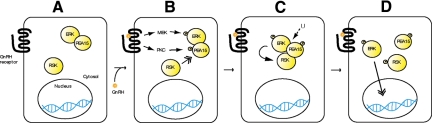
PEA-15 represents a novel point of convergence of the PKC and MAPK/ERK pathways under GnRH stimulation. The three entities form a key logic ‘AND’ gate.
Abstract
In the pituitary gonadotropes, both protein kinase C (PKC) and MAPK/ERK signaling cascades are activated by GnRH. Phosphoprotein-enriched in astrocytes 15 (PEA-15) is a cytosolic ERK scaffolding protein, which is expressed in LβT2 gonadotrope cells. Pharmacological inhibition of PKC and small interfering RNA-mediated silencing of Gαq/11 revealed that GnRH induces accumulation of phosphorylated PEA-15 in a PKC-dependent manner. To investigate the potential role of PEA-15 in GnRH signaling, we examined the regulation of ERK subcellular localization and the activation of ribosomal S6 kinase, a substrate of ERK. Results obtained by cellular fractionation/Western blot analysis and immunohistochemistry revealed that GnRH-induced accumulation of phosphorylated ERK in the nucleus was attenuated when PEA-15 expression was reduced. Conversely, in the absence of GnRH stimulation, PEA-15 anchors ERK in the cytosol. Our data suggest that GnRH-induced nuclear translocation of ERK requires its release from PEA-15, which occurs upon PEA-15 phosphorylation by PKC. Additional gene-silencing experiments in GnRH-stimulated cells demonstrated that ribosomal S6 kinase activation was dependent on both PEA-15 and PKC. Furthermore, small interfering RNA-mediated knockdown of PEA-15 caused a reduction in GnRH-stimulated expression of early response genes Egr2 and c-Jun, as well as gonadotropin FSHβ-subunit gene expression. PEA-15 knockdown increased LHβ and common α-glycoprotein subunit mRNAs, suggesting a possible role in differential regulation of gonadotropin subunit gene expression. We propose that PEA-15 represents a novel point of convergence of the PKC and MAPK/ERK pathways under GnRH stimulation. PKC, ERK, and PEA-15 form an AND logic gate that shapes the response of the gonadotrope cell to GnRH.
GnRH is a hypothalamic peptide that plays a pivotal role in the control of mammalian reproductive function. Upon binding to its receptor expressed at the surface of the pituitary gonadotrope cells, GnRH stimulates the synthesis and release of gonadotropins LH and FSH, which in turn promote gametogenesis and sex steroid production in the ovaries and testes. The development of the immortalized gonadotrope cell lines αT3–1 and LβT2 has greatly expanded our understanding of GnRH signaling (1–5). The interaction between GnRH and its receptor, a member of the G-protein coupled receptor family, initiates several intracellular signaling cascades, such as the protein kinase C (PKC)- and MAPK/ERK-dependent pathways. Activation of the PKC-dependent pathway occurs via the Gαq/11 heterotrimeric protein complex, whereas the MAPK/ERK cascade is induced partially through PKC activation (reviewed in Refs. 6–8). ERK phosphorylates cytoplasmic targets, which include the family of 90-kDa ribosomal S6 kinases (RSK) (9). Additionally, activated ERK translocates from the cytoplasm to the nucleus and was shown to phosphorylate nuclear transcription factors such as ETS domain-containing protein Elk-1 (Elk1) in vivo and early growth response protein (Egr)-1 in vitro, both of which are involved in the induction of gonadotropin gene expression (10–13). It was previously reported that GnRH-induced activation of ERK is partially mediated by PKC and calcium influx and that ERK nuclear translocation requires PKC signaling (8, 14). Several mechanisms by which GnRH may activate ERK have been described. For instance, in αT3-1 cells challenged with GnRH, PKC mediates transactivation of the epidermal growth factor (EGF) receptor, which engages in the Ras/Raf/ERK signaling cascade (15). The same research group implicated two matrix metalloproteinases in EGF receptor transactivation and ERK activation by GnRH in αT3-1 cells (16). In contrast, formation of a calcium-dependent Pyk2-dependent multiprotein superstructure was reported to mediate GnRH-induced ERK activation and nuclear translocation in pituitary gonadotropes (11). Recently, GnRH was reported to activate ERK in a multiprotein signaling complex in a Src-dependent fashion (17). Thus, there is a need to further characterize the distinct signaling components that may be involved in GnRH-dependent ERK activation and to delineate the molecular mechanisms underlying its nuclear translocation.
Several scaffold/adaptor proteins were reported to modulate MAPK signaling (for review, see Refs. 18–21). The importance of such scaffolds resides in the fact that they target MAPK kinase (MEK)/ERK to specific substrates by regulating the kinetics, intensity, and localization of MEK/ERK signaling, thereby promoting a specific response. Phosphoprotein-enriched in astrocytes-15 kDa (PEA-15) is predominantly found in the brain (22, 23) but is also expressed in the pituitary (22, 23). Originally characterized as a major substrate for PKC in astrocytes, PEA-15 has two main phosphorylation sites: a PKC site at Ser104 and a calcium/calmodulin-dependent protein kinase II at Ser116 (23, 24). PEA-15 has been highly conserved throughout the evolution of vertebrates (22) and has been implicated in a variety of biological processes, including modulation of apoptosis (25–35), astrocyte migration (36), glucose transport (26, 37, 38), and cell proliferation (39, 40). Various potential PEA-15 interaction partners were identified by yeast two-hybrid assays, including ERK (41, 42), p90RSK (43, 44), and MEK1 (45). PEA-15 apparently binds both the active and inactive forms of ERK (42, 46). Several studies demonstrated suggest that PEA-15 may contribute to the regulation of ERK cytoplasmic sequestration (40–42, 45, 47). Hence, overexpression of PEA-15 prevents ERK nuclear translocation (41, 45). PEA-15 was proposed to mediate ERK cytosolic sequestration by inhibiting ERK binding to nucleoporin (45); moreover, the nuclear export sequence of PEA-15 may keep the complex out of the nucleus (41). ERK-PEA-15 interaction is controlled by the phosphorylation status of PEA-15 at Ser104. PKC-dependent phosphorylation of PEA-15 at Ser104 blocks ERK binding to PEA-15, thereby restoring normal ERK function, which is to promote cell proliferation (42). Phosphorylation of PEA-15 also appears to require the activity of another factor, which has yet to be identified (47). Furthermore, binding of PEA-15 to the D-recruitment site of ERK was suggested to inhibit ERK interaction with its substrates Elk1 and Ets1 (46, 48).
In the present study, RNA expression data from microarray analysis revealed the expression of PEA-15 in the gonadotrope-derived LβT2 cell line (data not shown). We thus investigated the potential involvement of PEA-15 in GnRH signaling in those cells. In particular, we examined the impact of PEA-15 on ERK spatiotemporal localization.
Results
GnRH induces the accumulation of phosphorylated PEA-15 in LβT2 cells in a PKC-dependent manner
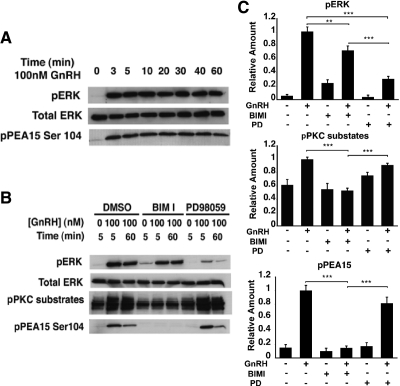
To determine whether GnRH activates PEA-15 in the LβT2 cells, we performed a time-course experiment and analyzed the protein levels of phospho-PEA-15 by Western blotting. Total ERK was used as a control for protein loading, whereas phospho-ERK was used as a control for GnRH-induced ERK activation. Accumulation of phosphorylated PEA-15 after GnRH exposure was rapid and sustained for 1 h (Fig. 1A). We next sought to identify the signaling pathway(s) involved in GnRH-induced PEA-15 activation. GnRH-stimulated cells were pretreated with either the PKC inhibitor bisindolylmaleimide-I (BIMI) or the MEK inhibitor PD98059. BIMI blocked the accumulation of phospho-PEA-15 (P < 0.005; Fig. 1, B and C), whereas PD98059 had no significant effect, strongly suggesting that PEA-15 activation was PKC dependent. As expected, GnRH-induced phosphorylation of PKC substrates was significantly decreased in the presence of BIMI but not PD98059 (Fig. 1, B and C). ERK phosphorylation declined dramatically with pretreatment by PD98059 (by 70%; P < 0.005) but was only moderately reduced in the presence of BIMI (by 25%; P < 0.01), consistent with an earlier report (8).
Fig. 1.
GnRH induces PEA-15 phosphorylation via PKC in LβT2 cells. A, Time course of GnRH-stimulated ERK and PEA-15 activation in LβT2 cells. Total ERK was used as a loading control. LβT2 cells were serum starved overnight before treatment with 100 nm GnRH for the indicated periods of time. Whole-cell lysates were subjected to a Western blot analysis using phospho-ERK- and phospho-PEA-15 (Ser104)-specific antibodies. Total ERK was used as a loading control. B, Effect of pharmacological inhibitors on GnRH-induced ERK and PEA-15 activation. LβT2 cells were serum starved overnight, pretreated with the following inhibitors for 30 min: 10 μm BIMI or 50 μm PD98059 and stimulated with 100 nm GnRH for either 5 or 60 min. Whole-cell lysates were subjected to a Western blot analysis using phospho-ERK-, phospho-PKC substrates, and phospho-PEA-15 (Ser104)-specific antibodies. Total ERK was used as a loading control. C, Quantification of Western blot (B) densitometry, plotted as mean ± sem. Results of triplicate experiments were combined for analysis. The y-axis shows band intensities relative to the positive control (no inhibitor, + GnRH). Two-way ANOVA; ***, P <0.005, **, P <0.01. PD, PD98059.
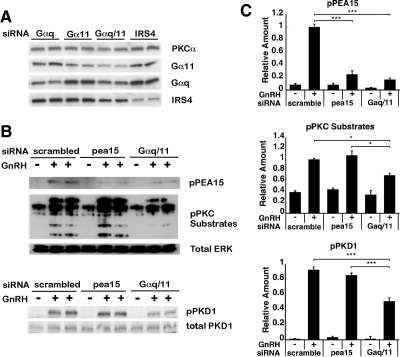
To confirm that PEA-15 activation was mediated by PKC, we used small interfering RNA (siRNA) silencing to down-regulate the expression of Gαq and Gα11, both of which are upstream activators of PKC (49). The extent of gene knockdown was assessed by Western blot analysis and quantification of the Western blot analysis. The protein level of Gαq was reduced by 39% after Gαq siRNA knockdown, as compared with its expression level after siRNA knockdown of the unrelated protein insulin receptor substrate 4 (IRS4) (Fig. 2A). Similarly, expression of Gα11 was reduced by 40%. Combining Gαq and Gα11 siRNAs resulted in a comparable 40% reduction of Gαq and Gα11 protein expression (Fig. 2A). Gαq/11 siRNA knockdown led to an approximately 60% reduction of the corresponding mRNAs (data not shown). Notably, no cross-reaction was observed between the two Gα-targeting siRNAs. To determine transfection efficacy, as well as knockdown efficiency of PEA-15 siRNA, cells were transfected with a green fluorescent protein (GFP)-expressing construct, and the percentage of transfected cells (i.e. transfection efficacy) was determined using fluorescence microscopy: the calculated percentage of GFP-transfected cells was 30% (data not shown). With regard to knockdown efficiency, PEA-15 expression levels were examined by immunohistochemistry and quantitative real-time PCR (Supplemental Figs. 1 and 2), and knockdown specificity was evaluated using cells stably expressing GFP (Supplemental Figs. 3 and 4). Data provided evidence for a specific and significant down-regulation of PEA-15 expression by PEA-15 siRNA: the PEA-15 transcript levels were reduced by at least 60% compared with control cells (Supplemental Fig. 1C) and protein levels by 70–80% (Supplemental Fig. 2B).
Fig. 2.
Effect of siRNA-mediated silencing of PEA-15 and Gαq/11 on GnRH-induced PEA-15 phosphorylation and PKC activity. A, Knockdown efficiency of Gα proteins in LβT2 cells. The LβT2 cells were transfected with Gαq, Gα11, Gαq/11, or IRS4 siRNA using the nucleofection method, as described in Materials and Methods. Whole-cell lysates were subjected to a Western blot analysis using Gα11-, Gαq-, and IRS4-specific antibodies. PKCα was used as loading control. B, LβT2 cells were serum starved overnight, transfected with scrambled, PEA-15, or Gαq/11 siRNA and stimulated with 100 nm GnRH for 5 min. Whole-cell lysates were subjected to a Western blot analysis using phospho-PEA-15- and phospho-PKC substrate-specific antibodies (upper blot) or using a phospho-PKD1-specific antibody (lower blot). Total ERK and total PKD1 were used as loading controls for the upper and the lower blots, respectively. C, Quantification of Western blot (B) densitometry, plotted as mean ± sem. Results of triplicate experiments were combined for analysis. The y-axis shows band intensities relative to the positive control (scrambled siRNA, + GnRH). Two-way ANOVA, n = 8; ***, P < 0.005; *, P < 0.05.
Phosphorylation of PEA-15 was reduced by 80% by Gαq/11 siRNA knockdown (P < 0.005; Fig. 2, B and C). Similarly, PEA-15 siRNA knockdown decreased the level of phospho-PEA-15 by 75% (P < 0.005). As expected, phosphorylation of PKC substrates in response to GnRH stimulus was significantly decreased by Gαq/11 siRNA knockdown (P < 0.05; Fig. 2, B and C). Likewise, phosphorylation of protein kinase D1 (PKD1), which is known to be activated by specific PKC isoforms (for review, see Ref. 50) was reduced by 50% by Gαq/11 siRNA knockdown (P < 0.005; Fig. 2, B and C). Consequently, our results supported the formulation that PEA-15 is phosphorylated by PKC upon GnRH stimulation.
The accumulation of phosphorylated ERK in the nucleus in response to GnRH depends on PEA-15 expression
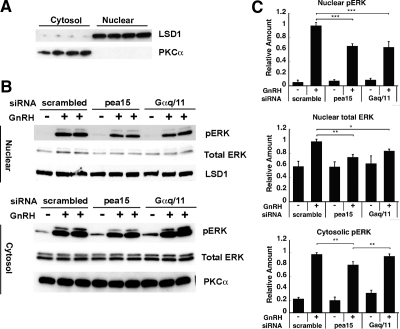
We next sought to examine the potential role of PEA-15 in the regulation of ERK subcellular localization. To this aim, LβT2 cells were transfected with siRNA targeting PEA-15 and stimulated with GnRH; the cytosolic and nuclear fractions derived from those cells were separated, and ERK and phospho-ERK protein levels were monitored by Western blot. The near absence of cross-contamination between expression of the cytosolic marker PKCα and that of the nuclear marker lysine (K)-specific demethylase 1 (LSD1) demonstrated efficient separation of the cytosolic and nuclear fractions (Fig. 3A). GnRH induced a large increase in phospho-ERK in the nucleus as well as in the cytosol and a modest increase in nuclear total ERK, illustrating ERK phosphorylation and translocation to the nucleus under GnRH stimulation. We observed a significant decrease (34%; P < 0.005) in nuclear phospho-ERK in cells knocked down for PEA-15, as compared with control cells (scrambled siRNA; Fig. 3, B and C). Nuclear total ERK and cytosolic phospho-ERK were decreased by 26 and 20% (P < 0.01), respectively. These findings indicate that the increase in nuclear phospho-ERK and phospho-ERK translocation itself may depend, at least in part, on PEA-15.
Fig. 3.
Effect of PEA-15 and Gαq/11 silencing on GnRH-induced ERK activation in the nucleus vs. cytosol. A, Isolation of the nuclear and cytoplasmic fractions of LβT2 cells. Cells were fractionated into nuclear and cytosolic extracts. Aliquots of the nuclear and cytoplasmic fractions were subjected to a Western blot analysis using an LSD1- and a PKCα-specific antibody, respectively. LSD1 is a nuclear marker; PKCα is a cytosolic marker. B, LβT2 cells were serum starved overnight, transfected with scrambled, PEA-15, or Gαq/11 siRNA and stimulated with 100 nm GnRH for 5 min. Cells were fractionated into nuclear and cytoplasmic extracts, and the aliquots of the nuclear and the cytoplasmic fractions were subjected to a Western blot analysis using phospho-ERK and LSD1-specific antibodies (upper blot) or using phospho-ERK- and PKCα-specific antibodies (lower blot). LSD1 was used as a loading control for the upper blot, whereas PKCα was used in the lower blot. C, Quantification of Western blot (B) densitometry, plotted as mean ± sem. Results of triplicate experiments were combined for analysis. The y-axis shows band intensities relative to the positive control (scrambled siRNA, + GnRH). Two-way ANOVA, n = 8; ***, P < 0.005; **, P < 0.01; *, P < 0.05.
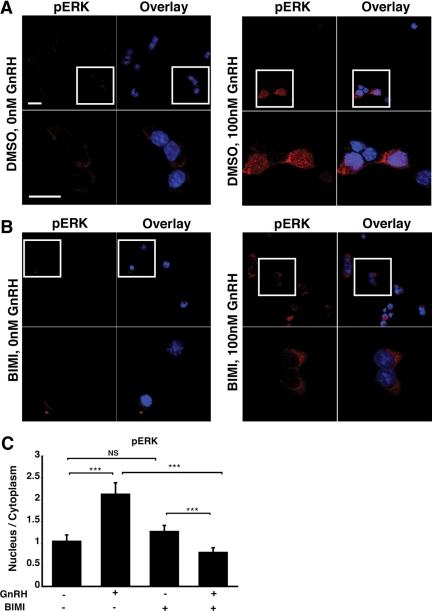
Cells knocked down for Gαq/11 exhibited a decrease in nuclear phospho-ERK, which was comparable with that observed in cells knocked down for PEA-15. In contrast, Gαq/11 knockdown produced no change in cytosolic phospho-ERK level (Fig. 3, B and C). Moreover, immunohistochemical analysis revealed that phospho-ERK was mainly localized in the cytosol of GnRH-stimulated cells pretreated with a pharmacological PKC inhibitor, whereas it was present in both the cytosol and nucleus of nonpretreated cells (Fig. 4). Thus, our data indicate that activation of nuclear ERK, but not cytosolic ERK, is dependent on PKC. Our results corroborate those of Liu et al., showing that accumulation of nuclear phospho-ERK in response to GnRH is PKC dependent (8).
Fig. 4.
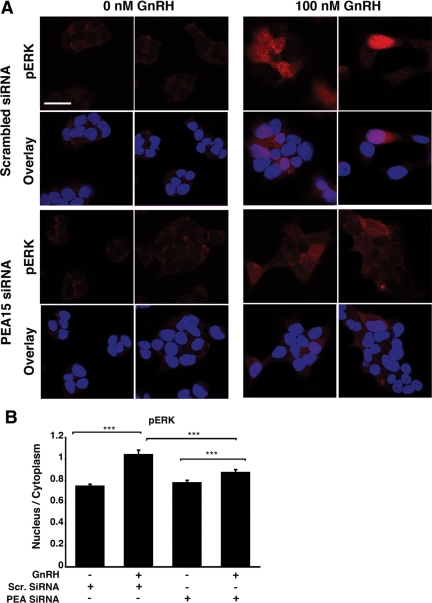
GnRH-induced accumulation of phospho-ERK in the nucleus is PKC- dependent. LβT2 cells were serum starved overnight, pretreated with either dimethyl sulfoxide (DMSO) (A) or 10 μm BIMI (B) for 30 min and stimulated or not with 100 nm GnRH for 5 min. Cells were then fixed and stained with an anti-phospho-ERK antibody (red) and DAPI (blue), as indicated in Materials and Methods. The boxed areas are shown at high magnification. Scale bar, 20 μm. C, Single-cell quantification of phospho-ERK fluorescence using the 3D-CatFISH image analysis suite (70, 71). The y-axis displays the nucleus to cytoplasm fluorescence ratio. NS, Nonsignificant. Two-way ANOVA; ***, P < 0.005.
To confirm the involvement of PEA-15 in the augmentation of nuclear phospho-ERK, we performed an immunohistochemical analysis of phospho-ERK in PEA-15 siRNA-transfected cells stimulated with GnRH. The immunohistochemistry data shown in Fig. 5A revealed that phospho-ERK increased in the nucleus of GnRH-stimulated cells, and this increase was reduced significantly in the presence of PEA-15 siRNA (by 19%; P < 0.005; Fig. 5B; see also Supplemental Figs. 5 and 6). Altogether, our data indicate that PEA-15 plays an important role in ERK nuclear translocation upon GnRH stimulation and that PKC is involved in ERK activation solely in the nucleus.
Fig. 5.
Effect of PEA-15 silencing on GnRH-induced nuclear accumulation of phospho-ERK. A, LβT2 cells were serum starved overnight, transfected with either scrambled (top panel) or PEA-15 siRNA (bottom panel) and stimulated or not with 100 nm GnRH for 5 min. Cells were then fixed and stained with an anti-phospho-ERK antibody (red) and DAPI (blue), as described in Materials and Methods. B, Single-cell quantification of phospho-ERK fluorescence using the 3D-CatFISH image analysis suite (70, 71). The y-axis displays the nucleus to cytoplasm fluorescence ratio. ANOVA, n = 62; ***, P < 0.005. Single-cell fluorescence distribution is also presented in Supplemental Fig. 5 (using GFP siRNA as a control) and Supplemental Fig. 6 (using scrambled siRNA as a control). Scr, Scrambled.
In the absence of GnRH stimulation, PEA-15 hinders the increase of ERK in the nucleus
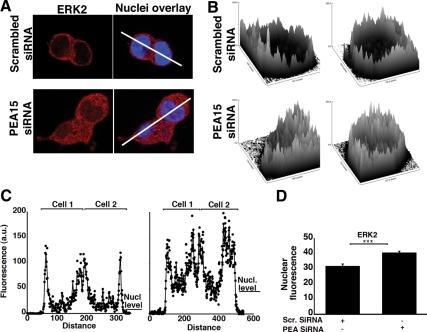
To further delineate the role of PEA-15 in the gonadotropes, we examined its effect on ERK subcellular localization in the absence of GnRH stimulus. We observed a significant increase in nuclear ERK in cells treated with PEA-15 siRNA, as compared with control cells, in which nuclear ERK was barely detected by immunohistochemistry (Fig. 6, A and C). Nuclear ERK was increased in knocked down cells, suggesting that PEA-15 prevents ERK nuclear translocation in unstimulated gonadotropes. Single-cell experiments corroborated those observations, showing a 25% increase in the nuclear ERK fluorescence level in the presence of PEA-15 siRNA (P < 0.005; Fig. 6D and Supplemental Fig. 7). Our data thus support the concept that PEA-15 sequesters ERK in the cytosol of unstimulated gonadotropes.
Fig. 6.
PEA-15 impedes the accumulation of ERK in the nucleus of nonstimulated cells. A, Resting LβT2 cells were transfected with either scrambled or PEA-15 siRNA. The cells were then fixed and stained with an anti-ERK2 antibody (red) and DAPI (blue), as described in Materials and Methods. White lines identify cross-sections in which fluorescence signal intensity was quantified in C. B, Surface plot analysis of ERK2 fluorescence from A using the Image J image processing program (NIH). C, Quantification of ERK2 fluorescence in the cross-sections indicated in A, using the Image J image processing program. Left plot, Scrambled siRNA; right plot, PEA-15 siRNA. D, Single-cell quantification of ERK2 nuclear fluorescence using the 3D-CatFISH image analysis suite (70, 71). Student t test, n = 102; ***, P < 0.005. Single-cell fluorescence distribution is also presented in Supplemental Fig. 7.
Activation of RSK, a substrate of ERK in GnRH-activated LβT2 cells, is dependent on both PEA-15 and PKC
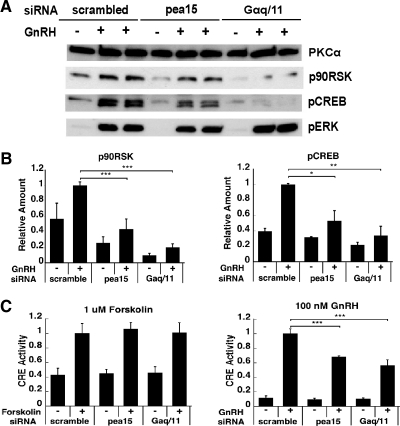
Because PEA-15 was previously shown to interact with RSK2 and act as an intermediary between ERK and its substrate RSK2 (44, 51), we speculated that PEA-15 may regulate RSK activation in GnRH-stimulated gonadotropes. To test this hypothesis, we analyzed the effect of PEA-15 siRNA knockdown on GnRH-induced accumulation of phospho-RSK. The increase in phospho-RSK after GnRH stimulation was significantly attenuated in the presence of PEA-15 siRNA and Gαq/11 siRNA (by 56 and 80%, respectively; P < 0.005; Fig. 7, A and B), indicating the PEA-15 and PKC reliance of GnRH-induced phospho-RSK accumulation. Moreover, GnRH-induced accumulation of phospho-cAMP-response element-binding protein (CREB), which is a direct substrate of RSK, was significantly diminished by siRNA knockdown of either PEA-15 (47% reduction; P < 0.05) or Gαq/11 (65% reduction; P < 0.01; Fig. 7, A and B). The effect of PEA-15 knockdown on GnRH-induced phospho-ERK in whole-cell lysates is small but significant (data not shown). This small effect is because nuclear phospho-ERK generation is much more affected by PEA-15 knockdown (Figs. 3 and 5), and most phospho-ERK is localized to the cytoplasm (Supplemental Fig. 8). Further supporting the involvement of RSK in GnRH-mediated ERK signaling, phospho-RSK accumulation was impeded by the MEK/ERK inhibitor U0126 (Supplemental Fig. 9). We also studied the effect of PEA-15 knockdown on cAMP response element (CRE) activity to further substantiate our observations on phospho-CREB accumulation. PEA-15 knockdown reduced GnRH-induced CRE activity but not forskolin (a PKA activator)-induced CRE activity (Fig. 7C). Thus, our data support an effect of PEA-15 on GnRH-induced RSK activation, thereby affecting the activation of RSK downstream targets CREB and CRE in a PKA-independent manner. On the whole, our data indicate that GnRH-induced activation of RSK requires both PEA-15 and PKC activities.
Fig. 7.
Effect of PEA-15 and Gαq/11 silencing on GnRH-induced RSK activation. A, LβT2 cells were serum starved overnight, transfected with scrambled, PEA-15, or Gαq/11 siRNA and stimulated or not with 100 nm GnRH for 5 min. Whole-cell lysates were subjected to a Western blot analysis using phospho-CREB-, phospho-RSK-, and phospho-ERK-specific antibodies. PKCα was used as a loading control. The small reductions in basal phospho-CREB and phospho-RSK seen in the blot shown were not reproducible in multiple experiments. B, Quantification of Western blot (A) densitometry, plotted as mean ± sem. The results of triplicate experiments were combined for analysis. The y-axis shows band intensities relative to positive control (scrambled siRNA, + GnRH). For GnRH-stimulated conditions: two-way ANOVA, n = 6; ***, P < 0.005; **, P < 0.01; *, P < 0.05; for basal conditions, two-way ANOVA, n = 3. C, Effects of PEA-15 and Gαq/11 knockdowns on CRE reporter activity. After being serum starved overnight, LβT2 cells were cotransfected with scrambled, PEA-15, or Gαq/11 siRNA, a CRE Firefly luciferase reporter plasmid and a thymidine kinase-Renilla luciferase reporter plasmid and stimulated with either 1 μm forskolin or 100 nm GnRH for 6 h. Results are presented as the average of two experiments, calculated as Firefly/Renilla luciferase activity, and plotted as mean ± sem. Two-way ANOVA, n = 10; ***, P < 0.005.
PEA-15 regulates the expression of early response genes and gonadotropin subunit genes in GnRH-activated LβT2 cells
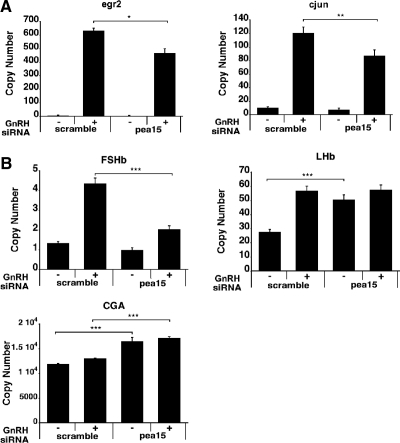
To study the role of PEA-15 in GnRH-stimulated gene regulation, we examined the effect of PEA-15 siRNA knockdown on GnRH-induced expression of early response genes Egr2 and c-Jun, both of which are CRE- dependent. Egr2 and c-Jun were described as essential genes for the induction of LHβ and FSHβ expression by GnRH (52–54). We observed a significant decrease in the mRNA levels of both genes after siRNA knockdown (26% for Egr2 and 28% for c-Jun; Fig. 8A). We next examined the effect of PEA-15 siRNA knockdown on GnRH-induced expression of gonadotropin subunit genes. We found that PEA-15 siRNA knockdown causes a significant decrease in GnRH-stimulated FSHβ transcript levels (by 54%; P < 0.005), whereas it leads to an increase in LHβ and common α-glycoprotein subunit (CGA) mRNAs (Fig. 8B). Thus, our results underscore the overall involvement of PEA-15 in the regulation of gene expression in GnRH-stimulated gonadotrope cells.
Fig. 8.
Downstream effects of PEA-15 knockdown in GnRH-stimulated cells. A, Effect of PEA-15 knockdown on early response gene expression. LβT2 cells were serum starved overnight, transfected with either scrambled or PEA-15 siRNA, and stimulated or not with 1 nm GnRH for 45 min. Transfections were carried out in the Nucleofector 96-well shuttle using the cell line optimization 96-well nucleofector kit (SG nucleoporation buffer) and nucleofector program DS-137 (Lonza Walkersville Inc.). The RNA copy numbers of Egr2 and c-Jun were determined by qPCR. Student t test, n = 4; **, P < 0.01; *, P < 0.05. B, Effect of PEA-15 knockdown on gonadotropin subunit gene expression. LβT2 cells were serum starved overnight, transfected with either scrambled or PEA-15 siRNA, and stimulated or not with 1 nm GnRH for 2 h; the medium was then removed and replaced with fresh medium to remove GnRH, and cells were incubated for another 4 h. Transfections were carried out in the nucleofector 96-well shuttle using the cell line optimization 96-well nucleofector kit (SG nucleoporation buffer) and nucleofector program DS-137 (Lonza Walkersville Inc.). RNA copy numbers of FSHβ, LHβ, and CGA were determined by qPCR. Two-way ANOVA, n = 12; ***, P < 0.005.
PEA-15 interacts with RSK, and this association requires PKC-mediated phosphorylation of PEA-15
We next sought to characterize the interaction of PEA-15 with PKC, ERK, and RSK. To this end, heterologous human embryonic kidney (HEK) 293 cells were transfected with an expression construct of hemagglutinin (HA)-tagged PEA-15 and treated with the PKC activator phorbol-12-myristate-13-acetate (PMA). The coimmunoprecipitation results, which were visualized by Western blot with an anti-HA antibody, supported an interaction between PEA-15 and RSK1 in PKC-activated cells (Supplemental Fig. 10, right panel). Although PMA increased the association of PEA-15 and RSK1 in transfected HEK293 cells, it did not cause a detectable decrease in the association with ERK (Supplemental Fig. 10, right panel). This apparent lack of dependence of the interaction of PEA-15 and ERK on the phosphorylation status of PEA-15 may result from nonphysiological expression levels of transfected PEA-15, as suggested in a previous study (51).
Because interaction experiments need to be interpreted cautiously in an overexpression system, additional controls were performed to test further the role of PKC and the specificity of the PEA-15 and RSK1 interaction. In cells transfected with a PEA-15 mutant construct carrying a SertoAla mutation at the PKC phosphorylation site, this interaction was abolished. The interaction of PEA-15 was RSK isoform-specific because no coimmunoprecipitation with RSK2 was found (data not shown). Analysis of the whole-cell lysates was carried out in parallel to verify that the levels of expression of PEA-15 and mutant PEA-15 were comparable and that the total amount of RSK and ERK remained constant (Supplemental Fig. 10, left panel). As expected, phospho-PEA-15 was increased by PMA treatment in cells transfected with wild-type PEA-15, whereas it was absent from cells transfected with mutant PEA-15 (Supplemental Fig. 10, left panel). Overall, these data suggest that PEA-15 can recruit RSK in a PKC-dependent manner.
Discussion
MAPK and PKC are important components of the complex GnRH-modulated signaling network in the gonadotrope (8, 11, 14–17). We implicate the PEA-15 scaffold protein in gonadotropes as mediating a novel cross talk mechanism between the PKC and MAPK/ERK pathways.
Our findings are consistent with previous work showing that activation of nuclear ERK is PKC dependent (8). Previous studies have identified PEA-15 as contributing to ERK subcellular localization and ELK1 activation in NIH 3T3 cells (41). Our results suggest that PEA-15 interacts with ERK and tethers it in the cytosol under resting conditions (Fig. 9A). Upon GnRH stimulation, both PKC- and MEK are activated independently; PKC and MEK phosphorylate PEA-15 and ERK, respectively (Fig. 9B). Once PEA-15 is phosphorylated, RSK is recruited to PEA-15. RSK recruitment promotes its phosphorylation by ERK (Fig. 9C). RSK phosphorylation mediates its dissociation from ERK (55), and ERK is released from phospho-PEA-15. ERK release might involve additional factors that remain to be identified (42, 47). ERK then translocates to the nucleus, in which it phosphorylates nuclear substrates such as Elk1; similarly, activated RSK translocates to the nucleus to phosphorylate substrates such as CREB (Fig. 9D) (56, 57). PEA-15 and ERK thus function as a computer AND logic gate, which takes two inputs and produces a positive output if and only if both inputs are true. Similarly the PEA-15/ERK gate requires both PKC and MAPK activation (inputs) to generate nuclear phospho-ERK and phospho-RSK (output).
Fig. 9.
Proposed model for PEA-15 mediation of ERK nuclear translocation and RSK activation. A, In resting gonadotrope cells, PEA-15 and ERK interact, retaining ERK in the cytoplasm. B, GnRH stimulation leads to the independent activation of the PKC and ERK pathways. PKC phosphorylates PEA-15, and its phosphorylation recruits RSK to PEA-15. C, The recruitment of RSK to PEA-15 increases the local concentration of RSK in the vicinity of ERK, thereby promoting RSK phosphorylation by ERK. D, After RSK phosphorylation, ERK dissociates from the RSK-PEA-15 complex and enters the nucleus, activating its nuclear substrates, which include ELK1. The activated RSK translocates to the nucleus, in which it phosphorylates its own substrates CREB and activating transcription factor 1. U, Unknown factor(s).
PEA-15 not only controls ERK activity but also has a modulatory effect on GnRH-induced early response and gonadotropin subunit gene expression. Surprisingly, PEA-15 gene silencing had a differential effect on gonadotropin subunit gene expression, causing a marked decrease in GnRH-stimulated FSHβ transcript levels and an increase in LHβ and CGA mRNA basal levels. Notably, gonadotropin subunit gene expression is differentially regulated by different patterns of GnRH stimulation (58). Thus, it is possible that PEA-15 plays a role in the differential regulation of gonadotropin subunit genes.
It is possible that other scaffold proteins and/or some unknown factors participate in ERK nuclear translocation besides PEA-15. Proteins that were reported to interact with ERK in the cytoplasm and contribute to its spatiotemporal subcellular localization in specific cell types include β-arrestin (20, 21, 59–62), kinase suppressor of Ras (21), IQ motif containing GTPase activating protein 1 (21), and MEK binding partner 1 (63). Therefore, even though several receptors at the plasma membrane may activate the same intracellular cascade(s), signaling specificity occurs most probably via the integration of distinct signaling pathways and the spatiotemporal modulation of signaling components. Dual-specificity phosphatases (DUSP), DUSP2 and DUSP4, inactivate and anchor ERK2, whereas DUSP1 dephosphorylates ERK in the nucleus but allows it to return to the cytosol for reactivation (64–66). Based on studies in GnRH receptor-expressing Hela cells, DUSPs are anticipated to play a regulatory role in the gonadotropes (64). Pyk2 was identified as a scaffold for the assembly of a signaling complex that links between GnRH-induced intracellular calcium release and Ras-dependent activation of the ERK pathway in the gonadotrope cells (11).
Because Gαq/11 siRNA knockdown resulted in a significant decrease in the phosphorylation of PKC substrates and PKD1 and a decline in the levels of phosphorylated PEA-15, we deduced that activation of PEA-15 by GnRH was PKC- dependent. Furthermore, we demonstrated that PEA-15 is involved in ERK nuclear translocation, and others previously showed that the PEA15-ERK interaction is controlled by the phosphorylation status of PEA-15 at the PKC site (42). However, Gαq/11 is also known to activate the calcium/calmodulin-dependent pathway (67), which could potentially contribute to PEA-15 activation under GnRH stimulation.
In the current study, siRNA-mediated depletion of either PEA-15 or Gαq/11 substantially reduced GnRH-induced increase in phospho-RSK, indicating that RSK activation was at least partially dependent on PEA-15 and PKC. Further supporting these observations, we obtained similar inhibitory effects on GnRH-induced phospho-CREB levels as well as on the promoter activity of a heterologous CRE reporter plasmid. Our results are in keeping with previous cotransfection experiments in COS-7 cells, showing that phosphorylation of RSK2- and CREB-mediated transcription were augmented with increasing amounts of PEA-15; however, RSK2 phosphorylation and CREB-mediated transcription were inhibited at higher levels of PEA-15, presumably due to some dominant-negative effect (44, 51). We believe that our findings uncover a gonadotrope-specific regulatory mechanism, wherein PEA-15 acts as a positive regulator of RSK activity under GnRH stimulation. To examine the potential interaction between PEA-15, ERK, and RSK, LβT2 cells were initially transfected with a PEA-15-expressing construct due to the low level of expression of endogenous PEA-15. Transfection of either wild-type or mutant PEA-15-expressing vector was toxic to the LβT2 cells. Because PEA-15 is known to exert antiapoptotic actions (25, 28, 32, 35), this apparent toxicity was surprising. Using a heterologous expression system, we confirmed the interaction between PEA-15 and RSK and the requirement of PEA-15 phosphorylation at Ser104 for this interaction. Overall, our findings are consonant with those of Vaidyanathan et al. (51) and support the idea that PEA-15 forms a complex with ERK and most likely RSK in the gonadotropes.
There are more than 1500 receptors in the human genome (68), and more than 50 different types of receptors are expressed in the pituitary gonadotropes, as determined by quantitative real-time PCR (data not shown). Interestingly, many receptors expressed in the gonadotropes, such as the insulin receptor, the EGF, and fibroblast growth factor receptors, and the GnRH receptor activate the MAPK/ERK pathway. The chemistry underlying the information transfer in the cell relies on both chemical modification, e.g. phosphorylation, and dynamic changes in the aggregation of reactants, e.g. membrane association or scaffolding protein association. The use of signaling modules allows the cell to distinguish upstream stimuli and to generate diverse responses and use a limited number of signaling components. The function of PEA-15 in the gonadotrope illustrates how these dual elements of modification and localization can be used to decode specific patterns of receptor-mediated signaling.
Materials and Methods
Materials
BIMI, PD98059 (2′-amino-3′-methoxyflavone), and U0126 [1,4-diamino-2,3-dicyano-1,4-bis(2-aminophenylthio)butadiene] were purchased from Calbiochem (La Jolla, CA). Dimethyl sulfoxide was obtained from Sigma Aldrich (St. Louis, MO). Antibodies purchased from Cell Signaling Technology (Beverly, MA) were: mouse monoclonal antiphospho-ERK antibody, rabbit polyclonal antitotal ERK, rabbit polyclonal antiphospho-p90RSK Ser380, rabbit polyclonal antiphospho-PEA-15 Ser104, rabbit polyclonal antitotal PEA-15 (used in immunohistochemistry), rabbit polyclonal antiphospho-PKC substrates (targeting phospho-serine containing substrates of conventional PKC), phosphorylated, rabbit polyclonal antiphospho-PKD, rabbit polyclonal antitotal PKD, rabbit polyclonal antiphospho-CREB, and rabbit monoclonal anti-LSD1. Rabbit polyclonal antitotal RSK1 antibody, and mouse monoclonal anti-ERK2 were from Santa Cruz Biotechnology (Santa Cruz, CA). Mouse monoclonal anti-PKCα was from BD Transduction Laboratory (BD Biosciences, San Jose, CA). Rabbit polyclonal anti-total PEA-15 obtained from Abcam (Cambridge, MA) was used in Western blot analysis. The HA probe and horseradish peroxidase-coupled antibodies were from Santa Cruz Biotechnology, whereas secondary 568-fluorophore was from Invitrogen (Carlsbad, CA).
Cell culture
LβT2 cells obtained from Professor Pamela Mellon (University of California, San Diego, San Diego, CA) were maintained at 37 C in a humidified air atmosphere of 5% CO2 in phenol-red free DMEM (Mediatech, Herndon, VA) supplemented with 10% fetal bovine serum (FBS; Gemini, Calabasas, CA) and l-glutamine (Gibco Invitrogen, Carlsbad, CA). For immunohistochemical assays, 200,000 cells were seeded on poly-d-lysine (Sigma Aldrich)-pretreated glass coverslips [18 × 18 mm (Fisher Scientific, Pittsburgh, PA)] in six-well plates. The cells were synchronized in 0.5% charcoal-treated FBS (CT-g; HyClone Laboratories, Inc., Logan, UT), l-glutamine, and 25 mm HEPES (Mediatech).
Immunohistochemistry
LβT2 cells were stimulated for the time periods specified in the appropriate figure legends with either GnRH (100 nm) diluted in DMEM with 0.5% CT-FBS, l-glutamine and 25 mm HEPES or vehicle at 37 C. For synchronization purposes, cells were maintained on a heat pad from the first time point up to 5 min and then transferred to a tissue-culture incubator for longer exposures. The protocol is described elsewhere (69). Briefly, cells were fixed in 4% formaldehyde (Ultra Pure EM grade; Polysciences, Warrington, PA) for 30 min at room temperature (RT), permeabilized in 0.2% Triton X-100 PBS (Triton; Sigma Aldrich) for 10 min at RT, and quenched in 50 mm NH4Cl (Sigma Aldrich) for 5 min at RT and blocked in PBS/0.1% Tween 20/5% BSA (Roche Diagnostics, Mannheim, Germany) for 1 h at RT. Primary antibodies (1:1000) were added and incubated overnight at 4 C. Secondary 568 fluorophore-coupled antibodies were added (1:1000) for 2 h at RT. After washing and 4′,6′-diamidino-2-phenylindole counterstaining (DAPI; 0.1μg/ml; Sigma Aldrich), coverslips were mounted in ProLong Gold Antifade Reagent (Invitrogen).
Confocal microscopy and image analysis
Fluorescent microscopy and subsequent image analysis were performed as described previously (69). Briefly, a Zeiss LSM510-META inverted confocal laser-scanning microscope (Peabody, MA) was used for imaging. Lasers and filters were set up for imaging Alexa-568 and DAPI. The exposure settings were determined empirically for each channel from control background and unchanged within an experiment. At least 10 images of nonoverlapping areas were assayed for each condition in each experiment.
Digital images were analyzed in a custom automated image analysis suite called 3D-CatFISH (70, 71) as described elsewhere (69). First, the cell nuclei were segmented using DAPI with the enhanced three-dimensional watershed algorithm (72) followed by model-based object merging (73). Then a desired region of interest was defined based on geometric distance for each segmented nucleus, and specific signals were quantified in nucleus and cytoplasm. Each image was visually inspected for possible segmentation errors. The results were exported in Excel (Richmond, CA) for further analysis.
To determine fluorescence distribution, digital images were analyzed using Image J version 1.37 (National Institutes of Health, Bethesda, MD). For plot profiles, a line was drawn across the cells and the profile calculated using a plot profile function. The list of data was exported into KaleidaGraph (Synergy Software, Reading, PA) for plotting of the profiles. For surface profiles, the cells were circled and the surface plotted in Image J using the surface plot function.
Western blot analysis
LβT2 cells (2.5 million) were grown in 35-mm dishes and synchronized in low serum for 24 h before GnRH treatment. After Nonidet P-40 lysis (20 mm Tris-HCl, 1% Nonidet P-40, NaCl) and centrifugation, 20 μg/well supernatant was loaded onto 10–20% Tris-HCl ready gradient gel (no. 161-1160; Bio-Rad Laboratories, Hercules, CA) and electrophoresed 1.5 h at 100 V. After transfer to H-Bond membrane (Hybond ECL; Amersham, Buckinghamshire, UK), blocking for 1 h with 5% nonfat dry milk (Bio-Rad) in Tris-buffered saline and 1% Tween 20 was followed by overnight incubation in primary antibody (1:000) at 4 C. Incubation with the secondary antibody (1:5000) coupled to peroxidase (Santa Cruz Biotechnology) was performed at RT for 45 min, followed by repeated washings with Tris-buffered saline and 1% Tween 20. Immunoreactive proteins were visualized with enhanced chemiluminescence (Amersham Pharmacia Biotech, Piscataway, NJ) according to the manufacturer's recommendations. In each case, the blots were stripped and reprobed for the total protein or a control protein to control loading amounts. All immunoblots were quantified by densitometry using the Image J version 1.37 software (NIH).
Quantitative real-time PCR (qPCR)
For quantitative real-time PCR experiments, cells were seeded in 12-well plates at 750,000 cells/well. The medium was replaced 24 h later with DMEM containing 25 mm HEPES (Mediatech), 0.5% CT-FBS (HyClone Laboratories), and glutamine. On the next day, the cells were treated with 100 nm GnRH or vehicle and were returned to the CO2 incubator for 40 min, at which point the medium was replaced with 360 μl lysis buffer [4 m guanidinium thiocyanate, 25 mm sodium citrate (pH 7.0), 0.5% N-lauroyl-sarcosine, and 0.1 m 2-mercaptoethanol]. RNA was isolated according to the method of Chomczynski and Sacchi (74). Total RNA was isolated with the StrataPrep96 kit (Stratagene, La Jolla, CA). After reverse transcription of 1μg of RNA, the samples were diluted 1:20 in distilled H2O. Later, SYBR green qPCR assays were performed (40 cycles) using 5μl of cDNA template and 5μl of master mix containing the specific primers for the targeted gene (egr2 or RPS11) and the required qPCR buffers. The results were exported as cycle threshold values for subsequent analysis. Three biological replicates were done. From the three-replicates measurements of each biological sample, the mean, sd, and fold change to vehicle treatment were estimated and normalized to RPS11. Primer sequences were as follows: egr2/S, TGTTAACAGGGTCTGCATGTG; egr2/A, AGCGGCAGTGACATTGAAG; c-jun/S, TGAAAGCTGTGTCCCCTGTC; c-jun/A, ATCACAGCACATGCCACTTC; rps11/S, CGTGACGAAGATGAAGATGC; rps11/A, GCACATTGAATCGCACAGTC; pea15/S, CCTGCCTTAACTCTCACACCTC; pea15/A, ACCCTGTCTCCTCCCTCTTC; FSHb/S, TGGAGACTCTGGCATGATTG; FSHb/A, GAGTTGAGCAGCCTAACCTT; LHb/S, TGTCCTAGCATGGTCCGAGT; LHb/A, CCCCCACAGTCAGAGCTACT; CGA/S, TAGGAGCCCCCATCTACCAG; CGA/A, GCTACAGTGGCACTCCGTAT.
siRNA interference
LβT2 cells (5 million) were transfected with siRNAs in eletroporation cuvettes using the Amaxa Cell Line Nucleofector Kit L, following the manufacturer's instructions (Lonza Walkersville Inc., Walkersville, MD). Briefly, 1 μg PEA-15, IRS4, Gαq, Gα11, or scrambled siRNA (Dharmacon, Thermo Fisher Scientific, Denver, CO) were transfected with or without GFP (Lonza Walkersville Inc.) using 100 μl buffer L using Nucleofector program A-023. Immediately after transfection, cells were incubated in fresh medium for 72 h. Cells were then harvested by trypsinization and pelleted by centrifugation.
Isolation of nuclear and cytosolic fractions
NE-PER nuclear and cytoplasmic extraction reagents (Pierce, Thermo Fisher Scientific, Rockford, IL) was used for the biochemical separation of nuclear and cytoplasmic fractions of LβT2 cells according to the manufacturer's instructions. Briefly, 0.5 mg/ml benzamidine, 2 μg/ml aprotinin, 2 μg/ml leupeptin, and either 0.75 mm or 2 mm phenylmethylsulfonyl fluoride (Sigma Aldrich) were added to the cell lysis buffer for the inhibition of protease activity. Halt phosphatase inhibitor cocktail 100x (Thermo Fisher Scientific) was added to the cell lysis buffer for phosphatase activity inhibition.
Coimmunoprecipitation assay
HEK293 cells (6 million) were seeded on a 60-mm cell culture dish containing 5 ml DMEM supplemented with 10% FBS. Twenty-four hours after cell seeding, wild-type or mutant DNA constructs (47) were introduced into the cells using Lipofectamine 2000 (Invitrogen) following the manufacturer's instruction in which 1μg of PEA-15 DNA construct was introduced per transfection. After the transfection, cells were allowed to grow for 72 h in a 5% CO2 incubator at 37 C. To harvest cells, 200 μl ice-cold Nonidet P-40 lysis buffer (20 mm Tris-HCl, 1% Nonidet P-40, NaCl) was added to each cell culture dish, and cell lysate was collected and incubated on ice for 10 min. The cell lysate was centrifuged at 10,000 rpm for 10 min, and supernatant was saved. Anti-HA antibody conjugated with agarose beads (Sigma Aldrich) was added to the saved supernatant, and the mixture was gently shaken at 4 C overnight. The agarose resign complexed with HA-PEA-15 and was precipitated by centrifugation. The beads were then washed several times with ice-cold 1× PBS. The protein-protein interaction patterns between HA-PEA15, RSK, and ERK were analyzed by Western blot.
Luciferase assay
One microgram of CRE or serum response element Firefly luciferase reporter (panomics, no. LR0093 and no. LR0072), 0.1 μg thymidine kinase Renilla luciferase reporter (internal control), and 1 μg siRNA were cotransfected into 5 million LβT2 cells in eletroporation cuvettes using the Amaxa Cell Line Nucleofector Kit L and Nucleofector program A-023 (Lonza Walkersville Inc.). After the transfection, 0.25 million LβT2 cells were added to each well of 24-well cell culture plates containing 1 ml DMEM supplemented with 10% FBS. Forty-eight hours after transfection, the media were exchanged to 1 ml DMEM supplemented with 10% CT-FBS to remove steroid hormones in the media. Seventy-two hours after transfection, cells were stimulated with 100 nm GnRH for 6 h to induce CRE or steroid-responsive element Firefly luciferase reporter expression. Sirius single-tube luminometer (Berthold Detection Systems, Huntsville, AL) was used for the luminescence signal detection, and a dual-luciferase reporter assay system (Promega, Madison, WI) provided the substrates for the Firefly and Renilla luciferases.
Statistical analysis
Statistical calculations were performed using the GraphPad Prism statistical software package version 5 (GraphPad Inc., San Diego, CA). Data were analyzed for normality followed by calculation of ANOVA. Statistical significance was set as indicated in each figure legend with at least a P < 0.05.
Supplementary Material
Acknowledgments
We thank Dr. Mark Ginsberg for kindly providing the PEA-15 expression vector constructs.
This work was supported by National Institutes of Health Grant Grant DK46943.
Disclosure Summary: S.G.C., F.R.-Z., H.P., and B.R. have nothing to declare. S.C.S. is an inventor on U.S. Patents no. 5,985,583 and no. 5,750,366 and has received royalties for these patents.
Footnotes
- BIMI
- bisindolylmaleimide I
- CGA
- common α-glycoprotein subunit
- CRE
- cAMP response element
- CREB
- cAMP-responsive element-binding protein
- CT-FBS
- charcoal-treated FBS
- DAPI
- 4′,6-diamidino-2-phenylindole
- DUSP
- dual-specificity phosphatase
- EGF
- epidermal growth factor
- Egr
- early growth response
- Elk1
- ETS domain-containing protein Elk-1
- FBS
- fetal bovine serum
- GFP
- green fluorescent protein
- HA
- hemagglutinin
- HEK
- human embryonic kidney
- IRS4
- insulin receptor substrate 4
- LSD1
- lysine (K)-specific demethylase 1
- MEK
- MAPK kinase
- PEA-15
- phosphoprotein-enriched in astrocyte 15
- PKC
- protein kinase C
- PKD1
- protein kinase D1
- PMA
- phorbol-12-myristate-13-acetate
- qPCR
- quantitative real-time PCR
- RSK
- ribosomal S6 kinase
- RT
- room temperature
- siRNA
- small interfering RNA.
References
- 1. Alarid ET, Windle JJ, Whyte DB, Mellon PL. 1996. Immortalization of pituitary cells at discrete stages of development by directed oncogenesis in transgenic mice. Development 122:3319–3329 [DOI] [PubMed] [Google Scholar]
- 2. Pernasetti F, Vasilyev VV, Rosenberg SB, Bailey JS, Huang HJ, Miller WL, Mellon PL. 2001. Cell-specific transcriptional regulation of follicle-stimulating hormone-β by activin and gonadotropin-releasing hormone in the LβT2 pituitary gonadotrope cell model. Endocrinology 142:2284–2295 [DOI] [PubMed] [Google Scholar]
- 3. Thomas P, Mellon PL, Turgeon J, Waring DW. 1996. The LβT2 clonal gonadotrope: a model for single cell studies of endocrine cell secretion. Endocrinology 137:2979–2989 [DOI] [PubMed] [Google Scholar]
- 4. Turgeon JL, Kimura Y, Waring DW, Mellon PL. 1996. Steroid and pulsatile gonadotropin-releasing hormone (GnRH) regulation of luteinizing hormone and GnRH receptor in a novel gonadotrope cell line. Mol Endocrinol 10:439–450 [DOI] [PubMed] [Google Scholar]
- 5. Windle JJ, Weiner RI, Mellon PL. 1990. Cell lines of the pituitary gonadotrope lineage derived by targeted oncogenesis in transgenic mice. Mol Endocrinol 4:597–603 [DOI] [PubMed] [Google Scholar]
- 6. Fink MY, Pincas H, Choi SG, Nudelman G, Sealfon SC. 2010. Research resource: gonadotropin-releasing hormone receptor-mediated signaling network in LβT2 cells: a pathway-based web-accessible knowledge base. Mol Endocrinol 24:1863–1871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ruf F, Fink MY, Sealfon SC. 2003. Structure of the GnRH receptor-stimulated signaling network: insights from genomics. Front Neuroendocrinol 24:181–199 [DOI] [PubMed] [Google Scholar]
- 8. Liu F, Austin DA, Mellon PL, Olefsky JM, Webster NJ. 2002. GnRH activates ERK1/2 leading to the induction of c-fos and LHβ protein expression in LβT2 cells. Mol Endocrinol 16:419–434 [DOI] [PubMed] [Google Scholar]
- 9. Frödin M, Gammeltoft S. 1999. Role and regulation of 90 kDa ribosomal S6 kinase (RSK) in signal transduction. Mol Cell Endocrinol 151:65–77 [DOI] [PubMed] [Google Scholar]
- 10. Aplin AE, Stewart SA, Assoian RK, Juliano RL. 2001. Integrin-mediated adhesion regulates ERK nuclear translocation and phosphorylation of Elk-1. J Cell Biol 153:273–282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Maudsley S, Naor Z, Bonfil D, Davidson L, Karali D, Pawson AJ, Larder R, Pope C, Nelson N, Millar RP, Brown P. 2007. Proline-rich tyrosine kinase 2 mediates gonadotropin-releasing hormone signaling to a specific extracellularly regulated kinase-sensitive transcriptional locus in the luteinizing hormone β-subunit gene. Mol Endocrinol 21:1216–1233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Roberson MS, Misra-Press A, Laurance ME, Stork PJ, Maurer RA. 1995. A role for mitogen-activated protein kinase in mediating activation of the glycoprotein hormone α-subunit promoter by gonadotropin-releasing hormone. Mol Cell Biol 15:3531–3539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhang F, Lin M, Abidi P, Thiel G, Liu J. 2003. Specific interaction of Egr1 and c/EBPβ leads to the transcriptional activation of the human low density lipoprotein receptor gene. J Biol Chem 278:44246–44254 [DOI] [PubMed] [Google Scholar]
- 14. Mulvaney JM, Zhang T, Fewtrell C, Roberson MS. 1999. Calcium influx through L-type channels is required for selective activation of extracellular signal-regulated kinase by gonadotropin-releasing hormone. J Biol Chem 274:29796–29804 [DOI] [PubMed] [Google Scholar]
- 15. Grosse R, Roelle S, Herrlich A, Höhn J, Gudermann T. 2000. Epidermal growth factor receptor tyrosine kinase mediates Ras activation by gonadotropin-releasing hormone. J Biol Chem 275:12251–12260 [DOI] [PubMed] [Google Scholar]
- 16. Roelle S, Grosse R, Aigner A, Krell HW, Czubayko F, Gudermann T. 2003. Matrix metalloproteinases 2 and 9 mediate epidermal growth factor receptor transactivation by gonadotropin-releasing hormone. J Biol Chem 278:47307–47318 [DOI] [PubMed] [Google Scholar]
- 17. Dobkin-Bekman M, Naidich M, Rahamim L, Przedecki F, Almog T, Lim S, Melamed P, Liu P, Wohland T, Yao Z, Seger R, Naor Z. 2009. A pre-formed signaling complex mediates GnRH-activated ERK-phosphorylation of paxillin and FAK at focal adhesions in LβT2 gonadotrope cells. Mol Endocrinol 23:1850–1864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kolch W. 2005. Coordinating ERK/MAPK signalling through scaffolds and inhibitors. Nat Rev Mol Cell Biol 6:827–837 [DOI] [PubMed] [Google Scholar]
- 19. Caunt CJ, Finch AR, Sedgley KR, McArdle CA. 2006. GnRH receptor signalling to ERK: kinetics and compartmentalization. Trends Endocrinol Metab 17:308–313 [DOI] [PubMed] [Google Scholar]
- 20. DeWire SM, Ahn S, Lefkowitz RJ, Shenoy SK. 2007. β-Arrestins and cell signaling. Annu Rev Physiol 69:483–510 [DOI] [PubMed] [Google Scholar]
- 21. Sacks DB. 2006. The role of scaffold proteins in MEK/ERK signalling. Biochem Soc Trans 34:833–836 [DOI] [PubMed] [Google Scholar]
- 22. Danziger N, Yokoyama M, Jay T, Cordier J, Glowinski J, Chneiweiss H. 1995. Cellular expression, developmental regulation, and phylogenic conservation of PEA-15, the astrocytic major phosphoprotein and protein kinase C substrate. J Neurochem 64:1016–1025 [DOI] [PubMed] [Google Scholar]
- 23. Araujo H, Danziger N, Cordier J, Glowinski J, Chneiweiss H. 1993. Characterization of PEA-15, a major substrate for protein kinase C in astrocytes. J Biol Chem 268:5911–5920 [PubMed] [Google Scholar]
- 24. Kubes M, Cordier J, Glowinski J, Girault JA, Chneiweiss H. 1998. Endothelin induces a calcium-dependent phosphorylation of PEA-15 in intact astrocytes: identification of Ser104 and Ser116 phosphorylated, respectively, by protein kinase C and calcium/calmodulin kinase II in vitro. J Neurochem 71:1307–1314 [DOI] [PubMed] [Google Scholar]
- 25. Condorelli G, Trencia A, Vigliotta G, Perfetti A, Goglia U, Cassese A, Musti AM, Miele C, Santopietro S, Formisano P, Beguinot F. 2002. Multiple members of the mitogen-activated protein kinase family are necessary for PED/PEA-15 anti-apoptotic function. J Biol Chem 277:11013–11018 [DOI] [PubMed] [Google Scholar]
- 26. Condorelli G, Vigliotta G, Iavarone C, Caruso M, Tocchetti CG, Andreozzi F, Cafieri A, Tecce MF, Formisano P, Beguinot L, Beguinot F. 1998. PED/PEA-15 gene controls glucose transport and is overexpressed in type 2 diabetes mellitus. EMBO J 17:3858–3866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Estellés A, Charlton CA, Blau HM. 1999. The phosphoprotein protein PEA-15 inhibits Fas- but increases TNF-R1-mediated caspase-8 activity and apoptosis. Dev Biol 216:16–28 [DOI] [PubMed] [Google Scholar]
- 28. Kitsberg D, Formstecher E, Fauquet M, Kubes M, Cordier J, Canton B, Pan G, Rolli M, Glowinski J, Chneiweiss H. 1999. Knock-out of the neural death effector domain protein PEA-15 demonstrates that its expression protects astrocytes from TNFα-induced apoptosis. J Neurosci 19:8244–8251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zvalova D, Formstecher E, Fauquet M, Canton B, Chneiweiss H. 2001. Keeping TNF-induced apoptosis under control in astrocytes: PEA-15 as a ‘double key’ on caspase-dependent and MAP-kinase-dependent pathways. Prog Brain Res 132:455–467 [DOI] [PubMed] [Google Scholar]
- 30. Renault F, Formstecher E, Callebaut I, Junier MP, Chneiweiss H. 2003. The multifunctional protein PEA-15 is involved in the control of apoptosis and cell cycle in astrocytes. Biochem Pharmacol 66:1581–1588 [DOI] [PubMed] [Google Scholar]
- 31. Hill JM, Vaidyanathan H, Ramos JW, Ginsberg MH, Werner MH. 2002. Recognition of ERK MAP kinase by PEA-15 reveals a common docking site within the death domain and death effector domain. EMBO J 21:6494–6504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Trencia A, Fiory F, Maitan MA, Vito P, Barbagallo AP, Perfetti A, Miele C, Ungaro P, Oriente F, Cilenti L, Zervos AS, Formisano P, Beguinot F. 2004. Omi/HtrA2 promotes cell death by binding and degrading the anti-apoptotic protein ped/pea-15. J Biol Chem 279:46566–46572 [DOI] [PubMed] [Google Scholar]
- 33. Mizrak SC, Renault-Mihara F, Párraga M, Bogerd J, van de Kant HJ, López-Casas PP, Paz M, del Mazo J, de Rooij DG. 2007. Phosphoprotein enriched in astrocytes-15 is expressed in mouse testis and protects spermatocytes from apoptosis. Reproduction 133:743–751 [DOI] [PubMed] [Google Scholar]
- 34. Siegelin MD, Habel A, Gaiser T. 2008. Epigalocatechin-3-gallate (EGCG) downregulates PEA15 and thereby augments TRAIL-mediated apoptosis in malignant glioma. Neurosci Lett 448:161–165 [DOI] [PubMed] [Google Scholar]
- 35. Eckert A, Böck BC, Tagscherer KE, Haas TL, Grund K, Sykora J, Herold-Mende C, Ehemann V, Hollstein M, Chneiweiss H, Wiestler OD, Walczak H, Roth W. 2008. The PEA-15/PED protein protects glioblastoma cells from glucose deprivation-induced apoptosis via the ERK/MAP kinase pathway. Oncogene 27:1155–1166 [DOI] [PubMed] [Google Scholar]
- 36. Renault-Mihara F, Beuvon F, Iturrioz X, Canton B, De Bouard S, Léonard N, Mouhamad S, Sharif A, Ramos JW, Junier MP, Chneiweiss H. 2006. Phosphoprotein enriched in astrocytes-15 kDa expression inhibits astrocyte migration by a protein kinase CΔ-dependent mechanism. Mol Biol Cell 17:5141–5152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Vigliotta G, Miele C, Santopietro S, Portella G, Perfetti A, Maitan MA, Cassese A, Oriente F, Trencia A, Fiory F, Romano C, Tiveron C, Tatangelo L, Troncone G, Formisano P, Beguinot F. 2004. Overexpression of the ped/pea-15 gene causes diabetes by impairing glucose-stimulated insulin secretion in addition to insulin action. Mol Cell Biol 24:5005–5015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Miele C, Raciti GA, Cassese A, Romano C, Giacco F, Oriente F, Paturzo F, Andreozzi F, Zabatta A, Troncone G, Bosch F, Pujol A, Chneiweiss H, Formisano P, Beguinot F. 2007. PED/PEA-15 regulates glucose-induced insulin secretion by restraining potassium channel expression in pancreatic β-cells. Diabetes 56:622–633 [DOI] [PubMed] [Google Scholar]
- 39. Gervais M, Dugourd C, Muller L, Ardidie C, Canton B, Loviconi L, Corvol P, Chneiweiss H, Monnot C. 2006. Akt down-regulates ERK1/2 nuclear localization and angiotensin II-induced cell proliferation through PEA-15. Mol Biol Cell 17:3940–3951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Bartholomeusz C, Itamochi H, Nitta M, Saya H, Ginsberg MH, Ueno NT. 2006. Antitumor effect of E1A in ovarian cancer by cytoplasmic sequestration of activated ERK by PEA15. Oncogene 25:79–90 [DOI] [PubMed] [Google Scholar]
- 41. Formstecher E, Ramos JW, Fauquet M, Calderwood DA, Hsieh JC, Canton B, Nguyen XT, Barnier JV, Camonis J, Ginsberg MH, Chneiweiss H. 2001. PEA-15 mediates cytoplasmic sequestration of ERK MAP kinase. Dev Cell 1:239–250 [DOI] [PubMed] [Google Scholar]
- 42. Renganathan H, Vaidyanathan H, Knapinska A, Ramos JW. 2005. Phosphorylation of PEA-15 switches its binding specificity from ERK/MAPK to FADD. Biochem J 390:729–735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zhang Y, Redina O, Altshuller YM, Yamazaki M, Ramos J, Chneiweiss H, Kanaho Y, Frohman MA. 2000. Regulation of the expression of phospholipase D1 and D2 by PEA-15, a novel protein that interacts with them. J Biol Chem 275:35224–35232 [DOI] [PubMed] [Google Scholar]
- 44. Vaidyanathan H, Ramos JW. 2003. RSK2 activity is regulated by its interaction with PEA-15. J Biol Chem 278:32367–32372 [DOI] [PubMed] [Google Scholar]
- 45. Whitehurst AW, Robinson FL, Moore MS, Cobb MH. 2004. The death effector domain protein PEA-15 prevents nuclear entry of ERK2 by inhibiting required interactions. J Biol Chem 279:12840–12847 [DOI] [PubMed] [Google Scholar]
- 46. Callaway K, Rainey MA, Dalby KN. 2005. Quantifying ERK2-protein interactions by fluorescence anisotropy: PEA-15 inhibits ERK2 by blocking the binding of DEJL domains. Biochim Biophys Acta 1754:316–323 [DOI] [PubMed] [Google Scholar]
- 47. Krueger J, Chou FL, Glading A, Schaefer E, Ginsberg MH. 2005. Phosphorylation of phosphoprotein enriched in astrocytes (PEA-15) regulates extracellular signal-regulated kinase-dependent transcription and cell proliferation. Mol Biol Cell 16:3552–3561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Callaway K, Abramczyk O, Martin L, Dalby KN. 2007. The anti-apoptotic protein PEA-15 is a tight binding inhibitor of ERK1 and ERK2, which blocks docking interactions at the D-recruitment site. Biochemistry 46:9187–9198 [DOI] [PubMed] [Google Scholar]
- 49. Liu F, Usui I, Evans LG, Austin DA, Mellon PL, Olefsky JM, Webster NJ. 2002. Involvement of both G(q/11) and G(s) proteins in gonadotropin-releasing hormone receptor-mediated signaling in LβT2 cells. J Biol Chem 277:32099–32108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Rykx A, De Kimpe L, Mikhalap S, Vantus T, Seufferlein T, Vandenheede JR, Van Lint J. 2003. Protein kinase D: a family affair. FEBS Lett 546:81–86 [DOI] [PubMed] [Google Scholar]
- 51. Vaidyanathan H, Opoku-Ansah J, Pastorino S, Renganathan H, Matter ML, Ramos JW. 2007. ERK MAP kinase is targeted to RSK2 by the phosphoprotein PEA-15. Proc Natl Acad Sci USA 104:19837–19842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Wang Y, Fortin J, Lamba P, Bonomi M, Persani L, Roberson MS, Bernard DJ. 2008. Activator protein-1 and smad proteins synergistically regulate human follicle-stimulating hormone β-promoter activity. Endocrinology 149:5577–5591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Coss D, Jacobs SB, Bender CE, Mellon PL. 2004. A novel AP-1 site is critical for maximal induction of the follicle-stimulating hormone beta gene by gonadotropin-releasing hormone. J Biol Chem 279:152–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Lawson MA, Tsutsumi R, Zhang H, Talukdar I, Butler BK, Santos SJ, Mellon PL, Webster NJ. 2007. Pulse sensitivity of the luteinizing hormone β promoter is determined by a negative feedback loop Involving early growth response-1 and Ngfi-A binding protein 1 and 2. Mol Endocrinol 21:1175–1191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Roux PP, Richards SA, Blenis J. 2003. Phosphorylation of p90 ribosomal S6 kinase (RSK) regulates extracellular signal-regulated kinase docking and RSK activity. Mol Cell Biol 23:4796–4804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Shaywitz AJ, Greenberg ME. 1999. CREB: a stimulus-induced transcription factor activated by a diverse array of extracellular signals. Annu Rev Biochem 68:821–861 [DOI] [PubMed] [Google Scholar]
- 57. Xing J, Ginty DD, Greenberg ME. 1996. Coupling of the RAS-MAPK pathway to gene activation by RSK2, a growth factor-regulated CREB kinase. Science 273:959–963 [DOI] [PubMed] [Google Scholar]
- 58. Bédécarrats GY, Kaiser UB. 2003. Differential regulation of gonadotropin subunit gene promoter activity by pulsatile gonadotropin-releasing hormone (GnRH) in perifused LβT2 cells: role of GnRH receptor concentration. Endocrinology 144:1802–1811 [DOI] [PubMed] [Google Scholar]
- 59. DeFea KA, Zalevsky J, Thoma MS, Déry O, Mullins RD, Bunnett NW. 2000. β-Arrestin-dependent endocytosis of proteinase-activated receptor 2 is required for intracellular targeting of activated ERK1/2. J Cell Biol 148:1267–1281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Scott MG, Pierotti V, Storez H, Lindberg E, Thuret A, Muntaner O, Labbé-Jullié C, Pitcher JA, Marullo S. 2006. Cooperative regulation of extracellular signal-regulated kinase activation and cell shape change by filamin A and β-arrestins. Mol Cell Biol 26:3432–3445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Luttrell LM, Roudabush FL, Choy EW, Miller WE, Field ME, Pierce KL, Lefkowitz RJ. 2001. Activation and targeting of extracellular signal-regulated kinases by β-arrestin scaffolds. Proc Natl Acad Sci USA 98:2449–2454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Rozenfeld R, Devi LA. 2007. Receptor heterodimerization leads to a switch in signaling: β-arrestin2-mediated ERK activation by μ-Δ opioid receptor heterodimers. FASEB J 21:2455–2465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Caunt CJ, Finch AR, Sedgley KR, McArdle CA. 2006. Seven-transmembrane receptor signalling and ERK compartmentalization. Trends Endocrinol Metab 17:276–283 [DOI] [PubMed] [Google Scholar]
- 64. Armstrong SP, Caunt CJ, McArdle CA. 2009. Gonadotropin-releasing hormone and protein kinase C signaling to ERK: spatiotemporal regulation of ERK by docking domains and dual-specificity phosphatases. Mol Endocrinol 23:510–519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Caunt CJ, Armstrong SP, Rivers CA, Norman MR, McArdle CA. 2008. Spatiotemporal regulation of ERK2 by dual specificity phosphatases. J Biol Chem 283:26612–26623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Caunt CJ, Rivers CA, Conway-Campbell BL, Norman MR, McArdle CA. 2008. Epidermal growth factor receptor and protein kinase C signaling to ERK2: spatiotemporal regulation of ERK2 by dual specificity phosphatases. J Biol Chem 283:6241–6252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Haisenleder DJ, Ferris HA, Shupnik MA. 2003. The calcium component of gonadotropin-releasing hormone-stimulated luteinizing hormone subunit gene transcription is mediated by calcium/calmodulin-dependent protein kinase type II. Endocrinology 144:2409–2416 [DOI] [PubMed] [Google Scholar]
- 68. Venter JC, Adams MD, Myers EW, Li PW, Mural RJ, Sutton GG, Smith HO, Yandell M, Evans CA, Holt RA, Gocayne JD, Amanatides P, Ballew RM, Huson DH, Wortman JR, Zhang Q, Kodira CD, Zheng XH, Chen L, Skupski M, Subramanian G, Thomas PD, Zhang J, Gabor Miklos GL, Nelson C, Broder S, Clark AG, Nadeau J, McKusick VA, Zinder N, Levine AJ, Roberts RJ, Simon M, Slayman C, Hunkapiller M, Bolanos R, Delcher A, Dew I, Fasulo D, Flanigan M, Florea L, Halpern A, Hannenhalli S, Kravitz S, Levy S, Mobarry C, Reinert K, Remington K, Abu-Threideh J, Beasley E, et al. 2001. The sequence of the human genome. Science 291:1304–1351 [DOI] [PubMed] [Google Scholar]
- 69. Ruf F, Park MJ, Hayot F, Lin G, Roysam B, Ge Y, Sealfon SC. 2006. Mixed analog/digital gonadotrope biosynthetic response to gonadotropin-releasing hormone. J Biol Chem 281:30967–30978 [DOI] [PubMed] [Google Scholar]
- 70. Chawla MK, Lin G, Olson K, Vazdarjanova A, Burke SN, McNaughton BL, Worley PF, Guzowski JF, Roysam B, Barnes CA. 2004. 3D-catFISH: a system for automated quantitative three-dimensional compartmental analysis of temporal gene transcription activity imaged by fluorescence in situ hybridization. J Neurosci Methods 139:13–24 [DOI] [PubMed] [Google Scholar]
- 71. Lin G, Chawla MK, Olson K, Guzowski JF, Barnes CA, Roysam B. 2005. Hierarchical, model-based merging of multiple fragments for improved three-dimensional segmentation of nuclei. Cytometry A 63:20–33 [DOI] [PubMed] [Google Scholar]
- 72. Lin G, Adiga U, Olson K, Guzowski JF, Barnes CA, Roysam B. 2003. A hybrid 3D watershed algorithm incorporating gradient cues and object models for automatic segmentation of nuclei in confocal image stacks. Cytometry A 56:23–36 [DOI] [PubMed] [Google Scholar]
- 73. Lin G, Stewart CV, Roysam B, Fritzsche K, Yang G, Tanenbaum HL. 2004. Predictive scheduling algorithms for real-time feature extraction and spatial referencing: application to retinal image sequences. IEEE Trans Biomed Eng 51:115–125 [DOI] [PubMed] [Google Scholar]
- 74. Chomczynski P, Sacchi N. 1987. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem 162:156–159 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.