koff. In our study, the surface concentrations of peptides were within the range of recommended immobilization values; the permanent increases in RU after immobilization of each SP ligand were 108.0 for SPa, 58.4 for SPb, and 45.4 for SPc, which correspond to 108, 58.4, and 45.4 pg/mm2, respectively. The measured koff values for analyte binding to all peptide surfaces were similar, suggesting that variations in surface concentration in this range do not substantially alter dissociation. The koff values for VEGF were only two to five times slower than those of monomeric analytes, further suggesting a minor role, if any, for bivalent binding of the VEGF under these conditions. The kon values for analyte binding to SPb and SPc were not higher than those for binding to SPa, which suggests that excess carboxylates present on the chip surface do not cause significant non-specific binding.
Among the three sulfated peptides, the SPa peptide showed, in general, the highest affinities toward the HBPs, despite the fact that it is the shortest peptide with the fewest sulfated residues. In comparisons of the three SP peptides, the fewer number of sulfated residues in the SPa (two) may reduce steric crowding of these important residues versus that experienced by the sulfated residues in the SPb and SPc peptides (four and three, respectively). The smaller size of the SPa may also result in a lower loss in conformational entropy upon binding and contribute to its improved binding relative to the other SPs. The carboxylate and hydroxyl groups in the SPs should also contribute to the binding event, given the known importance of these functional groups in modulating binding by heparin via electrostatic and hydrogen bonding interactions [7,15,18,48,56]; indeed, the reduced affinity of SPc (particularly as indicated via SPR) toward the HBPs may in part result from its fewer carboxylate and hydroxyl side chains relative to SPa and SPb, as well as from the presentation of only three of the four sulfate groups on the peptide.
Another observation from these investigations is that the PF4ZIP peptide had the highest binding affinity of the HBPs toward all sulfated peptides. This result is consistent with results from the heparin affinity chromatography and SPR experiments in our previous reports (included in Figs. 2 and 3) [66,69], and may result from differences in the presentation of positive charges on these HBPs. In general, the ATIII and HIP peptides show similar affinities for the SPs (although this is not perfectly consistent in the data), which may be expected given their similar size and charge. It has been suggested that the heparin-binding domains in ATIII and in HIP adopt a helical conformation that arranges the positive sidechains along the face of the helix for optimized heparin binding [19,39,60,61]. However, the sulfated peptide molecules in our study do not drive such a conformational change (CD data not shown), and therefore the binding of the short HBPs to the SPs may be reduced relative to that of PF4ZIP owing to the suboptimal presentation of the basic residues. In contrast, the PF4ZIP peptide exists as coiled-coil helical dimer under our experimental conditions (concentrations greater than 3.5 μM) [69]. SPR experiments with PF4ZIP as the analyte were done in the concentration range of 5–200 μM, as below 5 μM, the PF4ZIP did not bind measurably to the chip surface, suggesting that the monomeric form of the PF4ZIP does not have significant affinity for the SP peptides. In the dimer, the basic residues are accessible, having been positioned to mimic the presentation of lysines in human platelet factor 4 [5]. The accessibility of the lysines for binding between the SPs and the helical PF4ZIP may therefore minimize any entropic costs of reorganization [40], resulting in a more favorable free energy of binding. Additionally, the HBPs generally have lower affinity for the SPs than they do for heparin, which likely results from a combination of the lack of conformational change for optimized presentation of basic residues (for ATIII and HIP) and from the smaller size of the SPs than the reported binding regions in heparin for ATIII, HIP, and PF4 [26,39,69].
Interestingly, the ITC studies of the binding of PF4ZIP with SPa suggested the presence of two binding events, which was not indicated in the SPR data. The ITC experiment was done at a PF4ZIP concentration of 0.2 mM; this greater concentration was necessary to generate a reliable binding isotherm. This concentration of PF4ZIP was sufficiently high that the coiled-coil peptide exists as a dimer [69]; four lysine sidechains, which play an important role in heparin binding, are displayed on the two chains [5]. Given that PF4 is reported to bind to an oligosaccharide unit in heparin that contains 16–20 saccharides [26], it is possible that the PF4ZIP heparin-binding domain is sufficiently large to accommodate more than one of the short SPa, and the two pairs of Lys may therefore provide two binding sites for SPa peptides on the PF4ZIP peptide. The appropriateness of these determined values is suggested by the fact that the calculated first dissociation constant, KD1, was in the same order of magnitude as the KD value from the SPR experiment (5.27 μM), suggesting that the SPR measurement captures the highest affinity binding event. The lack of a measured KD2 in the SPR experiments most likely results from the fact that the SPR experiments were conducted with the monovalent SPa on the chip surface, thus only the first binding event may be detected.
4.3. Comparisons of affinities between SPs and VEGF
The trends in the binding of SPs to VEGF, as determined via SPR, also followed the ALC results and are consistent with the previously reported SPR results for the VEGF–SPa interaction. The KD of SPa and VEGF165 (0.907 μM) measured here is in good agreement with the KD value (3.1 μM) reported by Maynard and Hubbell for binding of the peptide SYSO3DYSO3G with VEGF165 [42], as well as with the estimated KD for the binding of heparin and VEGF165 (5.5 μM) based on affinity chromatography [16,32,58]. The slight difference in the values may be caused by differences in the experimental conditions or by the impact of the longer glycine linker in the peptide here. In the previously reported experiments, biotinylated VEGF165 was immobilized on an SPR chip through non-covalent interaction, and the sulfated peptide was injected as an analyte, in contrast to our experiments in which the VEGF was injected as an analyte across a sulfated peptide-modified surface.
Similar structural considerations as those mentioned above may play a role in the higher affinity of the SPa for VEGF165, relative to those measured for the other sulfated peptides. The SPa has a sufficient number of amino acids to span the shortest positively charged region of the heparin-binding domain in VEGF165 [42], and its smaller size may allow unhindered approach to the binding domain in VEGF165, in comparison to the SPb or SPc which may be hindered due to unfavorable repulsive interactions near the heparin-binding site in VEGF. For example, Fairbrother et al. have suggested that the heparin-binding domain of VEGF is likely to include Arg13, Arg14, Lys15, Lys30, Arg35, Arg39, and Arg46, with potential contributions from Lys52, Arg54, Arg55 (supporting information). If the SPs also bind at this domain (in the region of highest positive charge density which includes Arg13, Arg14, Arg46 and Arg49), the improvement in binding affinity of the smaller SPa relative to the longer peptides, SPb and SPc, may result from unfavorable enthalpic interactions between the SPb and SPc and the neighboring Glu12 (repulsive interaction) and His30 (steric interference). These interactions may not have a large impact on the binding of heparin owing to the greater number of energetically favorable contacts between heparin and the entire heparin-binding domain in VEGF. While detailed experiments to confirm the preceding speculations are outside the scope of this initial report, the data nevertheless indicate that distinct structural differences in the SPs result in measurable differences in binding affinities for both VEGF165 and HBPs.
4.4. Summary
Despite the slight differences in the exact trends in the ALC and SPR experiments, in general, the PF4ZIP shows the highest affinity toward sulfated peptides, and the SPa shows the strongest affinity to the HBPs. These data for the sulfated peptides provide additional support for Maynard and Hubbell's suggestion that the YSO3-X-YSO3 motif, where X is an arbitrary amino acid, is important in the binding to VEGF165. Although the SPb and SPc carry the same YSO3-X-YSO3 motif and double the number of sulfate groups, their lower affinity to all HBPs and VEGF may result from steric interference, unfavorable repulsion, or unfavorable entropic considerations due to their longer lengths. Incorporation of the SPa as a surrogate for heterogeneous heparin in hydrogels is therefore promising for improving hydrogel homogeneity and protein distribution in the gel. In addition, preliminary data (not shown) indicate that the SPa does not have any significant anticoagulant effects, while retaining its heparin-like binding to growth factors. Thus the application of SPa in biomaterials applications would offer the advantages of its small size, good binding to multiple partners, and a low potential for unwanted anticoagulative effects.
The useful binding affinities demonstrated by the SPs toward multiple heparin-binding partners are consistent with the fact that short sequences in heparin are sufficient to bind ATIII and HIP [39,69] and suggest the general utility of SPa and potentially other similarly short sulfated peptides. Interestingly, the SPb peptide is suggested to show the best discrimination in the relative binding between the various HBPs and VEGF165, with the same trends as those for the binding of these HBPs to heparin. Although the exact origins of the differences in binding between these molecules are not yet known, these data illustrate that short sulfated peptides may be capable of showing differential binding to multiple binding partners. While it is true that none of the SPs showed binding to HBPs as strong as those between the HBPs and heparin, affinities are sufficiently high to anticipate successful use of these peptides in materials assembly. The affinities observed for SPb and SPc, although not higher than that of SPa, may also have utility in controlled delivery of proteins, and sulfated peptides of higher affinity could be realized via an expanded set of combinatorial libraries and screening to provide information about useful motifs in peptide design. Specifically, owing to the general utility of solid-phase peptide synthesis and combinatorial library methods, prospects appear promising for the identification of multiple sulfated peptide sequences with high-affinity and selective binding to specific growth factors, which will permit manipulation of individual delivery rates.
5. Conclusions
In the present work we have investigated the binding affinities between three sulfated peptides, including the previously reported VEGF165-binding peptide SPa, and heparin-binding peptides. The shortest peptide, SPa (SYSO3DYSO3), had the highest binding affinities to heparin-binding peptides and VEGF165 in spite of its lowest number of sulfate groups per molecule. Of the heparin-binding peptides, the PF4ZIP peptide had highest binding affinities to all sulfated peptides, which was consistent with its highest heparin binding affinity. The SPa and PF4ZIP are predicted to successfully mediate hydrogel formation between four-arm star PEGs modified with the peptides, as the peptides exhibit a similar binding affinity as that reported for binding partners that we have shown support hydrogel formation (e.g., LWMH with HIP or ATIII). A four-arm star PEG-SPa should also form hydrogels in presence of dimeric VEGF165 and perhaps other dimeric, heparin-binding growth factors. Owing to the increased homogeneity of the SPa relative to the structurally and chemically heterogeneous heparin or LMWH, it is expected that hydrogels formed will be more homogeneous with improved mechanical properties that can be more easily attributed to the SPa and PF4ZIP or VEGF165 interaction. The SPs studied all showed interactions of various affinities to the different HBPs, indicating their potential utility in binding multiple partners. The identification of multiple sulfated peptides with distinct binding affinities for select growth factors is also suggested as a potential and promising route in the design of hydrogels.
Supplementary Material
Acknowledgments
This work was financially supported by the National Institutes of Health (1 RO1 EB003172-01) and the Arnold and Mabel Beckman Foundation. The project described was also supported in part by grant numbers 1-P20-RR17716-01 and 1-P20-RR015588 (both for instrument facilities) from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH). We are very grateful to Prof. Heather D. Maynard (Department of Chemistry & Biochemistry, University of California, Los Angeles) for very helpful discussions on the synthesis and characterization of sulfated peptides and Prof. Christopher J. Roberts (Department of Chemical Engineering, University of Delaware) for advice on the ITC experiments. Le Zhang (Department of Materials Science and Engineering, University of Delaware) is thanked for providing the PF4ZIP peptide and the data regarding binding affinities of heparin to various targets. We also thank Dr. Andreas H. Zisch (Department of Obstetrics and Gynecology, University Hospital Zurich, Switzerland) for providing the pRSET-VEGF165 plasmid.
Footnotes
Publisher's Disclaimer: This article was published in an Elsevier journal. The attached copy is furnished to the author for non-commercial research and education use, including for instruction at the author's institution, sharing with colleagues and providing to institution administration. Other uses, including reproduction and distribution, or selling or licensing copies, or posting to personal, institutional or third party websites are prohibited. In most cases authors are permitted to post their version of the article (e.g. in Word or Tex form) to their personal website or institutional repository. Authors requiring further information regarding Elsevier's archiving and manuscript policies are encouraged to visit: http://www.elsevier.com/copyright
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.peptides. 2007.08.010.
REFERENCES
- 1.Baeg G, Lin X, Khare N, Baumgartner S, Perrimon N. Heparan sulfate proteoglycans are critical for the organization of the extracellular distribution of Wingless. Development. 2001;128:87–94. doi: 10.1242/dev.128.1.87. [DOI] [PubMed] [Google Scholar]
- 2.Bagdy D, Barabas E, Gráf L, Petersen TE, Magnusson S. Hirudin. Methods Enzymol. 1976;45:669–78. doi: 10.1016/s0076-6879(76)45057-7. [DOI] [PubMed] [Google Scholar]
- 3.Bernfield M, Gotte M, Park PW, Reizes O, Fitzgerald ML, Lincecum J, et al. Functions of cell surface-heparan sulfate proteoglycans. Annu Rev Biochem. 1999;68:729–77. doi: 10.1146/annurev.biochem.68.1.729. [DOI] [PubMed] [Google Scholar]
- 4.Bollhagen R, Schmiedberger M, Barlos K, Grell E. A new reagent for the cleavage of fully protected peptides synthesized on 2-chlorotrityl chloride resin. J Chem Soc Chem Commun. 1994;22:2559–60. [Google Scholar]
- 5.Butcher DJ, Kowalska MA, Li S, Luo Z, Shan S, Lu Z, et al. A natural motif approach to protein design: a synthetic leucine zipper peptide mimics the biological function of the platelet factor 4 protein. FEBS Lett. 1997;409:183–7. doi: 10.1016/s0014-5793(97)00504-8. [DOI] [PubMed] [Google Scholar]
- 6.Campos SV, Miranda LP, Meldal M. Preparation of novel O-sulfated amino acid building blocks with improved acid stability for Fmoc-based solid-phase peptide synthesis. J Chem Soc Perkin Trans. 2002;5:682–6. [Google Scholar]
- 7.Capila I, Linhardt RJ. Heparin–protein interactions. Angew Chem Int Ed. 2002;41:390–412. doi: 10.1002/1521-3773(20020201)41:3<390::aid-anie390>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 8.Chan WC, White PD. Fmoc solid phase peptide synthesis: a practical approach. 1 ed. Oxford University Press Inc.; New York: 2000. [Google Scholar]
- 9.Chen J, Jo S, Park K. Polysaccharide hydrogels for protein drug delivery. Carbohyd Polym. 1995;28:69–76. [Google Scholar]
- 10.Choi YK, Bae YH, Kim SW. Star-shaped poly(ether–ester) block copolymers: synthesis, characterization, and their physical properties. Macromolecules. 1998;31:8766–74. [Google Scholar]
- 11.Dam TK, Brewer CF. Thermodynamic studies of lectin–carbohydrate interactions by isothermal titration calorimetry. Chem Rev. 2002;102:387–430. doi: 10.1021/cr000401x. [DOI] [PubMed] [Google Scholar]
- 12.Dam TK, Gabius HJ, André S, Kaltner H, Lensch M, Brewer CF. Galectins bind to the multivalent glycoprotein Asialofetuin with enhanced affinities and a gradient of decreasing binding constants. Biochemistry. 2005;44:12564–71. doi: 10.1021/bi051144z. [DOI] [PubMed] [Google Scholar]
- 13.Danesi R, Del BIanchiF S, Soldani P, Campagni A, La RoccaF RV, Myers CE, et al. Suramin inhibits bFGF-induced endothelial cell proliferation and angiogenesis in the chick chorioallantoic membrane. Br J Cancer. 1993;68:932–8. doi: 10.1038/bjc.1993.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Edelman ER, Nugent MA, Smith LT, Karnovsky MJ. Basic fibroblast growth factor enhances the coupling of intimal hyperplasia and proliferation of vasa vasorum in injured rat arteries. J Clin Invest. 1992;89:465–73. doi: 10.1172/JCI115607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ernst S, Venkataraman G, Sasisekharan V, Langer R, Cooney CL, Sasisekharan R. Pyranose ring flexibility. Mapping of physical data for iduronate in continuous conformational space. J Am Chem Soc. 1998;120:2099–107. [Google Scholar]
- 16.Fairbrother WJ, Champe MA, Christinger HW, Keyt BA, Starovasnik MA. Solution structure of the heparin-binding domain of vascular endothelial growth factor. Structure. 1998;6:637–48. doi: 10.1016/s0969-2126(98)00065-3. [DOI] [PubMed] [Google Scholar]
- 17.Farzan M, Mirzabekov T, Kolchinsky P, Wyatt R, Cayabyab M, Gerard NP, et al. Tyrosine sulfation of the amino terminus of CCR5 facilitates HIV-1 entry. Cell. 1999;96:667–76. doi: 10.1016/s0092-8674(00)80577-2. [DOI] [PubMed] [Google Scholar]
- 18.Fath MA, Wu X, Hileman RE, Linhardt RJ, Kashem MA, Nelson RM, et al. Interaction of secretory leukocyte protease inhibitor with heparin inhibits proteases involved in asthma. J Biol Chem. 1998;273:13563–9. doi: 10.1074/jbc.273.22.13563. [DOI] [PubMed] [Google Scholar]
- 19.Ferran DS, Sobel M, Harris RB. Design and synthesis of a helix heparin-binding peptide. Biochemistry. 1992;31:5010–6. doi: 10.1021/bi00136a014. [DOI] [PubMed] [Google Scholar]
- 20.Firsching A, Nickel P, Mora P, Allolio B. Antiproliferative and angiostatic activity of suramin analogues. Cancer Res. 1995;55:4957–61. [PubMed] [Google Scholar]
- 21.Folkman J, Weisz PB, Joullié MM, Li WW, Ewing WR. Control of angiogenesis with synthetic heparin substitutes. Science. 1989;243:1490–3. doi: 10.1126/science.2467380. [DOI] [PubMed] [Google Scholar]
- 22.Gospodarowicz D, Cheng J. Heparin protects basic and acidic FGF from inactivation. J Cell Physiol. 1986;128:475–84. doi: 10.1002/jcp.1041280317. [DOI] [PubMed] [Google Scholar]
- 23.Green RJ, Frazier RA, Shakesheff KM, Davies MC, Roberts CJ, Tendler SJB. Surface plasmon resonance analysis of dynamic biological interactions with biomaterials. Biomaterials. 2000;21:1823–35. doi: 10.1016/s0142-9612(00)00077-6. [DOI] [PubMed] [Google Scholar]
- 24.Hortin G, Tollefsen DM, Strauss AW. Identification of two sites of sulfation of human heparin cofactor II. J Biol Chem. 1986;261:15827–30. [PubMed] [Google Scholar]
- 25.Hosang M. Suramin binds to platelet-derived growth factor and inhibits its biological activity. J Cell Biochem. 1985;29:265–73. doi: 10.1002/jcb.240290310. [DOI] [PubMed] [Google Scholar]
- 26.Ibel K, Poland GA, Baldwin JP, Pepper DS, Luscombe M, Holbrook JJ. Low-resolution structure of the complex of human blood platelet factor 4 with heparin determined by small-angle neutron scattering. BBA/Protein Struct Mol Enzymol. 1986;870:58–63. doi: 10.1016/0167-4838(86)90008-7. [DOI] [PubMed] [Google Scholar]
- 27.Iozzo RV. Matrix proteoglycans: from molecular design to cellular function. Annu Rev Biochem. 1998;67:609–52. doi: 10.1146/annurev.biochem.67.1.609. [DOI] [PubMed] [Google Scholar]
- 28.Ishida K, Wierzba MK, Teruya T, Simizu S, Osada H. Novel heparan sulfate mimetic compounds as antitumor agents. Chem Biol. 2004;11:367–77. doi: 10.1016/j.chembiol.2004.02.015. [DOI] [PubMed] [Google Scholar]
- 29.Ishihara M, Sato M, Hattori H, Saito Y, Yura H, Ono K, et al. Heparin-carrying polystyrene (HCPS)-bound collagen substratum to immobilize heparin-binding growth factors and to enhance cellular growth. J Biomed Mater Res. 2001;56:536–44. doi: 10.1002/1097-4636(20010915)56:4<536::aid-jbm1125>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 30.Jørgensen KE, Møller JV. Use of flexible polymers as probes of glomerular pore size. Am J Physiol Renal Physiol. 1979;236:103–11. doi: 10.1152/ajprenal.1979.236.2.F103. [DOI] [PubMed] [Google Scholar]
- 31.Kehoe JW, Bertozzi CR. Tyrosine sulfation: a modulator of extracellular protein–protein interactions. Chem Biol. 2000;7:R57–61. doi: 10.1016/s1074-5521(00)00093-4. [DOI] [PubMed] [Google Scholar]
- 32.Keyt BA, Berleau LT, Nguyen HV, Chen H, Heinsohn H, Vandlen R, et al. The carboxyl-terminal domain(111–165) of vascular endothelial growth factor is critical for its mitogenic potency. J Biol Chem. 1996;271:7788–95. doi: 10.1074/jbc.271.13.7788. [DOI] [PubMed] [Google Scholar]
- 33.Lee AC, Yu VM, Lowe JB, III, Brenner MJ, Hunter DA, Mackinnon SE, et al. Controlled release of nerve growth factor enhances sciatic nerve regeneration. Exp Neurol. 2003;184:295–303. doi: 10.1016/s0014-4886(03)00258-9. [DOI] [PubMed] [Google Scholar]
- 34.Liao I-C, Wan ACA, Yim EKF, Leong KW. Controlled release from fibers of polyelectrolyte complexes. J Control Release. 2005;104:347–58. doi: 10.1016/j.jconrel.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 35.Lin X, Perrimon N. Role of heparan sulfate proteoglycans in cell–cell signaling in Drosophila. Matrix Biol. 2000;19:303–7. doi: 10.1016/s0945-053x(00)00073-1. [DOI] [PubMed] [Google Scholar]
- 36.Liu S, Hoke D, Julian J, Carson DD. Heparin/heparan sulfate (HP/HS) interacting protein (HIP) supports cell attachment and selective, high affinity binding of HP/HS. J Biol Chem. 1997;272:25856–62. doi: 10.1074/jbc.272.41.25856. [DOI] [PubMed] [Google Scholar]
- 37.Liu S, Julian J, Carson DD. A peptide sequence of heparin/heparan sulfate (HP/HS)-interacting protein supports selective, high affinity binding of HP/HS and cell attachment. J Biol Chem. 1998;273:9718–26. doi: 10.1074/jbc.273.16.9718. [DOI] [PubMed] [Google Scholar]
- 38.Liu S, Smith SE, Julian J, Rohde LH, Karin NJ, Carson DD. cDNA cloning and expression of HIP, a novel cell surface heparan sulfate/heparin-binding protein of human uterine epithelial cells and cell lines. J Biol Chem. 1996;271:11817–23. doi: 10.1074/jbc.271.20.11817. [DOI] [PubMed] [Google Scholar]
- 39.Liu S, Zhou F, Höök M, Carson DD. A heparin-binding synthetic peptide of heparin/heparan sulfateinteracting protein modulates blood coagulation activities. PNAS. 1997;94:1739–44. doi: 10.1073/pnas.94.5.1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mammen M, Choi S-K, Whitesides GM. Polyvalent interactions in biological systems: implications for design and use of multivalent ligands and inhibitors. Angew Chem Int Ed. 1998;37:2754–94. doi: 10.1002/(SICI)1521-3773(19981102)37:20<2754::AID-ANIE2754>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 41.Maraganore JM, Chao B, Joseph ML, Jablonski J, Ramachandran KL. Anticoagulant activity of synthetic hirudin peptides. J Biol Chem. 1989;264:8692–8. [PubMed] [Google Scholar]
- 42.Maynard HD, Hubbell JA. Discovery of a sulfated tetrapeptide that binds to vascular endothelial growth factor. Acta Biomaterialia. 2005;1:451–9. doi: 10.1016/j.actbio.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 43.Mukherjee S, Ghosh RN, Maxfield FR. Endocytosis. Physiol Rev. 1997;77:759–803. doi: 10.1152/physrev.1997.77.3.759. [DOI] [PubMed] [Google Scholar]
- 44.Mulloy B, Forster MJ, Jones C, Davies DB. N.m.r. and molecular-modelling studies of the solution conformation of heparin. Biochem J. 1993;293:849–58. doi: 10.1042/bj2930849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nimni ME. Polypeptide growth factors: targeted delivery systems. Biomaterials. 1997;18:1201–25. doi: 10.1016/s0142-9612(97)00050-1. [DOI] [PubMed] [Google Scholar]
- 46.Pöschel KA, Bucha E, Esslinger H-U, Nörtersheuser P, Jansa U, Schindler S, et al. Pharmacodynamics and pharmacokinetics of polyethylene glycol-hyrudin in patients with chronic renal failure. Kidney Int. 2000;58:2478–84. doi: 10.1046/j.1523-1755.2000.00431.x. [DOI] [PubMed] [Google Scholar]
- 47.Qiu LY, Bae YH. Polymer architecture and drug delivery. Pharm Res. 2006;23:1–30. doi: 10.1007/s11095-005-9046-2. [DOI] [PubMed] [Google Scholar]
- 48.Raman R, Venkataraman G, Ernst S, Sasisekharan V, Sasisekharan R. Structural specificity of heparin binding in the fibroblast growth factor family of proteins. PNAS. 2003;100:2357–62. doi: 10.1073/pnas.0437842100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Roghani M, Mansukhani A, Dell'Era P, Bellosta P, Basilico C, Rifkin D, et al. Heparin increases the affinity of basic fibroblast growth factor for its receptor but is not required for binding. J Biol Chem. 1994;269:3976–84. [PubMed] [Google Scholar]
- 50.Sakiyama-Elbert SE, Hubbell JA. Development of fibrin derivatives for controlled release of heparin-binding growth factors. J Control Release. 2000;65:389–402. doi: 10.1016/s0168-3659(99)00221-7. [DOI] [PubMed] [Google Scholar]
- 51.Salmivirta M, Lidholt K, Lindahl U. Heparan sulfate: a piece of information. FASEB J. 1996;10:1270–9. doi: 10.1096/fasebj.10.11.8836040. [DOI] [PubMed] [Google Scholar]
- 52.Schuck P. Use of surface plasmon resonance to probe the equilibrium and dynamic aspects of interactions between biological macromolecules. Annu Rev Biophys Biomol Struct. 1997;26:541–66. doi: 10.1146/annurev.biophys.26.1.541. [DOI] [PubMed] [Google Scholar]
- 53.Seal BL, Panitch A. Physical polymer matrices based on affinity interactions between peptides and polysaccharides. Biomacromolecules. 2003;4:1572–82. doi: 10.1021/bm0342032. [DOI] [PubMed] [Google Scholar]
- 54.Seal BL, Panitch A. Viscoelastic behavior of environmentally sensitive biomimetic polymer matrices. Macromolecules. 2006;39:2268–74. [Google Scholar]
- 55.Shaffer CB, Critchfield FH, Carpenter CP. Renal excretion and volume distribution of some polyethylene glycols in the dog. Am J Physiol. 1947;152:93–9. doi: 10.1152/ajplegacy.1947.152.1.93. [DOI] [PubMed] [Google Scholar]
- 56.Sinaÿ P. Active fragments of natural polysaccharides. Pure Appl Chem. 1989;61:481–3. [Google Scholar]
- 57.Stone SR, Hofsteenge J. Kinetics of the inhibition of thrombin by hirudin. Biochemistry. 1986;25:4622–8. doi: 10.1021/bi00364a025. [DOI] [PubMed] [Google Scholar]
- 58.Thompson LD, Pantoliano MW, Springer BA. Energetic characterization of the basic fibroblast growth factor–heparin interaction: identification of the heparin binding domain. Biochemistry. 1994;33:3831–40. doi: 10.1021/bi00179a006. [DOI] [PubMed] [Google Scholar]
- 59.Torchilin VP, Lukyanov AN. Peptide and protein drug delivery to and into tumors: challenges and solutions. Drug Discov Today. 2003;8:259–66. doi: 10.1016/s1359-6446(03)02623-0. [DOI] [PubMed] [Google Scholar]
- 60.Tyler-Cross R, Sobel M, Marques D, Harris RB. Heparin binding domain peptides of antithrombin III: analysis by isothermal titration calorimetry and circular dichroism spectroscopy. Protein Sci. 1994;3:620–7. doi: 10.1002/pro.5560030410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tyler-Cross R, Sobel M, McAdory LE, Harris RB. Structure-function relations of antithrombin III-heparin interactions as assessed by biophysical and biological assays and molecular modeling of peptide-pentasaccharide-docked complexes. Arch Biochem Biophys. 1996;334:206–13. doi: 10.1006/abbi.1996.0448. [DOI] [PubMed] [Google Scholar]
- 62.Ueki M, Watanabe S, Ishii Y, Okunaka O, Uchino K, Saitoh T, et al. Synthesis and anti-HIV activity of nonatyrosine N- and O1-9-decasulfate. Bioorg Med Chem. 2001;9:477–86. doi: 10.1016/s0968-0896(00)00269-8. [DOI] [PubMed] [Google Scholar]
- 63.Waltenberger J, Mayr U, Frank H, Hombach V. Suramin is a potent inhibitor of vascular endothelial growth factor. A contribution to the molecular basis of its antiangiogenic action. J Mol Cell Cardiol. 1996;28:1523–9. doi: 10.1006/jmcc.1996.0142. [DOI] [PubMed] [Google Scholar]
- 64.Wissink MJB, Beernink R, Poot AA, Engbers GHM, Beugeling T, van Aken WG, et al. Improved endothelialization of vascular grafts by local release of growth factor from heparinized collagen matrices. J Control Release. 2000;64:103–14. doi: 10.1016/s0168-3659(99)00145-5. [DOI] [PubMed] [Google Scholar]
- 65.Yagami T, Kitagawa K, Aida C, Fujiwara H, Futaki S. Stabilization of a tyrosine O-sulfate residue by a cationic functional group: formation of a conjugate acid–base pair. J Pept Res. 2000;56:239–49. doi: 10.1034/j.1399-3011.2000.00746.x. [DOI] [PubMed] [Google Scholar]
- 66.Yamaguchi N, Chae B-S, Zhang L, Kiick KL, Furst EM. Rheological characterization of polysaccharide-poly(ethylene glycol) star copolymer hydrogels. Biomacromolecules. 2005;6:1931–40. doi: 10.1021/bm0500042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yamaguchi N, Kiick KL. Polysaccharide-poly(ethylene glycol) star copolymer as a scaffold for the production of bioactive hydrogels. Biomacromolecules. 2005;6:1921–30. doi: 10.1021/bm050003+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yamaguchi N, Zhang L, Chae B-S, Palla CS, Furst EM, Kiick KL. Growth factor mediated assembly of cell receptor-responsive hydrogels. J Am Chem Soc. 2007;129:3040–1. doi: 10.1021/ja0680358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhang L, Furst EM, Kiick KL. Manipulation of hydrogel assembly and growth factor delivery via the use of peptide–polysaccharide interactions. J Control Release. 2006;114:130–42. doi: 10.1016/j.jconrel.2006.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zisch AH, Lutolf MP, Ehrbar M, Raeber GP, Rizzi SC, Davies N, et al. Cell-demanded release of VEGF from synthetic, biointeractive cell-ingrowth matrices for vascularized tissue growth. FASEB J. 2003;17:2260–2. doi: 10.1096/fj.02-1041fje. [DOI] [PubMed] [Google Scholar]
- 71.Zisch AH, Schenk U, Schense JC, Sakiyama-Elbert SE, Hubbell JA. Covalently conjugated VEGF-fibrin matrices for endothelialization. J Control Release. 2001;72:101–13. doi: 10.1016/s0168-3659(01)00266-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.