Reduced somatostatin receptor sst2A internalization by the biased agonists SOM230 and KE108 results from both decreased receptor phosphorylation and a destabilization of the receptor-arrestin complex.
Abstract
The somatostatin receptor subtype 2A (sst2A) mediates many of somatostatin's neuroendocrine actions and is the primary therapeutic target for the stable somatostatin analogs used to inhibit hormone secretion by pituitary and gastroenteropancreatic tumors. Two new multireceptor targeting somatostatin analogs currently under clinical investigation, the multisomatostatin receptor agonist cyclo-[diaminoethylcarbamoyl-HydroxyPro-Phenylglycine-D-Trp-Lys-(4-O-benzyl)Tyr-Phe] (SOM230) (Pasireotide) and pan-somatostatin receptor agonist Tyr-cyclo-[D-diaminobutyric acid-Arg-Phe-Phe-D-Trp-Lys-Thr-Phe] (KE108), behave as functionally selective ligands at the sst2A receptor, mimicking some of somatostatin's actions but antagonizing others. Further, SOM230 and KE108 are less able to induce receptor internalization than somatostatin, indicating that they exhibit functional selectivity for receptor regulation as well as signaling. Here, we identify agonist-specific differences in the molecular events regulating sst2A receptor endocytosis. SOM230 and KE108 were less potent and less effective than somatostatin at stimulating sst2A receptor phosphorylation at two pairs of residues, Ser341/343 and Thr353/354. Only the pattern of Thr353/354 phosphorylation correlated with receptor internalization, consistent with the known importance of Thr phosphorylation for sst2A receptor endocytosis. As expected, arrestin recruitment to membrane receptors was reduced with SOM230 and KE108. In addition, both receptor dephosphorylation and receptor recycling occurred more rapidly with SOM230 and KE108 than with somatostatin. Surprisingly, however, SOM230 and KE108 also altered sst2A internalization in a phosphorylation-independent manner, because these analogs were less effective than somatostatin at stimulating the endocytosis of a phosphorylation-negative receptor mutant. These results show that the decreased receptor internalization produced by SOM230 and KE108 compared with somatostatin result from phosphorylation-independent effects as well as reduced site-specific receptor phosphorylation and receptor-arrestin association.
The two active forms of the neuroendocrine peptide somatostatin, namely somatostatin-14 (SS14) and SS28, act through a family of five G protein-coupled receptors (GPCR) to carry out their essential physiological functions, which include inhibition of hormone secretion by the pituitary gland and endocrine pancreas, inhibition of neurotransmitter release in the central and peripheral nervous system, and inhibition of exocrine secretion in the gastrointestinal tract. Because somatostatin receptors are often highly expressed in human neuroendocrine tumors, they have been targeted for both therapeutic and diagnostic applications (1–5). The somatostatin receptor subtype 2A (sst2A) is the most abundant and widely distributed somatostatin receptor expressed in neuroendocrine cancers. This receptor exhibits high affinity for octreotide and lanreotide, the first metabolically stable somatostatin analogs introduced into clinical practice to inhibit hormone secretion from pituitary and gastroenteropancreatic neuroendocrine tumors (2, 3, 5). The sst2A receptor also shows high affinity for 111In-diethylene triamine penta-acetic acid-octreotide (Octreoscan), the first radiolabeled somatostatin analog approved for the detection of somatostatin receptor-containing tumors by γ-camera scintigraphy (1, 4). However, these three analogs show no activity at sst1 and sst4 receptors and bind to the sst3 and sst5 receptor subtypes only weakly (2, 3, 6). Perhaps as a result of this receptor selectivity, octreotide and lanreotide are not universally effective against somatostatin receptor-expressing tumors: many neuroendocrine tumors are either resistant to therapy with octreotide and lanreotide from the start or develop resistance after chronic treatment. Because neuroendocrine tumors often express multiple sst receptor subtypes, new somatostatin analogs with high affinity for several somatostatin receptors are being developed in an effort to provide more effective medical therapies for octreotide and lanreotide resistant tumors. Two such multireceptor ligands are cyclo-[diaminoethylcarbamoyl-HydroxyPro-Phenylglycine-D-Trp-Lys-(4-O-benzyl)Tyr-Phe] (SOM230) or Pasireotide, which binds with high affinity to sst1, sst2, sst3, and sst5 (7), and Tyr-cyclo-[D-diaminobutyric acid-Arg-Phe-Phe-D-Trp-Lys-Thr-Phe] (KE108), which binds to all five sst receptor subtypes with nanomolar affinity (8). The goal in developing these multireceptor binding analogs was to mimic the action of the native hormone at all somatostatin receptors. Indeed, encouraging early studies showed that both SOM230 and KE108 potently inhibited adenylyl cyclase, the classic signaling pathway linked to all sst receptor subtypes (8–10).
Unexpectedly, however, SOM230 and KE108 did not always mimic the effect of somatostatin nor did they couple sst receptors to all the effector systems activated by the native hormone (11, 12). For example, in AR42J pancreatic acinar cells, which express sst2A receptors endogenously (13), somatostatin increased intracellular calcium accumulation and ERK phosphorylation, whereas SOM230 and KE108 antagonized these effects, even though all three peptides inhibited adenylyl cyclase to the same extent (12). Mechanistic studies showed that SOM230 and KE108 activated the coupling of sst2A receptors only to pertussis toxin sensitive G proteins, whereas the native peptide stimulated receptor coupling to both Gi/o-mediated inhibition of adenylyl cyclase and to pertussis toxin-insensitive signaling pathways (12).
The property of synthetic agonists to mimic only a subset of the actions produced by the native hormone at a particular receptor is now recognized as a general feature of GPCR signaling and has been termed functional selectivity. Compounds that direct a receptor to activate only select components of its biological repertoire have been named biased or protean agonists (14, 15). Functional selectivity has garnered a great deal of attention recently, not only because it illuminates basic aspects of GPCR behavior but also because of its significance for drug action and drug development (14, 16, 17). Such ligand-specific signaling is explained by the ability of agonists to stabilize distinct receptor conformations that then determine the differential interaction of the receptor with downstream transducing and regulatory proteins. As a result, the relative potencies or efficacies of agonists for particular biological end points may differ in a cell, even though their actions are triggered by a common receptor (14, 15). In fact, functionally selective agonists have been identified for several sst receptor subtypes (reviewed in Ref. 18).
In addition to signaling, GPCR regulation is also sensitive to the nature of the activating agonist and is instrumental in determining the dynamics and duration of the cellular response to drug stimulation. In fact, the relative activities of somatostatin analogs to regulate sst2A receptor internalization and G protein activation were shown to vary widely, characteristic of biased agonism (10). Among the 11 ligands examined in our previous study, SOM230 and KE108 were shown to be much less potent than SS14, SS28, or octreotide at inducing sst2A receptor endocytosis (10), an observation that was subsequently confirmed independently (19). However, the specific molecular events responsible for such differences are not clear.
Although the mechanisms involved in sst2A receptor internalization remain to be fully characterized, the basic steps are understood in some detail. After the binding of agonists, the activated sst2A receptor is rapidly phosphorylated by GPCR kinases (GRK) both in differentiated cell lines in culture and in human tumors in situ (13, 20–22). A combined approach, involving biochemical mapping, site directed mutagenesis, and phosphosite-specific antibodies, showed that at least five residues in the C terminus of the sst2A receptor become phosphorylated within minutes of hormone binding: namely Ser341, Ser343, Ser348, Thr353, and Thr354 (21). The two nonvisual arrestins, β-arrestin-1 and β-arrestin-2, are then recruited to the activated, phosphorylated sst2A receptor at the plasma membrane, and as with other class B GPCR, the two arrestins remain associated with the receptor in endosomal vesicles after receptor internalization by clathrin-mediated endocytosis (10, 23). Both arrestin association with the sst2A receptor and rapid agonist-stimulated receptor internalization require Thr phosphorylation but are independent of Ser phosphorylation (23). However, Ser and Thr phosphorylation are both necessary for sst2A receptor desensitization (23). Thus, different phosphorylated residues in the sst2A receptor have distinct biological roles. After removal of agonist, most of the internalized receptor is rapidly dephosphorylated (46) and recycled to the cell surface, ready for a second round of stimulation.
Our objective in this study was to elucidate the mechanisms involved in agonist selective regulation of sst2A receptor trafficking, focusing on the two biased agonists SOM230 and KE108. To this end, we first compared the effect of SOM230, KE108, and SS14 on the phosphorylation of the sst2A receptor at multiple specific Ser/Thr residues and examined the relationship between site-specific phosphorylation and receptor endocytosis. Next, we determined how arrestin-receptor association was affected by the nature of the activating agonist and its consequences for receptor dephosphorylation and trafficking. Finally, we determined whether differences in agonist-stimulated receptor internalization were entirely dependent on altered receptor phosphorylation.
Results
Differential effects of agonists on sst2A receptor internalization in pancreatic acinar cells
We previously reported that SOM230 and KE108 showed functional selectivity at the endogenously expressed sst2A receptor in AR42J cells and were unable to activate the full spectrum of signaling pathways regulated by the native hormone (12). To determine whether these analogs showed differential effects on sst2A receptor internalization, we compared the effects of SOM230, KE108, and SS14 on receptor endocytosis in this pancreatic cell line. Figure 1 shows that sst2A receptors are localized primarily at the surface of untreated AR42J cells. Exposure of cells to 100 nm SS14 triggered extensive receptor internalization within 2 min. By 5 min of hormone treatment, most of the receptors were internalized, and by 15 min, cell surface receptors were barely discernable, and the receptors had concentrated in a perinuclear region characteristic of distribution in the trans-Golgi network (TGN). This behavior resembles that previously seen in HEK293 cells, where treatment with somatostatin for 20 min led to the colocalization of sst2A receptors with mannose 6-phosphate receptors, a TGN marker (10).
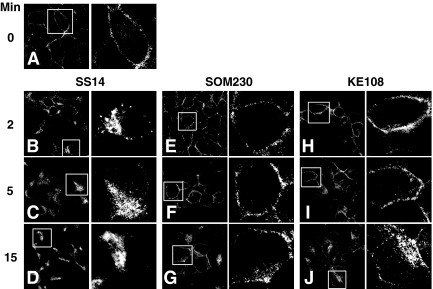
Fig. 1.
Agonist-specific effects on sst2A receptor internalization in pancreatic cells. AR42J cells were incubated at 37 C with 100 nm SS14, 1 μm SOM230, or 1 μm KE108 for the times shown. After agonist treatment, cells were rinsed with cold PBS, fixed, and permeabilized as described in Materials and Methods. sst2A receptors were immuno-labeled with receptor antibody followed with DTAF-conjugated donkey antirabbit IgG, and receptor distribution was then assessed by confocal microscopy. The images shown are representative of results from at least three independent experiments. The right panel of each pair of images shows an enlargement of a typical cell observed in the left panel. Although SS14 elicits pronounced receptor internalization within 2 min, SOM230 and KE108 have a much smaller effect on receptor endocytosis with substantial membrane staining still evident after 15 min of peptide treatment.
Treatment of AR42J cells with 1 μm SOM230 or KE108 produced much less receptor internalization than did SS14 (Fig. 1). In contrast to the vesicular receptors seen after SS14 treatment, sst2A receptors remained primarily associated with the plasma membrane after 2 min of analog exposure. Longer analog stimulation increased the accumulation of intracellular sst2A receptors, but in contrast to SS14, significant receptor density remained at the cell surface even after 15 min. These results demonstrate that despite their full efficacy to inhibit adenylyl cyclase in AR42J cells (12), SOM230 and KE108 did not stimulate sst2A receptor internalization as effectively as SS14.
Differential effects of agonists on sst2A receptor internalization in CHO-K1 cells
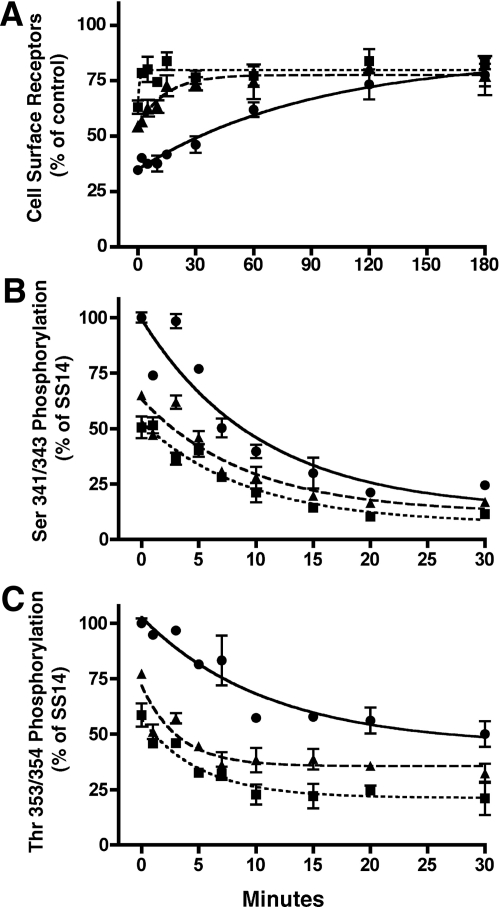
To elucidate the mechanisms responsible for the reduced ability of SOM230 and KE108 to induce sst2A receptor internalization, we took advantage of the CHO-K1 cell model that we used previously to determine the importance of site-specific receptor phosphorylation for sst2A receptor endocytosis (23). Because the relative potencies and efficacies of SS14, SOM230 and KE108 vary depending on the response measured (10, 12), we first compared the dose-response characteristics for analog inhibition of cAMP accumulation and sst2A receptor internalization (Fig. 2). All three peptides were full agonists for inhibition of adenylyl cyclase (Fig. 2A and Table 1). SS14 was the most potent with an EC50 of 0.73 nm, whereas SOM230 and KE108 exhibited lower potencies of 17.5 and 12.1 nm, respectively (Table 1) (10).
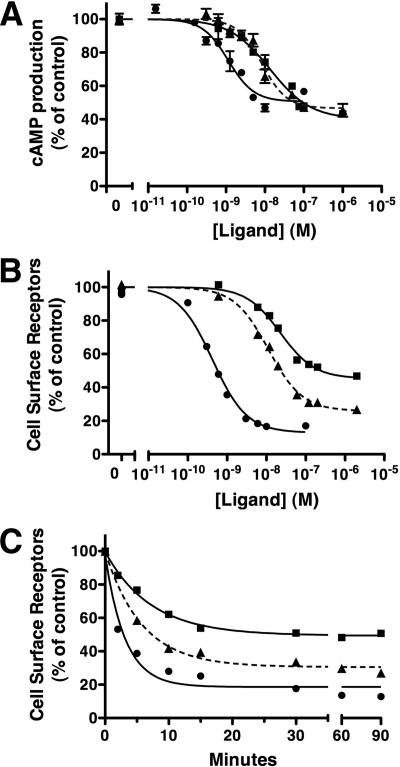
Fig. 2.
Agonist-specific effects on sst2A receptor signaling and internalization in CHO cells. A, CHO-sst2A cells, preincubated with [3H]adenine for 3 h at 37 C, were subsequently incubated with 1 mm IBMX for 5 min. Cells were then treated for 10 min at 37 C with 10 μm forskolin in the presence of different concentrations of SS14 (●), KE108 (▴), or SOM230 (■). Reactions were stopped by the addition of 5% trichloroacetic acid, and intracellular cAMP was measured as described in Materials and Methods. Cyclic AMP in each group is expressed as a percentage of that measured in cells treated with forskolin alone. B and C, CHO-sst2A cells were preincubated with HA antibody to label cell surface receptors and then treated at 37 C with varying concentrations of SS14 (●), KE108 (▴), or SOM230 (■) for 30 min (B) or with 100 nm SS14 (●), 1 μm KE108 (▴), or 1 μm SOM230 (■) for varying times (C). After fixation with paraformaldehyde, surface receptors were quantitated by ELISA as described in Materials and Methods. Receptors remaining at the cell surface after peptide treatment are expressed as a percentage of untreated control cells. Dose-response data were fit to a one-component sigmoidal curve using least squares nonlinear regression analysis with GraphPad Prism. The rates of receptor internalization were calculated using nonlinear regression curve fitting to a one-phase exponential decay. Each panel shows the mean ± sem from multiple wells in a single experiment and is representative of at least three independent experiments summarized in Tables 1 and 2. Where not shown, error bars fell within the size of the symbols.
Table 1.
Dose dependence for somatostatin analog-induced sst2A receptor signaling and internalization
| Analog | Internalization |
cAMP inhibition |
||
|---|---|---|---|---|
| EC50 (nm) | Max response (% of SS14) | EC50 (nm) | Max response (% of SS14) | |
| SS14 | 0.4 ± 0.1 | 100 | 0.73 ± 0.13 | 100 |
| KE108 | 23.6 ± 4.1 | 90.2 ± 1.7 | 12.1 ± 3.3 | 100 |
| SOM230 | 23.3 ± 5.7 | 70.2 ± 3.5 | 17.5 ± 5.1 | 100 |
Dose responses for inhibition of forskolin-stimulated cAMP production and stimulation of receptor endocytosis were determined as described in Materials and Methods and shown in Fig 2. The values for EC50 and maximal (Max) effect were calculated by nonlinear regression analysis of sigmoidal dose-response curves as described under Materials and Methods and are given as the mean ± sem of the values obtained in at least three independent experiments. Values for cAMP inhibition were from Ref. 10.
In parallel with their inhibitory effects on cAMP production, KE108 and SOM230 were also less potent than SS14 at inducing receptor endocytosis (Fig. 2B). In fact, the EC50 values for inhibition of cAMP production and stimulation of receptor internalization agreed closely for all three peptides (Table 1). Notably, however, maximal concentrations of SOM230 and KE108 were unable to stimulate sst2A receptor internalization to the same extent as SS14.
Figure 2C shows the time course of receptor endocytosis after stimulation of CHO-sst2A cells with 100 nm SS14, 1 μm SOM230, and 1 μm KE108 (Fig. 2C). There was no measurable difference in the rate of receptor endocytosis after SS14 and analog stimulation (t1/2 ≈ 4 min), and the steady state level of cell surface receptors was maintained for at least 90 min in the continued presence of all three agonists (Fig. 2C and Table 2). Again, however, the extent of receptor internalization at steady state was less after SOM230 and KE108 treatment than after SS14 exposure (Fig. 2C and Table 2).
Table 2.
Analog-induced sst2A receptor internalization
| Analog | Half-life (min) | Maximal internalization (% of total) |
|---|---|---|
| SS14 | 4.3 ± 0.7 | 82.8 ± 1.5 |
| KE108 | 4.5 ± 0.2 | 74.2 ± 0.9 |
| SOM230 | 4.4 ± 0.3 | 46.0 ± 3.2 |
The rate of sst2A receptor endocytosis following stimulation of CHO-sst2A cells with 100 nm SS14, 1 μm SOM230, or 1 μm KE108 was measured as described in Fig 2. Values for the half-time and extent of receptor internalization were determined by nonlinear regression analysis of three to seven independent experiments. Data are presented as the mean ± sem. Statistical analysis was carried out using Dunnett's multiple comparison test and showed that maximal internalization with SOM230 (P < 0.01) and KE108 (P < 0.05) both differed significantly from that produced by SS14.
Together, these results demonstrate that SOM230 and KE108 act as full agonists for inhibition of cAMP production but behave as partial agonists for receptor endocytosis.
Differential effects of agonists on site-specific sst2A receptor phosphorylation
Our earlier studies showed that the sst2A receptor is phosphorylated on multiple Ser and Thr residues after SS14 stimulation and that distinct phosphorylation sites are involved in receptor internalization, desensitization, and arrestin binding (23, 24). Using phosphosite-specific receptor antibodies, we identified Ser341/343 and Thr353/354 in the receptor C terminus as substrates for GPCR GRK-dependent phosphorylation after SS14 treatment (21). To determine whether compromised receptor phosphorylation could be responsible for the reduced ability of SOM230 and KE108 to induce sst2A receptor endocytosis, we compared the effect of these analogs on receptor internalization and site-specific receptor phosphorylation measured under identical conditions.
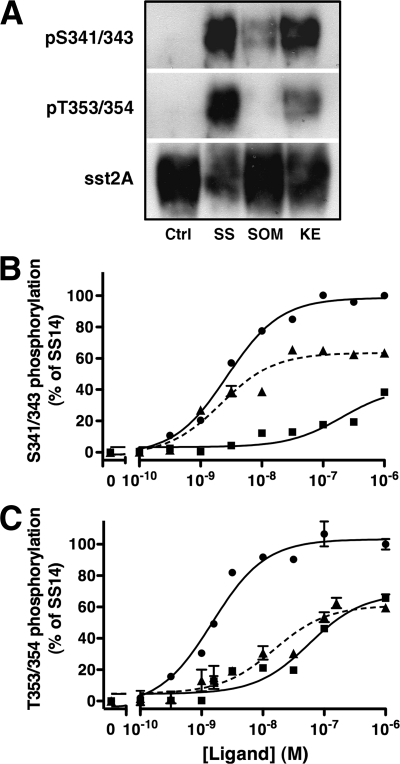
We first examined sst2A receptor phosphorylation by immunoblotting receptors prepared from analog treated CHO-sst2A cells according to our previously established protocol (21) using two phosphosite-specific receptor antibodies: 2C12, which recognizes phosphorylated Ser341/343, and 1A2, which recognizes phosphorylated Thr353/354. Treatment of cells with 10 nm SS14 for 30 min stimulated sst2A receptor phosphorylation at Thr353/354 to a much greater extent than did 10 nm SOM230 or KE108 (Fig. 3A). Surprisingly, however, 10 nm KE108 was almost as effective as SS14 at stimulating Ser341/343 phosphorylation, and both peptides were more effective than SOM230 (Fig. 3A). To accurately quantitate analog-induced receptor phosphorylation, we measured the dose-dependent effects of each peptide on site-specific receptor phosphorylation with a whole-cell ELISA (Fig. 3, B and C, and Table 3). CHO-sst2A cells were incubated with varying concentrations of each agonist for 30 min at 37 C, fixed, and permeabilized. Receptor phosphorylation was then measured with ELISA using the two phosphosite-specific antibodies (Fig. 3, B and C, and Table 3). Although SOM230 and KE108 were both less efficacious than SS14 at stimulating phosphorylation at all receptor phosphosites (P < 0.01) (Table 3), they differed markedly in their effect at different residues. Whereas SS14 increased the phosphorylation of Ser341/343 and Thr353/354 with similar potencies (EC50 = 2.5 and 1.1 nm, respectively; P > 0.05), SOM230 and KE108 produced strikingly different patterns of receptor phosphorylation (Fig. 3 and Table 3). KE108 was substantially more potent at stimulating phosphorylation of Ser341/343 (EC50 = 2.5 nm) than Thr353/354 (EC50 = 17.3 nm) (P < 0.01), whereas SOM230 was more potent at stimulating Thr353/354 phosphorylation (EC50 = 49.5 nm) than Ser341/343 phosphorylation (EC50 > 200 nm; P < 0.01) (Fig. 3 and Table 3). These unexpected results demonstrate that the phosphorylation of neighboring residues in the sst2A receptor can be regulated independently.
Fig. 3.
Agonists-specific patterns of sst2A receptor phosphorylation. A, CHO-sst2A cells were incubated at 37 C for 30 min with 10 nm SS14, KE108, or SOM230. Cells were then solubilized, and equal protein samples from each treatment group were purified by adsorption to wheat germ agglutinin-agarose followed by SDS-PAGE and sequential immunoblotting with the phosphosite-specific antibodies shown and then with HA antibody to show receptor loading. B and C, CHO-sst2A cells were incubated at 37 C for 30 min with varying concentration of SS14 (●), KE108 (▴), or SOM230 (■). Cells were then fixed, and receptor phosphorylation at Ser341/343 (B) or Thr353/354 (C) was measured by whole-cell ELISA using phosphosite-specific antibodies. Receptor phosphorylation is expressed as a percent of the response produced by 1 μm SS14. Nonlinear regression curve fitting was performed using GraphPad Prism. Each panel shows the mean ± sem from a single experiment and is representative of at least three independent experiments, summarized in Table 3.
Table 3.
Site-specific stimulation of sst2A receptor phosphorylation by somatostatin analogs
| Analog | Receptor phosphorylation |
|||
|---|---|---|---|---|
| Ser341/343 |
Thr353/354 |
|||
| EC50 (nm) | Max response (% of SS14) | EC50 (nm) | Max response (% of SS14) | |
| SS14 | 2.5 ± 0.6 | 100 | 1.1 ± 0.1 | 100 |
| KE108 | 2.5 ± 0.5 | 65.2 ± 3.1 | 17.3 ± 1.5 | 66.5 ± 11.6 |
| SOM230 | >200 | 48.4 ± 9.7 | 49.5 ± 1.1 | 74.8 ± 2.8 |
The dose response for agonist stimulation of sst2A receptor phosphorylation was measured at Ser341/343 and Thr353/354 as described in Fig 3. The values for EC50 and maximal (Max) effect were calculated by nonlinear regression analysis of sigmoidal dose-response curves as described under Materials and Methods and are given as the mean ± sem of the values obtained in at least three independent experiments. The effect of each analog on receptor phosphorylation at distinct sites was analyzed using ANOVA and Dunnett's multiple comparison test. The EC50 values for Ser341/343 phosphorylation differed significantly from the EC50 values for Thr353/354 phosphorylation for both SOM230 (P < 0.05) and KE108 (P < 0.01) but not for SS14.
To determine the relationship between receptor internalization and receptor phosphorylation, we compared the effect of each analog to stimulate site-specific phosphorylation (Fig. 3 and Table 3) with its ability to induce receptor endocytosis (Fig. 2 and Table 1). The potencies of KE108 and SOM230 to promote Ser341/343 phosphorylation differed significantly from their potencies to stimulate receptor endocytosis (P ≤ 0.01, Dunnett's multiple comparison test). In contrast, The EC50 values for Thr353/354 phosphorylation and internalization were indistinguishable for both analogs (P > 0.05).
Together, these results demonstrate that SOM230 and KE108 have distinct effects on sst2A receptor phosphorylation at neighboring phosphorylation sites. Further, although phosphorylation of Thr353/354 correlates closely with receptor endocytosis for both analogs, phosphorylation of Ser341/343 does not.
Agonist-specific interaction between sst2A receptors and β-arrestin
We previously showed that a phosphorylation negative sst2A receptor mutant (Phos−/sst2A) exhibits markedly reduced agonist-stimulated endocytosis and arrestin binding compared with the wild-type (WT) receptor, indicating that SS14-stimulated receptor phosphorylation is required for both β-arrestin association with the receptor and rapid receptor internalization (23). Because of their different effects on sst2A receptor phosphorylation, we next determined the effect of SOM230, KE108, and SS14 on sst2A receptor-arrestin binding.
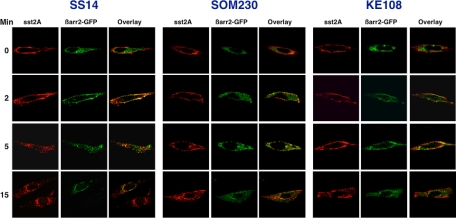
CHO-K1 cells were transfected with the sst2A receptor and enhanced green fluorescent protein (EGFP)-β-arrestin-2 and then cultured for 48 h. Cells were subsequently incubated with hemagglutinin (HA) antibody on ice to label cell surface receptors and then stimulated at 37 C with maximal concentrations of different agonists. After fixation, permeabilization, and immunolabeling, cells were examined by confocal fluorescence microscopy to determine the distribution of the sst2A receptor (red) and EGFP-β-arrestin-2 (green) after various times of peptide treatment (Fig. 4). Under basal conditions (t = 0), the receptor was localized at the plasma membrane, whereas β-arrestin-2 was distributed throughout the cytosol. After exposure of cells to each agonist for 2 min, β-arrestin-2 was translocated from the cytosol to the cell surface, where it often colocalized with the receptor in punctate fluorescent clusters. However, the extent of arrestin translocation was greater after 2 min of SS14 stimulation than after the same treatment with SOM230 or KE108: SS14 stimulation usually depleted the cytosol of arrestin, whereas incubation with SOM230 or KE108 only partially reduced cytosolic arrestin (Fig. 4). Longer exposure to the native peptide (5 and 15 min) caused endocytosis of the sst2A receptor-β-arrestin complex first into cytoplasmic vesicles and subsequently into a perinuclear compartment. Substantial colocalization of the receptor and arrestin was observed during SS14-stimulated receptor internalization even after 15 min of treatment. In contrast, although SOM230 and KE108 also stimulated sst2A receptor internalization, the receptor containing vesicles remained distributed throughout the cytosol and did not concentrate around the nucleus (Fig. 4). Moreover, although some arrestin colocalization was observed with the internalized receptor after analog treatment, substantial cytosolic arrestin was evident at all times.
Fig. 4.
Agonist selective trafficking of the sst2A receptor and EGFP-β-arrestin-2. CHO-K1 cells were transfected with HA-tagged sst2A receptor and EGFP-β-arrestin-2 as described in Materials and Methods and grown for 48 h. Cells were then incubated with HA antibody for 2 h on ice to label cell surface receptors, washed to remove unbound antibody, and treated with 100 nm SS14, 1 μm SOM230, or 1 μm KE108 at 37 C for the times shown. After fixation and permeabilization, cells were incubated with Alexa Fluor 568-conjugated antimouse IgG. Images of antibody-labeled sst2A receptors (red) and EGFP-tagged arrestin (green) from the same cell were acquired with a Nikon A1 confocal microscope. The merged images depict receptor and β-arrestin-2 colocalization (yellow). Shown are representative cells from at least three independent experiments.
These experiments show that SS14, SOM230, and KE108 had a similar effect on sst2A receptor internalization in CHO cells (Fig. 4) as in AR42J cells (Fig. 1). However, although all three agonists stimulated some recruitment of β-arrestin-2 to receptors, the extent of receptor-arrestin association varied with the agonist.
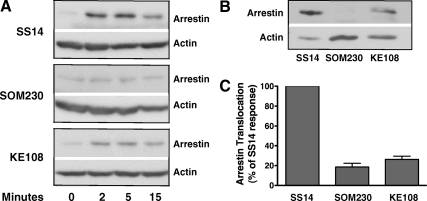
Because immunofluorescence microscopy provides only a qualitative assessment of receptor-arrestin association, we next used a translocation assay based on subcellular fractionation to quantitate β-arrestin binding after agonist stimulation (Fig. 5). CHO-K1 cells, transiently transfected with HA-sst2A and FLAG-tagged β-arrestin-2, were stimulated with a maximal concentration of each agonist for varying times. Cell lysates were then subjected to ultracentrifugation, and isolated membranes were analyzed by SDS-PAGE and immunoblotting for β-arrestin-2. Treatment of cells with 100 nm SS14 led to a large increase in membrane-associated β-arrestin-2 within 2 min, and arrestin remained membrane bound even after 15 min of SS14 stimulation (Fig. 5A), similar to the results observed by immunofluorescence microscopy (Fig. 4A). Much less β-arrestin-2 was associated with the receptor after stimulation with KE108, and β-arrestin recruitment was barely detectable after SOM230 stimulation (Fig. 5A).
Fig. 5.
Agonist-induced membrane translocation of β-arrestin-2. CHO cells were transiently transfected with FLAG-β-arrestin-2 and sst2A receptor. After 48 h, cells were incubated at 37 C with 100 nm SS14, 1 μm SOM230, or 1 μm KE108 for the times shown. After cell lysis, membranes were purified by centrifugation as described in Materials and Methods, and the membrane fraction was subjected to SDS-PAGE. Membrane-associated β-arrestin-2 and actin were detected by incubating each blot with anti-FLAG and antiactin antibodies. Band intensities were quantitated from scanned autoradiograms using Scion Image (version 1.63). A, Time course of arrestin translocation to membranes after peptide treatment in representative experiments. The actin band provides a loading control. B, Direct comparison of membrane-associated β-arrestin-2 after treatment of cells with 100 nm SS14, 1 μm SOM230, or 1 μm KE108 for 5 min in a representative experiment. C, Mean ± sem (n = 6) for membrane-bound arrestin after treatment of cells for 5 min with different analogs in experiments like the one shown in B. The ratio of arrestin to actin was calculated in each sample and then expressed as a percentage of the arrestin/actin ratio observed in samples treated with SS14 for 5 min.
We next directly compared the amount of β-arrestin bound to the receptor after stimulating cells with agonist for 5 min, the half-time for receptor internalization. As shown in Fig. 5, B and C, incubation with 1 μm SOM230 produced very little membrane accumulation of β-arrestin-2, whereas KE108 had a slightly greater effect although still substantially less than observed with SS14. Overall, these results parallel the reduced colocalization of receptor and β-arrestin observed by immunofluorescence microscopy after analog treatment (Fig. 4).
Together, the results show that SOM230 and KE108 are less able to recruit arrestin to the sst2A receptor than SS14, just as they are less effective at stimulating receptor phosphorylation.
Agonist dependence of sst2A receptor recycling and dephosphorylation
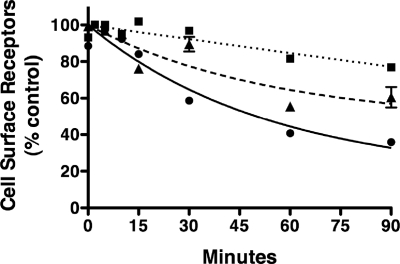
The stability of the receptor-arrestin complex is thought to be an important determinant for GPCR recycling (25, 26). Therefore, we next examined the rates of sst2A receptor recycling after treatment with SS14, SOM230, and KE108 (Fig. 6A). CHO-sst2A cells were first incubated with a maximal concentration of each agonist for 30 min to induce receptor internalization. Receptor recycling was then initiated by removing the agonist and adding fresh medium containing an sst2A receptor antagonist [3-(4-chlorophenyl)alanine-cyclo[DCys-Tyr-DTrp-NMethyl-Lys-Thr-Cys]-3-(2-naphtyl)alanine-NH2 (Coy-14)]. Cells were incubated at 37 C for various times to allow internalized receptors to recycle to the cell surface and then fixed to permit measurement of surface receptors by ELISA. As shown in Fig. 6A, the rate of sst2A receptor recycling was strikingly dependent on the agonist used to induce receptor endocytosis. In multiple independent experiments, the half-time for sst2A receptor recycling was 42.3 min (n = 6) after treatment with 100 nm SS14, 10.7 min (n = 5) after incubation with 1 μm KE108, and 4.8 min (n = 4) after incubation with SOM230 (Table 4). Thus, receptor recycling occurs four to nine times more quickly after treatment with KE108 and SOM230 than after SS14 stimulation.
Fig. 6.
Rate of sst2A receptor recycling and dephosphorylation after treatment with different agonists. CHO-sst2A cells were incubated for 30 min at 37 C in the absence of agonist or in the presence of 100 nm SS14 (●), 1 μm KE108 (▴), or 1 μm SOM230 (■). After peptide treatment, cells were washed and incubated at 37 C in fresh media without agonist but in the presence of 100 nm of the antagonist Coy-14. A, After different periods of recovery, cells were fixed with paraformaldehyde, and cell surface receptors were quantitated with anti-HA antibodies by ELISA as described in Materials and Methods. Receptors in treated samples were expressed as a percentage of receptors in untreated control cultures. Shown are replicate samples from a single experiment. Where not shown, error bars fell within the size of the symbols. B and C, After the incubation with antagonist, cells were fixed, and receptor phosphorylation was measured at Ser341/343 (B) or Thr353/354 (C) using the phosphosite-specific antibodies in a whole-cell ELISA. Receptor phosphorylation in treated samples was expressed as a percentage of the phosphorylation observed after treatment with SS14 for 30 min. The graphs represent the mean ± sem of triplicate samples in a representative experiment. In all three panels, rates of receptor dephosphorylation or receptor recovery were calculated using nonlinear regression curve fitting to a one-phase exponential curve with GraphPad Prism. Results from three to six independent experiments are summarized in Table 4.
Table 4.
Rates of analog-induced sst2A receptor recycling and dephosphorylation
| Analog | Receptor Recycling (min) | Receptor dephosphorylation |
|
|---|---|---|---|
| Ser341/343 (min) | Thr353/354 (min) | ||
| SS14 | 42.3 ± 7.4 | 6.5 ± 0.5 | 7.9 ± 0.3 |
| KE108 | 10.7 ± 1.9 | 5.8 ± 0.5 | 3.0 ± 0.5 |
| SOM230 | 4.8 ± 2.1 | 5.5 ± 0.5 | 2.7 ± 0.5 |
The rates of sst2A receptor recycling and receptor dephosphorylation were measured as described in Fig. 6 after stimulation of CHO-sst2A cells for 30 min with 100 nm SS14, 1 μm KE108, or 1 μm SOM230. Values for half-times were calculated by nonlinear regression analysis of three to six independent experiments, and data are presented as the mean ± sem.
Arrestin dissociation from phosphorylated receptors has been shown to occur within seconds after agonist removal and precedes receptor dephosphorylation (27, 28). Therefore, we next determined whether differences in the stability of the receptor-arrestin complex influenced the rate of sst2A receptor dephosphorylation. As in the receptor recycling experiments, CHO-sst2A cells were incubated with a maximal concentration of each agonist for 30 min to induce receptor phosphorylation, after which receptor dephosphorylation was initiated by removing agonist and adding antagonist-containing medium. After different times of incubation, residual receptor phosphorylation was measured by the whole-cell ELISA using phosphosite-specific antibodies (Fig. 6, B and C, and Table 4). After SS14 treatment, pSer341/343 and pThr353/354 were dephosphorylated with half-times of 6.5 and 7.9 min, respectively (Fig. 6 and Table 4). The rate of pSer341/343 dephosphorylation was unaffected by the agonist used (Fig. 6B and Table 4). In contrast, pThr353/354 dephosphorylation occurred faster after stimulation with KE108 (t1/2 = 3.0 min) and SOM230 (t1/2 = 2.7 min) than after SS14 treatment (7.9 min) (Fig. 6C and Table 4). These studies show that the nature of the agonist has a much more dramatic effect on receptor recycling than on receptor dephosphorylation (Fig. 6 and Table 4). Because the sst2A receptor is dephosphorylated more rapidly than it is recycled after SS14 and KE108 treatment (Table 4), receptor dephosphorylation is not the rate limiting step in receptor recycling with these peptides. In contrast, the rates of dephosphorylation and recycling are similar with SOM230, such that receptor dephosphorylation now appears to be rate limiting for receptor recycling. These results demonstrate agonist selective regulation of a critical step in sst2A receptor trafficking.
Phosphorylation-independent effect of agonists on sst2A receptor internalization
Although internalization of the phosphorylation negative sst2A receptor is compromised, SS14 is still able to stimulate the endocytosis of this receptor mutant, albeit to a much smaller extent than seen with the WT receptor (23). To determine whether phosphorylation-independent effects played a role in the impaired ability of KE108 and SOM230 to induce sst2A receptor internalization, we next examined the effect of these analogs on the endocytosis of the phosphorylation negative sst2A receptor.
CHO cells stably expressing Phos−/sst2A were incubated with maximal concentrations of SS14, SOM230, or KE108, and the rate of receptor internalization was assessed by ELISA (Fig. 7). As previously observed (23), SS14 treatment led to the internalization of the Phos−/sst2A receptor at a rate (t1/2 = 32.9 ± 3.6 min) much reduced from that of the WT receptor (t1/2 = 4.3 ± 0.7 min). Surprisingly, SOM230 and KE108 were substantially less effective at stimulating the internalization of the Phos−/sst2A receptor than was SS14 (Fig. 7). These results demonstrate that the reduced effect of SOM230 and KE108 on sst2A receptor internalization do not result entirely from changes in sst2A receptor phosphorylation. Rather, differences in the conformation of the ligand-bound receptor must exert an effect, either directly or indirectly, on agonist-induced sst2A receptor endocytosis.
Fig. 7.
Rate of agonist-induced internalization of a phosphorylation negative sst2A receptor mutant. CHO cells stably expressing the phosphorylation negative sst2A receptor, Phos−/sst2A, were incubated for the times shown at 37 C with 100 nm SS14 (●), 1 μm KE108 (▴), or 1 μm SOM230 (■). Cell surface receptors were then measured by ELISA as described in Materials and Methods and expressed as a percent of untreated control cells. Shown is the mean ± sem of cell surface receptors determined in replicate samples in a single experiment, representative of two to five independent experiments. Where not visible, error bars fell within the symbols. Nonlinear regression curve fitting to a one-phase exponential decay was performed using GraphPad Prism. The half-times for receptor internalization from multiple independent experiments was 32.9 ± 3.6 min with SS14 (n = 5) and greater than 10 h with KE108 and SOM230.
Discussion
GPCR trafficking is a critical component of receptor regulation and signaling, because it modulates cellular responsiveness to hormones and drugs (26, 29, 30). Thus, understanding the mechanisms of ligand-directed sst2A receptor endocytosis is essential for the effective use of somatostatin analogs to control the secretion and growth of neuroendocrine tumors with continued efficacy. The somatostatin analogs SOM230 and KE108 were developed to mimic the effects of the native peptide at multiple sst receptor subtypes (8, 9). However, these analogs do not mimic SS14 signaling at the sst2A receptor but instead behave as biased agonists (12). Here, we demonstrate that SOM230 and KE108 were less effective than SS14 at inducing sst2A receptor internalization in both cultured pancreatic AR42J cells, which express the sst2A receptor endogenously, and in sst2A receptor-transfected CHO-K1 cells. These results parallel previous observations showing that iv administration of SOM230 produced less sst2A receptor internalization in vivo than did [Tyr(3), Thr(8)]-octreotide (31). In studies aimed at understanding the mechanisms responsible for analog-specific regulation of sst2A receptor endocytosis, we found that SOM230 and KE108 were less effective than SS14 at stimulating receptor phosphorylation. Unexpectedly, and in striking contrast to the native peptide, analog stimulation of sst2A receptor phosphorylation differed between phosphorylation sites. Although the potency of SOM230 and KE108 to induce sst2A phosphorylation at Thr353/354 correlated closely with their respective potencies to induce endocytosis, their effect on Ser341/343 phosphorylation did not. SOM230 and KE108 were also less effective than SS14 at inducing arrestin recruitment to membrane receptors, consistent with the importance of Thr phosphorylation for sst2A receptor-arrestin association (23). Interestingly, however, the compromised effect of SOM230 and KE108 on receptor phosphorylation did not completely account for the reduced receptor internalization observed with these analogs, because SOM230 and KE108 did not stimulate the endocytosis of a phosphorylation negative sst2A receptor as effectively as the native peptide. Moreover, receptor recycling occurred much more rapidly at the cessation of SOM230 and KE108 stimulation than after SS14 treatment. These observations suggest that SOM230 and KE108 stabilize different receptor structures than SS14. Consistent with current understanding of biased agonism, such ligand-specific receptor conformations could produce the observed differences in agonist-stimulated endocytosis of the Phos−/sst2A receptor and in the kinetics of receptor recycling after receptor dephosphorylation. Thus, our studies provide new insights into the complex mechanisms involved in agonist-specific regulation of the sst2A receptor and should help guide the development of more effective somatostatin analogs for tumor treatment.
Most GPCR are regulated by GRK-catalyzed phosphorylation at multiple intracellular sites, and there is growing evidence that such multisite phosphorylation is required for the finely tuned coordination of distinct signaling events initiated by phosphorylation of individual sites (32–34). However, little is known about how the phosphorylation of specific residues in GPCR can be independently regulated. Early studies showed that agonist-induced phosphorylation of the sst2A receptor exhibits just such complexity, occurring on Ser and Thr in both the C-terminal tail and third intracellular loop of the receptor (13, 24). Although not all the sst2A receptor residues phosphorylated upon agonist stimulation have been identified, recent studies have shown that somatostatin stimulation increases phosphorylation of at least six amino acids in the C terminus: namely Ser at positions 341, 343, and 348 and Thr at position 353, 354, and 356 (21, 35). Therefore, we used a whole-cell ELISA with phosphosite-specific antibodies to quantitatively compare the effects of SOM230, KE108, and SS14 on agonist-stimulated sst2A receptor phosphorylation at two neighboring pairs of residues: Ser341/343 and Thr353/354. SS14 was equipotent at stimulating sst2A receptor phosphorylation at both sets of phosphorylation sites. Surprisingly, however, SOM230 and KE108 produced strikingly different effects on sst2A receptor phosphorylation at different residues. KE108 was seven times more potent at stimulating Ser341/343 phosphorylation than Thr353/354 phosphorylation, such that at concentrations between 1 and 10 nm, substantial Ser341/343 phosphorylation was observed with a minimal increase in Thr353/354 phosphorylation (Fig. 3). In contrast, SOM230 was much more potent at stimulating Thr353/354 phosphorylation than Ser341/343 phosphorylation, such that at concentrations between 10 and 100 nm, SOM230 increased Thr353/354 phosphorylation without affecting Ser341/343 phosphorylation (Fig. 3). Thus, depending on the agonist and its concentration, sst2A receptor phosphorylation will occur on one pair of residues without a corresponding change in the phosphorylation state of the other. It remains to be determined whether this striking specificity results from the action of different GRK subtypes, from differential accessibility of specific phosphosites to GRK when the receptor is occupied by biased agonists, or from unidentified protein-protein interactions that regulate site-specific receptor phosphorylation.
Comparing the potencies of biased agonists for stimulating receptor internalization and site-specific receptor phosphorylation showed that agonist-induced receptor endocytosis paralleled phosphorylation on Thr353/354 but was dissociated from phosphorylation on Ser341/343. This observation is consistent with the results from our previous mutagenesis studies, which showed that Thr residues in the sst2A receptor C tail are essential for SS14 stimulation of rapid receptor internalization, whereas Ser residues are not required (23). Together, these results indicate that the deficiency in agonist-stimulated Thr phosphorylation could be responsible for the reduced effects of SOM230 and KE108 on sst2A internalization. In fact, a previous study came to this conclusion based on the observation that after a 5-min treatment, a maximal concentration of SOM230 produced less Thr phosphorylation of the sst2A receptor than did SS14 (35). To test this conclusion directly, we determined whether biased agonists altered sst2A receptor internalization independently of receptor phosphorylation. If the reduced effect of SOM230 and KE108 on sst2A receptor internalization was due entirely to their inability to fully stimulate receptor phosphorylation, then these analogs should have the same effect as SS14 on the internalization of a phosphorylation negative receptor in which all agonist stimulated phosphorylation sites have been mutated (21). Our previous studies showed that SS14 is able to induce the internalization of the Phos−/sst2A receptor mutant although internalization occurs much more slowly than observed with the WT receptor (23), and this observation was confirmed here: SS14-stimulated endocytosis of the Phos−/sst2A receptor was far slower (t1/2 = 32.9 ± 3.6 min) than that of the WT receptor (t1/2 = 4.3 ± 0.7 min). Unexpectedly, the internalization of the Phos−/sst2A receptor was strikingly dependent on the stimulating agonist: the rate of Phos−/sst2A receptor endocytosis was much slower after SOM230 and KE108 treatment than after SS14 stimulation. These agonist-specific effects on the internalization of the Phos−/sst2A receptor must arise directly or indirectly from differences in the conformation of the agonist-receptor complex, because the Phos−/sst2A receptor is not phosphorylated upon agonist binding (23). Therefore, our studies show that reduced receptor endocytosis after SOM230 and KE108 treatment is not entirely due to a deficiency in sst2A receptor phosphorylation.
GPCR, like the sst2A receptor, which traffic through clathrin-dependent endocytic pathways, can be divided into two classes, A and B, based on the characteristics of agonist-induced arrestin-receptor association and the rate of receptor recycling (25, 26, 36). Although arrestin binds to both types of receptors, the receptor-arrestin complex is less stable with class A than class B receptors, and these differences in stability determine postendocytic receptor sorting (25, 26, 37). Thus class A receptors, which associate weakly with arrestin after agonist stimulation, typically undergo rapid recycling to the plasma membrane after agonist removal. In contrast, class B receptors, which remain tightly associated with arrestin in endocytic vesicles, recycle to the cell surface much more slowly. We show here that the ligand determines whether the sst2A receptor exhibits class A or class B characteristics. As we described previously, and confirm here, SS14 stimulates the formation of a stable complex between β-arrestins and the sst2A receptor, and this complex is maintained as the receptor is endocytosed and traffics to the TGN (10, 23). Furthermore, receptor recycling is rather slow after SS14 removal (Fig. 6). Thus, when stimulated by the native ligand, the sst2A receptor behaves as a class B GPCR. In contrast, SOM230 and KE108 induce less receptor-arrestin association than SS14, and the receptor is recycled quickly after analog removal, behavior that is typical of class A receptors. The pattern of arrestin recruitment observed here with SOM230 and KE108 is similar to what we reported previously with the nonpeptide sst2A receptor agonist L-779,976 (10) and suggest that agonist-specific regulation of sst2A receptor-arrestin association may be usefully manipulated for improved therapeutic effect. However, because arrestins can initiate G protein-independent signaling events (26, 29, 38–40), such altered receptor-arrestin interactions are likely to have multiple consequences for the biological actions of somatostatin analogs (41).
After agonist-stimulated receptor internalization, some GPCR must be dephosphorylated before they can be recycled to the plasma membrane, whereas others can be recycled in the phosphorylated state. We show that after stimulation with SS14 and subsequent ligand removal, the sst2A receptor is dephosphorylated (t1/2 ≈ 7 min) long before it is recycled (t1/2 = 42 min) (Table 4). Although the rate of receptor dephosphorylation at Ser341/343 was unchanged after treatment with SOM230 and KE108, the dephosphorylation of Thr353/354 was increased about 3-fold, perhaps as a consequence of the reduced stability of the receptor-arrestin complex. Even so, the rate of receptor dephosphorylation is still faster than receptor recycling after stimulation with KE108. However, because receptor recycling occurs so quickly after SOM230 stimulation, the rates of sst2A receptor dephosphorylation and recycling are indistinguishable after stimulation with this particular analog (Table 4). Thus, receptor dephosphorylation may be rate limiting for sst2A recycling after SOM230 treatment, although it is much faster than receptor recycling with KE108 and SS14.
The molecular mechanisms responsible for the striking analog dependence of receptor recycling are unclear but cannot be explained by differences in rates of receptor dephosphorylation. Because the net loss of plasma membrane receptors depends on both the rate of recycling and the rate of internalization (42), differences in the kinetics of sst2A receptor recycling must contribute to the reduced receptor internalization at steady state with SOM230 and KE108. Moreover, because receptor recycling plays a critical role in the reactivation of desensitized GPCR, faster recycling kinetics with SOM230 and KE108 is likely to impact cellular response to chronic stimulation.
In summary, we show here that the biased agonists SOM230 and KE108 are both less potent and less effective than SS14 at stimulating sst2A receptor internalization. This impairment appears to result both from differences in receptor phosphorylation and from phosphorylation-independent effects and impacts multiple molecular events in receptor trafficking. We show that SOM230 and KE108 regulate sst2A receptor phosphorylation and dephosphorylation at specific phosphosites differentially. Although phosphorylation at Thr353/354 correlates with receptor endocytosis, phosphorylation at Ser341/343 does not. Although compromised, the amount of receptor phosphorylation appears sufficient to recruit β-arrestin to the activated receptor with both analogs, albeit to a lesser extent than with SS14. However, the receptor recycles far more rapidly after cessation of SOM230 and KE108 than SS14 treatment. These studies illuminate an unexpected complexity to sst2A receptor regulation. The future challenge will be to identify those agonist-dependent changes that can be best exploited for the development of more effective and longer-acting somatostatin analogs for therapy.
Materials and Methods
Reagents and antibodies
Cell culture media and reagents were purchased from Sigma-Aldrich (St. Louis, MO), unless otherwise specified. Fetal bovine serum was from Atlanta Biologicals, Inc., (Lawrenceville, GA). SS14 and KE108 (8) were purchased from Bachem (Torrance, CA). The somatostatin analogs SOM230 (43) and Coy-14 (44) were generously provided by Novartis, Inc., (Basel, Switzerland) and Jean E. Rivier, respectively. The generation and characterization of the phosphorylation site-specific antibodies have been described in detail (21, 46). The specificity of each antibody was determined by three complementary assays: peptide competition ELISA using phospho and nonphospho peptides, immunoblotting of WT receptors and receptors mutated in target Ser/Thr phosphorylation sites, and intact cell ELISA of WT and phosphorylation negative receptors (21, 46). Together, these assays have demonstrated that the antibodies specifically recognize either phosphorylated Ser341/343 (monoclonal antibody 2C12) or phosphorylated Thr353/354 (monoclonal antibody 1A2), respectively, in the sst2A receptor. Phosphorylation-independent sst2A receptor antibodies (NB100-74537) were purchased from Novus Biologicals (Littleton, CO).
Plasmids
We previously described the generation of plasmids encoding the HA-tagged WT rat sst2A receptor as well as a phosphorylation negative receptor mutant (Phos−/sst2A), in which all the Ser and Thr residues in the third loop and C terminus of the receptor were mutated to Ala (21, 23). Addition of the extracellular HA-epitope tag does not affect any of the functional properties of the receptor, including ligand binding affinity, receptor internalization, or inhibition of cAMP production (10). The EGFP-β-arrestin-2 chimera was constructed by excising the rat β-arrestin-2 gene from a plasmid generously provided by Marc Caron (Duke University, Durham, NC) and inserting it into the pEGFP-N3 vector (CLONTECH, BD Biosciences, Palo Alto, CA) to produce a hybrid protein with EGFP attached to the C terminus of β-arrestin-2 (10). The FLAG-tagged bovine β-arrestin-2 (pcA3NFL6) was generously provided by Vsevolod Gurevich (Vanderbilt University, Nashville, TN). All plasmids were sequenced to ensure accuracy.
Cell culture
The rat AR42J pancreatic acinar cell line (CRL-1492; American Type Culture Collection, Manassas, VA) was cultured as described previously (13). The generation of clonal CHO cell lines stably expressing either the WT (CHO-sst2A) or phosphorylation negative (CHO-Phos−/sst2A) rat sst2A receptors has been described previously (23). CHO cells were cultured in Ham's F-12 medium containing 10% fetal bovine serum and 0.25 mg/ml of G-418 at 37 C and 5% CO2. Experimental cultures were plated in medium without G-418 1 or 2 d before internalization or phosphorylation studies. For experiments involving transient transfection, CHOK1 cells obtained from the American Type Culture Collection were cultured without G-418 in the same culture medium as the stable cell lines.
Quantitation of receptor internalization and recycling
Cell surface sst2A receptors were measured by an ELISA using a colorimetric peroxidase assay as described previously (10). Briefly, CHO-K1 cells stably expressing sst2A receptors with an HA-epitope tag at the amino terminus were grown overnight in medium without G-418. The next day, cells were incubated for 1 h at room temperature with primary antibody (HA.11 anti-HA monoclonal; Covance, Berkeley, CA) to label cell surface receptors. After washing to remove unbound antibody, cells were treated with somatostatin analogs for the times indicated in Ham's F-12 medium containing 20 mm HEPES (pH 7.4) and 5 mg/ml lactalbumin hydrolysate (F12LH). To terminate agonist stimulation, cells were placed on ice, washed with cold PBS (pH 7.4), and fixed with 3% paraformaldehyde in PBS for 10 min. After a 30-min incubation with 1% BSA (fraction V) in PBS to block nonspecific binding, cells were incubated with secondary antibody (horseradish peroxidase-conjugated goat antimouse IgG; Kirkegaard & Perry Laboratories, Inc., Gaithersburg, MD) for 1 h. A colorimetric peroxidase substrate, either 2,2′-azino-bis(3-ethylbenzthiazoline-6-sulfonic acid (Roche Diagnostics, Indianapolis, IN) or 3,3′,5,5′-tetramethylbenzidine substrate (TMB) (Pierce, Rockford, IL) was then added for 5–45 min. The reaction with TMB was terminated with 10% sulfuric acid. The optical density of the colorimetric end product was measured by absorbance at 405 nm for 2,2′-azino-bis(3-ethylbenzthiazoline-6-sulfonic acid or at 450 nm for TMB using a Precision microplate reader (Molecular Devices, Union City, CA). The background was determined either with CHO-sst2A cells by omitting the primary antibody or with CHO-K1 cells not expressing sst2A receptors and was substracted from experimental points. Surface receptor remaining after agonist treatment was calculated as the absorbance in treated cells expressed as a percentage of the absorbance in control cells not exposed to agonist. In the absence of agonist, there was no change in cell surface receptors during the incubation period.
Receptor recycling was determined after a 30-min pretreatment of cells with agonist as described above. After peptide stimulation cells were rapidly washed with PBS to remove excess ligand and then incubated at 37 C in the presence of 100 nm antagonist (Coy-14) for various times to permit receptor recovery at the cell surface. At the end of each recovery period, cells were washed with PBS, fixed in 3% paraformaldehyde for 10 min, and then incubated with anti-HA antibody. Cell surface receptors were subsequently measured by ELISA as described above. Surface receptors at each time point were calculated as the absorbance measured in pretreated cells expressed as a percentage of the absorbance in untreated control cells.
Intracellular cAMP accumulation
The effect of somatostatin analogs on forskolin-stimulated cAMP accumulation was determined as previously described (10). Briefly, cells plated in 24-well plates were incubated for 3 h at 37 C with 3.5 μCi/ml [2-3H]adenine (24 Ci/mmole; PerkinElmer Life and Analytical Sciences, Boston, MA) in F12LH. Cells were then washed to remove the unincorporated 3H-adenine, preincubated with 1 mm of the phosphodiesterase inhibitor 1-methyl-3-isobutylxanthine (IBMX) for 5 min, and then treated for 10 min at 37 C with somatostatin receptor ligands in the presence of 10 μm forskolin (Calbiochem, La Jolla, CA) and 1 mm IBMX. The reaction was terminated by the addition of cold stop buffer (5% trichloroacetic acid, 0.1 mm unlabeled cAMP, and 20 mm ATP). 32P-cAMP (∼5000 cpm in 100 μl of water) was then added to provide an internal control for recovery. The amount of [3H]cAMP formed in the assay was determined by sequential chromatography through Dowex 50 and neutral alumina columns. Each point represents the mean ± sem of triplicate wells.
Receptor phosphorylation and dephosphorylation
Receptor phosphorylation was detected by immunoblotting of receptor preparations with phosphosite-specific antibodies using a slightly modified protocol from that described previously (21). Briefly, CHO-sst2A cells cultured to 70–80% confluency in 60-mm dishes were stimulated with SS14, KE108, or SOM230 at 37 C in F12LH for the times shown. Cells were then washed with cold PBS and scraped into HEPES buffered saline (0.15 m NaCl, 20 mm HEPES (pH 7.4), 5 mm EDTA, and 3 mm EGTA) containing 4 mg/ml dodecyl β-maltoside and protease and phosphatase inhibitors. Receptors were then purified by wheat germ agglutinin agarose beads as previously described (21). Denatured protein samples were resolved in 10% sodium dodecyl sulfate-polyacrylamide gels, transferred to polyvinylidene difluoride membranes, and blotted sequentially with 1A2 and 2C12 phosphorylation site-specific antibodies and then with HA antibody as a loading control.
A whole-cell ELISA was used to quantitated receptor phosphorylation in intact cells using the same phosphorylation site-specific antibodies (21, 46). CHO cells stably expressing receptors were grown in 24-well culture plates, washed with F12LH, and treated with analogs for 30 min at 37 C. Cells were then rinsed on ice with cold PBS and fixed with prechilled (−20 C) methanol:acetone (1:1) on ice for 10 min. After drying, cells were washed with PBS and blocked for 1 h at room temperature with 5% milk in RIPS buffer [150 mm NaCl, 50 mm Tris, 1 mm EDTA, 10 mm NaF, 100 nm sodium orthovanadate, 0.5% sodium deoxycholate, and 1% Triton X-100 (pH 8.0)]. Dishes were then incubated with either the 2C12 or 1A2 phosphorylation site-specific antibody overnight at 4 C. The next day, cells were washed first with 0.05% Tween-20 in PBS, then PBS, and then incubated with horseradish peroxidase-conjugated goat antimouse IgG for 1 h at room temperature. Colorimetric product was generated using TMB as instructed by the manufacturer. The reaction was terminated after 5–20 min by the addition of 10% sulfuric acid, and absorbance was measured at 450 nm with a Precision microplate reader. Assay background was determined in samples without primary phospho-specific antibody. No signal is observed with either CHO-K1 cells, which do not express endogenous sst2A receptors, or CHO-sst2A cells without SS14 stimulation.
To measure receptor dephosphorylation, CHO-sst2A cells were treated at 37 C with maximal concentrations of SS14, SOM230, or KE108 for 30 min as described above, washed, and then incubated in fresh F12LH medium in the presence of 100 nm antagonist (Coy-14) for the times shown. The dephosphorylation incubation was stopped by placing the cells on ice and fixing with cold methanol/acetone (1:1) for 10 min. The level of receptor phosphorylation was then determined with the in-cell ELISA as above.
Immunofluorescence confocal microscopy
The trafficking of endogenous sst2A receptors was examined before and after treatment of pancreatic AR4-2J cells with somatostatin analogs (13). AR4-2J cells were seeded at a density of 8 × 104 cells per chamber in eight-chamber glass slides (Nalge Nunc International, Rochester, NY) precoated with 50 μg/ml fibronectin or 0.1 mg/ml poly-l-ornithine (Sigma-Aldrich). After overnight culture, cells were incubated with agonists for the times shown, washed with PBS, then processed for confocal microscopy using a modified protocol from that previously reported (10, 23). Briefly, cells were fixed with freshly prepared 3% paraformaldehyde in PBS for 20 min at room temperature and permeabilized with 0.1% Triton X-100 in PBS for 30 min on ice. Cells were then incubated sequentially at room temperature for 1 h with anti-sst2A antibody (NB100-74537; Novus Biologicals), with normal donkey serum, and finally with dichlorotriazinyl amino fluorescein (DTAF)-conjugated AffiniPure F(ab′)2 fragment donkey antirabbit IgG (heavy and light chains) (Jackson ImmunoResearch, West Grove, PA). The slides were mounted with ProLong Gold antifade mounting reagent (Invitrogen, Eugene, OR) and stored in the dark at 4 C.
The codistribution of EGFP-β-arrestin-2 and sst2A receptors was evaluated in CHO-K1 cells by confocal microscopy. Cells were seeded at a density of 1–4 × 104 cells per chamber in precoated eight-chamber glass slides. The next day, cells were transfected with 0.6 μg of HA-tagged sst2A receptor and 0.12 μg of EGFP-β-arrestin-2 plasmid DNA, using FuGENE HD transfection reagent (Roche Diagnostics). Two days after transfection, cells were incubated with anti-HA antibody on ice for 2 h to label surface receptors and then treated with SS14 or analogs at 37 C for various times. After agonist stimulation, cells were processed for confocal microscopy as described above, except that normal goat serum was used to block nonspecific antibody binding, and Alexa Fluor 568-conjugated goat antimouse IgG (Invitrogen) was used as secondary antibody to detect HA-tagged receptors.
Fluorescent images were viewed with a ×60 objective lens using a Nikon A1 confocal microscope (Nikon, Melville, NY) with an excitation filter of 488 nm and an emission filter of 500–550 nm for EGFP and DTAF and excitation and emission filters of 561 and 570–620 nm, respectively, for Alexa Fluor 568. Only CHO cells expressing both receptor and arrestin were selected for study. Images from at least eight to 10 cells were recorded in each treatment group in each experiment. The images shown are representative of observations in four independent experiments.
Cytosol to membrane translocation of β-arrestin-2
Somatostatin analog-stimulated translocation of β-arrestin from the cytosol to the membrane fraction was analyzed using a subcellular fractionation protocol described previously (45). CHO-K1 cells were plated at a density of 1 × 106 cells/100-mm dish, grown overnight, and then transfected with 15 μg of HA-sst2A receptor and 5 μg of FLAG-β-arrestin-2 plasmid DNA. Forty-eight hours after transfection, cells were stimulated with 100 nm SS14, 1 μm SOM230, or 1 μm KE108 at 37 C for the times indicated. After a cold PBS wash, cells were scraped into cold 1,4-piperazinediethane sulfonic acid (PIPES) buffer [10 mm PIPES, 100 mm KCl, 3 mm NaCl, and 3.5 mm MgCl2 (pH 7.0) containing 50 μg/ml phenylmethylsulfonyl fluoride, 1 μg/ml pepstatin A, 10 μg/ml leupeptin, and 5 μg/ml aprotinin], homogenized with a Dounce homogenizer (10 strokes), and then sonicated with a VibraCell sonicator (Sonics and Materials, Inc., Danbury, CT). Cell nuclei were removed by centrifugation at 1000 × g for 20 min at 4 C. The supernatant was loaded onto a discontinuous gradient of 50, 35, and 20% of sucrose in PIPES buffer and centrifuged at 160,000 × g for 2 h at 4 C. Membrane fractions were collected from the 20–35% sucrose interphase, diluted with PIPES buffer, and pelleted by centrifugation at 160,000 × g for 15 min at 4 C. Membranes were resuspended in PIPES buffer containing 1% Triton X-100, and the protein content was determined using the bicinchoninic acid protein assay (Pierce). In some experiments, membranes were isolated from postnuclear supernatants by direct centrifugation at 100,000 × g rather than by purification through sucrose gradients, with similar results. Protein samples were analyzed by SDS-PAGE and immunoblotting with monoclonal anti-FLAG M2 antibody (Sigma-Aldrich) to detect FLAG-β-arrestin. Blots were then stripped and reprobed with monoclonal antiactin antibody (Sigma-Aldrich) as a loading control. Chemiluminescent detection was performed with enhanced chemiluminescence Western blotting reagent (Amersham, GE Heathcare, Buckingamshire, UK). Exposed films were scanned, and immunoreactive signal in each lane was quantitated using Scion Image software from the National Institutes of Health (Scion Corp., Frederick, MD). In each agonist-treated sample, the immunoreactivity of the FLAG band was calculated as a fraction of the intensity of the actin signal and then expressed as a percentage of the signal obtained from SS14-treated samples loaded on the same gel.
Statistical analysis and curve fitting
Unless stated otherwise, figures show data expressed as the mean ± sem from a single experiment and are representative of multiple independent experiments. The half-times for agonist-induced receptor internalization and recycling were obtained by nonlinear regression curve fitting to a one-phase exponential rate curve using Prism version 4.0 (GraphPad Software, San Diego, CA). Values for EC50 and maximal response were calculated by least-squares nonlinear regression analysis of dose-response curves fit to a one-component sigmoidal curve with a Hill coefficient of −1. Summary tables show mean ± sem values calculated using individual values from multiple independent experiments. Statistical analysis was carried out using Dunnett's multiple comparison test. P values less than 0.05 were considered statistically significant.
Acknowledgments
We thank Ms. Weiley Liu for expert assistance with the cAMP experiments, Dr. James Broughman for help with confocal microscopy, and Dr. Vsevolod Gurevich and Dr. Marc Caron for arrestin cDNA.
This work was supported by the National Institute of Arthritis, Diabetes, Digestive, and Kidney Disease Grant DK032234 (to A.S.).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- Coy-14
- 3-(4-Chlorophenyl)alanine-cyclo[DCys-Tyr-DTrp-NMethyl-Lys-Thr-Cys]-3-(2-naphtyl)alanine-NH2
- DTAF
- dichlorotriazinyl amino fluorescein
- EGFP
- enhanced green fluorescent protein
- F12LH
- Ham's F-12 medium containing 20 mm HEPES (pH 7.4) and 5 mg/ml lactalbumin hydrolysate
- GPCR
- G protein-coupled receptor
- GRK
- GPCR kinase
- HA
- hemagglutinin
- IBMX
- 1-methyl-3-isobutylxanthine
- KE108
- Tyr-cyclo-[D-diaminobutyric acid-Arg-Phe-Phe-D-Trp-Lys-Thr-Phe]
- PIPES
- 1,4-piperazinediethane sulfonic acid
- SOM230
- cyclo-[diaminoethylcarbamoyl-HydroxyPro-Phenylglycine-D-Trp-Lys-(4-O-benzyl)Tyr-Phe]
- SS14
- somatostatin-14
- sst2A
- somatostatin receptor subtype 2A
- TGN
- trans-Golgi network
- TMB
- 3,3′,5,5′-tetramethylbenzidine substrate
- WT
- wild type.
References
- 1. Reubi JC, Maecke HR. 2008. Peptide-based probes for cancer imaging. J Nucl Med 49:1735–1738 [DOI] [PubMed] [Google Scholar]
- 2. Colao A, Faggiano A, Pivonello R. 2010. Somatostatin analogues: treatment of pituitary and neuroendocrine tumors. Prog Brain Res 182:281–294 [DOI] [PubMed] [Google Scholar]
- 3. De Martino MC, Hofland LJ, Lamberts SW. 2010. Somatostatin and somatostatin receptors: from basic concepts to clinical applications. Prog Brain Res 182:255–280 [DOI] [PubMed] [Google Scholar]
- 4. Kwekkeboom DJ, Kam BL, van Essen M, Teunissen JJ, van Eijck CH, Valkema R, de Jong M, de Herder WW, Krenning EP. 2010. Somatostatin-receptor-based imaging and therapy of gastroenteropancreatic neuroendocrine tumors. Endocr Relat Cancer 17:R53–R73 [DOI] [PubMed] [Google Scholar]
- 5. Modlin IM, Pavel M, Kidd M, Gustafsson BI. 2010. Review article: somatostatin analogues in the treatment of gastroenteropancreatic neuroendocrine (carcinoid) tumours. Aliment Pharmacol Ther 31:169–188 [DOI] [PubMed] [Google Scholar]
- 6. Schonbrunn A. 2004. Somatostatin receptors. In: Lennarz WJ, Lane MD. eds. Encyclopedia of biological chemistry. Oxford: Elsevier; 55–60 [Google Scholar]
- 7. Bruns C, Lewis I, Briner U, Meno-Tetang G, Weckbecker G. 2002. SOM230: a novel somatostatin peptidomimetic with broad somatotropin release inhibiting factor (SRIF) receptor binding and a unique antisecretory profile. Eur J Endocrinol 146:707–716 [DOI] [PubMed] [Google Scholar]
- 8. Reubi JC, Eisenwiener KP, Rink H, Waser B, Mäcke HR. 2002. A new peptidic somatostatin agonist with high affinity to all five somatostatin receptors. Eur J Pharmacol 456:45–49 [DOI] [PubMed] [Google Scholar]
- 9. Schmid HA, Schoeffter P. 2004. Functional activity of the multiligand analog SOM230 at human recombinant somatostatin receptor subtypes supports its usefulness in neuroendocrine tumors. Neuroendocrinology 80(Suppl 1):47–50 [DOI] [PubMed] [Google Scholar]
- 10. Liu Q, Cescato R, Dewi DA, Rivier J, Reubi JC, Schonbrunn A. 2005. Receptor signaling and endocytosis are differentially regulated by somatostatin analogs. Mol Pharmacol 68:90–101 [DOI] [PubMed] [Google Scholar]
- 11. Cervia D, Langenegger D, Schuepbach E, Cammalleri M, Schoeffter P, Schmid HA, Bagnoli P, Hoyer D. 2005. Binding and functional properties of the novel somatostatin analogue KE 108 at native mouse somatostatin receptors. Neuropharmacology 48:881–893 [DOI] [PubMed] [Google Scholar]
- 12. Cescato R, Loesch KA, Waser B, Mäcke HR, Rivier JE, Reubi JC, Schonbrunn A. 2010. Agonist-biased signaling at the sst2A receptor: the multi-somatostatin analogs KE108 and SOM230 activate and antagonize distinct signaling pathways. Mol Endocrinol 24:240–249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Elberg G, Hipkin RW, Schonbrunn A. 2002. Homologous and heterologous regulation of somatostatin receptor 2. Mol Endocrinol 16:2502–2514 [DOI] [PubMed] [Google Scholar]
- 14. Kenakin T, Miller LJ. 2010. Seven transmembrane receptors as shapeshifting proteins: the impact of allosteric modulation and functional selectivity on new drug discovery. Pharmacol Rev 62:265–304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Urban JD, Clarke WP, von Zastrow M, Nichols DE, Kobilka B, Weinstein H, Javitch JA, Roth BL, Christopoulos A, Sexton PM, Miller KJ, Spedding M, Mailman RB. 2007. Functional selectivity and classical concepts of quantitative pharmacology. J Pharmacol Exp Ther 320:1–13 [DOI] [PubMed] [Google Scholar]
- 16. Kenakin T. 2008. Functional assays as prismatic views of drug activity: relevance to new drug discovery. J Recept Signal Transduct Res 28:109–125 [DOI] [PubMed] [Google Scholar]
- 17. Mailman RB. 2007. GPCR functional selectivity has therapeutic impact. Trends Pharmacol Sci 28:390–396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Schonbrunn A. 2008. Selective agonism in somatostatin receptor signaling and regulation. Mol Cell Endocrinol 286:35–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lesche S, Lehmann D, Nagel F, Schmid HA, Schulz S. 2009. Differential effects of octreotide and pasireotide on somatostatin receptor internalization and trafficking in vitro. J Clin Endocrinol Metab 94:654–661 [DOI] [PubMed] [Google Scholar]
- 20. Hipkin RW, Friedman J, Clark RB, Eppler CM, Schonbrunn A. 1997. Agonist-induced desensitization, internalization, and phosphorylation of the sst2a somatostatin receptor. J Biol Chem 272:13869–13876 [DOI] [PubMed] [Google Scholar]
- 21. Liu Q, Bee MS, Schonbrunn A. 2009. Site specificity of agonist and second messenger-activated kinases for somatostatin receptor subtype 2A (sst2A) phosphorylation. Mol Pharmacol 76:68–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Liu Q, Reubi JC, Wang Y, Knoll BJ, Schonbrunn A. 2003. In vivo phosphorylation of the somatostatin 2A receptor in human tumors. J Clin Endocrinol Metab 88:6073–6079 [DOI] [PubMed] [Google Scholar]
- 23. Liu Q, Dewi DA, Liu W, Bee MS, Schonbrunn A. 2008. Distinct phosphorylation sites in the SST2A somatostatin receptor control internalization, desensitization, and arrestin binding. Mol Pharmacol 73:292–304 [DOI] [PubMed] [Google Scholar]
- 24. Hipkin RW, Wang Y, Schonbrunn A. 2000. Protein kinase C activation stimulates the phosphorylation and internalization of the sst2A somatostatin receptor. J Biol Chem 275:5591–5599 [DOI] [PubMed] [Google Scholar]
- 25. Moore CA, Milano SK, Benovic JL. 2007. Regulation of receptor trafficking by GRKs and arrestins. Annu Rev Physiol 69:451–482 [DOI] [PubMed] [Google Scholar]
- 26. Luttrell LM, Gesty-Palmer D. 2010. Beyond desensitization: physiological relevance of arrestin-dependent signaling. Pharmacol Rev 62:305–330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Krasel C, Bünemann M, Lorenz K, Lohse MJ. 2005. β-Arrestin binding to the β2-adrenergic receptor requires both receptor phosphorylation and receptor activation. J Biol Chem 280:9528–9535 [DOI] [PubMed] [Google Scholar]
- 28. Tran TM, Friedman J, Baameur F, Knoll BJ, Moore RH, Clark RB. 2007. Characterization of β2-adrenergic receptor dephosphorylation: comparison with the rate of resensitization. Mol Pharmacol 71:47–60 [DOI] [PubMed] [Google Scholar]
- 29. Sorkin A, von Zastrow M. 2009. Endocytosis and signalling: intertwining molecular networks. Nat Rev Mol Cell Biol 10:609–622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Calebiro D, Nikolaev VO, Persani L, Lohse MJ. 2010. Signaling by internalized G-protein-coupled receptors. Trends Pharmacol Sci 31:221–228 [DOI] [PubMed] [Google Scholar]
- 31. Waser B, Cescato R, Tamma ML, Maecke HR, Reubi JC. 2010. Absence of somatostatin SST(2) receptor internalization in vivo after intravenous SOM230 application in the AR42J animal tumor model. Eur J Pharmacol 644:257–262 [DOI] [PubMed] [Google Scholar]
- 32. Tobin AB. 2008. G-protein-coupled receptor phosphorylation: where, when and by whom. Br J Pharmacol 153(Suppl 1):S167–S176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zidar DA, Violin JD, Whalen EJ, Lefkowitz RJ. 2009. Selective engagement of G protein coupled receptor kinases (GRKs) encodes distinct functions of biased ligands. Proc Natl Acad Sci USA 106:9649–9654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Busillo JM, Armando S, Sengupta R, Meucci O, Bouvier M, Benovic JL. 2010. Site-specific phosphorylation of CXCR4 is dynamically regulated by multiple kinases and results in differential modulation of CXCR4 signaling. J Biol Chem 285:7805–7817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Pöll F, Lehmann D, Illing S, Ginj M, Jacobs S, Lupp A, Stumm R, Schulz S. 2010. Pasireotide and octreotide stimulate distinct patterns of sst2A somatostatin receptor phosphorylation. Mol Endocrinol 24:436–446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Oakley RH, Laporte SA, Holt JA, Caron MG, Barak LS. 2000. Differential affinities of visual arrestin, β arrestin1, and β arrestin2 for G protein-coupled receptors delineate two major classes of receptors. J Biol Chem 275:17201–17210 [DOI] [PubMed] [Google Scholar]
- 37. Gurevich VV, Gurevich EV. 2006. The structural basis of arrestin-mediated regulation of G-protein-coupled receptors. Pharmacol Ther 110:465–502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. DeWire SM, Ahn S, Lefkowitz RJ, Shenoy SK. 2007. β-Arrestins and cell signaling. Annu Rev Physiol 69:483–510 [DOI] [PubMed] [Google Scholar]
- 39. Xiao K, Sun J, Kim J, Rajagopal S, Zhai B, Villén J, Haas W, Kovacs JJ, Shukla AK, Hara MR, Hernandez M, Lachmann A, Zhao S, Lin Y, Cheng Y, Mizuno K, Ma'ayan A, Gygi SP, Lefkowitz RJ. 2010. Global phosphorylation analysis of β-arrestin-mediated signaling downstream of a seven transmembrane receptor (7TMR). Proc Natl Acad Sci USA 107:15299–15304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Gurevich VV, Gurevich EV, Cleghorn WM. 2008. Arrestins as multi-functional signaling adaptors. Handb Exp Pharmacol 15–37 [DOI] [PubMed] [Google Scholar]
- 41. Rajagopal S, Rajagopal K, Lefkowitz RJ. 2010. Teaching old receptors new tricks: biasing seven-transmembrane receptors. Nat Rev Drug Discov 9:373–386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Koenig JA, Edwardson JM. 1997. Endocytosis and recycling of G protein-coupled receptors. Trends Pharmacol Sci 18:276–287 [DOI] [PubMed] [Google Scholar]
- 43. Weckbecker G, Briner U, Lewis I, Bruns C. 2002. SOM230: a new somatostatin peptidomimetic with potent inhibitory effects on the growth hormone/insulin-like growth factor-I axis in rats, primates, and dogs. Endocrinology 143:4123–4130 [DOI] [PubMed] [Google Scholar]
- 44. Rajeswaran WG, Hocart SJ, Murphy WA, Taylor JE, Coy DH. 2001. Highly potent and subtype selective ligands derived by N-methyl scan of a somatostatin antagonist. J Med Chem 44:1305–1311 [DOI] [PubMed] [Google Scholar]
- 45. Huttenrauch F, Nitzki A, Lin FT, Höning S, Oppermann M. 2002. β-Arrestin binding to chemokine receptor CCR5 requires multiple C-terminal receptor phosphorylation sites and involves a conserved Asp-Arg-Tyr sequence motif. J Biol Chem 277:30769–30777 [DOI] [PubMed] [Google Scholar]
- 46. Ghosh M, Schonbrunn A. 2011. Differential temporal and spatial regulation of somatostatin receptor phosphorylation and dephosphorylation. J Biol Chem 286:13561–13573 [DOI] [PMC free article] [PubMed] [Google Scholar]