Leptin communicates with the neuroendocrine reproductive axis via multiple populations of LepRb neurons that lie afferent to both Kiss1 and GnRH neurons.
Abstract
Negative energy balance and insufficient adipose energy stores decrease the production of leptin, thereby diminishing the leptin-supported secretion of GnRH from the hypothalamus and promoting decreased reproductive function. Leptin acts via its receptor (LepRb) to support the neuroendocrine reproductive axis, but the nature and location of the relevant LepRb neurons remain poorly understood. Possibilities include the direct or indirect action of leptin on hypothalamic GnRH neurons, or on kisspeptin (Kiss1) neurons that are major regulators of GnRH neurons. To evaluate these potential mechanisms, we employed immunohistochemical analysis of the female brain from various molecular mouse models and sheep. Our analysis revealed no LepRb in GnRH neurons or in anteroventral periventricular Kiss1 neurons, and very limited (0–6%) colocalization with arcuate nucleus Kiss1 cells, suggesting that leptin does not modulate reproduction by direct action on any of these neural populations. LepRb neurons, primarily in the hypothalamic ventral premammillary nucleus and a subregion of the preoptic area, lie in close contact with GnRH neurons, however. Furthermore, an unidentified population or populations of LepRb neurons lie in close contact with arcuate nucleus and anteroventral periventricular Kiss1 neurons. Taken together, these findings suggest that leptin communicates with the neuroendocrine reproductive axis via multiple populations of LepRb neurons that lie afferent to both Kiss1 and GnRH neurons.
The hormone leptin plays a central role in the control of reproduction by energy status (1, 2). Secreted by adipose tissue in proportion to fat stores, leptin communicates the sufficiency of energy reserves via the functional form of the leptin receptor (LepRb) on specialized populations of neurons in the central nervous system (CNS) (3–5). The decreased leptin levels associated with diminished fat reserves or elevated energy flux promote increased feeding and energy conservation, in part by blocking energy expenditure on functions not required for survival (1). Conversely, sufficient leptin permits behaviors and processes that require energy, as well as suppressing feeding.
Leptin serves as a permissive metabolic signal for reproduction, and minimum leptin levels are required to initiate puberty and for the maintenance of reproductive capacity and the promotion of reproductive behaviors (6–9). Leptin-deficient (Lepob/ob) mice display characteristics similar to the starvation response (even in the face of nutrient excess and obesity), including hypothalamic infertility, which is reversed by leptin administration (1–2, 10). Leptin also reverses the hypogonadotropic hypogonadism associated with reduced body fat (1, 2, 10–12). Thus, leptin serves to link energy homeostasis and the neuroendocrine reproductive axis. Importantly, CNS leptin action is sufficient to overcome reproductive dysfunction in low leptin states (13–15). Leptin modulates pulsatile gonadotropin secretion from the anterior pituitary via the regulation of hypothalamic GnRH neurons (2, 12, 16, 17). Because previous observations suggest that hypothalamic GnRH neurons do not express LepRb (16–17), leptin presumably mediates the hypothalamic control of reproduction indirectly, by acting upstream of GnRH neurons.
Hypothalamic neurons that express the neuropeptide, kisspeptin (Kiss1) have emerged as important modulators of GnRH neurons and the reproductive axis. Kiss1 signals through the GPR54 G protein-coupled receptor (18, 19), which is expressed by most GnRH neurons (20–22). Mutations of Kiss1 or GPR54 result in delayed or absent pubertal maturation, low circulating gonadotropin levels, and hypogonadotropic hypogonadism in humans (23) and mice (22, 24–27). In female rodents, Kiss1-containing neurons lie in the hypothalamic arcuate (ARC) and in the anteroventral periventricular (AVPV) nuclei (males have few AVPV Kiss1 neurons) (21, 28–30). The majority of ARC Kiss1 neurons also coexpress neurokinin B [also known as tachykinin 2 (Tac2)] and dynorphin A, and have been termed “KNDy” neurons (31–33).
Kiss1 mRNA levels are regulated by nutritional status; fasting suppresses Kiss1 expression in rodents (34, 35). Furthermore, Lepob/ob mice demonstrate decreased hypothalamic Kiss1 expression, which is partially reversed by systemic leptin replacement (30). Central administration of leptin also increases Kiss1 mRNA levels in other models of decreased energy stores, including streptozotocin-induced diabetic rats (36). Although these data suggest that leptin modulates Kiss1 neurons, several reports disagree on the extent of LepRb expression in Kiss1 neurons and the potential mechanism of this regulation (30, 37). The mechanisms by which LepRb-expressing neurons interact with GnRH and/or Kiss1 neurons to potentially influence reproduction remain incompletely understood, however. We hypothesize that leptin acts upstream of GnRH and Kiss1 neurons on multiple populations of cells to modulate these neurons indirectly. Here, we map the neural pathways by which LepRb neurons may regulate Kiss1 and GnRH neurons to address this hypothesis.
Materials and Methods
Experimental animals
Mice.
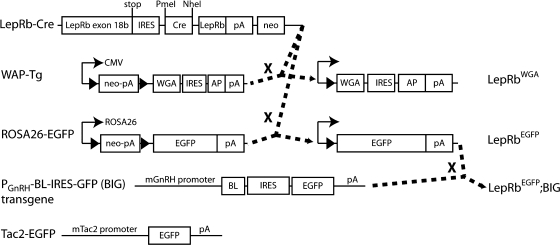
The generation of Leprcre/cre (LepRbCre), LepRbEGFP, and LepRbWGA mice has been described previously (38,39)(Fig. 1). Transgenic BIG mice, described previously (40), were obtained from Drs. Linda Buck and Ulrich Boehm and propagated in our colony. LepRbEGFP; BIG mice were generated by intercrossing LepRbEGFP and BIG mice (Fig. 1). Tac2-enhanced green fluorescent protein (EGFP) BAC transgenic mice [015495-UCD/STOCK Tg (Tac2-EGFP)381Gsat] were obtained from Mouse Mutant Regional Resource Center and propagated in our colony. All care and procedures for mice were according to guidelines approved by the University of Michigan Committee on the Use and Care of Animals.
Fig. 1.
Genetic systems to probe the connections between LepRb neurons and the neuroendocrine reproductive axis. LepRb-cre mice were crossed with either ROSA26-EGFP mice or with WAP-Tg mice to produce LepRbEGFP or LepRbWGA mice, respectively. LepRbEGFP mice were crossed with BIG transgenic animals to produce LepRbEGFP;BIG mice. Tac2-EGFP mice are BAC transgenic animals that express EGPF from the Tac2 promoter. LoxP sites are indicated by solid black arrowheads.
Sheep.
Adult, gonadal-intact Suffolk ewes were maintained at the Sheep Research Facility of the University of Michigan in an open barn with daily feeding and free access to water. Animals for this study were taken off feed 2 d before receiving either iv injections of either leptin (1 mg/kg) or vehicle. All experimental procedures were approved by the University of Michigan Animal Care and Use Committee.
Materials
Leptin for the mouse experiments was the generous gift of Amylin Pharmaceuticals, Inc. (San Diego, CA). For sheep experiments, recombinant human leptin was obtained from the National Hormone and Peptide Program of the National Institute of Diabetes and Digestive and Kidney Diseases.
Perfusion and immunolabeling
Mice.
Where noted, some animals received stereotaxic intracerebroventricular injection of 10 μg of colchicine 2 d before euthanasia to aid in the detection of neuronal cell bodies. Perfusion and immunohistochemistry were performed as described (41) on adult, postpubertal female mice. Briefly, mice were deeply anesthetized with a lethal dose of pentobarbital (150 mg/kg, ip) and transcardially perfused with sterile PBS followed by 10% neutral buffered formalin. Brains were removed, postfixed overnight in 10% formalin, and then dehydrated in a 30% sucrose solution. Brains were sectioned into 30-μm coronal slices on a microtome, collected in four consecutive series, and stored at −20 C in cryoprotectant. Brain sections were washed, blocked in normal donkey serum, and then incubated in primary antibodies overnight. For pSTAT3 staining, sections were pretreated sequentially in 1% H2O2/1% NaOH, 0.6% glycine, and 0.3% sodium dodecyl sulfate, before blocking in normal donkey serum and incubating with primary antibodies. Labeling was visualized by immunofluorescent secondary detection using species-specific Alexa 488 or 568 antibodies (1:200, Invitrogen, Carlsbad, CA). Sections were mounted on slides and coverslipped with Prolong antifade mounting medium (Invitrogen). Primary antibodies used for these studies included: chicken anti-GFP (green fluorescent protein) (1:1000, Abcam, Inc., Cambridge, MA), goat anti-WGA (wheat germ agglutinin) (1:1000, Vector), rabbit anti-Kiss1 (1:2000, Chemicon, Temecula, CA), rabbit anti-GnRH (1:1000; Abcam), and rabbit anti-pSTAT3 (1:1000, Cell Signaling Technology, Inc., Danvers, MA).
Sheep.
Two hours after receiving either leptin (1 mg/kg, iv) or vehicle injections, ewes were euthanized via an iv overdose of sodium phenobarbital (∼2 g in 7ml saline; Sigma, St. Louis, MO). After they had stopped breathing, the head was perfused through both carotid arteries with 6 liters of fixative (4% paraformaldehyde in 0.1 m phosphate buffer containing 0.1% sodium nitrite). After perfusion, brains were removed, and preoptic area (POA)/hypothalamic tissue was dissected out. The tissue was then infiltrated with 30% sucrose, and coronal sections (45 μm thick) were cut on a freezing microtome and stored at −20 C in cryoprotectant (30% ethylene glycol, 1% polyvinylpyrrolidone, 30% sucrose in sodium phosphate buffer) until processing.
A dual-immunoperoxidase procedure was performed on a series of every sixth POA and ARC section in each animal to determine colocalization of leptin-induced pSTAT3 in Kiss1 neurons. For pSTAT3 staining, sections were preincubated in 30% hydrogen peroxide (catalog item H325; Fisher Scientific, Pittsburgh, PA) in methanol for 10 min to eliminate endogenous peroxidase activity and permeablilize the sections. After washing, sections were then incubated in rabbit polyclonal antibody against pSTAT3 (1:100; Cell Signaling Technology) for 17 h at room temperature (RT). This was followed by incubations (1 h at RT) with biotinylated goat antirabbit IgG (1:500; Jackson Immunoresearch Laboratories, Inc., West Grove, PA) and avidin-biotin-horseradish peroxidase complex (ABC-elite; 1:250; 1 h; Vector Laboratories, Burlingame, CA). Labeling was visualized using nickel-enhanced diaminobenzidine (Sigma) as chromagen, producing a black/purple reaction product. For detection of Kiss1, sections were next incubated in polyclonal rabbit antiKiss1 10 serum (1:75,000; 17 h at RT; catalog no. 564, gift from A. Caraty, Université Tours, Nouzilly, France), which has been previously validated for use in sheep tissue (31, 42). After incubation in primary antiserum, tissue was labeled (1 h at RT) with biotinylated goat antirabbit IgG (1:500; Jackson Immunoresearch Laboratories, Inc.), and then incubated in ABC-elite (1:500; 1 h at RT; Vector Laboratories). Kiss1 was visualized using unenhanced diaminobenzidine as chromagen, which produced a brown reaction product. Sections were then mounted onto plus-charged slides, air dried, dehydrated, and coverslipped using DPX mountant (Electron Microscopy Sciences, Hatfield, PA). Control sections in which either primary antibody was omitted resulted in complete elimination of staining for the corresponding antigen. In addition, preabsorption of the diluted antibody with 5–10 μg/ml purified pSTAT3 peptide (catalog no. 1195, Cell Signaling) for 17 h at RT completely eliminated all pSTAT3 staining.
Data collection and analysis
Mice.
Slides were analyzed by fluorescent microscopy using an Olympus BX-51 microscope equipped with a DP30BW camera (Olympus Corp., Lake Success, NY) and filters for Alexa 488 or Alexa 568. Using Adobe Photoshop software (Adobe Systems, San Jose CA), images were overlaid in different red-green-blue channels to reveal possible overlap between red and green channels.
Sheep.
For each ewe, the numbers of single and dual labeled Kiss1 and pSTAT3-positive cells were counted under bright field illumination in sections through the POA and the middle division of the ARC (the level containing the greatest number of Kiss1 cells) by an independent investigator blinded to the animal number or group. A cell was considered dual labeled when a brown Kiss1 cytoplasm surrounded a black pSTAT3-positive nucleus. Images of immunostained sections were captured using a digital camera (Optronics Engineering, Goleta, CA) attached to a Leica DMRD microscope (Leica Microsystems GmbH, Wetzlar, Germany).
Results
Lack of LepRb in GnRH neurons
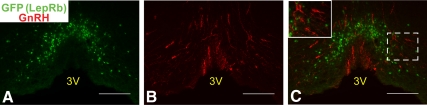
Previous reports used in situ hybridization (ISH) to examine the potential expression of LepRb in GnRH neurons, demonstrating an absence of detectable Leprb mRNA in GnRH cells of the hypothalamus (16, 17). Given the difficulty of sensitively detecting the modest levels of endogenous Leprb mRNA, however, we used the robust detection of LepRb neurons by EGFP staining in LepRbEGFP mice (38, 43, 44) to reexamine the possibility of LepRb/GnRH colocalization in females (Fig. 2). Although this analysis revealed the previously noted presence of EGFP-immunoreactive (IR) LepRb neurons in the POA (43, 45, 46), and many nearby GnRH neurons, we observed no colocalization of EGFP- and GnRH-IR anywhere in the brain, confirming that GnRH neurons do not contain LepRb and cannot directly respond to leptin. These data suggest that leptin must regulate GnRH neurons by acting on upstream neurons.
Fig. 2.
Hypothalamic GnRH neurons do not express LepRb. Representative images of GFP-IR (panel A, green), GnRH-IR (panel B, red), and merged (panel C) of the POA from colchicine-treated LepRbEGFP animals. Inset represents digital zoom of the area indicated by white dashed box in panel C. Scale bars, 100 μm. 3V, Third ventricle.
GnRH neurons receive input from LepRb neurons
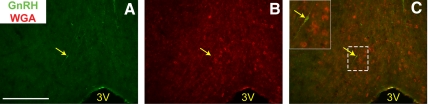
In LepRbWGA mice (in which LepRb neurons express the trans-synaptic tracer, WGA) (39), WGA-IR reveals LepRb neurons and the neurons that lie in synaptic contact with LepRb neurons. To determine whether there is close contact between LepRb and GnRH neurons, we examined the potential colocalization of WGA-IR and GnRH-IR in female LepRbWGA mice (Fig. 3). This analysis revealed the presence of POA GnRH neurons that also contained WGA-IR. Because GnRH neurons do not express LepRb, the accumulation of WGA in GnRH neurons indicates close contact between LepRb and GnRH neurons.
Fig. 3.
GnRH neurons lie in close contact with LepRb neurons. Representative images of GnRH-IR (panel A, green), WGA-IR (panel B, red) and merged (panel C) in the POA of a LepRbWGA mouse. Inset represents digital zoom of the area indicated by white dashed box in panel C. Yellow arrows indicate colocalization of GnRH-IR and WGA-IR. Scale bar, 100 μm. 3V, Third ventricle.
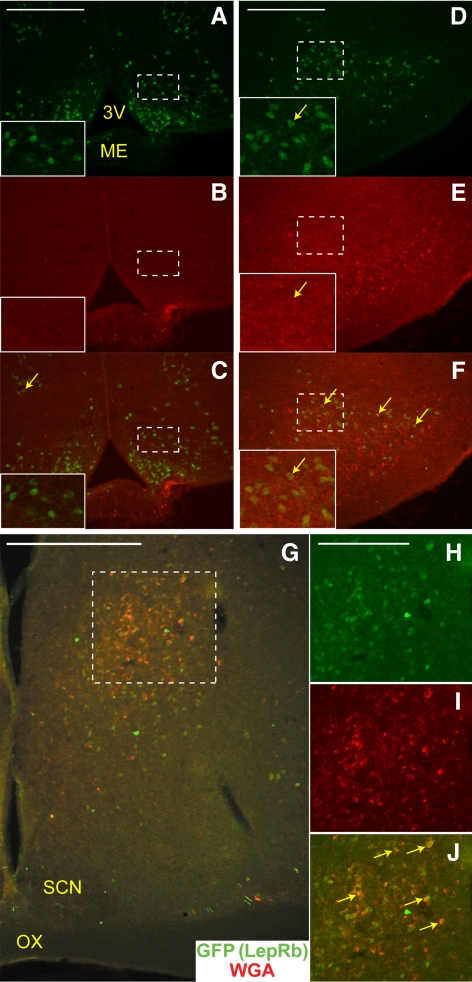
Although LepRbWGA mice reveal synaptic connections between LepRb and GnRH neurons, WGA is expressed in LepRb neurons throughout the brain in this model, and thus we cannot use these animals to identify which LepRb populations might synapse with GnRH neurons. We thus used transgenic BIG mice (40), in which GnRH neurons express the WGA-related trans-synaptic tracer, barley lectin (BL). Similarly to WGA, the release of BL into the synapse permits its accumulation in efferent and afferent neurons; thus, in BIG mice, WGA-IR (which detects BL) identifies neurons pre- and postsynaptic to GnRH neurons. To detect LepRb neurons that lie in close contact with GnRH neurons, we bred BIG mice to LepRbEGFP mice, producing LepRbEGFP;BIG mice, in which WGA-IR identifies neurons that contact GnRH neurons and EGFP-IR identifies LepRb neurons (Fig. 1). We examined the brains from these animals for neurons containing WGA- and EGFP-IR. As reported previously (43), EGFP-IR in these animals revealed many LepRb neurons in a number of hypothalamic nuclei, including the ARC and dorsomedial portion of the ventromedial hypothalamic nucleus (Fig. 4, A–C), dorsomedial hypothalamic nucleus, and lateral hypothalamic area (data not shown), ventral premammillary (PMv) (Fig. 4, D–F), and POA (Fig. 4, G–J). Despite the large number of EGFP-IR LepRb neurons in the ARC and VMH, few WGA-containing cells were detected, and almost no neurons containing both WGA- and EGFP-IR were observed. As previously shown, the PMv contained large populations of LepRb neurons and WGA-IR neurons, and some of the PMv LepRb neurons contained WGA-IR, suggesting their intimate connection with GnRH neurons. Within the POA, a cluster of EGFP-IR LepRb neurons in the striohypothalamic nucleus (StHy) extensively colocalized with WGA-IR, suggesting that these LepRb neurons lie in contact with GnRH cells. Because BL and WGA pass retrogradely as well as anterogradely, the presence of WGA-IR cannot definitively distinguish the efferent vs. afferent nature of the contact between neurons. In the BIG mice, however, the absence of GnRH-IR axons in the region of the PMv and the StHy/POA neurons that colocalize EGFP and WGA-IR (Ref. 40 and data not shown) suggests that these LepRb populations lie upstream of GnRH neurons, consistent with the notion that these LepRb neurons regulate GnRH neurons (rather than GnRH neurons modulating LepRb neurons).
Fig. 4.
LepRb neurons that lie in close contact with GnRH neurons. Representative images of GFP-IR (panels A, D, and H, green), WGA-IR (panels B, E, and I, red) and merged (panels C, F, G, and J) in the Arc (A–C), the PMv (D–F), and in the StHy (G–J) of a LepRbEGFP;BIG mouse. H–J, Enlarged view of area indicated by dashed box in panel G. Yellow arrows indicate colocalization of GFP-IR and WGA-IR. Insets represent digital zoom of the areas indicated by white dashed boxes. Scale bars, 200 μm (panels A, D, and H) and 500 μm (panel G). 3V, Third ventricle; ME, median eminence; SCN, suprachiasmatic nucleus; OX, optic chiasm.
Few Kiss1 neurons contain LepRb
Recent data have suggested a crucial role for Kiss1 neurons in the modulation of GnRH neurons and the neuroendocrine reproductive axis, including a role in the response to alterations in energy balance and/or leptin action (30, 33). A number of groups have therefore examined the potential for LepRb expression in Kiss1 neurons. Although most reports suggest that the AVPV Kiss1 neurons do not contain LepRb, LepRb expression has been reported in up to 40% of ARC Kiss1 neurons in the mouse (30). To address this issue directly, we employed several of our molecular mouse models to facilitate the detection of the neurons in question, as well as examining colocalization in ewes, where the immunohistochemical detection of neuropeptides (such as Kiss1) is more robust than in mice (Figs. 5 and 6).
Fig. 5.
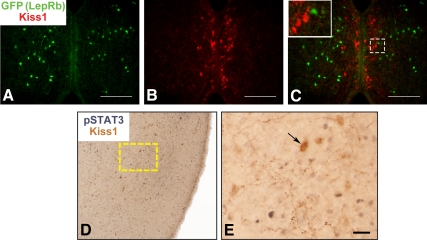
Kiss1 neurons of the AVPV/preoptic region do not express LepRb. Representative images of GFP-IR (panel A, green), Kiss1-IR (panel B, red), and merged (panel C) in the AVPV of a colchicine-treated LepRbEGFP mouse (A–C). Inset represents digital zoom of the area indicated by dashed box in panels C. D, and E, Representative images of Kiss1-IR (brown) and leptin-induced pSTAT3-IR (black) in the ovine POA. E, Enlarged image of dashed box in panel D. Arrows denote examples of Kiss1-IR neurons. Scale bars, 100 μm (panels A–C); 25 μm (panel E).
Fig. 6.
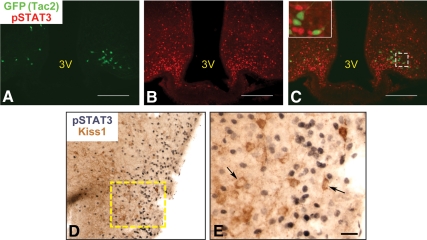
Minimal functional LepRb in ARC Tac2/Kiss1 neurons. Representative images of GFP-IR (panel A, green), leptin-induced pSTAT3-IR (panel B, red) and merged (panel C) in the AARC of a Tac2-EGFP mouse. Inset represents digital zoom of the area indicated by dashed box. D and E, Representative images of Kiss1-IR (brown) and leptin-induced pSTAT3-IR (black) in the ovine ARC. E, Enlarged image of dashed box in panel D. Arrows denote examples of Kiss1-IR neurons. Scale bars, 100 μm (panels A–C); 25 μm (panel E). 3V, Third ventricle.
We initially examined the potential for LepRb expression in AVPV Kiss1 neurons (Fig. 5). Because AVPV Kiss1 neurons in the female mouse brain are robustly detected by IHC, we examined the potential colocalization of EGFP- and Kiss1-IR in the AVPV of female LepRbEGFP mice (Fig. 5, A–C). Although this analysis revealed the presence of EGFP-IR LepRb neurons in the region lateral to the AVPV Kiss1 neurons, it revealed no evidence of LepRb/Kiss1 colocalization within the AVPV. To confirm this in a separate species, we also examined the potential colocalization of leptin-induced STAT3 phosphorylation (pSTAT3-IR; a marker of direct LepRb activation) (47–49) in the POA of the ewe (which contains scattered Kiss1 neurons, but not in a nucleus that is homologous in location to the AVPV) (Fig. 5, D and E) (33). This analysis revealed no pSTAT3-IR in Kiss1 neurons of the ovine POA, suggesting that AVPV/POA Kiss1 neurons do not contain functional LepRb in either species. Although leptin stimulates multiple intracellular signaling pathways via LepRb, most are difficult to detect by IHC and may be modulated indirectly by leptin, whereas activated LepRb directly promotes pSTAT3 (50).
Kiss1-IR in the mouse ARC revealed copious fibers, but very few cell bodies, even in colchicine-treated animals (data not shown, and see Fig. 7D), rendering it impossible to thoroughly examine potential LepRb/Kiss1 colocalization by using Kiss1-IR in LepRbEGFP mice. ARC Kiss1 neurons coexpress Tac2 (also known as neurokinin B) and dynorphin A (hence, KNDy neurons); similarly, ARC Tac2 neurons coexpress Kiss1 (31, 32). We thus obtained transgenic Tac2-EGFP mice, in which EGFP expression robustly identifies Tac2 neurons, to permit the detection of the ARC Kiss1/KNDy neurons by EGFP-IR. We examined the potential colocalization of ARC EGFP-IR and leptin-stimulated pSTAT3-IR in the brains of female Tac2-EGFP mice (Fig. 6, A–C). We counted all EGFP-IR neurons in the ARC of three female mice and determined the percentage that colocalized with pSTAT3-IR; although this analysis revealed some colocalization, only 5–6% of EGFP-positive neurons in the Tac2-EGFP ARC contained detectable pSTAT3, with most sections exhibiting no double-labeled cells. Given the poor detection of Kiss1 soma in the murine ARC, it was difficult to examine the extent of colocalization between Tac2-EGFP and Kiss1 in the ARC, so we also used the robust detection of Kiss1-IR in the ovine ARC to examine the potential colocalization between Kiss1-IR and leptin-stimulated pSTAT3-IR (Fig. 6, D and E). As was the case for Kiss1 cells of the ovine POA, we found no evidence of pSTAT3- and Kiss1-IR colocalization in the ARC of the ewe. Thus, these data suggest that the vast majority of ARC Kiss1/KNDy neurons in mice and sheep do not express LepRb and cannot directly respond to leptin.
Fig. 7.
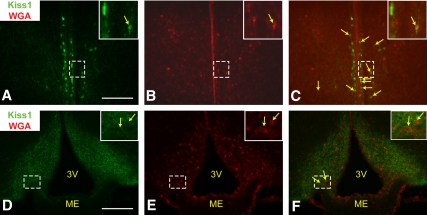
LepRb neurons lie in close contact with AVPV and Arc Kiss1 neurons. Representative images of Kiss1-IR (panels A and D, green) and WGA-IR (panels B and E, red) and merged (panels C and F) in the AVPV (panels A–C) and ARC (panels D–F) of a LepRbWGA mouse. Yellow arrows indicate colocalization of Kiss1-IR and WGA-IR. Inset represents digital zoom of the area indicated by dashed box. Scale bars, 200 μm. 3V, Third ventricle; ME, median eminence.
Some Kiss1 neurons lie in contact with LepRb neurons
The predominant lack of LepRb/Kiss1 colocalization demonstrated above suggests that leptin modulates Kiss1 neurons by indirect (e.g. trans-synaptic) means. To obtain further evidence for such a mechanism, we used our LepRbWGA mice to examine the potential accumulation of WGA in AVPV and ARC Kiss1 neurons (Fig. 7). This analysis demonstrated the colocalization of WGA- and Kiss1-IR in AVPV cells (Fig. 7, A–C), suggesting that a set or sets of LepRb neurons lie in close contact with this population of Kiss1 neurons. Although our analysis of the ARC was limited by the poor detection of Kiss1 soma by Kiss1-IR (Fig. 7D), we detected WGA-IR in many of the identifiable Kiss1-IR soma in this analysis (Fig. 7, D–F), consistent with the notion that ARC Kiss1 neurons lie in close contact with some LepRb neurons.
Discussion
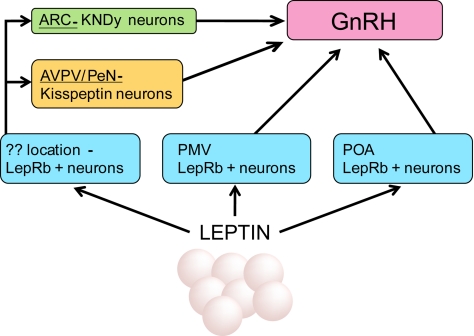
Using a number of molecular mouse models, we have examined multiple potential neural mechanisms by which leptin may regulate the neuroendocrine reproductive axis. We found that neither GnRH neurons nor AVPV Kiss1 neurons express LepRb, and that very few ARC KNDy neurons express LepRb and directly respond to leptin. Each of these neuronal groups known to be crucial for the CNS control of reproduction lie in synaptic contact with LepRb neurons, however, and the trans-synaptic regulation of Kiss1 and GnRH neurons by LepRb neurons represents a reasonable mechanism for the regulation of neuroendocrine reproductive function by leptin. Although our current models do not permit the identification of the LepRb population(s) that synapse with Kiss1 neurons, our data suggest that StHy/POA and PMv LepRb neurons directly innervate GnRH neurons (Fig. 8).
Fig. 8.
Multiple indirect routes for the regulation of GnRH neurons by leptin. Leptin acts at multiple sites to indirectly modulate GnRH neurons. Some LepRb neurons in the PMv lie in close contact with GnRH neurons; a large number of LepRb neurons in the StHy nucleus of the POA also contact GnRH neurons. Kiss1 neurons are thought to be major regulators of GnRH neurons. No AVPV Kiss1 neurons and very few ARC Kiss1 neurons express LepRb; therefore the majority of their regulation by leptin is likely to be indirect. Kiss1 neurons receive inputs from LepRb neurons, although the population(s) of LepRb neurons that lie in contact with Kiss1 neurons remains unknown.
Taken together, our data also suggest that the control of the neuroendocrine reproductive axis by leptin is unlikely to be mediated by a single population of LepRb neurons, but rather relies on the concerted action of multiple sets of LepRb neurons (including those in the PMv, the StHy/POA, and potentially others) acting via at least two downstream mechanisms (i.e. via Kiss1 and GnRH neurons). Although our negative findings in this area cannot unequivocally rule out the possibility that LepRb neurons in the brain stem or elsewhere contact GnRH neurons, we have no evidence to support this idea. Although a potential role for StHy/POA LepRb neurons in reproductive control has not been examined, numerous observations suggest roles for PMv LepRb neurons in neuroendocrine reproductive function (51, 52). Not only do PMv LepRb neurons respond to sexual cues and lie in close contact with GnRH neurons (38, 52), but PMv lesions blunt the ability of exogenous leptin to support reproduction in low leptin states (52). Because the PMv is also known as a point for the control of reproductive behavior, it is also possible that leptin action via PMv neurons controls these behaviors (8), in addition to neuroendocrine reproductive function. A potential role for ARC proopiomelanocortin neurons in the control of reproduction has also been proposed (53); the lack of substantial ARC WGA-IR in BIG mice may indicate that proopiomelanocortin neurons do not directly innervate GnRH soma (54), but might instead innervate and modulate the function of Kiss1 neurons (55).
Although our data regarding the lack of LepRb expression in GnRH neurons and AVPV Kiss1 neurons are consistent with most reports in the literature, some confusion exists regarding the extent of potential LepRb expression in ARC Kiss1/KNDy neurons. An initial report using ISH suggested that as many as 40% of mouse ARC Kiss1 neurons contain Leprb mRNA (30). More recently, another group estimated that 20% of mouse ARC neurons containing Kiss1 mRNA colocalize with leptin-induced pSTAT3-IR and that deletion of LepRb from Kiss1 neurons decreases the amount of colocalization (52). Using genetic markers for Kiss1 neurons and pSTAT3 colocalization, this same group suggested that approximately 10% of murine ARC Kiss1 neurons contain functional LepRb, however (37). In contrast, our data suggest 5–6% of ARC Tac2 KNDy neurons contain functional LepRb in the mouse, and we were unable to detect functional LepRb in ovine ARC Kiss1 neurons. Given the modest anatomical resolution of ISH and the density of LepRb neurons in the ARC, it is possible that the studies using ISH in the ARC could overestimate the extent of LepRb/Kiss1 coexpression, given that some neurons that appear colocalized could merely overlie each other. The extent of functional LepRb/Kiss1 coexpression in the murine ARC is thus likely to lie on the lower end of the available estimates (in the range of 5–10%) and may be lower in other species (such as the sheep). Indeed, the deletion of LepRb from Kiss1 neurons promoted no detectable change in metabolic or reproductive function in mice (52). Thus, these genetic data are consistent with the notion that the amount of functional LepRb/Kiss1 coexpression is negligible and support the notion that leptin likely acts trans-synaptically to modulate Kiss1 neurons.
Collectively, our present data elucidate mechanisms by which leptin may modulate the neuroendocrine reproductive axis; leptin acts upstream of both Kiss1 and GnRH neurons, via multiple population(s) of leptin-responsive neurons. In addition to determining the specific roles for PMv and StHy LepRb neurons in reproductive control, it will be crucial in the future to identify the populations of LepRb neurons that project to Kiss1 neurons and examine their roles in reproductive homeostasis.
Acknowledgments
We thank members of the Myers laboratory, especially Rebecca Leshan, for discussions and experimental support throughout, and Z. Z. Zhang for technical assistance. We thank Amylin Pharmaceuticals for the generous gift of leptin.
This work was supported by National Institutes of Health (NIH) Grants DK057768 and DK078056 (to M.G.M.); R01 HD39916 and P01 HD44232 (Project 2) (to M.N.L.); grants from the American Diabetes Association and American Heart Association, and the Marilyn H. Vincent Foundation (to M.G.M.); the Reproductive Studies Program training grant (NIHT32 HD007048) (to G.W.L.); and the Systems and Integrative Biology training grant (NIH T32 GM008322)(to M.G.Y.) at the University of Michigan. Core support was provided by The University of Michigan Cancer Center (NIH Grant CA46592) and the University of Michigan Diabetes Research and Training Center (NIH Grant DK20572).
Present Address for G.W.L.: US Environmental Protection Agency, Toxicity Assessment Division, MD-72 Endocrine Toxicology Branch, Research Triangle Park, North Carolina 27711.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- ARC
- Arcuate nucleus
- AVPV
- anteroventral periventricular nucleus
- BL
- barley lectin
- CNS
- central nervous system
- EGFP
- enhanced green fluorescent protein
- IR
- immunoreactive
- ISH
- in situ hybridization
- Kiss1
- kisspeptin
- LepRb
- leptin receptor
- PMv
- ventral premammillary
- POA
- preoptic area
- RT
- room temperature
- StHy
- striohypothalamic nucleus
- Tac2
- tachykinin 2
- WGA
- wheat germ agglutinin.
References
- 1. Ahima RS, Prabakaran D, Mantzoros C, Qu D, Lowell B, Maratos-Flier E, Flier JS. 1996. Role of leptin in the neuroendocrine response to fasting. Nature 382:250–252 [DOI] [PubMed] [Google Scholar]
- 2. Chehab FF, Lim ME, Lu R. 1996. Correction of the sterility defect in homozygous obese female mice by treatment with the human recombinant leptin. Nat Genet 12:318–320 [DOI] [PubMed] [Google Scholar]
- 3. Friedman JM, Halaas JL. 1998. Leptin and the regulation of body weight in mammals. Nature 395:763–770 [DOI] [PubMed] [Google Scholar]
- 4. Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. 1994. Positional cloning of the mouse obese gene and its human homologue. Nature 372:425–432 [DOI] [PubMed] [Google Scholar]
- 5. Halaas JL, Gajiwala KS, Maffei M, Cohen SL, Chait BT, Rabinowitz D, Lallone RL, Burley SK, Friedman JM. 1995. Weight-reducing effects of the plasma protein encoded by the obese gene. Science 269:543–546 [DOI] [PubMed] [Google Scholar]
- 6. Hill JW, Elmquist JK, Elias CF. 2008. Hypothalamic pathways linking energy balance and reproduction. Am J Physiol Endocrinol Metab 294:E827–E832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Louis GW, Myers MG., Jr 2007. The role of leptin in the regulation of neuroendocrine function and CNS development. Rev Endocr Metab Disord 8:85–94 [DOI] [PubMed] [Google Scholar]
- 8. Klingerman CM, Krishnamoorthy K, Patel K, Spiro AB, Struby C, Patel A, Schneider JE. 2010. Energetic challenges unmask the role of ovarian hormones in orchestrating ingestive and sex behaviors. Horm Behav 58:563–574 [DOI] [PubMed] [Google Scholar]
- 9. Schneider JE, Casper JF, Barisich A, Schoengold C, Cherry S, Surico J, DeBarba A, Fabris F, Rabold E. 2007. Food deprivation and leptin prioritize ingestive and sex behavior without affecting estrous cycles in Syrian hamsters. Horm Behav 51:413–427 [DOI] [PubMed] [Google Scholar]
- 10. Barash IA, Cheung CC, Weigle DS, Ren H, Kabigting EB, Kuijper JL, Clifton DK, Steiner RA. 1996. Leptin is a metabolic signal to the reproductive system. Endocrinology 137:3144–3147 [DOI] [PubMed] [Google Scholar]
- 11. Frederich RC, Hamann A, Anderson S, Löllmann B, Lowell BB, Flier JS. 1995. Leptin levels reflect body lipid content in mice: evidence for diet-induced resistance to leptin action. Nat Med 1:1311–1314 [DOI] [PubMed] [Google Scholar]
- 12. Nagatani S, Guthikonda P, Thompson RC, Tsukamura H, Maeda KI, Foster DL. 1998. Evidence for GnRH regulation by leptin: leptin administration prevents reduced pulsatile LH secretion during fasting. Neuroendocrinology 67:370–376 [DOI] [PubMed] [Google Scholar]
- 13. Campfield LA, Smith FJ, Guisez Y, Devos R, Burn P. 1995. Recombinant mouse OB protein: evidence for a peripheral signal linking adiposity and central neural networks. Science 269:546–549 [DOI] [PubMed] [Google Scholar]
- 14. de Luca C, Kowalski TJ, Zhang Y, Elmquist JK, Lee C, Kilimann MW, Ludwig T, Liu SM, Chua SC., Jr 2005. Complete rescue of obesity, diabetes, and infertility in db/db mice by neuron-specific LEPR-B transgenes. J Clin Invest 115:3484–3493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Watanobe H. 2002. Leptin directly acts within the hypothalamus to stimulate gonadotropin-releasing hormone secretion in vivo in rats. J Physiol 545:255–268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Finn PD, Cunningham MJ, Pau KY, Spies HG, Clifton DK, Steiner RA. 1998. The stimulatory effect of leptin on the neuroendocrine reproductive axis of the monkey. Endocrinology 139:4652–4662 [DOI] [PubMed] [Google Scholar]
- 17. Quennell JH, Mulligan AC, Tups A, Liu X, Phipps SJ, Kemp CJ, Herbison AE, Grattan DR, Anderson GM. 2009. Leptin indirectly regulates gonadotropin-releasing hormone neuronal function. Endocrinology 150:2805–2812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kotani M, Detheux M, Vandenbogaerde A, Communi D, Vanderwinden JM, Le Poul E, Brézillon S, Tyldesley R, Suarez-Huerta N, Vandeput F, Blanpain C, Schiffmann SN, Vassart G, Parmentier M. 2001. The metastasis suppressor gene KiSS-1 encodes kisspeptins, the natural ligands of the orphan G protein-coupled receptor GPR54. J Biol Chem 276:34631–34636 [DOI] [PubMed] [Google Scholar]
- 19. Muir AI, Chamberlain L, Elshourbagy NA, Michalovich D, Moore DJ, Calamari A, Szekeres PG, Sarau HM, Chambers JK, Murdock P, Steplewski K, Shabon U, Miller JE, Middleton SE, Darker JG, Larminie CG, Wilson S, Bergsma DJ, Emson P, Faull R, Philpott KL, Harrison DC. 2001. AXOR12, a novel human G protein-coupled receptor, activated by the peptide KiSS-1. J Biol Chem 276:28969–28975 [DOI] [PubMed] [Google Scholar]
- 20. Han SK, Gottsch ML, Lee KJ, Popa SM, Smith JT, Jakawich SK, Clifton DK, Steiner RA, Herbison AE. 2005. Activation of gonadotropin-releasing hormone neurons by kisspeptin as a neuroendocrine switch for the onset of puberty. J Neurosci 25:11349–11356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Irwig MS, Fraley GS, Smith JT, Acohido BV, Popa SM, Cunningham MJ, Gottsch ML, Clifton DK, Steiner RA. 2004. Kisspeptin activation of gonadotropin releasing hormone neurons and regulation of KiSS-1 mRNA in the male rat. Neuroendocrinology 80:264–272 [DOI] [PubMed] [Google Scholar]
- 22. Messager S, Chatzidaki EE, Ma D, Hendrick AG, Zahn D, Dixon J, Thresher RR, Malinge I, Lomet D, Carlton MB, Colledge WH, Caraty A, Aparicio SA. 2005. Kisspeptin directly stimulates gonadotropin-releasing hormone release via G protein-coupled receptor 54. Proc Natl Acad Sci USA 102:1761–1766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Seminara SB, Messager S, Chatzidaki EE, Thresher RR, Acierno JS, Jr, Shagoury JK, Bo-Abbas Y, Kuohung W, Schwinof KM, Hendrick AG, Zahn D, Dixon J, Kaiser UB, Slaugenhaupt SA, Gusella JF, O'Rahilly S, Carlton MB, Crowley WF, Jr, Aparicio SA, Colledge WH. 2003. The GPR54 gene as a regulator of puberty. N Engl J Med 349:1614–1627 [DOI] [PubMed] [Google Scholar]
- 24. Funes S, Hedrick JA, Vassileva G, Markowitz L, Abbondanzo S, Golovko A, Yang S, Monsma FJ, Gustafson EL. 2003. The KiSS-1 receptor GPR54 is essential for the development of the murine reproductive system. Biochem Biophys Res Commun 312:1357–1363 [DOI] [PubMed] [Google Scholar]
- 25. Kauffman AS, Park JH, McPhie-Lalmansingh AA, Gottsch ML, Bodo C, Hohmann JG, Pavlova MN, Rohde AD, Clifton DK, Steiner RA, Rissman EF. 2007. The kisspeptin receptor GPR54 is required for sexual differentiation of the brain and behavior. J Neurosci 27:8826–8835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lapatto R, Pallais JC, Zhang D, Chan YM, Mahan A, Cerrato F, Le WW, Hoffman GE, Seminara SB. 2007. Kiss1−/− mice exhibit more variable hypogonadism than Gpr54−/− mice. Endocrinology 148:4927–4936 [DOI] [PubMed] [Google Scholar]
- 27. d'Anglemont de Tassigny X, Fagg LA, Dixon JP, Day K, Leitch HG, Hendrick AG, Zahn D, Franceschini I, Caraty A, Carlton MB, Aparicio SA, Colledge WH. 2007. Hypogonadotropic hypogonadism in mice lacking a functional Kiss1 gene. Proc Natl Acad Sci USA 104:10714–10719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gottsch ML, Cunningham MJ, Smith JT, Popa SM, Acohido BV, Crowley WF, Seminara S, Clifton DK, Steiner RA. 2004. A role for kisspeptins in the regulation of gonadotropin secretion in the mouse. Endocrinology 145:4073–4077 [DOI] [PubMed] [Google Scholar]
- 29. Kinoshita M, Tsukamura H, Adachi S, Matsui H, Uenoyama Y, Iwata K, Yamada S, Inoue K, Ohtaki T, Matsumoto H, Maeda K. 2005. Involvement of central metastin in the regulation of preovulatory luteinizing hormone surge and estrous cyclicity in female rats. Endocrinology 146:4431–4436 [DOI] [PubMed] [Google Scholar]
- 30. Smith JT, Acohido BV, Clifton DK, Steiner RA. 2006. KiSS-1 neurones are direct targets for leptin in the ob/ob mouse. J Neuroendocrinol 18:298–303 [DOI] [PubMed] [Google Scholar]
- 31. Goodman RL, Lehman MN, Smith JT, Coolen LM, de Oliveira CV, Jafarzadehshirazi MR, Pereira A, Iqbal J, Caraty A, Ciofi P, Clarke IJ. 2007. Kisspeptin neurons in the arcuate nucleus of the ewe express both dynorphin A and neurokinin B. Endocrinology 148:5752–5760 [DOI] [PubMed] [Google Scholar]
- 32. Navarro VM, Gottsch ML, Chavkin C, Okamura H, Clifton DK, Steiner RA. 2009. Regulation of gonadotropin-releasing hormone secretion by kisspeptin/dynorphin/neurokinin B neurons in the arcuate nucleus of the mouse. J Neurosci 29:11859–11866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lehman MN, Coolen LM, Goodman RL. 2010. Minireview: kisspeptin/neurokinin B/dynorphin (KNDy) cells of the arcuate nucleus: a central node in the control of gonadotropin-releasing hormone secretion. Endocrinology 151:3479–3489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Castellano JM, Navarro VM, Fernández-Fernández R, Nogueiras R, Tovar S, Roa J, Vazquez MJ, Vigo E, Casanueva FF, Aguilar E, Pinilla L, Dieguez C, Tena-Sempere M. 2005. Changes in hypothalamic KiSS-1 system and restoration of pubertal activation of the reproductive axis by kisspeptin in undernutrition. Endocrinology 146:3917–3925 [DOI] [PubMed] [Google Scholar]
- 35. Luque RM, Kineman RD, Tena-Sempere M. 2007. Regulation of hypothalamic expression of KiSS-1 and GPR54 genes by metabolic factors: analyses using mouse models and a cell line. Endocrinology 148:4601–4611 [DOI] [PubMed] [Google Scholar]
- 36. Castellano JM, Navarro VM, Fernández-Fernández R, Roa J, Vigo E, Pineda R, Dieguez C, Aguilar E, Pinilla L, Tena-Sempere M. 2006. Expression of hypothalamic KiSS-1 system and rescue of defective gonadotropic responses by kisspeptin in streptozotocin-induced diabetic male rats. Diabetes 55:2602–2610 [DOI] [PubMed] [Google Scholar]
- 37. Cravo RM, Margatho LO, Osborne-Lawrence S, Donato J, Jr, Atkin S, Bookout AL, Rovinsky S, Frazão R, Lee CE, Gautron L, Zigman JM, Elias CF. 2011. Characterization of Kiss1 neurons using transgenic mouse models. Neuroscience 173:37–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Leshan RL, Louis GW, Jo YH, Rhodes CJ, Münzberg H, Myers MG., Jr 2009. Direct innervation of GnRH neurons by metabolic- and sexual odorant-sensing leptin receptor neurons in the hypothalamic ventral premammillary nucleus. J Neurosci 29:3138–3147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Leshan RL, Opland DM, Louis GW, Leinninger GM, Patterson CM, Rhodes CJ, Münzberg H, Myers MG., Jr 2010. Ventral tegmental area leptin receptor neurons specifically project to and regulate cocaine- and amphetamine-regulated transcript neurons of the extended central amygdale. J Neurosci 30:5713–5723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Boehm U, Zou Z, Buck LB. 2005. Feedback loops link odor and pheromone signaling with reproduction. Cell 123:683–695 [DOI] [PubMed] [Google Scholar]
- 41. Münzberg H, Jobst EE, Bates SH, Jones J, Villanueva E, Leshan R, Björnholm M, Elmquist J, Sleeman M, Cowley MA, Myers MG., Jr 2007. Appropriate inhibition of orexigenic hypothalamic arcuate nucleus neurons independently of leptin receptor/STAT3 signaling. J Neurosci 27:69–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Smith JT, Coolen LM, Kriegsfeld LJ, Sari IP, Jaafarzadehshirazi MR, Maltby M, Bateman K, Goodman RL, Tilbrook AJ, Ubuka T, Bentley GE, Clarke IJ, Lehman MN. 2008. Variation in kisspeptin and RFamide-related peptide (RFRP) expression and terminal connections to gonadotropin-releasing hormone neurons in the brain: a novel medium for seasonal breeding in the sheep. Endocrinology 149:5770–5782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Patterson CM, Leshan RL, Jones JC, Myers MG., Jr 2011. Molecular mapping of mouse brain regions innervated by leptin receptor-expressing cells, Brain Res 1348:18–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Leinninger GM, Jo YH, Leshan RL, Louis GW, Yang H, Barrera JG, Wilson H, Opland DM, Faouzi MA, Gong Y, Jones JC, Rhodes CJ, Chua S, Jr, Diano S, Horvath TL, Seeley RJ, Becker JB, Munzberg H, Myers MG., Jr 2009. Leptin acts via leptin receptor-expressing lateral hypothalamic neurons to modulate the mesolimbic dopamine system and suppress feeding. Cell Metab 10:89–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Elmquist JK, Bjørbaek C, Ahima RS, Flier JS, Saper CB. 1998. Distributions of leptin receptor mRNA isoforms in the rat brain. J Comp Neurol 395:535–547 [PubMed] [Google Scholar]
- 46. Scott MM, Lachey JL, Sternson SM, Lee CE, Elias CF, Friedman JM, Elmquist JK. 2009. Leptin targets in the mouse brain. J Comp Neurol 514:518–532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Banks AS, Davis SM, Bates SH, Myers MG., Jr 2000. Activation of downstream signals by the long form of the leptin receptor. J Biol Chem 275:14563–14572 [DOI] [PubMed] [Google Scholar]
- 48. Münzberg H, Huo L, Nillni EA, Hollenberg AN, Bjørbaek C. 2003. Role of signal transducer and activator of transcription 3 in regulation of hypothalamic proopiomelanocortin gene expression by leptin. Endocrinology 144:2121–2131 [DOI] [PubMed] [Google Scholar]
- 49. White DW, Kuropatwinski KK, Devos R, Baumann H, Tartaglia LA. 1997. Leptin receptor (OB-R) signaling. Cytoplasmic domain mutational analysis and evidence for receptor homo-oligomerization. J Biol Chem 272:4065–4071 [DOI] [PubMed] [Google Scholar]
- 50. Robertson SA, Leinninger GM, Myers MG., Jr 2008. Molecular and neural mediators of leptin action. Physiol Behav 94:637–642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Donato J, Jr, Silva RJ, Sita LV, Lee S, Lee C, Lacchini S, Bittencourt JC, Franci CR, Canteras NS, Elias CF. 2009. The ventral premammillary nucleus links fasting-induced changes in leptin levels and coordinated luteinizing hormone secretion. J Neurosci 29:5240–5250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Donato J, Jr, Cravo RM, Frazão R, Gautron L, Scott MM, Lachey J, Castro IA, Margatho LO, Lee S, Lee C, Richardson JA, Friedman J, Chua S, Jr, Coppari R, Zigman JM, Elmquist JK, Elias CF. 2011. Leptin's effect on puberty in mice is relayed by the ventral premammillary nucleus and does not require signaling in Kiss1 neurons. J Clin Invest 121:355–368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Backholer K, Bowden M, Gamber K, Bjørbaek C, Iqbal J, Clarke IJ. 2010. Melanocortins mimic the effects of leptin to restore reproductive function in lean hypogonadotropic ewes. Neuroendocrinology 91:27–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Rondini TA, Baddini SP, Sousa LF, Bittencourt JC, Elias CF. 2004. Hypothalamic cocaine- and amphetamine-regulated transcript neurons project to areas expressing gonadotropin releasing hormone immunoreactivity and to the anteroventral periventricular nucleus in male and female rats. Neuroscience 125:735–748 [DOI] [PubMed] [Google Scholar]
- 55. Backholer K, Smith JT, Rao A, Pereira A, Iqbal J, Ogawa S, Li Q, Clarke IJ. 2010. Kisspeptin cells in the ewe brain respond to leptin and communicate with neuropeptide Y and proopiomelanocortin cells. Endocrinology 151:2233–2243 [DOI] [PubMed] [Google Scholar]