The development of stronger, more specific therapies for castration-recurrent prostate cancer depends upon gaining a more complete understanding of ligand-independent activity of the androgen receptor.
Abstract
Advanced prostate tumors, which are androgen dependent, are often initially treated in the clinic with hormone ablation therapy, either through surgical castration or administration of small-molecule antiandrogens. Most tumors respond favorably to these treatments, exhibiting regression of the tumor, amelioration of symptoms, and a decrease of prostate-specific antigen in patient sera. However, with time, the majority of tumors recur in a more aggressive, castration-resistant (CR) phenotype. Currently, no effective treatment exists for this stage of the cancer, and patients ultimately succumb to metastatic disease. The androgen receptor (AR), which is a member of the nuclear hormone receptor superfamily of proteins, is the transcription factor that is responsible for mediating the effects of androgens upon target tissues, and it has been demonstrated to play a central role in the development and progression of prostate cancer. Despite CR tumor cells being able to continue to grow after hormonal therapy in which testosterone and dihydrotestosterone are markedly reduced, they still require the expression and activity of the AR. The AR can become transactivated in this low-androgen environment through a number of different mechanisms, including amplification and mutation of the receptor, cross talk with other signaling pathways, and altered regulation by coregulatory proteins. This review will summarize the most current data regarding non-ligand-mediated activation of the AR in prostate cancer cells. Developing work in this field aims to more clearly elucidate the signals that drive AR activity independently of androgens in CR disease so that better therapeutic targets can be developed for patients with this stage of highly aggressive prostate carcinoma.
As the second leading cause of cancer deaths in American men, prostate cancer is a persistent, significant challenge for clinicians and researchers (1). Prostate tumors initially develop and progress in an androgen-dependent (AD) form, and clinical castration either through orchiectomy or chemical administration has been a standard treatment for more than half a century. Initially, tumors respond well to antiandrogen therapy, regressing significantly along with symptoms. However, after a median time of approximately 18–36 months, most tumors regrow and are characterized by a more aggressive, androgen depletion-independent or castration-resistant (CR) phenotype. Until fairly recently, these relapsed tumors were characterized as being androgen independent. However, a paradigm shift occurred upon reports by a number of groups demonstrating that despite being surgically or chemically castrated, very low levels of androgens are still detectable in the tissues and serum of advanced patients (2, 3). The current nomenclature of CR prostate cancer indicates that these advanced tumors are resistant to the castration therapy, but not necessarily 100% free from androgens. Despite being resistant to androgen ablation therapy, it was observed that a large proportion of CR prostate tumors maintain expression of a functional androgen receptor (AR), the nuclear hormone receptor and transcription factor that functions to regulate the expression of genes that are associated with androgenic development and gland formation (4). It is now a central tenet that the expression and activity of the AR is still required for survival and growth of many types of CR prostate cancers and that the development of better methods to inhibit the activity and/or expression of the AR would greatly increase the quality of life for many patients (5). Another central hallmark of prostate cancer is that its development and progression is marked by vast heterogeneity. Prostate tumors often arise from multifocal lesions within the same gland and exhibit large variability in gene expression and response to therapy, not only from patient to patient but also between different lesions from the same patient (6). As more evidence is obtained with regard to the complicated interactions that drive prostate tumor growth, it becomes more apparent that better therapeutic options are required for patients, both before and after the development of CR disease. Thus, a thorough mechanistic understanding of how the receptor functions to promote cell proliferation and tumor growth has been a key area of prostate cancer research.
The AR is required for the proliferation and survival of prostate cancer cells, and it is active in CR disease (5). As a transcription factor, the AR regulates the expression of thousands of both coding and noncoding RNA targets (4, 7). This transactivation of the AR can occur through a number of non-mutually-exclusive mechanisms, including increased expression of the receptor; generation of mutations in the receptor, which can change its canonical regulation; and altered action of coregulatory proteins, through cross talk with other signaling pathways or through the action of constitutively active splice variants. The specific mechanism of AR activation has grown increasingly complicated over the past decade and what was originally considered to be a fairly simple ligand-receptor activation pathway is turning out to be a much more complex system with multiple inputs, cross talk, and redundancy. The first generation of true antiandrogen molecules used in the clinic, including bicalutamide (Casodex) and flutamide, aimed at inhibiting AR transcriptional activity by specifically targeting ligand binding to the AR (8, 9). The prevention of direct ligand binding inhibits AR-driven gene expression and cell proliferation, but transactivation of the receptor by other factors renders these treatments ineffective in CR disease. Furthermore, there is a growing body of evidence that the AR has significant signaling effects outside of the nucleus and in regulating target gene expression, both in the presence and absence of androgen. This nongenomic activity of the AR has profound implications in terms of prostate cancer development and potential therapy but will not be discussed here because it has been expertly reviewed elsewhere (10–12). This minireview will summarize the current state of research regarding the induction of AR transcriptional activity in the absence of androgen in prostate cancer cells and give perspective into those aspects of this regulation, which might be best exploited for the development of future therapeutic intervention. The AR remains a crucial factor in the promotion of CR prostate cancer, and through the inhibition of one or more of the transactivation pathways described in this review, more effective therapies can be created to improve the quality of life and reduce tumor and metastasis growth for patients with terminal disease.
Extracellular Peptide Signaling: Growth Factors and Cytokines
In the prostate, the AR mediates androgenic signals that primarily arise from extracellular sources, namely the testes and adrenal glands. Testosterone passively diffuses across the prostate cell membrane, where it is then converted to the more active metabolite, dihydrotestosterone (DHT) by the 5α-reductase enzyme. DHT binds to the AR ligand-binding domain (LBD) with high affinity, inducing conformational changes that lead to activation of the receptor (4). These paracrine signals are an integral part of prostate development and tissue maintenance and play an important role in the development of prostate carcinoma. The effect of paracrine signals on AR activity is not limited to those of androgens, however. Extracellular peptide signals, in the form of growth factors and cytokines, can promote AR activity, although not through the same ligand-binding mechanism as DHT. External signals other than androgens can also affect AR activity, although not through the same ligand-binding mechanism as DHT. These peptides stimulate the activity of cognate membrane-bound protein receptors, such as receptor tyrosine kinases and G protein-coupled receptors, thus initiating intracellular signaling cascades that can lead to AR transcriptional activity downstream, even in low levels of androgen. Stimulation of AR transcriptional activity from these cascades can result from ultimate modification of the AR itself, by augmenting the activity of AR coactivators, or both.
Interleukins
The action of IL, especially IL-6 and IL-8, in the promotion of CR cancer has been an active area of research for almost two decades. IL-6 is a multifunctional cytokine produced by many cell types including prostate cells, immune cells, and osteoblasts. The predominant effect of IL-6 in many cell types is the stimulation of Janus kinase (JAK) signaling and activation of signal transducers and activators of transcription (STAT) proteins, especially STAT3; however, activation of MAPK and phosphatidylinositol-3 kinase (PI3K) can also occur depending on the context (13–15). Chronic local inflammation in the prostate (proliferative inflammatory atrophy) can promote up-regulation of inflammatory signaling factors that can drive the development and progression of prostate tumors. Thus, the mechanism by which these chemokines can stimulate AR transcriptional activity is an active area of research (16). The levels of IL-6 and its soluble receptor, IL-6sR, in circulating plasma is increased in patients with aggressive metastatic disease and can be predictive of recurrence after treatment of localized cancer (17). Also, IL-6 can be produced in an autocrine manner in CR prostate cells by overexpression of atypical protein kinase C (aPKCλ/ι) (18). IL-6 can transactivate the AR in prostate cancer cells; however, the effect of IL-6 on ligand-independent AR activation, tumor formation, and CR growth varies depending on the status of the AR as well as other interacting signaling pathways (19, 20). The effect of IL-6 treatment on LAPC-4 cells, which express a wild-type AR and in MDA-PCa-2b cells, which express a double-mutant AR (substitutions at L701H and T877A) is agonistic, whereas IL-6 inhibits ligand-independent AR activity in T877A AR-expressing LNCaP cells (20). In the presence of androgen, IL-6 treatment of LNCaP cells impairs transcription of prostate-specific antigen (PSA), via a mechanism that prevents recruitment of the histone acetyltransferase coactivator p300 to the PSA promoter (21). In contrast, chronic stimulation of LNCaP cells with IL-6 results in stimulation of AR via STAT signaling, thus inducing PSA expression, even in the absence of androgen (22). STAT3 interacts directly with amino acids 234–558 of the AR (23), an interaction that is dependent upon phosphorylation of STAT3 at Ser-722 (24). Interestingly, IL-6 stimulation of AR activity in this context can be inhibited by a form of selenium chemopreventative, sodium selenite (25). The effect of IL-6 is further diversified via downstream MAPK activation and sarcoma-related kinase (Src) from growth factor receptors, and the cross talk of these pathways can also promote AR activity. In LNCaP cells, IL-6 treatment induces tyrosine phosphorylation of growth factor receptors ErbB2/neu and ErbB3 but not the related family member ErbB1/epidermal growth factor receptor (EGFR) (26). This phosphorylation event is mediated by the interaction of ErbB2 with the gp130 subunit of the IL-6 receptor, demonstrating that signal diversity can be generated by associations between receptors (26). Activated Src can phosphorylate AR at Tyr-534, thereby promoting its transcriptional activity in LNCaP and LAPC-4 cells (27, 28). MAPK signaling after IL-6 induction also leads in part to phosphorylation of another AR coregulator, steroid receptor coregulator-1 (SRC-1), which is required for optimal ligand-independent activation of the AR by IL-6 (29).
Recently, IL-8 was also found to promote AR transcriptional activity. Treatment of androgen-dependent LNCaP and CR 22Rv1 cells with recombinant IL-8 in the absence of serum resulted in cell growth in the absence and presence of androgen, concomitant with increased PSA mRNA expression (30). Whether the mechanism of activation of the AR by IL-8 is similar to that of IL-6 has yet to be elucidated; however, their specific receptors and varying functions suggests that similar but distinct mechanisms exist for each factor. At least in cell culture systems, the contrasting effects of IL in AD vs. CR backgrounds on AR activity suggests that these paracrine factors may be significant promoters of AR activity in CR disease. Especially when coupled with increasing evidence that proliferative inflammatory atrophy, prostatic intraepithelial neoplasia, and other manifestations of inflammatory prostate tissue can correlate with development of prostate carcinoma (31, 32), the IL may be a strong candidate target for therapeutic intervention.
Growth factors
The ability of mitogenic growth factors such as EGF and IGF to stimulate intracellular signaling that cross talks with those of the AR has been extensively reviewed elsewhere (33). As extracellular signals, these stimuli can be produced in an autocrine or paracrine manner, thus greatly increasing the potential for tumor cells to receive proliferative stimulation. In many systems, full activation of the AR by androgen requires the activation of at least one of these signaling pathways. By targeting coregulators, transcriptional machinery, and the AR itself, these mitogenic signals can amplify the activity of the AR in a low-ligand environment, via genomic and nongenomic mechanisms.
The expression of EGF and its receptor (EGFR) are increased in patients with advanced, aggressive prostate cancer, and both molecules have been implicated in the development and progression of many other kinds of tumors (33). A major signaling effect of EGF stimulation and subsequent EGFR activation is initiation of the proliferative MAPK pathway and its associated effectors (34). Evidence of the interaction of EGF stimulation on AR activity was simply demonstrated over a decade ago. In one study, treatment of ectopic AR-expressing DU-145 cells (CR prostate cancer cells isolated from a brain metastasis that do not express an endogenous AR) with EGF in the absence of androgen induces an androgen-responsive luciferase promoter to a similar extent as with androgen treatment, an effect that is inhibited by the AR antagonist bicalutamide (35). In prostate cancer cells that express endogenous AR, such as LNCaP and LAPC-4, treatment with EGF in the absence of androgen induces phosphorylation of the AR at Tyr-267 and -534 by Src and Ack1 kinases (28). This effect is similar to that of other growth factors such as heregulin/neuregulin (NRG-1) and growth-arrest specific 6 (Gas-6) (a ligand for the Mer receptor tyrosine kinase; named for its expression in monocytes and tissues of epithelial and reproductive origin) treatment, which also induce AR phosphorylation and activity, through activation of the Cdc42-related kinase Ack1 (28). EGF can also induce IL-6 up-regulation in prostate cancer cells (24). As described in the previous section, IL-6 activation promotes AR transcriptional activity in the absence of androgen and demonstrates how autocrine/paracrine signals can synergize to promote transactivation of the AR in the scenario of CR prostate cancer. Inhibition of receptor tyrosine kinase activity is currently in clinical application as a supplementary treatment for CR disease, and future work will determine whether their continued use as a combination therapy can inhibit the growth of advanced metastatic carcinoma (36).
IGF-I promotes cell growth, survival, and differentiation of prostate cells. The expression of both IGF-I and its receptor are increased in advanced cancers, especially in metastases (37). In addition to being a target of AR transcriptional activity in prostate epithelial cells, IGF-I receptor can induce intracellular signaling cascades that regulate phosphorylation of the AR, thus affecting its transcriptional activation both in the presence and absence of androgen (37). Activation of androgen-responsive promoters after 50 ng/ml IGF-I treatment occurs to the same extent as with androgen in ectopic AR-expressing DU-145 cells (35). Treatment with the keratinocyte growth factor has a similar effect (35). Additionally, the cross talk between IGF and AR is apparent in other cell types, including nontransformed cells (33). The data reported thus far indicate that the action of IGF and keratinocyte growth factor on AR activity largely appears to be a general phenomenon and not specific to the scenario of CR disease. It is due to this aspect that these pathways are not the most eligible of candidates for therapeutic targeting in advanced disease, because the effect may not be limited to tumor cells.
Neuroendocrine (NE) cells support normal function and differentiation of the prostate gland. There is increasing, and conflicting, evidence for their implication in the development of prostate carcinoma, as reviewed elsewhere (38). At the core of the debate is whether NE cells promote CR disease by supporting AR activity in nearby epithelial-derived tumor cells or whether NE cells are the progenitor cell of a subset of CR tumors. It is also hypothesized that some tumor cells take on a more NE-like phenotype in response to therapy or during tumor progression. The AR may be a critical factor in maintaining epithelial-like characteristics in tumor cells, because silencing of AR in LNCaP cells results in a shift to a more NE-like phenotype (39). What is quite apparent from the literature is that secretions by NE cells can promote cancer cell proliferation in a paracrine manner in some tumors. One of the main factors secreted by NE cells is bombesin/gastrin-releasing peptide (GRP). Treatment of LNCaP and LAPC-4 cells with GRP in the absence of androgen induces AR phosphorylation at Tyr-534, via Src activation, and induces subsequent AR-mediated transcription (28). Overexpression of GRP is also sufficient to confer LNCaP xenograft tumor growth in castrated mice, in accordance with the observed AR transactivation (40). In the future, it will be of particular interest to determine how GRP and other secreted NE factors promote the growth of CR disease and whether tumors that exhibit increased amounts of NE cells are derived from epithelial prostate cells or whether the disease transitions into a more neuroendocrine phenotype over time. At the current moment, however, the relationship between NE cells and the development of CR disease is only vaguely understood, and more basic research is needed to determine whether these factors can be targeted to control CR tumor growth.
Intracellular Kinase Signaling
The many extracellular signals discussed in the previous section represent just one level of complexity when it comes to the transactivation of the AR in the absence of androgens. These factors can work alone or in concert with each other to initiate intracellular kinase signaling cascades that target cell proliferation machinery, survival signals, transcription initiation, etc. There are over 500 putative protein kinase genes in the human genome (41). Thus, the complexity and variety of signals that can be transduced is enormous. Kinase signal cascades are rapidly transduced and are mediated through specific protein-protein interactions. Alterations to the activity of some kinases, especially to those with oncogenic or tumor-suppressive functions, has the potential to promote malignant signaling and drive the development and progression of many kinds of cancers, including prostate (42). In the context of CR prostate cancer, even slight modifications in intracellular kinase activity might have significant influence on AR activity while still having a modest effect of the survival or proliferation of the cell in general. Furthermore, as important mediators of extracellular signals, it is possible to postulate that multiple upstream factors can signal through the same kinase or family of kinases, which means that inhibiting the interaction of one kinase with the AR could inhibit multiple paracrine signals. However, specifically designing therapies to prevent protein-protein interactions or phosphorylation events is difficult and requires vigorous validation and confirmation of specificity. At the very least, understanding the main intracellular kinases responsible for promoting ligand-independent AR activity provides a more complete picture to understand how transactivation of the AR might occur in advanced CR disease.
The MAPK pathway is a powerful oncogenic signaling modality that has been implicated in many kinds of cancers, including prostate carcinoma. As described in an earlier section, many extracellular stimuli can lead to activation of the MAPK signaling network, thus promoting synergistic promulgation of a strong proliferative signal. The many effectors of MAPK signals provide hundreds of potential regulators of AR activity, only a few of which have been characterized, including Src and p42/44 ERK.
The Src kinase is a potent oncogene that is activated in many kinds of tumors. In addition to phosphorylating effector proteins, it can also specifically phosphorylate the AR at Tyr-534, a modification that induces transcriptional activity by promoting nuclear translocation and DNA binding in the absence of androgen (27, 43). Stimulation of Src activity results from a number of stimuli, including EGF, as described earlier. The activity of Src on AR transactivation is modulated, at least in part, through its binding partner and associated scaffolding protein receptor for activated C kinase 1 (RACK1) (43). Treatment of C4-2 cells with the Src inhibitor protein phosphatase 2 in androgen-free conditions results in a decreased activation of mouse mammary tumor virus and PSA luciferase reporters as well as reduced recruitment of the AR to the endogenous PSA gene enhancer region (44). Src inhibition in this manner also affects ligand-dependent AR activity.
Another key effector of MAPK signal propagation is p42/44 (ERK2 and ERK1, respectively). The expression of the AR itself is regulated, in part, by this branch of the MAPK pathway, because treatment of LNCaP and C4-2 cells with the MAPK kinase inhibitor UO126 strongly reduces the amount of AR protein in both the presence and absence of androgen (45). As mentioned earlier, extracellular signals such as IL-6 induce MAPK activation (23). G protein-related factors have also been recently discovered to regulate AR activity through stimulation of the MAPK cascade. The expression of Vav3, a Rho guanosine triphosphate guanine nucleotide exchange factor, is up-regulated in prostate cancer and has been demonstrated to enhance EGF/IGF activation of the AR in the absence of androgen (46). This signaling pathway induces nuclear localization of the AR via the activation Rho guanosine triphosphatase Rac1, whose downstream signaling includes members of the MAPK pathway, including ERK (46).
The involvement of the phosphatase and tensin homolog (PTEN) and Akt/protein kinase B signaling in prostate cancer has been well documented (47). PTEN is one of the most frequently mutated and/or deleted tumor suppressors in human cancers, and its expression is often lost or reduced in advanced metastatic tumors compared with primary prostate tumors (48). Loss of the regulatory phosphatase activity by PTEN results in unchecked PI3K activity (initiated by a number of transmembrane receptors), which generates the secondary messenger phospholipid phosphatidylinositol 3,5-triphosphate (PIP3) from phosphatidylinositol 4,5-diphosphate (PIP2). PIP3 presence in the cell membrane recruits the pleckstrin homology domains of a number of signaling kinases, including Akt/protein kinase B. Recruitment of Akt by PIP3 leads to conformational change that allows activation to occur by the transfer of a phosphate moiety by the constitutively active phosphoinositide-dependent kinase 1 and 2. Activated Akt has a number of downstream effects, including survival/apoptosis evasion, proliferation, and protein synthesis/cell growth (47). Akt has been shown to have both agonistic and antagonistic influence on AR activity, either through direct phosphorylation of the receptor or through the action of downstream effectors, in both the presence and absence of androgen (49–51). One target of Akt is forkhead box O1 (FOXO1), whose primary role, when active, is to induce the transcription of target genes that will induce apoptosis. Akt phosphorylation of FOXO1 excludes it from the nucleus, thus preventing cell death. FOXO1 is also thought to have a functionality that is not dependent upon its transcriptional activity. Ectopic expression of FOXO1 in PTEN-null prostate cancer cells inhibits androgen-independent activation of the AR (52). Interestingly, silencing of FOXO1 in PTEN-positive cells increases the basal activity of the AR and can sensitize the receptor to lower levels of androgens and by IL-6 (52). The mechanism of this regulation is still unclear; however, it appears that it is at least somewhat dependent on the action of a putative AR coregulator, histone deacetylase 3 (52).
Signaling through nuclear factor κ-light chain enhancer of activated B cells (NF-κB) in most cell types results in prosurvival signaling whose effects include differentiation, proliferation, and evasion of apoptosis. Blocking of NF-κB by small interfering RNA (siRNA)-mediated knockdown of the IκBα (nuclear factor of κ light polypeptide gene enhancer in B-cells inhibitor, α) inhibitor inhibits AR activation in prostate cancer cells in vitro (53). Thus, constitutive NF-κB activity maintains high levels of the AR in the nucleus, which promotes constant transcription of proliferative genes. NF-κB/p52 can activate the AR in the absence of androgen in LAPC-4, LNCaP, and C4-2 cells. The exact mechanism for this is not clearly understood; however, it appears that at least part of the transactivation is due to NF-κB direct interaction with the NH2 domain of the AR, which allows for recruitment of coactivators like p300 and augmented binding to the promoter regions of AR-targeted genes (54). This action of NF-κB drives constitutive activity of the AR, because siRNA knockdown of endogenous p52 in C4-2 cells abolished transcription of AR target genes (54).
Protein kinase A, whose activity is regulated by intracellular cAMP levels, also can modulate AR activity in the absence of androgens. When prostate cancer cells are treated with the inhibitor forskolin (a naturally derived molecule that activates adenylyl cyclase, thus increasing the intracellular levels of cAMP and consequently protein kinase A), AR-driven transcription is increased from AR-responsive promoters, an effect that is quenched by the addition of AR antagonists bicalutamide and flutamide (55, 56). Also, Aurora-A, a kinase involved in mitosis progression, can phosphorylate the AR at Thr-282 and Ser-293, leading to transactivation of the receptor (57). Aurora-A expression is elevated in prostate tumors compared with normal tissue. This, coupled with its ability to modify the AR, might promote CR cell growth (57). As mentioned above, the Cdc42-associated kinase Ack1 has also been identified as a transactivator of AR activity. A downstream mediator of a number of receptor tyrosine kinases, Ack1 promotes cell survival in part by targeting the tumor suppressor Wwox for degradation and activating AKT (58). Ack1 can directly interact with the AR and can specifically phosphorylate the AR at Tyr-267, a modification that has been described to be required for optimal AR recruitment to target genes at low- to no-androgen conditions (59). Tyrosine-phosphorylated AR was found in eight of 18 CR prostate tumor samples, and the expression correlated with Ack1 expression in those same tumors (59). LNCaP xenografts that overexpress ectopic Ack1 demonstrate significantly increased growth in castrated mice, which is inhibited by the kinase inhibitor dasatinib (28). Treatment of prostate cancer cells with the Ack1-specific inhibitor AIM-100 after stimulation with EGF prevents phosphorylation of AR at Tyr-267 as well as binding of AR to PSA, NK3 homeobox 1, and transmembrane protease, serine 2 promoters in the absence of androgen (60).
Intracellular kinase signaling is a critical mediator of signals that promote cancer cell proliferation and survival; however, their involvement is made even more complicated when their effect on the AR is considered. Although there is no denying that the ability of kinases such as Src, ERK, Ack1, and NF-κB to directly modify the AR and promote its activity in CR disease provides attractive targets for potential intervention, this line of approach may be fraught with pitfalls. Redundancy between pathways, which is increasingly understood to be the norm rather than the exception, makes achieving specificity and durable inhibition difficult to achieve. With continued investigation into the interplay between these pathways and the effect of individual kinase activity upon the AR, a combinatorial therapeutic approach might be designed that will inhibit AR transactivation in the subset of CR tumors that exhibit increased kinase activity.
Augmentation by coregulators
The role of coregulators in the transactivation of the AR in prostate cancer has been extensively reviewed elsewhere (61). Here, we will focus upon a few key findings that were published since that review, because some of them add much to the understanding of how coregulators might regulate the AR. Heat-shock protein 90 (Hsp90) is one of the main chaperone proteins that is associated with the AR in the cytoplasm. In addition to serving as a binding partner and promoting the stability of AR, Hsp90 has also been demonstrated to hold the AR in a permissible conformation that allows for efficient binding to DHT (62). Interestingly, other chaperones as well as the histone deacetylase HDAC6 have roles in modulating the activity of the AR. Knockdown of HDAC6 in C4-2 cells inhibits the nuclear localization of endogenous AR and inhibits expression of PSA (63). Furthermore, silencing of HDAC6 in these cells inhibits androgen-dependent and -independent cell growth, both in vitro and in a castrated nude mouse xenograft model (63). FK506-binding protein (FKBP51), a cochaperone of Hsp90 and a member of the immunophilin class of proteins, is overexpressed in a LAPC-4 androgen-independent xenograft model of prostate cancer; however the enhancement of AR transcriptional activity observed with FKBP51 in this model appears to occur only in the presence of androgen (64). The histone methyltransferase SET-domain containing protein-9 (SET9) was very recently identified as a novel coregulator of the AR (65). SET9 is able to methylate the AR on Lys-632, a modification that facilitates N-C interaction of the receptor and promotes recruitment to AR-regulated genes. The expression of SET9 is increased in prostate cancer specimens compared with benign prostatic hyperplasia; however, more work is needed to determine whether increased expression of SET9 is exhibited by CR tumors and whether SET9 can promote transactivation of the AR in the absence of DHT. Other members of the HDAC family have also been described to regulate the activity of the AR in the presence and absence of androgens. Recently, histone deacetylase 3 was demonstrated to cooperate with FOXO1 to inhibit AR-mediated transcription in LNCaP cells and suggests a mechanism by which FOXO1 can promote AR activity in the absence of ligand (52).
Intrinsic Activation of the AR
N-C interaction
As a large, 919-amino-acid, multidomain nuclear hormone receptor, the activity of the AR is subject to regulation not only by other factors and signals but also via intramolecular mechanisms. This complex aspect of AR regulation as well as a comprehensive overview of the activity and function of the different domains of the AR can be found in Ref. 66. Approximately half of the ligand-independent transcriptional activity of the AR in CR prostate cancer cells is conferred by the amino-terminal WxxLF domain, which can interact with the AR LBD. However, this domain is not necessary for conferring ligand-independent activity (67). The WxxLF motif is located in a region that was originally identified as Tau-5 (transactivation unit-5), and along with a similar region called Tau-1, it was found that these regions are necessary and sufficient for the intrinsic activity of the AR N-terminal domain (NTD) and activity of the AR (68). However, when nullifying mutations are introduced into Tau-1 and Tau-5 in full-length AR, it is completely inactive, even in the presence of androgen (68). Thus, abolishing the transactivation potential of the AR NTD has putative consequences in curtailing AR-driven CR growth. Expression of AR NTD decoy molecules, comprised of an amino-terminal portion of the AR comprising amino acids 1–558 in LNCaP xenografts decreased growth of the tumor and the abundance of serum PSA after castration (69). The proposed mechanism suggests that these N-terminal decoy molecules bind to interacting proteins that inhibit activation of endogenous AR; however, the specific factors retained by the decoys were not identified. Changes in AR intramolecular interactions can also modulate the nuclear export and import of the receptor. The movement of the AR into the nucleus is largely dependent upon the nuclear localization signal (NLS) present in the hinge region of the receptor; however, a portion of that regulation is also found within the NTD. The NTD and DNA-binding domain (DBD) have ligand-independent nuclear import ability, whereas the LBD exhibits ligand-dependent activity (70). Two additional NLS exist in the NTD in LBD with distinct pathways for import: the NLS of the DBD is Ran and importin α/β dependent, whereas the NLS of the NTD and LBD are Ran dependent but importin α/β independent (70).
Mutations
Activating mutations of the AR have the capacity to create a promiscuous receptor, alter interactions with coregulatory proteins, and impair intramolecular N-C interactions (71). Approximately 70 different missense mutations have been described in patient samples, with varying consequences upon AR activity. The mutations and their effects have been expertly reviewed by others (71, 72). Three LBD mutations that are commonly found in patient tumors, H874Y, T877A, and T877S, impart additional capacity for AR binding to motifs associated with coregulator proteins as well as stimulating the expression of AR target genes in addition to being activated by selective AR modulators, antiandrogens, and other hormones when compared with the wild-type AR (73).
AR variants
The recent identification of multiple kinds of AR variants and their role in promoting CR growth in prostate cancer cells has ignited much interest in how such variants might promote increased AR activity in the absence of androgen (74–77). Early evidence suggests that expression of some AR variant in prostate tumors is associated with poor clinical outcome (75). The contribution of AR variants to the promotion of CR prostate cancer is still uncertain, especially because the predominance of variant expression has yet to be completely described. In one study, endogenous expression of variant mRNA in patient samples and xenografts is much lower (∼0.1–2.5%) relative to full-length AR mRNA (77). In contrast, another study found the expression of variant protein (ARv567es) to be significantly higher in two of three LuCaP xenografts examined, and variant mRNA of any form increased in CR patient metastases (41 of 69) compared with normal tissue samples (10 of 36) (76). Moreover, the constitutive activity attributed to some variants may still rely on the presence of the full-length AR to mediate binding of AR to DNA and stimulate transcription (77). Two such variants, human AR-V7, identified in CR prostate cancer specimens, and mouse AR-V4, from the murine Myc-CaP cell line, demonstrate ligand-free activation of a 4xARE-Luc promoter as well as promoting CR LNCaP tumor growth. The activity of these variants still depends on the presence of a full-length AR, although treatment with the AR antagonist MDV3100 or siRNA knockdown of full-length AR inhibited transcription and androgen-free growth (77). At least in the CWR22R xenograft model, heterodimers of full-length AR with variants was not found by coimmunoprecipitation (78). Full-length AR and the ARv567es variant do interact when coexpressed in AR-null prostate cancer cells (76). As reviewed in Ref. 79, the majority of the variants described to date are characterized by the loss of significant portions of the DBD and LBD (C-terminal), which means that in addition to losing motifs for ligand-dependent regulation, they also lose key motifs for degradation. When ectopically expressed in PC-3 cells, the AR-V7 variant is localized in the nuclei and is constitutively active in the absence of androgen (75). Localization of AR-V7 is cytoplasmic in many benign and AD tissues but is found in the nucleus in CR tumors (78). Interestingly, comparison of the transcriptome of variants with that of full-length AR suggests a significant amount of target specificity for each variant (76–78). There is new hope with a new generation of AR antagonists, including MDV 3100 and EPI-001, which demonstrate more effective inhibition of AR activity. The mechanism of antagonism for each has yet to be fully described, but preliminary reports are promising with regard to their specificity and activity. EPI-001 targets the NTD of the AR (80), whereas MDV 3100 binds to the LBD (81), similarly to bicalutamide. The ability of these molecules to inhibit AR transcriptional activity and demonstrate clinical efficacy in a phase I/II trial of patients with CR tumors, in the case of MDV 3100, suggests that ligand-directed therapeutics might still have a place in future treatment regimes. EPI-001 treatment in AD models including LNCaP xenografts in noncastrated hosts and the LTL313 renal capsule xenograft model leads to effective and specific inhibition of AR activity (80). Because these molecules are still in their nascency, it will be interesting to see how their activities hold up in future clinical trials and especially whether they are effective in AD models and whether they are amenable to combination with other therapies to provide a greater effect. Should they be effective in early AD as they seem to be in CR tumors, it might provide a favorable alternative to current antiandrogen therapy and perhaps generate longer-term remission from a more thorough inhibition of AR activity.
Conclusions
The realization that many prostate tumors are still dependent on AR activity after androgen ablation has provided a paradigm shift in our understanding of the role of the AR in progression of prostate cancer. A number of cellular mechanisms have been described whereby the AR can be activated after androgen ablation. Thus, these mechanisms, as summarized in Fig. 1, are able to significantly contribute to the progression of advanced disease, either alone or, more likely, in concert with each other. The challenge for researchers and clinicians now is to find the right balance of therapeutic modalities to inhibit tumor cell growth or, preferably, to induce specific tumor cell death for patients with terminal CR disease. This balance might be best achieved through combined inhibition of upstream factors that are at play in a specific patient, such as those from paracrine factors or elevated intracellular kinase activity, with more potent inhibition of the AR molecule itself. The first generations of antiandrogen therapies have proven to be effective against AD disease; however, their efficacy is nullified once the tumor achieves castration resistance. A new generation of AR antagonists holds much clinical promise because they are reported to be stronger and more durable inhibitors of transcriptional activity than current compounds used in the clinic. The hope for these new antagonists, used alone or in concert with other treatments to target the pathways discussed in this review, is high because perhaps more complete inhibition of the AR would yield longer remission of AD as well as be effective to destroy CR prostate tumors.
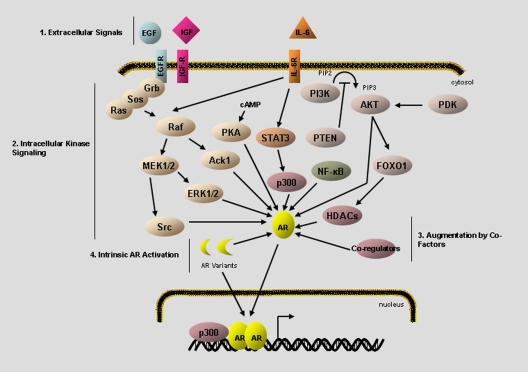
Fig. 1.
Summary of alternative activation pathways for the AR. After the emergence of CR disease, the AR can be transactivated in the absence, or in very low levels, of DHT. Transactivation of the receptor leads to regulation of genomic targets in a manner comparable to that of canonical AR activation. The AR transcriptome regulates a myriad of cellular processes including proliferation, lipid production, survival, and secretion. Activating signals arise from several, non-mutually-exclusive mechanisms including 1) extracellular peptide signals such as EGF and IL-6, 2) up-regulation of intracellular kinase pathways, such as PI3K/Akt and MAPK activation, 3) altered activity of coregulatory proteins, and 4) intrinsic activation of the AR itself through deregulated intramolecular interaction or through expression of constitutively active AR variants.
Acknowledgments
Disclosure Summary: The authors have nothing to disclose.
This work was supported by the National Cancer Institute (Grants CA125747, CA121277, and CA091956) and the T.J. Martell Foundation to D.J.T.
NURSA Molecule Pages:
Nuclear Receptors: AR;
Coregulators: SRC-1;
Ligands: Bicalutamide | Flutamide | Dihydrotestosterone | Testosterone.
Footnotes
- AD
- Androgen dependent
- AR
- androgen receptor
- CR
- castration resistant
- DBD
- DNA-binding domain
- DHT
- dihydrotestosterone
- EGFR
- epidermal growth factor receptor
- FOXO1
- forkhead box O1
- GRP
- gastrin-releasing peptide
- Hsp90
- heat-shock protein 90
- LBD
- ligand-binding domain
- NE
- neuroendocrine
- NF-κB
- nuclear factor κ-light chain enhancer of activated B cells
- NLS
- nuclear localization signal
- NTD
- N-terminal domain
- PI3K
- phosphatidylinositol-3 kinase
- PIP3
- phosphatidylinositol 3,5-triphosphate
- PSA
- prostate-specific antigen
- PTEN
- phosphatase and tensin homolog
- SET9
- SET-domain containing protein-9
- Src
- sarcoma-related kinase
- STAT
- signal transducers and activators of transcription.
References
- 1. Jemal A, Siegel R, Xu J, Ward E. 2010. Cancer Statistics, 2010. CA Cancer J Clin 60:277–300 [DOI] [PubMed] [Google Scholar]
- 2. Geller J. 1995. Prolonging survival in metastatic prostate cancer: the case for adrenal androgens: overview and summary of therapeutic controversies in prostatic cancer. J Clin Endocrinol Metab 80:1074–1078 [DOI] [PubMed] [Google Scholar]
- 3. Mohler JL. 2008. Castration-recurrent prostate cancer is not androgen-independent. Adv Exp Med Biol 617:223–234 [DOI] [PubMed] [Google Scholar]
- 4. Lamont KR, Tindall DJ. 2010. Androgen regulation of gene expression. Adv Cancer Res 107:137–162 [DOI] [PubMed] [Google Scholar]
- 5. Debes JD, Tindall DJ. 2002. The role of androgens and the androgen receptor in prostate cancer. Cancer Lett 187:1–7 [DOI] [PubMed] [Google Scholar]
- 6. Andreoiu M, Cheng L. 2010. Multifocal prostate cancer: biologic, prognostic, and therapeutic implications. Hum Pathol 41:781–793 [DOI] [PubMed] [Google Scholar]
- 7. Dehm SM, Tindall DJ. 2006. Molecular regulation of androgen action in prostate cancer. J Cell Biochem 99:333–344 [DOI] [PubMed] [Google Scholar]
- 8. Scher HI, Liebertz C, Kelly WK, Mazumdar M, Brett C, Schwartz L, Kolvenbag G, Shapiro L, Schwartz M. 1997. Bicalutamide for advanced prostate cancer: the natural versus treated history of disease. J Clin Oncol 15:2928–2938 [DOI] [PubMed] [Google Scholar]
- 9. Eisenberger MA, Blumenstein BA, Crawford ED, Miller G, McLeod DG, Loehrer PJ, Wilding G, Sears K, Culkin DJ, Thompson IM, Jr, Bueschen AJ, Lowe BA. 1998. Bilateral orchiectomy with or without flutamide for metastatic prostate cancer. N Engl J Med 339:1036–1042 [DOI] [PubMed] [Google Scholar]
- 10. Bennett NC, Gardiner RA, Hooper JD, Johnson DW, Gobe GC. 2010. Molecular cell biology of androgen receptor signalling. Int J Biochem Cell Biol 42:813–827 [DOI] [PubMed] [Google Scholar]
- 11. Foradori CD, Weiser MJ, Handa RJ. 2008. Non-genomic actions of androgens. Front Neuroendocrinol 29:169–181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Papadopoulou N, Papakonstanti EA, Kallergi G, Alevizopoulos K, Stournaras C. 2009. Membrane androgen receptor activation in prostate and breast tumor cells: molecular signaling and clinical impact. IUBMB Life 61:56–61 [DOI] [PubMed] [Google Scholar]
- 13. Blaszczyk N, Masri BA, Mawji NR, Ueda T, McAlinden G, Duncan CP, Bruchovsky N, Schweikert HU, Schnabel D, Jones EC, Sadar MD. 2004. Osteoblast-derived factors induce androgen-independent proliferation and expression of prostate-specific antigen in human prostate cancer cells. Clin Cancer Res 10:1860–1869 [DOI] [PubMed] [Google Scholar]
- 14. Culig Z, Bartsch G. 2006. Androgen axis in prostate cancer. J Cell Biochem 99:373–381 [DOI] [PubMed] [Google Scholar]
- 15. Culig Z, Steiner H, Bartsch G, Hobisch A. 2005. Interleukin-6 regulation of prostate cancer cell growth. J Cell Biochem 95:497–505 [DOI] [PubMed] [Google Scholar]
- 16. Singh RK, Sudhakar A, Lokeshwar BL. 2010. Role of chemokines and chemokine receptors in prostate cancer development and progression. J Cancer Sci Ther 2:89–94 [DOI] [PubMed] [Google Scholar]
- 17. Shariat SF, Andrews B, Kattan MW, Kim J, Wheeler TM, Slawin KM. 2001. Plasma levels of interleukin-6 and its soluble receptor are associated with prostate cancer progression and metastasis. Urology 58:1008–1015 [DOI] [PubMed] [Google Scholar]
- 18. Ishiguro H, Akimoto K, Nagashima Y, Kojima Y, Sasaki T, Ishiguro-Imagawa Y, Nakaigawa N, Ohno S, Kubota Y, Uemura H. 2009. aPKCγ/ι promotes growth of prostate cancer cells in an autocrine manner through transcriptional activation of interleukin-6. Proc Natl Acad Sci USA 106:16369–16374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hobisch A, Eder IE, Putz T, Horninger W, Bartsch G, Klocker H, Culig Z. 1998. Interleukin-6 regulates prostate-specific protein expression in prostate carcinoma cells by activation of the androgen receptor. Cancer Res 58:4640–4645 [PubMed] [Google Scholar]
- 20. Malinowska K, Neuwirt H, Cavarretta IT, Bektic J, Steiner H, Dietrich H, Moser PL, Fuchs D, Hobisch A, Culig Z. 2009. Interleukin-6 stimulation of growth of prostate cancer in vitro and in vivo through activation of the androgen receptor. Endocr Relat Cancer 16:155–169 [DOI] [PubMed] [Google Scholar]
- 21. Jia L, Choong CS, Ricciardelli C, Kim J, Tilley WD, Coetzee GA. 2004. Androgen receptor signaling: mechanism of interleukin-6 inhibition. Cancer Res 64:2619–2626 [DOI] [PubMed] [Google Scholar]
- 22. Debes JD, Comuzzi B, Schmidt LJ, Dehm SM, Culig Z, Tindall DJ. 2005. p300 regulates androgen receptor-independent expression of prostate-specific antigen in prostate cancer cells treated chronically with interleukin-6. Cancer Res 65:5965–5973 [DOI] [PubMed] [Google Scholar]
- 23. Ueda T, Bruchovsky N, Sadar MD. 2002. Activation of the androgen receptor N-terminal domain by interleukin-6 via MAPK and STAT3 signal transduction pathways. J Biol Chem 277:7076–7085 [DOI] [PubMed] [Google Scholar]
- 24. Aaronson DS, Muller M, Neves SR, Chung WC, Jayaram G, Iyengar R, Ram PT. 2007. An androgen-IL-6-Stat3 autocrine loop re-routes EGF signal in prostate cancer cells. Mol Cell Endocrinol 270:50–56 [DOI] [PubMed] [Google Scholar]
- 25. Gazi MH, Gong A, Donkena KV, Young CYF. 2007. Sodium selenite inhibits interleukin-6-mediated androgen receptor activation in prostate cancer cells via upregulation of c-Jun. Clin Chim Acta 380:145–150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Qiu Y, Ravi L, Kung HJ. 1998. Requirement of ErbB2 for signalling by interleukin-6 in prostate carcinoma cells. Nature 393:83–85 [DOI] [PubMed] [Google Scholar]
- 27. Guo Z, Dai B, Jiang T, Xu K, Xie Y, Kim O, Nesheiwat I, Kong X, Melamed J, Handratta VD, Njar VC, Brodie AM, Yu LR, Veenstra TD, Chen H, Qiu Y. 2006. Regulation of androgen receptor activity by tyrosine phosphorylation. Cancer Cell 10:309–319 [DOI] [PubMed] [Google Scholar]
- 28. Liu Y, Karaca M, Zhang Z, Gioeli D, Earp HS, Whang YE. 2010. Dasatinib inhibits site-specific tyrosine phosphorylation of androgen receptor by Ack1 and Src kinases. Oncogene 29:3208–3216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ueda T, Mawji NR, Bruchovsky N, Sadar MD. 2002. Ligand-independent activation of the androgen receptor by interleukin-6 and the role of steroid receptor coactivator-1 in prostate cancer cells. J Biol Chem 277:38087–38094 [DOI] [PubMed] [Google Scholar]
- 30. Seaton A, Scullin P, Maxwell PJ, Wilson C, Pettigrew J, Gallagher R, O'Sullivan JM, Johnston PG, Waugh DJJ. 2008. Interleukin-8 signaling promotes androgen-independent proliferation of prostate cancer cells via induction of androgen receptor expression and activation. Carcinogenesis 29:1148–1156 [DOI] [PubMed] [Google Scholar]
- 31. Rajarubendra N, Lawrentschuk N, Bolton DM, Klotz L, Davis ID. 10 November 2010. Prostate cancer immunology: an update for urologists. BJU Int 10.1111/j.1464-410X.2010.09820.x [DOI] [PubMed] [Google Scholar]
- 32. Sutcliffe S, Platz EA. 2008. Inflammation and prostate cancer: a focus on infections. Curr Urol Rep 9:243–249 [DOI] [PubMed] [Google Scholar]
- 33. Zhu ML, Kyprianou N. 2008. Androgen receptor and growth factor signaling cross-talk in prostate cancer cells. Endocr Relat Cancer 15:841–849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lemmon MA, Schlessinger J. 2010. Cell signaling by receptor tyrosine kinases. Cell 141:1117–1134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Culig Z, Hobisch A, Cronauer MV, Radmayr C, Trapman J, Hittmair A, Bartsch G, Klocker H. 1994. Androgen receptor activation in prostatic tumor cell lines by insulin-like growth factor-I, keratinocyte growth factor, and epidermal growth factor. Cancer Res 54:5474–5478 [PubMed] [Google Scholar]
- 36. Stavridi F, Karapanagiotou EM, Syrigos KN. 2010. Targeted therapeutic approaches for hormone-refractory prostate cancer. Cancer Treat Rev 36:122–130 [DOI] [PubMed] [Google Scholar]
- 37. Wu JD, Haugk K, Woodke L, Nelson P, Coleman I, Plymate SR. 2006. Interaction of IGF signaling and the androgen receptor in prostate cancer progression. J Cell Biochem 99:392–401 [DOI] [PubMed] [Google Scholar]
- 38. Komiya A, Suzuki H, Imamoto T, Kamiya N, Nihei N, Naya Y, Ichikawa T, Fuse H. 2009. Neuroendocrine differentiation in the progression of prostate cancer. Int J Urol 16:37–44 [DOI] [PubMed] [Google Scholar]
- 39. Wright ME, Tsai MJ, Aebersold R. 2003. Androgen receptor represses the neuroendocrine transdifferentiation process in prostate cancer cells. Mol Endocrinol 17:1726–1737 [DOI] [PubMed] [Google Scholar]
- 40. Yang JC, Ok JH, Busby JE, Borowsky AD, Kung HJ, Evans CP. 2009. Aberrant activation of androgen receptor in a new neuropeptide- autocrine model of androgen-insensitive prostate cancer. Cancer Res 69:151–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Manning G, Whyte DB, Martinez R, Hunter T, Sudarsanam S. 2002. The protein kinase complement of the human genome. Science 298:1912–1934 [DOI] [PubMed] [Google Scholar]
- 42. Blume-Jensen P, Hunter T. 2001. Oncogenic kinase signalling. Nature 411:355–365 [DOI] [PubMed] [Google Scholar]
- 43. Kraus S, Gioeli D, Vomastek T, Gordon V, Weber MJ. 2006. Receptor for activated C kinase 1 (RACK1) and Src regulate the tyrosine phosphorylation and function of the androgen receptor. Cancer Res 66:11047–11054 [DOI] [PubMed] [Google Scholar]
- 44. Asim M, Siddiqui IA, Hafeez BB, Baniahmad A, Mukhtar H. 2008. Src kinase potentiates androgen receptor transactivation function and invasion of androgen-independent prostate cancer C4-2 cells. Oncogene 27:3596–3604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Agoulnik IU, Bingman WE, 3rd, Nakka M, Li W, Wang Q, Liu XS, Brown M, Weigel NL. 2008. Target gene-specific regulation of androgen receptor activity by p42/p44 mitogen-activated protein kinase. Mol Endocrinol 22:2420–2432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Lyons LS, Rao S, Balkan W, Faysal J, Maiorino CA, Burnstein KL. 2008. Ligand-independent activation of androgen receptors by Rho GTPase signaling in prostate cancer. Mol Endocrinol 22:597–608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Sarker D, Reid AH, Yap TA, de Bono JS. 2009. Targeting the PI3K/AKT pathway for the treatment of prostate cancer. Clin Cancer Res 15:4799–4805 [DOI] [PubMed] [Google Scholar]
- 48. Suzuki H, Freije D, Nusskern DR, Okami K, Cairns P, Sidransky D, Isaacs WB, Bova GS. 1998. Interfocal heterogeneity of PTEN/MMAC1 gene alterations in multiple metastatic prostate cancer tissues. Cancer Res 58:204–209 [PubMed] [Google Scholar]
- 49. Lin HK, Yeh S, Kang HY, Chang C. 2001. Akt suppresses androgen-induced apoptosis by phosphorylating and inhibiting androgen receptor. Proc Natl Acad Sci USA 98:7200–7205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Mellinghoff IK, Vivanco I, Kwon A, Tran C, Wongvipat J, Sawyers CL. 2004. HER2/neu kinase-dependent modulation of androgen receptor function through effects on DNA binding and stability. Cancer Cell 6:517–527 [DOI] [PubMed] [Google Scholar]
- 51. Wen Y, Hu MC, Makino K, Spohn B, Bartholomeusz G, Yan DH, Hung MC. 2000. HER-2/neu promotes androgen-independent survival and growth of prostate cancer cells through the Akt pathway. Cancer Res 60:6841–6845 [PubMed] [Google Scholar]
- 52. Liu P, Li S, Gan L, Kao TP, Huang H. 2008. A transcription-independent function of FOXO1 in inhibition of androgen-independent activation of the androgen receptor in prostate cancer cells. Cancer Res 68:10290–10299 [DOI] [PubMed] [Google Scholar]
- 53. Jin RJ, Lho Y, Connelly L, Wang Y, Yu X, Saint Jean L, Case TC, Ellwood-Yen K, Sawyers CL, Bhowmick NA, Blackwell TS, Yull FE, Matusik RJ. 2008. The nuclear factor-κB pathway controls the progression of prostate cancer to androgen-independent growth. Cancer Res 68:6762–6769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Nadiminty N, Lou W, Sun M, Chen J, Yue J, Kung HJ, Evans CP, Zhou Q, Gao AC. 2010. Aberrant activation of the androgen receptor by NF-κB2/p52 in prostate cancer cells. Cancer Res 70:3309–3319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Nazareth LV, Weigel NL. 1996. Activation of the human androgen receptor through a protein kinase A signaling pathway. J Biol Chem 271:19900–19907 [DOI] [PubMed] [Google Scholar]
- 56. Sadar MD. 1999. Androgen-independent induction of prostate-specific antigen gene expression via cross-talk between the androgen receptor and protein kinase A signal transduction pathways. J Biol Chem 274:7777–7783 [DOI] [PubMed] [Google Scholar]
- 57. Shu SK, Liu Q, Coppola D, Cheng JQ. 2010. Phosphorylation and activation of androgen receptor by Aurora-A. J Biol Chem 285:33045–33053 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 58. Mahajan K, Mahajan NP. 2010. Shepherding AKT and androgen receptor by Ack1 tyrosine kinase. J Cell Physiol 224:327–333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Mahajan NP, Liu Y, Majumder S, Warren MR, Parker CE, Mohler JL, Earp HS, Whang YE. 2007. Activated Cdc42-associated kinase Ack1 promotes prostate cancer progression via androgen receptor tyrosine phosphorylation. Proc Natl Acad Sci USA 104:8438–8443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Mahajan K, Challa S, Coppola D, Lawrence H, Luo Y, Gevariya H, Zhu W, Chen YA, Lawrence NJ, Mahajan NP. 2010. Effect of Ack1 tyrosine kinase inhibitor on ligand-independent androgen receptor activity. Prostate 70:1274–1285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Heemers HV, Tindall DJ. 2007. Androgen receptor (AR) coregulators: a diversity of functions converging on and regulating the AR transcriptional complex. Endocr Rev 28:778–808 [DOI] [PubMed] [Google Scholar]
- 62. Georget V, Térouanne B, Nicolas JC, Sultan C. 2002. Mechanism of antiandrogen action: key role of Hsp90 in conformational change and transcriptional activity of the androgen receptor. Biochemistry 41:11824–11831 [DOI] [PubMed] [Google Scholar]
- 63. Ai J, Wang Y, Dar JA, Liu J, Liu L, Nelson JB, Wang Z. 2009. HDAC6 regulates androgen receptor hypersensitivity and nuclear localization via modulating Hsp90 acetylation in castration-resistant prostate cancer. Mol Endocrinol 23:1963–1972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Ni L, Yang CS, Gioeli D, Frierson H, Toft DO, Paschal BM. 2010. FKBP51 promotes assembly of the Hsp90 chaperone complex and regulates androgen receptor signaling in prostate cancer cells. Mol Cell Biol 30:1243–1253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Gaughan L, Stockley J, Wang N, McCracken SR, Treumann A, Armstrong K, Shaheen F, Watt K, McEwan IJ, Wang C, Pestell RG, Robson CN. 2011. Regulation of the androgen receptor by SET9-mediated methylation. Nucleic Acids Research 39:1266–1279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Claessens F, Denayer S, Van Tilborgh N, Kerkhofs S, Helsen C, Haelens A. 2008. Diverse roles of androgen receptor (AR) domains in AR-mediated signaling. Nucl Recept Signal 6:e008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Dehm SM, Regan KM, Schmidt LJ, Tindall DJ. 2007. Selective role of an NH2-terminal WxxLF motif for aberrant androgen receptor activation in androgen depletion-independent prostate cancer cells. Cancer Res 67:10067–10077 [DOI] [PubMed] [Google Scholar]
- 68. Callewaert L, Van Tilborgh N, Claessens F. 2006. Interplay between two hormone-independent activation domains in the androgen receptor. Cancer Res 66:543–553 [DOI] [PubMed] [Google Scholar]
- 69. Quayle SN, Mawji NR, Wang J, Sadar MD. 2007. Androgen receptor decoy molecules block the growth of prostate cancer. Proc Natl Acad Sci USA 104:1331–1336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Kaku N, Matsuda K-i, Tsujimura A, Kawata M. 2008. Characterization of nuclear import of the domain-specific androgen receptor in association with the importin α/β and Ran-guanosine 5′-triphosphate systems. Endocrinology 149:3960–3969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Brooke GN, Bevan CL. 2009. The role of androgen receptor mutations in prostate cancer progression. Curr Genomics 10:18–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Culig Z, Klocker H, Bartsch G, Hobisch A. 2001. Androgen receptor mutations in carcinoma of the prostate: significance for endocrine therapy. Am J Pharmacogenomics 1:241–249 [DOI] [PubMed] [Google Scholar]
- 73. Brooke GN, Parker MG, Bevan CL. 2008. Mechanisms of androgen receptor activation in advanced prostate cancer: differential co-activator recruitment and gene expression. Oncogene 27:2941–2950 [DOI] [PubMed] [Google Scholar]
- 74. Dehm SM, Schmidt LJ, Heemers HV, Vessella RL, Tindall DJ. 2008. Splicing of a novel androgen receptor exon generates a constitutively active androgen receptor that mediates prostate cancer therapy resistance. Cancer Res 68:5469–5477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Hu R, Dunn TA, Wei S, Isharwal S, Veltri RW, Humphreys E, Han M, Partin AW, Vessella RL, Isaacs WB, Bova GS, Luo J. 2009. Ligand-independent androgen receptor variants derived from splicing of cryptic exons signify hormone-refractory prostate cancer. Cancer Res 69:16–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Sun S, Sprenger CC, Vessella RL, Haugk K, Soriano K, Mostaghel EA, Page ST, Coleman IM, Nguyen HM, Sun H, Nelson PS, Plymate SR. 2010. Castration resistance in human prostate cancer is conferred by a frequently occurring androgen receptor splice variant. J Clin Invest 120:2715–2730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Watson PA, Chen YF, Balbas MD, Wongvipat J, Socci ND, Viale A, Kim K, Sawyers CL. 2010. Constitutively active androgen receptor splice variants expressed in castration-resistant prostate cancer require full-length androgen receptor. Proc Natl Acad Sci USA 107:16759–16765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Guo Z, Yang X, Sun F, Jiang R, Linn DE, Chen H, Chen H, Kong X, Melamed J, Tepper CG, Kung HJ, Brodie AM, Edwards J, Qiu Y. 2009. A novel androgen receptor splice variant is up-regulated during prostate cancer progression and promotes androgen depletion-resistant growth. Cancer Res 69:2305–2313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Nacusi LP, Tindall DJ. 2009. Androgen receptor abnormalities in castration-recurrent prostate cancer. Expert Rev Endocrinol Metab 4:417–422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Andersen RJ, Mawji NR, Wang J, Wang G, Haile S, Myung JK, Watt K, Tam T, Yang YC, Bañuelos CA, Williams DE, McEwan IJ, Wang Y, Sadar MD. 2010. Regression of castrate-recurrent prostate cancer by a small-molecule inhibitor of the amino-terminus domain of the androgen receptor. Cancer Cell 17:535–546 [DOI] [PubMed] [Google Scholar]
- 81. Tran C, Ouk S, Clegg NJ, Chen Y, Watson PA, Arora V, Wongvipat J, Smith-Jones PM, Yoo D, Kwon A, Wasielewska T, Welsbie D, Chen CD, Higano CS, Beer TM, Hung DT, Scher HI, Jung ME, Sawyers CL. 2009. Development of a second-generation antiandrogen for treatment of advanced prostate cancer. Science 324:787–790 [DOI] [PMC free article] [PubMed] [Google Scholar]