Transgenic expression of the MATER autoantigen in antigen-presenting cells significantly mitigates autoimmune oophoritis in mice with implications for diagnosis and tolerance induction in human autoimmune primary ovarian insufficiency.
Abstract
Primary ovarian insufficiency (POI) resulting from ovarian autoimmunity is a poorly understood clinical condition lacking in effective treatments. Understanding the targets of the autoimmune response and induction of ovarian-specific tolerance would allow development of focused therapies to preserve fertility in an at-risk population. MATER (maternal antigen that embryos require) is a known ovarian autoantigen targeted in autoimmune syndromes of POI. We attempt to induce ovarian-specific tolerance via transgenic expression of the MATER antigen on potentially tolerogenic antigen-presenting cells (APC), which typically present antigen via the major histocompatibility complex (MHC) class II molecule. We hypothesize that expression of MATER in a MHC class II-dependent manner on APC can mediate induction of ovarian tolerance.
We utilized a well-characterized murine model of ovarian autoimmunity, whereby oophoritis develops after d 3 neonatal thymectomy (NTx). Wild-type and transgenic mice, carrying an MHC Class II-driven Mater gene (IE-Mater), were subjected to NTx and assessed for evidence of autoimmune oophoritis. After disease induction by NTx, female mice carrying the IE-Mater transgene had significant reductions in histological oophoritis (56%) and circulating ovarian autoantibodies (28%) compared with wild-type females (94% and 82%, respectively). Incidence of other autoimmunity was unaffected as assessed by antinuclear autoantibodies. Transgenic expression of MATER in APC can induce antigen-specific tolerance with a significant reduction in ovarian autoimmunity. Lack of complete disease protection suggests that other antigens may also play a role in autoimmune oophoritis. As a known autoantigen in the human APS1 (autoimmune polyglandular syndrome type 1), which is associated with POI, MATER may represent a relevant target for future diagnostic and therapeutic clinical interventions.
Primary ovarian insufficiency (POI), also known as premature menopause and premature ovarian failure, represents a significant cause of morbidity and reduced fertility affecting approximately 1% of women in the United States by age 40. POI is diagnosed in women less than 40 yr of age by the presence of oligo/amenorrhea for 4 months or more with at least two serum FSH levels in the menopausal range (separated by at least 1 month). Clinical manifestations of the disease are quite variable, with intermittent ovarian function in 50% of women and even occasional conception after diagnosis, suggesting a continuum of impaired ovarian function more appropriately described as POI (1, 2). The majority of cases of POI have an unclear etiology, but growing evidence suggests that autoimmune impairment of ovarian function may be a significant contributor.
SCA-POI (steroidogenic cell autoimmunity as a mechanism of POI) is characterized clinically by oophoritis with lymphocytic infiltrates into growing follicles and relative sparing of primordial follicles. Although disease is thought to be T cell mediated, autoantibodies to specific steroidogenic enzyme antigens are characteristically seen in these patients (3, 4), suggesting cognate antigen recognition. However, the relevance of specific ovarian antigens to disease pathogenesis remains unclear, leaving us with limited diagnostic or therapeutic tools to intervene in a population whose ovarian function might be preserved with timely therapy. Fortunately, work in experimental mouse models has provided insight into the mechanisms of ovarian autoimmunity.
In multiple strains of inbred mice, thymectomy at d 3 after birth induces a variety of organ-specific autoimmune diseases, notably oophoritis and ovarian failure in 90% of C57Bl/6xA/J F1 mice (5–7). Autoimmunity in the neonatal thymectomy (NTx) model develops in part through an imbalance between regulatory and effector T cells. Disease arises from the relative depletion of CD4+CD25+ regulatory T cells (Tregs), which emigrate from the thymus after d 3, and replacement of Tregs in NTx mice prevents autoimmune disease (8). Like women with SCA-POI, female mice that undergo NTx develop lymphocytic infiltrates and autoantibodies to several ovarian targets. The predominant, earliest, and most robust antibody response occurs against oocyte proteins, suggesting a possible equivalent mechanism for human autoimmune primary ovarian insufficiency targeted against oocyte proteins, or what might be termed “OA-POI.”
Characterization of the ovarian autoantibodies that develop in NTx mice allowed the identification of a novel ovarian antigen known as MATER (maternal antigen that embryos require) (9). Mater encodes a 125-kDa protein that is highly expressed in oocytes. Inactivation of the mouse Mater gene causes embryos from Mater knockout mothers to arrest at the two-cell stage and eventually degenerate, indicating that Mater is required for embryonic development after fertilization (10). The human homolog for mouse Mater also shows largely ovarian-specific expression (11). MATER is also known as NACHT leucine-rich-repeat protein 5 (NALP5, now also known as NLRP5), a member of the CATERPILLER family of proteins that have roles in immunity, cell death, and inflammatory responses (12). Recent work has also identified NALP5 as a potential autoantigen in patients with autoimmune polyglandular syndrome type 1 (APS1) who are affected with autoimmune parathyroid disease (13). Notably, many patients with APS1 with NALP5 autoantibodies also had POI. Thus, MATER/NALP5 represents a putative target of ovarian disease in women with autoimmune-mediated POI.
Work in the NTx model has demonstrated that the ability of polyclonal Tregs to suppress autoimmunity is disease specific and dependent on exposure to endogenous autoantigen in the periphery (7). Additionally, expression of self-antigen in the context of MHC class II in the thymus and the periphery has been shown to mediate immune tolerance in several models of autoimmunity (14). We hypothesized that expression of MATER in a MHC class II-dependent manner could mediate induction of ovarian tolerance. We have thus taken advantage of the mouse MHC II I-E transgenic system to drive expression of MATER in order to assess its effect on tolerance induction. Here we show that transgenic expression of MATER in the thymus and secondary lymphoid system is sufficient to mediate a significant reduction in autoimmune oophoritis. Suppression of autoimmunity is disease- and antigen-specific, as demonstrated by the continued detection of other autoantibodies. In those cases where oophoritis persists, autoantibodies to other ovarian proteins are detected, suggesting that multiple antigens may play a role in the pathogenesis of autoimmune oophoritis in this model. Together, these results indicate that MATER is a significant pathogenic antigen in NTx-induced ovarian autoimmunity and an attractive candidate antigen for the development of diagnostic testing, as well as for induction of tolerance in human autoimmune oophoritis.
Materials and Methods
Mice
Experiments were performed under an approved animal protocol in compliance with the Animal Welfare Act and National Institutes of Health (NIH) guidelines for animal care and research. Wild-type C57BL/6J and A/J mice were obtained from the Frederick Cancer Resource Facility (National Cancer Institute, Frederick, MD), and transgenic mice were generated in house. Wild-type and transgenic female C57BL/6J mice were mated with wild-type male A/J mice to generate F1 (B6AF1) mice for NTx (15, 16) at age 2–4 d. Sham operations were identical but left the thymus intact. Sera were collected by tail bleeds at age 6–10 wk. Incompletely thymectomized mice were identified on necropsy and excluded from analysis.
Transgene DNA and transgenic mouse generation
A plasmid (pIE/Mater/BGH-2) carrying the IE-Mater transgene (∼6.5 kb) was constructed in pBluescript KS II vector (Stratagene, La Jolla, CA) to contain the promoter region of the mouse MHC Class II I-Eακ gene [1.92 kb, nucleotides (nt) −39 to −1964; gift of Dr. Frank Alderuccio at Monash University, Victoria, Australia], a nearly full-length mouse Mater cDNA (nt 74–3438), and a DNA fragment (∼310 bp) encoding bovine GH (BGH) polyadenylation and terminal signals (pRc/CMV; Invitrogen, Carlsbad, CA) (see Fig. 2A). After restriction digest and agarose gel electrophoresis, the transgene fragment was purified (QIAGEN DNA purification kit; QIAGEN, Valencia, CA) for pronuclear injection to generate transgenic mice in a standard protocol. Briefly, DNA was dissolved in buffer (10 mm Tris-HCl, pH 7.5; 0.1 mm EDTA) and injected (5–10 ρl at 1 ng/ml) into the male pronucleus of one-cell zygotes (C57BL/6J). Mice delivered from foster mothers (C57BL/6J) were screened for founders that carried the IE-Mater transgene by Southern hybridization and PCR.
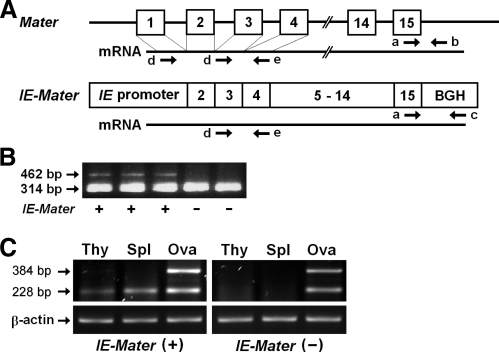
Fig. 2.
IE-Mater transgene and its expression. A, Schematic representation of mouse Mater (upper) and the IE-Mater transgene (lower). Exons are numbered and shown as open boxes with black lines between exons representing intronic sequence (drawing not to scale). Solid lines under each gene represent mRNA transcripts. Arrows indicate the directions (5′ to 3′) and positions of the primers used for PCR genotyping (a, b, and c) and RT-PCR (d and e). Note that the omission of exon 1 from the IE-Mater transgene allows differential amplification of transcripts from endogenous Mater (two products) compared with IE-Mater (single product) on RT-PCR. Exons 1 and 3 encode repetitive sequences within the protein, and loss of exon 1 results in an N-terminal truncation of 25 amino acids in the transgenic MATER antigen. B, PCR for genotyping mice. Three primers (a, b, and c) were used in PCR amplification of genomic tail DNA for genotyping. Primers a and b target the endogenous Mater gene to produce a 314-bp product, whereas the primers a and c target the IE-Mater transgene to produce a 462-bp product. Genotypes (IE-Mater + or −) are indicated on the bottom of the panel. C, RT-PCR for transgene expression. The mRNA was isolated from the thymus (Thy), spleen (Spl), and ovary (Ova) of the IE-Mater transgenic mice (left) and wild-type mice (right) for RT (RT) using oligo-dT primers. PCR were carried out using Mater-specific primers (d and e). Two PCR products (228 bp and 384 bp) were amplified from ovarian cDNA (Ova) of both IE-Mater transgenic (left) and wild-type (right) mice because the forward primer sequence (d) is repeated in both exons 1 and 3. Because the IE-Mater transgene lacks exon 1, only one PCR product (228 bp) was obtained from cDNA of the IE-Mater transgenic mouse thymus (Thy) and spleen (Spl) in the left panel, indicating transgenic but not endogenous expression of Mater in nonovarian lymphoid tissue.
PCR and genotyping mice
At age 3–4 wk mice were genotyped by Southern blot of genomic tail DNA to confirm the IE-Mater transgene in the first several generations. Thereafter, mice were genotyped by PCR of tail DNA. Three primers were included in each PCR to differentially amplify fragments from the endogenous Mater gene (314 bp) or the IE-Mater transgene (462 bp). One forward primer (primer a, 5′-GATGAAGATGACCGAAACTG-3′; nt 3312–3331) was specific for sequence within Mater exon 15, whereas two reverse primers were complementary to sequence immediately after Mater exon 15 (primer b, 5′-CCAGCACTGGAATTACAAGC-3′) and the BGH polyadenylation/terminal signals (primer c, 5′-ATTCCGCCTCAGAAGCCATAG-3′), respectively (see Fig. 2A). PCR were carried out in 15 μl volumes (95 C, 5 min; 35 cycles of 95 C, 30 min, 60 C, 30 min and 72 C, 60 min; and 72 C, 10 min) using a DNA polymerase kit (Invitrogen) and visualized by standard agarose (2%) gel electrophoresis.
RT-PCR
RNA was extracted from thymus, spleen, and ovaries of wild-type (C57BL/6J) and IE-Mater transgenic mice (QIAGEN RNeasy Mini Kit with RNase-Free DNase Set) followed by reverse transcription (RT) (QIAGEN Omniscript RT Kit) of the purified mRNA with oligo dT18 primers. PCR amplification after RT reactions was performed for Mater and β-actin. Sequences of primers specific for Mater transcripts were 5′-CAATGGGTCCTCCAGAAAAA-3′ (primer d, forward, nt 7–26, and nt 163–182) and 5′-ATGTCTGCTCCTGCTCTGGT-3′(primer e, reverse, nt 371–390). Because forward primer sequences repeat in exon 1 (nt 7–26) and exon 3 (nt 163–182), and the IE-Mater transgene does not contain exon 1, two PCR products (228 and 384 bp) were amplified from the normal endogenous Mater transcripts whereas only one PCR product (228 bp) was amplified from the transgene transcripts. β-actin transcripts (324 bp) were amplified using primers 5′-GCTCTAGACTTCGAGCAGGAGA-3′ (forward, nt 738–759) and 5′-GATCTTCATGGTGCTAGG-3′(reverse, nt 1044–1061).
Ovarian histology
Mouse ovaries were fixed in 4% neutralized formalin and paraffin embedded. Hematoxylin and eosin-stained ovarian sections (8 μm thickness) were scored in a blinded manner by light microscopy for the presence of autoimmune oophoritis, based on the system of Tung and colleagues (17, 18) with modification for numbers of follicles observed. Oophoritis was graded from 0–4 (0, no inflammation and normal follicular development; 1, focal inflammation in interstitial space and slightly reduced follicular number; 2 and 3, increasing multifocal inflammatory foci and/or granuloma between and within ovarian follicles and/or reduced number of developing follicles; and 4, loss of ovarian follicles and ovarian atrophy). Ovaries with lymphoplasmacytic infiltration and/or fibrosis with atrophy and depletion of ovarian follicles were regarded as positive for autoimmune oophoritis (AO positive = scores 1– 4).
Indirect immunofluorescence
Ovaries from wild-type and Mater null mice were frozen, sectioned at 8 μm thickness, and fixed with cold acetone for 1 min. After washing with PBS buffer (pH 7.4) three times and incubation with blocking buffer (5% dry milk in PBS) for 1 h, sections were incubated with mouse sera (1:10 dilution) for 1 h at room temperature. After extensive washing, sections were incubated with a fluorescein isothiocyanate (FITC)-conjugated goat antimouse IgG antibody (1:1000 dilution; Sigma-Aldrich, St. Louis, MO). Immunofluorescence was visualized under a Leica fluorescence microscope. Sera from normal mice or the sham-operated mice served as negative controls.
Immunoblotting
Oocytes were dissected from ovaries of wild-type mice at age of 3–5 wk. Samples (12 oocytes each) were dissolved in Laemmli buffer (Bio-Rad Laboratories, Inc., Hercules, CA) and resolved by gel electorphoresis (4–12% NuPAGE gel system, Invitrogen). Gels were transferred onto nitrocellulose membranes at 100 V, and the membranes were blocked (5% dry milk in PBS) at room temperature for 1 h. The blot was incubated with the individual serum diluted at 1:50 with 5% dry milk in PBS containing 0.1% Tween-20 at 4 C overnight and then washed with PBS containing 0.1% Tween-20. Blots were incubated with secondary (fluorescein-conjugated goat antimouse IgG at 1:600) and tertiary (alkaline phosphate-conjugated antifluorescein at 1:2500) antibodies per instructions of the ECF kit (GE Healthcare, Piscataway, NJ). Immunoblots were developed with the ECF substrate [2′-(2-benzothiazoyl)-6′-hydroxybenzothiazole phosphate] and detected with a fluoroimager (FLA-2000, FUJI, Japan). Rabbit antimouse MATER antibody was used as a positive control.
ELISA
Antinuclear antibodies (ANA) in mouse sera were measured by ELISA (kit, Alpha Diagnostic, San Antonio, TX) per the manufacturer's protocol. Briefly, sera were diluted (1:100) with the provided sample buffer, and 100-μl aliquots were assayed in duplicate in a 96-well plate format. After incubation with diluted goat antimouse IgG (heavy and light chains)-horseradish peroxidase conjugate (1X), antibodies were detected by addition of chromogenic substrate (3,3′,5,5′-tetramentylbenzidine). Absorbance at 450 nm was measured using an automatic microplate reader (PerkinElmer, Shelton, CT).
Statistical analysis
Comparisons were made by the Wilcoxon Rank Sum Test using Sigma Stat for Windows, version 2.03 (SPSS, Inc., Chicago, IL).
Results
Multiple ovarian antigenic targets
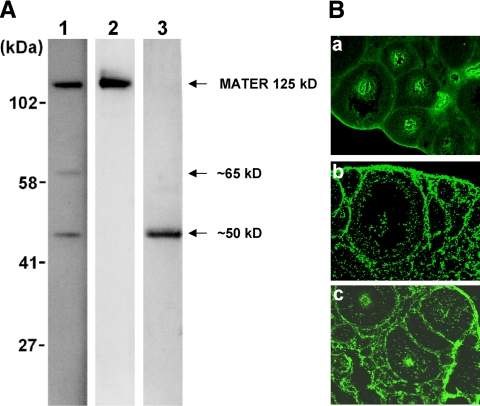
Individual mice with NTx-induced autoimmune oophoritis produced antibodies against oocyte proteins, including either MATER alone or MATER and other unknown proteins (molecular mass, ∼50 and ∼65 kDa) (Fig. 1A). Previous studies have confirmed the identity of the 125-kDa band as MATER (9). In a few animals antibodies to other oocyte proteins were detected without MATER autoreactivity. Outside of MATER, other oocyte proteins targeted in mice with NTx-induced oophoritis remain to be identified. Autoreactivity is not limited to the oocyte in autoimmune oophoritis. On indirect immunofluorescence staining, serum from some mice also demonstrated antibodies to other ovarian compartments, including thecal and stromal cells (Fig. 1B), in a few cases without oocyte autoantibodies (panel b). Thus, multiple ovarian antigens appear to be targeted by disease in NTx-induced oophoritis. In the present study, we aimed to determine the pathogenic role of MATER autoimmunity in this model.
Fig. 1.
Multiple ovarian antigen targets are recognized by sera from mice with autoimmune oophoritis. A, Oocyte antibodies by immunoblotting. Representative blots are shown. Each blot (lane) contains protein lysate of 12 oocytes isolated from wild-type mouse ovaries. Sera from mice with autoimmune oophoritis induced by NTx were individually incubated with each of the blots. Molecular masses (kDa) are indicated on the left of the panel. Arrows indicate MATER at 125 kDa and other unidentified proteins with molecular masses of approximately 50 and 65 kDa. B, Ovarian antibodies by indirect immunofluorescence. Mouse ovarian frozen sections were individually incubated with sera from mice with autoimmune oophoritis induced by NTx. Autoantibodies were detected by a FITC-conjugated goat antimouse IgG antibody. Mice produced antibodies against oocytes alone (a), theca and stroma cells alone (b), or both (c). Scale bar, 100 μm.
Generation of IE-Mater transgenic mice
We generated transgenic mice that would induce immune tolerance to MATER by an approach similar to that used to induce tolerance to the β-subunit of the H/K ATPase in the NTx model of autoimmune gastritis (19). The 5′-flanking region of the I-Eακ gene, one of the major MHC class II genes in mice, was used as a promoter to drive Mater expression (Fig. 2A). Transcriptional regulation of I-Eακ gene has been well characterized, and approximately 2 kb upstream of the cap site is sufficient to direct correct expression of MHC II in thymic epithelium as well as in peripheral B cells, macrophages, and dendritic cells (20, 21). We generated a transgenic mouse line that carries the IE-Mater transgene in the C57BL/6J background. Transgenic founders and transgene transmission in the first several generations were confirmed by Southern hybridization (data not shown). Thereafter, mice were genotyped by PCR (Fig. 2B), with differential amplification products for the endogenous Mater gene (314 bp) and IE-Mater transgene (462 bp). The IE-Mater transgene appeared to be stable, and the transgenic mice have been maintained for more than 5 yr.
We analyzed mRNA expression of the IE-Mater transgene in the thymus and spleen of the transgenic mice by RT-PCR (Fig. 2C). After reverse transcription (RT), we performed PCR using primers that could differentiate between endogenous Mater or transgenic IE-Mater transcripts. Because the forward primer recognizes repeat sequences in exons 1 and 3, two PCR products (228 bp and 384 bp) were amplified from the ovarian cDNA of both the transgenic and wild-type mice (Fig. 2C, lanes 3 and 6). Because the IE-Mater transgene lacks exon 1, only one PCR product (228 bp) was amplified from the cDNA of the transgenic mouse thymus and spleen, indicating that expression of Mater transcripts was driven by the IE promoter in the thymic epithelium and splenic APC of the IE-Mater transgenic mice. As controls for RT-PCR, β-actin expression was confirmed in all tissues, and PCR products were not found in the absence of RT (data not shown).
We made efforts to detect MATER protein in the lymphoid tissue of the IE-Mater transgenic mice. However, using a specific antibody against MATER, we were unable to detect MATER in thymus and spleen by either immunohistochemistry or immunoblotting (data not shown). Thus, the protein expression derived from the transgene mRNA was low or may undergo processing with production of epitopes that are not recognized by our antibodies.
Reduction of ovarian autoimmunity in IE-Mater transgenic mice
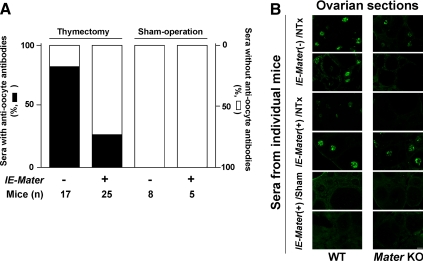
Littermates were thymectomized or sham-operated and assessed for the development of antioocyte antibodies, anti-MATER antibodies, and autoimmune oophoritis. As expected, at 6 wk of age most of the thymectomized wild-type mice generated autoantibodies against oocyte proteins (82.4%, n = 17; Fig. 3A). Strikingly, the incidence of autoantibodies against oocyte proteins in NTx-treated IE-Mater transgenic mice was significantly lower than in the wild-type mice after NTx (28%, n = 25; P = 0.01). None of the sham-operated wild-type mice (n = 8) or IE-Mater transgenic mice (n = 5) produced antibodies against oocyte proteins. Of note, some sera from both wild-type and IE-Mater transgenic mice with NTx-induced autoimmune oophoritis showed autoreactivity to oocyte sections from Mater null mice, suggesting that other oocyte protein(s), in addition to MATER, are involved in the development of disease in this model (Fig. 3B).
Fig. 3.
Antioocyte autoantibodies in wild-type and IE-Mater transgenic mice. A, Incidence. Based on immunofluorescence on frozen ovarian sections (B) and confirmation with the immunoblotting assay, the sera from 82% of the wild-type (n = 17) and 28% of the IE-Mater transgenic (n = 25) NTx-treated mice contained autoantibodies against oocytes. Thus the incidence of antioocyte antibodies was significantly reduced in the thymectomized IE-Mater transgenic mice (P = 0.01). None of the wild-type (n = 8) and IE-Mater transgenic (n = 5) mice with sham operations produced antioocyte antibodies. B, Representative indirect immunofluorescence. Immune sera from mice with confirmed autoimmune oophoritis are capable of reacting with oocyte proteins other than MATER. Shown are results using sera from NTx-treated mice that had histologically confirmed autoimmune oophoritis: wild-type mice (rows 1 and 2) and IE-Mater transgenic mice (rows 3 and 4). Oocyte autoantibodies were detected using FITC-conjugated goat antimouse IgG as the secondary antibody on wild-type frozen mouse ovarian sections (left column) and Mater knockout (KO) mouse frozen ovarian sections (right column). Results using sera from mice without autoimmune oophoritis are also shown (rows 5 and 6). Negative controls with the secondary antibody alone in both wild-type and Mater null ovarian sections gave little fluorescence (data not shown). Scale bar, 100 μm. WT, Wild type.
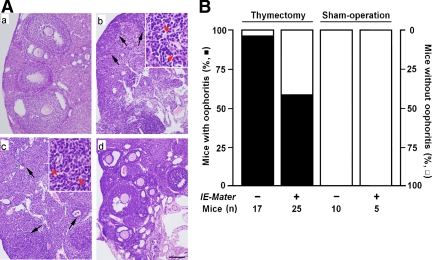
Figure 4A illustrates representative sections contrasting sham-operated normal mouse ovarian histology (a) with NTx-induced autoimmune oophoritis (b and c). Oophoritis is evidenced by lymphocytic infiltration and disruption of normal ovarian architecture. Ovaries were harvested at 8–10 wk of age. As shown in Fig. 4B, 94% of the wild-type mice developed autoimmune oophoritis (n = 17) after NTx as previously reported (5–7). However, only 56% of the NTx-treated IE-Mater transgenic mice developed autoimmune oophoritis (n = 25). Sham operations did not cause oophoritis in either wild-type (n = 10) or IE-Mater transgenic (n = 5) mice. Although disease protection is not seen in all mice, the incidence of NTx-induced autoimmune oophoritis is remarkably reduced in the mice carrying the IE-Mater transgene (P < 0.02). Thus, Mater expression under control of the IE promoter significantly mitigates the development of autoimmune oophoritis induced by NTx.
Fig. 4.
Incidence of autoimmune oophoritis after NTx in wild-type and IE-Mater transgenic mice. A, Histopathology of autoimmune oophoritis after NTx. Representative sections of ovarian histology with hematoxylin and eosin staining are shown for sham-operated wild-type mice (a), thymectomized wild-type mice (b), and thymectomized IE-Mater transgenic mice (c and d) at age 10 wk. Lymphocytic infiltration and ovarian follicular destruction indicating autoimmune oophoritis were observed in most of the thymectomized wild-type (b) and some of the thymectomized IE-Mater transgenic (c) mice. Note the lack of primordial follicles in these sections (b and c). Areas of infiltration are indicated with black arrowheads, and insets show the presence of lymphocytes and plasma cells on higher magnification (panels b and c, red arrowheads). However, the ovarian histology of many of the thymectomized IE-Mater transgenic mice (d) appeared normal. Scale bar, 100 μm. B, Incidence of autoimmune oophoritis in mice after NTx. Based on histological evaluations, 94% of the thymectomized wild-type mice (n = 17) and 56% of the thymectomized IE-Mater transgenic mice (n = 25) developed autoimmune oophoritis (solid bars), and thus the incidence of developing autoimmune oophoritis was significantly reduced (P < 0.02). All of the wild-type (n = 10) and IE-Mater transgenic mice (n = 5) with sham operations showed no evidence for oophoritis (open bars).
Specificity of immune tolerance to MATER in IE-Mater transgenic mice
Reduction in overall ovarian autoimmunity by both histology and oocyte autoantibody measures is seen in IE-Mater transgenic mice undergoing NTx. However, because disease protection is incomplete, we wished to examine the antigen-specific contribution of MATER to these two disease measures. In all NTx-treated mice that develop oophoritis, a pronounced response is seen against MATER antigen. Immunoblotting was performed to define the oocyte protein targets of the autoantibody response in mice with autoimmune oophoritis. In affected wild-type mice with positive antioocyte antibodies (n = 14, see Fig. 3A), more than 93% of the sera recognized MATER (data not shown). Although the predominant response is against MATER, individual sera positive for anti-MATER antibodies in some cases contained autoantibodies against other unidentified oocyte proteins, consistent with our initial data (see Fig. 1).
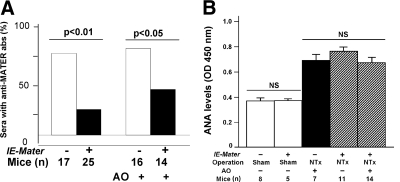
MATER-specific antibodies were seen in 76.5% of thymectomized wild-type mice (n = 17), whereas only 24% of NTx-treated IE-Mater transgenic mice (n = 25) had positive MATER autoantibodies (P < 0.01; Fig. 5A, left bars). When considering only those mice with autoimmune oophoritis, IE-Mater transgenic mice still had a significantly lower incidence of anti-MATER antibodies (42.9%, n = 14; P < 0.05; Fig. 5A, right bars) compared with wild-type mice (81.3%, n = 16). Furthermore, NTx-treated IE-Mater transgenic mice that were protected from oophoritis did not produce detectable antibodies against oocyte proteins, including MATER (n = 11; data not shown).
Fig. 5.
Effect of NTx with regard to development of anti-MATER and ANA in wild-type and IE-Mater transgenic mice. A, Anti-MATER antibodies: Based on immunoblotting assay, 77% of the thymectomized wild-type (n = 17) and 24% of the thymectomized IE-Mater transgenic (n = 25) mice produced anti-MATER antibodies in their sera. Eighty one percent of the wild-type (n = 16) and 42.9% of the IE-Mater transgenic (n = 14) mice with autoimmune oophoritis (AO) after thymectomy produced anti-MATER antibodies in their sera. The IE-Mater transgenic mice that failed to develop autoimmune oophoritis after NTx (n = 11) did not produce antibodies against either MATER or other oocyte proteins (data not shown). B, ANA levels in the mouse sera were detected by ELISA. Bar graphs (mean ± sd) illustrate absorption at OD450 nm and reflect the serum levels of ANA. Indication of genotype, surgical operation, autoimmune oophoritis (AO), and numbers of mice for each group are listed below the graphic bars. Basal levels of ANA in the sera of wild-type and IE-Mater transgenic mice with sham operations (open bars) were the same. Levels of ANA were elevated in the sera of mice after NTx, but appeared to be the same among the wild-type (n = 7) and IE-Mater transgenic (n = 14) mice with autoimmune oophoritis and the IE-Mater transgenic mice (n = 11) without autoimmune oophoritis. abs, Antibodies; NS, P value not significant).
The presence of the IE-Mater transgene in NTx-treated mice significantly reduces the development of anti-MATER autoantibodies, suggesting that the transgenic mice develop specific immune tolerance to MATER. To exclude the possibility that the decreased autoimmunity to MATER in the transgenic mice resulted from generalized impairment of the immune response, rather than antigen-specific tolerance, we measured ANA, another marker for NTx-induced autoimmunity (22, 23). Both NTx-treated wild-type (n = 7) and IE-Mater transgenic (n = 25) mice had similar elevations of ANA in their sera (Fig. 5B). Furthermore, NTx-treated IE-Mater mice without oophoritis and anti-MATER autoantibodies (n = 11) had elevations of ANA comparable to wild-type (n = 7) and transgenic (n = 14) mice with oophoritis. Sham-operated wild-type (n = 5) and IE-Mater transgenic (n = 8) mice both had similarly low ANA levels. In summary, expression of the IE-Mater transgene mitigated the development of anti-MATER antibodies and oophoritis, without effect on ANA elevations after NTx. Thus, immune tolerance to MATER does not result from generalized impairment of the immune response but is specifically induced in the IE-Mater transgenic mice through MHC class II-dependent expression of antigen.
Discussion
Immunological tolerance is established by both central and peripheral mechanisms, beginning with the process of negative selection in the thymus. Thymic medullary epithelial cells express high levels of MHC class II and present an array of tissue-specific self-antigens to developing T cells. Autoreactive T cells that recognize self-antigens in the context of binding to MHC are then deleted. Because thymic deletion is incomplete, additional mechanisms of T cell tolerance in the periphery, such as suppression by Tregs or induction of anergy by tolerogenic APC, are also important to prevent autoimmunity (14). Within peripheral lymphoid organs, presentation of self-antigens by subsets of MHC class II-expressing dendritic cells, B cells, and macrophages are thought to be capable of inducing T cell tolerance. By driving MATER expression under the I-E promoter, we have targeted self-antigen expression to antigen-presenting cell populations within the thymus and the peripheral lymphoid system. This is supported by the detection of IE-Mater transcript in the thymus and spleen of transgenic animals. Despite NTx, the expression of MATER in APC is sufficient to mediate a significant reduction in experimentally induced autoimmune oophoritis.
Oophoritis in the NTx model is primarily T cell mediated, as evidenced by adoptive transfer of disease by T cells from NTx mice and abrogation of disease by transfer of regulatory T cells (8, 24, 25). Ovarian disease is evident shortly after the first estrus with lymphocytic infiltrates most prominent in the theca of developing follicles (6). Early in disease, primordial and early small follicles are relatively spared and do not react with serum from NTx-treated mice on indirect immunofluorescence; however, by 8 wk of age most ovaries are reduced to a fibrous band of tissue lacking primordial follicles (6). MATER is first detected in oocytes of later-stage small follicles (Stage 3a) that are also among the initial targets of disease (26, 27). Previous studies have demonstrated that multiple ovarian antigens are targeted in NTx-induced autoimmune oophoritis (9, 27). Consistent with this, we also observed production of autoantibodies to multiple oocyte proteins in addition to MATER, as well as to steroidogenic cells. Many autoimmune diseases are known to generate multiple autoantibodies, and it remains unclear whether these arise through epitope spreading of autoantibody responses later in disease pathogenesis, or reflect the specificity of the initiating autoimmune response. Although MATER was originally identified through the NTx-induced humoral response, the induction of tolerance through self-antigen expression indicates that it is a pathogenic target of T cells in autoimmune oophoritis.
Disease protection by tolerance induction to a single antigen requires knowledge of the initiating antigenic stimulus as well as understanding of the often complex pattern of epitope spreading. IE-driven expression of the H/K ATPase β-subunit is sufficient to induce disease-specific tolerance and abrogate development of NTx-induced autoimmune gastritis (19). In the NOD mouse model of type 1 diabetes, transgenic expression of proinsulin II under the IE promoter confers protection from diabetes (28). In contrast, IE-driven expression of islet-specific glucose-6-phosphatase catalytic subunit related protein (IGRP), another well-characterized diabetes antigen, resulted in loss of islet-infiltrating IGRP-specific T cells but did not prevent disease, suggesting that IGRP is a downstream secondary epitope (29). In tolerized I-E Mater females that undergo NTx, ovarian disease is mitigated despite the persistence of ANA, illustrating the antigen specificity of the effect without generalized immune impairment. The lack of complete suppression of autoimmune oophoritis by transgenic expression of MATER indicates that: 1) it is not the sole initiating pathogenic antigen in this model; 2) other ovarian antigens may also contribute significantly to disease pathogenesis in the absence of MATER autoimmuity; or 3) the induction of tolerance was not complete in this model.
Given that NTx promotes a wide array of autoimmunity, it is remarkable that induction of tolerance to a single antigen can result in a significant reduction of organ-specific disease. The mechanism by which transgenic expression of MATER mediates tolerance remains unclear. Induction of T cell anergy in effector cells by expression of MATER in tolerogenic MHC class II+ APC is one possibility. Thymic expression of MATER in MHC II+ medullary thymic epithelial cells may also mediate some early deletion of autoreactive T cells before thymectomy. Alternatively, expression of MATER antigen first in the thymus and also in the spleen and lymph nodes may promote the development and maturation, respectively, of antigen-specific Tregs.
In the treatment of clinical autoimmunity, targeted interventions rather than global immunosuppression may soon be a reality. Our results have indicated that despite the presence of multiple ovarian targets in NTx-induced oophoritis, MATER is a pathogenic target of T cells that generate significant antigen-specific tolerance in the context of MHC class II. The identification of a human MATER homolog (NALP5) and the association of NALP5 autoantibodies with primary ovarian insufficiency in some women with APS1 disease implicates MATER autoimmunity as a potential pathogenic mechanism in human autoimmune oophoritis (13). Further studies into the mechanism of antigen-specific tolerance induction to MATER antigen will be important in determining whether MATER could ultimately serve as a relevant target for the development of diagnostic and therapeutic intervention in ovarian autoimmunity.
Acknowledgments
We thank Dr. Eric Lee at the National Institute of Child Health and Human Development for assistance with DNA injection to generate the IE-Mater transgenic mice.
This work was supported in part by the Intramural Research Program, National Institute of Child Health and Human Development, National Institutes of Health. L.M.N. is a commissioned officer in the United States Public Health Service. M.H.C. is supported by funding from the National Institute of Child Health and Human Development, National Institutes of Health (Grant K08HD058599).
Disclosure Summary: L.M.N. and Z.B.T. report being inventors on United States patents directed to MATER (a potential antigen in autoimmune POI) and no other potential conflicts of interest relevant to this article. The remaining authors have nothing to disclose.
Footnotes
- ANA
- Antinuclear antibodies
- APC
- antigen-presenting cell
- APS1
- autoimmune polyglandular syndrome type 1
- FITC
- fluorescein isothiocyanate
- IGRP
- islet-specific glucose-6-phosphatase catalytic subunit related protein
- MATER
- maternal antigen that embryos require
- MHC
- major histocompatibility complex
- NALP5
- NACHT leucine-rich-repeat protein 5
- NTx
- neonatal thymectomy
- POI
- primary ovarian insufficiency
- RT
- reverse transcription
- Treg
- regulatory T cell.
References
- 1. Welt CK. 2008. Primary ovarian insufficiency: a more accurate term for premature ovarian failure. Clin Endocrinol (Oxf) 68:499–509 [DOI] [PubMed] [Google Scholar]
- 2. Nelson LM. 2009. Clinical practice. Primary ovarian insufficiency. N Engl J Med 360:606–614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bats AS, Barbarino PM, Bene MC, Faure GC, Forges T. 2008. Local lymphocytic and epithelial activation in a case of autoimmune oophoritis. Fertil Steril 90:849.e5–e8 [DOI] [PubMed] [Google Scholar]
- 4. Bakalov VK, Anasti JN, Calis KA, Vanderhoof VH, Premkumar A, Chen S, Furmaniak J, Smith BR, Merino MJ, Nelson LM. 2005. Autoimmune oophoritis as a mechanism of follicular dysfunction in women with 46,XX spontaneous premature ovarian failure. Fertil Steril 84:958–965 [DOI] [PubMed] [Google Scholar]
- 5. Kojima A, Tanaka-Kojima Y, Sakakura T, Nishizuka Y. 1976. Spontaneous development of autoimmune thyroiditis in neonatally thymectomized mice. Lab Invest 34:550–557 [PubMed] [Google Scholar]
- 6. Taguchi O, Nishizuka Y, Sakakura T, Kojima A. 1980. Autoimmune oophoritis in thymectomized mice: detection of circulating antibodies against oocytes. Clin Exp Immunol 40:540–553 [PMC free article] [PubMed] [Google Scholar]
- 7. Samy ET, Parker LA, Sharp CP, Tung KSK. 2005. Continuous control of autoimmune disease by antigen-dependent polyclonal CD4+CD25+ regulatory T cells in the regional lymph node. J Exp Med 202:771–781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Asano M, Toda M, Sakaguchi N, Sakaguchi S. 1996. Autoimmune disease as a consequence of developmental abnormality of a T cell subpopulation. J Exp Med 184:387–396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tong ZB, Nelson LM. 1999. A mouse gene encoding an oocyte antigen associated with autoimmune premature ovarian failure. Endocrinology 140:3720–3726 [DOI] [PubMed] [Google Scholar]
- 10. Tong ZB, Gold L, Pfeifer KE, Dorward H, Lee E, Bondy CA, Dean J, Nelson LM. 2000. Mater, a maternal effect gene required for early embryonic development in mice. Nat Genet 26:267–268 [DOI] [PubMed] [Google Scholar]
- 11. Tong ZB, Bondy CA, Zhou J, Nelson LM. 2002. A human homologue of mouse Mater, a maternal effect gene essential for early embryonic development. Hum Reprod 17:903–911 [DOI] [PubMed] [Google Scholar]
- 12. Ting JP, Kastner DL, Hoffman HM. 2006. CATERPILLERs, pyrin and hereditary immunological disorders. Nat Rev Immunol 6:183–195 [DOI] [PubMed] [Google Scholar]
- 13. Alimohammadi M, Björklund P, Hallgren A, Pöntynen N, Szinnai G, Shikama N, Keller MP, Ekwall O, Kinkel SA, Husebye ES, Gustafsson J, Rorsman F, Peltonen L, Betterle C, Perheentupa J, Akerström G, Westin G, Scott HS, Holländer GA, Kämpe O. 2008. Autoimmune polyendocrine syndrome type 1 and NALP5, a parathyroid autoantigen. N Engl J Med 358:1018–1028 [DOI] [PubMed] [Google Scholar]
- 14. Mueller DL. 2010. Mechanisms maintaining peripheral tolerance. Nat Immunol 11:21–27 [DOI] [PubMed] [Google Scholar]
- 15. East J, Parrott DM. 1962. Operative techniques for newborn mice using anaesthesia by cooling. J Endocrinol 24:249–250 [DOI] [PubMed] [Google Scholar]
- 16. Sjodin K, Dalmasso AP, Smith JM, Martinez C. 1963. Thymectomy in newborn and adult mice. Transplantation 1:521–525 [DOI] [PubMed] [Google Scholar]
- 17. Garza KM, Agersborg SS, Baker E, Tung KS. 2000. Persistence of physiological self antigen is required for the regulation of self tolerance. J Immunol 164:3982–3989 [DOI] [PubMed] [Google Scholar]
- 18. Suri-Payer E, Wei K, Tung K. 2001. The day-3 thymectomy model for induction of multiple organ-specific autoimmune diseases. In: Coligan JE, Bierer BE, Margulies DH, Shevach EM, Strober W, Coico R. eds. Current protocols in immunology. New York: Wiley; 15.16.1–15.16.12 [DOI] [PubMed] [Google Scholar]
- 19. Alderuccio F, Toh BH, Tan SS, Gleeson PA, van Driel IR. 1993. An autoimmune disease with multiple molecular targets abrogated by the transgenic expression of a single autoantigen in the thymus. J Exp Med 178:419–426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Le Meur M, Gerlinger P, Benoist C, Mathis D. 1985. Correcting an immune-response deficiency by creating E α gene transgenic mice. Nature 316:38–42 [DOI] [PubMed] [Google Scholar]
- 21. van Ewijk W, Ron Y, Monaco J, Kappler J, Marrack P, Le Meur M, Gerlinger P, Durand B, Benoist C, Mathis D. 1988. Compartmentalization of MHC class II gene expression in transgenic mice. Cell 53:357–370 [DOI] [PubMed] [Google Scholar]
- 22. Smith HR, Green DR, Raveche ES, Smathers PA, Gershon RK, Steinberg AD. 1982. Studies of the induction of anti-DNA in normal mice. J Immunol 129:2332–2334 [PubMed] [Google Scholar]
- 23. Smith HR, Chused TM, Smathers PA, Steinberg AD. 1983. Evidence for thymic regulation of autoimmunity in BXSB mice: acceleration of disease by neonatal thymectomy. J Immunol 130:1200–1204 [PubMed] [Google Scholar]
- 24. Sakaguchi S, Fukuma K, Kuribayashi K, Masuda T. 1985. Organ-specific autoimmune diseases induced in mice by elimination of T cell subset. I. Evidence for the active participation of T cells in natural self-tolerance; deficit of a T cell subset as a possible cause of autoimmune disease. J Exp Med 161:72–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sakaguchi S, Takahashi T, Nishizuka Y. 1982. Study on cellular events in postthymectomy autoimmune oophoritis in mice. I. Requirement of Lyt-1 effector cells for oocytes damage after adoptive transfer. J Exp Med 156:1565–1576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tong ZB, Gold L, De Pol A, Vanevski K, Dorward H, Sena P, Palumbo C, Bondy CA, Nelson LM. 2004. Developmental expression and subcellular localization of mouse MATER, an oocyte-specific protein essential for early development. Endocrinology 145:1427–1434 [DOI] [PubMed] [Google Scholar]
- 27. Alard P, Thompson C, Agersborg SS, Thatte J, Setiady Y, Samy E, Tung KS. 2001. Endogenous oocyte antigens are required for rapid induction and progression of autoimmune ovarian disease following day-3 thymectomy. J Immunol 166:4363–4369 [DOI] [PubMed] [Google Scholar]
- 28. French MB, Allison J, Cram DS, Thomas HE, Dempsey-Collier M, Silva A, Georgiou HM, Kay TW, Harrison LC, Lew AM. 1997. Transgenic expression of mouse proinsulin II prevents diabetes in nonobese diabetic mice. Diabetes 46:34–39 [DOI] [PubMed] [Google Scholar]
- 29. Krishnamurthy B, Dudek NL, McKenzie MD, Purcell AW, Brooks AG, Gellert S, Colman PG, Harrison LC, Lew AM, Thomas HE, Kay TWH. 2006. Responses against islet antigens in NOD mice are prevented by tolerance to proinsulin but not IGRP. J Clin Invest 116:3258–3265 [DOI] [PMC free article] [PubMed] [Google Scholar]