SynCAM1, a synaptic cell adhesion molecule, is expressed in astrocytes and mediates astrocyte-GnRH adhesive communication, an action regulated by erbB4 signaling.
Abstract
We previously identified synaptic cell adhesion molecule 1 (SynCAM1) as a component of a genetic network involved in the hypothalamic control of female puberty. Although it is well established that SynCAM1 is a synaptic adhesion molecule, its contribution to hypothalamic function is unknown. Here we show that, in addition to the expected neuronal localization illustrated by its presence in GnRH neurons, SynCAM1 is expressed in hypothalamic astrocytes. Cell adhesion assays indicated that SynCAM is recognized by both GnRH neurons and astrocytes as an adhesive partner and promotes cell-cell adhesiveness via homophilic, extracellular domain-mediated interactions. Alternative splicing of the SynCAM1 primary mRNA transcript yields four mRNAs encoding membrane-spanning SynCAM1 isoforms. Variants 1 and 4 are predicted to be both N and O glycosylated. Hypothalamic astrocytes and GnRH-producing GT1-7 cells express mainly isoform 4 mRNA, and sequential N- and O-deglycosylation of proteins extracted from these cells yields progressively smaller SynCAM1 species, indicating that isoform 4 is the predominant SynCAM1 variant expressed in astrocytes and GT1-7 cells. Neither cell type expresses the products of two other SynCAM genes (SynCAM2 and SynCAM3), suggesting that SynCAM-mediated astrocyte-astrocyte and astrocyte-GnRH neuron adhesiveness is mostly mediated by SynCAM1 homophilic interactions. When erbB4 receptor function is disrupted in astrocytes, via transgenic expression of a dominant-negative erbB4 receptor form, SynCAM1-mediated adhesiveness is severely compromised. Conversely, SynCAM1 adhesive behavior is rapidly, but transiently, enhanced in astrocytes by ligand-dependent activation of erbB4 receptors, suggesting that erbB4-mediated events affecting SynCAM1 function contribute to regulate astrocyte adhesive communication.
In a previous study (1), we identified synaptic cell adhesion molecule 1 (SynCAM1) as a subordinate gene of a regulatory gene network postulated to operate in the hypothalamus. It was postulated that the main function of this network is to facilitate and integrate cellular and cell-cell communication programs required for the acquisition of female reproductive competence (1, 2). The network is composed of genes that control diverse cellular function but share the common feature of having been earlier identified as involved in tumor suppression/tumor formation. SynCAM1, also known as nectin-like protein 3 (Necl2) or Ig superfamily4 (IGSF4), was originally described as tumor suppressor in lung cancer-1 (TSLC1) (3, 4). SynCAM1 mRNA abundance increases in the hypothalamus of peripubertal monkeys as compared with juvenile animals (1) and interfering with SynCAM1 signaling delays puberty in mice (5), suggesting that in both species the onset of female puberty is accompanied by hypothalamic activation of SynCAM1 synthesis.
The SynCAM family of adhesive proteins is encoded by four vertebrate-specific genes that share a high degree of homology and structural conservation among themselves and across species (6). One of these genes encodes SynCAM1, a protein that plays an important role in central nervous system development because it drives synaptic formation (7, 8), induces functional differentiation of presynaptic terminals (9), and establishes adhesive contacts between neuronal growth cones and target neurites (10).
SynCAM1 contains an extracellular domain with three Ig-like domains, an extracellular juxtamembranous region subjected to alternative splicing, a single transmembrane domain, and a short intracellular domain endowed with two protein-protein interaction motifs. The intracellular domain is subdivided into a juxtamembranous motif able to interact with members of the protein 4.1 family and a carboxy terminus sequence predicted to interact with proteins containing a PDZ [postsynaptic density protein (PSD95); Drosophila disc large tumor suppressor (DlgA), and zonula occludens-1 protein (zo-1) domain] (6, 11, 12). Alternative splicing in the extracellular juxtamembrane region of SynCAM1 generates five isoforms (SynCAM1 1–5) (6) with different molecular properties (6, 7). Four of them are membrane-spanning (isoforms 1–4), and one corresponds to a secreted protein (isoform 5). Although the Ig-like domains of all SynCAM1 isoforms are predicted to be heavily N glycosylated, only the extracellular juxtamembranous domain of isoforms 1 and 4 is also predicted to be O glycosylated (6, 13, 14). Little is known regarding the physiological consequences of O-glycosylation, but evidence exists that N-glycosylation of the first two Ig-like domains is important to regulate SynCAM1 adhesive properties (13). Although it is well established that SynCAM1 is a major synaptic adhesive protein, we made the unexpected finding (5) that the hypothalamic content of SynCAM1 is reduced in mice in which puberty is delayed by the astrocyte-specific expression of a truncated erythroblastosis B (erbB) 4 receptor (15, 16). This decrease was found to be prominent in hypothalamic astrocytes, suggesting that SynCAM1 may not only play a role in synaptic organization, but also be involved in facilitating astrocyte-dependent adhesive communication. Here we report that SynCAM1, expressed in astrocytes of the neuroendocrine brain and GnRH neurons, mediates both astrocyte-to-astrocyte and astrocyte-GnRH neuron adhesiveness. We also show that both GnRH-producing cells and hypothalamic astrocytes express the same alternatively spliced form of SynCAM1 mRNA (isoform 4), resulting in a SynCAM1 protein that is N and O glycosylated in both cell types. Using both SynCAM1-specific antibodies and pleio-SynCAM antibodies that recognize SynCAM2 and -3 in addition to SynCAM1, we show that the product of the SynCAM1 gene is the major, if not the only, SynCAM species expressed in GnRH producing cells and hypothalamic astrocytes. It is therefore likely that both astrocyte-astrocyte and astrocyte-GnRH neuron adhesions are mediated by homophilic SynCAM1 interactions, instead of the heterophilic SynCAM1/2 interactions known to promote interneuronal synaptic organization in the developing brain (13). Lastly, our results also show that SynCAM1 adhesive behavior is functionally coupled to astrocytic erbB4 receptor function. Ligand-dependent activation of astrocytic erbB4 receptors results in a rapid, but transient, increase in SynCAM1 adhesive behavior. Conversely, disruption of astrocytic erbB4 receptor function leads to loss of SynCAM1-mediated adhesiveness.
Materials and Methods
Animals
In this study we used wild-type (WT) mice of the FvB strain and transgenic (GFAP-DNerbB4) mice carrying a dominant-negative (DN) form of the erbB4 receptor under the control of the human glial fibrillary acidic protein (GFAP) promoter. They were used in accordance with National Institutes of Health guidelines for the Care and Use of Laboratory Animals. All experimental protocols were approved by the Animal Care and Use Committee of the Oregon National Primate Research Center.
SynCAM deglycosylation and Western blotting
SynCAM protein N- and O-linked deglycosylation was performed on protein lysates from cultured GT1-7 cells and hypothalamic astrocytes, using the enzymatic CarboRelease kit (QABio, Palm Desert, CA), according to the manufacturer's protocol. Briefly, for N-deglycosolation, 100 μg of protein extract was incubated with PNGaseF (5 mU) for 3 h at 37 C. For N- plus O-deglycosolation, 100 μg of protein extract was simultaneously incubated with PNGaseF (5 mU), O-glycosidase (1.25 mU), sialidase (5 mU), β-galactosidase (50 mU), and glucosaminidase (1 mU) for 3 h at 37 C. Enzymatic deglycosolation was confirmed by running 30 μg of N-deglycosolated, N- plus O-deglycosolated, and native (nondeglycosolated) protein on a SDS-PAGE gel, transferred to polyvinylidene difluoride membranes, and probed for either SynCAM1 using antibody 3E1 (1:5000) or SynCAM1, SynCAM2, and SynCAM3 using pleio-SynCAM antibodies (1:1000). The reactions were developed with enhanced chemiluminescence using the Western lightning chemiluminescence substrate (PerkinElmer Life Sciences, Boston, MA).
Fusion protein isolation
For adhesion assays, we used vectors containing the human Fc portion of human IgG fused to the WT extracellular domain of SynCAM1 (SynCAM1-Fc) or to the extracellular SynCAM1 domain lacking the three SynCAM1 IgG extracellular subdomains (7). We refer to this construct as ΔECD-Fc. A plasmid (pCMV-VIgL-C) encoding amino acids 1–48 of rat neurexin-1α (including the signal peptide and 18 amino acids of the NH2-terminal) fused to the hinge region of human IgG1-Fc (generously provided by Dr. Thomas Südhof, Stanford University, School of Medicine, Palo Alto, CA) was used as an additional control (17). We refer to this construct as ΔNRX-Fc. Fusion proteins were generated and purified using SynCAM1-Fc, ΔECD-Fc, and ΔNRX-Fc expression plasmids transiently transfected into Chinese hamster ovary cells. The resulting fusion proteins were purified from the cell supernatants using protein G-sepharose columns. Each column (PolyPrep chromatography columns; Bio-Rad Laboratories, Hercules, CA) contained 2 ml of protein G slurry (Sigma, St. Louis, MO). The collected fractions were dialyzed overnight against three changes of PBS, and the proteins were concentrated with an Amicon Ultra-15 column (Millipore, Billerica, MA). The integrity and relative purity of the fusion proteins was determined by Western blotting. The purified proteins were size fractionated on a 10% Tris-glycine gel (Invitrogen, Carlsbad, CA) and transferred to polyvinylidene difluoride membranes (Millipore). After a blocking step in 5% milk (1 h at room temperature), the Fc component of each protein was detected using goat polyclonal antibodies to human Fc (1:1000; Sigma). The relative purity of the proteins was confirmed by silver staining (SilverSnap Stain Kit II; Pierce, Rockford, IL) of the gels.
Adhesion of SynCAM1-coated beads to hypothalamic astrocytes and GT1-7 neurons
To more precisely estimate the ability of GT1-7 cells and astrocytes to adhere to isolated SynCAM1, red dyed 6-μm polystyrene beads were coated with either SynCAM1-Fc or ΔECD-Fc fusion protein. Polystyrene red-dyed microspheres (Polysciences Inc., Warrington, PA) were coated with either purified SynCAM1-Fc or ΔECD-Fc fusion protein. Twenty microliters of uncoated beads were washed three times with 0.2 m borate buffer (pH 8.5), resuspended in 500 μl of borate buffer containing 20 μg of goat antihuman IgG (Fc specific) antibody (Sigma), and rocked overnight at 4 C. The beads were pelleted at 9000 rpm for 10 min at room temp and the supernatant was discarded. The beads were then washed three times with 0.2 m borate buffer (pH 8.5) containing 0.3% immunoglobulin-free BSA (Sigma) and were resuspended in 500 μl BSA-borate buffer containing 0.5 μg of purified fusion protein (either SynCAM1-Fc or ΔECD-Fc) for 3 h at room temperature. The coated beads were then washed three times in BSA-borate buffer and resuspended in 100 μl of this buffer. For adhesion studies using GT1-7 cells, poly-l-lysine-coated glass coverslips (22 × 22 mm; Fisher Scientific, Fair Lawn, NJ) were placed into six-well culture dishes and seeded with 4 × 105 GT1-7 cells/well. The cells were grown for 24 h in DMEM containing 10% fetal calf serum. At this time, 40 μl of beads coated with either SynCAM1-Fc or ΔECD-Fc were suspended in 1 ml of phenol red-free DMEM without fetal calf serum and added to the cultured cells. One hour later, the suspension was removed, and the cells were gently washed three times with PBS and then fixed with either 4% paraformaldehyde and PBS (pH 7.4) for 10 min at room temperature or 95% methanol-5% glacial acetic acid solution for 10 min at −20 C for either bead quantification/scanning electron microscopy (SEM) or SynCAM immunohistofluorescence studies, respectively. For adhesion studies using WT and GFAP-DNerbB4 hypothalamic astrocytes, the cells were seeded on poly-l-lysine-coated glass coverslips (round 12 mm diameter; Ted Pella Inc., Redding, CA) in 12-well culture dishes at 1.25 × 105 cells/well and grown in DMEM/F12 containing 10% donor calf serum at 37 C until they reached 80–90% confluency. The medium was then replaced with astrocyte-defined medium (ADM) (18, 19). Twenty-four hours later, this medium was removed, the cells were briefly washed with PBS, and 10 μl of coated beads (either SynCAM1-Fc or ΔECD-Fc) suspended in 500 μl of ADM containing neuregulin-β1 (NRGβ1; 3 nm) or ADM alone were added to each well and incubated for one of three different times (10, 20, and 40 min) at 37 C. The cultures were then washed three times with PBS and fixed as described above for GT1-7 neurons. Before quantifying the number of beads adhered to either GT1-7 cells or astrocytes, the cells were stained with Texas Red-X Phalloidin (Molecular Probes-Invitrogen Corp., Carlsbad, CA) to visualize the actin cytoskeleton and consequently the surface area occupied by the cell. The cells were permeabilized by incubation in potassium PBS-0.5% Triton X-100 for 15 min and then stained with phalloidin (1:40) for 45 min at room temperature. Nuclei were stained with Hoechst 33258 (1:10,000, Molecular Probes-Invitrogen) and the coverslips were mounted onto Superfrost microscope slides using Perm Fluor aqueous mountant (Thermo Electron Corp., Pittsburgh, PA) for imaging analysis. Imaging was performed using a Marianas imaging workstation and Slidebook 4.2 software (Intelligent Imaging Innovations, Denver, CO). The Stereology module of Slidebook was used for the unbiased selection of uniformly spaced random fields based on the image of the entire coverslip, acquired as a montage of low-magnification images. Each field was acquired using a ×20 (NA0.8) objective with transmitted light (for bead visualization) and epifluorescence for Texas-Red and Hoechst. Exported images were analyzed using ImageJ software (National Institutes of Health, Bethesda, MD) equipped with the cell counter and Grid2 plug-ins. First, the image series were merged using the Texas Red, Hoechst, and inverted transmitted light channels as the final red, blue, and green channels, respectively. A counting grid, with a uniform distribution of counting boxes measuring 100,000 pixels, was overlaid onto each field and the number of beads adhered to the stained cells was determined using stereology counting rules (only beads overlapping cell surface and completely included into the field or touching the top and right borders were counted). The number of beads adhered to the cells was determined for each field, then averaged for the coverslip. The data for each treatment group were expressed as a fold change from the negative control ΔECD-Fc or nonstimulated groups.
Scanning electron microscopy
An impact freeze drying technique (20) was used to examine GT1-7 neuron cultures by SEM. A spring-activated impact device (17) was not used to avoid crushing the culture. A cylindrical brass chill block 0.5-in. thick and 1.25-in.-diameter with a flat polished surface capped with an optically flat sapphire disc 1.0 in. in diameter and 0.25 in. thick was placed in a 100-ml polypropylene beaker and then submerged in liquid nitrogen by filling the beaker, replenishing the liquid nitrogen until boiling ceased, indicating that the chill block was at liquid nitrogen temperature. The beaker sits in a gallon-size styrofoam bucket to isolate it thermally from the surroundings including the ambient air. The liquid nitrogen was then allowed to evaporate until the surface of the chill block was exposed. The coverslips were placed in three deionized water rinses for 1 min each to remove PBS. The excess water was wicked away at the edge of the coverslips with a filter paper before dropping the coverslips on the chill block culture, side up to preserve the three-dimensional nature of the cultures as seen in the images. The beaker, residual liquid nitrogen, chill block, and coverslip were placed in a bell jar evaporator, which was pumped to high vacuum with a mechanical pump followed by pumping with a turbomolecular pump to the low 10−6 Torr range. Care was taken in transferring the beaker to the evaporator, ensuring that nitrogen gas is continuously blown into the beaker to isolate the specimen and chill block from water containing ambient air so that frost does not form on the specimen before vacuum pump down. The specimen was allowed to dry overnight at high vacuum followed by coating with 10–20 nm of titanium (Ti). The beaker was also placed on two microscope slides in the evaporator to minimize thermal contact with metal parts. The specimens were then examined in a Hitachi S-4160 cold field emitter SEM with a 1- to 2-nm resolution. The coverslips were at approximately 45° tilt to the electron beam. A 3-KV accelerating voltage was used. The secondary electron signal was used to generate the images.
Statistical analysis
Quantitative data were analyzed using SigmaStat 3.1 software (Systat Software Inc., San Jose, CA). The data were first subjected to a normality test and an equal variance test. Data that passed these two tests were then analyzed as follows: comparison of two groups was performed with the Student's t test, and data sets containing more than two groups were analyzed with one-way ANOVA followed by Student-Newman-Keuls multiple test for individual means. Data that failed either the normality or equal variance test were analyzed by nonparametric methods such as the Mann-Whitney rank sum test (two groups), the Kruskal-Wallis one-way ANOVA on ranks followed by Student-Newman-Keuls method of pair-wise multiple comparison procedure (multiple groups). The null hypothesis was rejected at the P = 0.05 level for all analyses.
Supplemental material and methods
A detailed account of cell culture procedures, antibodies used, immunohistofluorescence, protein extraction, SynCAM polysialylation, RNA extraction and RT-PCR, cell adhesion to SynCAM1 substrates, and adhesion of GT1-7 cells to hypothalamic astrocytes is provided as Supplemental Material and Methods (published on The Endocrine Society's Journals Online web site at http://endo.endojournals.org).
Results
SynCAM1 is expressed in hypothalamic astrocytes and GnRH neurons
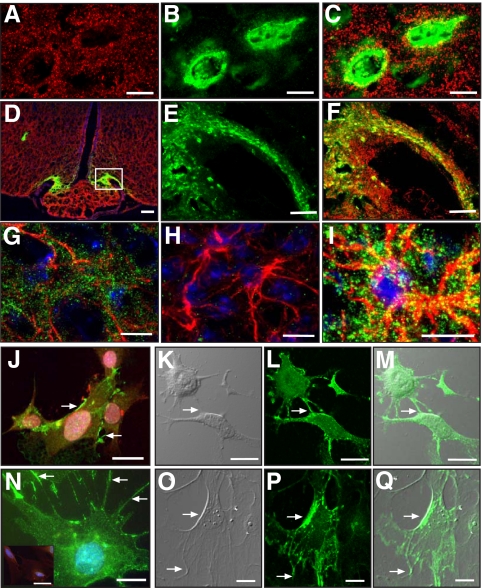
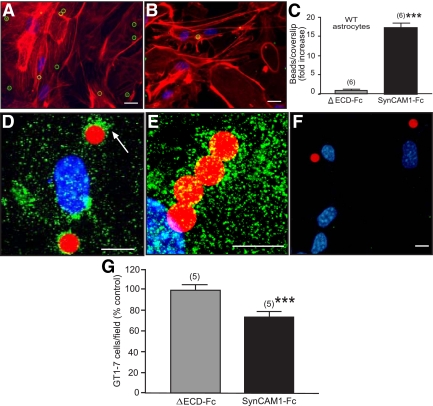
Because SynCAM is not only expressed in neurons (7) but also appears to be present in some glial populations (21), we sought to determine whether hypothalamic astrocytes or only GnRH neurons express SynCAM1. Using immunostaining of brain sections from mice that express green fluorescent protein (GFP) in GnRH neurons (22) and pleio-SynCAM antibodies, we found that the overall pattern of SynCAM immunostaining in the hypothalamus (Fig. 1, A and D) is identical to the punctate, synaptic-like pattern previously described in brain using similar antibodies (7). The relationship between the SynCAM and GFP immunoreactive signals (Fig. 1, A–F) indicates that SynCAM are extensively associated with both GnRH neuron cell bodies in the preoptic area (SynCAM, red, Fig. 1A; GnRH, green, Fig. 1B; merged images in Fig. 1C) and to cellular elements of the median eminence (Fig. 1D), which include GnRH nerve terminals (GnRH, green, Fig. 1E; merged images in Fig. 1F). Double staining for SynCAM and GFAP, a marker for astrocytes, showed that SynCAM are also abundant in hypothalamic astrocytes (Fig. 1, G and I), which appear to contain more SynCAM than glial cells of other brain regions (21). No SynCAM staining was observed in hypothalamic tissues subjected to the immunohistochemical reaction with GFAP antibodies in the absence SynCAM antibodies (Fig. 1H). In both GnRH neurons and astrocytes, SynCAM has a surface, punctate pattern of expression suggestive of an abundance of SynCAM in points of cell-cell contact. Although some of these contacts in GnRH neurons are likely synaptic (7), some others may represent glia-neuron adhesive interactions. To better define this pattern of cellular distribution, we analyzed the localization of SynCAM in cultured cells. Immunostaining of GnRH-producing cells (GT1-7 cell line) showed that the protein is concentrated at points of neuron-to-neuron contact (Fig. 1J, arrows). Consistent with the in vivo observations, hypothalamic astrocytes in culture also show an abundance of SynCAM immunostaining, which can be seen more prominently in astrocytic processes and points of cell-cell contact (Fig. 1N, arrows). No staining was observed in astrocytes subjected to the immunohistochemical reaction without SynCAM antibodies (inset, Fig. 1N). Differential interference contrast (DIC) microscopy confirmed that SynCAM protein accumulates at points of cell-to-cell and cell-to-culture well surface contact in GnRH neurons (DIC, Fig. 1K; SynCAM, green, Fig. 1L; merged images in Fig. 1M) and hypothalamic astrocytes (DIC, Fig. 1O; SynCAM, green, Fig. 1P; merged images in Fig. 1Q).
Fig. 1.
SynCAM immunoreactivity in the female mouse hypothalamus (late juvenile period, 28 d of age) is extensively associated with astrocytes and GnRH neurons. A, SynCAM (red). B, GFP-expressing GnRH neurons (green). C, Merged image of single confocal sections. D, Merged confocal image shows that SynCAM (red) is extensively distributed throughout the hypothalamus and is associated with GnRH nerve terminals (green) in the median eminence. E and F, Zoomed confocal images of the area demarcated by the white box in D showing GnRH nerve terminals (E, green) and merged SynCAM/enhanced GFP images (F). G, Merged projection of confocal images showing SynCAM immunoreactivity (green) associated with GFAP-labeled astrocytes (red). H, Hypothalamic tissues incubated with only GFAP antibodies (red). I, Zoomed merged projection of confocal images showing SynCAM immunoreactivity (green) associated with GFAP-labeled astrocytes (red). J, GT1-7 cells stained with GnRH antibodies (red) show intense SynCAM staining (green) in regions of cell contact (arrows). K–M, Projection of confocal images showing GT1-7 adhesion points using DIC (arrow, K) and the corresponding cellular distribution of SynCAM (green, L). The merged image is shown in M. N, Projection of confocal images showing SynCAM immunoreactivity (green, points of abundance denoted by arrows) in cultured hypothalamic astrocytes. The inset in N shows astrocytes stained only with GFAP antibodies (red) and Hoescht. O and P, Projection of confocal images showing astrocyte adhesion points using DIC (arrow, O) and the corresponding cellular distribution of SynCAM (green, P). Q, Merged image of O and P. Bars (A–C), 10 μm; (D), 100 μm; (E and F), 20 μm; (G–J), 10 μm; (K–M), 15 μm; (N), 10 μm; (O and P), 20 μm. Cell nuclei in G–I and N (blue) are stained with Hoescht.
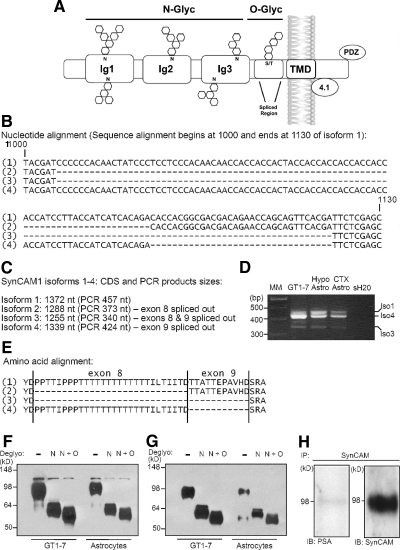
Hypothalamic astrocytes and GnRH-secreting cells express a SynCAM1 isoform that is N and O glycosylated, but not polysialylated
SynCAM1 consists of an extracellular domain containing three Ig-like domains, a transmembrane domain, and an intracellular domain endowed with two signaling motifs. The Ig-like motifs can be N glycosylated, and the juxtamembrane region subjected to alternative splicing, resulting in isoforms that can be O glycosylated (Fig. 2A). Alternative splicing in the extracellular juxtamembrane region of SynCAM1 generates five isoforms (SynCAM1 1–5) (6) with different molecular properties (6, 7). Four of them are membrane spanning (isoforms 1–4), and one corresponds to a secreted protein (isoform 5). We therefore used PCR amplification followed by sequencing to identify which of the four membrane-spanning isoforms (Fig. 2, B and C) are present in GT1-7 cells and hypothalamic astrocytes. In GT1-7 cells, isoform 4 is the most abundant, whereas hypothalamic astrocytes express comparable levels of isoforms 1 and 4 (Fig. 2D). Both cell types also contain traces of isoforms 2 and 3. Resembling GT1-7 cells, cerebrocortical astrocytes express mostly isoform 4, with progressively lower levels of isoforms 1, 3, and 2, respectively (Fig. 2D). Further insight into the SynCAM1 variants expressed in GT1-7 cells and hypothalamic astrocytes came from an analysis of the glycosylation status of each protein isoform. As discussed earlier by one of us (6), isoforms 1–4 (amino acid alignment shown in Fig. 2E) are predicted to be N-linked glycosylated (http://www.cbs.dtu.dk/services/NetNGlyc/), but only isoforms 1 and 4 are predicted to also be O-linked glycosylated (http://www.cbs.dtu.dk/services/NetOGlyc/). Extracts from hypothalamic astrocytes and GT1-7 cells were subjected to either N-linked or N- plus O-linked deglycosylation followed by Western blot analysis using either the monoclonal antibody 3E1 that specifically recognizes SynCAM1 (13) or the pleio-SynCAM antibodies that recognize the SynCAM2 and -3 gene products (7) in addition to SynCAM1. A decrease in molecular weight is an indicator of deglycosylation. We found that GT1-7 and hypothalamic astrocytes contain only a single detectable SynCAM species of the correct molecular mass (95–100 kDa) that is heavily N- and O-linked glycosylated (Fig. 2F). Parallel blotting with pleio-SynCAM antibodies yielded an almost identical pattern of intact and deglycosylated SynCAM species (Fig. 2G), indicating that both astrocytes and GT1-7 cells express SynCAM1 and not SynCAM2 or -3. The presence of N-glycans suggests that the adhesive properties of SynCAM1 in hypothalamic astrocytes could be modulated by polysialylation (23). To determine whether astrocytic SynCAM1 is subjected to polysialylation, we performed immunoprecipitation assays using pleio-SynCAM antibodies. Western blot analysis of the immunoprecipitated proteins using mouse monoclonal antibody 5A5 that recognizes polysialic acid showed a near complete lack of SynCAM1 polysialylation (Fig. 2H). These results indicate that the predominant, if not the only, SynCAM gene expressed in cultured hypothalamic astrocytes and GnRH producing GT1–7 cells is SynCAM1. They also indicate that both cell types display a similar profile of SynCAM1 isoform expression in which the isoforms 4 and 1 are most abundant.
Fig. 2.
The predominant SynCAM species in GT1-7 cells and hypothalamic astrocytes is the N- and O-glycosylated SynCAM1 isoform 4. A, Illustration depicting the structure of SynCAM1 with three extracellular Ig-like domains with potential N-glycosylation sites, a juxtamembranous alternative splicing domain with potential O-glycosylation sites, transmembrane domain, and intracellular protein 4.1 and PDZ interaction domains. B, Nucleotide alignment depicting the boundaries of the alternatively spliced exons 8 and 9 in SynCAM1. Alternative use of these exons gives origin to the SynCAM1 isoforms 1–4. C, Sizes of the complete coding sequence for SynCAM1 isoforms 1–4 (1327, 1288, 1255, and 1399 nt, respectively) and the expected product size after PCR amplification (457, 373, 340, and 424 nt, respectively). D, Gel profiles of SynCAM1 mRNA isoforms expressed in GT1-7 cells, hypothalamic astrocytes, and cortical astrocytes. E, Corresponding amino acid alignment of the alternative splice domain of SynCAM1 isoforms 1–4. F, SynCAM1 is N and O linked glycosylated in GT1-7 and hypothalamic astrocytes. Monoclonal antibody 3E1 that specifically detects SynCAM1 recognizes a protein species that has an identical pattern of migration in SDS-PAGE in both GT1-7 neurons and hypothalamic astrocytes after N-linked and N + O-linked deglycosylation. G, An identical protein species showing the same N-linked and N + O-linked deglycosylation patterns is detected in GT1-7 cells and hypothalamic astrocytes using the pleio-SynCAM rabbit polyclonal antibodies, suggesting that both cell types only express SynCAM1. H, Immunoprecipitation (IP) of protein extracts from hypothalamic astrocytes with pleio-SynCAM antibodies followed by blotting with a mouse monoclonal antibody (5A5) that recognizes polysialic acid (PSA; left panel). The Western blot was reprobed using pleio-SynCAM antibodies (right panel). CTX, Cerebral cortex; Hypo, hypothalamus; Astro, astrocytes; MM, molecular markers.
Astrocytes and GT1-7 cells recognize SynCAM1 as an adhesive substrate
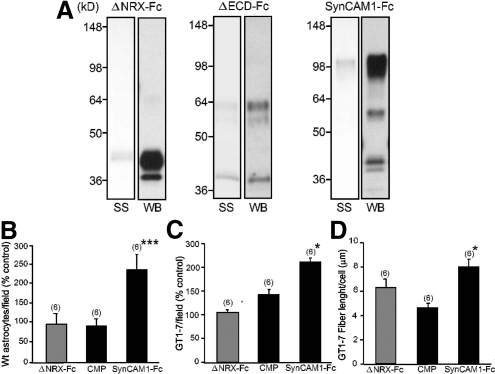
The pattern of SynCAM1 expression in the hypothalamus and its known function as an adhesion molecule suggested an involvement of SynCAM1 in mediating the adhesion of hypothalamic neurons and astrocytes. To examine this possibility, we first used an adhesion assay that uses glass coverslips coated with Fc fusion proteins (24) containing either the entire extracellular domain of SynCAM1 (SynCAM1-Fc) (7) or the mutated extracellular domain of rat neurexin-1α (ΔNRX-Fc) (17). The extracellular Ig domains of SynCAM1 (Fig. 2A) mediate the adhesive properties of the protein. These fusion proteins were produced in COS-7 cells, purified from their conditioned media, analyzed by gel electrophoresis and Western blot (Fig. 3A), and then used in the adhesion assay. Both WT astrocytes and GT1-7 cells adhered more to surfaces coated with SynCAM1-Fc than to either ΔNRX-Fc or proteins purified from CMP [culture medium proteins conditioned by COS-7 cells transfected with a control plasmid (7)] (Fig. 3, B and C). Furthermore, GT1-7 cells seeded onto SynCAM1-Fc-coated surfaces extended longer neurites than cells seeded on the control surfaces (Fig. 3D).
Fig. 3.
SynCAM1 serves as an adhesion molecule for hypothalamic astrocytes and GT1-7 cells. A, Silver stain (SS) and corresponding Western blot (WB), using goat polyclonal antibodies to human Fc, identifies the purified fusion proteins used for adhesion assays. B and C, WT hypothalamic astrocytes (B) and GT1-7 cells (C) bind preferentially to the intact extracellular domain of SynCAM1 (SynCAM1-Fc). Adhesion assays were performed by plating astrocytes or GT1-7 cells on coverslips coated with fusion proteins containing the WT extracellular domain of SynCAM1 (SynCAM1-Fc), purified CMP, or the mutated extracellular domain of Nrx1 lacking IgG domains (ΔNrx-Fc). D, GT1-7 cells preferentially extend neurites when seeded onto SynCAM1-Fc-coated coverslips compared with neurite outgrowth on CMP or ΔNrx-Fc. ***, P < 0.01 and *, P < 0.05 vs. ΔNrx-Fc and CMP groups. Numbers in parentheses on top of bars represent the number of coverslips analyzed for each group, and vertical lines are sem.
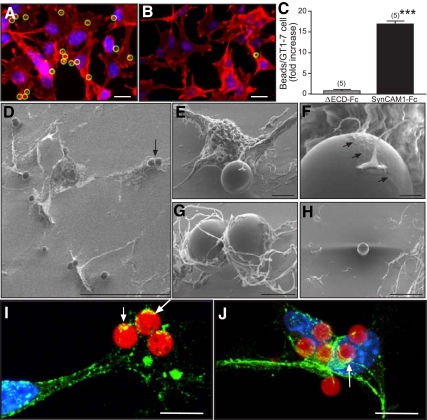
To further explore the phenomenon of SynCAM1-mediated adhesion in GT1-7 cells and astrocytes, we used a different, more dynamic assay that assesses the ability of cells to adhere polystyrene microbeads coated with either SynCAM1-Fc or with a SynCAM1 protein lacking the Ig domains of the extracellular domain (ΔECD-Fc). Although numerous SynCAM1-Fc beads associated with the GnRH neuronal GT1-7 cells (Fig. 4A), neuronal interactions with ΔECD-Fc-coated beads were minimal (Fig. 4B), with a majority of analyzed fields having no adherent beads (P < 0.01 vs. the group exposed to SynCAM1-Fc) (Fig. 4C). To more accurately identify the contact points of SynCAM1 adhesion to GT1-7 cells, we used SEM (Fig. 4, D–H) and immunohistofluorescence (Fig. 4, I and J). In keeping with the aforementioned findings, SEM of GnRH-producing cells revealed numerous SynCAM1-Fc beads attached to neurons (Fig. 4D). Higher magnification SEM of a single GT1-7 cell revealed that, within 45 min, the neuron extends long processes that reach and trap a SynCAM1-Fc bead (Fig. 4E). Examination at higher magnification shows that neuronal contact with a SynCAM1-Fc bead was accompanied by the formation of a biofilm, which coats the bead and is evident by the ruffling across the upper bead surface adjacent to the neuron adhesion point (Fig. 4F, arrows). Other beads are surrounded by numerous processes that adhere to the beads via multiple contact points (Fig. 4G). No interaction of neurons with ΔECD-Fc beads were detected (Fig. 4H). SynCAM immunostaining with pleio-SynCAM antibodies revealed an abundance of SynCAM immunoreactive material (green) in the regions in which the neuronal processes contact the SynCAM1-Fc beads (red) (Fig. 4, I and J, examples denoted by arrows).
Fig. 4.
GT1-7 cells recognize SynCAM1 as an adhesive partner. A, Many SynCAM1-Fc-coated polystyrene beads (green circles) adhere to GT1-7 cells, stained with phalloidin (red). B, Few, if any, ΔECD-Fc-coated beads (green circle) adhered to GT1-7 cells. C, Quantification of the data illustrated in A and B. Numbers above bars are number of coverslips per group (20–35 fields per coverslip). ***, P < 0.01 vs. ΔECD-Fc group. Numbers in parentheses on top of bars represent the number of coverslips analyzed for each group, and vertical lines are sem. D, Low-magnification SEM image of SynCAM1-Fc-coated beads adhering to GT1-7 neurons. The arrow depicts the two beads shown at higher magnification in G. E, Secondary electron image of an individual neuron contacting a SynCAM1-Fc bead via fine processes, including a process bearing a growth cone. F, Higher magnification of the contact shown in E demonstrating that the neuronal processes generate a biofilm (arrows) that spreads over the region of the microsphere surrounding the adhesion point. Wavy distortion on the upper right is due to charging (electrostatic field). G, Higher-magnification image of the two SynCAM1-Fc beads from D. H, Secondary electron image of a ΔECD-Fc bead adhered to the poly-l-lysine coating, near GT1-7 neurons, but not adhering to them. I and J, Overlaid projection of confocal images showing that the neuronal processes trapping SynCAM1-Fc-coated beads (red) are rich in SynCAM1 (green). Arrows point to sites of close contact between neuronal processes and the microspheres. Cell nuclei stained with Hoechst are seen in blue. Bars (A and B), 20 μm; (D), 60 μm; (E), 4 μm; (F), 1 μm; (G), 2 μm; (H), 15 μm; (I and J), 10 μm.
These studies demonstrate that, consistent with its strong adhesive properties, SynCAM1 mediates a rapid (<45 min) change in membrane organization that is manifested through both extensive process remodeling and SynCAM redistribution. The dynamics of this process is comparable with the SynCAM adhesion complex assembly in hippocampal neurons (8, 13). Hypothalamic astrocytes showed a similar response to contact with SynCAM1-Fc beads compared with the GT1-7 cells. SynCAM1-Fc beads adhered significantly (P < 0.01) more to astrocytes than ΔECD-Fc control beads (Fig. 5, A–C). Interestingly, 45 min after overlaying the beads on WT astrocytes, endogenous SynCAM1 immunoreactivity (green) appeared clustered in the regions of contact with SynCAM1-Fc beads (Fig. 5, D and E; example denoted by arrow), suggesting that contact with a SynCAM1-containing bead leads to a redistribution of SynCAM on the glial cell surface. No staining was detected in the absence of SynCAM antibodies (Fig. 5F). These results are consistent with SynCAM1 functions in adhesion of both hypothalamic astrocytes and GnRH neurons. Finally, to determine whether SynCAM1 is important for the adhesion of GnRH neurons to astrocytes, we seeded GT1-7 cells expressing enhanced GFP on a lawn of hypothalamic astrocytes in the presence of the soluble extracellular domain of SynCAM1 (SynCAM1-Fc) or soluble ΔECD-Fc. Neuron-astrocyte attachment was significantly lower when SynCAM1-Fc was present (Fig. 5G), suggesting that some of the interactions used by SynCAM1 to mediate GnRH neuron adhesiveness to glial cells are homophilic.
Fig. 5.
SynCAM1 is recognized as an adhesive partner by astrocytes and mediates astrocytes-GT1-7 cell adhesiveness. A, Image illustrating the adhesion of SynCAM1-Fc beads (green circles) to WT hypothalamic astrocytes. B, Lack of adhesiveness of ΔECD-Fc control beads to WT hypothalamic astrocytes. In both cases the astrocytes are identified by phalloidin staining (red). C, Quantification of the changes in adhesiveness depicted in A and B. D and E, Overlaid projections of confocal images illustrating the accumulation of SynCAM immunoreactivity (green, highlighted by white arrow in D) around SynCAM1-Fc coated beads (red) in WT astrocytes. F, Absence of immunoreactive material in cultures incubated without SynCAM antibodies. Only SynCAM1-Fc-coated beads (red) and Hoechst stained nuclei (blue) are visible. G, GT1-7 cell adhesion to WT astrocytes is reduced by the presence of soluble SynCAM1. GT1-7 cells expressing GFP were suspended in medium containing either SynCAM1-Fc (5 μg/ml) or ΔECD-Fc (5 μg/ml) and plated onto a monolayer of WT astrocytes for 24 h. Adhesion of the experimental SynCAM1-Fc groups is expressed as fold change relative to the ΔECD-Fc control group. Numbers in the parentheses are number of coverslips per treatment. Vertical lines represent sem. ***, P < 0.01 vs. ΔECD-Fc control. Bars (A and B), 20 μm; (D–F), 10 μm.
NRG1-erbB4 signaling results in dynamic changes in SynCAM1-mediated astrocytic adhesiveness
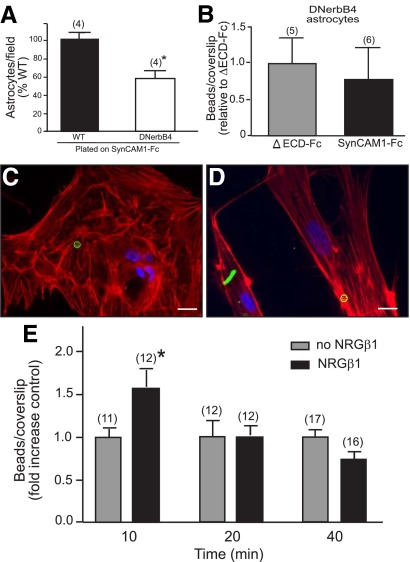
Considering our data in the companion paper (5) linking erbB4 receptor activity to SynCAM1 function, we sought to understand the consequences that NRG1-erbB signaling has on SynCAM1 adhesion. First, we assessed SynCAM1-mediated adhesive properties in astrocytes with disrupted erbB4 receptor function (GFAP-DNerbB4). We observed that astrocytes purified from the hypothalamus of GFAP-DNerbB4 mice adhered less than WT astrocytes to coverslips coated with the SynCAM1-Fc substrate (Fig. 6A) and do not preferentially bind SynCAM1-Fc-coated beads over ΔECD-Fc control beads (Fig. 6, B–D). Next, we assessed the ability of WT hypothalamic astrocytes to adhere SynCAM1-Fc-coated beads after NRGβ1-dependent stimulation of erbB receptors. Because hypothalamic astrocytes do no express erbB3 (19), NRG1 stimulation is expected to activate only erbB4 receptors and erbB4/erbB2 receptor complexes. The presence of NRGβ1 (3 nm) in the medium containing SynCAM1-Fc beads resulted in a rapid increase in number of SynCAM1-Fc beads adhering to the astrocytes compared with nonstimulated controls (Fig. 6E). This increase was surprisingly transient because it was no longer seen after 20 min of exposure. Paralleling this brief NRGβ1-dependent increase in SynCAM1-mediated adhesiveness, NRGβ1 applied to hypothalamic astrocytes caused a rapid (5–10 min) increase in erbB4 receptor phosphorylation, with levels returning to baseline values by 20 min (data not shown).
Fig. 6.
SynCAM1-mediated adhesion in hypothalamic astrocytes is coupled to erbB4 receptor activation. A, SynCAM1-Fc-coated beads adhere less to GFAP-DNerbB4 hypothalamic astrocytes than to WT astrocytes. The number of adherent WT or GFAP-DNerbB4 astrocytes to SynCAM1-Fc was determined 18 h after seeding. *, P < 0.05 vs. adhered WT astrocytes. B, Adhesion of SynCAM1-Fc-coated beads to GFAP-DNerbB4 astrocytes is not different from that of ΔECD-Fc-coated beads. SynCAM1-Fc adhesion is expressed relative to the ΔECD-Fc control group. C and D, Images illustrating the absence of adhesion of both SynCAM1-Fc beads (C) and ΔECD-Fc control beads (D) to GFAP-DNerbB4 astrocytes. In both cases, beads are shown in green, and the astrocytes are identified by phalloidin staining (red). E, SynCAM1-mediated adhesiveness in hypothalamic astrocytes is transiently increased after exposure of the cells to NRGβ1 (3 nm). SynCAM1-Fc-coated beads were presented to WT astrocytes for 10, 20, and 40 min in the presence (black bars) or absence (gray bars) of NRGβ1. At each time point, the data are expressed as a fold change of SynCAM1-Fc-coated beads adhered relative to the nonstimulated control. Numbers in parentheses are number of coverslips per treatment. Vertical lines represent sem. *, P < 0.05 vs. nonstimulated controls. Bars (C and D), 15 μm.
Discussion
SynCAM1, a cell adhesion protein with signaling capabilities (3, 25), was previously shown to facilitate neuron-to-neuron communication by promoting synaptic formation (7, 8) and specifying the organization of excitatory synapses (9, 13). The present results indicate that the functions of SynCAM1 in brain are not limited to neurons. Instead, we found that SynCAM1 is also expressed in astrocytes of the neuroendocrine brain, in which it serves as an adhesive substrate for cell-cell communication. Our results also show that SynCAM1 uses its extracellular domain to promote glia-to-glia adhesiveness and the adhesion of astroglial cells to at least one type of secretory neuron in the neuroendocrine brain. These neurons secrete GnRH, which plays a central role in the control of sexual maturation and adult reproductive function. The physiological importance of astrocytic SynCAM1 is evidenced by the finding that mice carrying an astrocyte-specific DN form of SynCAM1 have delayed puberty, disrupted estrous cyclicity, and reduced fecundity (5). These observations make clear that astrocyte-specific loss of SynCAM1 function adversely impacts the ability of GnRH neurons to control reproductive function.
GnRH neurons and hypothalamic astrocytes share the common feature of expressing the SynCAM1 gene. Immunoblot analysis using antibodies that recognize SynCAM1 specifically, and antibodies also able to identify SynCAM2 and -3, revealed that neither astrocytes nor GT1-7 cells contain detectable levels of SynCAM2 or -3. Whether they express SynCAM4, the most divergent member of the SynCAM family (6), remains to be determined. Additional analysis revealed that the most abundant SynCAM1 variant present in both astrocytes and GT1-7 cells is isoform 4, which is N and O glycosylated. These findings suggest that these cells may use not only an identical SynCAM gene product but also the same alternatively spliced variant of that gene for adhesive communication. There is now solid evidence that the protein products of all four SynCAM family genes are abundantly expressed in neurons of both the developing an adult brain (10, 21), in which they function as synaptic adhesion molecules able to promote synaptic assembly (7), enhance excitatory synaptic transmission (8, 9, 13), and organize functional axodendritic synaptic contacts (10). It is also clear that they exhibit a heterophilic preference for interaction so that SynCAM1 adheres more avidly to SynCAM2 than to other SynCAM1 molecules (13), and SynCAM3 preferentially forms adhesive complexes by assembling with SynCAM4 (13, 21). Because SynCAM1–4 display divergent patterns of expression in different brain regions (21), it would appear likely that the relative prevalence of two given SynCAM subtypes is the decisive factor determining which partners are used by adjacent cells. This is well illustrated by the recently described heterophilic interaction of SynCAM1, abundantly expressed in axons of the peripheral nervous system, with SynCAM4 (Necl4) present in Schwann cells (26, 27). Because SynCAM1 also displays strong homophilic adhesiveness (7, 13), adjacent cells with an abundance of SynCAM1 would be expected to use hemophilic SynCAM1/SynCAM1 for cell-cell adhesive interactions. By showing that SynCAM1 is the most abundant species expressed in both GnRH neurons and hypothalamic astrocytes, our results suggest that homophilic SynCAM1/SynCAM1 interactions play a significant role in the process by which astrocytes of the endocrine brain engage in glia-glia and glia-GnRH neuron adhesive communication. It is possible, however, that SynCAM1 also interacts in these cells with SynCAMs that were either not recognized by the antibodies used (SynCAM4) or were not detected by our Western blot analysis due to their low abundance (SynCAM2 and 3).
These considerations notwithstanding, our results leave open the likely possibility that GnRH neurons may engage in heterophilic interactions with neuronal subsets expressing SynCAM2 as shown to occur in other neuronal populations of the brain (13, 21). Similar interactions may occur between astrocytes and SynCAM2-expressing neurons. The striking predominance of SynCAM1 isoform 4 in both GT1-7 cells and hypothalamic astrocytes suggests that GnRH neurons and astrocytes use similar posttranscriptional and posttranslational strategies to modulate SynCAM1-mediated adhesiveness. They use the same alternatively spliced form, and this form appears to be similarly glycosylated. This is likely to impact the protein's adhesive capability because the first two Ig-like domains of SynCAM1 contain N-glycosylation sites shown to be required for efficient SynCAM-mediated cell-cell interaction (13, 28). Additionally, N-glycans attached to the first Ig-like domain are the target of polysialylation, which, instead of facilitating SynCAM/SynCAM interactions, abolishes homophilic adhesiveness (23). Because polysialylation only targets the Ig-like domain furthest away from the cell membrane, and this reaction is carried out by polysialyltransferases, which are also transmembrane proteins, it has been speculated that polysialylation requires proper separation of the N-glycan acceptor from the cell membrane (23). The size of the extracellular juxtamembranous domain of SynCAM1 isoforms 4 and 1 may provide the spacing necessary for modulation of this polysialylation repressive function, thereby enhancing SynCAM1 homophilic interactions. Specific studies are required to examine this possibility.
It may be contended that the predominance of SynCAM1 isoform 4 in GT1-7 cells and cultured astrocytes merely reflects the fact that GT1-7 cells are immortalized neurons, and the astrocytes are cells in primary culture. Arguing against this view is the finding that in both cell types SynCAM1 is heavily glycosylated, a feature that is established in brain by the end of the second week of postnatal life (13). These observations suggest that isoform 4 is indeed the alternatively spliced form most abundantly expressed in GnRH neurons in vivo. However, it is possible that subsets of astrocytes in situ may express either other SynCAM1 isoforms or the products of SynCAM2 and SynCAM3 genes.
Astrocytic SynCAM1-mediated adhesiveness increases rapidly after ligand-dependent activation of erbB4 receptors. Because these receptors become physically associated with SynCAM1 upon neuregulin stimulation (5), it appears clear that astrocytes respond to erbB4 receptor activation not only with changes in intracellular signaling but also with changes in cell adhesiveness. Importantly, these changes are not sustained, implying the existence of a dynamic, minute-to-minute regulatory mechanism linking erbB4 receptors to plastic changes in astrocytic adhesive engagement to neighboring cells. The biochemical and molecular mechanisms underlying erbB4-dependent changes in SynCAM1 mediated adhesives remain to be defined.
Altogether, our results suggest that SynCAM1 is an important component of adhesive communication between astrocytes and GnRH neurons in the neuroendocrine brain and that in contrast to the heterophilic interactions mediating SynCAM-dependent synaptic adhesiveness throughout the brain, astrocyte-GnRH neuron adhesive communication is mediated by SynCAM1/SynCAM1 homophilic interactions. The contribution of SynCAM1-mediated adhesiveness to the reproductive phenotype seen in mutant mice with disrupted astrocytic SynCAM1 function (5) remains to be elucidated.
Supplementary Material
Acknowledgments
We thank Dr. Anda Cornea for her valuable assistance with imaging and Ms. Maria Costa for expert technical help with immunohistochemical procedures.
This work was supported by Grants MH-65438 and HD25123 from the National Institutes of Health (NIH), Eunice Kennedy Shriver National Institute of Child Health and Human Development/NIH through Cooperative Agreement U54 HD18185 as part of the Specialized Cooperative Centers Program in Reproduction and Infertility Research, National Institute of Child Health and Human Development/NIH, and Grant RR000163 for the operation of the Oregon National Primate Research Center (to S.R.O.); National Institutes of Health Grant R01 DA018928 (to T.B.); and a NARSAD Young Investigator Award (to T.B.). U.S.S. was supported by NIH Training Grants T32 HD007133 and T32 DK007680.
Current address for J.M.: Materials and Manufacturing Research Institute, Portland State University, 20000 NW Walker Road, Beaverton, Oregon 97006.
Current address for A.E.M.: Picower Institute for Learning and Memory, Massachusetts Institute of Technology, 77 Massachusetts Avenue, Room 46-4235, Cambridge, Massachusetts 02139.
Current address for U.S.S.: Legacy Emanuel Hospital and Health Center, Legacy Research, 1225 NE 2nd Avenue, Portland, Oregon 97232.
Disclosure Summary: The authors have nothing to report.
Footnotes
- ADM
- Astrocyte-defined medium
- CMP
- culture medium protein
- DIC
- differential interference contrast
- DN
- dominant-negative
- DNerbB4
- transgenic carrying a DN form of the erbB4 receptor under the control of the human GFAP
- ΔECD-Fc
- extracellular SynCAM1 domain lacking the three SynCAM1 IgG extracellular subdomains
- erbB
- erythroblastosis B
- GFAP
- glial fibrillary acidic protein
- GFP
- green fluorescent protein
- NRGβ1
- neuregulin-β1
- ΔNRX-Fc
- mutated extracellular domain of rat neurexin-1α
- SEM
- scanning electron microscopy
- SynCAM1
- synaptic cell adhesion molecule 1
- SynCAM1-Fc
- human Fc portion of human IgG fused to the WT extracellular domain of SynCAM1
- WT
- wild type.
References
- 1. Roth CL, Mastronardi C, Lomniczi A, Wright H, Cabrera R, Mungenast AE, Heger S, Jung H, Dubay C, Ojeda SR. 2007. Expression of a tumor-related gene network increases in the mammalian hypothalamus at the time of female puberty. Endocrinology 148:5147–5161 [DOI] [PubMed] [Google Scholar]
- 2. Ojeda SR, Lomniczi A, Mastronardi C, Heger S, Roth C, Parent AS, Matagne V, Mungenast AE. 2006. Minireview: the neuroendocrine regulation of puberty: is the time ripe for a systems biology approach? Endocrinology 147:1166–1174 [DOI] [PubMed] [Google Scholar]
- 3. Kuramochi M, Fukuhara H, Nobukuni T, Kanbe T, Maruyama T, Ghosh HP, Pletcher M, Isomura M, Onizuka M, Kitamura T, Sekiya T, Reeves RH, Murakami Y. 2001. TSLC1 is a tumor-suppressor gene in human non-small-cell lung cancer. Nat Genet 27:427–430 [DOI] [PubMed] [Google Scholar]
- 4. Fukami T, Satoh H, Fujita E, Maruyama T, Fukuhara H, Kuramochi M, Takamoto S, Momoi T, Murakami Y. 2002. Identification of the Tslc1 gene, a mouse orthologue of the human tumor suppressor TSLC1 gene. Gene 295:7–12 [DOI] [PubMed] [Google Scholar]
- 5. Sandau US, Mungenast AE, Alderman Z, Sardi P, Fogel AI, Taylor B, Parent A-S, Biederer T, Ojeda SR. 2011. SynCAM1, a synaptic adhesion molecule, is expressed in astrocytes and contributes to erbB4 receptor-mediated control of female sexual development. Endcrinology 152:2364–2376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Biederer T. 2006. Bioinformatic characterization of the SynCAM family of immunoglobulin-like domain-containing adhesion molecules. Genomics 87:139–150 [DOI] [PubMed] [Google Scholar]
- 7. Biederer T, Sara Y, Mozhayeva M, Atasoy D, Liu X, Kavalali ET, Südhof TC. 2002. SynCAM, a synaptic adhesion molecule that drives synapse assembly. Science 297:1525–1531 [DOI] [PubMed] [Google Scholar]
- 8. Robbins EM, Krupp AJ, Perez de Arce K, Ghosh AK, Fogel AI, Boucard A, Sudhof TC, Stein V, Biederer T. 2010. SynCAM 1 adhesion dynamically regulates synapse number and impacts plasticity and learning. Neuron 68:894–906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sara Y, Biederer T, Atasoy D, Chubykin A, Mozhayeva MG, Südhof TC, Kavalali ET. 2005. Selective capability of SynCAM and neuroligin for functional synapse assembly. J Neurosci 25:260–270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Stagi M, Fogel AI, Biederer T. 2010. SynCAM 1 participates in axo-dendritic contact assembly and shapes neuronal growth cones. Proc Natl Acad Sci USA 107:7568–7573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hoover KB, Bryant PJ. 2000. The genetics of the protein 4.1 family: organizers of the membrane and cytoskeleton. Curr Opin Cell Biol 12:229–234 [DOI] [PubMed] [Google Scholar]
- 12. Hung AY, Sheng M. 2002. PDZ domains: structural modules for protein complex assembly. J Biol Chem 277:5699–5702 [DOI] [PubMed] [Google Scholar]
- 13. Fogel AI, Akins MR, Krupp AJ, Stagi M, Stein V, Biederer T. 2007. SynCAMs organize nascent synapses through heterophilic adhesion. J Neurosci 27:12516–12530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fogel AI, Li Y, Giza J, Wang Q, Lam TT, Modis Y, Biederer T. 2010. N-glycosylation at the SynCAM (synaptic cell adhesion molecule) immunoglobulin interface modulates synaptic adhesion. J Biol Chem 285:34864–34874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mungenast AE, Parent A, Chen SS, Goodlett D, Aebersold R, Corfas G, Ojeda SR. The synaptic adhesion molecule SynCAM is associated with ERBB4 dysregulation in the hypothalamus of mice with a delayed onset of puberty. Proc 2003 Abstract Viewer, Society for Neuroscience, Washington, DC, 2003. Program no. 281 20, Online [Google Scholar]
- 16. Ojeda SR, Lomniczi A, Sandau US. 2008. Glial-gonadotrophin hormone (GnRH) neurone interactions in the median eminence and the control of GnRH secretion. J Neuroendocrinol 20:732–742 [DOI] [PubMed] [Google Scholar]
- 17. Sugita S, Saito F, Tang J, Satz J, Campbell K, Südhof TC. 2001. A stoichiometric complex of neurexins and dystroglycan in brain. J Cell Biol 154:435–445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ma YJ, Berg-von der Emde K, Moholt-Siebert M, Hill DF, Ojeda SR. 1994. Region-specific regulation of transforming growth factor α (TGFα) gene expression in astrocytes of the neuroendocrine brain. J Neurosci 14:5644–5651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ma YJ, Hill DF, Creswick KE, Costa ME, Cornea A, Lioubin MN, Plowman GD, Ojeda SR. 1999. Neuregulins signaling via a glial erbB2/erbB4 receptor complex contribute to the neuroendocrine control of mammalian sexual development. J Neurosci 19:9913–9927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Block SM. 1998. Construction of optical tweezers. In: Spector DL, Goldman RD, Leinwand LA. eds. Cells: a laboratory manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 81.1–81.14 [Google Scholar]
- 21. Thomas LA, Akins MR, Biederer T. 2008. Expression and adhesion profiles of SynCAM molecules indicate distinct neuronal functions. J Comp Neurol 510:47–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Suter KJ, Song WJ, Sampson TL, Wuarin JP, Saunders JT, Dudek FE, Moenter SM. 2000. Genetic targeting of green fluorescent protein to gonadotropin-releasing hormone neurons: characterization of whole-cell electrophysiological properties and morphology. Endocrinology 141:412–419 [DOI] [PubMed] [Google Scholar]
- 23. Galuska SP, Rollenhagen M, Kaup M, Eggers K, Oltmann-Norden I, Schiff M, Hartmann M, Weinhold B, Hildebrandt H, Geyer R, Mühlenhoff M, Geyer H. 2010. Synaptic cell adhesion molecule SynCAM 1 is a target for polysialylation in postnatal mouse brain. Proc Natl Acad Sci USA 107:10250–10255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Peles E, Nativ M, Campbell PL, Sakurai T, Martinez R, Lev S, Clary DO, Schilling J, Barnea G, Plowman GD, Grumet M, Schlessinger J. 1995. The carbonic anhydrase domain of receptor tyrosine phosphatase β is a functional ligand for the axonal cell recognition molecule contactin. Cell 82:251–260 [DOI] [PubMed] [Google Scholar]
- 25. Gomyo H, Arai Y, Tanigami A, Murakami Y, Hattori M, Hosoda F, Arai K, Aikawa Y, Tsuda H, Hirohashi S, Asakawa S, Shimizu N, Soeda E, Sakaki Y, Ohki M. 1999. A 2-Mb sequence-ready contig map and a novel immunoglobulin superfamily gene IGSF4 in the LOH region of chromosome 11q23.2. Genomics 62:139–146 [DOI] [PubMed] [Google Scholar]
- 26. Spiegel I, Adamsky K, Eshed Y, Milo R, Sabanay H, Sarig-Nadir O, Horresh I, Scherer SS, Rasband MN, Peles E. 2007. A central role for Necl4 (SynCAM4) in Schwann cell-axon interaction and myelination. Nat Neurosci 10:861–869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Maurel P, Einheber S, Galinska J, Thaker P, Lam I, Rubin MB, Scherer SS, Murakami Y, Gutmann DH, Salzer JL. 2007. Nectin-like proteins mediate axon Schwann cell interactions along the internode and are essential for myelination. J Cell Biol 178:861–874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Fogel AI, Li Y, Giza J, Wang Q, Lam TT, Modis Y, Biederer T. 2010. N-Glycosylation at the SynCAM (Synaptic Cell Adhesion Molecule) Immunoglobulin Interface Modulates Synaptic Adhesion. J Biol Chem 285:34864–34874 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.