Mice genetically deficient in NK-1R gain less weight in response to high fat feeding, providing evidence for an important role of NK-1R in the development of obesity.
Abstract
Peripheral administration of a specific neurokinin-1 receptor (NK-1R) antagonist to mice leads to reduced weight gain and circulating levels of insulin and leptin after high-fat diet (HFD). Here, we assessed the contribution of substance P (SP) and NK-1R in diet-induced obesity using NK-1R deficient [knockout (KO)] mice and extended our previous findings to show the effects of SP-NK-1R interactions on adipose tissue-associated insulin signaling and glucose metabolic responses. NK-1R KO and wild-type (WT) littermates were fed a HFD for 3 wk, and obesity-associated responses were determined. Compared with WT, NK-1 KO mice show reduced weight gain and circulating levels of leptin and insulin in response to HFD. Adiponectin receptor mRNA levels are higher in mesenteric fat and liver in NK-1 KO animals compared with WT, after HFD. Mesenteric fat from NK-1R KO mice fed with HFD has reduced stress-activated protein kinase/c-Jun N-terminal kinase and protein kinase Cθ activation compared with WT mice. After glucose challenge, NK-1R KO mice remove glucose from the circulation more efficiently than WT and pair-fed controls, suggesting an additional peripheral effect of NK-1R-mediated signaling on glucose metabolism. Glucose uptake experiments in isolated rat adipocytes showed that SP directly inhibits insulin-mediated glucose uptake. Our results further establish a role for SP-NK-1R interactions in adipose tissue responses, specifically as they relate to obesity-associated pathologies such as glucose intolerance and insulin resistance. Our results highlight this pathway as an important therapeutic approach for type 2 diabetes.
Substance P (SP), an 11-amino acid peptide first isolated by Chang and Leeman (1), is expressed in the central nervous system and the periphery by numerous cell types, including afferent sensory neurons and inflammatory cells (2–8). The high affinity SP receptor neurokinin-1 receptor (NK-1R) is also expressed on several cell types, including neurons, epithelial cells, adipocytes, and immune cells (3, 5, 9–13). In the intestine, SP regulates motility (14), mucosal permeability (15), ion transport, and proliferation of colonic epithelial cells (16, 17), as well as inflammatory responses (18).
Obesity research has intensified in recent years mainly due to its associated comorbidities. These range from insulin resistance (and the development of noninsulin-dependent diabetes mellitus) and the metabolic syndrome to various types of cancer (19). Glucose uptake by target cells represents an important endpoint of insulin action, because impairment of this system leads to diabetes. Numerous proinflammatory pathways have been shown to induce impairment of insulin signaling. We have recently demonstrated the ability of SP to induce proinflammatory responses in human mesenteric preadipocytes via binding to NK-1R receptor (9). The proinflammatory potential of SP (9), coupled with the beneficial effects of NK-1R pharmacologic antagonism on weight gain and glucose clearance (20), led us to investigate whether SP can directly affect insulin sensitivity in isolated fat cells.
A proposed mechanism for the impairment of insulin signaling by cytokines and fatty acids involves a number of stress-activated kinases such as c-Jun N-terminal kinase (JNK) and protein kinase C (PKC). JNK is activated by extracellular signals, such as cytokines and fatty acids, as well as intracellular signals like stress of the endoplasmic reticulum (21). The strongest evidence on the importance of JNK activation on insulin resistance comes from studies that demonstrate a protective effect of JNK deficiency on insulin receptor substrate 1 serine phosphorylation and the maintenance of normal insulin signaling in obese mice (22). Extracellular fatty acids also activate PKCθ, and the role of PKCθ in the development of insulin resistance is well documented (23–25). Collectively, either alone or by interacting with each other, these kinases lead to impaired intracellular insulin signaling in response to either cytokines or circulating free fatty acids, both central characteristics of obesity. In addition, adiponectin is one of the molecules that mediate fat tissue-associated effects on insulin resistance. Adiponectin is an adipokine that exerts its effects by interacting with adiponectin receptor (AdipoR)1 and AdipoR2 (26). Changes in the levels of adiponectin and/or its receptors are associated with both obesity and diabetes mellitus (27, 28).
Here, we examined the contribution of SP and NK-1R in high-fat diet (HFD)-induced obesity using NK-1R-deficient [knockout (KO)] mice and tested the possibility that SP directly affects glucose uptake in isolated adipocytes. We show that, compared with wild-type (WT) mice, NK-1R-deficient mice demonstrate reduced weight gain in response to HFD. In addition, NK-1R KO mice clear circulating glucose more efficiently after glucose challenge even in comparison with paired-fed (PF) littermates with similar overall weights. More importantly, we show that genetic NK-1R deficiency is associated with decreased JNK and PKCθ activation in the mesenteric fat after HFD and that SP while increasing the activating phosphorylation of these kinases also inhibits insulin-induced glucose uptake in rat adipocytes. Thus, SP via NK-1R may promote weight gain and directly modulate decreases in glucose uptake, normally associated with increased adiposity. Our results identify SP-NK-1R interactions as potential targets for therapeutic intervention for the prevention of insulin resistance and type 2 diabetes.
Research Design and Methods
Animals and treatments
NK-1R KO mice were generated by targeted disruption of the NK-1R gene in embryonic stem cells as we previously described (29). The animals for these experiments were generated at the University of California at Los Angeles facility from heterozygous breeding pairs, and animal genotype was verified by PCR using the following primers: WT, forward-atggataacgtccttcctgtgg and reverse-ctgtcaaaggccacagctgtca; KO, forward-agaggctattcggctatgact and reverse-ccacacccagccggccacagt. Three groups of male weight-matched littermate mice (12–15 wk of age) were studied. Mice for these experiments were backcrossed for more than nine generations. The first group included WT mice and the second group NK-1R KO mice. A third group, termed PF, was comprised of WT animals that received the equivalent amount of food (grams) that their designated KO-paired animals had consumed during the previous 24-h period. Such a pairing allows for the direct assessment of the effect of food consumption in the overall weight changes observed in the NK-1R KO group. All mice were fed with HFD (45% Kcal from fat; Research Diets, Inc., New Brunswick, NJ) and were weighed and had their food replaced daily. After 3 wk, mice were killed, and tissues were collected. We performed a 3-wk feeding protocol, because the weight gain rates of mice of the C57BL/6 background are reduced after the animal weight reaches 45–50 g, and this reduction could influence the results of the study. The Institutional Animal Care and Use Committee of the David Geffen School of Medicine at University of California at Los Angeles approved all procedures.
Measurement of nonesterified fatty acids (NEFA)
For the measurement of NEFA in the serum of mice, we used the HR Series NEFA-HR(2) kit (Wako Diagnostics, Richmond, VA). Changes in color were detected using the SpectraMax Plate reader (Molecular Devices, Sunnyvale, CA).
Isolation of rat epididymal adipocytes
Two to five grams of epididymal fat tissue were obtained from each rat. The tissue was then be placed into sterile 50-ml polypropylene tubes containing 15 ml of collagenase solution (1 mg of collagenase, 1 ml of PBS, 3 ml of solution, and 1 g of tissue) and minced within the tube to a fine consistency. The solution was then vortexed thoroughly and the tubes placed in a 37 C shaking water bath (100 rpm) until the tissue pieces were dissolved. The solution was vortexed once more and filtered through a double gauze-containing funnel. The homogenates were centrifuged at 1000 rpm for 10 min, and the top fatty layer was placed in separate tubes and washed 3× with PBS (this is the adipocyte-containing layer).
Determination of mRNA levels using real-time quantitative-PCR (TaqMan assay)
Hundred nanograms of RNA isolated from liver and whole mesenteric fat tissue homogenates were reverse transcribed into cDNA using the TaqMan One-step reverse transcription-PCR kit (Applied Biosystems, Foster City, CA) and incubated with dual fluorogenic probes (Applied Biosystems). Glyceraldehyde-3-phosphate dehydrogenase was used as an endogenous control and was detected using dual labeled fluorogenic probe (5′-FAM/3′-MGB probe; Applied Biosystems). mRNA levels for adiponectin as well as AdipoR were quantified using a fluorogenic 5′-nuclease PCR assay (30) with a 7500 Fast Real-Time PCR sequence detection system (Applied Biosystems). Duplicate reactions of each standard or sample were incubated for 2 min at 50 C, denatured for 10 min at 95 C, and subjected to 40 cycles of annealing at 55 C for 20 sec, extension at 60 C for 1 min followed by denaturation at 95 C for 15 sec.
Western immunoblotting
For immunoblots, proteins (15–40 μg) from mesenteric fat depots were separated by electrophoresis in a 10% polyacrylamide gel. Protein samples were mixed with sample buffer (3×; Cell Signaling, Beverly, MA) and denatured by boiling. Samples were electrophoresed at 100–150 V for 1.5 h or longer until the dye migrated to the bottom of the gel. The separating gel was equilibrated in transfer buffer (20 mm Tris-HCl, 150 mm glycine, 20% methanol, and 0.1% sodium dodecyl sulfate) for 10 min. The proteins were then transferred to Polyvinylidene difluoride membranes (Millipore, Billerica, MA) at 4 C. All membrane incubations were carried out at room temperature with rocking. The membranes were blocked for 1 h at room temperature in blocking buffer (Tris-buffered saline, 5% nonfat dry milk, and 0.1% Tween 20) and then incubated with primary antibodies against PKCθ, c-Jun NH(2)-terminal kinase [stress-activated protein kinase (SAPK)/JNK] (Cell Signaling), and actin (Santa Cruz Biotechnology, Inc., Santa Cruz, CA), in blocking buffer, overnight at 4 C. Horseradish peroxidase-conjugated secondary antibodies in blocking buffer were used (Santa Cruz Biotechnology, Inc.). The proteins were visualized and quantified with FujiFilm LAS-4000 Luminescent Image Analyzer (FujiFilm Corp., Edison, NJ) using Supersignal West Pico chemiluminescent substrate (Pierce, Rockford, IL).
3H-2-deoxy-D-glucose uptake
Primary Fisher 344 (n = 6) rat adipocytes from epididymal fat depots were isolated as described (31), placed in 1.5 ml Eppendorf tubes, and transferred to serum-free DMEM 4 h before experiments. Cells were washed with glucose-free KRH medium [ 121 mm NaCl, 4.9 mm KCl, 1.2 mm MgSO4, 0.33 mm CaCl2, and 12 mm HEPES acid (pH 7.4)] and treated with 100 nm insulin (Sigma, St. Louis, MO) with or without SP (10−7) or carrier for 15 min. 3H-2-deoxy-D-glucose/2-deoxy-D-glucose cocktail (specific activity, 6.25 mCi/mmol) was added for 3.5 min at 37 C. Before insulin treatment, cells in the appropriate groups were pretreated with 6-diphenylmethyl-5-(5-isopropyl-2-methoxybenzylamino)-1-azabicyclo(2.2.2)octane-3-carboxylic acid (CJ 12,255) (10−6), SP600125 (JNK inhibitor, 50 nm; CalBiochem, San Diego, CA) or bisindolylmaleimide I (PKC inhibitor, 50 nm; CalBiochem). Glucose uptake was stopped by washing the cells with ice-cold KRH with 25 mm glucose and 10 μm cytochalasin B for three times. Cells were then collected in glucose-free KRH with 0.1% sodium dodecyl sulfate and were subjected to liquid scintillation counting in EcoLume (ICN Biomedicals, Costa Mesa, CA). Rats were used for this experiment instead of mice due to the small number of cells we can obtain from the significantly smaller mouse epididymal fat depots. Radioactive glucose levels were corrected for the total adipocyte protein content per tube.
Glucose tolerance tests (GTT)
Mice were fasted overnight (1700–0800 h) and tested for glucose tolerance at the final day of the experiment. Dextrose (Sigma) was injected ip (1 g/kg), and tail vein blood glucose was measured at 0, 15, 30, 120, and 240 min using the ACCU-CHECK Advantage meter (Roche, Nutley, NJ).
Hormonal measurements
Leptin, insulin, and adiponectin serum levels (fasted) were measured in duplicate by EIA (Millipore).
Statistical analysis
Results were analyzed using the Prism professional statistics software program (GraphPad Software, Inc., San Diego, CA). ANOVA and nonparametric Mann-Whitney test (for comparisons between two groups) were used for intergroup comparisons. In our glucose clearance studies, to statistically compare the entire time course of glucose clearance, we used a nonlinear method, Generalized Estimating Equations (Liang and Zeger, 1986), which considers unbalanced longitudinal data with repeated measures to statistically evaluate group differences over time. Differences were considered to be significant when P < 0.05.
Results
NK-1R KO mice gain less weight in response to HFD compared with WT littermates
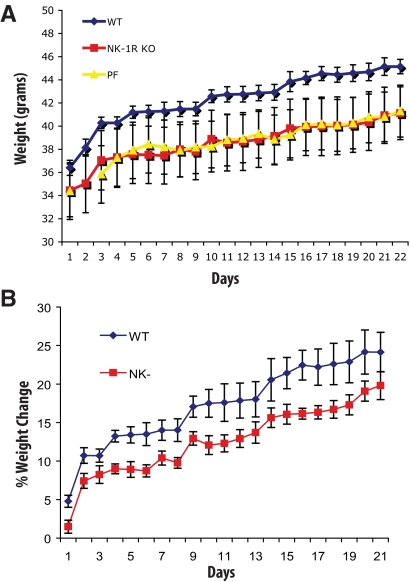
Administration of the NK-1R nonpeptide receptor antagonist CJ 12,255 leads to reduced weight gain in response to high-fat feeding as well as to increased weight loss in models of diet-induced and genetic obesity (ob/ob) in mice (20). Here, we used NK-1R KO mice to investigate the effects of NK-1R deficiency in response to HFD as well as weight-associated metabolic responses. After 3 wk of HFD feeding, NK-1R KO mice accumulate less weight compared with WT littermates (d 16–18, P < 0.01) (Fig. 1A). By d 17, WT mice gain approximately 6% more weight compared with NK-1R KO mice (WT, 22.2% vs. NK-1R KO, 16.3%; P < 0.05) (Fig. 1B). PF mice gain similar weight to NK-1-KO littermates, indicating an effect of SP signaling through NK-1R on food uptake (Fig. 1A). Indeed, when daily food consumption is measured, NK-1R KO mice show reduced food uptake in comparison with their WT littermates (average 2.91 vs. 3.11 g/d, P < 0.01) (detailed daily consumption depicted in Supplemental Fig. 1, published on The Endocrine Society's Journals Online web site at http://endo.endojournals.org).
Fig. 1.
NK-1R KO mice demonstrate less weight gain in response to high-fat feeding. Mice (n = 6 per group) matched for weight were fed with HFD and weighed daily for 3 wk. Mice were then killed, and tissues were collected. A, NK-1R KO mice gain less weight in response to high-fat feeding in comparison with their WT littermates (P < 0.01) but not in comparison with the PF mice. B, WT mice gain on average approximately 6% more weight when compared with their NK-1R KO littermates (WT, 22.2% vs. NK-1R KO, 16.3%; P < 0.05). *, P < 0.05.
NK-1R KO mice have lower circulating leptin and insulin levels after HFD compared with WT littermates
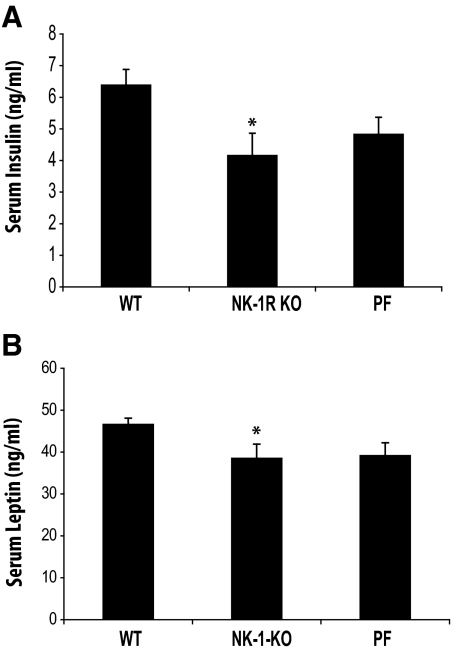
Obesity is associated with increased circulating levels of both insulin and leptin that are linked with the development of type 2 diabetes in obese subjects (32). Thus, we measured the circulating levels of insulin and leptin to determine whether the weight differences in response to HFD between WT and NK-1R KO mice reflect similar changes in the levels of these two factors. Our results show that, compared with WT, NK-1R KO mice have lower levels of insulin and leptin (P < 0.05 for both; Fig. 2, A and B, respectively). Both insulin and leptin levels are also significantly lower in PF mice compared with WT (P < 0.05) (Fig. 2, A and B), suggesting that this response is likely due to the lesser weight gain in NK-1R KO mice. No differences in the serum levels of NEFA were observed (data not shown), and thus, they cannot account for the changes in circulating insulin levels between the NK-1R KO and WT groups.
Fig. 2.
NK-1R KO mice have lower levels of circulating insulin and leptin after HFD compared with WT littermates. Mice (n = 7–8 per group) matched for weight were fed with HFD for 3 wk. Mice were then killed, blood was collected, and leptin and insulin serum levels were measured using an immunoassay. A, Decreased weight in NK-1R KO and PF mice is associated with reduced levels of insulin (P < 0.05) when compared with WT littermates. B, Decreased weight in NK-1R KO and PF animals is associated with reduced levels of leptin (P < 0.05) compared with WT littermates. *, P < 0.05.
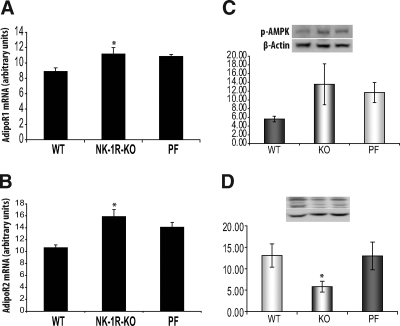
NK-1R KO mice have increased AdipoR mRNA in liver and mesenteric adipose tissue after HFD
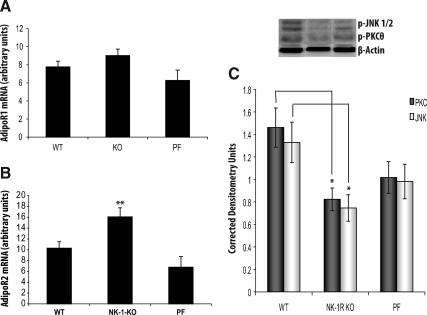
We tested the effects of NK-1R ablation in the expression of AdipoR in mesenteric fat (a major tissue for adiponectin production). Although mRNA expression of AdipoR2 is higher in the mesenteric fat depots of NK-1R KO animals (P < 0.05, n = 7) (Fig. 3B), there is no difference in the expression of AdipoR1 in the same depots between NK-1R KO and WT mice (Fig. 3A). Interestingly, NK-1R KO mesenteric depot AdipoR2 mRNA levels are also higher when compared with PF animals (P < 0.01) (Fig. 3B), suggesting a SP-NK-1R-specific signaling effect on fat tissue. There are no changes in the mesenteric fat depot mRNA and the circulating levels of adiponectin or in the activation of Akt between NK-1R KO and WT mice (data not shown).
Fig. 3.
NK-1R KO mice show increased AdipoR2 levels in mesenteric adipose tissue and changes in the activation of PKCθ and SAPK/JNK after HFD compared with WT littermates. Mice (n = 7–8 per group) matched for weight were fed with HFD for 3 wk. Mice were then killed; RNA was isolated from liver and whole mesenteric fat tissue homogenates, reverse-transcribed into cDNA, and incubated with dual fluorogenic probes for real-time PCR analysis. A, There is no difference in the expression of AdipoR1 in mesenteric fat depots between NK-1R KO and WT mice (P = 0.11). B, The mRNA expression of AdipoR2 was higher in mesenteric fat depots of NK-1R KO animals when compared with both WT and PF groups (P < 0.05). C, NK-1R KO mice have reduced SAPK/JNK (light bars) and PKCθ (dark bars) phosphorylation in the mesenteric fat depot compared with WT littermates. Densitometric analysis of PKCθ and JNK phosphorylation levels in the mesenteric adipose tissue (P = 0.013 and P < 0.05, respectively). *, P < 0.05 and **, P < 0.01.
NK-1R KO mice have lower levels of activated SAPK/JNK and PKCθ in mesenteric fat depots after HFD compared with WT littermates
Activation of the SAPK/JNK pathway is implicated in the inhibition of insulin signaling during inflammation and stress via serine phosphorylation of insulin receptor substrate 1, which blocks insulin receptor signal transduction (22, 33). NK-1R KO mice were fed HFD for 3 wk, and at the end of the experiment, whole mesenteric fat was removed and total protein was isolated. Our results show that mesenteric fat depots from NK-1R KO mice after HFD have reduced SAPK/JNK and PKCθ activation compared with WT mice (P < 0.05 and P = 0.013, for SAPK/JNK and PKCθ, respectively) (Fig. 3C). Such changes may contribute to the improved ability of NK-1R KO animals to clear glucose from the circulation (see figure 5).
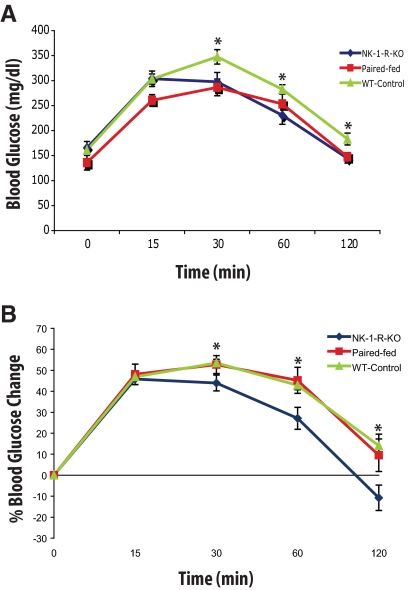
Fig. 5.
Changes in plasma glucose levels after glucose challenge in NK-1R KO mice compared with PF and WT mice after HFD. Mice were fed HFD for 3 wk. Mice were then fasted overnight and injected ip with dextrose (1 g/kg body weight). Blood glucose levels were monitored using a glucometer. A, NK-1R KO animals circulating glucose levels stabilize within 30 min after glucose challenge, whereas in WT and PF animals, glucose levels continue to increase (P < 0.01 and P < 0.05, green and red line, respectively; 15 vs. 30 min glucose). NK-1R KO blood glucose levels are lower after 60 min of glucose challenge and until the end of the experiment (P < 0.05 for both time points) in comparison with their WT and PF littermates. B, Calculation of the percent change in blood glucose levels showing that NK-1R KO mice are more efficient in removing glucose from the blood and sustain lower blood glucose levels than both their WT and PF littermates 30 min after challenge (P < 0.05 for the 30- and 60-min time points, P < 0.01 for the 120-min time point). *, P < 0.05.
NK-1R KO mice have increased AdipoR mRNA and increased AMP-activated protein kinase (AMPK) activation in liver after HFD
Here, we examined whether NK-1R deficiency alters the expression of adiponectin and its receptors primarily in liver (a primary target tissue for adiponectin action). We observed increased mRNA expression of both AdipoR1 (P < 0.05) (Fig. 4A) and AdipoR2 (P < 0.01) (Fig. 4B) receptors in the NK-1R KO mice compared with WT mice, suggesting increased sensitivity to adiponectin signaling in this tissue. AMPK has been shown as a downstream target of AdipoR activation and is thought to promote the beneficial effects of adiponectin-AdipoR signaling on insulin sensitization in the liver. Here, we show that NK-1R-KO mice have borderline significant increases (P ∼ 0.06, n = 6) (Fig. 4C) in activated AMPK levels compared with WT littermates in contrast to Akt, where no changes in the activated levels of this kinase were observed (data not shown). PF littermates do not demonstrate different responses in AMPK activation in comparison to both other groups (Fig. 4C). As in the case of mesenteric fat, activated JNK levels decreased in the livers of NK-1R KO mice (P < 0.05, n = 7) (Fig. 4D).
Fig. 4.
Hepatic levels of both AdipoR1 and AdipoR2 mRNA and AMPK activation increase in NK-1R KO mice. Mice (n = 7–8 per group) matched for weight were fed with HFD for 3 wk. Liver RNA was isolated to form cDNA and incubated with dual fluorogenic probes for real-time PCR analysis. The mRNA levels of both (A) AdiporR1 and (B) AdipoR2 were elevated in livers from NK-1R KO mice compared with WT (P < 0.05 and P < 0.01, respectively) but not PF littermates. C, Activated AMPK (a downstream effector of AdipoR signaling) levels also increased in the livers of NK-1R KO mice (although borderline P ∼ 0.06). D, NK-1R KO mice have reduced levels of activated JNK in their livers compared with WT but not to PF mice (P < 0.05). *, P < 0.05 and **, P < 0.01.
Improved responses to glucose challenge in NK-1R KO mice compared with WT and PF littermates after HFD
Obesity is linked to impaired insulin signaling, leading to reduced glucose tissue uptake and the development of hyperglycemia and type 2 diabetes (21). We next investigated whether reduced weight gain in NK-1R KO mice is also associated with improved glucose metabolism. As shown in Fig. 5A, NK-1R KO mice achieve a plateau of their circulating glucose levels as early as 15 min after challenge, whereas WT and PF mice continue to increase their levels (P < 0.01 and P < 0.05, green and red line, respectively; 15 vs. 30 min glucose) (Fig. 5A). Furthermore, 60 min after glucose challenge and until the end of the experiment, circulating glucose levels remain lower in NK-1 KO animals compared with WT (P < 0.05 for both 60 and 120 min) (Fig. 5A). The differences between NK-1R KO and WT mice in glucose metabolism become more apparent during the GTT, when glucose levels during GTT are expressed as percentage of the values at time 0, (P < 0.05 for the 30- and 60-min time points, P < 0.01 for the 120-min time point) (Fig. 5B). Interestingly, using a nonlinear model that considers repeated glucose measurements, NK-1R KO animals also show more efficient circulating glucose clearance than their PF counterparts (P < 0.05) (Fig. 5B), suggesting an additional, perhaps peripheral, NK-1R-dependent effect on glucose uptake. Fasting glucose levels were similar among groups (Fig. 5A).
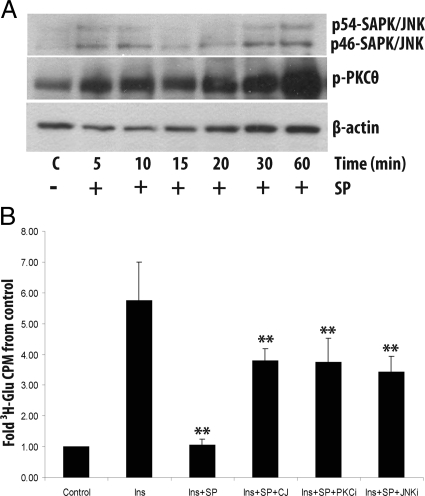
SP inhibits insulin-induced glucose uptake in rat epididymal adipocytes
Freshly isolated, noncultured adipocytes were obtained from rat epididymal fat depots. SP treatment of these cells led to a significant increase in both JNK and PKCθ phosphorylation starting at 5 min and peaking after 60 min of treatment (P < 0.05 and P < 0.01, respectively) (Fig. 6A). In addition, SP exposure decreases glucose uptake in response to insulin stimulation (P < 0.01, n = 6) (Fig. 6B) similar to the levels of untreated, control cells. Inhibition of NK-1R signaling with the specific inhibitor CJ 12,255 abolishes this effect (P < 0.01, n = 6) (Fig. 6B, fourth bar). Interestingly, pretreatment with inhibitors for JNK and PKC produced similar effects on SP-induced decrease in glucose uptake as NK-1R inhibition (P < 0.01 and P < 0.05, n = 6 and 5, respectively) (Fig. 6B). These results provide evidence implicating SP in the inhibition of insulin signaling and the subsequent regulation of glucose uptake in adipocytes. Because we previously showed that adipocytes express NK-1R (9), it is likely that SP mediates this response by interacting with NK-1R.
Fig. 6.
SP inhibits insulin-stimulated glucose uptake in freshly isolated rat adipocytes. A, Primary rat adipocytes from epididymal fat depots were treated with SP for different time points, and the levels of phosphorylated JNK and PKCθ were detected using Western blot analysis. There is a significant increase in phosphorylation of both JNK and PKCθ within 5 min of SP treatment with a peak phosphorylation at time 60 min (P < 0.05 and P < 0.01, respectively). B, Primary rat epididymal adipocytes were treated with insulin (Ins) or vehicle (Control). Then in different reactions SP, CJ 12,255, and PKC (PKCi) and JNK (JNKi) inhibitors were added. Cells were treated with a 3H-2-deoxy-D-glucose/2-deoxy-D-glucose cocktail, and glucose uptake was measured by quantifying radioactivity in a liquid scintillation counter. Treatment with insulin (100 nm) results in a robust increase of glucose uptake (P < 0.01, n = 6). Treatment with SP (10−7) reduces glucose uptake in response to insulin stimulation (P < 0.01, n = 6, insulin vs. Ins+SP columns), an effect that is blocked by the NK-1R antagonist CJ 12,255 (P < 0.01, n = 6). Inhibition of both JNK and PKC has similar inhibitory effects (P < 0.01 and P < 0.05, n = 6 and 5, respectively). **, P < 0.01.
Discussion
We report here that mice genetically deficient in NK-1R gain less weight in response to high-fat feeding (Fig 1), providing evidence for an important role of NK-1R in the development of obesity. The differences in weight accumulation in these mice are also associated with lower circulating levels of leptin and insulin (Fig 2), suggesting reduced adiposity in NK-1R KO mice. Along these lines, NK-1R KO mice exhibit more efficient blood glucose clearing ability when compared with both their WT and PF littermates after glucose challenge (Fig. 5). Such a response could, at least in part, be attributed to weight and adiposity changes in NK-1R mice, especially because insulin-dependent glucose uptake is closely associated with such changes. However, the disparity in glucose clearing ability between the NK-1R and PF groups (with similar weights) suggests a direct obesity-independent effect of SP on glucose metabolism.
NK-1R KO mice have lower levels of activated PKCθ and JNK kinases in the mesenteric fat after HFD (Fig 3C). The same is true for JNK activation in the liver (Fig. 4D). Because these signaling molecules are known for their ability to promote insulin resistance (22, 23, 34), decreases in both PKCθ and JNK kinases may contribute to the improvement in glucose clearance observed in NK-1R KO mice in response to HFD. This notion is further supported by our demonstration of the ability of SP to increase the activating phosphorylation of both PKCθ and JNK in primary rat epididymal adipocytes (Fig. 6A) and the blocking of SP inhibition on insulin-induced glucose uptake in the same cells via pharmacologic inhibition of these two kinases (Fig. 6B). In agreement with this, previous data from our group also demonstrated the ability of SP to induce activation of PKCθ in human mesenteric preadipocytes (35). Furthermore, as we mentioned above, we show that SP directly reduces insulin-mediated glucose uptake (Fig. 6B), providing strong evidence for an inhibitory role for SP in insulin signaling. These results show that SP effects on intracellular molecules involved in the regulation of insulin signaling in adipocytes may at least be part of the mechanism by which SP/NK-1R signaling controls weight accumulation and related metabolic changes.
Adiponectin and AdipoR are linked to insulin sensitization pathways (36). Thus, our observed increases in mRNA levels of AdipoR1 and AdipoR2 in the liver (Fig. 4), and AdipoR2 in fat (Fig. 3), may reflect significant changes in the ability of these tissues to respond to adiponectin. In the case of liver, we did observe borderline increases in AMPK activation in NK-1R KO animals (Fig. 4C), an observation that was not consistent in fat underlying potential discrepancy in the mode of SP action in liver compared with fat. Considering again the short duration of the study, these effects (which are in accordance to the moderate changes in weigh) are likely to magnify during conditions of chronic obesity as is the common case in humans. Because alterations in the hepatic responses to adiponectin affect the development of insulin resistance (36, 37), further studies on the participation of SP and NK-1R in the regulation of AdipoR expression and function could represent an exciting avenue of future research.
Early studies showed higher SP levels in the plasma of obese children compared with healthy controls (38). In addition, SP via NK-1R activation directly stimulates preadipocytes (9, 35), whereas administration of a NK-1R antagonist to mice reduces weight accumulation in mouse models with diet-induced or genetic obesity (20).
The approximately 6% less weight gain observed in NK-1R KO mice after HFD is significant, if one considers the short duration of the experiments as well as the higher weight of the animals at the initiation of the experiment, making it difficult to observe dramatic weight changes. Similar HFD-associated weight gain between NK-1R KO and PF mice suggests that SP-NK-1R interactions may be involved in the control of appetite at the brain level consistent with our previous findings (20). We understand that genetic NK-1R deficiency may alter activation of alternative pathways involved in the responses seen in our study. However, in the absence of adipose tissue-specific markers for targeted molecule deletion, the use of the NK-1R KO mouse model enables us to evaluate the NK-1R-specific adipose tissue-associated effects on glucose metabolism in vivo.
Although differences in glucose clearance (Fig. 4) point to SP-dependent adipose effects on insulin sensitization, further studies are required to investigate the potential effects of genetic NK-1R ablation on the levels of centrally acting orexigenic neuropeptides, such as neuropeptide Y or agouti-related peptide that are implicated in appetite regulation (39). In support of this notion, we have previously demonstrated an orexigenic effect of SP that could, at least in part, be attributed on its effects in the expression of hypothalamic peptides involved in appetite control (20).
Of particular interest is our observation that, after glucose challenge, NK-1R KO mice clear glucose from their circulation more efficiently than their WT as well as their PF littermates (Fig. 4), despite the fact that PF animals exhibit comparable weight gain after high-fat feeding (Fig. 1A). In addition, PF mice have comparable leptin and insulin levels as the NK-1R KO mice but respond to glucose challenge similarly to WT mice. These two observations exclude the weight and adipose differences between these groups as the sole factor contributing to this effect. Thus, SP may exert peripheral effects in adipose tissue that may interfere with insulin signaling and influence the ability of mice to clear glucose from the circulation. If indeed such effects are in place, NK-1R deficiency may be responsible for the improved glucose clearance exhibited by NK-1R KO animals (Fig. 4).
Thus our data suggest a dual effect of NK-1R deletion on glucose metabolism, one indirect, through appetite-dependent reduction in weight gain, and one direct, through effects on intracellular effectors of insulin-dependent glucose uptake in adipocytes. Thus, removal of the strong direct effects of SP-NK-1R interaction on insulin-induced signaling are mainly responsible for the glucose metabolism-associated responses observed in NK-1R KO mice, because the changes in weight and food intake, although significant, are modest.
Our previous studies demonstrated that human mesenteric preadipocytes respond to SP by increased IL-8 secretion in an nuclear factor κB-dependent manner (9). Interestingly, IL-8 may interfere with insulin signaling-related responses, because lack of its receptor in the bone marrow of mice protects from the development of insulin resistance during diet-induced obesity (40). SP exerts proproliferative and antiapoptotic effects on human mesenteric preadipocytes (35), suggesting that this peptide may affect fat mass. Furthermore, in a mouse model of diet-induced obesity, pharmacologic NK-1R antagonism reduces weight and improves insulin sensitivity (20). Such data further support the ability of SP to trigger insulin-related responses in adipose tissue and affect the regulation of glucose metabolism. Although the source of SP within the fat depots has yet to be recognized, studies have demonstrated that white adipose tissue received sensory innervation, and the neuropeptide itself has been traced within fat depots in animal models (41, 42). Further studies assessing the possibility that SP exerts direct effects of fat cells that may influence insulin resistance and adipocyte-associated lipid metabolism are required.
Overall, our data provide strong evidence for an important role of SP-NK-1R interactions in the development of adipose tissue-associated glucose metabolism in response to high-fat, Western type, diet. We demonstrate that SP inhibits insulin-stimulated glucose uptake in adipocytes. Considering the central role of adipose tissue in the development of insulin resistance, and in particular the visceral compartment, the direct effects of SP on insulin-stimulated glucose uptake provide evidence for a previously unrecognized SP response and identifies this peptide as a potential target molecule for future therapeutic approaches against insulin resistance and the development of type 2 diabetes.
Supplementary Material
Acknowledgments
We thank Dr. Craig Gerard from Harvard Medical School for his input in the creation of NK-1R KO mice.
This work was supported by National Institutes of Health Grants DK 47343, 1RC1 DK 086150-01 (to C.P.), and DK 56891 (to J.L.K.), a Research Fellowship Award from the Crohn's and Colitis Foundation of America, Inc. (I.K.), a Medical Research Award from the Broad Foundation (I.K.), and the Pilot Feasibility Grant from the Boston Obesity Nutrition Research Center 5 P30 DK 46200-15 (to E.K.).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AdipoR
- Adiponectin receptor
- AMPK
- AMP-activated protein kinase
- CJ 12,255
- 6-diphenylmethyl-5-(5-isopropyl-2-methoxybenzylamino)-1-azabicyclo(2.2.2)octane-3-carboxylic acid
- HFD
- high-fat diet
- GTT
- glucose tolerance test
- JNK
- c-Jun N-terminal kinase
- KO
- knockout
- NEFA
- nonesterified fatty acid
- NK-1R
- neurokinin-1 receptor
- PF
- paired fed
- PKC
- protein kinase C
- SAPK
- stress-activated protein kinase
- SP
- substance P
- WT
- wild type.
References
- 1. Chang MM, Leeman SE. 1970. Isolation of a sialogogic peptide from bovine hypothalamic tissue and its characterization as substance P. J Biol Chem 245:4784–4790 [PubMed] [Google Scholar]
- 2. Aliakbari J, Sreedharan SP, Turck CW, Goetzl EJ. 1987. Selective localization of vasoactive intestinal peptide and substance P in human eosinophils. Biochem Biophys Res Commun 148:1440–1445 [DOI] [PubMed] [Google Scholar]
- 3. Ho WZ, Lai JP, Zhu XH, Uvaydova M, Douglas SD. 1997. Human monocytes and macrophages express substance P and neurokinin-1 receptor. J Immunol 159:5654–5660 [PubMed] [Google Scholar]
- 4. Holzer P. 1988. Local effector functions of capsaicin-sensitive sensory nerve endings: involvement of tachykinins, calcitonin gene-related peptide and other neuropeptides. Neuroscience 24:739–768 [DOI] [PubMed] [Google Scholar]
- 5. Lai JP, Douglas SD, Ho WZ. 1998. Human lymphocytes express substance P and its receptor. J Neuroimmunol 86:80–86 [DOI] [PubMed] [Google Scholar]
- 6. Lundberg JM. 1996. Pharmacology of cotransmission in the autonomic nervous system: integrative aspects on amines, neuropeptides, adenosine triphosphate, amino acids and nitric oxide. Pharmacol Rev 48:113–178 [PubMed] [Google Scholar]
- 7. Pascual DW, Bost KL. 1990. Substance P production by P388D1 macrophages: a possible autocrine function for this neuropeptide. Immunology 71:52–56 [PMC free article] [PubMed] [Google Scholar]
- 8. Patak EN, Pennefather JN, Story ME. 2000. Effects of tachykinins on uterine smooth muscle. Clin Exp Pharmacol Physiol 27:922–927 [DOI] [PubMed] [Google Scholar]
- 9. Karagiannides I, Kokkotou E, Tansky M, Tchkonia T, Giorgadze N, O'Brien M, Leeman SE, Kirkland JL, Pothoulakis C. 2006. Induction of colitis causes inflammatory responses in fat depots: evidence for substance P pathways in human mesenteric preadipocytes. Proc Natl Acad Sci USA 103:5207–5212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pothoulakis C, Castagliuolo I, Leeman SE, Wang CC, Li H, Hoffman BJ, Mezey E. 1998. Substance P receptor expression in intestinal epithelium in clostridium difficile toxin A enteritis in rats. Am J Physiol 275:G68–G75 [DOI] [PubMed] [Google Scholar]
- 11. Stewart-Lee A, Burnstock G. 1989. Actions of tachykinins on the rabbit mesenteric artery: substance P and [Glp6,L-Pro9]SP6-11 are potent agonists for endothelial neurokinin-1 receptors. Br J Pharmacol 97:1218–1224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tsuchida K, Shigemoto R, Yokota Y, Nakanishi S. 1990. Tissue distribution and quantitation of the mRNAs for three rat tachykinin receptors. Eur J Biochem 193:751–757 [DOI] [PubMed] [Google Scholar]
- 13. Tansky MF, Pothoulakis C, Leeman SE. 2007. Functional consequences of alteration of N-linked glycosylation sites on the neurokinin 1 receptor. Proc Natl Acad Sci USA 104:10691–10696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Holzer P, Holzer-Petsche U. 1997. Tachykinins in the gut. Part I. Expression, release and motor function. Pharmacol Ther 73:173–217 [DOI] [PubMed] [Google Scholar]
- 15. Pothoulakis C, Castagliuolo I, LaMont JT, Jaffer A, O'Keane JC, Snider RM, Leeman SE. 1994. CP-96,345, a substance P antagonist, inhibits rat intestinal responses to Clostridium difficile toxin A but not cholera toxin. Proc Natl Acad Sci USA 91:947–951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Castagliuolo I, Valenick L, Liu J, Pothoulakis C. 2000. Epidermal growth factor receptor transactivation mediates substance P-induced mitogenic responses in U-373 MG cells. J Biol Chem 275:26545–26550 [DOI] [PubMed] [Google Scholar]
- 17. Riegler M, Castagliuolo I, So PT, Lotz M, Wang C, Wlk M, Sogukoglu T, Cosentini E, Bischof G, Hamilton G, Teleky B, Wenzl E, Matthews JB, Pothoulakis C. 1999. Effects of substance P on human colonic mucosa in vitro. Am J Physiol 276:G1473–G1483 [DOI] [PubMed] [Google Scholar]
- 18. Koon HW, Pothoulakis C. 2006. Immunomodulatory properties of substance P: the gastrointestinal system as a model. Ann NY Acad Sci 1088:23–40 [DOI] [PubMed] [Google Scholar]
- 19. Lazar MA. 2005. How obesity causes diabetes: not a tall tale. Science 307:373–375 [DOI] [PubMed] [Google Scholar]
- 20. Karagiannides I, Torres D, Tseng YH, Bowe C, Carvalho E, Espinoza D, Pothoulakis C, Kokkotou E. 2008. Substance P as a novel anti-obesity target. Gastroenterology 134:747–755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hotamisligil GS. 2006. Inflammation and metabolic disorders. Nature 444:860–867 [DOI] [PubMed] [Google Scholar]
- 22. Hirosumi J, Tuncman G, Chang L, Görgün CZ, Uysal KT, Maeda K, Karin M, Hotamisligil GS. 2002. A central role for JNK in obesity and insulin resistance. Nature 420:333–336 [DOI] [PubMed] [Google Scholar]
- 23. Kim JK, Fillmore JJ, Sunshine MJ, Albrecht B, Higashimori T, Kim DW, Liu ZX, Soos TJ, Cline GW, O'Brien WR, Littman DR, Shulman GI. 2004. PKC-theta knockout mice are protected from fat-induced insulin resistance. J Clin Invest 114:823–827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cai D, Yuan M, Frantz DF, Melendez PA, Hansen L, Lee J, Shoelson SE. 2005. Local and systemic insulin resistance resulting from hepatic activation of IKK-β and NF-κB. Nat Med 11:183–190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yuan M, Konstantopoulos N, Lee J, Hansen L, Li ZW, Karin M, Shoelson SE. 2001. Reversal of obesity- and diet-induced insulin resistance with salicylates or targeted disruption of Ikkβ. Science 293:1673–1677 [DOI] [PubMed] [Google Scholar]
- 26. Yamauchi T, Kamon J, Ito Y, Tsuchida A, Yokomizo T, Kita S, Sugiyama T, Miyagishi M, Hara K, Tsunoda M, Murakami K, Ohteki T, Uchida S, Takekawa S, Waki H, Tsuno NH, Shibata Y, Terauchi Y, Froguel P, Tobe K, Koyasu S, Taira K, Kitamura T, Shimizu T, Nagai R, Kadowaki T. 2003. Cloning of adiponectin receptors that mediate antidiabetic metabolic effects. Nature 423:762–769 [DOI] [PubMed] [Google Scholar]
- 27. Antoniades C, Antonopoulos AS, Tousoulis D, Stefanadis C. 2009. Adiponectin: from obesity to cardiovascular disease. Obesity Reviews 10:269–279 [DOI] [PubMed] [Google Scholar]
- 28. Weyer C, Funahashi T, Tanaka S, Hotta K, Matsuzawa Y, Pratley RE, Tataranni PA. 2001. Hypoadiponectinemia in obesity and type 2 diabetes: close association with insulin resistance and hyperinsulinemia. J Clin Endocrinol Metab 86:1930–1935 [DOI] [PubMed] [Google Scholar]
- 29. Bozic CR, Lu B, Höpken UE, Gerard C, Gerard NP. 1996. Neurogenic amplification of immune complex inflammation. Science 273:1722–1725 [DOI] [PubMed] [Google Scholar]
- 30. Holland PM, Abramson RD, Watson R, Gelfand DH. 1991. Detection of specific polymerase chain reaction product by utilizing the 5′–3′ exonuclease activity of Thermus aquaticus DNA polymerase. Proc Natl Acad Sci USA 88:7276–7280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Karagiannides I, Tchkonia T, Dobson DE, Steppan CM, Cummins P, Chan G, Salvatori K, Hadzopoulou-Cladaras M, Kirkland JL. 2001. Altered expression of C/EBP family members results in decreased adipogenesis with aging. Am J Physiol Regul Integr Comp Physiol 280:R1772–R1780 [DOI] [PubMed] [Google Scholar]
- 32. Caro JF, Sinha MK, Kolaczynski JW, Zhang PL, Considine RV. 1996. Leptin: the tale of an obesity gene. Diabetes 45:1455–1462 [DOI] [PubMed] [Google Scholar]
- 33. Aguirre V, Uchida T, Yenush L, Davis R, White MF. 2000. The c-Jun NH(2)-terminal kinase promotes insulin resistance during association with insulin receptor substrate-1 and phosphorylation of Ser(307). J Biol Chem 275:9047–9054 [DOI] [PubMed] [Google Scholar]
- 34. Hotamisligil GS, Peraldi P, Budavari A, Ellis R, White MF, Spiegelman BM. 1996. IRS-1-mediated inhibition of insulin receptor tyrosine kinase activity in TNF-α- and obesity-induced insulin resistance. Science 271:665–668 [DOI] [PubMed] [Google Scholar]
- 35. Gross K, Karagiannides I, Thomou T, Koon HW, Bowe C, Kim H, Giorgadze N, Tchkonia T, Pirtskhalava T, Kirkland JL, Pothoulakis C. 2009. Substance P promotes expansion of human mesenteric preadipocytes through proliferative and antiapoptotic pathways. Am J Physiol Gastrointest Liver Physiol 296:G1012–G1019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kadowaki T, Yamauchi T, Kubota N, Hara K, Ueki K, Tobe K. 2006. Adiponectin and adiponectin receptors in insulin resistance, diabetes, and the metabolic syndrome. J Clin Invest 116:1784–1792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Yamauchi T, Kadowaki T. 2008. Physiological and pathophysiological roles of adiponectin and adiponectin receptors in the integrated regulation of metabolic and cardiovascular diseases. Int J Obes 32(Suppl 7):S13–S18 [DOI] [PubMed] [Google Scholar]
- 38. Baroncelli GI, Bertelloni S, Buggiani B, Papini A, Gualtieri M, Saggese G. 1989. Evidence of increased levels of substance P in obese children. Funct Neurol 4:183–184 [PubMed] [Google Scholar]
- 39. Badman MK, Flier JS. 2005. The gut and energy balance: visceral allies in the obesity wars. Science 307:1909–1914 [DOI] [PubMed] [Google Scholar]
- 40. Neels JG, Badeanlou L, Hester KD, Samad F. 2009. Keratinocyte-derived chemokine in obesity: expression and role in adipose macrophage infiltration and glucose homeostasis. J Biol Chem 284:20692–20698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bartness TJ, Shrestha YB, Vaughan CH, Schwartz GJ, Song CK. 2010. Sensory and sympathetic nervous system control of white adipose tissue lipolysis. Mol Cell Endocrinol 318:34–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Shi H, Song CK, Giordano A, Cinti S, Bartness TJ. 2005. Sensory or sympathetic white adipose tissue denervation differentially affects depot growth and cellularity. Am J Physiol-Reg I 288:R1028–R1037 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.