Ubc9 and PIAS1 are coactivators for SF-1-mediated transcription of steroidogenic enzymes beyond sumoylation enzyme activities and play a role in cortisol-producing adenomas.
Abstract
Steroidogenic factor-1 (SF-1) is a nuclear orphan receptor, which is essential for adrenal development and regulation of steroidogenic enzyme expression. SF-1 is posttranslationally modified by small ubiquitin-related modifier-1 (SUMO-1), thus mostly resulting in attenuation of transcription. We investigated the role of sumoylation enzymes, Ubc9 and protein inhibitors of activated STAT1 (PIAS1), in SF-1-mediated transcription of steroidogenic enzyme genes in the adrenal cortex. Coimmunoprecipitation assays showed that both Ubc9 and PIAS1 interacted with SF-1. Transient transfection assays in adrenocortical H295R cells showed Ubc9 and PIAS1 potentiated SF-1-mediated transactivation of reporter constructs containing human CYP17, CYP11A1, and CYP11B1 but not CYP11B2 promoters. Reduction of endogenous Ubc9 and PIAS1 by introducing corresponding small interfering RNA significantly reduced endogenous CYP17, CYP11A1, and CYP11B1 mRNA levels, indicating that they normally function as coactivators of SF-1. Wild type and sumoylation-inactive mutants of Ubc9 and PIAS1 can similarly enhance the SF-1-mediated transactivation of the CYP17 gene, indicating that the coactivation potency of Ubc9 and PIAS1 is independent of sumoylation activity. Chromatin immunoprecipitation assays demonstrated that SF-1, Ubc9, and PIAS1 were recruited to an endogenous CYP17 gene promoter in the context of chromatin in vivo. Immunohistochemistry and Western blotting showed that SF-1, Ubc9, and PIAS1 were expressed in the nuclei of the human adrenal cortex. In cortisol-producing adenomas, the expression pattern of SF-1 and Ubc9 were markedly increased, whereas that of PIAS1 was decreased compared with adjacent normal adrenals. These results showed the physiological roles of Ubc9 and PIAS1 as SF-1 coactivators beyond sumoylation enzymes in adrenocortical steroidogenesis and suggested their possible pathophysiological roles in human cortisol-producing adenomas.
Steroidogenic factor-1 (SF-1; Ad4BP/NR5A1) belongs to the NR5A subfamily of orphan nuclear receptors that bind DNA with high affinity as monomers (1, 2). SF-1 is essential for the development and function of the hypothalamic-pituitary-adrenal and -gonadal axes (3–10). Targeted disruption of SF-1 in mice resulted in adrenal and gonadal agenesis (11), absence of the ventromedial hypothalamic nucleus (12, 13), and impaired expression of pituitary gonadotropins (14). The ability of SF-1 to transactivate target genes has been shown to be regulated by posttranslational modification such as phosphorylation (15, 16) and acetylation (17, 18) and protein-protein interactions (19–23). The sumoylation of SF-1 also occurs at two lysine residues, which mostly suppresses its transcriptional activity (24–28).
In the transcriptional regulation of steroidogenic P450 genes, our studies have suggested that two nuclear orphan receptors, SF-1 and chicken ovalbumin upstream promoter-transcription factors (COUP-TF), play pivotal roles (29–32). Recently we identified Ubc9 and protein inhibitors of activated STAT1 (PIAS1) (33), which are sumoylation conjugating enzyme and ligase, respectively, as COUP-TFI-interacting proteins. We have shown that COUP-TFI activates human CYP11B2 gene transcription in cooperation with Ubc9 and PIAS1, thus resulting in aldosterone secretion in the adrenal zona glomerulosa cells (34).
During the studies (33, 34), we found that Ubc9 and PIAS1 also markedly interact with SF-1. Herein in the present study, we showed another function of Ubc9 and PIAS1 in SF-1-mediated transcription of steroidogenic P450 genes in human adrenal cell. We have also studied the expression of these factors in human adrenals including adrenocortical tumors. This study indicates that SF-1, Ubc9, and PIAS1 may play possible pathophysiological roles in the steroidogenesis of cortisol-producing adenomas.
Materials and Methods
Plasmid constructs
Constructs of pcDNA3.1/His-Ubc9, pcDNA3.1/His-Ubc9 (C93S), pcDNA3.1/His-PIAS1, and pcDNA3.1/His-PIAS1 (C351S) were described previously (33, 34). pRc/RSV-SF-1/Ad4BP and p3xFlag-CMV-10-PIAS1 are generous gifts of Dr. Ken-ichirou Morohashi (Kyushu University, Fukuoka, Japan). p4xGal4-tk-Luc is a generous gift of Dr. Bert W. O'Malley (Baylor College of Medicine, Houston, TX) (33, 34). Gal4 and Gal4-SF-1 expression plasmids are generous gifts of Dr. James Larry Jameson (Northwestern University, Chicago, IL). A transient expression system using a luciferase reporter gene was used to characterize the Ad4BP/SF-1 binding site present in the hCYP11B1 (35, 36), hCYP11B2 (37), hCYP17 (35, 38), and hCYP11A1 (35, 39) gene promoters as shown previously. phRL-null was purchased from Promega (Fitchburg, WI).
Cell culture, transient transfections, and luciferase assays
H295R cells, derived from human adrenocortical carcinoma, were routinely maintained in DMEM/F-12 (Invitrogen, Carlsbad, CA) supplemented with 2.5% NuSerum (Collaborative Bio, Bedford, MA) and 1% insulin-transferrin-selenium culture supplement (BD Bioscience, Franklin Lakes, NJ). COS-7 cells were routinely maintained in DMEM supplemented with 10% fetal bovine serum (Invitrogen). Twenty-four hours before transfection, 5 × 104 cells of COS-7 cells or 1 × 105 cells of H295R cells per well of a 24-well dish were plated in the medium. All transfections except small interfering RNA (siRNA) were carried out using Lipofectamine 2000 (Invitrogen) with indicated amount of expression plasmids, according to the manufacturer's guideline. Transfected H295R and COS-7 cells were harvested 48 and 24 h, respectively, after transfection, and each cell extract was assayed for both Firefly and Renilla luciferase activities with a dual-luciferase reporter assay system (Promega). Relative luciferase activity was determined as ratio of Firefly/Renilla luciferase activities, and data are expressed as the mean (±sd) of triplicate values obtained from a representative experiment that was independently repeated at least three times.
Adrenal cell siRNA electroporation
H295R cells were electroporated using Amaxa System (Gaithersburg, MD) as shown previously (40) and transfected with siRNA for Ubc9 (s14591, s14590), PIAS1 (s16285, s16286), and control siRNA (am4611), all purchased from Applied Biosystems (Branchburg, NJ). Transfected cells were plated at a density of 2 million cells/well onto six-well dishes. Experiments were repeated a minimum of three times.
RNA extraction and Quantitative RT-PCR
After 48 h of transfection, total RNA was extracted using the RNeasy minikit (QIAGEN, Valencia, CA), and concentration and purity of the RNA were checked spectroscopically using a Nanodrop spectrophotometer (Nano Drop Technologies, Wilmington, DE). One microgram of total RNA was reverse transcribed using TaqMan reverse transcription reagents (Applied Biosystems). Primers for SF1 (Hs00610436), Ubc9 (Hs00163336), PIAS1 (HS00184008), CYP17 (Hs00164375), CYP11A1 (Hs00167984), CYP11B1 (HS01596404), COUP-TF1 (Hs00818842), and epidermal growth factor receptor (EGFR; Hs01076092) were purchased from Applied Biosystems. Quantitative PCR was performed using the ABI 7700 sequence detector (Applied Biosystems) following the reaction parameters recommended by the manufacturer, using 25 μl of total volume consisting of universal PCR master mix (Applied Biosystems), primers/probe mix, and cDNA. Quantification of 18S levels (Applied Biosystems) was used to normalize samples. In all experiments, the relative gene expression was calculated using standard curves generated by the use of serial dilution of one sample yielding correlation coefficients larger than 0.98. Standard and sample values were determined in duplicate from three independent experiments.
Antibodies
For Western blot analysis, antihuman SF-1 mouse monoclonal antibody was obtained from Perseus Proteomics (Tokyo, Japan), and goat polyclonal Ubc9 antibody and rabbit polyclonal PIAS1 antibodies were purchased from Abcam (Cambridge, MA). Mouse anti-α-tubulin antibody was obtained from Calbiochem (Darmstadt, Germany).
For immunoprecipitation, mouse monoclonal anti-Xpress IgG (Invitrogen) and mouse anti-Flag M2 monoclonal antibody (Sigma-Aldrich, St. Louis, MO) were used.
For the chromatin immunoprecipitation (ChIP) assay, rabbit antimouse SF-1 antibody (Upstate Biotechnology, Millipore, MA), rabbit antihuman Ubc9 (H-81) antibody, goat antihuman PIAS1 (C-20) antibody, and normal goat IgG (Santa Cruz Biotechnology, CA) were used.
Western blot analysis and coimmunoprecipiation assays
The cells were lysed with lysis buffer [10 mm Tris-HCl (pH 8.0), 150 mm NaCl, 1% Triton X-100, 5 mm EDTA, and 2 mm phenylmethylsulfonyl fluoride], and Western blots were performed before the immunoprecipitation (IP) steps to confirm protein expression by corresponding antibodies as described previously (33, 34, 41). The same samples for the Western blots were diluted to 1 ml in IP buffer [20 mm Tris-HCl (pH 7.5), 150 mm NaCl, 10 mm dithiothreitol, 5 ng/μl aprotinin, 0.5 mm phenylmethylsulfonyl fluoride, and 0.1% Tween 20] and precleared with protein G plus-agarose beads (Santa Cruz Biotechnology), and antibodies were added for 1 h. Immune complexes were adsorbed to protein G plus-agarose beads and washed four times in IP buffer. Proteins were then separated on 12.5% polyacrylamide gels and transferred onto Hybond ECL nitrocellulose membranes (Amersham, Harrisburg, PA). The primary antibodies used for immunoprecipitation were anti-Flag (Sigma) or anti-Xpress (Invitrogen) antibodies, and antibodies used for the Western blots were anti-SF-1 (Perseus Proteomics), anti-PIAS1 (Abcam), anti-Ubc9 (Abcam), anti-Flag, anti-Xpress antibodies.
ChIP assay
ChIP assay was performed as described previously (34, 41). The cross-linked sheared chromatin solution of H295R cells was used for immunoprecipitation with 3 μg of anti-SF-1 antibody, anti-Ubc9 antibody, anti-PIAS1 antibody or normal IgG. The immunoprecipitated DNAs were purified by phenol/chloroform extraction, precipitated by ethanol, and amplified by PCR using primers flanking the Ad4 region or control region as follows: CYP17-Ad4 sense primer, 5′-AGT TGA GCC AGC CCT TGA G-3′ (−169/+45); CYP17-Ad4 antisense primer, 5′-GCA GGC AAG ATA GAC AGC AG-3′ (−169/+45); CYP17-control sense primer, 5′-GTC GTT AAG GGC TAC-3′ (+5968/+6168); CYP17-control antisense primer, 5′-CCG CTG GAT TCA AGA-3′ (+5968/+6168). DNA samples with serial dilution were amplified by PCR to determine the linear range for the amplification (data not shown).
Histology and immunohistochemistry
Formalin-fixed tissues were embedded in paraffin, sectioned at 6 mm, and mounted on silane-coated slides. For histological examination, sections were dewaxed, rehydrated, and stained with hematoxylin and eosin. For immunohistochemistry, sections were dewaxed, rehydrated, and endogenous peroxidase blocked using 3% (vol/vol) hydrogen peroxidase in PBS for 5 min. After washing in water, the sections were subjected to microwave antigen retrieval in 0.01 m citrate buffer. Thereafter they were washed in PBS and blocked with a blocking solution containing 5% BSA in PBS for 30 min. They were subsequently incubated overnight at 4 C with primary antibodies diluted appropriately with the blocking solution. Primary antibodies for immunohistochemistry included anti-SF-1, anti-PIAS1, and anti-Ubc9. After two washes in PBS, immunoreactivities were detected using a Vectastatin ABC Elite kit (Vector Laboratories, Burlingame, CA) and a Vecta DAB substrate kit (Vector Laboratories). As negative controls, sections were incubated with the preimmune or control serum in place of the primary antibody.
Human adrenal specimens
We investigated six cases of aldosterone-producing adenomas and six cases of cortisol-producing adenomas. The clinical and pathological diagnoses were based on the clinical practice guidelines by The Endocrine Society (42, 43) using hormone evaluations, computed tomography, adrenal scintigraphy, and adrenal venous sampling. The patients with aldosterone-producing adenomas had symptoms of primary aldosteronism, such as hypertension, hypokalemia, increased aldosterone concentration, and suppressed plasma renin activity. The patients with cortisol-producing adenomas had symptoms of Cushing's syndrome with increased cortisol concentrations and suppressed ACTH concentrations. The protocols for this study were approved by the Ethics Committee of Keio University School of Medicine (Tokyo, Japan). Informed consent from each patient was obtained before collection of any tissue specimens examined in this study. When laparoscopic unilateral adrenalectomy was performed, adrenal tumor tissue was immediately frozen in liquid nitrogen and stored at −80 C until processing. Each adrenal tumor tissue was homogenated with 0.25 m sucrose, 50 mm Tris-HCl (pH 7.5), 5 mm magnesium chloride, 25 mm potassium chloride buffer containing protease inhibitor, and 0.5 mm phenylmethylsulfonyl fluoride. Protein lysate was taken and protein concentrations were determined by the Lowry method using the Bio-Rad DC protein assay kit (Bio-Rad Laboratories, Hercules, CA). Twenty micrograms of proteins from each sample were used for Western blot as described above.
Statistics
All experiments were performed in triplicate several times. The error bars in the graphs of the individual experiments correspond to the sd of the triplicate values. Statistical analysis was performed using Student's t test for unpaired comparisons (SPSS Statistics, version 17.0.1; Chicago, IL).
Results
SF-1 interacted with Ubc9 and PIAS1 in mammalian cells
We have previously shown that nuclear orphan receptor COUP-TFI specifically interacts with Ubc9 and PIAS1, thus resulting in transcriptional activation of the human CYP11B2 gene (33, 34). During these studies, we also found that both Ubc9 and PIAS1 markedly interact with SF-1 in yeast two-hybrid assays. Based on previous reports (24–28) that SF-1 is sumoylated by Ubc9 and PIAS family proteins, the interaction between SF-1 and Ubc9 and PIAS1 was obviously expected.
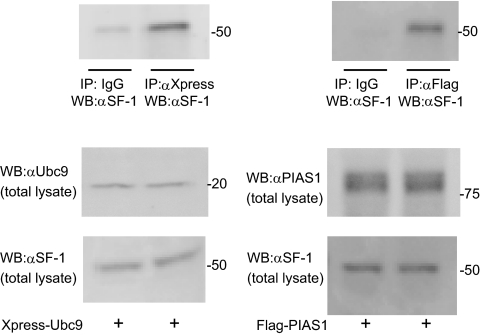
To confirm the physical interaction between SF-1 and Ubc9 or PIAS1 in mammalian cells, coimmunoprecipitation assays were performed (Fig. 1). H295R cells were transfected with mammalian expression plasmids of Xpress-tagged Ubc9 or Flag-tagged PIAS1. Polyclonal anti-Xpress or IgG was first used to precipitate the protein complexes containing Ubc9, and the presence of SF-1 protein in these complexes was subsequently examined by immunoblotting with a polyclonal anti-SF-1 antibody. The presence of SF-1 protein was markedly detected in the cellular lysates with IP by anti-Xpress antibody, not by IgG, indicating a specific interaction of Ubc9 with SF-1 (left top panel, Fig. 1). Similarly, SF-1 interacted with Flag-tagged PIAS1 (right top panel, Fig. 1). These data indicate that endogenous SF-1 interacts with Ubc9 and PIAS1, which were ectopically overexpressed in H295R cells. As expected, these findings confirmed previous reports that both Ubc9 and PIAS1 specifically interact with SF-1 in mammalian cells.
Fig. 1.
Interaction of Ubc9 and PIAS-1 with SF-1 in H295R cells. H295R cells were transfected with Xpress-tagged Ubc9 or Flag-tagged PIAS1. Whole-cell extracts were subjected to IP with anti-Xpress, anti-Flag antibody, or IgG and immunoprecipitates were subsequently analyzed by Western blotting (WB) with anti-SF-1 (top panel). Expression levels of overexpressed Ubc9 or PIAS1 (middle panels) and endogenous SF-1 (bottom panel) in total lysate were analyzed by Western blotting.
SF-1-mediated transactivation of the human CYP17 by Ubc9 and PIAS1
To investigate the functional effects of interaction between SF-1 and Ubc9 and PIAS1, transient transfection assays were performed (Fig. 2). Several steroidogenic P450 genes, including human CYP17, CYP11A1, CYP11B1, and CYP11B2, which contain the consensus binding sites of SF-1 in their proximal 5′-flanking DNA, were used.
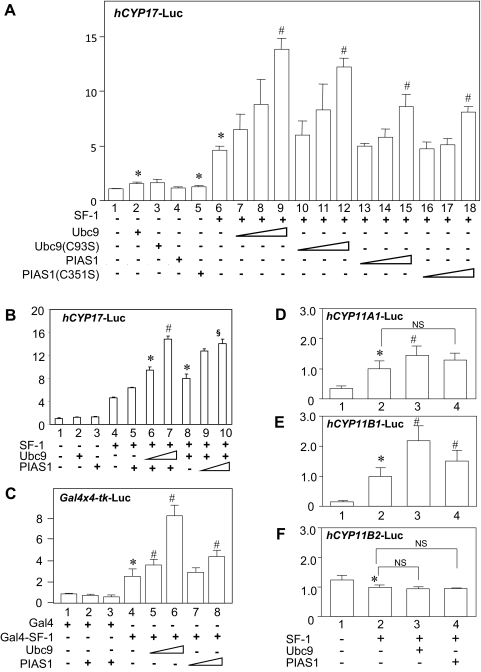
Fig. 2.
Effects of Ubc9 and PIAS1 on SF-1-mediated transcriptional activation. A, Ubc9 and PIAS1 enhance SF-1-mediated transcriptional activities of human CYP17 promoter in H295R cells. H295R cells were transfected with 610 ng of total DNA for each well of the 24-well dish, including 300 ng of hCYP17-Luc reporter, 100 ng of pRc/RSV-SF-1 or empty vector (pRc/RSV), increasing amounts (50, 100, and 200 ng) of pcDNA3.1/His-Ubc9 (or C93S mutants), or pcDNA3.1/His-PIAS1 (or C351S mutants) or empty vector (pcDNA3.1/His) along with 10 ng of phRL-null. B, Synergistic effects of Ubc9 and PIAS1 on the SF-1-mediated CYP17 transactivation. H295R cells were transfected with 300 ng of hCYP17-Luc reporter, 100 ng of pRc/RSV-SF-1, 100 ng of pcDNA3.1/His-PIAS1 along with 100 or 200 ng of pcDNA3.1/His-Ubc9 (lanes 5–7), or 100 ng of pcDNA3.1/His-Ubc9 along with 100 or 200 ng of pcDNA3.1/His-PIAS1 (lanes 8–10), and 10 ng of phRL-null. Empty vectors were used to make total DNA 610 ng for each well of 24-well dishes. C, Ubc9 and PIAS1 enhance Gal4-SF-1-mediated transcriptional activities of Gal4-responsive Gal4 × 4-tk-Luc promoter in COS-7 cells. COS-7 cells were transfected with 610 ng of total DNA for each well of the 24-well dish, including 300 ng of Gal4 × 4-tk-Luc reporter, 100 ng of Gal4-SF-1 or Gal4, and increasing amounts (100 and 200 ng) of pcDNA3.1/His-Ubc9 and/or pcDNA3.1/His-PIAS1 along with 10 ng of phRL-null. D–F, Effects of Ubc9 and PIAS1 on CYP11A1, CYP11B1, and CYP11B2 gene promoters. H295R cells were transfected with 610 ng of total DNA for each well of the 24-well dish, including 300 ng of hCYP11A1-Luc (D), hCYP11B1-Luc (E) or hCYP11B2-Luc (F) reporter, 100 ng of pRc/RSV-SF-1 or empty vector (pRc/RSV), 200 ng of pcDNA3.1/His-Ubc9 or pcDNA3.1/His-PIAS1 or empty vector (pcDNA3.1/His) along with 10 ng of phRL-null. Forty-eight hours after transfection, cells were harvested and the extracts were measured luciferase activities. All transfection efficiency was normalized using corresponding Renilla activity. Assays were performed with at least triplicate samples. Statistically significant differences are indicated as follows: *, P < 0.05 vs. bar 1; #, P < 0.05 vs. bar 6 (A), bar 5 (B), bar 4 (C), or bar 2 (D–F); §, P < 0.05 vs. bar 8 (B).
First, we examined the effects of SF-1 on human CYP17 gene transcription. Human adrenocortical H295R cells were cotransfected with a reporter construct of CYP17 promoter and SF-1 expression plasmid and further cotransfected with increasing concentrations of expression vectors encoding Ubc9 or PIAS1 (Fig. 2A). Expression levels of each protein were investigated with Western blotting, and they correspond to transfected amounts of each plasmid DNA (Supplemental Fig. 1, published on The Endocrine Society's Journals Online web site at http://endo.endojournals.org). In the absence of transfected SF-1, overexpression of Ubc9 or PIAS1 had minimal effects, although partially significant (lanes 2 and 5, Fig. 2A), on the transcriptional activity mediated by endogenous SF-1. In contrast, when SF-1 was overexpressed, the CYP17 promoter was significantly activated by SF-1 (lane 6, Fig. 2A) as shown previously (38). Coexpression of Ubc9 or PIAS1 potentiated SF-1-mediated transactivation of the CYP17 promoter in a dose-dependent manner with activity increasing to 3.0- or 1.9-fold above basal SF-1 activation levels, respectively (lanes 7–9 and 13–15, Fig. 2A). These findings indicate that both Ubc9 and PIAS1 can enhance SF-1-mediated transactivation of the CYP17 promoter, dependent on the expression level of SF-1. Cotransfection of both Ubc9 and PIAS1 with SF-1 showed that their transactivating effect on the CYP17 promoter activity was synergistic (Fig. 2B). We also investigated the effect of Ubc9 and PIAS1 on SF-1-mediated activity using heterologous Gal4 DNA-binding site promoter and Gal4-fused SF-1 in COS-7 cells (Fig. 2C). Overexpression of Gal4-SF-1 increased transactivation of a reporter containing four copies of Gal4 DNA-binding domain by 2-fold (lane 4, Fig. 2C). Coexpression of Ubc9 or PIAS1 potentiated the Gal4-SF-1-mediated activity by 4- or 2-fold, respectively (lanes 6 and 8, Fig. 2C). These data also suggest that both Ubc9 and PIAS1 potentiate Gal4-SF-1-mediated transcription of the Gal4-responsive tk promoter, but the extent of potentiation by ectopic expression of Ubc9 and PIAS1 varied, depending on the reporter construct.
The functions of Ubc9 and PIAS1 as coactivators for the SF-1 mediated CYP17 gene transactivation are independent of sumoylation enzyme activity
Ubc9 and PIAS1 are known as enzymes that are responsible for sumoylation (SUMO), SUMO-conjugating enzyme (E2) conjugating, and SUMO-ligase (E3) ligating enzyme, respectively. To study whether sumoylation activity of Ubc9 and PIAS1 is required for coactivation of SF-1, transient transfection assays were also performed using the sumoylation-inactive mutant Ubc9 (C93S) and PIAS1 (C351S) as shown previously (33, 34, 41) (Fig. 2A). The cysteine 93 of the Ubc9 is a residue to which SUMO protein is attached. Therefore, a mutant Ubc9 (C93S) was used as an E2 SUMO-conjugating enzyme-inactive mutant. The cysteine 351 of the PIAS1, which is located within the RING finger-like domains of the protein, is a critical residue for the E3 SUMO ligase activity. Therefore, a mutant PIAS1 (C351S) was used as an E3 SUMO ligase-inactive mutant. The results showed that coexpression of Ubc9 (C93S) or PIAS1(C351S) also potentiated the transcriptional activity of wild-type SF-1 in a similar extent to coexpression of wild-type proteins (lanes 10–12 and 16–18, Fig. 2A), indicating that sumoylation activity is not required for SF-1 coactivation function by Ubc9 and PIAS1.
Ubc9 and PIAS1 potentiated SF-1-mediated transactivation of the human CYP11A1 and CYP11B1 but not CYP11B2
Next, we investigated the effects of Ubc9 and PIAS1 on SF-1-mediated transcription of CYP11A1, CYP11B1, or CYP11B2 genes that were also essential for the steroidogenesis in the adrenal cortex. We similarly cotransfected H295R cells with a reporter construct containing these gene promoters and expression plasmids of SF-1, Ubc9, and PIAS1 (Fig. 2, D–F). As expected, SF-1 activated the CYP11A1 and CYP11B1 promoter activities (lane 2, Fig. 2, D and E) (35, 44). Coexpression of Ubc9 potentiated SF-1-mediated transactivation of the CYP11A1 and CYP11B1 reporter by 1.5- and 2.2-fold, respectively, which was similarly observed in the CYP17 promoter (lane 6, Fig. 2A). Coexpression of PIAS1 similarly potentiated SF-1-mdiated transactivation of the CYP11B1 reporter by 1.5-fold, although there was minimal effect on CYP11A1 activity (lane 4, Fig. 2, D and E).
It was previously reported that SF-1 represses reporter gene activity of aldosterone synthase (CYP11B2) (35, 45). In this study, the activity of the CYP11B2 reporter was also decreased (lane 2, Fig. 2F). Coexpression of Ubc9 or PIAS1 did not affect the SF-1-mediated transcription of the CYP11B2 reporter significantly (lanes 3 and 4, Fig. 2F).
To summarize, these data indicate that Ubc9 and PIAS1 enhance SF-1-mediated transactivation of CYP17, CYP11A1, and CYP11B1 but not CYP11B2 genes, which SF-1 does not transactivate.
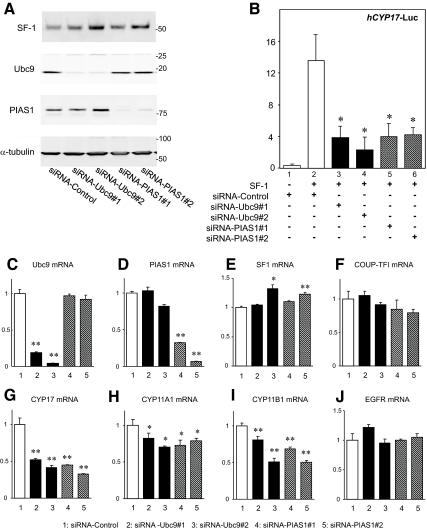
If Ubc9 and PIAS1 function as coactivators of SF-1, reducing the endogenous levels of each protein should decrease the transcriptional activity by SF-1. To investigate this, we used siRNA of Ubc9 and PIAS1 (Fig. 3). Cotransfection of two sets of Ubc9 siRNA (siRNA-Ubc9 #1 and #2) or PIAS1 siRNA (siRNA-PIAS1 #1 and #2) effectively reduced endogenous levels of the target proteins, but these siRNAs had no effect on the levels of SF-1 or α-tubulin protein as shown in Western blotting (Fig. 3A). On the other hand, using negative control (siRNA-control), there were no significant changes in the Ubc9 and PIAS1 protein levels. Using these siRNAs, the SF-1-mediated transactivation of the CYP17 promoter was significantly decreased in H295R (Fig. 3B). These findings indicate that endogenous Ubc9 and PIAS1 normally function as transcriptional coactivators for the SF-1-mediated CYP17 transactivation in H295R cells. To further confirm the coactivation potency of Ubc9 and PIAS1 on SF-1-mediated transcription, effects of siRNAs for Ubc9 or PIAS1 on endogenous SF-1-target gene expression were investigated with real-time RT-PCR. Cotransfection of two sets of Ubc9 and PIAS1 siRNAs again effectively reduced each mRNA levels, respectively (Fig. 3, C and D). In parallel with these treatments, endogenous CYP17, CYP11A1, and CYP11B1 mRNA levels were significantly decreased (Fig. 3, G-I), but the SF-1 mRNA level was slightly increased, and COUP-TFI and EGFR mRNA levels were not affected (Fig. 3, E, F, and J). These data clearly indicate that endogenous Ubc9 and PIAS1 normally function as coactivators of SF-1.
Fig. 3.
Endogenous Ubc9 and PIAS1 are coactivators for the SF-1 target gene expression. H295R cells were transfected with siRNA for Ubc9 or PIAS1. Two sets of siRNA for Ubc9 (#1 for s14591, #2 for s14590) and PIAS1 (#1 for s16285, #2 for s16286) or siRNA-control (am4611) were introduced. A, Western blot analysis was used to confirm the knockdown of Ubc9 and PIAS1 in transfected cells. B, Knockdown of Ubc9 or PIAS1 protein reduces the SF-1-mediated transactivation of the CYP17-Luc reporter. H295R cells in 24-well dishes were transiently transfected with 20 pmol of each siRNA. Forty-eight hours after siRNA transfection, plasmid DNA including 100 ng of pRc/RSV-SF-1, 300 ng of CYP17-Luc, and 10 ng of phRL-null were transfected consecutively. After an additional 24 h, the cell extracts were assayed for luciferase activity. Transfection efficiency was normalized using phRL-null reporter gene activity. Assays were performed with triplicate samples. Statistically significant difference is indicated as follows: *, P < 0.05 vs. bar 2. C–J, Effects of Ubc9 or PIAS1 knockdown on mRNA expression of Ubc9 (C), PIAS1 (D), SF-1 (E), COUP-TFI (F), CYP17 (G), CYP11A1 (H), CYP11B1 (I), and EGFR (J). H295R cells in six-well dishes were transfected using the Amaxa system with 30 nm siRNA. After 48 h, total RNA was extracted and reverse transcribed. The gene expression of Ubc9, PIAS1, SF-1, COUP-TFI, CYP17, CYP11A1, CYP11B1, and EGFR were assayed with quantitative real-time RT-PCR analysis. Statistically significant difference compared with siRNA-control is indicated as follows: *, P < 0.05; **, P < 0.01.
SF-1, Ubc9, and PIAS1 are specifically recruited to the Ad4 sites of human CYP17 gene promoter
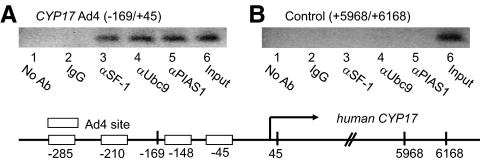
To investigate whether the SF-1-Ubc9-PIAS1 complex is recruited together to the endogenous CYP17 gene promoter in the chromatin context of H295R cells, we used a ChIP assay (Fig. 4). SF-1, Ubc9, and PIAS1 were overexpressed in H295R cells, and the cross-linked, sheared chromatin preparations were subjected to IP with various antibodies. The precipitated DNA was analyzed by PCR amplification of the Ad4 sites of the CYP17 promoter (−169/+45) or the 3′-untranslated control region (+5968/+6168). Antibodies against SF-1, Ubc9, and PIAS1 efficiently immunoprecipitated the Ad4 of the CYP17 promoter, but normal IgG and no antibody failed to precipitate the CYP17 promoter (Fig. 4A). In contrast to the Ad4 sites, the control region of the CYP17 gene was not detected in association with SF-1, Ubc9, and PIAS1 (Fig. 4B). These data highlight a finding that SF-1, Ubc9, and PIAS1 are recruited to a native SF-1-responsive promoter to form a functional transcriptional complex in the context of chromatin in vivo.
Fig. 4.
SF-1, Ubc9 and PIAS1 were recruited to endogenous CYP17 promoter containing the SF-1 binding sites in H295R cells. SF-1, Xpress-Ubc9, and Flag-PIAS1 were overexpressed in H295R cells, and sheared chromatin was immunoprecipitated with anti-SF-1, anti-Xpress, anti-Flag, or normal IgG. The coprecipitated DNA was amplified by PCR, using primers to amplify the CYP17 promoter containing the Ad4 site or the 3′-untranslated control region. The ChIP assay shows SF-1, Ubc9, and PIAS1 interact with the −169/+45 DNA segment containing Ad4 site (A, lanes 3–6) but not with the +5968/+6168 region of the 3′-untranslated region (B, lanes 3–6) in H295R cells. Ab, Antibody.
Expression of SF-1, Ubc9, and PIAS1 in human adrenal cortex and adrenocortical adenomas
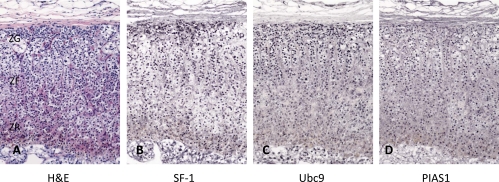
We previously showed that Ubc9 and PIAS1 are exclusively expressed in the nuclei of the zona glomerulosa cells of rat adrenal cortex (34). However, in the human adrenal cortex that was resected with renal cell carcinoma, the expression of these proteins was highly detected in the nuclei of the zonae fasciculata-reticularis as well as zona glomerulosa cells (Fig. 5). As shown previously (46), SF-1 was diffusely distributed in the nuclei of all three zones of the adrenal cortex (Fig. 5B). Immunohistochemical stainings in serial sections showed that SF-1, Ubc9, and PIAS1 were apparently colocalized in the nuclei of normal adrenal cortex, suggesting that these proteins are possibly involved in adrenocortical steroidogenesis.
Fig. 5.
Immunohistochemistry for SF-1, Ubc9, and PIAS1 in human adrenal glands. Sections show hematoxylin and eosin staining (A) and immunohistochemical stainings of SF-1 (B), Ubc9 (C), and PIAS1 (D). Zona glomerulosa (ZG), zona fasciculate (ZF), and zona reticularis (ZR) are shown in A. Original magnification, ×20.
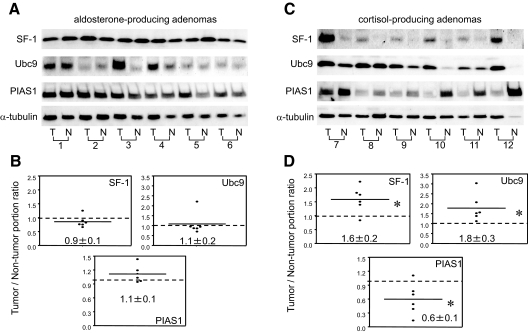
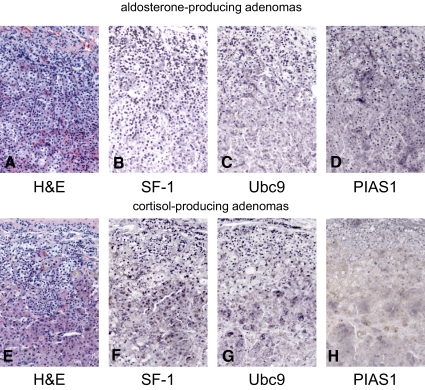
Next, we investigated the level of expression of SF-1, Ubc9, and PIAS1 in aldosterone- (n = 6) and cortisol-producing adrenal cortical adenomas (n = 6) (Table 1 and Fig. 6). The expression level of each protein was corrected by dividing a level of expression of α-tubulin. The comparison of expression of each protein between tumor and nontumor portions was shown as a tumor/nontumor portion ratio (Fig. 6, B and D). In six cases of aldosterone-producing adenomas, levels of expression of SF-1, Ubc9, and PIAS1 were comparable between tumor and nontumor portions (Fig. 6, A and B). Immunohistochemical analysis also demonstrated that SF-1, Ubc9, and PIAS1 were widely distributed in aldosterone-producing adenomas as well as nontumorous adjacent adrenal cortical cells (case 1 of Table 1 and Figs. 6, A and B, and 7, A–D).
Table 1.
Clinical features of patients with primary aldosteronism and Cushing's syndrome
| Case | Age | Sex | Tumor size (mm) | BP (mmHg) | Aldosterone (pg/ml) | Cortisol (μg/dl) | Active renin (pg/ml) | ACTH (pg/ml) | Na (mEq/liter) | K (mEq/liter) |
|---|---|---|---|---|---|---|---|---|---|---|
| Primary aldosteronism | ||||||||||
| 1 | 30 | M | 15 | 140/90 | 280 | 13.2 | <2.5 | 43.0 | 143.4 | 3.5 |
| 2 | 46 | M | 6 | 138/84 | 333 | 9.6 | <2.5 | 33.0 | 143.2 | 3.7 |
| 3 | 42 | M | 15 | 148/92 | 278 | 18.2 | <2.5 | 43.0 | 142.5 | 2.5 |
| 4 | 33 | F | 14 | 164/90 | 127 | 17.7 | <2.5 | 38.0 | 141.4 | 4.2 |
| 5 | 39 | F | 12 | 150/86 | 294 | 12.9 | 3.5 | 24.0 | 142.6 | 2.7 |
| 6 | 36 | M | 10 | 146/86 | 174 | 8.4 | <2.5 | 14.0 | 145.8 | 3.0 |
| Cushing's syndrome | ||||||||||
| 7 | 39 | F | 30 | 130/84 | 38 | 20.2 | 5.0 | <5 | 140.7 | 4.0 |
| 8 | 63 | F | 35 | 124/84 | 110 | 20.0 | 5.6 | <5 | 147.7 | 4.0 |
| 9 | 57 | F | 25 | 130/100 | 86 | 24.3 | 8.6 | <5 | 142.7 | 3.8 |
| 10 | 61 | F | 26 | 150/105 | 86 | 24.3 | 8.6 | <5 | 142.7 | 3.8 |
| 11 | 43 | F | 32 | 150/100 | 186 | 21.1 | 10.1 | <5 | 146.4 | 3.1 |
| 12 | 52 | F | 28 | 160/100 | 58 | 15.5 | <2.5 | <5 | 144.4 | 3.3 |
BP, Blood pressure; M, male; F, female.
Fig. 6.
Expression of SF-1, Ubc9, and PIAS1 in hormone-producing adenomas. Western blot analysis shows SF-1, Ubc9, PIAS1, and α-tubulin expression in aldosterone-producing adenomas (A) and cortisol-producing adenomas (C). Each adrenal tissue was separated into the tumorous tissue (T) and their nontumorous adjacent adrenal tissue (N). Each expression was normalized by α-tubulin and the lower figures (B and D) show the ratio of the normalized expression of tumorous tissue to the normalized expression of the nontumorous adjacent adrenal tissue. *, P < 0.05 vs. the nontumorous adjacent adrenal tissue.
Fig. 7.
Immunohistochemistry for SF-1, Ubc9, and PIAS1 in an aldosterone-producing adenoma (A–D) and a cortisol-producing adenoma (E–H). Sections show hematoxylin and eosin (H&E) staining (A and E), SF-1 expression (B and F), Ubc9 expression (C and G), and PIAS1 expression (D and H). Original magnification, ×20.
In contrast, in cortisol-producing adenomas, the level of expression of SF-1 and Ubc9 was increased in the tumor portion compared with the adjacent nontumor portion by 1.5- and 1.8-fold, respectively (Fig. 6, C and D). The level of expression of PIAS1 was reciprocally decreased in the cortisol-producing adenomas compared with the adjacent nontumor portion by 0.6-fold. Immunohistochemical analysis showed that SF-1, Ubc9, and PIAS1 were widely distributed in cortisol-producing adenomas (case 7 of Table 1 and Fig. 7, E–H) as well as nontumorous adjacent adrenal cortical cells (data not shown). It was difficult to quantitate expression levels of these proteins between tumor and nontumor portions by immunohistochemical analysis; therefore, we compared expression levels by Western blotting (Fig. 6). Taken together with the expression profile of SF-1, Ubc9, and PIAS1 in the human adrenals, we postulate that dysregulated expression of SF-1, Ubc9, and PIAS1 is associated with the excessive production of cortisol in cortisol-producing adenomas.
To study whether excessive glucocorticoid may affect the level of expression of these proteins in cortisol-producing adenomas, we therefore treated H295R cells with the increasing concentrations (10−9 to 10−5 m) of dexamethasone for 12 h and investigated the endogenous expression levels of SF-1, Ubc9, and PIAS1 with Western blotting. Although the results may not be applicable to the effect of long-term treatment of glucocorticoid, our data showed that none of these proteins were changed, even though the cells were under the treatment beyond the physiological dose of dexamethasone (Supplemental Fig. 2).
Discussion
This paper showed that both Ubc9 and PIAS1 interact with SF-1 and function as coactivators of SF-1 in steroidogenic enzyme P450 gene transcription in a promoter-specific manner in human adrenocortical H295R cells. Although SF-1 is known to be sumoylated by Ubc9 and PIAS1, thus resulting in attenuation of receptor-dependent transcription (24–28), our results demonstrated another new function of these proteins as SF-1 coactivators beyond sumoylation enzyme activity. Furthermore, localization of these proteins in the nuclei of human adrenal cortex was also commonly observed, indicating that our observation is potentially relevant to physiological adrenocortical steroidogenesis. Interestingly, unique expression profiles of these factors, increased expression of SF-1 and Ubc9 and decreased expression of PIAS1 in cortisol-producing adenomas suggest possible pathophysiological roles of these factors in this particular type of adrenocortical tumors.
Ubc9 and PIAS1 function as transcriptional coactivators of SF-1
First of all, both Ubc9 and PIAS1 interacted with SF-1 in mammalian cells. Second, both Ubc9 and PIAS1 enhanced the transactivation of three reporter constructs, such as CYP17, CYP11A1, and CYP11B1, dependent on the expression level of SF-1. Third, reduction of endogenous Ubc9 and PIAS1 by small interfering RNA decreased SF-1-mediated expression of the CYP17, CYP11A1, and CYP11B1 but not other genes, such as COUP-TFI or EGFR mRNA, indicating that endogenous Ubc9 and PIAS1 contribute to SF-1-mediated transactivation. Fourth, ChIP assays clearly showed that SF-1, Ubc9, and PIAS1 were recruited to a native SF-1-regulated CYP17 gene promoter, demonstrating functional coupling between SF-1 and Ubc9 and PIAS1. Therefore, Ubc9 and PIAS1 have all the characteristics expected for transcriptional coactivators of SF-1. In addition, these proteins were colocalized in the nuclei of all zones of human adrenal cortical cells. Based on these data, Ubc9 and PIAS1 can function as coactivators of SF-1 in human adrenal cortex.
Function of sumoylation of SF-1
SF-1 is modified by SUMO-1 via three consecutive enzyme reactions including SUMO-E1 activating enzyme, a SAE1 (SUMO-activating enzyme subunit 1)-SAE2 heterodimer, SUMO-E2 conjugating enzyme Ubc9 and SUMO-E3 ligase, PIAS family proteins, thus resulting in attenuation of transcription. The exact molecular mechanisms have been unknown how sumoylation of SF-1 results in transcriptional repression. There are several reports to identify proteins that interact with sumoylated SF-1. A DEAD-box RNA helicase, DP103, was shown to directly interact with sumoylated SF-1, and the interaction was involved in transcriptional repression by protein sumoylation (27). Furthermore, a recent study identified androgen receptor interacting protein 4, a member of the Rad54 chromatin remodeling family, as a factor that interacts with sumoylated SF-1, and androgen receptor interacting protein 4 may be a cofactor that modulates SUMO-mediated fine-tuning of transcriptional repression (28). In contrast, sumoylation of SF-1 was shown to result in coordinated protein modification, such as reducing CDK7-mediated serine 203 phosphorylation (47) and reduction of DNA binding activity of SF-1 (26).
In the present study, overexpression of Ubc9 (C93S) and PIAS1 (C351S), which are loss-of-sumoylation mutants, also enhanced SF-1-mediated transactivation of the CYP17 reporter. These findings indicate that the coactivation function of Ubc9 and PIAS1 is independent of their sumoylation activities. It is still possible that ectopic expression of Ubc9 and PIAS1 may modify not only SF-1 but also one or more other cellular factors involved in SF-1-mediated transactivation, but these data indicate that sumoylation enzyme activity may minimally contribute to the coactivation function of these proteins. In fact, there are other examples that Ubc9 and PIAS1 can function as cofactors of nuclear receptor-mediated transactivation mediated by mineralocorticoid receptor (41), androgen receptor (48, 49), and COUP-TFI (33, 34). Because the spatiotemporal regulation of these dual functions is unknown, our current study highlights a novel mechanism of Ubc9 and PIAS1 for sumoylation-independent transcriptional regulation of SF-1.
Role of SF-1, Ubc9, and PIAS1 in human adrenal cortex and adrenocortical adenomas
In YAC transgenic mice carrying the rat Sf-1 gene locus (50), Sf-1 overexpression triggered adrenocortical tumorigenesis (51). In human adult adrenocortical tumors, some comparative genomic hybridization studies also showed frequent amplification of the region harboring the SF-1 gene (52). Levels of expression of SF-1 mRNA or protein were shown to be increased or normal compared with normal control adrenals in human adrenocortical tumors, depending on the methods used to measure SF-1 expression (31, 46, 53). The present study showed that levels of expression of SF-1 protein were significantly increased in cortisol-producing adenomas than nontumorous adjacent adrenals, suggesting an implication of an increased SF-1 dosage in adrenocortical tumorigenesis in patients with Cushing's syndrome. A recent report (54) showed that SF-1 inverse agonists of the isoquinolinone class are able to reverse the effect of increased SF-1 dosage on the proliferation of the H295R adrenocortical cell line. These data suggest the potential clinical utility of molecules targeting SF-1 in the therapy of adrenocortical tumors.
We have previously shown that Ubc9 and PIAS1 are exclusively localized in the rat adrenal zona glomerulosa cells in which aldosterone is selectively synthesized (34). However, this specific expression profile was observed only in rat but not mouse or human adrenal gland. The present results showed that SF-1, Ubc9, and PIAS-1 were widely localized in not only zona glomerulosa but also zonae fasciculata-reticularis of the human adrenal cortex. Moreover, coactivation function of Ubc9 and PIAS1 on SF-1 was observed in CYP17, CYP11A1, and CYP11B1 but not CYP11B2 genes. Therefore, one would consider that a complex of SF-1, Ubc9, and PIAS1 is involved in cortisol synthesis in adrenal zonae fasciculata-reticularis cells, whereas both Ubc9 and PIAS1 are positive regulators of aldosterone synthesis through a complex formation of COUP-TFI in adrenal zona glomerulosa cells (34). In functioning adrenal tumors, it is shown that the excessive steroid hormone production results from aberrant expression of specific steroidogenic enzymes (30, 31, 55); for example, CYP17 is overexpressed in cortisol-producing adenomas and CYP11B2 is overexpressed in aldosterone-producing adenomas. In this study, the unique expression profiles that increased expression of SF-1 and Ubc9 in cortisol-producing adenomas may be associated with excessive cortisol secretion from cortisol-producing adenomas.
There is another possibility of the unique expression profiles in cortisol-producing adenomas because both Ubc9 and PIAS1 are shown to play crucial roles in tumor development. A recent study demonstrated that Ubc9 is associated with breast tumor growth independent of its sumoylation enzyme activity (56). PIAS family proteins are reported to be associated with colon tumor (57) and lung cancer (58). Therefore, aberrant expression of SF-1, Ubc9, and PIAS1 may play important roles in adrenocortical tumorigenesis, particularly cortisol-producing adenomas. Of course, unique expression of Ubc9 and PIAS1 may relate to specific protein sumoylation processes in cortisol-producing adenomas, which should be clarified in the future study.
There are several limitations in this study. First of all, although we showed another functional aspect of Ubc9 and PIAS1 as coactivators of SF-1, the molecular mechanism has not been clear. Although we confirmed the DNA binding of SF-1 was not changed with Ubc9 or PIAS1 (data not shown), it might recruit another coactivator, such as steroid receptor coactivator-1 when Ubc9 and PIAS1 enhance SF-1-mediated transcription in a similar manner to mineralocorticoid receptor and COUP-TFI (34, 41). Second, we investigated six cases of aldosterone-producing adenomas and six of cortisol-producing adenomas; however, the numbers of cases are limited. In the future study, it should be investigated how aberrant expression of SF-1, Ubc9, and PIAS1 is reflected in cortisol-producing adenomas, such as tumor growth and excessive cortisol production. Third, we are aware that nontumor portion is not the exact control compared with the tumor. However, we cannot collect the same number of normal adrenal as control and further studies are needed to clear these limitations.
In summary, the present study showed that Ubc9 and PIAS1 function as SF-1 coactivators beyond sumoylation enzyme function in adrenocortical steroidogenesis and also suggested that SF-1, Ubc9, and PIAS1 play some pathophysiological roles in human cortisol-producing adenomas.
Supplementary Material
Acknowledgments
We are grateful to Dr. Ming-Jer Tsai, Dr. Sophia Tsai, Dr. Bert W. O'Malley, Dr. James Larry Jameson, and Dr. Ken-ichirou Morohashi for plasmid contribution.
This work was supported by a Grant-in-Aid for Scientific Research from the Japan Society for the Promotion of Science (to H.S. and I.K.), an investigator-initiated research grant from Bayer Pharmaceuticals Inc. (to H.S.), Pfizer Pharmaceuticals Inc. (to H.S.), a Health Labor Science Research Grant for Disorders of Adrenocortical Hormone Production from the Ministry of Health, Labor, and Welfare, Japan (to H.S.), Keio Gijuku Fukuzawa Memorial Fund for the Advancement of Education and Research (to H.S. and I.K.), Keio Gijuku Academic Development Funds (to H.S.), Nateglinide Memorial Toyoshima Research and Education Fund (to H.S.), National Institutes of Health Grant DK43140 (to W.E.R.), and a grant from the Smoking Research Foundation (to H.I. and H.S.).
Disclosure Summary: All authors have nothing to disclose.
Footnotes
- ChIP
- Chromatin immunoprecipitation
- COUP-TF
- chicken ovalbumin upstream promoter-transcription factor
- E2
- SUMO-conjugating enzyme
- E3
- SUMO-ligase
- EGFR
- epidermal growth factor receptor
- IP
- immunoprecipitation
- PIAS1
- protein inhibitors of activated STAT1
- SF-1
- steroidogenic factor-1
- siRNA
- small interfering RNA
- STAT1
- signal transducers and activators of transcription 1
- SUMO-1
- small ubiquitin-related modifier-1.
References
- 1. Morohashi K, Honda S, Inomata Y, Handa H, Omura T. 1992. A common trans-acting factor, Ad4-binding protein, to the promoters of steroidogenic P-450s. J Biol Chem 267:17913–17919 [PubMed] [Google Scholar]
- 2. Rice DA, Mouw AR, Bogerd AM, Parker KL. 1991. A shared promoter element regulates the expression of three steroidogenic enzymes. Mol Endocrinol 5:1552–1561 [DOI] [PubMed] [Google Scholar]
- 3. Achermann JC, Ozisik G, Ito M, Orun UA, Harmanci K, Gurakan B, Jameson JL. 2002. Gonadal determination and adrenal development are regulated by the orphan nuclear receptor steroidogenic factor-1, in a dose-dependent manner. J Clin Endocrinol Metab 87:1829–1833 [DOI] [PubMed] [Google Scholar]
- 4. Bland ML, Fowkes RC, Ingraham HA. 2004. Differential requirement for steroidogenic factor-1 gene dosage in adrenal development versus endocrine function. Mol Endocrinol 18:941–952 [DOI] [PubMed] [Google Scholar]
- 5. Fatchiyah, Zubair M, Shima Y, Oka S, Ishihara S, Fukui-Katoh Y, Morohashi K. 2006. Differential gene dosage effects of Ad4BP/SF-1 on target tissue development. Biochem Biophys Res Commun 341:1036–1045 [DOI] [PubMed] [Google Scholar]
- 6. Hammer GD, Parker KL, Schimmer BP. 2005. Minireview: transcriptional regulation of adrenocortical development. Endocrinology 146:1018–1024 [DOI] [PubMed] [Google Scholar]
- 7. Ikeda Y, Shen WH, Ingraham HA, Parker KL. 1994. Developmental expression of mouse steroidogenic factor-1, an essential regulator of the steroid hydroxylases. Mol Endocrinol 8:654–662 [DOI] [PubMed] [Google Scholar]
- 8. Morohashi KI. 1999. Gonadal and extragonadal functions of Ad4BP/SF-1: developmental aspects. Trends Endocrinol Metab 10:169–173 [DOI] [PubMed] [Google Scholar]
- 9. Schimmer BP, White PC. 2010. Minireview: steroidogenic factor 1: its roles in differentiation, development, and disease. Mol Endocrinol 24:1322–1337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhao L, Bakke M, Hanley NA, Majdic G, Stallings NR, Jeyasuria P, Parker KL. 2004. Tissue-specific knockouts of steroidogenic factor 1. Mol Cell Endocrinol 215:89–94 [DOI] [PubMed] [Google Scholar]
- 11. Luo X, Ikeda Y, Parker KL. 1994. A cell-specific nuclear receptor is essential for adrenal and gonadal development and sexual differentiation. Cell 77:481–490 [DOI] [PubMed] [Google Scholar]
- 12. Ikeda Y, Luo X, Abbud R, Nilson JH, Parker KL. 1995. The nuclear receptor steroidogenic factor 1 is essential for the formation of the ventromedial hypothalamic nucleus. Mol Endocrinol 9:478–486 [DOI] [PubMed] [Google Scholar]
- 13. Tran PV, Lee MB, Marín O, Xu B, Jones KR, Reichardt LF, Rubenstein JR, Ingraham HA. 2003. Requirement of the orphan nuclear receptor SF-1 in terminal differentiation of ventromedial hypothalamic neurons. Mol Cell Neurosci 22:441–453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zhao L, Bakke M, Parker KL. 2001. Pituitary-specific knockout of steroidogenic factor 1. Mol Cell Endocrinol 185:27–32 [DOI] [PubMed] [Google Scholar]
- 15. Desclozeaux M, Krylova IN, Horn F, Fletterick RJ, Ingraham HA. 2002. Phosphorylation and intramolecular stabilization of the ligand binding domain in the nuclear receptor steroidogenic factor 1. Mol Cell Biol 22:7193–7203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sewer MB, Waterman MR. 2003. CAMP-dependent protein kinase enhances CYP17 transcription via MKP-1 activation in H295R human adrenocortical cells. J Biol Chem 278:8106–8111 [DOI] [PubMed] [Google Scholar]
- 17. Chen WY, Juan LJ, Chung BC. 2005. SF-1 (nuclear receptor 5A1) activity is activated by cyclic AMP via p300-mediated recruitment to active foci, acetylation, and increased DNA binding. Mol Cell Biol 25:10442–10453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jacob AL, Lund J, Martinez P, Hedin L. 2001. Acetylation of steroidogenic factor 1 protein regulates its transcriptional activity and recruits the coactivator GCN5. J Biol Chem 276:37659–37664 [DOI] [PubMed] [Google Scholar]
- 19. Børud B, Hoang T, Bakke M, Jacob AL, Lund J, Mellgren G. 2002. The nuclear receptor coactivators p300/CBP/cointegrator-associated protein (p/CIP) and transcription intermediary factor 2 (TIF2) differentially regulate PKA-stimulated transcriptional activity of steroidogenic factor 1. Mol Endocrinol 16:757–773 [DOI] [PubMed] [Google Scholar]
- 20. Dammer EB, Leon A, Sewer MB. 2007. Coregulator exchange and sphingosine-sensitive cooperativity of steroidogenic factor-1, general control nonderepressed 5, p54, and p160 coactivators regulate cyclic adenosine 3′,5′-monophosphate-dependent cytochrome P450c17 transcription rate. Mol Endocrinol 21:415–438 [DOI] [PubMed] [Google Scholar]
- 21. Li LA, Chiang EF, Chen JC, Hsu NC, Chen YJ, Chung BC. 1999. Function of steroidogenic factor 1 domains in nuclear localization, transactivation, and interaction with transcription factor TFIIB and c-Jun. Mol Endocrinol 13:1588–1598 [DOI] [PubMed] [Google Scholar]
- 22. Monté D, DeWitte F, Hum DW. 1998. Regulation of the human P450scc gene by steroidogenic factor 1 is mediated by CBP/p300. J Biol Chem 273:4585–4591 [DOI] [PubMed] [Google Scholar]
- 23. Zhou D, Quach KM, Yang C, Lee SY, Pohajdak B, Chen S. 2000. PNRC: a proline-rich nuclear receptor coregulatory protein that modulates transcriptional activation of multiple nuclear receptors including orphan receptors SF1 (steroidogenic factor 1) and ERRα1 (estrogen related receptor α-1). Mol Endocrinol 14:986–998 [DOI] [PubMed] [Google Scholar]
- 24. Chen WY, Lee WC, Hsu NC, Huang F, Chung BC. 2004. SUMO modification of repression domains modulates function of nuclear receptor 5A1 (steroidogenic factor-1). J Biol Chem 279:38730–38735 [DOI] [PubMed] [Google Scholar]
- 25. Komatsu T, Mizusaki H, Mukai T, Ogawa H, Baba D, Shirakawa M, Hatakeyama S, Nakayama KI, Yamamoto H, Kikuchi A, Morohashi K. 2004. Small ubiquitin-like modifier 1 (SUMO-1) modification of the synergy control motif of Ad4 binding protein/steroidogenic factor 1 (Ad4BP/SF-1) regulates synergistic transcription between Ad4BP/SF-1 and Sox9. Mol Endocrinol 18:2451–2462 [DOI] [PubMed] [Google Scholar]
- 26. Campbell LA, Faivre EJ, Show MD, Ingraham JG, Flinders J, Gross JD, Ingraham HA. 2008. Decreased recognition of SUMO-sensitive target genes following modification of SF-1 (NR5A1). Mol Cell Biol 28:7476–7486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lee MB, Lebedeva LA, Suzawa M, Wadekar SA, Desclozeaux M, Ingraham HA. 2005. The DEAD-box protein DP103 (Ddx20 or Gemin-3) represses orphan nuclear receptor activity via SUMO modification. Mol Cell Biol 25:1879–1890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ogawa H, Komatsu T, Hiraoka Y, Morohashi K. 2009. Transcriptional suppression by transient recruitment of ARIP4 to sumoylated nuclear receptor Ad4BP/SF-1. Mol Biol Cell 20:4235–4245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Shibata H, Ando T, Suzuki T, Kurihara I, Hayashi K, Hayashi M, Saito I, Kawabe H, Tsujioka M, Mural M, Saruta T. 1998. Differential expression of an orphan receptor COUP-TFI and corepressors in adrenal tumors. Endocr Res 24:881–885 [DOI] [PubMed] [Google Scholar]
- 30. Shibata H, Ando T, Suzuki T, Kurihara I, Hayashi K, Hayashi M, Saito I, Murai M, Saruta T. 1998. COUP-TFI expression in human adrenocortical adenomas: possible role in steroidogenesis. J Clin Endocrinol Metab 83:4520–4523 [DOI] [PubMed] [Google Scholar]
- 31. Shibata H, Ikeda Y, Mukai T, Morohashi K, Kurihara I, Ando T, Suzuki T, Kobayashi S, Murai M, Saito I, Saruta T. 2001. Expression profiles of COUP-TF, DAX-1, and SF-1 in the human adrenal gland and adrenocortical tumors: possible implications in steroidogenesis. Mol Genet Metab 74:206–216 [DOI] [PubMed] [Google Scholar]
- 32. Shibata H, Kurihara I, Kobayashi S, Yokota K, Suda N, Saito I, Saruta T. 2003. Regulation of differential COUP-TF-coregulator interactions in adrenal cortical steroidogenesis. J Steroid Biochem Mol Biol 85:449–456 [DOI] [PubMed] [Google Scholar]
- 33. Kobayashi S, Shibata H, Kurihara I, Yokota K, Suda N, Saito I, Saruta T. 2004. Ubc9 interacts with chicken ovalbumin upstream promoter-transcription factor I and represses receptor-dependent transcription. J Mol Endocrinol 32:69–86 [DOI] [PubMed] [Google Scholar]
- 34. Kurihara I, Shibata H, Kobayashi S, Suda N, Ikeda Y, Yokota K, Murai A, Saito I, Rainey WE, Saruta T. 2005. Ubc9 and Protein inhibitor of activated STAT 1 activate chicken ovalbumin upstream promoter-transcription factor I-mediated human CYP11B2 gene transcription. J Biol Chem 280:6721–6730 [DOI] [PubMed] [Google Scholar]
- 35. Bassett MH, Zhang Y, Clyne C, White PC, Rainey WE. 2002. Differential regulation of aldosterone synthase and 11β-hydroxylase transcription by steroidogenic factor-1. J Mol Endocrinol 28:125–135 [DOI] [PubMed] [Google Scholar]
- 36. Wang XL, Bassett M, Zhang Y, Yin S, Clyne C, White PC, Rainey WE. 2000. Transcriptional regulation of human 11β-hydroxylase (hCYP11B1). Endocrinology 141:3587–3594 [DOI] [PubMed] [Google Scholar]
- 37. Clyne CD, Zhang Y, Slutsker L, Mathis JM, White PC, Rainey WE. 1997. Angiotensin II and potassium regulate human CYP11B2 transcription through common cis-elements. Mol Endocrinol 11:638–649 [DOI] [PubMed] [Google Scholar]
- 38. Hanley NA, Rainey WE, Wilson DI, Ball SG, Parker KL. 2001. Expression profiles of SF-1, DAX1, and CYP17 in the human fetal adrenal gland: potential interactions in gene regulation. Mol Endocrinol 15:57–68 [DOI] [PubMed] [Google Scholar]
- 39. Hu MC, Chou SJ, Huang YY, Hsu NC, Li H, Chung BC. 1999. Tissue-specific, hormonal, and developmental regulation of SCC-LacZ expression in transgenic mice leads to adrenocortical zone characterization. Endocrinology 140:5609–5618 [DOI] [PubMed] [Google Scholar]
- 40. Nogueira EF, Rainey WE. 2010. Regulation of aldosterone synthase by activator transcription factor/cAMP response element-binding protein family members. Endocrinology 151:1060–1070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Yokota K, Shibata H, Kurihara I, Kobayashi S, Suda N, Murai-Takeda A, Saito I, Kitagawa H, Kato S, Saruta T, Itoh H. 2007. Coactivation of the N-terminal transactivation of mineralocorticoid receptor by Ubc9. J Biol Chem 282:1998–2010 [DOI] [PubMed] [Google Scholar]
- 42. Funder JW, Carey RM, Fardella C, Gomez-Sanchez CE, Mantero F, Stowasser M, Young WF, Jr, Montori VM. 2008. Case detection, diagnosis, and treatment of patients with primary aldosteronism: an endocrine society clinical practice guideline. J Clin Endocrinol Metab 93:3266–3281 [DOI] [PubMed] [Google Scholar]
- 43. Nieman LK, Biller BM, Findling JW, Newell-Price J, Savage MO, Stewart PM, Montori VM. 2008. The diagnosis of Cushing's syndrome: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab 93:1526–1540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lan HC, Li HJ, Lin G, Lai PY, Chung BC. 2007. Cyclic AMP stimulates SF-1-dependent CYP11A1 expression through homeodomain-interacting protein kinase 3-mediated Jun N-terminal kinase and c-Jun phosphorylation. Mol Cell Biol 27:2027–2036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ye P, Nakamura Y, Lalli E, Rainey WE. 2009. Differential effects of high and low steroidogenic factor-1 expression on CYP11B2 expression and aldosterone production in adrenocortical cells. Endocrinology 150:1303–1309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Sasano H, Shizawa S, Suzuki T, Takayama K, Fukaya T, Morohashi K, Nagura H. 1995. Ad4BP in the human adrenal cortex and its disorders. J Clin Endocrinol Metab 80:2378–2380 [DOI] [PubMed] [Google Scholar]
- 47. Yang WH, Heaton JH, Brevig H, Mukherjee S, Iñiguez-Lluhi JA, Hammer GD. 2009. SUMOylation inhibits SF-1 activity by reducing CDK7-mediated serine 203 phosphorylation. Mol Cell Biol 29:613–625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Poukka H, Aarnisalo P, Karvonen U, Palvimo JJ, Jänne OA. 1999. Ubc9 interacts with the androgen receptor and activates receptor-dependent transcription. J Biol Chem 274:19441–19446 [DOI] [PubMed] [Google Scholar]
- 49. Gross M, Yang R, Top I, Gasper C, Shuai K. 2004. PIASy-mediated repression of the androgen receptor is independent of sumoylation. Oncogene 23:3059–3066 [DOI] [PubMed] [Google Scholar]
- 50. Karpova T, Presley J, Manimaran RR, Scherrer SP, Tejada L, Peterson KR, Heckert LL. 2005. A FTZ-F1-containing yeast artificial chromosome recapitulates expression of steroidogenic factor 1 in vivo. Mol Endocrinol 19:2549–2563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Doghman M, Karpova T, Rodrigues GA, Arhatte M, De Moura J, Cavalli LR, Virolle V, Barbry P, Zambetti GP, Figueiredo BC, Heckert LL, Lalli E. 2007. Increased steroidogenic factor-1 dosage triggers adrenocortical cell proliferation and cancer. Mol Endocrinol 21:2968–2987 [DOI] [PubMed] [Google Scholar]
- 52. Dohna M, Reincke M, Mincheva A, Allolio B, Solinas-Toldo S, Lichter P. 2000. Adrenocortical carcinoma is characterized by a high frequency of chromosomal gains and high-level amplifications. Genes Chromosomes Cancer 28:145–152 [PubMed] [Google Scholar]
- 53. Bassett MH, Mayhew B, Rehman K, White PC, Mantero F, Arnaldi G, Stewart PM, Bujalska I, Rainey WE. 2005. Expression profiles for steroidogenic enzymes in adrenocortical disease. J Clin Endocrinol Metab 90:5446–5455 [DOI] [PubMed] [Google Scholar]
- 54. Doghman M, Cazareth J, Douguet D, Madoux F, Hodder P, Lalli E. 2009. Inhibition of adrenocortical carcinoma cell proliferation by steroidogenic factor-1 inverse agonists. J Clin Endocrinol Metab 94:2178–2183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Shibata H, Kobayashi S, Kurihara I, Saito I, Saruta T. 2003. Nuclear receptors and co-regulators in adrenal tumors. Horm Res 59(Suppl 1):85–93 [DOI] [PubMed] [Google Scholar]
- 56. Zhu S, Sachdeva M, Wu F, Lu Z, Mo YY. 2010. Ubc9 promotes breast cell invasion and metastasis in a sumoylation-independent manner. Oncogene 29:1763–1772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Coppola D, Parikh V, Boulware D, Blanck G, Ogata Y, Osaki T, Naka T, Iwahori K, Furukawa M, Nagatomo I, Kijima T, Kumagai T, Yoshida M, Tachibana I, Kawase I. 2009. Substantially reduced expression of PIAS1 is associated with colon cancer development overexpression of PIAS3 suppresses cell growth and restores the drug sensitivity of human lung cancer cells in association with PI3-K/Akt inactivation. J Cancer Res Clin Oncol 135:1287–1291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Ogata Y, Osaki T, Naka T, Iwahori K, Furukawa M, Nagatomo I, Kijima T, Kumagai T, Yoshida M, Tachibana I, Kawase I. 2006. Overexpression of PIAS3 suppresses cell growth and restores the drug sensitivity of human lung cancer cells in association with PI3-K/Akt inactivation. Neoplasia 8:817–825 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.