Basal cells positive for LEF1, on WNT/β-catenin pathway, are not dependent upon androgen during fetal prostate development. WNT/Lef1-positive basal progenitors can repopulate the luminal compartment.
Abstract
Lymphoid enhancer-binding factor (LEF)1 is a major mediator and a target in canonical Wnt/β-catenin pathway. Interactions between the androgen receptor (AR) and canonical Wnt pathways have been implicated in the development of the genitourinary organs. Here, we investigated the localization and role of LEF1-positive cells during development of the prostate gland in human and in the murine model. We show that during human prostate development, LEF1 is restricted to the basal epithelial layer of the urogenital sinus. During mouse development, Lef1 is also present in the urogenital mesenchyme in addition to the basal epithelial layer of the urogenital sinus. In the course of elongation and branching of the prostatic ducts, Lef1 is localized to the proliferating epithelium at the distal tips of the buds. Notably, during branching morphogenesis, domains of Lef1 and AR are mutually exclusive. We further employed the TOPGAL reporter strain to examine the dynamics of Wnt signaling in the context of prostate regression upon a 7-d treatment with a competitive AR inhibitor, bicalutamide. We found that Wnt/Lef1-positive basal cells are not dependent upon androgen for survival. Furthermore, upon bicalutamide treatment, Wnt/Lef1-positive basal progenitors repopulated the luminal compartment. We conclude that Wnt/Lef1 activity identifies an androgen-independent population of prostate progenitors, which is important for embryonic development and organ maintenance and regeneration in the adult.
The prostate is a male-specific sex-accessory exocrine gland. The prostate develops during embryogenesis from the epithelium and mesenchyme of the urogenital sinus (UGS) (1, 2). Prostate buds evaginate at embryonic day (E)16.5 in the mouse at distinct dorso-ventral domains of the UGS (1, 3). With increasing gestational age, the prostatic ducts elongate into the UGS mesenchyme and undergo branching morphogenesis, which continues postnatally (4–7). After postnatal day (P)5 in the mouse, the prostate epithelium is compartmentalized into the basal compartment expressing cytokeratin (CK)5 and CK14 and the luminal compartment expressing CK8 and CK18. Subsequently, with a surge of androgens at puberty, the luminal compartment terminally differentiates into a prostate-specific secretory epithelium (1). Both the murine and human fetal prostates develop by a similar process of branching morphogenesis (8). However, there are some key differences between the prostates of humans and the mouse. Firstly, the mouse prostate is divided into anatomically distinct lobes, anterior, ventral, dorsal, and lateral, which are separate entities that remain recognizable into adulthood (9–11). In contrast, the adult human prostate is described as consisting of zones, defined by their location in the gland, which are morphologically indistinguishable but vary in their secretory properties and predisposition to benign and malignant tumors (12–14). Secondly, the adult mouse prostate gland contains comparatively less fibroblast stroma and more smooth muscle tissue than the human gland (15–17). Studies by us and others show that morphogenesis of the prostate gland is directed by several developmental ligands: sonic hedgehog (18), fibroblast growth factor 10 (19), bone morphogenetic proteins 4 and 7 (3, 20), and activity of the Notch-Delta cell fate selection system (3, 21, 22). Recent studies also suggest an important function for canonical Wnt/β-catenin signaling in maintaining prostate growth in androgen-dependent (23) and in androgen-independent conditions (24, 25). Prostate growth, cell survival, and differentiation are also dependent on signaling by the androgen receptor (AR) in response to testosterone secreted by the male testis. Specifically, it has been proposed that a testosterone derivative, dihydrotestosterone, binds to the AR, primarily in the UGS mesenchyme (26–28), and its effect on the prostate epithelium is mediated by a paracrine mechanism. Mesenchymally expressed fibroblast growth factor 7 and fibroblast growth factor 10 have been suggested to mediate androgen-dependent prostate growth (29, 30). Regulation of AR expression and signaling is complex, involving positive and negative control by multiple processes (31). In particular, we have previously reported that lymphoid enhancer-binding factor (LEF)1 can regulate AR expression in prostate cancer (32).
Canonical Wnt signaling is mediated by a family of T-cell factor (TCF)/LEF1 proteins encoded by the LEF1 and Tcf1–Tcf4 genes in the mouse and human (33). Upon activation of canonical Wnt signaling, the nuclear localized β-catenin binds to a LEF/TCF factor, and together, they activate transcription of Wnt target genes (34–37). LEF1 is also a transcriptional target of canonical Wnt signaling and, thus, a component of a positive feedback loop in the canonical Wnt signaling network. LEF1/β-catenin signaling has been shown to function during hair bud formation (38) and during development and cancer of the mammary gland (39, 40). In addition, LEF1 has been implicated in prostate tumorigenesis (3, 41–45). Recent studies also indicate an interaction between LEF1 and AR in bone maintenance (46). Because AR is essential for growth, differentiation, and survival of the prostate gland, we decided to investigate the interactions between AR and Wnt/Lef1 signaling during embryonic and postnatal prostate development and in the adult organ. Interestingly, we found that the Wnt/Lef1 pathway functions to promote prostate growth independently of AR signaling. In particular, our studies show that Wnt/Lef1 activity identifies a population of basal progenitors with their survival, proliferation, and differentiation independent of AR signaling.
Results
LEF1 and AR designate distinct cell populations in the human fetal prostate
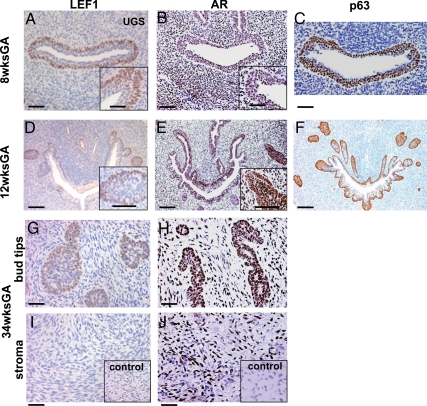
Because little is known of Wnt activity in human prostate development, we first examined localization of LEF1 protein in human fetal prostate. In total, 19 human fetal prostates were collected from 9 to 42 wk of gestation, ranging from first to third trimesters. Prostates were formalin fixed and embedded in paraffin. Distribution of LEF1 was analyzed by immunohistochemistry (Fig. 1, A, D, G, and I). We found that LEF1 protein is strongly expressed in the basal layer of the UGS epithelium (Fig. 1A) and colocalized with the p63-positive basal progenitor population (Fig. 1C). At the initiation of prostate budding, strong AR expression was detected in the luminal epithelium of the UGS and in the urogenital mesenchyme (Fig. 1B). There was negligible AR expression in the basal urogenital epithelium positive for LEF1 (Fig. 1A compared with Fig. 1B). At 12 wk of gestation, prostatic epithelium first appeared as solid epithelial buds emerging from the UGS (Fig. 1D). At this stage, strong LEF1 was detected at the edges of the prostate buds (Fig. 1, D and inset) and colocalized with the p63-positive basal population (Fig. 1F). We also detected LEF1, at low levels, in the canalized epithelial ducts (Fig. 1D). In contrast, at 12 wk, AR was detected throughout the UGS, prostate epithelium, and stroma (Fig. 1E). During prostatic branching at 34 wk, LEF1 was consistently localized to the prostate tips (Fig. 1G). No LEF1 was detected in the prostatic stroma (Fig. 1I). In contrast, AR was strongly expressed throughout the prostate epithelium (Fig. 1H) and stroma (Fig. 1J).
Fig. 1.
LEF1 activity in the human embryonic prostate. Sections of the human embryonic UGS at 8 wk of gestational age (GA) (A–C), 12-wk GA (D–F), and 34-wk GA (G–J) were immunolabeled for LEF1 (A and inset, D and inset, G, and I), AR (B and inset, E and inset, H, and J), and p63 (C and F). Dorsal side is up. IgG negative control is included as insets in I and J. Scale bars: 100 μm (all panels), 50 μm (insets in A, B, D, and E).
Lef1 colocalizes with p63 and Ki67-positive (Ki67+) basal epithelium in the embryonic murine prostates
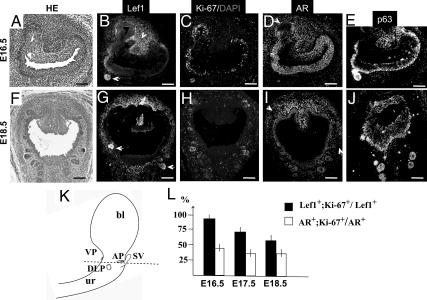
To analyze Lef1 function during embryonic and postnatal prostate development, we employed a murine model. At the initiation of murine prostate formation, at E16.5 (of 19.3–19.6 d of mouse gestation), Lef1 was localized to the nuclei in the developing prostate buds, the basal UGS epithelium, and to the dorsal urogenital mesenchyme (Fig. 2B, arrowhead). During elongation of the prostatic ducts at E17.5 (Supplemental Fig. 1C, published on The Endocrine Society's Journals Online web site at http://mend.endojournals.org) and E18.5 (Fig. 2G), Lef1 persisted in the prostate tips, in the basal UGS epithelium, and in the periepithelial mesenchyme (Fig. 2G and Supplemental Fig. 1C). We further confirmed that during fetal development of the mouse, as in human (Fig. 1), Lef1-positive (Lef1+) epithelium coincided with the p63-positive basal population (Fig. 2, B, E, G, and J, and Supplemental Fig. 1, C and F). At all embryonic stages, AR was detected in the UGS epithelium and mesenchyme (Fig. 2, D and I, and Supplemental Fig. 1E). Notably, AR expression was not detected in the Lef1+ buds (Fig. 2, I and G, and Supplemental Fig. 1, E and C). We further examined the proliferative properties of Lef1+ and AR-positive (AR+) epithelial populations by labeling adjacent prostate sections with the cell cycle marker, Ki67 (Fig. 2, B–D and G–I), and calculated the percentages of Lef1+/Ki67+ and AR+/Ki67+ cells (Fig. 2L). We found that Lef1+ cells presented a population with high density of cell divisions (Fig. 2L). At E16.5, 89.5 ± 3.2% of Lef1+ cells were Ki67+; at E17.5, 67.8 ± 2.8% of Lef1+ cells were Ki67+; and at E18.5, 56.3 ±3.7% of prostate epithelium were Lef1+/Ki67+ (Fig. 2L). In contrast, only 43.7 ± 2.1% of AR+ cells were proliferative at E16.5 (Fig. 2L), and the portion of AR+ dividing cells decreased to 33.6 ±2.6% at E17.5 and E18.5 (Fig. 2L). Thus, throughout embryonic development, the rates of cell proliferation in the Lef1+ prostate epithelium were significantly higher than in the AR+ epithelial compartment.
Fig. 2.
Localization of Lef1, AR, p63, and Ki67 in the embryonic murine prostate. Transverse sections of the embryonic UGS at E16.5 (A–E) and E18.5 (F–J) stained with eosin and hematoxylin (HE) (A and F) and labeled for Lef1 (B and G), Ki67 (C and H), AR (D and I), p63 (E and J), and the nuclei stained with 4′,6-diamidino-2-phenylindole (DAPI, C and H). In the emerging prostate buds and distal tips, Lef1 is up-regulated (B and G, arrows), and AR is down-regulated (D and I, arrows). Lef1 expression is also present in the dorsal UGS mesenchyme at E16.5 (arrowhead) and in all pereipithelial mesenchyme at E18.5 (G). Scale bars, 100 μm. K, Schematic representation of the embryonic UGS. bl, Bladder; ur, urethra; SV, seminal vesicles; AP, anterior prostate buds; DLP, dorso-lateral prostate buds; VP, ventral prostate buds. The dashed line indicates the plane of transverse sections in A–J. L, Graphic presentation of the relative percentage ratios of proliferating cells positive for Lef1 (Lef1+;Ki67+/Lef1+, black bar) and AR (AR+;Ki67+/AR+, white bar) in the embryonic prostate epithelium. P < 0.05. See Supplemental Fig. 1 for E17.5 UGS sections labeled for Lef1 and Ki67.
Lef1 persists in a subset of CK14+/CK5+ epithelium during postnatal prostate branching and differentiation
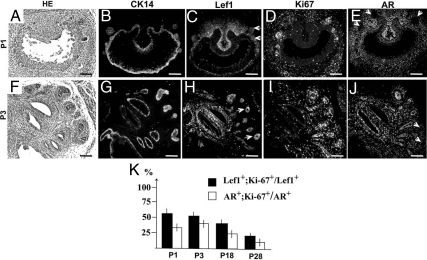
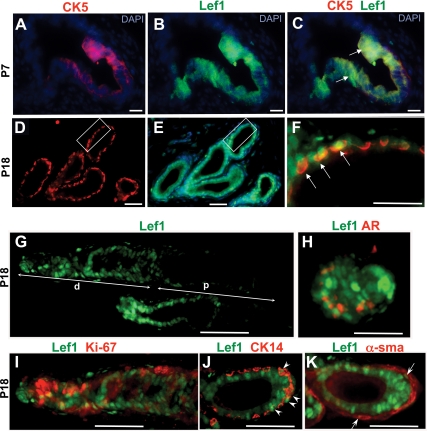
We further examined Lef1 localization from P1 to P28. From P1 to P5, murine prostate undergoes extensive branching and forms canalized prostate ducts. During branching, Lef1 was localized to the distal prostate tips (Fig. 3, C and H). Interestingly, substantial Lef1 expression persisted in the murine urogenital mesenchyme (Fig. 3, C and H). In the postnatal and young adult prostates, Lef1+ epithelium represented a fraction of the basal epithelium positive for CK14+ (Fig. 3, B, C, G, and H, and Fig. 4J). Lef1+ epithelium also highly colocalized with CK5 (Fig. 4, C and F).
Fig. 3.
Localization of Lef1 during postnatal prostate development. Sections of postnatal murine prostates at P1 (A–E) and P3 (F–J) stained with eosin and hematoxylin (A and F) and immunolabeled for CK14 (B and G), Lef1 (C and H), Ki67 (D and I), and AR (E and J). Scale bars, 100 μm. K, Graphic presentation of the percentage ratios of Lef1+;Ki67+/Lef1+ (black bar) and AR+;Ki67+/AR+ cells (white bar) during postnatal prostate development. P < 0.05. See Fig. 4 and Supplemental Fig. 2 for Lef1 and Ki67-labeled sections in P18 and P28 prostate.
Fig. 4.
Localization of Lef1 in differentiating prostate at P7 and P18. A–F, Immunolabeling of dorsal prostate sections at P7 and P18 for CK5 (A, C, D, and F) and Lef1 (B, C, E, and F) shows colocalization in the prostate epithelium (arrows in C and F). DAPI, 4′,6-diamidino-2-phenylindole. C, Overlay of adjacent sections in A and B. D, Overlay of the framed area on adjacent sections in D and E shown at higher resolution. G–K, Sections of P18 anterior prostate labeled for Lef1 and overlaid with adjacent sections labeled for AR (H), Ki67 (I), CK14 (J), and smooth muscle α-actin (α-sma) (K). G, High levels of Lef1 are present in the distal (d) ducts and tips. Interspersed Lef1+ cells are detected in the proximal (p) ducts. H, Most cells at the bud tips are Lef1-positive (green) and AR-negative (red). I, Lef1+ epithelium shows high percentage of Ki67-positive cells. J and K, Lef1 localized to CK14+ epithelium is pointed with arrowheads (J) and to the smooth muscle cells with arrows (K). Scale bars: 30 μm (A–F) and 100 μm (G–K).
Lef1 marks the dividing cell population in the postnatal prostate
Immunolabeling for Ki67 showed that 53.9 ± 2.0% of Lef1+ prostate epithelial cells at P1 (Fig. 3, C, D, and K), and 51.0 ± 1.7%, at P3 (Fig. 3, H, I, and K), are in active cell cycle. In contrast, only 30.7 ± 3.5% of AR+ epithelial cells were positive for Ki67 at P1 (Fig. 3, D, E, and K), and 36.4 ± 2.9% at P3 (Fig. 3, I–K). Starting at P5, the epithelium of the prostate gland undergoes differentiation into the luminal and basal compartments. Basal prostate epithelium is characterized by expression of CK5 and CK14, whereas luminal epithelium expresses CK8 and CK18 (1). Analysis of murine prostate at P18 (Fig. 4) and P28 (Supplemental Fig. 2) showed persistent Lef1 activity in a subset of proliferating basal epithelium (Fig. 4, D–F and I) and in a few smooth muscle cells (Fig. 4K). In the prostate epithelium, 38.4 ± 3.1% of Lef1+ cells continued cell divisions at P18 (Figs. 3K and 4K) and 20.5 ± 2.8% at P28 (Fig. 3K and Supplemental Fig. 2E). In contrast, only 23.7 ± 1.6% of AR+ epithelial cells were also Ki67+ at P18 (Figs. 3K and 4, H and I), and only 11 ± 3% were Ki67+ at P28 (Fig. 3K and Supplemental Fig. 2, D and E). In young adult prostates, the rate of cell divisions in the Lef1+ epithelium was 2-fold higher than in the AR-responsive cells (P < 0.05) (Fig. 3K).
Upon androgen-dependent prostate regression, Wnt/Lef1+ cells repopulate the luminal prostate compartment
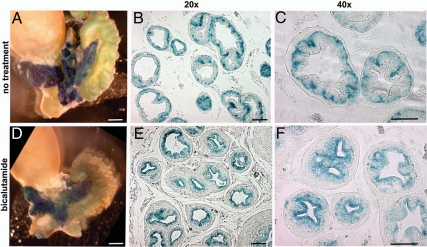
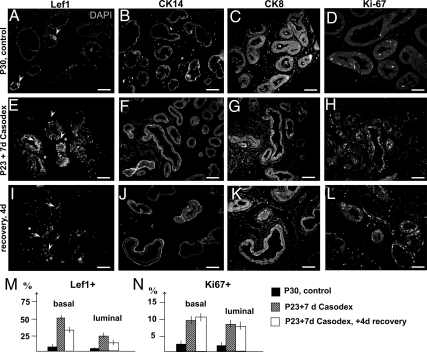
To determine the role of canonical Wnt signaling during the AR-dependent prostate growth and differentiation at puberty, we employed the mouse reporter strain for canonical Wnt signaling, TOPGAL (Fig. 5). TOPGAL is a transgenic strain made by an insertion of the bacterial β-galactosidase gene preceded by three Lef/TCF binding sites into the Rosa26 locus (47). In TOPGAL mice, binding of the Lef1/β-catenin complex to the transgene reporter results in TOPGAL activity (i.e. β-galactosidase activity), which is easily identified by X-gal staining (Fig. 5). Examination of the control P30 TOPGAL prostates revealed normal TOPGAL activity in all prostate lobes and a particularly strong X-gal staining at the prostatic tips (Fig. 5A). Further analysis of sections revealed that in untreated P30 prostates, TOPGAL activity and Lef1 protein are localized to the basal epithelium (Figs. 5, B and C, and 6, A and B). We further determined that fate of TOPGAL-positive (Fig. 5) and Lef1+ cells (Fig. 6) upon prostate involution due to inhibition of AR signaling by a competitive AR inhibitor, bicalutamide (Casodex; AstraZeneca, Newark, NJ). We analyzed TOPGAL activity (Fig. 5, D–F) and distribution of Lef1 (Fig. 6E) in prostates from TOPGAL males treated for 7 d with bicalutamide administered at 30 mg/kg·d in drinking water. Bicalutamide treatment resulted in a 42 ± 3% decrease in prostate mass (Fig. 5D compared with Fig. 5A). Notably, TOPGAL activity persisted in the remaining prostate tissue (Fig. 5D). Interestingly, bicalutamide treatment resulted in a redistribution of the TOPGAL-positive cells from the basal (Fig. 5, B and C) to the luminal compartment (Fig. 5, E and F). Bicalutamide treatment also resulted in appearance of Lef1 protein in the luminal epithelium (Fig. 6E, arrow) and in a few cells in the periductal stroma (Fig. 6E, asterisk). We also noted an increase in the density of CK14+ basal epithelium in bicalutamide-treated (Fig. 6F) and recovering (Fig. 6J) prostates compared with the untreated controls (Fig. 6B). We further quantified the percentages of the Lef1+ and Ki67+ positive epithelial cells in the treated and untreated prostates (Fig. 6, M and N). We found that bicalutamide treatment resulted in a 73 ± 6% increase in Lef1+ epithelial cells, including a 48 ± 6% increase in the basal population, and a 25 ± 6% increase in the luminal population, which showed no Lef1+ cells before treatment (Fig. 6M, and Fig. 6E compared with Fig. 6A). Bicalutamide treatment also resulted in an increase in Ki67+ cells in the basal and luminal epithelium (Fig. 6N, and Fig. 6H compared with Fig. 6D). Only 3.0 ± 0.8% basal and 2.0 ± 0.5% luminal cells were positive for Ki67 in the untreated P30 prostates (Fig. 6, N and D). Bicalutamide treatment resulted in a 7 ± 3% increase in Ki67+ epithelium in the basal compartment and a 6 ± 1.1% increase in cell divisions in the luminal compartment (Fig. 6N). These results show that androgen-dependent prostate regression results in increased accumulation of Lef1 in the prostate epithelium, concomitant with increased cell proliferation. Interestingly, prostate involution resulted in appearance of Lef1 and TOPGAL activity in the luminal epithelium (Figs. 5, E and F, and 6E) positive for CK8 (Fig. 6G and Supplemental Fig. 3). This indicates that in the absence of androgen signaling, and subsequent prostate involution, Lef1+ basal progenitors are induced to repopulate the luminal compartment. We further examined distribution of Lef1 in the recovering prostates, 4 d after cessation of bicalutamide treatment (Fig. 6, I–N). We found that 4 d into recovery, Lef1+ population decreased by 33 ± 6% in the basal epithelium and by 21 ± 6% in the luminal epithelium (Fig. 6M), indicating a decrease in Wnt signaling. We found no significant difference in rates of cell proliferation in prostate immediately after treatment and 4 d into recovery (Fig. 6H, L and N).
Fig. 5.
Wnt activity in the normal and bicalutamide-treated adult prostates. A–C, Control prostate samples from P30 TOPGAL-untreated males. A, UGS tissues stained with X-gal in whole mount show strong LacZ activity in the prostate lobes. Prostate tips are indicated with arrowheads. B and C, Sections of untreated ventral prostate lobes at ×20 (A) and ×40 (B) magnification show LacZ-positive cells in the basal prostate epithelium. D–F, In contrast, bicalutamide-treated prostates show LacZ activity in the luminal epithelium. P23 TOPGAL males were given 30 mg/kg·d of Casodex (AstraZeneca) in drinking water for 7 d, then prostates were stained with X-gal in whole mount (D), embedded in paraffin, and sectioned (E and F). Scale bars, 50 μm.
Fig. 6.
Localization of Lef1+ cells in bicalutamide-treated prostates. A–K, Sections of ventral prostates lobes from P30 control-untreated males (A–D), male siblings treated for 7 d with Casodex (30 mg/kg·d in drinking water) starting at P23 (E–H), and male siblings treated with bicalutamide and analyzed after 4 d of recovery (I–L). Sections were immunolabeled for Lef1 (A, E, and I) and nuclei stained for 4′,6-diamidino-2-phenylindole (DAPI) (A); then for CK14 (B, F, and J), CK8 (C, G, and K), and Ki67 (D, H, and L). A, In untreated prostates, Lef1 localized to the basal epithelium (arrowhead). In Casodex-treated prostates (E and I), Lef1 was detected in the basal (arrowheads) and luminal (arrows) epithelium and also in periepithelial mesenchyme (asterisk). Scale bars, 100 μm. M and N, Percentages of the Lef1+ (M) and Ki67+ (N) cells in the control (black bar), treated (stripped bar), and recovering prostates (white bar). P < 0.05.
Discussion
Recent studies uncovered the importance of canonical Wnt signaling and the interactions between the Wnt and AR pathways during sexual differentiation of the urogenital organs, such as the urethra (48, 49). Complex relationships have also been observed between Wnt and AR signaling in normal prostate homeostasis and in prostate cancer (24, 32). The Wnt pathway can effectively promote prostate growth independent of AR signaling. In turn, nuclear β-catenin can facilitate AR signaling and modulate AR targets (25, 50–52).
In this study, we demonstrate that canonical Wnt signaling is active during development of the human and murine prostates. We show that LEF1 is present in the fetal prostate from the earliest stages of gland development. LEF1 was first detected before prostate bud formation, in the basal layer of the UGS epithelium in human and in the mouse. We show that LEF1 and AR expression are initiated in nonoverlapping compartments in the human UGS. Specifically, AR expression is initiated in the luminally positioned cells in the human UGS. Furthermore, during prostate formation and branching, LEF1 is localized to the tips of the prostatic buds and the periphery of the solid prostatic ducts. It is an intriguing finding that during prostate growth, proliferating cells are not only localizing to the prostate tips but are also to the periphery of the solid prostatic ducts, suggesting a role in ductal morphogenesis. In contrast, we detected only few proliferating cells within the internal epithelium of the canalized ducts, suggesting that this epithelium is replenished by migration from the peripheral layers. Further studies are needed to determine the roles of the canonical and, possibly, noncanonical Wnt pathways in these processes.
We show that during murine prostate development, Lef1 defines a subpopulation of basal epithelial cells positive for p63, CK14, and CK5, the latter a putative progenitor/stem cell marker in the mammary and submandibular glands (53, 54). Interestingly, we detected that a substantial, 12% Lef1+ cell population persists in adult murine prostates at 3 months of age (Supplemental Fig. 4). Thus, further studies should determine if Lef1 colocalizes with all or some of the prostate stem cell markers or with the Notch1-positive transient progenitor population (3, 21, 22).
Interestingly, during development of the murine prostate, high levels of Lef1 were also detected in the urogenital mesenchyme, and Lef1 activity persisted within a small number of smooth muscle cells in 1-month-old postnatal prostate glands. In contrast, human fetal prostates showed negligible LEF1 accumulation in the mesenchyme. Further studies are needed to determine whether mesenchymal Wnt activity may contribute to the differences in development of the human and mouse prostatic stroma. We also detected subtle differences in the dynamics of AR expression in the human and mouse prostates. In the mouse UGS, AR expression was observed throughout the UGS epithelium before epithelial bud formation and, subsequently, was excluded from the prostate buds during branching morphogenesis. Notably, at most stages in prostate development in human and mouse, AR expression was excluded from the LEF1+ domains. Thus, our data indicate that, in contrast to prostate cancer, during prostate development, LEF1 does not induce AR expression directly.
An important finding resulting from our studies is that in the adult prostate, canonical Wnt/Lef1 signaling labels a portion of androgen-independent basal progenitors, which can repopulate the luminal compartment in the absence of androgen signaling. Lef1+ basal epithelium was maintained in bicalutamide-treated prostates. We used bicalutamide instead of surgical castration for this study due to its noninvasive nature as well as the advantage of allowing us to observe prostate regeneration and protein expression in prostate cells upon weaning from bicalutamide. Our results on prostate regression upon a 7-d treatment are very similar to prostate regression upon castration (23, 55). Most interestingly, we show that upon pharmacological inhibition of AR signaling, the Wnt/Lef1+ basal progenitors repopulate the luminal compartment. This androgen-independent prostate regeneration is accompanied by proliferation of Wnt/Lef1+ cells in both basal and luminal compartments. These results strongly indicate that Wnt/Lef1 signaling can induce partial prostate regeneration in the absence of androgen signaling. In support of our observations on the role of Wnt signaling in prostate regrowth, Wnt/Lef1/β-catenin signaling has a well-characterized function in promoting cell proliferation, in particular, by inducing cyclin D1 and c-myc (56). Notably, endogenous Wnt signaling is not sufficient to prevent prostate involution in the absence of AR activity.
In summary, our studies indicate an important role for Wnt/Lef1 signaling in prostate development in the mouse and human and in homeostasis of the adult organ in murine model. We show that the Lef1+ basal progenitors are induced to undergo cell proliferation upon inhibition of androgen signaling and can properly repopulate the basal and luminal compartments. Mouse mutants deficient for Lef1 survive up to 2 wk postnatally and also show defects in mammary gland development (57). Thus, it would be important to examine prostate specification and branching morphogenesis in the embryonic and postnatal Lef1−/− prostates and in Lef1−/− subrenal grafts. A thorough analysis of the contribution of canonical Wnt signaling to prostate development also needs to consider that Lef1 loss function can be compensated by Tcf1–Tcf4, which are expressed in the caudal part of the embryo (58). To determine the composite effect of the canonical Wnt signaling in prostate development and in adult maintenance, further studies should address the effect of prostate-specific inactivation of β-catenin using the conditional β-catenin knockout strain (59) and the prostate-specific Cre-recombinase (60, 61). An important advantage in studying the Lef1 null prostates vs. the β-catenin conditional knockout mice is that deletion of β-catenin would not only abrogate the canonical Wnt signaling but would also compromise the structure of the adherens junctions and cell adhesion.
Materials and Methods
Human tissues
All human samples were used with approval of the New York University Institutional Review Board. Prostate specimens from human fetuses at gestational ages 8–34 wk, respectively, were obtained after surgical abortion, performed for reasons unrelated to this investigation, or from autopsy. Informed consent was obtained by the consulting the obstetrician for all specimens. Gestational age was estimated from the date of last menstrual period as well as from sonographic measurements of crown to rump and foot length. Specimens were formalin fixed, paraffin embedded, and serially sectioned at 5 μm.
Mouse strains, X-gal staining, and bicalutamide treatment
Animal work was conducted under New York University School of Medicine Institutional Animal Care and Use Committee approved animal protocol. Studies were conducted using C3H mice purchased from Taconic (Germantown, NY), and TOPGAL reporter strain [stock Tg(Fos-lacZ)34Efu/J; no. 004623] was obtained from The Jackson Laboratory (Bar Harbor, ME). E0.5 was defined as the noon of the first day when the vaginal plug was first detected. In addition, embryos were staged by morphological appearance using the Atlas of Mouse Development (62). Fetal and postnatal mouse prostates were dissected, fixed, embedded in paraffin and sectioned at 4 μm as described previously (3).
TOPGAL strain is a transgenic reporter strain for the canonical Wnt signaling, made by an insertion of the bacterial β- galactosidase gene preceded by three Lef/TCF binding sites in the Rosa26 locus (46). β-Galactosidase activity was determined by X-gal staining as described (63).
Bicalutamide treatment was initiated at P23. Six P23 males were given 30 mg/kg·d of Casodex (AstraZeneca) dissolved in 500 ml of drinking water for 7 d. Control P23 males were given drinking water. For analysis of prostate recovery, Casodex-treated males were given clean drinking water for 4 d and then analyzed. Experiments under all conditions were repeated three times. Statistical significance of the calculations was determined by the Student's t test. P < 0.05 was considered significant.
Immunofluorescence and immunohistochemistry
Antibodies for Lef1 (catalogue no. 2230; Cell Signaling, Danvers, MA), Ki67 (clone K2; Ventana Medical Systems, Tucson, AZ), AR (Santa Cruz Biotechnology, Inc., Santa Cruz, CA), p63 (catalogue no. 3239; Abcam, Cambridge, MA), CK14 (catalogue no. PRB-155P; Covance, Princeton, NJ), and CK5 [catalogue no. 356M (Cell Marque, Rocklin, CA) and catalogue no. PRB-160P (Covance)] were used as 1:100 dilution. Preimmune serum was used as negative control. The specificity of Lef1 antibodies was confirmed with Lef1 small interfering RNA knockdown as described previously (32). Immunofluorescent analysis of the murine prostates with antibodies for p63 was performed using tyromide-enhanced immunofluorescence (Tyromide Amplification System kit #23; Molecular Probes, Eugene, OR) as described previously (3). Immunofluorescent imaging for Lef1, Ki67, AR, p63, CK14, and CK5 on mouse tissues was performed using the Zeiss Axioplan 2 fluorescent microscope (Carl Zeiss MicroImaging, Thornwood, NY). Quantitation of Ki67 and p63-positive cells was carried out on a set of six independent sections from six murine prostates at each stage indicated. Statistical significance of the calculations was determined by the Student's t test. P < 0.05 was considered significant. Immunohistochemistry for LEF1 and AR on human fetal tissue was performed using single label immunohistochemistry on a NexES automated immunostainer as described previously (32) with appropriate negative and positive controls. The suitability of the fetal human prostatic tissues was assessed by CK AE1/AE3 and vimentin immunostaining for the preservation of antigenicity of markers (data not shown). Cases with apparent autolysis or congenital anomalies were excluded.
Supplementary Material
Acknowledgements
This work was supported by Department of Defense Prostate Cancer Research Program, Veterans Affairs Merit Award, New York University School of Medicine Clinical and Translational Science Institute (1UL1RR029893), and a New York University Urology Center of Excellence grant (P.L.); Department of Urology support and the National Institute of Diabetes, Digestive, and Kidney Diseases Grant DK068007 (to I.G.); American Recovery and Reinvestment Act salary support (X.W.); the China Fellowship Council Predoctoral Fellowship 2008617069 (to K.X.); the National Center for Research Resources, National Institutes of Health Grant 1UL1RR029893 from (to G.D.); and DOD fellowship award (Y.R.L.).
Disclosure Summary: The authors have nothing to disclose.
NURSA Molecule Pages:
Nuclear Receptors: AR;
Ligands: Dihydrotestosterone | Testosterone | Bicalutamide.
Footnotes
- AR
- Androgen receptor
- AR+
- AR positive
- CK
- cytokeratin
- E
- embryonic day
- Ki67+
- Ki67 positive
- LEF
- human lymphoid enhancer-binding factor
- Lef
- murine lymphoid enhancer-binding factor
- Lef1+
- Lef1 positive
- P
- postnatal day
- TCF
- T-cell factor
- UGS
- urogenital sinus
- Wnt
- vertebrate homologues of the Drosophila Wingless (Wg) gene.
References
- 1. Marker PC, Donjacour AA, Dahiya R, Cunha GR. 2003. Hormonal, cellular, and molecular control of prostatic development. Dev Biol 253:165–174 [DOI] [PubMed] [Google Scholar]
- 2. Bhatia-Gaur R, Donjacour AA, Sciavolino PJ, Kim M, Desai N, Young P, Norton CR, Gridley T, Cardiff RD, Cunha GR, Abate-Shen C, Shen MM. 1999. Roles for Nkx3.1 in prostate development and cancer. Gene Dev 13:966–977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Grishina IB, Kim SY, Ferrara C, Makarenkova HP, Walden PD. 2005. BMP7 inhibits branching morphogenesis in the prostate gland and interferes with Notch signaling. Dev Biol 288:334–347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Timms BG, Mohs TJ, Didio LJ. 1994. Ductal budding and branching patterns in the developing prostate. J Urol 151:1427–1432 [DOI] [PubMed] [Google Scholar]
- 5. Xue Y, Smedts F, Ruijter ET, Debruyne FM, de la Rosette JJ, Schalken JA. 2001. Branching activity in the human prostate: a closer look at the structure of small glandular buds. Eur Urol 39:222–231 [DOI] [PubMed] [Google Scholar]
- 6. Xue Y, Sonke G, Schoots C, Schalken J, Verhofstad A, de la Rosette J, Smedts F. 2001. Proliferative activity and branching morphogenesis in the human prostate: a closer look at pre- and postnatal prostate growth. Prostate 49:132–139 [DOI] [PubMed] [Google Scholar]
- 7. Lee C. 1997. Biology of the prostatic ductal system. In: Naz RK. ed. Prostate: basic and clinical aspects. Boca Raton, FL: CRC Press; 53–71 [Google Scholar]
- 8. Freestone SH, Marker P, Grace OC, Tomlinson DC, Cunha GR, Harnden P, Thomson AA. 2003. Sonic hedgehog regulates prostatic growth and epithelial differentiation. Dev Biol 264:352–362 [DOI] [PubMed] [Google Scholar]
- 9. Chang CP, McDill BW, Neilson JR, Joist HE, Epstein JA, Crabtree GR, Chen F. 2004. Calcineurin is required in urinary tract mesenchyme for the development of the pyeloureteral peristaltic machinery. J Clin Invest 113:1051–1058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. McNeal JE. 1988. Normal histology of the prostate. Am J Surg Pathol 12:619–633 [DOI] [PubMed] [Google Scholar]
- 11. Epstein JI. 1997. Non-neoplastic diseases of the prostate. In: Bostwick DG, Eble JN. eds. Urologic surgical pathology. St. Louis, MO: Mosby; 307–340 [Google Scholar]
- 12. Epstein JI, Walsh PC, Sauvageot J, Carter HB. 1997. Use of repeat sextant and transition zone biopsies for assessing extent of prostate cancer. J Urol 158:1886–1890 [DOI] [PubMed] [Google Scholar]
- 13. Lowsley OS. 1912. The development of the human prostate gland with reference to the development of other structures at the neck of the urinary bladder. Am J Anat 13:299–349 [Google Scholar]
- 14. Brooks B, Miller GJ. 1986. Evaluation of prostatic-cancer histology and grade distribution—experience with the Colorado central cancer registry. Prostate 8:139–150 [DOI] [PubMed] [Google Scholar]
- 15. Shappell SB, Masumori N, Thomas T, Case T, Pau M, Kasper S, Matusik RJ. 2003. Transgenic mouse models of prostate carcinoma: anatomic, histopathologic, and molecular considerations. In: Lalani EN, Abel PD. eds. Prostate cancer: scientific and clinical aspects: bridging the gap. 1st ed Hackensack, NJ: Imperial College Press; 245–319 [Google Scholar]
- 16. Hayashi N, Sugimura Y, Kawamura J, Donjacour AA, Cunha GR. 1991. Morphological and functional heterogeneity in the rat prostatic gland. Biol Reprod 45:308–321 [DOI] [PubMed] [Google Scholar]
- 17. Jesik CJ, Holland JM, Lee C. 1982. An anatomic and histologic study of the rat prostate. Prostate 3:81–97 [DOI] [PubMed] [Google Scholar]
- 18. Podlasek CA, Barnett DH, Clemens JQ, Bak PM, Bushman W. 1999. Prostate development requires Sonic hedgehog expressed by the urogenital sinus epithelium. Dev Biol 209:28–39 [DOI] [PubMed] [Google Scholar]
- 19. Donjacour AA, Thomson AA, Cunha GR. 2003. FGF-10 plays an essential role in the growth of the fetal prostate. Dev Biol 261:39–54 [DOI] [PubMed] [Google Scholar]
- 20. Lamm ML, Podlasek CA, Barnett DH, Lee J, Clemens JQ, Hebner CM, Bushman W. 2001. Mesenchymal factor bone morphogenetic protein 4 restricts ductal budding and branching morphogenesis in the developing prostate. Dev Biol 232:301–314 [DOI] [PubMed] [Google Scholar]
- 21. Wang XD, Shou J, Wong P, French DM, Gao WQ. 2004. Notch1-expressing cells are indispensable for prostatic branching morphogenesis during development and re-growth following castration and androgen replacement. J Biol Chem 279:24733–24744 [DOI] [PubMed] [Google Scholar]
- 22. Wang XD, Leow CC, Zha J, Tang Z, Modrusan Z, Radtke F, Aguet M, de Sauvage FJ, Gao WQ. 2006. Notch signaling is required for normal prostatic epithelial cell proliferation and differentiation. Dev Biol 290:66–80 [DOI] [PubMed] [Google Scholar]
- 23. Ontiveros CS, Salm SN, Wilson EL. 2008. Axin2 expression identifies progenitor cells in the murine prostate. Prostate 68:1263–1272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wang G, Wang J, Sadar MD. 2008. Crosstalk between the androgen receptor and β-catenin in castrate-resistant prostate cancer. Cancer Res 68:9918–9927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yu X, Wang Y, Jiang M, Bierie B, Roy-Burman P, Shen MM, Taketo MM, Wills M, Matusik RJ. 2009. Activation of β-catenin in mouse prostate causes HGPIN and continuous prostate growth after castration. Prostate 69:249–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sugimura Y, Cunha GR, Donjacour AA. 1986. Morphogenesis of ductal networks in the mouse prostate. Biol Reprod 34:961–971 [DOI] [PubMed] [Google Scholar]
- 27. Shannon JM, Cunha GR. 1983. Autoradiographic localization of androgen binding in the developing mouse prostate. Prostate 4:367–373 [DOI] [PubMed] [Google Scholar]
- 28. Takeda H, Mizuno T, Lasnitzki I. 1985. Autoradiographic studies of androgen-binding sites in the rat urogenital sinus and postnatal prostate. J Endocrinol 104:87–92 [DOI] [PubMed] [Google Scholar]
- 29. Cunha GR, Chung LW. 1981. Stromal-epithelial interactions–I. Induction of prostatic phenotype in urothelium of testicular feminized (Tfm/y) mice. J Steroid Biochem 14:1317–1324 [DOI] [PubMed] [Google Scholar]
- 30. Lu W, Luo YD, Kan M, McKeehan WL. 1999. Fibroblast growth factor-10—a second candidate stromal to epithelial cell andromedin in prostate. J Biol Chem 274:12827–12834 [DOI] [PubMed] [Google Scholar]
- 31. Wang LG, Johnson EM, Kinoshita Y, Babb JS, Buckley MT, Liebes LF, Melamed J, Liu XM, Kurek R, Ossowski L, Ferrari AC. 2008. Androgen receptor overexpression in prostate cancer linked to Pur α loss from a novel repressor complex. Cancer Res 68:2678–2688 [DOI] [PubMed] [Google Scholar]
- 32. Li Y, Wang L, Zhang M, Melamed J, Liu X, Reiter R, Wei J, Peng Y, Zou X, Pellicer A, Garabedian MJ, Ferrari A, Lee P. 2009. LEF1 in androgen-independent prostate cancer: regulation of androgen receptor expression, prostate cancer growth, and invasion. Cancer Res 69:3332–3338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Novak A, Dedhar S. 1999. Signaling through β-catenin and Lef/Tcf. Cell Mol Life Sci 56:523–537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Arce L, Yokoyama NN, Waterman ML. 2006. Diversity of LEF/TCF action in development and disease. Oncogene 25:7492–7504 [DOI] [PubMed] [Google Scholar]
- 35. Cadigan KM, Nusse R. 1997. Wnt signaling: a common theme in animal development. Genes Dev 11:3286–3305 [DOI] [PubMed] [Google Scholar]
- 36. Barolo S. 2006. Transgenic Wnt/TCF pathway reporters: all you need is Lef? Oncogene 25:7505–7511 [DOI] [PubMed] [Google Scholar]
- 37. Kikuchi A, Kishida S, Yamamoto H. 2006. Regulation of Wnt signaling by protein-protein interaction and post-translational modifications. Exp Mol Med 38:1–10 [DOI] [PubMed] [Google Scholar]
- 38. Jamora C, DasGupta R, Kocieniewski P, Fuchs E. 2003. Links between signal transduction, transcription and adhesion in epithelial bud development. Nature 422:317–322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Boras-Granic K, Chang H, Grosschedl R, Hamel PA. 2006. Lef1 is required for the transition of Wnt signaling from mesenchymal to epithelial cells in the mouse embryonic mammary gland. Dev Biol 295:219–231 [DOI] [PubMed] [Google Scholar]
- 40. Hatsell S, Rowlands T, Hiremath M, Cowin P. 2003. β-Catenin and Tcfs in mammary development and cancer. J Mammary Gland Biol Neoplasia 8:145–158 [DOI] [PubMed] [Google Scholar]
- 41. Lustig B, Behrens J. 2003. The Wnt signaling pathway and its role in tumor development. J Cancer Res Clin Oncol 129:199–221 [DOI] [PubMed] [Google Scholar]
- 42. Nusse R. 2005. Wnt signaling in disease and in development. Cell Res 15:28–32 [DOI] [PubMed] [Google Scholar]
- 43. Reya T, Clevers H. 2005. Wnt signalling in stem cells and cancer. Nature 434:843–850 [DOI] [PubMed] [Google Scholar]
- 44. Yardy GW, Brewster SF. 2005. Wnt signalling and prostate cancer. Prostate Cancer P D 8:119–126 [DOI] [PubMed] [Google Scholar]
- 45. Barker N, Clevers H. 2000. Catenins, Wnt signaling and cancer. Bioessays 22:961–965 [DOI] [PubMed] [Google Scholar]
- 46. Noh T, Gabet Y, Cogan J, Shi Y, Tank A, Sasaki T, Criswell B, Dixon A, Lee C, Tam J, Kohler T, Segev E, Kockeritz L, Woodgett J, Müller R, Chai Y, Smith E, Bab I, Frenkel B. 2009. Lef1 haploinsufficient mice display a low turnover and low bone mass phenotype in a gender- and age-specific manner. PLoS One 4:e5438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. DasGupta R, Fuchs E. 1999. Multiple roles for activated LEF/TCF transcription complexes during hair follicle development and differentiation. Development 126:4557–4568 [DOI] [PubMed] [Google Scholar]
- 48. Miyagawa S, Moon A, Haraguchi R, Inoue C, Harada M, Nakahara C, Suzuki K, Matsumaru D, Kaneko T, Matsuo I, Yang L, Taketo MM, Iguchi T, Evans SM, Yamada G. 2009. Dosage-dependent hedgehog signals integrated with Wnt/β-catenin signaling regulate external genitalia formation as an appendicular program. Development 136:3969–3978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Lin C, Yin Y, Long F, Ma L. 2008. Tissue-specific requirements of β-catenin in external genitalia development. Development 135:2815–2825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Liu S, Vinall RL, Tepper C, Shi XB, Xue LR, Ma AH, Wang LY, Fitzgerald LD, Wu Z, Gandour-Edwards R, deVere White RW, Kung HJ. 2008. Inappropriate activation of androgen receptor by relaxin via β-catenin pathway. Oncogene 27:499–505 [DOI] [PubMed] [Google Scholar]
- 51. Truica CI, Byers S, Gelmann EP. 2000. β-Catenin affects androgen receptor transcriptional activity and ligand specificity. Cancer Res 60:4709–4713 [PubMed] [Google Scholar]
- 52. Placencio VR, Sharif-Afshar AR, Li X, Huang H, Uwamariya C, Neilson EG, Shen MM, Matusik RJ, Hayward SW, Bhowmick NA. 2008. Stromal transforming growth factor-β signaling mediates prostatic response to androgen ablation by paracrine Wnt activity. Cancer Res 68:4709–4718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Böcker W, Bürger H, Buchwalow IB, Decker T. 2005. Ck5-positive cells are precursor cells of glandular and myoepithelial cell lineages in the human breast epithelium. A new cell concept as a basis for a better understanding of proliferative breast disease? Verh Dtsch Ges Pathol 89:45–47 [PubMed] [Google Scholar]
- 54. Lombaert IM, Hoffman MP. 2010. Epithelial stem/progenitor cells in the embryonic mouse submandibular gland. Front Oral Biol 14:90–106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Wang X, Kruithof-de Julio M, Economides KD, Walker D, Yu H, Halili MV, Hu YP, Price SM, Abate-Shen C, Shen MM. 2009. A luminal epithelial stem cell that is a cell of origin for prostate cancer. Nature 461:495–500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Jesse S, Koenig A, Ellenrieder V, Menke A. 2010. Lef-1 isoforms regulate different target genes and reduce cellular adhesion. Int J Cancer 126:1109–1120 [DOI] [PubMed] [Google Scholar]
- 57. van Genderen C, Okamura RM, Fariñas I, Quo RG, Parslow TG, Bruhn L, Grosschedl R. 1994. Development of several organs that require inductive epithelial-mesenchymal interactions is impaired in Lef-1-deficient mice. Gene Dev 8:2691–2703 [DOI] [PubMed] [Google Scholar]
- 58. Galceran J, Fariñas I, Depew MJ, Clevers H, Grosschedl R. 1999. Wnt3a(−/−)-like phenotype and limb deficiency in Lef1(−/−)Tcf1(−/−) mice. Gene Dev 13:709–717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Brault V, Moore R, Kutsch S, Ishibashi M, Rowitch DH, McMahon AP, Sommer L, Boussadia O, Kemler R. 2001. Inactivation of the β-catenin gene by Wnt1-Cre-mediated deletion results in dramatic brain malformation and failure of craniofacial development. Development 128:1253–1264 [DOI] [PubMed] [Google Scholar]
- 60. Wu XT, Wu J, Huang JP, Powell WC, Zhang JF, Matusik RJ, Sangiorgi FO, Maxson RE, Sucov HM, Roy-Burman P. 2001. Generation of a prostate epithelial cell-specific Cre transgenic mouse model for tissue-specific gene ablation. Mech Develop 101:61–69 [DOI] [PubMed] [Google Scholar]
- 61. Birbach A, Casanova E, Schmid JA. 2009. A probasin-MerCreMer BAC allows inducible recombination in the mouse prostate. Genesis 47:757–764 [DOI] [PubMed] [Google Scholar]
- 62. Kaufman ME. 1999. The atlas of mouse development. Cambridge, UK: Academic Press, Harcourt Brace, Co. Publishers, University Press; 380–387 [Google Scholar]
- 63. Chang TH, Primig M, Hadchouel J, Tajbakhsh S, Rocancourt D, Fernandez A, Kappler R, Scherthan H, Buckingham M. 2004. An enhancer directs differential expression of the linked Mrf4 and Myf5 myogenic regulatory genes in the mouse. Dev Biol 269:595–608 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.