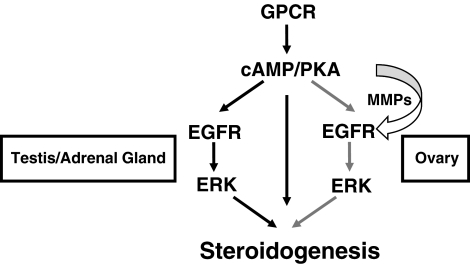
Steroidogenesis in all major tissues requires cross-talk between GPRC and EGFR receptors. In the ovary, this cross-talk is uniquely mediated by MMPs 2 and 9.
Abstract
Previous work has demonstrated that cross talk between G protein-coupled LH receptors and epidermal growth factor receptors (EGFR) is essential for LH-induced steroid production in ovarian follicles and testicular Leydig cells. Here we demonstrate that G protein-coupled receptor (GPCR)/EGFR cross talk is also required for ACTH-induced steroidogenesis in Y1 adrenal cells. Moreover, we confirm that the signaling pathway from GPCR to Erk activation is conserved in all three steroidogenic tissues. ACTH or LH induces Gαs, resulting in elevated cAMP and protein kinase A activation. cAMP/protein kinase A then triggers EGFR trans-activation, which promotes Erk signaling and subsequent steroidogenesis. Interestingly, although EGFR trans-activation is conserved in all three tissues, the specific mechanisms regulating this receptor cross talk differ. ACTH and LH trigger matrix metalloproteinase (MMP)-mediated release of EGFR ligands in adrenal and gonadal cells, respectively. However, this extracellular, ligand-dependent EGFR transactivation is required only for LH-induced steroidogenesis in ovarian follicles, reflecting the unique requirement of cell-cell cross talk for ovarian steroid production. Furthermore, MMP2 and MMP9 appear to regulate LH-induced steroidogenesis in mouse ovarian follicles, because a specific MMP2/9 inhibitor as well as the MMP2/9 inhibitor doxycycline suppress LH-induced follicular steroid production in vitro. Notably, although EGFR or MMP inhibition minimally affects estrous cycling in female mice, they attenuate ovarian steroidogenesis in response to LHR overstimulation in vivo. These results may have implications with regard to EGFR inhibitor use in various cancers as well as in polycystic ovarian syndrome, where excess LH-driven ovarian androgen production might be controlled by MMP2/9 inhibition.
Gonadal steroidogenesis in both the ovary and the testes is regulated in large part by the pituitary peptide LH. Interestingly, LH-induced steroid production in both gonadal tissues requires cross talk between G protein-coupled LH receptors (LHR) and epidermal growth factor receptors (EGFR) (1–5). This cross talk has been best characterized in testicular Leydig cells, where LH-induced steroidogenesis is regulated by the following linear pathway (3). First, LHR activation stimulates cAMP production and subsequent protein kinase A (PKA) signaling. Second, PKA triggers EGFR activation through a yet unknown mechanism, leading to activation of Erk. Third, Erk appears to up-regulate phosphorylation of the steroidogenic acute regulatory protein (StAR), leading to increased steroid production (3, 6). Of note, although EGFR trans-activation is necessary for full LH-induced steroidogenesis in Leydig cells, direct EGFR stimulation by EGF alone is not sufficient, suggesting that additional signaling pathways are used for full LH-induced steroid production [e.g. direct PKA effects on StAR or PKA-induced cAMP response element-binding protein (CREB) activation of StAR expression]. Furthermore, evidence suggests that PKA- mediated transactivation of the EGFR in Leydig cells can occur via both intracellular, ligand-independent signaling (3) as well as extracellular, ligand-dependent activation that requires matrix metalloproteinase (MMP)-mediated release of membrane-bound EGFR ligands (4). However, only intracellular, ligand-independent signaling appears to be required for the biological endpoint of steroid production (3).
Interestingly, LH and EGFR cross talk is conserved between the testes and ovary, as LH stimulation of granulosa cell lines leads to EGFR phosphorylation (5), and inhibition of EGFR signaling abrogates LH-induced steroid production in isolated ovarian follicles (1). However, in contrast to the testes, LH-induced EGFR activation and subsequent steroidogenesis requires MMP-mediated extranuclear EGFR activation in the ovary. Here LH stimulates MMP-mediated release of EGFR ligands such as amphiregulin and epiregulin in theca and mural granulosa cells, which in turn promote EGFR activation in cumulus (and possibly mural) granulosa cells. This paracrine signaling leads to cumulus cell expansion, oocyte maturation (7), and steroid production (1), thus serving as a critical means of communication between outer mural granulosa and theca cells and the inner cumulus granulosa cells and oocytes.
Although MMP-mediated LH/EGFR cross talk is known to be important for steroidogenesis in ovarian follicles, the details of this pathway have not yet been characterized. For example, the LH-induced signals that precede MMP activation and EGFR ligand release cross talk are not known, nor have the MMP activated by LH been identified. Furthermore, although clearly important for LH-induced follicular development (e.g. granulosa cell expansion) and oocyte maturation, the role of Erk in ovarian LH-induced steroidogenesis remains uncertain. In addition, whereas EGFR inhibition by AG1478 has been shown to down-regulate testosterone production in live adult male mice (3), the in vivo effects of EGFR or MMP inhibition in adult female mice has not been examined. Finally, given the conservation of LH/EGFR cross talk in the regulation of gonadal steroidogenesis, the importance of G protein-coupled receptor (GPCR)/EGFR cross talk in adrenal steroid production (the third major steroidogenic tissue) has yet to be examined.
Here we perform studies in isolated preovulatory ovarian follicles and in adult female mice to carefully characterize the LH-induced steroidogenic pathway in the ovary. We find that the linear pathway described in Leydig cells is conserved in the ovary and demonstrate that MMP2 and MMP9 might be important regulators of EGFR ligand release. We also show that, although chronic treatment with EGFR and MMP inhibitors does not alter estrous cycling in normal adult female mice, these inhibitors do attenuate LHR-induced steroid production in mice over-stimulated with gonadotropins. Finally, we show that ACTH-induced steroid production in Y1 mouse adrenal cells similarly requires cross talk between G protein-coupled ACTH receptors and EGFR, confirming the conservation of this pathway in all three major steroidogenic tissues.
Results
Erk signaling is necessary for LH-induced steroid production in mouse preovulatory follicles
Previous work showed that Erk signaling is important for LH-induced steroid production in Leydig cells (3). To examine the importance of Erk signaling in LH-induced ovarian steroidogenesis, freshly isolated preovulatory follicles were treated with the MAPK kinase (MEK) inhibitor U0126, and LH-induced steroid production was measured. MEK inhibition significantly blocked LH-induced steroid production from 30 min to 2 h (Fig. 1A). As expected, U0126 blocked LH-induced Erk signaling for the entire 2 h (Fig. 1B). These studies are consistent with recent work demonstrating that Erk signaling is essential in ovarian granulosa cells for the maintenance of proper female fertility (8).
Fig. 1.
Erk and EGFR signaling are necessary for LH-induced steroid production in preovulatory follicles. Preovulatory follicles from PMSG-primed female mice were preincubated with 0.01% DMSO, 20 μm U0126 (A and B), or 20 μm AG1478 (C and D) for 30 min. LH (0.2 mg/ml) was used to stimulate follicles for the indicated times in the presence of the indicated inhibitors or DMSO. Progesterone concentrations in the medium were measured by RIA (A and C), and cell lysates were collected at 2 h and analyzed by Western blot for phosphorylated and total Erk (P- and T-Erk) by Western blot (B and D). Experiments were repeated at least three times with similar results. Each bar represents the mean ± sem (n = 3). *, P < 0.05 by Student's t test relative to LH alone.
EGFR signaling is necessary for LH-induced steroidogenesis in mouse preovulatory follicles and is upstream of Erk activation
Several studies have shown the critical role that the EGFR plays in regulating normal follicular development, including oocyte maturation, cumulus cell expansion, and steroidogenesis (1, 7, 9, 10). Here we confirmed and more completely characterized the importance of EGFR signaling in LH-induced steroidogenesis in preovulatory follicles using pharmacological manipulation. Follicles were pretreated for 30 min with the EGFR inhibitor AG1478, followed by stimulation with LH or LH plus AG1478 for 30 min to 2 h. LH-induced progesterone production was inhibited by AG1478 throughout the time course of the experiment (Fig. 1C), indicating that EGFR plays an important role in short- and long-term LH-induced steroidogenesis in mouse follicles. Notably, LH-induced Erk phosphorylation at 2 h was abrogated by the EGFR inhibitor AG1478 (Fig. 1D), demonstrating that LH-induced Erk signaling is downstream of EGFR activation. Furthermore, previous work in various cell lines has demonstrated that this concentration of AG1478 specifically blocks EGFR- but not other receptor tyrosine kinase (RTK)-mediated Erk activation (3, 11).
MMP2 and MMP9 are important for LH-induced steroidogenesis in mouse follicles
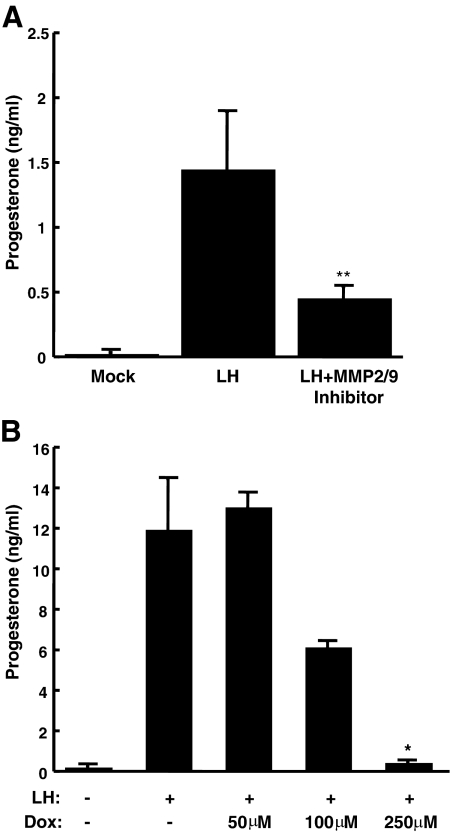
MMP activation is known to be important for the shedding of EGFR ligands and for granulosa cell expansion and oocyte maturation in the ovary (1, 7, 9, 12). Our previous study used Galardin, a broad-spectrum MMP inhibitor, to demonstrate the importance of MMP-mediated EGFR ligand release in ovarian steroid production (1). Here we attempted to narrow down the possible MMP that might be regulating LH-induced steroidogenesis in freshly isolated ovarian follicles. We first used two TNF-α converting enzyme inhibitors [TNF-α protease inhibitor (TAPI)-0 and TAPI-1; EMD Biosciences, San Diego, CA] neither of which had any effect on LH-induced steroidogenesis (TAPI-1 data are shown in Supplemental Fig. 1, published on The Endocrine Society's Journals Online web site at http://mend.endojournals.org). Because MMP2 and MMP9 are known to be expressed in the ovary and up-regulated in response to LH (13), we used the compound MMP2/9 inhibitor I (EMD Biosciences). This molecule is a potent and relatively specific inhibitor of MMP2 and MMP9, with secondary potential effects on MMP3. Preovulatory follicles were pretreated with the MMP2/9 inhibitor for 30 min before stimulation with LH. Because MMP actions are very early after LH stimulation, we focused on the early time point of 30 min. In fact, LH-induced steroidogenesis was attenuated by the MMP2/9 inhibitor at 30 min (Fig. 2A), suggesting that MMP2 and -9 might play major roles in regulating LH-induced steroidogenesis in preovulatory follicles.
Fig. 2.
MMP2 and MMP9 are important for LH-induced steroidogenesis in mouse follicles. Preovulatory follicles from PMSG-primed female mice were pretreated with 0.01% DMSO, 20 μm MMP2/9 inhibitor, or doxycycline (Dox) at the indicated concentrations for 30 min. Follicles were stimulated with 0.2 mg/ml LH or LH plus inhibitors or DMSO for 30 min (A) or 2 h (B). Progesterone levels in the medium were measured by RIA. Similar experiments were repeated at least three times with comparable results. Each bar represents the mean ± sem (n = 4 for the MM2/9 inhibitor, A; and n = 3 for doxycycline, B). *, P < 0.05 by Student's t test relative to LH alone; **, P = 0.08 by Student's t test relative to LH alone.
Doxycycline inhibits LH-induced steroidogenesis in mouse follicles
Interestingly, the common antibiotic doxycycline is a known MMP2/9 inhibitor that has been used to treat several disease states that involve MMP2/9 actions (14–19). Therefore, we tested whether doxycycline inhibited LH-induced steroidogenesis in preovulatory follicles. Similar to previous in vitro studies showing that doxycycline had an EC50 for MMP2 and MMP9 in the 100-μm range (20), we found that 50 μm doxycycline had minimal effect on LH-induced steroid production, whereas 100 and 250 μm doxycycline treatment inhibited steroidogenesis by 50 and nearly 100%, respectively (Fig. 2B). The ability of doxycycline, as well as the specific MMP2/9 inhibitor, to block LH-induced steroid production in primary preovulatory follicles further implicates MMP2 and MMP9 as potential regulators of LHR/EGFR cross talk.
PKA signaling is necessary for LH-induced steroidogenesis in mouse follicles and occurs upstream of EGFR and Erk signaling
What are the LH-induced signals that promote MMP activation in follicles? Notably, our laboratory has shown that cAMP and subsequent PKA signaling are critical activators of LH-induced steroid production in Leydig cells of the testis (3). Accordingly, cAMP is known to regulate steroidogenesis in granulosa cells as well (21, 22). To examine the importance of cAMP and PKA in LH-induced steroidogenesis in ovarian follicles, PKA activity was inhibited by treatment with H89. As expected, H89 significantly reduced LH-induced steroid production in preovulatory follicles for up to 2 h (Fig. 3A), confirming that PKA is necessary for normal ovarian steroidogenesis. Importantly, H89 decreased Erk activation (Fig. 3B) in preovulatory follicles, whereas addition of EGF in the presence of LH and H89 rescued steroidogenesis (Fig. 3C), confirming that, as in Leydig cells, EGFR and Erk signaling is downstream of PKA in the LH-induced steroidogenic pathway.
Fig. 3.
PKA signaling is necessary for LH-induced steroidogenesis in mouse follicles and functions upstream of the Erk signaling. A and B, Preovulatory follicles from PMSF-primed female mice were preincubated with 0.01% DMSO or 10 μm H89 for 30 min. LH (0.2 mg/ml) was used to stimulate follicles for 30 min to 2 h in the presence or absence of H89. Progesterone concentrations were measured by RIA (A), and cell lysates were collected at 2 h and analyzed by Western blot for phosphorylated and total Erk (P- and T-Erk) (B). C, Follicles were pretreated with 0.01% DMSO or 10 μm H89 for 30 min and stimulated with 0.2 mg/ml LH or LH plus 200 ng/ml EGF for 2 h in the presence or absence of H89, and progesterone concentrations were measured by RIA. Experiments were repeated at least three times with similar results. Each bar represents the mean ± sem (n = 3). *, P < 0.05 by Student's t test relative to LH alone.
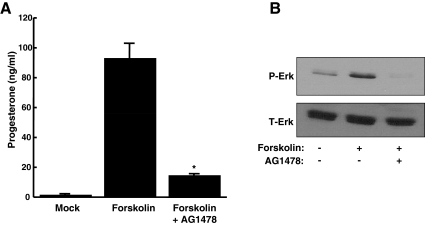
After demonstrating that PKA signaling was necessary for LH-induced steroidogenesis in ovarian follicles, we next determined whether cAMP was sufficient to induce steroid production. Preovulatory follicles were treated with forskolin to stimulate adenylyl cyclase and increase intracellular levels of cAMP. In fact, forskolin promoted progesterone production (Fig. 4A) as well as Erk signaling (Fig. 4B) at levels similar to that seen with LH stimulation. In addition, the EGFR inhibitor AG1478 abrogated forskolin-induced steroid production and Erk signaling at 2 h. Together, these data demonstrate that, as seen in Leydig cells, cAMP elevations are both necessary and sufficient for steroidogenesis in ovarian follicles, functioning upstream of EGFR and Erk signaling.
Fig. 4.
cAMP is sufficient to promote steroidogenesis in mouse follicles and signals upstream of the EGFR. Preovulatory follicles from PMSG-primed female mice were pretreated with 0.01% DMSO or 20 μm AG1478 for 30 min. Follicles were then stimulated for 2 h with 10 μm forskolin plus DMSO or AG1478. Progesterone concentrations in the medium were measured by RIA (A), and cell lysates were collected at 2 h and analyzed for phosphorylated and total Erk (P- and T-Erk) by Western blot (B). Experiments were repeated at least three times with similar results. Each bar represents the mean ± sem (n = 3). *, P < 0.05 by Student's t test relative to forskolin alone.
In vivo injection of MMP/EGFR inhibitors does not alter normal follicular development or estrous cycling in wild-type mice
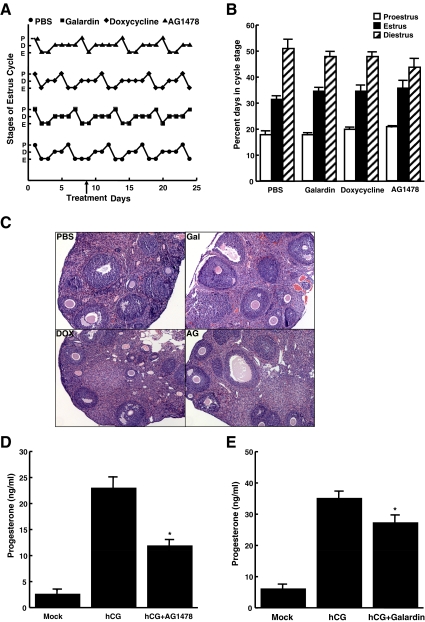
Although in vitro experiments demonstrated an important role for MMP in ovarian steroidogenesis and other processes (1, 7, 9), the importance of ovarian MMP in vivo has not been examined. We therefore studied how daily injection of Galardin or doxycycline affected normal female mouse cycling over several weeks. The estrous cycles of 9-wk-old female mice (four mice per condition) were examined by vaginal smears through two cycles, after which ip injections of dimethylsulfoxide (DMSO), 20 μm Galardin, 100 μm doxycycline, or 20 μm AG1478 were given daily for a total of 15 d. Surprisingly, estrous cycles of female mice were unaffected by both the EGFR inhibitor as well as the two MMP inhibitors (Fig. 5A shows one representative mouse from each group). In fact, under all conditions, mice spent 50% of days in diestrus, 30% in estrus, and 20% in proestrus (Fig. 5B). Furthermore, hematoxylin and eosin (H&E) staining of sectioned ovaries at the end of the 15 d were identical in all treatment groups, demonstrating that follicular development was grossly unaffected by the different treatments (Fig. 5C). These experiments indicate that under normal conditions, when proper LH and FSH levels drive normal follicular development and steroid production, both MMP and EGFR inhibitors appear to have no serious detrimental effects on estrous cycling.
Fig. 5.
MMP/EGFR inhibitors do not alter estrous cycling and follicular development but do attenuate hCG-induced steroidogenesis in vivo. Panel A, The estrous cycles of 9-wk-old female mice were analyzed by vaginal smears for two cycles. Mice were then injected daily with ip injections of vehicle, 20 μm Galardin, 20 μm AG1478, or 100 μm doxycycline for 15 d (starting at the point labeled treatment) and cycles tracked by vaginal smears. Shown are representative cycling for each treatment group (D, diestrus; P, proestrus; E, estrus). Panel B, Percentage of days spent in each estrous cycle stage. Data are represented as mean ± se (n = 4 mice). Panel C, Ovaries were removed after the 15th day of treatment and stained with H&E. AG, AG1478, DOX, doxycycline; Gal, Galardin. Panels D and E, Three-week-old (prepubertal) female mice were injected with 5 IU PMSG plus DMSO (Mock, hCG) or 5 IU PMSG plus 20 μm Galardin or 20 μm AG1478, and 12 h later, mice were injected a second time with either DMSO or inhibitors. At 48 h after PMSG injection, mice were injected with PBS (Mock), 1 IU hCG, 1 IU hCG plus 20 μm AG1478 (panel D), or 1 IU hCG plus 20 μm Galardin (panel E). At 8 h after hCG injection, mice were killed and serum progesterone levels measured by RIA. Each bar represents the mean ± sem [n = 8 (panel D), and n = 12 (panel E)]. *, P < 0.05 by Student's t test relative to hCG alone.
In vivo inhibition of steroidogenesis in hyperstimulated mice
Although MMP and EGFR inhibitors did not have a dramatic effect on normal cycling, we wondered whether they might suppress ovarian steroid production under conditions of excess LH stimulation. Prepubertal female mice were hyperstimulated by ip injections of pregnant mare serum gonadotropin (PMSG) followed by human chorionic gonadotropin (hCG), resulting in significant progesterone production compared with mice that received vehicle. Interestingly, injection of either AG1478 (EGFR inhibitor) or Galardin (MMP inhibitor), along with the gonadotropins, partially but significantly reduced hCG-induced serum progesterone levels (Fig. 5, D and E). Thus, pharmacological inhibition of MMP or the EGFR can indeed alter ovarian steroidogenesis in vivo when the system is overstimulated, as in the setting of excess gonadotropin stimulation. Notably, in vivo EGFR and MMP inhibition of hCG-induced steroidogenesis (Fig. 5, D and E) was less robust than LH-mediated steroid production in vitro (Figs. 1 and 2), most likely due to lower serum concentrations and potency of the inhibitors in live animals.
ACTH-induced steroidogenesis in Y1 adrenal cells requires MMP-independent cross talk between the ACTH receptor and the EGFR
After demonstrating that GPCR/EGFR cross talk is present in both gonads (although the mechanisms regulating EGFR trans-activation differ), we next focused on the third major steroidogenic tissue, the adrenal gland. To determine whether GPCR/EGFR cross talk was conserved in adrenal cells, we examined the mouse adrenal Y1 cell line. These cells produce steroid in response to ACTH; however, they lack the enzymatic machinery to metabolize steroids beyond progesterone; thus, here we measured ACTH-induced progesterone production. We first examined the role of EGFR signaling in ACTH-induced steroidogenesis. Y1 cells were pretreated for 30 min with vehicle or the EGFR inhibitor AG1478, followed by stimulation with ACTH or ACTH plus the indicated inhibitor. ACTH-induced progesterone production was markedly inhibited by the EGFR inhibitor AG1478 at 30 min (not shown) or 2 h (Fig. 6A), indicating that EGFR transactivation indeed plays an important role in ACTH-induced steroidogenesis. Similarly, the MEK inhibitor U0126 abrogated ACTH-induced steroid production at 2 h (Fig. 6B). Together, these results suggest that GPCR/EGFR cross talk, followed by Erk activation, is conserved in adrenal cells. However, as in Leydig cells (3), EGFR activation alone was not sufficient to promote steroid production, because treatment of Y1 cells with EGF alone did not promote significant steroidogenesis (Fig. 6A, last bar).
Fig. 6.
ACTH uses EGFR and Erk signaling to promote steroidogenesis in Y1 adrenal cells. Y1 mouse adrenal cells were pretreated with 0.01% DMSO, 20 μm AG1478 (A), 20 μm U0126 (B), or 20 μm Galardin (D) for 30 min before stimulation with 50 nm ACTH plus inhibitors or DMSO for 2 h. Progesterone levels in the media were measured by RIA (A, B, and D). For C, Y1 cells were treated with DMSO or 20 μm Galardin for 30 min followed by 2 h with 50 nm ACTH plus DMSO or Galardin. Medium was then removed and placed on A431 cells for 2 h. A431 lysates were then examined by Western blot for phosphorylated and total EGFR (P- and T-EGFR). The final lane represents A431 cells treated with fresh 50 nm ACTH in medium. All experiments were performed at least three times with similar results. For A and C, each point represents the mean ± sem (n = 3). *, P < 0.05 by Student's t test relative to ACTH alone.
MMP are critical for steroidogenesis in the ovary but are not essential for steroidogenesis in the testis (3). To test for MMP-mediated release of EGFR ligands, medium from Y1 cells treated with ACTH both in the presence and absence of the MMP inhibitor Galardin was added to A431 cells. A431cells are known to express very high levels of EGFR and are therefore very sensitive to EGFR ligands that might be released from the Y1 cells in response to ACTH. In fact, medium from ACTH-treated Y1 cells promoted EGFR phosphorylation in the A431 cells that was blocked by the addition of Galardin during the ACTH stimulation of the Y1 cells (Fig. 6C), suggesting the ACTH indeed triggered MMP-mediated release of EGFR ligands from the Y1 cells. However, although Galardin blocked ACTH-induced EGFR ligand release from Y1 cells, it had no effect on ACTH-induced steroidogenesis for up to 2 h (Fig. 6D), indicating that, as in Leydig cells (3, 4), extracellular (MMP-mediated) trans-activation of the EGFR occurs but is not necessary for ACTH-induced steroidogenesis in Y1 adrenal cells.
PKA signaling has been previously implicated in regulating EGFR signaling and steroidogenesis in other tissues. Its role in adrenal steroid production was therefore analyzed. Cells were pretreated with the PKA inhibitor H89 or vehicle for 30 min before stimulation with ACTH for 2 h. ACTH-induced steroid production was almost completely abrogated with H89 treatment at 2 h of stimulation (Fig. 7A), confirming its importance in regulating steroid production in adrenal cells.
Fig. 7.
cAMP/PKA signaling regulates steroidogenesis in Y1 cells via EGFR signaling. A and B, Y1 mouse adrenal cells were pretreated with 0.01% DMSO, 20 μm AG1478, 20 μm U0126, or 20 μm H89 for 30 min before stimulation with 50 nm ACTH plus inhibitors or DMSO for 2 h. A and B, Progesterone levels in the medium were measured by RIA (A) and Erk activation examined by Western blot (B). C, Y1 cells were treated with medium alone (Mock), 50 nm ACTH, or 10 μm cpTOME (gift from Alan Smrcka, University of Rochester) (24) for 30 min. Erk activation was then examined by Western blot. D, Cells were pretreated with 0.01% DMSO, 20 μm U0126, 20 μm AG1478, or 20 μm H89 for 30 min before stimulation with 10 μm forskolin plus inhibitors or DMSO for 2 h. Progesterone content in medium was measured by RIA. All experiments were performed at least three times with similar results. For A and C, each point represents the mean ± sem (n = 3). *, P < 0.05 by Student's t test relative to ACTH alone. P- and T-Erk, Phosphorylated and total Erk.
In Leydig cells and ovarian follicles, EGFR-mediated activation of Erk signaling occurs in response to LH. To analyze Erk signaling in adrenal cells, Y1 cell lysates were collected and analyzed for phosphorylated and total Erk at 2 h of ACTH stimulation with and without the indicated inhibitors. As expected, ACTH induced Erk phosphorylation (Fig. 7B) at 2 h. ACTH activation of Erk was essentially absent in the presence of the MEK inhibitor U0126 or the EGFR inhibitor AG1478, confirming that, similar to the testis and ovary, Erk activation is downstream of EGFR signaling. Surprisingly, however, Erk phosphorylation was less inhibited when PKA was blocked by H89 (Fig. 7B, last lane), suggesting the ACTH can activate EGFR/Erk signaling in a PKA-independent fashion. One possible means for ACTH to stimulate Erk independent of PKA signaling is through activation of exchange protein activated by cAMP (EPAC), which, in Y1 cells, is known to regulate kinase signaling in response to cAMP but not PKA (23). In fact, we find that the EPAC activator cpTOME (24) does indeed stimulate Erk activation in Y1 cells (Fig. 7C).
Finally, to directly increase cAMP levels and therefore analyze its role in steroidogenesis, cells were stimulated with forskolin. Cells treated with forskolin at 2 h induced similar levels of progesterone seen with ACTH stimulation. As with ACTH stimulation, treatment with 20 μm U0126, 20 μm AG1478, and 20 μm H89 blocked forskolin-induced steroidogenesis (Fig. 7D). Together, these data indicate that, as in Leydig cells and ovarian follicles, elevation of intracellular cAMP is sufficient to promote steroidogenesis in adrenal cells and serves as a proximal mediator of EGFR trans-activation.
Discussion
Many studies have demonstrated that GPCR can trans-activate RTK (25, 26). However, the majority of these experiments focused on intracellular signaling in cell lines rather than physiological processes; thus, the biological importance of GPCR/RTK cross talk has remained obscure. Our recent work (1–3), combined with the new data presented here, demonstrate that GPCR/EGFR cross talk is critical for the important physiological process of steroid production in all three major steroidogenic tissues: the testes, the ovary, and the adrenal gland. Beyond demonstrating the importance of GPCR/EGFR cross talk for a significant biological function, the requirement of EGFR signaling for steroid production brings forth the possibility that, if the potencies of the EGFR inhibitors currently being used to treat many cancers were great enough, then they could have the potential side effect of attenuating steroidogenesis. To date, no studies have closely followed steroid production in the setting of chronic EGFR inhibitor use in humans, but this may be an issue for future analysis.
Interestingly, nearly all of the LH- or ACTH-induced signals both upstream and downstream of EGFR activation are also shared between the three major steroidogenic tissues, suggesting a remarkable conservation of the entire signaling pathway that regulates steroid production. For example, we find that Erk1/2 signaling positively regulates LH- and ACTH-induced steroidogenesis in Leydig cells (3), ovarian follicles (Fig. 1), and adrenal cells (Figs. 6 and 7). These findings are consistent with previous signaling studies in Leydig and granulosa cells, where Erk is activated by LH (4–6, 27–30) or ACTH (29). Notably, other work in granulosa and theca cell lines suggested that, under some conditions, Erk signaling might actually inhibit steroidogenesis (28, 31). One reason for these disparate results may be that these latter cell lines were grown in culture and out of the context of an intact follicle. Here we used freshly isolated preovulatory follicle, where normal theca cell/granulosa cell/oocyte cross talk can still occur. Furthermore, these previous studies required long stimulation periods (up to 48 h) to see significant steroidogenesis, whereas we examined shorter time points that better reflect normal responses to LH (30–120 min shown here, but up to 6 h previously) (1).
How does EGFR/Erk signaling promote steroidogenesis? Others' (6) and our (1, 3) data suggest that EGFR/Erk might in part regulate steroid production by mediating posttranslational modification (phosphorylation) of StAR in the testes and ovary, respectively. Accordingly, ACTH did not induce significant changes in StAR protein expression in adrenal Y1 cells over the course of 2 h (Supplemental Fig. 2), suggesting that posttranslational modification of StAR is a conserved EGFR/Erk-mediated action in all steroidogenic tissue.
Importantly, although EGFR/Erk signaling is necessary for LH- and ACTH-triggered steroidogenesis in Leydig and adrenal cells, respectively, EGFR/Erk activation is not sufficient on its own for steroid production (3, 32, 33) (Fig. 6A). This implies the presence of two cAMP/PKA-mediated pathways required for steroid production in Leydig and adrenal cells: one that is EGFR dependent (Fig. 8, left pathway) and one that is EGFR independent (Fig. 8, middle pathway). The former involves the aforementioned Erk-mediated StAR phosphorylation, whereas the latter must involve an alternative cAMP/PKA-mediated pathway that includes direct StAR activation by PKA and/or CREB-mediated up-regulation of StAR or other steroidogenic enzymes. Interestingly, this alternative pathway likely exists in the cumulus granulosa cells of ovarian follicles as well, only it is activated by FSH during the priming phase with PMSG, thus allowing EGFR/Erk signaling to promote steroidogenesis on its own in primed ovarian follicles (1).
Fig. 8.
Model for steroidogenesis in the ovary, testes, and adrenal gland. In all three steroidogenic tissues, LH or ACTH binds its respective GPCR to activate Gαs and increase cAMP levels. Elevated cAMP then leads to PKA activation. In Leydig or adrenal cells, PKA then regulates two processes, both of which are required for normal steroidogenesis (black arrows). The first (middle arrow) likely involves activation of CREB and up-regulation of StAR and steroidogenic enzymes as well as direct activation of StAR through yet unknown mechanisms. The second (left black arrows) involves trans-activation of the EGFR, Erk activation, and subsequent StAR phosphorylation/activation. Although MMP are activated and EGFR ligands released, EGFR trans-activation occurs in an MMP- and ligand-independent, or intracellular, mechanism in Leydig and adrenal cells. In contrast, steroidogenesis requires cross talk between mural granulosa cells (which express LHR) and cumulus granulosa cells (which make progesterone and other steroids) in the ovary. Here, FSH activates PKA in cumulus granulosa cells to trigger the first signal (equivalent to the middle arrow). LH then triggers MMP-mediated release of EGFR ligands from mural granulosa cells, leading to ligand-dependent trans-activation of EGFR (right gray arrows), Erk activation, and StAR phosphorylation/activation in cumulus granulosa cells. Once activated, StAR promotes steroidogenesis in all three tissues.
In addition to placing Erk signaling downstream of EGFR activation in all three steroidogenic tissues, our studies also definitively place cAMP and PKA signaling upstream of EGFR activation (3) (Figs. 3, 4, and 7). Both ACTH and LHR are known to couple to Gαs, leading to elevated intracellular cAMP and PKA activation. As mentioned, cAMP and PKA are then thought to promote steroidogenesis mainly by increasing StAR expression and activity (34, 35).
How do cAMP elevations lead to EGFR activation in steroidogenic tissues? GPCR can transactivate the EGFR via both ligand-independent and ligand-dependent mechanisms. Ligand-independent transactivation of the EGFR may involve direct associations between GPCR and EGFR or direct phosphorylation of the EGFR by intracellular kinases such as PKA or Src (36–38). In contrast, ligand-dependent transactivation of the EGFR involves MMP-mediated release of EGFR ligands (e.g. amphiregulin, epiregulin, or heparin bound-EGF) that then activate the EGFR in a paracrine fashion (39, 40). How PKA activates MMP-mediated release of EGFR ligands is not yet known but likely involves stimulating intracellular proteases that cleave pro-MMP to their active forms.
Previous work demonstrated that LH promotes MMP activation and EGFR ligand release in both follicles (2, 9, 41) and Leydig cells (4). Furthermore, our data here demonstrate that ACTH promotes MMP-dependent release of EGFR ligands in Y1 cells (Fig. 6). However, although MMP-mediated release of EGFR ligands occurs in all three systems, we find that only ovarian GPCR-mediated steroidogenesis requires this ligand-dependent activation, because the MMP inhibitor Galardin blocked only LH-induced steroidogenesis in follicles. Thus, in Leydig and adrenal cells, where steroidogenesis occurs in one cell, intracellular (ligand-independent) mechanisms are all that is needed to promote EGFR trans-activation (Fig. 8). In contrast, in ovarian follicles, where LHR are located in mural granulosa cells but steroidogenesis occurs in cumulus granulosa cells, paracrine signaling via MMP-mediated EGFR ligand release is required for normal steroidogenesis.
The observation that ovarian steroidogenesis can be exclusively blocked through MMP inhibition begs the question as to whether MMP inhibition can be used to treat diseases where LH-induced steroidogenesis is up-regulated. One such example is polycystic ovarian syndrome (PCOS), where enhanced LH-induced testosterone production in the ovary leads to excess androgen production (42–44). To start examining this possibility, we narrowed down potential LH-activated MMP to MMP2 and MMP9. Although the specificity of the reagents is not absolute, results demonstrating that MMP2/MMP9 inhibitor I and doxycycline (also an MMP2/MMP9 inhibitor) (14, 16) blocked LH-induced steroidogenesis are consistent with previous observations that MMP2 and MMP9 are expressed in the ovary, are responsive to gonadotropins, and are elevated in patients with PCOS (13). Notably, MMP2- and MMP9-null female mice have reduced fertility (13). MMP2/9 double-null mice are viable (45), but their fertility has not been studied.
Unexpectedly, in vivo treatment with the MMP inhibitor Galardin or doxycycline had minimal effect on normal cycling in mice (Fig. 5), suggesting that MMP inhibition, at least at the doses used here, does not significantly suppress the hypothalamic/pituitary/gonadal axis. However, Galardin did partially block ovarian steroid production in response to overstimulation by LH (as is the scenario in patients with PCOS). Thus, using the appropriate dosing, MMP inhibition in vivo, perhaps with the common antibiotic doxycycline, might attenuate excessive LH-induced steroid production (as seen in PCOS) without further inhibiting normal cycling. Further investigations in a mouse model for PCOS (46) are currently underway to test this hypothesis.
Materials and Methods
Steroid production in preovulatory follicles
Experimental protocols involving mice were approved by the University of Texas Southwestern Medical Center and Rochester University Animal Care and Use Committee. Three-week-old C57BL/6J female mice (Jackson Laboratory, Bar Harbor, ME) were primed with 5 IU PMSG (Sigma Chemical Co., St. Louis, MO) by ip injection. Ovaries were harvested 40–44 h after PMSG injection. Ovaries were punctured with 30-gauge needles and follicles isolated in M2 medium (Millipore, Billerica, MA). Intact follicles were washed twice with M2 medium and treated with 0.2 mg/ml LH (Sigma) or 200 ng/ml EGF (Invitrogen, Carlsbad, CA) in M16 medium (Millipore). Follicles were pretreated with 20 μm AG1478, Galardin, MMP2/9 inhibitor I, H89, U0126 (EMD Biosciences), or vehicle for 30 min before and then throughout stimulation. Doxycycline (Sigma) treatment at the indicated concentrations was also performed 30 min before and throughout stimulation. Medium was collected as indicated and progesterone content measured as described below. Follicles were permeabilized and Erk activation detected as described below.
Steroid production in Y1 adrenal cells
The Y1 adrenal cell line (provided by P. Hinkle, University of Rochester, Rochester, NY) was grown in DMEM/F12 medium (GIBCO, Carlsbad, CA) supplemented with 15% fetal bovine serum (Invitrogen). Cells were seeded in 12-well plates followed by overnight serum starvation. Experiments were also performed without serum starvation, and similar results were obtained. Cells were treated with 20 μm AG1478, Galardin, H89, U0126 (EMD Biosciences), or vehicle for 30 min before and throughout stimulation with 50 nm ACTH (VWR, Radnor, PA). Culture medium from each treatment was sampled at 2 h after addition of ACTH.
Steroid RIA
Progesterone concentrations from culture medium or mouse serum were measured using a progesterone RIA kit (MP Biomedicals, Solon, OH). Culture medium collected was diluted 1:2 in diluent for all RIA.
Erk and EGFR activation and immunoblotting
Erk and EGFR immunoblotting assays were performed as described (1, 3, 11). Briefly, cultured follicles, Y1 cells, or A431 cells were washed with cold PBS and permeabilized in 200 μl 1× RIPA (Santa Cruz Biotechnology, Santa Cruz, CA) buffer supplemented with proteinase inhibitors, sodium orthovanadate, and phenylmethylsulfonyl fluoride. After clearing the lysates by microcentrifugation, an equal volume of 2× Laemmli sampler buffer containing 10% β-mercaptoethanol (Sigma) was added. Samples were then separated on 12.5% (Erk) or 7.5% (EGFR) SDS-polyacrylamide gels and transferred to Immobilon membranes (Millipore) at 100 V for 60 min, followed by Western blot analysis with anti-Erk and anti-phosphorylated Erk antibodies (9101, 9102; Cell Signaling Technology, Beverly, MA) or anti-EGFR or anti-phosphorylated EGFR antibodies (SC23420, SC-03; Santa Cruz Biotechnology). Blots were then treated with horseradish peroxidase-conjugated goat antirabbit (Bio-Rad, Hercules, CA) followed by ECL-Plus (GE Healthcare, Piscataway, NJ).
In vivo steroidogenesis assays
Four-week-old C57BL/6J female mice (Jackson Laboratory) were induced by ip injection with 1 IU PMSG plus DMSO vehicle, 20 μm AG1478, or 20 μm Galardin. Mice were then injected with the same inhibitors (or vehicle) 12 h later. At 44 h after PMSG treatment, mice were injected 1 IU hCG plus vehicle or the inhibitors above. Eight hours later, mice were anesthetized with Avertin (Sigma) before blood collection via cardiac puncture. Serum was extracted using serum separator tubes (BD microtainer) and progesterone levels measured by RIA.
In vivo injections of inhibitors into cycling female mice
Estrous cycles were tracked in 9-wk-old C57BL/6J female mice via vaginal smears (47). Mice were followed for two full cycles before daily ip injections were given for 15 d of 0.01% DMSO, 20 μm Galardin, 20 μm AG1478, or 100 μm doxycycline. After 15 d of tracking vaginal smears, mice were killed and ovaries collected. One ovary was sectioned and subjected to H&E staining. Sections were taken at intervals of 30 μm, and 5-μm paraffin-embedded sections were mounted on slides.
EGFR transactivation assay
A431 cells were used to detect ACTH-mediated release of EGFR ligands from Y1 cells. Y1 cells were treated for 30 min with DMSO or 20 μm Galardin followed by 2 h with 50 nm ACTH plus DMSO or Galardin for 2 h. As described previously (11), medium was removed from the Y1 cells and added to A431 cells (American Type Culture Collection, Manassas, VA) that had been serum starved overnight. After 2 h, A431 cells were permeabilized with RIPA and blotted for total and phosphorylated EGFR as described above. As a control, A431 cells were stimulated with fresh ACTH in DMEM/F12 medium.
Supplementary Material
Acknowledgments
We are grateful to Melanie Cobb (University of Texas Southwestern, Dallas, TX) for assisting with L.C.'s graduate education. We also thank Patty Hinkle (University of Rochester, Rochester, NY) for her assistance with this project.
This work was supported by National Institutes of Health Grant DK59913 (to S.R.H. and L.C.).
Disclosure Summary: The authors of this manuscript have nothing to disclose.
Footnotes
- CREB
- cAMP response element-binding protein
- DMSO
- dimethylsulfoxide
- EGFR
- epidermal growth factor receptor
- GPCR
- G protein-coupled receptor
- hCG
- human chorionic gonadotropin
- H&E
- hematoxylin and eosin
- LHR
- LH receptor
- MEK
- MAPK kinase
- MMP
- matrix metalloproteinase
- PCOS
- polycystic ovarian syndrome
- PKA
- protein kinase A
- PMSG
- pregnant mare serum gonadotropin
- RTK
- receptor tyrosine kinase
- StAR
- steroidogenic acute regulatory protein
- TAPI
- TNF-α protease inhibitor.
References
- 1. Jamnongjit M, Gill A, Hammes SR. 2005. Epidermal Growth Factor Receptor signaling is required for normal ovarian steroidogenesis and oocyte maturation. Proc Natl Acad Sci USA 102:16257–16262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Jamnongjit M, Hammes SR. 2006. Ovarian steroids: the good, the bad, and the signals that raise them. Cell Cycle 5:1178–1183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Evaul K, Hammes SR. 2008. Cross talk between G protein-coupled and epidermal growth factor receptors regulates gonadotropin-mediated steroidogenesis in Leydig cells. J Biol Chem 283:27525–27533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Shiraishi K, Ascoli M. 2008. A co-culture system reveals the involvement of intercellular pathways as mediators of the lutropin receptor (LHR)-stimulated ERK1/2 phosphorylation in Leydig cells. Exp Cell Res 314:25–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Andric N, Ascoli M. 2008. The luteinizing hormone receptor-activated extracellularly regulated kinase-1/2 cascade stimulates epiregulin release from granulosa cells. Endocrinology 149:5549–5556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Poderoso C, Maloberti P, Duarte A, Neuman I, Paz C, Maciel FC, Podesta EJ. 2009. Hormonal activation of a kinase cascade localized at the mitochondria is required for StAR protein activity. Mol Cell Endocrinol 300:37–42 [DOI] [PubMed] [Google Scholar]
- 7. Conti M, Hsieh M, Park JY, Su YQ. 2006. Role of the epidermal growth factor network in ovarian follicles. Mol Endocrinol 20:715–723 [DOI] [PubMed] [Google Scholar]
- 8. Fan HY, Liu Z, Shimada M, Sterneck E, Johnson PF, Hedrick SM, Richards JS. 2009. MAPK3/1 (ERK1/2) in ovarian granulosa cells are essential for female fertility. Science 324:938–941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Park JY, Su YQ, Ariga M, Law E, Jin SL, Conti M. 2004. EGF-like growth factors as mediators of LH action in the ovulatory follicle. Science 303:682–684 [DOI] [PubMed] [Google Scholar]
- 10. Jamnongjit M, Hammes SR. 2005. Oocyte maturation: the coming of age of a germ cell. Semin Reprod Med 23:234–241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sen A, O'Malley K, Wang Z, Raj GV, Defranco DB, Hammes SR. 2010. Paxillin regulates androgen- and epidermal growth factor-induced MAPK signaling and cell proliferation in prostate cancer cells. J Biol Chem 285:28787–28795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Panigone S, Hsieh M, Fu M, Persani L, Conti M. 2008. Luteinizing hormone signaling in preovulatory follicles involves early activation of the epidermal growth factor receptor pathway. Mol Endocrinol 22:924–936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Curry TE, Jr, Osteen KG. 2003. The matrix metalloproteinase system: changes, regulation, and impact throughout the ovarian and uterine reproductive cycle. Endocr Rev 24:428–465 [DOI] [PubMed] [Google Scholar]
- 14. Chung AW, Yang HH, Radomski MW, van Breemen C. 2008. Long-term doxycycline is more effective than atenolol to prevent thoracic aortic aneurysm in marfan syndrome through the inhibition of matrix metalloproteinase-2 and -9. Circ Res 102:e73–e85 [DOI] [PubMed] [Google Scholar]
- 15. Chaudhry K, Rogers R, Guo M, Lai Q, Goel G, Liebelt B, Ji X, Curry A, Carranza A, Jimenez DF, Ding Y. 2010. Matrix metalloproteinase-9 (MMP-9) expression and extracellular signal-regulated kinase 1 and 2 (ERK1/2) activation in exercise-reduced neuronal apoptosis after stroke. Neurosci Lett 474:109–114 [DOI] [PubMed] [Google Scholar]
- 16. Lee CZ, Xu B, Hashimoto T, McCulloch CE, Yang GY, Young WL. 2004. Doxycycline suppresses cerebral matrix metalloproteinase-9 and angiogenesis induced by focal hyperstimulation of vascular endothelial growth factor in a mouse model. Stroke 35:1715–1719 [DOI] [PubMed] [Google Scholar]
- 17. Sochor M, Richter S, Schmidt A, Hempel S, Hopt UT, Keck T. 2009. Inhibition of matrix metalloproteinase-9 with doxycycline reduces pancreatitis-associated lung injury. Digestion 80:65–73 [DOI] [PubMed] [Google Scholar]
- 18. Lindeman JH, Abdul-Hussien H, van Bockel JH, Wolterbeek R, Kleemann R. 2009. Clinical trial of doxycycline for matrix metalloproteinase-9 inhibition in patients with an abdominal aneurysm: doxycycline selectively depletes aortic wall neutrophils and cytotoxic T cells. Circulation 119:2209–2216 [DOI] [PubMed] [Google Scholar]
- 19. Palei AC, Zaneti RA, Fortuna GM, Gerlach RF, Tanus-Santos JE. 2005. Hemodynamic benefits of matrix metalloproteinase-9 inhibition by doxycycline during experimental acute pulmonary embolism. Angiology 56:611–617 [DOI] [PubMed] [Google Scholar]
- 20. Nicolescu AC, Holt A, Kandasamy AD, Pacher P, Schulz R. 2009. Inhibition of matrix metalloproteinase-2 by PARP inhibitors. Biochem Biophys Res Commun 387:646–650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Suh BS, Amsterdam A. 1990. Establishment of highly steroidogenic granulosa cell lines by cotransfection with SV40 and Ha-ras oncogene: induction of steroidogenesis by cyclic adenosine 3′-5′-monophosphate and its suppression by phorbol ester. Endocrinology 127:2489–2500 [DOI] [PubMed] [Google Scholar]
- 22. Kamei Y, Aoyama Y, Fujimoto T, Kenmotsu N, Kishi C, Koushi M, Sugano S, Morohashi K, Kamiyama R, Asakai R. 2005. A steroidogenic cell line with differentiation potential from mouse granulosa cells, transfected with Ad4BP and SV40 large T antigen genes. J Endocrinol 185:187–195 [DOI] [PubMed] [Google Scholar]
- 23. Holz GG, Kang G, Harbeck M, Roe MW, Chepurny OG. 2006. Cell physiology of cAMP sensor Epac. J Physiol 577:5–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Oestreich EA, Wang H, Malik S, Kaproth-Joslin KA, Blaxall BC, Kelley GG, Dirksen RT, Smrcka AV. 2007. Epac-mediated activation of phospholipase Cε plays a critical role in β-adrenergic receptor-dependent enhancement of Ca2+ mobilization in cardiac myocytes. J Biol Chem 282:5488–5495 [DOI] [PubMed] [Google Scholar]
- 25. Pierce KL, Luttrell LM, Lefkowitz RJ. 2001. New mechanisms in heptahelical receptor signaling to mitogen activated protein kinase cascades. Oncogene 20:1532–1539 [DOI] [PubMed] [Google Scholar]
- 26. Zwick E, Hackel PO, Prenzel N, Ullrich A. 1999. The EGF receptor as central transducer of heterologous signalling systems. Trends Pharmacol Sci 20:408–412 [DOI] [PubMed] [Google Scholar]
- 27. Shiraishi K, Ascoli M. 2006. Activation of the lutropin/choriogonadotropin receptor in MA-10 cells stimulates tyrosine kinase cascades that activate ras and the extracellular signal regulated kinases (ERK1/2). Endocrinology 147:3419–3427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Seger R, Hanoch T, Rosenberg R, Dantes A, Merz WE, Strauss JF, 3rd, Amsterdam A. 2001. The ERK signaling cascade inhibits gonadotropin-stimulated steroidogenesis. J Biol Chem 276:13957–13964 [DOI] [PubMed] [Google Scholar]
- 29. Gyles SL, Burns CJ, Whitehouse BJ, Sugden D, Marsh PJ, Persaud SJ, Jones PM. 2001. ERKs regulate cyclic AMP-induced steroid synthesis through transcription of the steroidogenic acute regulatory (StAR) gene. J Biol Chem 276:34888–34895 [DOI] [PubMed] [Google Scholar]
- 30. Cameron MR, Foster JS, Bukovsky A, Wimalasena J. 1996. Activation of mitogen-activated protein kinases by gonadotropins and cyclic adenosine 5′-monophosphates in porcine granulosa cells. Biol Reprod 55:111–119 [DOI] [PubMed] [Google Scholar]
- 31. Nelson-Degrave VL, Wickenheisser JK, Hendricks KL, Asano T, Fujishiro M, Legro RS, Kimball SR, Strauss JF, 3rd, McAllister JM. 2005. Alterations in mitogen-activated protein kinase kinase and extracellular regulated kinase signaling in theca cells contribute to excessive androgen production in polycystic ovary syndrome. Mol Endocrinol 19:379–390 [DOI] [PubMed] [Google Scholar]
- 32. Ascoli M, Euffa J, Segaloff DL. 1987. Epidermal growth factor activates steroid biosynthesis in cultured Leydig tumor cells without affecting the levels of cAMP and potentiates the activation of steroid biosynthesis by choriogonadotropin and cAMP. J Biol Chem 262:9196–9203 [PubMed] [Google Scholar]
- 33. Manna PR, Chandrala SP, Jo Y, Stocco DM. 2006. cAMP-independent signaling regulates steroidogenesis in mouse Leydig cells in the absence of StAR phosphorylation. J Mol Endocrinol 37:81–95 [DOI] [PubMed] [Google Scholar]
- 34. Wood JR, Strauss JF., 3rd 2002. Multiple signal transduction pathways regulate ovarian steroidogenesis. Rev Endocr Metab Disord 3:33–46 [DOI] [PubMed] [Google Scholar]
- 35. Strauss JF, 3rd, Kallen CB, Christenson LK, Watari H, Devoto L, Arakane F, Kiriakidou M, Sugawara T. 1999. The steroidogenic acute regulatory protein (StAR): a window into the complexities of intracellular cholesterol trafficking. Recent Prog Horm Res 54:369–394; discussion 394–395 [PubMed] [Google Scholar]
- 36. Daaka Y, Luttrell LM, Lefkowitz RJ. 1997. Switching of the coupling of the β2-adrenergic receptor to different G proteins by protein kinase A. Nature 390:88–91 [DOI] [PubMed] [Google Scholar]
- 37. Maudsley S, Pierce KL, Zamah AM, Miller WE, Ahn S, Daaka Y, Lefkowitz RJ, Luttrell LM. 2000. The β2-adrenergic receptor mediates extracellular signal-regulated kinase activation via assembly of a multi-receptor complex with the epidermal growth factor receptor. J Biol Chem 275:9572–9580 [DOI] [PubMed] [Google Scholar]
- 38. Tilley DG, Kim IM, Patel PA, Violin JD, Rockman HA. 2009. β- Arrestin mediates β1-adrenergic receptor-epidermal growth factor receptor interaction and downstream signaling. J Biol Chem 284:20375–20386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Drube S, Stirnweiss J, Valkova C, Liebmann C. 2006. Ligand-independent and EGF receptor-supported transactivation: lessons from β2-adrenergic receptor signalling. Cell Signal 18:1633–1646 [DOI] [PubMed] [Google Scholar]
- 40. Shah BH, Baukal AJ, Shah FB, Catt KJ. 2005. Mechanisms of extracellularly regulated kinases 1/2 activation in adrenal glomerulosa cells by lysophosphatidic acid and epidermal growth factor. Mol Endocrinol 19:2535–2548 [DOI] [PubMed] [Google Scholar]
- 41. Ashkenazi H, Cao X, Motola S, Popliker M, Conti M, Tsafriri A. 2005. Epidermal growth factor family members: endogenous mediators of the ovulatory response. Endocrinology 146:77–84 [DOI] [PubMed] [Google Scholar]
- 42. Adashi EY. 1988. Hypothalamic-pituitary dysfunction in polycystic ovarian disease. Endocrinol Metab Clin North Am 17:649–666 [PubMed] [Google Scholar]
- 43. Barontini M, García-Rudaz MC, Veldhuis JD. 2001. Mechanisms of hypothalamic-pituitary-gonadal disruption in polycystic ovarian syndrome. Arch Med Res 32:544–552 [DOI] [PubMed] [Google Scholar]
- 44. McCartney CR, Eagleson CA, Marshall JC. 2002. Regulation of gonadotropin secretion: implications for polycystic ovary syndrome. Semin Reprod Med 20:317–326 [DOI] [PubMed] [Google Scholar]
- 45. Perez SE, Cano DA, Dao-Pick T, Rougier JP, Werb Z, Hebrok M. 2005. Matrix metalloproteinases 2 and 9 are dispensable for pancreatic islet formation and function in vivo. Diabetes 54:694–701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Sullivan SD, Moenter SM. 2004. Prenatal androgens alter GABAergic drive to gonadotropin-releasing hormone neurons: implications for a common fertility disorder. Proc Natl Acad Sci USA 101:7129–7134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Sen A, Hammes SR. 2010. Granulosa cell-specific androgen receptors are critical regulators of ovarian development and function. Mol Endocrinol 24:1393–1403 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.