Abstract
Context
Although maternal stature has been associated with offspring mortality and health, the extent to which this association is universal across developing countries is unclear.
Objective
To examine the association between maternal stature and offspring mortality, underweight, stunting, and wasting in infancy and early childhood in 54 low- to middle-income countries.
Design, Setting, and Participants
Analysis of 109 Demographic and Health Surveys in 54 countries conducted between 1991 and 2008. Study population consisted of a nationally representative cross-sectional sample of children aged 0 to 59 months born to mothers aged 15 to 49 years. Sample sizes were 2 661 519 (mortality), 587 096 (underweight), 558 347 (stunting), and 568 609 (wasting) children.
Main Outcome Measures
Likelihood of mortality, underweight, stunting, or wasting in children younger than 5 years.
Results
The mean response rate across surveys in the mortality data set was 92.8%. In adjusted models, a 1-cm increase in maternal height was associated with a decreased risk of child mortality (absolute risk difference [ARD], 0.0014; relative risk [RR], 0.988; 95% confidence interval [CI], 0.987–0.988), underweight (ARD, 0.0068; RR, 0.968; 95% CI, 0.968–0.969), stunting (ARD, 0.0126; RR, 0.968; 95% CI, 0.967–0.968), and wasting (ARD, 0.0005; RR, 0.994; 95% CI, 0.993–0.995). Absolute risk of dying among children born to the tallest mothers (≥160 cm) was 0.073 (95% CI, 0.072–0.074) and to those born to the shortest mothers (<145 cm) was 0.128 (95% CI, 0.126–0.130). Country-specific decrease in the risk for child mortality associated with a 1-cm increase in maternal height varied between 0.978 and 1.011, with the decreased risk being statistically significant in 46 of 54 countries (85%) (α=.05).
Conclusion
Among 54 low- to middle-income countries, maternal stature was inversely associated with offspring mortality, underweight, and stunting in infancy and childhood.
Maternal stature is an important determinant of intrauterine growth restriction1 and low birth weight,2 birth weight and intrauterine growth restriction are predictors of subsequent mortality and growth failure.3 Because attained height reflects the health stock accumulated through social and environmental exposures during early childhood,4,5 maternal stature is a simple, stable, and useful marker for assessing intergenerational linkages in health. Maternal stature has been shown to predict offspring outcomes before or immediately after birth.6 However, evidence for whether risks associated with shorter maternal stature have a lasting influence on the offspring’s health during infancy and childhood is limited, or is restricted to small nonrepresentative samples, and inconclusive. In a large, nationally representative data set from India, an inverse association between maternal stature and both child mortality and growth failure was observed.7 It remains unclear the extent to which this association is present across a wider range of countries. Using the largest available, nationally representative, and comparable sample from 109 surveys in 54 low- to middle-income countries, with objective measurements of maternal stature and offspring anthropometry, we investigated the potential long-term effects of maternal stature on offspring mortality, underweight, stunting, and wasting in infancy and early childhood.
METHODS
Data Sources
Data for this study came from 109 Demographic and Health Surveys (DHS) conducted in 54 low-to middle-income countries between 1991 and 2008 (Table 1).8 The DHS are nationally representative household sample surveys measuring indicators of population, health, and nutrition, with special emphasis on maternal and child health.9 The target population in most DHS surveys was all women (or in some cases ever-married women)of reproductive age (15–49 years). A complete birth and death history was collected for each eligible woman’s children, including date of birth and when applicable age at death of each child. Anthropometric measurements on children were restricted to children born 5 years or less before the survey and alive at the time of the survey.9 Trained investigators weighed each child with a solar-powered scale accurate to within 100 g; maternal and child height were measured using an adjustable board calibrated in millimeters and theoretically accurate to 1 mm.10
Table 1.
Sample Size, Weighted Percentage of Child Mortality, Underweight, Stunting, and Wasting by Countrya
| Country, Survey Years | Mortality
|
Underweight
|
Stunting
|
Wasting
|
||||
|---|---|---|---|---|---|---|---|---|
| Sample Size | No. (Weighted %) | Sample Size | No. (Weighted %) | Sample Size | No. (Weighted %) | Sample Size | No. (Weighted %) | |
| Pooled | 2 661 519 | 312 533 (11.7) | 587 096 | 126 235 (21.5) | 558 347 | 214 120 (38.3) | 568 609 | 50 682 (8.9) |
|
| ||||||||
| Armenia, 2000, 2005 | 20 812 | 1093 (5.3) | 2774 | 90 (3.4) | 2699 | 437 (17.2) | 2722 | 87 (3.4) |
|
| ||||||||
| Azerbaijan, 2006 | 13 095 | 949 (7.2) | 2032 | 167 (8.6) | 1879 | 502 (25.8) | 1906 | 116 (6.9) |
|
| ||||||||
| Bangladesh, 1999–00, 1996–97, 2004, 2007 | 87 963 | 10 689 (12.2) | 21 590 | 9442 (44.5) | 20 222 | 10 109 (50.8) | 20 531 | 3214 (15.9) |
|
| ||||||||
| Benin, 1996, 2001, 2006 | 82 123 | 12 072 (14.6) | 19 889 | 4247 (21.0) | 18 547 | 7566 (40.6) | 18 952 | 1837 (9.6) |
|
| ||||||||
| Bolivia, 1994, 1998, 2003 | 71 221 | 8473 (12.0) | 18 054 | 1286 (6.7) | 17 525 | 5975 (32.6) | 17 831 | 425 (2.2) |
|
| ||||||||
| Brazil, 1996 | 9338 | 663 (6.4) | 4161 | 204 (4.5) | 3936 | 579 (12.7) | 3962 | 106 (2.6) |
|
| ||||||||
| Burkina Faso, 1992–93, 1998–99, 2003 | 72 529 | 13 310 (19.0) | 17 752 | 5822 (33.2) | 16 618 | 6968 (43.1) | 16 836 | 3058 (18.0) |
|
| ||||||||
| Cambodia, 2000, 2005 | 8709 | 5140 (12.4) | 7282 | 2594 (34.0) | 6892 | 3285 (45.6) | 7034 | 849 (12.4) |
|
| ||||||||
| Cameroon, 1998, 2004 | 40 376 | 2659 (13.0) | 5010 | 736 (16.1) | 4758 | 1658 (35.4) | 4869 | 311 (6.9) |
|
| ||||||||
| Central African Republic, 1994–95 | 20 731 | 1152 (13.3) | 2322 | 537 (22.9) | 2224 | 881 (39.5) | 2298 | 205 (8.9) |
|
| ||||||||
| Chad, 1996–97, 2004 | 33 060 | 5526 (17.4) | 10 226 | 3365 (33.8) | 9687 | 4140 (44.5) | 9885 | 1624 (16.0) |
|
| ||||||||
| Colombia, 1995, 2000, 2005 | 84 513 | 3077 (3.5) | 20 457 | 1148 (5.4) | 20 244 | 3483 (17.1) | 20 599 | 346 (1.5) |
|
| ||||||||
| Comoros, 1996 | 3770 | 426 (11.3) | 944 | 208 (22.0) | 897 | 348 (38.8) | 924 | 101 (10.9) |
|
| ||||||||
| Congo (Brazaville), 2005 | 15 901 | 1585 (10.4) | 3941 | 440 (12.0) | 3728 | 1049 (29.7) | 3793 | 273 (7.3) |
|
| ||||||||
| Congo (DRC), 2007 | 13 975 | 2021 (14.1) | 3545 | 863 (24.9) | 3121 | 1392 (44.2) | 3178 | 306 (9.8) |
|
| ||||||||
| Côte d’Ivoire, 1994, 1998–99 | 20 504 | 2769 (14.2) | 4905 | 935 (19.9) | 4753 | 1436 (31.4) | 4878 | 464 (9.7) |
|
| ||||||||
| Dominican Republic, 1996 | 17 854 | 1333 (7.2) | 3701 | 224 (4.8) | 3614 | 582 (13.8) | 3646 | 82 (2.0) |
|
| ||||||||
| Egypt, 2000, 2005, 2008 | 161 282 | 13 251 (7.9) | 33 440 | 1848 (5.3) | 31 210 | 7808 (24.0) | 31 718 | 1507 (4.8) |
|
| ||||||||
| Ethiopia, 2000, 2005 | 61 055 | 10 819 (17.7) | 13 043 | 4912 (39.5) | 12 286 | 6340 (55.2) | 12 511 | 1678 (12.4) |
|
| ||||||||
| Gabon, 2000 | 9965 | 727 (7.3) | 3407 | 348 (8.8) | 3332 | 971 (25.7) | 3414 | 137 (4.1) |
|
| ||||||||
| Ghana, 1998, 2003, 2008 | 34 260 | 3901 (10.6) | 8399 | 1596 (18.1) | 7964 | 2624 (31.9) | 8103 | 747 (8.9) |
|
| ||||||||
| Guatemala, 1995, 1998–99 | 35 632 | 3222 (8.3) | 12 414 | 2885 (21.2) | 11 979 | 7247 (54.4) | 12 188 | 441 (3.5) |
|
| ||||||||
| Guinea, 1999, 2005 | 28 154 | 5396 (19.2) | 7070 | 1501 (21.5) | 6749 | 2414 (35.9) | 6761 | 688 (10.5) |
|
| ||||||||
| Haiti, 1994–95, 2000, 2005–06 | 45 839 | 6170 (13.8) | 10 795 | 1946 (17.5) | 10 509 | 3218 (30.0) | 10 705 | 778 (7.5) |
|
| ||||||||
| Honduras, 2005 | 48 181 | 2485 (4.9) | 9095 | 952 (8.5) | 8977 | 3207 (29.9) | 9119 | 128 (1.3) |
|
| ||||||||
| India, 1998–99, 2005–06 | 482 378 | 46 624 (10.7) | 68 597 | 26 810 (43.7) | 64 028 | 29 173 (49.0) | 65 487 | 11 844 (19.7) |
|
| ||||||||
| Jordan, 1997, 2002, 2007 | 60 789 | 2122 (3.3) | 15 130 | 826 (4.5) | 14 509 | 1914 (11.9) | 14 732 | 577 (3.6) |
|
| ||||||||
| Kazakhstan, 1995, 1999 | 10 421 | 635 (6.2) | 1302 | 72 (5.6) | 1268 | 220 (15.3) | 1294 | 61 (4.9) |
|
| ||||||||
| Kenya, 1993, 1998, 2003 | 49 232 | 4804 (9.8) | 14 345 | 2634 (18.2) | 13 605 | 5086 (37.8) | 13 858 | 943 (6.5) |
|
| ||||||||
| Kyrgyz Republic, 1997 | 8528 | 721 (8.9) | 960 | 78 (8.2) | 937 | 297 (32.4) | 964 | 36 (3.2) |
|
| ||||||||
| Lesotho, 2004 | 7158 | 671 (9.0) | 1429 | 265 (17.8) | 1323 | 586 (42.8) | 1342 | 67 (4.9) |
|
| ||||||||
| Liberia, 2007 | 21 116 | 3461 (17.2) | 4378 | 836 (18.7) | 4164 | 1598 (38.4) | 4259 | 312 (7.5) |
|
| ||||||||
| Madagascar, 1997, 2003–04 | 31 690 | 3697 (12.5) | 7547 | 2520 (35.8) | 7158 | 3674 (53.9) | 7354 | 904 (13.0) |
|
| ||||||||
| Malawi, 1992, 2000, 2004 | 83 720 | 15 349 (18.8) | 21 403 | 4094 (20.5) | 19 751 | 10 260 (53.3) | 20 135 | 1194 (6.3) |
|
| ||||||||
| Mali, 1995–96, 2001, 2006 | 117 084 | 25 751 (22.4) | 25 449 | 7876 (30.7) | 24 113 | 9554 (39.4) | 24 494 | 4095 (16.5) |
|
| ||||||||
| Moldova, 2005 | 9629 | 320 (3.4) | 1352 | 43 (3.4) | 1271 | 136 (10.7) | 1284 | 61 (4.6) |
|
| ||||||||
| Morocco, 2003–04 | 31 854 | 2644 (8.1) | 5544 | 596 (10.0) | 5233 | 1178 (21.9) | 5297 | 548 (10.0) |
|
| ||||||||
| Mozambique, 1997, 2003 | 47 010 | 8227 (18.4) | 11 401 | 2475 (23.5) | 10 949 | 4996 (46.5) | 11 196 | 721 (7.1) |
|
| ||||||||
| Namibia, 1992, 2006–07 | 27 742 | 2030 (7.1) | 6306 | 1225 (19.4) | 6135 | 1964 (31.7) | 6134 | 508 (8.2) |
|
| ||||||||
| Nepal, 1996, 2001, 2006 | 67 641 | 9068 (13.1) | 15 202 | 6185 (41.2) | 14 692 | 8068 (54.7) | 15 029 | 1848 (12.6) |
|
| ||||||||
| Nicaragua, 1997–98, 2001 | 66 125 | 4621 (6.8) | 12 826 | 1199 (9.4) | 12 239 | 3619 (27.9) | 12 425 | 312 (2.6) |
|
| ||||||||
| Niger, 1998, 2006 | 34 424 | 7691 (24.7) | 7628 | 2998 (42.5) | 7280 | 3484 (51.2) | 7516 | 1386 (19.3) |
|
| ||||||||
| Nigeria, 2003 | 21 764 | 4243 (20.5) | 4609 | 1228 (27.0) | 4232 | 1766 (43.0) | 4319 | 473 (11.3) |
|
| ||||||||
| Peru, 1992, 1996, 2000, 2004 | 136 166 | 12 555 (8.4) | 36 181 | 2712 (6.2) | 35 225 | 12 611 (32.3) | 35 730 | 599 (1.4) |
|
| ||||||||
| Rwanda, 2000, 2005 | 41 957 | 6980 (16.9) | 9791 | 1826 (19.0) | 9420 | 4509 (49.1) | 9582 | 638 (6.8) |
|
| ||||||||
| Senegal, 2005 | 12 131 | 1619 (12.9) | 2810 | 420 (14.1) | 2749 | 587 (20.2) | 2797 | 248 (8.4) |
|
| ||||||||
| Swaziland, 2006 | 10 853 | 911 (8.2) | 2032 | 109 (5.6) | 1960 | 524 (27.4) | 1994 | 48 (2.4) |
|
| ||||||||
| Tanzania, 1996, 2004–05 | 46 306 | 5890 (13.0) | 12 532 | 2686 (20.9) | 12 123 | 5540 (46.1) | 12 345 | 755 (5.6) |
|
| ||||||||
| Togo, 1998 | 14 506 | 2068 (13.9) | 3645 | 906 (23.4) | 3490 | 1134 (31.0) | 3608 | 530 (13.8) |
|
| ||||||||
| Turkey, 1993, 1998, 2003 | 24 143 | 1932 (8.0) | 10 118 | 630 (6.2) | 9719 | 1895 (18.9) | 9871 | 238 (2.5) |
|
| ||||||||
| Uganda, 1995, 2000–01, 2006 | 46 219 | 6604 (14.7) | 12 070 | 2225 (19.5) | 11 632 | 4893 (43.7) | 11 898 | 692 (6.0) |
|
| ||||||||
| Uzbekistan, 1996 | 9397 | 551 (5.6) | 1049 | 144 (15.0) | 916 | 300 (34.2) | 939 | 104 (12.3) |
|
| ||||||||
| Zambia, 1996, 2001–02, 2007 | 61 335 | 9065 (14.8) | 16 314 | 3210 (19.2) | 15 568 | 7696 (49.1) | 15 887 | 872 (5.4) |
|
| ||||||||
| Zimbabwe, 1994, 1999, 2005–06 | 39 359 | 2771 (7.0) | 8898 | 1111 (12.2) | 8308 | 2639 (31.5) | 8476 | 560 (6.5) |
Tanzania refers to the United Republic of Tanzania and Guinea refers to the Republic of Guinea.
Due to coverage, comparability, and data quality, DHS is the primary reliable data source for measuring child mortality and undernutrition across developing countries.11–13 The DHS uses extensive interviewer training, standardized measurement tools and techniques, an identical core questionnaire, and instrument pretesting to ensure standardization and comparability across diverse sites and time.10 Teams responsible for data collection are monitored during fieldwork, including spot-checking and validation of completed questionnaires.10 Country reports detail pretesting and quality assurance measures by survey (see http://www.measuredhs.com/pubs/browse_type.cfm). The DHS is modular in structure, comprising a core questionnaire, a set of country-relevant modules, and country-specific variables. The DHS provides data with standardized variables across surveys and imputed dates of key events (see http://www.measuredhs.com/pubs/pdf/DHSG4/Recode4DHS.pdf).
The DHS uses a multistage stratified design with probabilistic sampling with each elementary unit having a defined probability of selection.14 Every survey was stratified by urban and rural status and additionally by country-specific geographic or administrative regions. eTable 1 (available at http://www.jama.com) describes each survey by country and year, along with sampling characteristics, response rates, and sample sizes. Detailed sampling plans are available from survey final reports at http://www.measuredhs.com/pubs/browse_type.cfm.
Study Population and Sample Size
For the pooled mortality analysis, the study population consisted of all children (n=3 395 212) born to mothers (n = 939 140) aged 15 to 49 years (eTable 2). There were 635 709 children (19%) whose mother’s height was intentionally not measured (eTable 1 shows height measurement protocol by survey). Among children whose mother’s height should have been measured, 92 839 (3%) did not have a height measure in the data; these cases were excluded. We also excluded children whose mothers had recorded heights of less than 100 cm or more than 200 cm, as these were considered improbable or outliers. Furthermore, children who had missing information on the covariates included in this analysis (n = 5145, <1%) were excluded. The final analytical sample for the mortality analysis was 2 661 519 children born to 751 912 mothers surveyed between 1991 and 2008 in 54 low- to middle-income countries (eTable 2).
For the anthropometric analysis, the study population comprised children (n=829 680) born 3 to 5 years preceding the survey to mothers (n=342 229) aged 15 to 49 years and alive at the time of the survey (eTable 3). There were 71 155 children (9% of the children sample) for whom maternal height was intentionally not measured. Among children whose mother’s height should have been measured, 23 677 (3%) did not have a height measure in the data and an additional 59 941 (8%) were missing data on covariates. In addition, 116 560 children (15%) had missing or biologically implausible height, 87 811 children (12%) had missing or biologically implausible weight, and 106 298 children (14%) had missing or biologically implausible weight for height. The World Health Organization (WHO) cutoffs for biological implausibility were used (eTable 3).15 The final analytical samples for the anthropometric analysis were 587 096 children born to 247 279 mothers for underweight, 558 347 children born to 234 604 mothers for stunting, and 568 609 children born to 239 628 mothers for wasting surveyed between 1991 and 2008 in 54 low- to middle-income countries (eTable 3).
Outcomes, Exposure, and Covariates
Offspring mortality was a binary variable; 1 if the child died between 0 to 59 months and 0 otherwise. Mortality by age categories was also assessed (<1 month [neonates], 1–11 months [infants], and 12–59 months [children]). Each variable was binary; 1 if the child died during that age interval and 0 otherwise. The risk set was all children alive at the beginning of the interval and the event was death during the interval.
Growth failure was defined as underweight, stunting, or wasting.3 Underweight z scores were calculated by subtracting from a child’s weight the median weight for a child of that age and sex and dividing by the SD of the weight for a child of that age and sex in the WHO reference population15; stunting was measured by subtracting from a child’s height the median height for a child of that age and sex and dividing by the SD of the height for a child of that age and sex in the WHO reference population15; and wasting was measured by subtracting from a child’s weight the median weight for a child of that height and sex and dividing by the SD of the weight for a child of that height and sex in the WHO reference population15 (see http://www.who.int/childgrowth/standards/en/). Software used for z score calculations is available at http://www.who.int/childgrowth/software/en/. Growth failure was defined as more than 2 SDs under WHO-growth standards15 on each dimension separately. We also calculated severe growth failure, defined as more than 3 SDs under WHO-growth standards.15
Maternal height was specified as a continuous and a categorical exposure with the following cutpoints: less than 145 cm, 145 to 149.9 cm, 150 to 154.9 cm, 155 to 159.9 cm, and 160.0 cm or more.
Sex, birth order, birth interval, birth year, and twin status of the child; mother’s age at birth of the child, education, occupation, and marital status; and household wealth and urban or rural residence were included as covariates (Table 2). Household wealth was defined in terms of ownership of material possessions,16 with each child assigned a wealth score based on a combination of different household characteristics that were weighted according to a factor analysis procedure. For this procedure, z scores were calculated for each indicator variable and a principal components analysis was performed using these z scores. For each household, the values of the indicator variables were multiplied by the factor loadings and summed to produce a standardized household index value with a mean of 0 and an SD of 1. This standardized score was then divided into quintiles for each country.17,18 In addition, we also derived an overall wealth quintile that was comparable across countries, based on assets common across surveys, which included whether the household had electricity, radio, or bicycle, and by major source of drinking water. We conducted a factor analysis on these 5 assets, using principal components analysis, and derived overall wealth quintiles.
Table 2.
Frequency and Percentage Distribution of Child Mortality, Underweight, Stunting, and Wasting by Maternal, Child, and Household Covariates
| Covariates | No. (%) of Participants
|
|||
|---|---|---|---|---|
| Mortality (n = 2 661 519) | Underweight (n = 587 096) | Stunting (n = 558 347) | Wasting (n = 568 609) | |
| Maternal | ||||
| Height, cm (continuous)a | 156 (7.2) | 156 (7.2) | 156 (7.2) | 156 (7.2) |
|
| ||||
| ≥160 | 715 535 (26.9) | 165 204 (28.1) | 156 769 (28.1) | 159 329 (28.0) |
|
| ||||
| 155–159.9 | 687 642 (25.8) | 154 596 (26.3) | 147 145 (26.4) | 149 809 (26.4) |
|
| ||||
| 150–154.9 | 673 568 (25.3) | 145 513 (24.8) | 138 793 (24.9) | 141 488 (24.9) |
|
| ||||
| 145–149.9 | 413 693 (15.5) | 86 152 (14.7) | 81 958 (14.7) | 83 554 (14.7) |
|
| ||||
| <145 | 171 081 (6.4) | 35 631 (6.1) | 33 682 (6.0) | 34 429 (6.1) |
|
| ||||
| Age at birth, y | ||||
| <17 | 142 278 (5.4) | 18 298 (3.1) | 17 313 (3.1) | 17 598 (3.1) |
|
| ||||
| 17–19 | 459 614 (17.3) | 77 784 (13.3) | 73 910 (13.2) | 75 287 (13.2) |
|
| ||||
| 20–24 | 920 332 (34.6) | 178 853 (30.5) | 170 068 (30.5) | 173 275 (30.5) |
|
| ||||
| 25–29 | 631 531 (23.7) | 145 305 (24.8) | 138 218 (24.8) | 140 769 (24.8) |
|
| ||||
| ≥30 | 507 764 (19.1) | 166 856 (28.4) | 158 838 (28.5) | 161 680 (28.4) |
|
| ||||
| Occupation | ||||
| Not working | 1 060 981 (39.9) | 254 963 (43.4) | 241 091 (43.2) | 245 702 (43.2) |
|
| ||||
| Working | 1 600 538 (60.1) | 332 133 (56.6) | 317 256 (56.8) | 322 907 (56.8) |
|
| ||||
| Marital status | ||||
| Unmarried | 277 426 (10.4) | 48 564 (8.3) | 46 569 (8.3) | 47 479 (8.4) |
|
| ||||
| Married | 2 383 957 (89.6) | 538 532 (91.7) | 511 778 (91.7) | 521 130 (91.7) |
|
| ||||
| Education | ||||
| None | 1 210 905 (45.5) | 222 711 (37.9) | 210 012 (37.6) | 213 951 (37.6) |
|
| ||||
| Primary | 826 169 (31.0) | 195 808 (33.4) | 187 303 (33.6) | 190 684 (33.5) |
|
| ||||
| Secondary or higher | 624 445 (23.5) | 168 577 (28.7) | 161 032 (28.8) | 163 974 (28.8) |
|
| ||||
| Child | ||||
| Birth order | ||||
| First | 711 464 (26.7) | 140 849 (24.0) | 133 852 (24.0) | 136 375 (24.0) |
|
| ||||
| Second | 598 027 (22.5) | 124 371 (21.2) | 118 356 (21.2) | 120 423 (21.2) |
|
| ||||
| Third | 444 918 (16.7) | 93 834 (16.0) | 89 288 (16.0) | 90 990 (16.0) |
|
| ||||
| Fourth | 311 953 (11.7) | 68 186 (11.6) | 64 843 (11.6) | 66 052 (11.6) |
|
| ||||
| ≥Fifth | 595 157 (22.4) | 159 856 (27.2) | 152 008 (27.2) | 154 769 (27.2) |
|
| ||||
| Birth interval, mo | ||||
| First child | 711 464 (26.7) | 140 849 (24.0) | 133 852 (24.0) | 136 375 (24.0) |
|
| ||||
| <24 | 620 528 (23.3) | 93 492 (15.9) | 89 025 (16.0) | 90 442 (15.9) |
|
| ||||
| 24–47 | 1 010 360 (38.0) | 244 749 (41.7) | 232 686 (41.7) | 237 041 (41.7) |
|
| ||||
| ≥48 | 318 600 (12.0) | 107 879 (18.4) | 102 663 (18.4) | 104 630 (18.4) |
|
| ||||
| Sex | ||||
| Male | 1 359 296 (51.1) | 297 401 (50.7) | 282 643 (50.6) | 288 102 (50.7) |
|
| ||||
| Female | 1 302 223 (48.9) | 289 695 (49.3) | 275 704 (49.4) | 280 507 (49.3) |
|
| ||||
| Twin | ||||
| Not twin | 2 602 444 (97.8) | 575 164 (98.0) | 547 150 (98.0) | 557 160 (98.0) |
|
| ||||
| Twin | 59 075 (2.2) | 11 932 (2.0) | 11 197 (2.0) | 11 449 (2.0) |
|
| ||||
| Age category, mo | ||||
| <1 | 112 930 (4.3) | 3443 (0.6) | 3512 (0.6) | 5800 (1.0) |
|
| ||||
| 1–12 | 240 838 (9.1) | 124 139 (21.8) | 113 721 (20.4) | 122 105 (20.8) |
|
| ||||
| >12 | 2 285 369 (86.9) | 441 027 (77.6) | 441 114 (79.0) | 459 191 (78.2) |
|
| ||||
| Age, moa | 22 (17.7) | 28 (16.9) | 28 (16.9) | 28 (16.8) |
|
| ||||
| Household | ||||
| Wealth quintile, within country | ||||
| First, poorest | 620 912 (23.3) | 140 794 (24.0) | 133 539 (23.9) | 136 085 (23.9) |
|
| ||||
| Second | 566 230 (21.3) | 125 884 (21.4) | 119 808 (21.5) | 121 941 (21.5) |
|
| ||||
| Third | 538 283 (20.2) | 118 173 (20.1) | 112 369 (20.1) | 114 475 (20.1) |
|
| ||||
| Fourth | 498 194 (18.7) | 108 144 (18.4) | 103 069 (18.5) | 104 962 (18.5) |
|
| ||||
| Fifth, richest | 437 900 (16.5) | 94 101 (16.0) | 89 562 (16.0) | 91 146 (16.0) |
|
| ||||
| Location | ||||
| Urban | 911 435 (34.2) | 204 156 (34.8) | 195 229 (35.0) | 198 620 (34.9) |
|
| ||||
| Rural | 1 750 084 (65.8) | 382 940 (65.2) | 363 118 (65.0) | 369 989 (65.1) |
Continuous height and child age are reported as mean (SD).
Analysis
Individual country files were created, combining multiple survey years, ensuring consistency of variable definitions across various survey years as well as consistency of region and primary sampling unit identifiers. These individual files were concatenated for the pooled analysis. Because prevalence of outcomes were more than 10%, we used a modified Poisson approach with robust error variance to model the binary outcomes associated with mortality and growth failure.19 We also estimated stratified models for neonates (<1 month), infants (1–11 months), and children (12–59 months) after testing for an interaction between maternal height and child’s age. We also conducted an analysis by child age for the anthropometric analysis. Results are presented as change in risks (relative to 1) for a 1-cm change in maternal height and, when maternal height is specified as categories, we report both the relative risks (RRs) and absolute probabilities. Absolute risk differences were calculated by fitting a normal model with an identity link.20 We also provided the absolute risk differences for maternal height categories calculated as differences in the absolute probabilities between the different maternal height categories compared with mothers in the tallest category. We accounted for clustering of children by mothers and primary sampling units in all analyses and included country and birth year fixed effects in pooled analyses. Country heterogeneity in the association between maternal stature and offspring outcomes were tested using Wald tests. Models were estimated by using STATA version 10SE.21 Statistical precision was ascertained by using 2-tailed Wald tests and exact P values, except when P<.05 are reported. Several ex-Soviet Republics had small numbers of respondents and events in the lowest education categories. For mortality analysis, education was not included for Kyrgyz Republic and education was included as a continuous variable in growth failure analysis for Kyrgyz Republic, Kazakhstan, Uzbekistan, and Moldova.
We conducted the following sensitivity analyses. First, to test for nonlinearity in the effect of maternal height and the appropriateness of the height categories, we conducted analyses with alternate categorizations of height. The analyses were also repeated removing the extreme (top and bottom 1%) outliers of maternal height. Second, in some populations with very early child-bearing, height could be affected by childbearing, which itself is associated with higher mortality risks. We conducted analysis restricted to women who had their first child after the age of 20 years. Third, because several of the study covariates were measured contemporaneously, a reduced form model, including only variables likely to be determined before the outcome (ie, age of mother at birth, education, and year-born), was estimated. Fourth, we estimated the pooled model without India, which comprised 15.4% of the total sample size, and where an association between maternal height and offspring mortality and growth failure has been shown.7 Fifth, given issues related to relative vs absolute wealth, we estimated pooled models with and without the global wealth index. Sixth, to test for recall bias in mortality, we conducted analysis restricted to births in the last 5 years preceding the survey. Seventh, because the survey period varies between 1991 and 2008, we tested if the main results hold in a restricted sample of countries surveyed after 2000. In addition, we compared prevalence of mortality, underweight, stunting, and wasting among women who did and did not report height, as well as comparing maternal heights among children who did and did not report child anthropometric measures as sensitivity tests for missing information.
Ethical Review
The DHS data collection procedures were approved by the ORC Macro (Calverton, Maryland) institutional review board as well as by the relevant body in each country that approves research studies on human subjects. Oral informed consent for the interview or survey was obtained from respondents by interviewers. This analysis was reviewed by the Harvard School of Public Health Institutional Review Board and was considered exempt from full review because the study was based on an anonymous public use data set with no identifiable information on the survey participants.
RESULTS
Mortality
The mean response rate across surveys in the mortality data set was 92.8%, ranging from 75.1% in Brazil (1996) to 98.8% in Egypt (2008) (eTable 1). The sample size was 2 661 519 for child mortality analysis, ranging from 3770 children in Comoros to 482 378 children in India (Table 1). Of 2 661 519 children in the data set, 312 553 died between the ages of 0 to 59 months, with a mortality prevalence of 11.7% (95% confidence interval [CI], 11.7%-11.8%) (Table 1). The prevalence of child mortality varied between 3.3% (Jordan) and 24.7% (Niger). Results are reported by country in an interactive information graphic (see http://www.jama.com). The covariate distribution across categories of maternal height was largely overlapping (eTable 4), although there were imbalances for location (70.4% rural for <145 cm vs 64.3% for >160 cm), local wealth quintile (31.9% in poorest quintile for <145 cm vs 20.4% for >160 cm), twin (1.3% prevalence of twins for <145 cm vs 3.0% for >160 cm), occupation (55.0% working for <145 cm vs 64.3% for >160 cm), and education (14.8% secondary or higher education for <145 cm vs 25.1% for >160 cm).
In the pooled analysis, a 1-cm increase in maternal height was associated with a decreased risk in offspring mortality in unadjusted(RR,0.983;95%CI,0.982–0.984)and adjusted(RR,0.988;95%CI, 0.987–0.988) models (Table 3). In adjusted models, compared with the tallest mothers (≥160 cm), each lower height category had substantially higher risk of child mortality, with children born to mothers of height shorter than 145 cm having an increased RR of 1.397(95% CI,1.373–1.422). The absolute probability of dying among children born to the tallest mothers (≥160 cm) was 0.073 (95%CI,0.072–0.074)and among those children born to the shortest mothers (<145 cm), absolute probability was 0.128 (95% CI, 0.126–0.130). The association between maternal height and offspring mortality was strongest among neonates (RR, 0.982; 95% CI, 0.981–0.983), followed by infants aged 1 to 11 months (RR, 0.988; 95% CI, 0.987–0.989) and weakest for children aged 12 to 59 months (RR, 0.990; 95% CI, 0.989–0.992).
Table 3.
Overall and Age-Stratified Unadjusted and Adjusted Changes in Risk, RR, AP, and DAP by Maternal Height for Offspring Mortality Among Children Younger Than 5 Yearsa
| No. of Events | Unadjusted
|
Adjusted
|
|||||
|---|---|---|---|---|---|---|---|
| RR (95% CI)b | AP (95% CI) | DAP | RR (95% CI)b | AP (95% CI) | DAP | ||
| Mortality, 0–59 mo | |||||||
| Maternal heightc | 0.983 (0.982–0.984) | 0.0020 (0.0019–0.0020) | 0.988 (0.987–0.988) | 0.0014 (0.0013–0.0015) | |||
|
| |||||||
| Maternal height, cm | |||||||
| ≥160 | 86 840 | 1 [Reference] | 0.077 (0.076–0.078) | 0 | 1 [Reference] | 0.073 (0.072–0.074) | 0 |
|
| |||||||
| 155–159.9 | 79 024 | 1.086 (1.074–1.098) | 0.114 (0.113–0.115) | 0.037 | 1.062 (1.051–1.072) | 0.098 (0.097–0.099) | 0.025 |
|
| |||||||
| 150–154.9 | 76 088 | 1.186 (1.173–1.200) | 0.122 (0.121–0.123) | 0.045 | 1.128 (1.116–1.141) | 0.102 (0.101–0.103) | 0.029 |
|
| |||||||
| 145–149.9 | 48 216 | 1.341 (1.323–1.360) | 0.138 (0.136–0.140) | 0.061 | 1.230 (1.213–1.246) | 0.111 (0.110–0.113) | 0.038 |
|
| |||||||
| <145 | 22 365 | 1.586 (1.558–1.615) | 0.166 (0.163–0.169) | 0.089 | 1.397 (1.373–1.422) | 0.128 (0.126–0.130) | 0.055 |
|
| |||||||
| Neonates, <1 mo | |||||||
| Maternal heightc | 0.979 (0.978–0.980) | 0.0008 (0.0008–0.0009) | 0.982 (0.981–0.983) | 0.0007 (0.0007–0.0008) | |||
|
| |||||||
| Maternal height, cm | |||||||
| ≥160 | 25 492 | 1 [Reference] | 0.026 (.0251–0.026) | 0 | 1 [Reference] | 0.023 (0.022–0.023) | 0 |
|
| |||||||
| 155–159.9 | 25 584 | 1.111 (1.089–1.135) | 0.041 (0.041–0.042) | 0.015 | 1.099 (1.077–1.112) | 0.034 (0.034–0.035) | 0.011 |
|
| |||||||
| 150–154.9 | 27 170 | 1.232 (1.206–1.259) | 0.045 (0.044–0.045) | 0.019 | 1.195 (1.170–1.221) | 0.037 (0.036–0.037) | 0.014 |
|
| |||||||
| 145–149.9 | 18 825 | 1.409 (1.374–1.445) | 0.051 (0.050–0.052) | 0.025 | 1.336 (1.303–1.370) | 0.041 (0.040–0.042) | 0.018 |
|
| |||||||
| <145 | 9349 | 1.718 (1.665–1.773) | 0.063 (0.061–0.065) | 0.037 | 1.584 (1.536–1.633) | 0.049 (0.048–0.051) | 0.026 |
|
| |||||||
| Infants, 1–11 mo | |||||||
| Maternal heightc | 0.983 (0.981–0.984) | 0.0007 (0.0006–0.0007) | 0.988 (0.987–0.989) | 0.0005 (0.0004–0.0005) | |||
|
| |||||||
| Maternal height, cm | |||||||
| ≥160 | 27 873 | 1 [Reference] | 0.026 (0.025–0.027) | 0 | 1 [Reference] | 0.025 (0.024–0.026) | 0 |
|
| |||||||
| 155–159.9 | 26 023 | 1.087 (1.067–1.108) | 0.039 (0.038–0.040) | 0.013 | 1.059 (1.039–1.079) | 0.033 (0.032–0.033) | 0.008 |
|
| |||||||
| 150–154.9 | 25 330 | 1.209 (1.185–1.234) | 0.042 (0.041–0.043) | 0.016 | 1.143 (1.120–1.166) | 0.035 (0.034–0.035) | 0.010 |
|
| |||||||
| 145–149.9 | 15 566 | 1.356 (1.323–1.389) | 0.047 (0.046–0.048) | 0.021 | 1.231 (1.201–1.261) | 0.038 (0.037–0.038) | 0.013 |
|
| |||||||
| <145 | 6980 | 1.587 (1.537–1.639) | 0.056 (0.055–0.058) | 0.030 | 1.385 (1.342–1.430) | 0.043 (0.042–0.044) | 0.018 |
|
| |||||||
| Children, 12–59 mo | |||||||
| Maternal heightc | 0.985 (0.984–0.986) | 0.0006 (0.0006–0.0007) | 0.990 (0.989–0.992) | 0.0004 (0.0003–0.0004) | |||
|
| |||||||
| Maternal height, cm | |||||||
| ≥160 | 33 475 | 1 [Reference] | 0.024 (0.023–0.024) | 0 | 1 [Reference] | 0.021 (0.021–0.022) | 0 |
|
| |||||||
| 155–159.9 | 27 417 | 1.081 (1.061–1.100) | 0.035 (0.034–0.035) | 0.011 | 1.050 (1.032–1.068) | 0.027 (0.027–0.028) | 0.006 |
|
| |||||||
| 150–154.9 | 23 588 | 1.158 (1.136–1.181) | 0.037 (0.036–0.037) | 0.013 | 1.090 (1.069–1.111) | 0.028 (0.028–0.029) | 0.007 |
|
| |||||||
| 145–149.9 | 13 825 | 1.326 (1.294–1.359) | 0.042 (0.041–0.043) | 0.018 | 1.192 (1.164–1.221) | 0.031 (0.030–0.031) | 0.010 |
|
| |||||||
| <145 | 6036 | 1.560 (1.509–1.613) | 0.050 (0.048–0.515) | 0.026 | 1.331 (1.287–1.375) | 0.035 (0.033–0.036) | 0.014 |
Abbreviations: AP, absolute probabilities; CI, confidence interval; DAP, differences in AP; RR, relative risk.
Adjusted models were adjusted for mother’s age at birth and education; child’s sex, birth order, preceding birth interval, twin status, and era-born; household income, urban/rural; and country fixed effects. AP for categorical height calculated at mean values of covariates; AP for continuous height is the decrease in probability of child mortality for a 1-cm increase in height. DAP calculated as AP minus AP of reference category. Interaction coefficients from sensitivity analysis (maternal height × neonate [referent]; maternal height × infant [RR, 1.011; 95% CI, 1.009–1.013; P<.001]; maternal height × child [RR, 1.031; 95% CI, 1.028–1.034; P<.001]).
All coefficients are P<.001.
Per 1-cm increase.
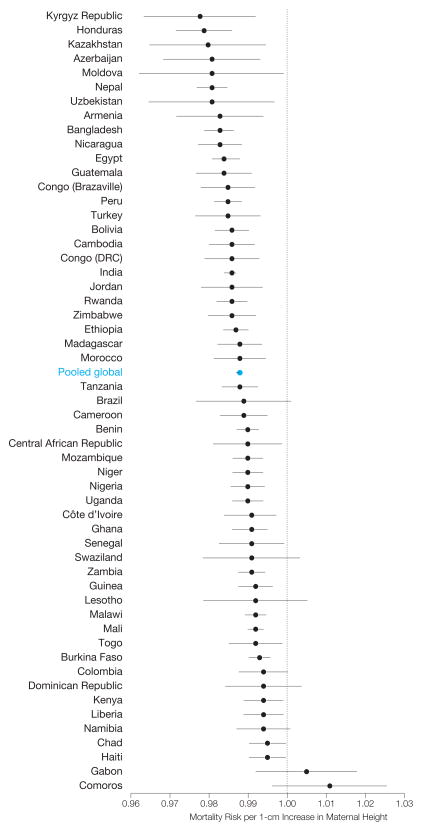
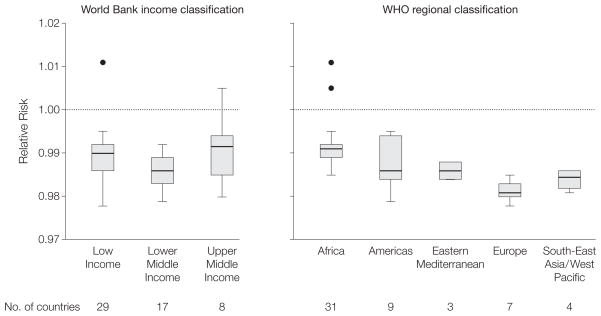
Country-specific risk of mortality by age 5 years for a 1-cm increase in maternal height ranged between 0.978 (95% CI, 0.964–0.992) for Kyrgyz Republic and 1.011 (95% CI, 0.996–1.026; P=.14) for Comoros. Mortality risk associated with increased height was decreased in 52 of 54 countries (96%) (ie, <1) and was statistically significant in 46 of those 54 countries (85%) (α=.05) (Figure 1). A joint Wald test of country-specific coefficients rejected the null hypothesis of equivalence, suggesting heterogeneity in the strength of the association between maternal height and offspring mortality across countries (χ2=165.37, P<.001) (eTable 5). The association between maternal height and offspring mortality remained among country groupings based on the World Bank income classification and WHO geographic regions (Figure 2).
Figure 1.
Country-Specific Adjusted Change in Offspring Mortality Risks for 1-cm Increase in Maternal Height Among Children Younger Than 5 Years
Plotted on log scale; error bars are 95% confidence intervals (CIs). Wald test for equivalence of coefficients rejected (χ2=165.37, P<.001). See eTable 5 for relative risks and CIs by country. Tanzania refers to the United Republic of Tanzania and Guinea refers to the Republic of Guinea. An interactive information graphic is available at http://www.jama.com.
Figure 2.
Box Plots of Relative Risk for the Association Between 1-cm Increase in Maternal Height and Offspring Mortality Among Children by World Bank Income Classification and WHO Regional Classification of Countries
WHO, World Health Organization; IQR, interquartile range. Plotted on log scale. For World Bank income classification, low income indicates $975 or less; lower middle income, $976–$3855; upper middle income, $3856–$11 905 (2008) gross national income per capita. Boxes indicate first and third quartiles with median lines; whiskers indicate first quartile minus 1.5 × IQR and third quartile + 1.5 × IQR; all values outside the whiskers are shown as filled circles (outliers). An interactive information graphic is available at http://www.jama.com.
Growth Failure
The sample size for the anthropometric analysis was 587 096, 558 347, and 568 609 children for underweight, stunting, and wasting, respectively; with sample sizes varying from 944 to 68 597 children for underweight, 897 to 64 028 children for stunting, and 924 to 65 487 children for wasting, in Comoros and India, respectively (Table 1). Of children in the sample, 21.5% were underweight (95% CI, 21.4%-21.6%), 38.3% were stunted (95% CI, 38.2%-38.5%), and 8.9% had wasting (95% CI, 8.8%-9.0%). The prevalence of underweight varied from 3.4% for Armenia to 44.5% for Bangladesh; stunting from 10.7% for Moldova to 55.2% for Ethiopia; and wasting from 1.3% for Honduras to 19.7% for India (Table 1). Results are reported by country in an interactive information graphic (see http://www.jama.com). The covariate distribution across categories of maternal height in the anthropometric data set was largely overlapping across covariates (eTable 6), and the patterns were similar to those reported earlier for the mortality data set.
In pooled adjusted models, a 1-cm increase in height was associated with a decreased risk in underweight (RR, 0.968; 95% CI, 0.968–0.969), stunting (RR, 0.968; 95% CI, 0.967–0.968), and wasting (RR, 0.994; 95% CI, 0.993–0.995) (Table 4). Compared with the tallest mothers (≥160 cm), each lower height category had substantially higher risk of offspring underweight and stunting, with the risk being highest for mothers shorter than 145 cm. Analysis stratified by child’s age showed no differential effect for underweight and stunting (eTable 7). An association was also observed between maternal height and severe growth failure (eTable 8). The RR for underweight, for a 1-cm increase in height, ranged from 0.930 (95% CI, 0.922–0.939) for Colombia to 0.991 (95% CI, 0.968–1.015; P=.40) for Uzbekistan, and was statistically significant in 50 of 54 countries (93%) (eFigure 1). For a 1-cm increase in height, the risk of stunting ranged from 0.926 (95% CI, 0.921–0.931) for Colombia to 0.993 (95% CI, 0.977–1.009; P=.39) for Azerbaijan, and was statistically significant in 52 of 54 countries (96%) (eFigure 2). The association between maternal height and wasting was weak and was statistically significant in only 11 of 54 countries (20%) (eFigure 3).
Table 4.
Unadjusted and Adjusted Changes in Risk, RR, AP, and DAP by Maternal Height for Offspring Underweight, Stunting, and Wasting Among Children Younger Than 5 Yearsa
| No. of Events | Unadjusted
|
Adjusted
|
|||||
|---|---|---|---|---|---|---|---|
| RR (95% CI)b | AP (95% CI) | DAP | RR (95% CI)b | AP (95% CI) | DAP | ||
| Underweight | |||||||
| Maternal heightc | 0.964 (0.963–0.965) | 0.0081 (0.0080–0.0083) | 0.968 (0.968–0.969) | 0.0068 (0.0067–0.0070) | |||
|
| |||||||
| Maternal height, cm | |||||||
| ≥160 | 26 871 | 1 [Reference] | 0.088 (0.087–0.090) | 0 | 1 [Reference] | 0.092 (0.090–0.093) | 0 |
|
| |||||||
| 155–159.9 | 29 142 | 1.272 (1.253–1.292) | 0.201 (0.198–0.203) | 0.113 | 1.241 (1.222–1.260) | 0.186 (0.184–0.189) | 0.094 |
|
| |||||||
| 150–154.9 | 33 171 | 1.545 (1.520–1.570) | 0.233 (0.230–0.236) | 0.145 | 1.467 (1.444–1.490) | 0.212 (0.209–0.215) | 0.120 |
|
| |||||||
| 145–149.9 | 24 298 | 1.912 (1.878–1.946) | 0.292 (0.288–0.297) | 0.204 | 1.751 (1.721–1.782) | 0.256 (0.252–0.260) | 0.164 |
|
| |||||||
| <145 | 12 753 | 2.453 (2.404–2.503) | 0.391 (0.383–0.398) | 0.303 | 2.152 (2.109–2.196) | 0.326 (0.320–0.332) | 0.234 |
|
| |||||||
| Stunting | |||||||
| Maternal heightc | 0.964 (0.963–0.964) | 0.0145 (0.0143–0.0147) | 0.968 (0.967–0.968) | 0.0126 (0.0124–0.0128) | |||
|
| |||||||
| Maternal height, cm | |||||||
| ≥160 | 44 816 | 1 [Reference] | 0.189 (0.187–0.191) | 0 | 1 [Reference] | 0.194 (0.192–0.196) | 0 |
|
| |||||||
| 155–159.9 | 50 762 | 1.271 (1.258–1.285) | 0.423 (0.419–0.427) | 0.243 | 1.242 (1.229–1.255) | 0.393 (0.389–0.396) | 0.199 |
|
| |||||||
| 150–154.9 | 56 642 | 1.544 (1.527–1.561) | 0.491 (0.487–0.495) | 0.311 | 1.475 (1.460–1.491) | 0.448 (0.444–0.452) | 0.254 |
|
| |||||||
| 145–149.9 | 40 877 | 1.922 (1.899–1.945) | 0.619 (0.613–0.625) | 0.439 | 1.779 (1.758–1.800) | 0.547 (0.542–0.553) | 0.353 |
|
| |||||||
| <145 | 21 023 | 2.393 (2.360–2.426) | 0.805 (0.795–0.815) | 0.625 | 2.132 (2.103–2.161) | 0.682 (0.673–0.690) | 0.488 |
|
| |||||||
| Wasting | |||||||
| Maternal heightc | 0.990 (0.988–0.991) | 0.0009 (0.0008–0.00010) | 0.994 (0.993–0.995) | 0.0005 (0.0004–0.0006) | |||
|
| |||||||
| Maternal height, cm | |||||||
| ≥160 | 14 402 | 1 [Reference] | 0.056 (0.054–0.058) | 0 | 1 [Reference] | 0.056 (0.054–0.057) | 0 |
|
| |||||||
| 155–159.9 | 12 687 | 1.051 (1.026–1.076) | 0.070 (0.069–0.072) | 0.014 | 1.029 (1.005–1.054)d | 0.064 (0.062–0.065) | 0.008 |
|
| |||||||
| 150–154.9 | 12 361 | 1.100 (1.072–1.128) | 0.073 (0.071–0.075) | 0.017 | 1.050 (1.024–1.077) | 0.065 (0.063–0.066) | 0.009 |
|
| |||||||
| 145–149.9 | 7866 | 1.197 (1.162–1.234) | 0.079 (0.077–0.081) | 0.023 | 1.109 (1.076–1.143) | 0.068 (0.664–0.070) | 0.012 |
|
| |||||||
| <145 | 3366 | 1.306 (1.256–1.358) | 0.087 (0.084–0.091) | 0.031 | 1.174 (1.129–1.222) | 0.073 (0.070–0.076) | 0.017 |
Abbreviations: AP, absolute probabilities; CI, confidence interval; DAP, differences in AP; RR, relative risk.
Adjusted models were adjusted for mother’s age at birth, marriage, occupation, and education; child’s sex, birth order, preceding birth interval, twin status, and era-born; household income, urban/rural; and country fixed effects. AP for categorical height calculated at mean values of covariates; AP for continuous height is the decrease in probability of child growth failure for a 1-cm increase in height. DAP calculated as AP minus AP of reference category.
All coefficients have P<.001.
Per 1-cm increase.
P<.05.
Sensitivity Analysis
We examined if there were substantial differences in the prevalence of mortality, underweight, stunting, and wasting among women who did and did not report maternal height, and observed considerable overlap in the prevalence distribution (eTable 9). No differences were found in maternal height among children who were measured and not measured for height or weight (eTable 10). The main results on mortality and growth failure appeared to be robust to sensitivity tests that included removing outliers, specifying maternal height in various ways to test for nonlinearity, excluding India from the overall analysis, restricting the analysis to mothers whose first born was after age 20 years, using overall wealth quintile, restricting the analysis to births in the last 5 years, restricting the analysis to surveys conducted after 2000, and to conditioning the association on only exogenous variables (eTable 7 and eTable 11). eTable 12 shows the mutually adjusted association between maternal height, select covariates and child mortality, underweight, stunting, and wasting.
COMMENT
Using large representative samples of children and mothers from 54 low-to middle-income countries, we observed a robust inverse association between maternal height and child mortality, underweight, and stunting. Our finding is suggestive of the importance of early life factors, not only for the subsequent health of a woman (as reflected in her attained stature)but also to her offspring’s health, and highlights the long-term effects of mother’s poor health stock. The weak association between wasting and maternal height may be because wasting reflects acute growth failure and is more likely to be influenced by contemporaneous factors as opposed to long-term effects of maternal health.
The association of maternal height and offspring health has a biomechanical plausibility (ie, shorter women have narrower pelves that increase the likelihood of cephalopelvic disproportion and obstructed labor)22,23 and a biological plausibility (ie, in shorter mothers who may have lower health stock, the supply of nutrients to the fetus may be inadequate, leading to intrauterine growth restriction and low birth weight, which can influence offspring health and survival).24 For mothers, limited nutrient supply at the cellular level during their development may lead to maintenance of basic metabolic functions taking precedence and resources being diverted away from growth, resulting in growth retardation and shortened stature.25 Furthermore, inflammation caused by infections has deleterious long-term health consequences,26 which may be transmitted to offspring. Because attained adult height reflects the stressful nutritional environment of the mother in early life,27 a plausible interpretation of an association between maternal stature and offspring health also reflects the intergenerational transfer of socioeconomic adversity.
Our study relies on DHS data that in general are considered to be of high quality and are often the only source of maternal and child health surveillance in developing countries.28 The comparability of the DHS data across countries and time, achieved through use of standardized questionnaires, manuals, field procedures, and technical support makes these data particularly appropriate for our study.28 Not-withstanding the strengths of the DHS data, recall bias in reporting offspring birth and death histories remains a potential concern.29 Furthermore, clustering of the age at death, particularly at 12 months, was a concern in country samples from sub-Saharan Africa.30 Despite objective measurements, a quality analysis of height data in 81 DHS surveys also showed clustering on whole and half centimeters, with some additional heaping at multiples of 5 and 10 cm.13 However, with the exception of Guatemala (1998–1999) and Bolivia (1994), the low levels of missing or implausible heights in almost all DHS surveys are taken as evidence that the quality of the recorded data be considered acceptable.13
The study findings should also be interpreted alongside the following caveats. First, the association between maternal height and offspring mortality, underweight, and stunting could arise due to unobserved factors common to both mother and child. Second, data constraints did not allow us to study how maternal height and child health are associated; restricting our assessment to whether there is an association and the extent to which it is common. Third, because women who may have died in childbirth are not observed, this could potentially lead to sampling bias in that causes of child mortality are linked to causes of maternal mortality with such women having a different covariate profile than those surviving. Fourth, contemporaneously measured covariates do not necessarily reflect the same covariates at the time of death of the child. In addition, although we show effect estimates pooled across countries, the assessment of the presence of an association between maternal height and offspring mortality, underweight, and stunting should be based on the consistency of the association in each country included in this study.
Our findings help resolve the mixed and inconclusive evidence observed in small nonrepresentative samples in a limited set of countries on the association between maternal height and child survival, with some suggesting a protective effect,31–35 while others show a null or reverse association.36–41 Besides confirming the overall inverse association between maternal stature and child mortality,42 our findings show that in more than 90% of the countries studied, children of shorter mothers were more likely to die, with robust association across different categories of country-level income as well as geographic regions. The consistent inverse association (both overall and country-specific) between maternal stature and child underweight and stunting is also novel as previous studies have been restricted to few countries, with small samples, and assessments of maternal stature largely restricted to childhood stunting.43–46
In conclusion, our study provides evidence that shorter maternal stature is a risk factor for offspring mortality, underweight, and stunting in developing countries. Underscoring the policy significance of this association, for child mortality, the effect of being in the lowest height category (relative to the tallest) was approximately 70% and 80% of the size of the effect of having no education or being in the poorest income quintile, respectively. For childhood underweight and stunting, maternal height was the most important factor, with an effect size about twice that of being in the lowest education category and 1.5 times that of being in the poorest quintile. This suggests the presence of an intergenerational transmission from mother’s own nutrition, disease, and socioeconomic circumstances during her childhood to her offspring’s health and mortality in their infancy and childhood.
Supplementary Material
Acknowledgments
Funding/Support: No direct financial support or funding was obtained to conduct this study. Dr Subramanian is supported by a career development grant NHLBI K25 HL081275 from the National Institutes of Health.
Role of the Sponsor: The National Institutes of Health and Macro International Inc did not participate in the design and conduct of the study, in the collection, analysis, and interpretation of the data, or in the preparation, review, or approval of the manuscript.
Footnotes
Author Contributions: Mr Özaltin had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Özaltin, Hill, Subramanian.
Acquisition of data: Özaltin.
Analysis and interpretation of data: Özaltin, Hill, Subramanian.
Drafting of the manuscript: Özaltin, Subramanian.
Critical revision of the manuscript for important intellectual content: Özaltin, Hill, Subramanian.
Statistical analysis: Özaltin, Hill.
Administrative, technical, or material support: Subramanian.
Study supervision: Subramanian.
Financial Disclosures: None reported.
Online-Only Material: eTables 1 through 12, eFigures 1 through 3, and an interactive information graphic are available at http://www.jama.com.
Additional Contributions: Macro International Inc, Washington, DC, provided access to the various Demographic and Health Surveys. David Canning, PhD (Economics and International Health, Harvard University, Boston, Massachusetts), and Christopher Murray, MD, DPhil (Institute for Health Metrics and Evaluation, University of Washington, Seattle), provided helpful discussions on the manuscript. Drs Canning and Murray did not receive any compensation for their contributions.
References
- 1.Kramer MS. The epidemiology of adverse pregnancy outcomes: an overview. J Nutr. 2003;133(5 suppl 2):1592S–1596S. doi: 10.1093/jn/133.5.1592S. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization. Maternal anthropometry and pregnancy outcomes: a WHO Collaborative Study. Bull World Health Organ. 1995;73(suppl):1–59. [PMC free article] [PubMed] [Google Scholar]
- 3.Black RE, Allen LH, Bhutta ZA, et al. Maternal and Child Undernutrition Study Group. Maternal and child undernutrition: global and regional exposures and health consequences. Lancet. 2008;371(9608):243–260. doi: 10.1016/S0140-6736(07)61690-0. [DOI] [PubMed] [Google Scholar]
- 4.Silventoinen K. Determinants of variation in adult body height. J Biosoc Sci. 2003;35(2):263–285. doi: 10.1017/s0021932003002633. [DOI] [PubMed] [Google Scholar]
- 5.Martorell R, Habicht JP. Growth in early childhood in developing countries. In: Falkner F, Tanner JM, editors. Human Growth: A Comprehensive Treatise. 3. New York, NY: Plenum Press; 1986. [Google Scholar]
- 6.Kelly A, Kevany J, de Onis M, Shah PMA WHO Collaborative. Study of maternal anthropometry and pregnancy outcomes. Int J Gynaecol Obstet. 1996;53(3):219–233. doi: 10.1016/0020-7292(96)02652-5. [DOI] [PubMed] [Google Scholar]
- 7.Subramanian SV, Ackerson LK, Davey Smith G, John NA. Association of maternal height with child mortality, anthropometric failure, and anemia in India. JAMA. 2009;301(16):1691–1701. doi: 10.1001/jama.2009.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. [Accessed February 1, 2010];Demographic and Health Surveys. http://www.measuredhs.com/
- 9.Rutstein SO, Rojas G. Guide to DHS Statistics. Calverton, MD: ORC Macro, MEASURE DHS+; 2003. [Google Scholar]
- 10.Macro O. Demographic and Health Survey Interviewer’s Manual. Calverton, MD: ORC Macro; 2006. [Google Scholar]
- 11.Wirth ME, Wirth E, Delamonica E, Sacks D, Balk A, Minujin A. Monitoring Health Equity in the MDGs: A Practical Guide. New York, NY: CIESIN/UNICEF; 2006. [Google Scholar]
- 12.Vaessen M. The potential of the demographic and health surveys (DHS) for the evaluation and monitoring of maternal and child health indicators. In: Khlat M, editor. Demographic Evaluation of Health Programmes (Proceedings) Paris, France: CICRED/UNFPA; 1996. [Google Scholar]
- 13.Pullum TW. An Assessment of the Quality of Data on Health and Nutrition in the DHS Surveys, 1993–2003. Calverton, MD: Macro International Inc; 2008. [Google Scholar]
- 14.Macro International Inc. Sampling Manual: DHS-III. Calverton, MD: Macro International Inc; 1996. [Google Scholar]
- 15.World Health Organization. WHO Child Growth Standards: Length/Height-for-Age, Weight-for-Age, Weight-for-Length, Weight-for-Height and Body Mass Index-for-Age: Methods and Development. Geneva, Switzerland: World Health Organization; 2006. [Google Scholar]
- 16.Filmer D, Pritchett LH. Estimating wealth with-out expenditure data—or tears: an application to educational enrollments in states of India. Demography. 2001;38(1):115–132. doi: 10.1353/dem.2001.0003. [DOI] [PubMed] [Google Scholar]
- 17.Gwatkin DR, Rustein S, Johnson K, Pande RP, Wagstaff A. Socioeconomic Differences in Health, Nutrition, and Population in India. Washington, DC: World Bank; 2000. [Google Scholar]
- 18.Rutstein SO, Johnson K. The DHS Wealth Asset Index. 6 Calverton, MD: ORC Macro; 2004. [Google Scholar]
- 19.Zou G. A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159(7):702–706. doi: 10.1093/aje/kwh090. [DOI] [PubMed] [Google Scholar]
- 20.Cheung YB. A modified least squares regression approach to the estimation of risk difference. Am J Epidemiol. 2007;166(11):1337–1344. doi: 10.1093/aje/kwm223. [DOI] [PubMed] [Google Scholar]
- 21.Statistical Software: Release 10SE [computer program] College Station, TX: Stata Corp; 2008. [Google Scholar]
- 22.Rush D. Nutrition and maternal mortality in the developing world. Am J Clin Nutr. 2000;72(1 suppl):212S–240S. doi: 10.1093/ajcn/72.1.212S. [DOI] [PubMed] [Google Scholar]
- 23.van Roosmalen J, Brand R. Maternal height and the outcome of labor in rural Tanzania. Int J Gynaecol Obstet. 1992;37(3):169–177. doi: 10.1016/0020-7292(92)90377-u. [DOI] [PubMed] [Google Scholar]
- 24.Hart N. Famine, maternal nutrition and infant mortality: a re-examination of the Dutch Hunger Winter. [DOI] [PubMed] [Google Scholar]
- 25.Martorell R. Body size, adaptation and function. Hum Organ. 1989;48(1):15–20. [Google Scholar]
- 26.Finch CE, Crimmins EM. Inflammatory exposure and historical changes in human life-spans. Science. 2004;305(5691):1736–1739. doi: 10.1126/science.1092556. [DOI] [PubMed] [Google Scholar]
- 27.Steckel RH. Biological measures of the standard of living. J Econ Perspect. 2008;22(1):129–152. doi: 10.1257/jep.22.1.129. [DOI] [PubMed] [Google Scholar]
- 28.Johnson K, Grant M, Khan S, Moore Z, Armstrong A, Sa Z. Fieldwork-Related Factors and Data Quality in the Demographic and Health Surveys Program. Calverton, MD: Macro International; 2009. [Google Scholar]
- 29.Boerma JT, Sommerfelt AE. Demographic and health surveys (DHS): contributions and limitations. World Health Stat Q. 1993;46(4):222–226. [PubMed] [Google Scholar]
- 30.Sullivan JM, Bicego GT, Rutstein SO. Systems IfRDM. An Assessment of DHS-I Data Quality: Vol Demographic and Health Surveys Methodological Reports No. 1 (December) Columbia, MD: Institute for Resource Development/Macro Systems Inc; 1990. Assessment of the quality of data used for the direct estimation of infant and child mortality in the Demographic and Health Surveys; pp. 113–143. [Google Scholar]
- 31.Allal N, Sear R, Prentice AM, Mace R. An evolutionary model of stature, age at first birth and reproductive success in Gambian women. Proc Biol Sci. 2004;271(1538):465–470. doi: 10.1098/rspb.2003.2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Baqui AH, Arifeen SE, Amin S, Black RE. Levels and correlates of maternal nutritional status in urban Bangladesh. Eur J Clin Nutr. 1994;48(5):349–357. [PubMed] [Google Scholar]
- 33.Mueller WH. Fertility and physique in a malnourished population. Hum Biol. 1979;51(2):153–166. [PubMed] [Google Scholar]
- 34.Pollet TV, Nettle D. Taller women do better in a stressed environment: height and reproductive success in rural Guatemalan women. Am J Hum Biol. 2008;20(3):264–269. doi: 10.1002/ajhb.20708. [DOI] [PubMed] [Google Scholar]
- 35.Sear R, Allal N, Mace R. Height, marriage and reproductive success in Gambian women. In: Alvard M, editor. Research in Economic Anthropology. New Milford, CT: Elsevier; 2004. pp. 203–224. [Google Scholar]
- 36.Devi MR, Kumari JR, Srikumari CR. Fertility and mortality differences in relation to maternal body size. Ann Hum Biol. 1985;12(5):479–484. doi: 10.1080/03014468500008031. [DOI] [PubMed] [Google Scholar]
- 37.Frisancho AR, Sanchez J, Pallardel D, Yanez L. Adaptive significance of small body size under poor socioeconomic conditions in Southern Peru. Am J Phys Anthropol. 1973;39(2):255–261. doi: 10.1002/ajpa.1330390216. [DOI] [PubMed] [Google Scholar]
- 38.Kirchengast S. Differential reproductive success and body size in! Kung San people from northern Namibia. Coll Antropol. 2000;24(1):121–132. [PubMed] [Google Scholar]
- 39.Lasker GW, Thomas R. Relationship between reproductive fitness and anthropometric dimensions in a Mexican population. Hum Biol. 1976;48(4):775–791. [PubMed] [Google Scholar]
- 40.Strickland SS. Functional consequences of adult malnutrition in developing countries: a review. J Physiol Anthropol Appl Human Sci. 2002;21(1):1–9. doi: 10.2114/jpa.21.1. [DOI] [PubMed] [Google Scholar]
- 41.Strickland SS, Tuffrey VR. Form and Function: A Study of Nutrition, Adaptation and Social Inequality in Three Gurung Villages of the Nepal Himalayas. London, England: Smith-Gordon; 1997. [Google Scholar]
- 42.Monden CW, Smits J. Maternal height and child mortality in 42 developing countries. Am J Hum Biol. 2009;21(3):305–311. doi: 10.1002/ajhb.20860. [DOI] [PubMed] [Google Scholar]
- 43.Behrman JR, Skoufias E. Correlates and determinants of child anthropometrics in Latin America: background and overview of the symposium. Econ Hum Biol. 2004;2(3):335–351. doi: 10.1016/j.ehb.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 44.Saleemi MA, Ashraf RN, Mellander L, Zaman S. Determinants of stunting at 6, 12, 24 and 60 months and postnatal linear growth in Pakistani children. Acta Paediatr. 2001;90(11):1304–1308. doi: 10.1080/080352501317130371. [DOI] [PubMed] [Google Scholar]
- 45.Sichieri R, Taddei JA, Everhart JE. Influence of parental height and sociodemographic factors on adolescent height in Brazil. J Adolesc Health. 2000;26(6):414–419. doi: 10.1016/s1054-139x(99)00004-x. [DOI] [PubMed] [Google Scholar]
- 46.Hernández-Díaz S, Peterson KE, Dixit S, et al. Association of maternal short stature with stunting in Mexican children: common genes vs common environment. Eur J Clin Nutr. 1999;53(12):938–945. doi: 10.1038/sj.ejcn.1600876. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.