Normal human prostate progenitor cells are estrogen targets and estradiol, in an androgen-supported milieu, is capable of transformation and malignant progression of human prostate epithelium.
Abstract
The present study sought to determine whether estrogens with testosterone support are sufficient to transform the normal human prostate epithelium and promote progression to invasive adenocarcinoma using a novel chimeric prostate model. Adult prostate stem/early progenitor cells were isolated from normal human prostates through prostasphere formation in three-dimensional culture. The stem/early progenitor cell status and clonality of prostasphere cells was confirmed by immunocytochemistry and Hoechst staining. Normal prostate progenitor cells were found to express estrogen receptor α, estrogen receptor β, and G protein-coupled receptor 30 mRNA and protein and were responsive to 1 nm estradiol-17β with increased numbers and prostasphere size, implicating them as direct estrogen targets. Recombinants of human prostate progenitor cells with rat urogenital sinus mesenchyme formed chimeric prostate tissue in vivo under the renal capsule of nude mice. Cytodifferentiation of human prostate progenitor cells in chimeric tissues was confirmed by immunohistochemistry using epithelial cell markers (p63, cytokeratin 8/18, and androgen receptor), whereas human origin and functional differentiation were confirmed by expression of human nuclear antigen and prostate-specific antigen, respectively. Once mature tissues formed, the hosts were exposed to elevated testosterone and estradiol-17β for 1–4 months, and prostate pathology was longitudinally monitored. Induction of prostate cancer in the human stem/progenitor cell-generated prostatic tissue was observed over time, progressing from normal histology to epithelial hyperplasia, prostate intraepithelial neoplasia, and prostate cancer with local renal invasion. These findings provide the first direct evidence that human prostate progenitor cells are estrogen targets and that estradiol in an androgen-supported milieu is a carcinogen for human prostate epithelium.
Prostate cancer is the most common noncutaneous cancer and the second leading cause of cancer deaths in North American men. Increasing evidence indicates that in addition to androgens, estrogens play key roles in prostate carcinogenesis and progression; however, the mechanisms are not fully understood (1–5). Progress toward understanding the role of sex steroids in the etiology of human prostate cancer has been hindered by the lack of a suitable laboratory research model (6). The only nonhuman mammals known to develop prostate cancer naturally are primates and dogs (7); however, both models are expensive and limited by long tumor latencies. Experimental rodent models have been developed and used extensively to elucidate the mechanisms of prostate carcinogenesis and include transgenic mouse (8, 9) and rat prostate cancers (10), spontaneous and chemically inducible Lobund-Wistar rat prostate cancer (11, 12), and hormone-inducible Dunning (13) and Noble rat prostate cancers (14). These models, however, are limited by their nonhuman origin, which restricts their direct application to humans. Prostate cancer models of human origin consist of primary cell or tissue slice cultures (15), multiple cancer cell lines used in vitro and in vivo as grafts (16, 17), as well as cancer tissue xenografts (18). However, these cells and tissues are derived from established prostate cancers that hamper their use in the investigation of initiating events and early transformation. To investigate these early steps in prostate carcinogenesis from normal cells to transformed epithelium, a model using normal human prostate cells is required. Currently, there is an unanswered need for such a model with transformation and malignant progression driven by steroid hormones.
Adult stem cells are found in adult tissues and act as a repair system by maintaining the normal turnover of the regenerative organ (19–21). Based on the stem cell hypothesis of cancer development, malignant tumors may originate from transformation of resident normal tissue stem cells that both self-renew and differentiate into abnormal progeny that continuously seed tumor growth (22, 23). Recent evidence indicates a special role of steroid hormones in the control of normal mammary stem cell function (24, 25) that implicates stem cells as the potential target during hormonal carcinogenesis.
The prostate gland is a ductal structure composed of basal, luminal, and a small number of neuroendocrine epithelial cells surrounded by stromal fibroblasts and smooth muscle cells. Adult prostate stem cells have recently been identified in human and rodent prostate tissue (22, 23, 26–28). This rare cell type self-renews and has potential to differentiate into the three epithelial cell types, an essential characteristic of a stem cell (29). Another characteristic of adult prostate stem/progenitor cells is their unique ability to form spheroids when cultured in an anchorage-independent, three-dimensional (3D) matrigel system where differentiated cells fail to survive (30–32). The prostate regenerating abilities of adult prostate stem cells isolated from rodents, patient samples, and human cancer cell lines have been documented by several laboratories using recombination with rat urogenital sinus mesenchyme (UGM) followed by in vivo growth as renal grafts in immunocompromised mice (26, 27, 33, 34). Taylor et al. (35) similarly reported the formation of human prostate-like tissues from human embryonic stem cells when mixed with rat UGM using a renal graft approach. However, an in vivo model of prostate tissue regeneration using normal human prostate adult stem cells as the starting material has not been reported to date.
In the current study, we first developed a method to isolate stem/progenitor cells from normal human prostates and generate normal human prostate-like tissue in vivo when combined with inductive rat UGM using a renal graft approach in nude mice. Prostatic hormonal carcinogenesis induced by combined testosterone (T) and estradiol-17β (E2) treatment for several months has been well established in both mouse (36, 37) and rat models (38, 39). Using this approach, we exposed the grafted mice containing chimeric human-rat prostate structures to elevated T+E2 via sc pellets. Over a 1- to 4-month-exposure period, the human prostate-like structures developed progressive disease from atypical hyperplasia to prostate intraepithelial neoplasia (PIN) and high-grade (HG) prostate cancer with local invasion. We have thus developed the first model of hormone-induced human prostate cancer initiation and progression from normal human prostate cells into full malignancy.
Materials and Methods
Animals
Timed pregnant Sprague Dawley rats were purchased from Harlan (Indianapolis, IN) and male nude mice from Charles River (Wilmington, MA). Animals were fed standard chow ad libitum. All studies were approved by the Institutional Animal Care Committee.
Cell and prostasphere cultures
Primary human prostate epithelial cells (PrEC) were obtained from normal healthy donors between 20 and 30 yr of age (Lonza Walkersville, Inc., Walkersville, MD) and maintained at 37 C in fibronectin-coated flasks using Prostate Epithelial Cell Growth Medium (PrEGM) (Lonza). Cells were passaged once and frozen for subsequent 3D cultures. The epithelial cultures lacked contamination by other cell types as revealed by immunohistochemistry (IHC) for p63 and vimentin (data not shown). LNCaP, PC3, and DU145 cells were cultured as described (5).
Prostasphere culture conditions were modifications of published protocols (33, 40, 41). Briefly, 1 × 105 PrEC cells were resuspended in 1:1 matrigel (BD Biosciences, Bedford, MA)/PrEGM (Lonza), plated in 12-well plates with 1 ml PrEGM, and cultured at 37 C in 5% CO2. Medium was replenished every 3 d, and prostasphere formation and growth were monitored by real-time imaging using a Zeiss Axiovert 200 inverted microscope with automated X-Y-Z stage and an Axiocam (Carl Zeiss MicroImaging, Inc., Thornwood, NY). For tissue recombination, matrigel was digested in dispase (1 mg/ml; Invitrogen, Carlsbad, CA) for 30 min. Spheres were resuspended in type I collagenase (190 U/ml) for 45 min, washed, resuspended in 0.05% trypsin/EDTA for 10 min, passaged 5–10 times through a 27-gauge needle, filtered (40 μm), and cells counted.
Generation of chimeric prostate-like structures through cell-tissue recombination and renal grafts
The urogenital sinus from embryonic d-17 rat fetuses was removed, digested, and UGM separated from urogenital epithelium as described (42). The UGM from one embryo was mixed with approximately 3000 dispersed human prostasphere cells, resuspended in 10 μl high growth factor matrigel, and incubated overnight on 1% agar with PrEGM in 5% CO2 at 37 C. Recombinants were grafted under the renal capsule of 7- to 8-wk-old male nude mice as described (43). Grafts with UGM or urogenital epithelium alone were used for controls. Recipient mice were supplemented with T containing SILASTIC (Dow Corning Corp., Midland, MI) capsules (0.5 cm) that produced serum T levels of 12.7 ng/ml at 1 month.
Induction of hormonal carcinogenesis
One month after renal grafting, prostate carcinogenesis was initiated in chimeric tissues through T+E2 treatment of the host. Mice were implanted with sc pellets containing 25 mg of T and 2.5 mg of E2 for sustained steroid delivery for 1–4 months. This 10:1 ratio promoted prostate cancer in recombinants with BPH-1 cells (37). One month after initiation of T+E2, several specimens were biopsied and grafts returned for an additional 1–3 months of T+E2. At killing, blood was collected, and grafts were processed for histologic examination as described (43). Serial sections for each tissue were examined by two board-certified pathologists and classified for prostate pathology according to criteria established by the Armed Forces Institute of Pathology. Control treatments included 13 mice with no T+E2 pellets that were killed at 1, 2, or 3 months and four mice given T implants alone for 3 months.
IHC and immunocytochemistry (ICC)
IHC was performed for positive identification of species origin of the cells, tissue type, and differentiation status of the grafts as previously described (43). ICC staining was performed on prostaspheres grown overnight on chamber slides to permit limited cell outgrowth and improve reagent penetration. Spheres were fixed in acetone/methanol (1:1) and immunostained as described (43). Antibodies used are provided in Supplemental Table 1, published on The Endocrine Society's Journals Online web site at http://endo.endojournals.org. Confocal imaging was conducted on a Zeiss LSM 510 META Confocal Microscope.
Hoechst exclusion assay and lineage labeling
A Hoechst exclusion assay was performed to confirm stem/progenitor cell status. Day-7 prostaspheres or two-dimensional (2D) PrEC cells were stained with 0.5 μg/ml Hoechst 33342 (H342; Sigma-Aldrich, Inc., St. Louis, MO) in Hanks' balanced salt solution buffer for 30 min at 37 C, washed, fixed, and imaged as described. As parallel controls, cells in separate wells were treated with verapamil (50 μm; Sigma-Aldrich, Inc.), an ABCG2 transporter inhibitor, for 10 min before H342 staining.
To assess prostasphere clonality, single PrEC cell suspensions were stained with high concentration H342 (5 μg/ml) in PrEGM for 45 min, washed, and mixed with equal amounts of unstained PrEC cells followed by 3D culture for 4–7 d. Prostaspheres were fixed, mounted in propidium iodide, and H342 signal was monitored using fluorescent microscopy.
Radioimmunoassay
Serum T and E2 levels were measured using murine RIA kits (TKTT2 for T, TKE21 for E2; Siemens Medical Solutions Diagnostics, Los Angeles, CA) at the Ligand Assay and Analysis Core Laboratory, Center for Research in Reproduction, University of Virginia (Charlottesville, VA). For T, sensitivity was 0.1 ng/ml, and the intra- and interassay coefficients of variance were 3.9 and 7.8%, respectively. For E2, sensitivity was 10 pg/ml, and the intra- and interassay coefficients of variance were 4.4 and 7.8%, respectively.
Quantitative real-time RT-PCR
Total RNA was isolated using TRIzol Reagent (Invitrogen). cDNA were synthesized using reverse transcriptase and random hexamers (Clontech Laboratories, Mountain View, CA). PCR in SYBR GreenER PCR Master-Mix (Invitrogen) were carried out using 7900HT Fast Real-time PCR System (Applied Biosystems, Carlsbad, CA). Primer sequences are provided in Supplemental Table 2. Individual mRNA levels were normalized to glyceraldehyde-3-phosphate dehydrogenase (GAPDH).
Statistic analysis
Data were analyzed using InStat ver. 3 (GraphPad Software, Inc., San Diego, CA) using Student's t test or ANOVA as appropriate, followed by post hoc tests. Values are expressed as mean ± sem, and P < 0.05 was considered significant.
Results
Isolation and enrichment of normal adult prostate stem/progenitor cells using a prostasphere culture system
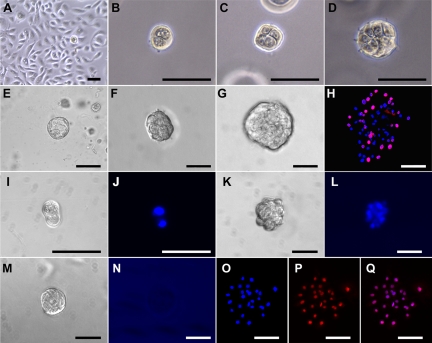
Primary cultures of PrEC cells from young normal organ donors had a doubling time of 16–24 h when grown in 2D, reaching confluence within 96 h (Fig. 1A). This was approximately 2-fold faster than proliferation rates of PrEC obtained from benign regions of surgical specimens from older prostate cancer patients used in preliminary studies (data not shown). After transfer of normal PrEC to 3D matrigel culture, a minor fraction (0.2–1.0%) of single PrEC cells survived and began sequential divisions to form two-, four-, and eight-cell structures that were at times asymmetric in size (Fig. 1, B–D). At culture d-3, early sphere formation was observed, and by d-4, the structures had grown to form small, solid spheroids of 20–40 cells, 25–40 μm in diameter (Fig. 1, E and F). The prostaspheres grew in size through continuous proliferation as visualized with bromodeoxyuridine labeling, reaching approximately 60- to 90-μm diameter with 100–300 cells at d-7 (Fig. 1, G and H). Continuous growth of spheroids through cell division was monitored in real time with video clips shown in Supplemental Fig. 1.
Fig. 1.
Isolation and growth of prostaspheres from primary cultures of normal human PrEC. A, Normal human PrEC grown in 2D culture were used as a source for stem/progenitor cells. A small fraction (0.2–1.0%) of PrEC was differentially selected and formed prostaspheres in a 3D matrigel culture system. At the early stage of prostasphere formation, single prostate progenitor cells divided and formed two- (B), four- (C), and eight-cell (D) structures. E, At d-3 of culture, early sphere formation was observed. F, Typical d-4 prostasphere 25–40 μm in diameter. G, Prostaspheres continued to grow, reaching approximately 60–90 μm in diameter by d-7. H, Day-7 prostasphere after 12-h bromodeoxyuridine incorporation (pink) revealed proliferation rates of 30–50% in the progenitor cells. Nuclei are counterstained with 4′,6-diamidino-2-phenylindole (DAPI) (blue). I–Q, To document clonal origin of prostaspheres from single cells, equal amounts of Hoechst-labeled and -unlabeled PrEC cells were mixed and placed in 3D culture. I and J, At culture d-2, a dividing progenitor cell at two-cell stage (I) transferred Hoechst stain to the daughter cell as seen using the blue fluorescent channel (J). K–L, Culture d-4 prostasphere with a brightfield (K) or blue fluorescent channel (L) shows all progenitor cells in the spheroid positive for Hoechst blue staining. M and N, Another prostasphere in the mixed culture at d-4 (M) was entirely negative under the blue fluorescent channel (N). O–Q, Day-4 prostaspheres from mixed 3D cultures were transferred to 2D culture for overnight attachment and outgrowth. Blue fluorescent channel viewing shows Hoechst-labeled nuclei (O), whereas red fluorescent channel viewing shows propidium iodide nuclear counterstain (P). When merged (Q), all cells in a single prostasphere are shown to be Hoechst positive (pink). Scale bar, 50 μm.
Hoechst dye at high concentration is retained by stem/progenitor cells and was used to confirm prostasphere clonality. Hoechst dye in single cells was passed onto daughter cells (Fig. 1, I and J) and detected in prostaspheres for 4–7 d (Fig. 1, K and L). Importantly, 3D cultures containing mixtures of Hoechst-labeled and -unlabeled cells revealed that individual prostaspheres were either entirely negative (Fig. 1, M and N) or Hoechst positive (Fig. 1, K and L and O–Q). No prostaspheres of mixed Hoechst-stained and -unstained cells were observed, indicating that prostaspheres are derived from single progenitor cells with self-renewal ability.
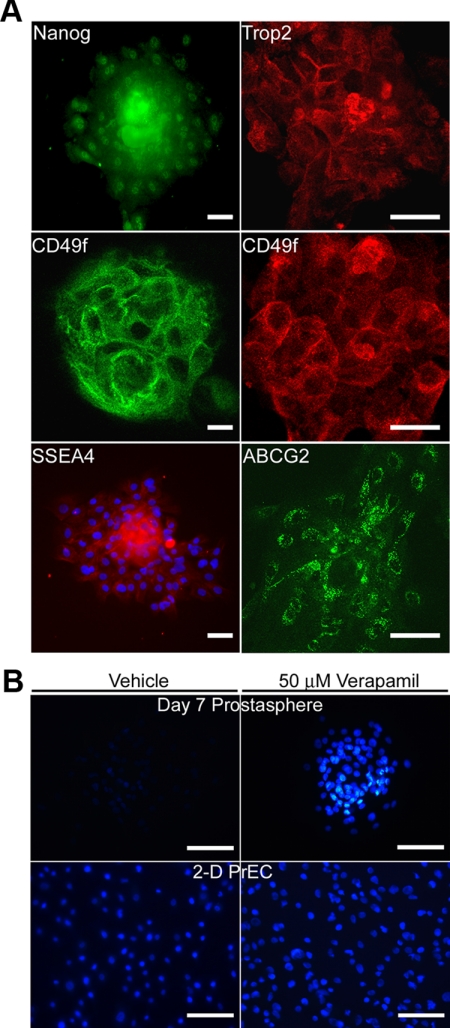
Previous work using flow cytometry showed that only epithelial cells with stem cell markers have the capacity to survive and form prostaspheres in 3D culture (32). To confirm that prostaspheres in the present study consist of stem/progenitor cell populations, d-7 prostaspheres were immunolabeled with stem cell markers, including Nanog, Trop2, CD49f, SSEA4, and ABCG2 (27, 44, 45). As shown in Fig. 2A, prostasphere cells were all positive for several combinations of these markers, indicating a relatively homogenous population of stem and early-stage progenitor cells. Further confirmation was obtained by Hoechst exclusion assay that examines the unique ability of stem/progenitor cells to exclude low concentration Hoechst dye due to functional ABCG2 transporter proteins expressed in these cells. Prostaspheres incubated in 0.5 μg/ml Hoechst were negative, a property that was lost upon preincubation with verapamil, an ABCG2 inhibitor (Fig. 2B). In contrast, parental PrEC cells retained Hoechst in the absence and presence of verapamil. Together, these findings confirm the stem/progenitor cell nature of the human d-7 prostasphere cells.
Fig. 2.
A, Immunofluorescent labeling of d-7 prostaspheres with Nanog, TROP2, CD49f, SSEA4, and ABCG2 confirms their stem/early progenitor cell characteristics. The majority of prostasphere cells were positive for membrane-associated TROP2, CD49f, ABCG2, SSEA4 [with blue 4′,6-diamidino-2-phenylindole (DAPI) counterstain], and nuclear Nanog. Confocal images are shown for TROP2, CD49f, and ABCG2, whereas fluorescent imaging of whole-mounted prostaspheres was employed for Nanog and SSEA4. In the latter, excess signal in the prostasphere center is a result of the dense cell compaction in the spheroid. Scale bar, 50 μm. B, Photomicrographs showing Hoechst exclusion by d-7 prostasphere cells and 2D PrEC in the absence or presence of 50 μm verapamil. Note that the exclusion of 0.5 μg/ml Hoechst dye by prostasphere cells (upper left) was blocked by verapamil which caused the retention of Hoechst dye (upper right), indicating stem/progenitor cell nature of the cells. In contrast, no significant Hoechst dye exclusion was found in the 2D culture PrEC cells (lower left), and there was no effect of verapamil on their dye retention (lower right). Scale bar, 50 μm.
Prostasphere steroid receptor expression and response to E2
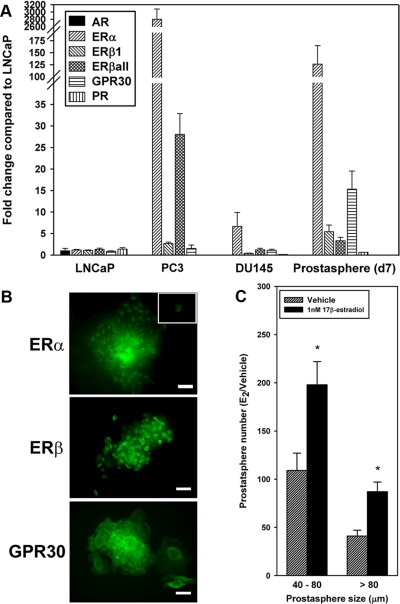
To determine whether isolated adult prostate progenitor cells have potential to respond to steroids, we examined the steroid receptor status of d-7 prostasphere cells by quantitative real-time RT-PCR and ICC. Steroid receptor mRNA levels are shown in Fig. 3A with expression normalized to LNCaP levels. Although prostate progenitor cells expressed androgen receptor (AR) mRNA at limit of detection levels and were AR negative at the protein level, they were positive for all known estrogen receptors (ER), including ERα, ERβ1, and G protein-coupled receptor (GPR)30. Importantly, the ER mRNA expression levels were markedly higher in normal progenitor cells relative to LNCaP cells with 6-fold higher ERβ1, 15-fold higher GPR30, and 125-fold higher ERα expression. In comparison, undifferentiated progenitor cells more closely resembled steroid receptor profiles in the androgen-independent cell lines PC3 and DU145 with elevated ER expression, minimal progesterone receptor (PR), and no AR mRNA. ER and GPR30 protein expression was confirmed by immunofluorescent staining. Although ERβ localized to the nucleus, ERα was found in the nucleus and cytoplasm whereas GPR30 localized to the membrane and cytoplasm in the majority of the progenitor cells (Fig. 3B). These findings suggest that normal human prostate progenitor cells have potential to respond to estrogen ligands.
Fig. 3.
Steroid receptor expression in d-7 prostaspheres and growth response of progenitor cells to E2. A, Steroid receptor mRNA levels in d-7 prostaspheres, LNCaP, and PC3 cell lines as determined by quantitative real-time PCR. Data are normalized to LNCaP levels (set as 1) after normalization of each sample to GAPDH. Although the prostate progenitor cells did not express AR, there was robust expression of ERα, ERβ1, GPR30, and PR. ERβ expression was measured using primers specific for ERβ1 as well as primers that amplify all ERβ isoforms (ERβall); n = 3 separate experiments. B, Immunofluorescent labeling of d-7 prostasphere cells for ERα, ERβ, or GPR30. ERα and ERβ primarily localized to the nucleus, whereas GPR30 localized to the cell membrane and, to a lesser degree, cytoplasm. The inset shows fluorescence with IgG substituted for primary antibody. Scale bar, 50 μm. C, Prostaspheres were grown for 7 d in a 3D matrigel culture in the absence or presence of 1 nm E2, and prostasphere numbers and sizes (40–80 μm, >80 μm diameter) were measured. Both the number of prostaspheres that formed as well as their size at d-7 were markedly increased in response to estrogen exposure. *, P < 0.05 vs. control; n = 6 separate experiments.
To directly test this possibility, prostaspheres were exposed to 1 nm E2 for 7 d as they formed and expanded in culture. E2 increased both the number of prostaspheres that formed by d-7 as well as their size, indicated by a greater proportion of prostaspheres more than 80 μm in diameter (Fig 3C). Thus the present results demonstrate that normal human prostate progenitor cells are responsive to estrogens with increased rates of self-renewal.
In vitro differentiation of normal prostaspheres
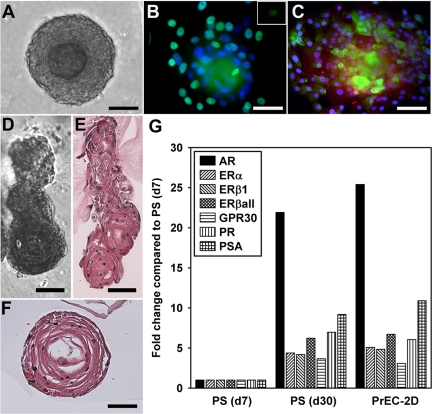
With continued prostasphere culture through d-10, the cells began to differentiate, forming double-layered prostaspheres 100–150 μm in diameter (Fig. 4A). To further characterize these cells, d-10 prostaspheres were immunolabeled for basal and luminal epithelial cell markers. Peripheral cells (outer layer) were positive for p63, a basal cell marker (Fig. 4B), whereas the innermost cells were p63-negative. In contrast, cells toward the spheroid center were positive for cytokeratin (CK)8 and NKX3.1 (Fig. 4C) indicating that interior prostasphere cells were differentiating toward a luminal cell phenotype. To further analyze the differentiation capacity for prostasphere cells in vitro, cultures were continued through d-30. During this period, prostaspheres frequently expanded laterally, forming branch-like structures (Fig. 4, D and E) with cross-sections exhibiting lumen-like formation in the prostasphere centers (Fig. 4F).
Fig. 4.
In vitro differentiation of normal prostaspheres with extended culture. A, By d-10 of culture, the cells located in the prostasphere center began to differentiate, forming a double-layered structure of 100–150 μm in diameter. B, Immunofluorescent labeling of d-10 prostaspheres with p63 (green) and 4′,6-diamidino-2-phenylindole (DAPI) nuclear stain (blue) shows that the basal cells (aqua-green) are located in the outer layer, whereas the more centrally located cells are p63 negative. The green signal in the compacted prostasphere center was nonspecific and appeared when IgG was substituted for primary antibody (inset). C, Immunofluorescent labeling of d-10 prostaspheres with CK8 (green), NKX3.1 (red-purple), and DAPI nuclear stain (blue). The positively stained cells are located toward the prostasphere center and represent the differentiated cells. D–F, Prostaspheres cultured through d-30 began to grow laterally, forming ductal-like structures (D and E) with lumen formation (F). Scale bar, 50 μm (A–F). G, Steroid receptor and PSA expression levels in d-7 and d-30 prostaspheres (PS) and in parental PrEC grown in 2D culture as measured by real-time RT-PCR. Data were normalized to GAPDH in each sample and expressed relative to d-7 prostasphere levels set as 1. Day-30 prostasphere cells and the primary epithelial cell cultures from human prostate expressed AR, higher levels of all ER and PR, as well as PSA when compared with the undifferentiated d-7 prostasphere cells.
Steroid receptor profiles of d-30 prostaspheres were measured and compared with expression levels in d-7 prostaspheres and to parental PrEC cells grown in 2D culture. In comparison with d-7 progenitor cells, d-30 prostaspheres expressed increased levels of AR and higher levels of all ER and PR (Fig. 4G). Furthermore, d-30 prostaspheres expressed prostate-specific antigen (PSA) mRNA documenting functional differentiation of luminal cells was initiated in vitro (Fig. 4G). Importantly, the steroid receptor profiles and PSA expression levels of the d-30 prostaspheres were nearly identical to their parental PrEC. Together, these results demonstrate that the normal adult prostate stem/progenitor cells isolated by prostasphere assay can differentiate into functional basal and luminal epithelial cells in vitro.
Regeneration of normal prostate-like tissue from human prostate stem/progenitor cells in vivo
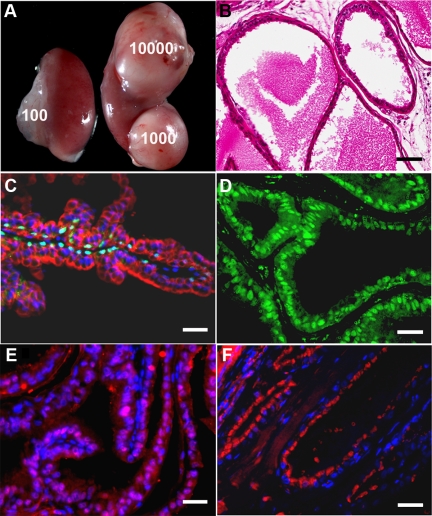
We investigated the differentiation and regenerative abilities of normal adult prostate progenitor cells by using an in vivo cell/tissue recombination and renal graft model (Supplemental Fig. 2). Day-7 prostasphere cells were used as the source of human prostate progenitor cells because characterizations confirmed their primary stem/progenitor status at this time point. By varying the number of progenitor cells used in the recombinants from 100 to 10,000 cells with a constant amount of UGM from a single embryo, renal grafts of increasing size were obtained after 4 wk of in vivo growth (Fig. 5A). Consequently, approximately 3000 progenitor cells were used for each graft in subsequent experiments. Histological examination of 4-wk graft sections by hematoxylin and eosin (H&E) staining showed formation of mature prostate-like structures with secretory material in the lumens (Fig. 5B). Immunofluorescent staining showed p63 expression in basal cells (Fig. 5C) and CK8/18 (Fig. 5C) and AR (Fig. 5D) in adluminal cells demonstrating cytodifferentiation of prostate progenitor cells into basal and luminal epithelial cells in vivo. The human origin and functional differentiation of the epithelium in chimeric grafts were confirmed by expression of human nuclear antigen (Fig. 5E) and secretion of PSA (Fig. 5F), a human-specific secretory product. All prostate grafts were confirmed as histologically normal by a board-certified pathologist.
Fig. 5.
Characterization of chimeric prostate tissue from normal human prostate progenitor cells. A, Increasing numbers (100–10,000) of prostate progenitor cells mixed with a constant amount of UGM produced grafts of increasing size. B, H&E staining of a 1-month graft shows normal glandular structure with prostatic histology. C, Immunofluorescent labeling with antibodies against p63 (green-aqua) and CK8/18 (red) with 4′,6-diamidino-2-phenylindole (DAPI)-stained nuclei confirms differentiation of cells into prostate basal and luminal epithelial cells. D, Nuclear immunostaining of AR (green) further confirms prostatic cytodifferentiation of the epithelium. E, The human origin of the epithelium in chimeric grafts was demonstrated by immunolabeling with human-specific antinuclear antigen (red stain; pink when merged with blue nuclear DAPI) and DAPI-labeled nuclei (blue) at 1 month. Note that stromal cells are negative for human nuclear antigen. F, Human origin and functional differentiation of the epithelium were further confirmed by immunostaining for PSA (red) in the luminal cells with DAPI-labeled nuclei. Scale bar, 50 μm.
Induction of epithelial hyperplasia, metaplasia, and carcinogenesis in normal chimeric prostate grafts with T+E2 exposure
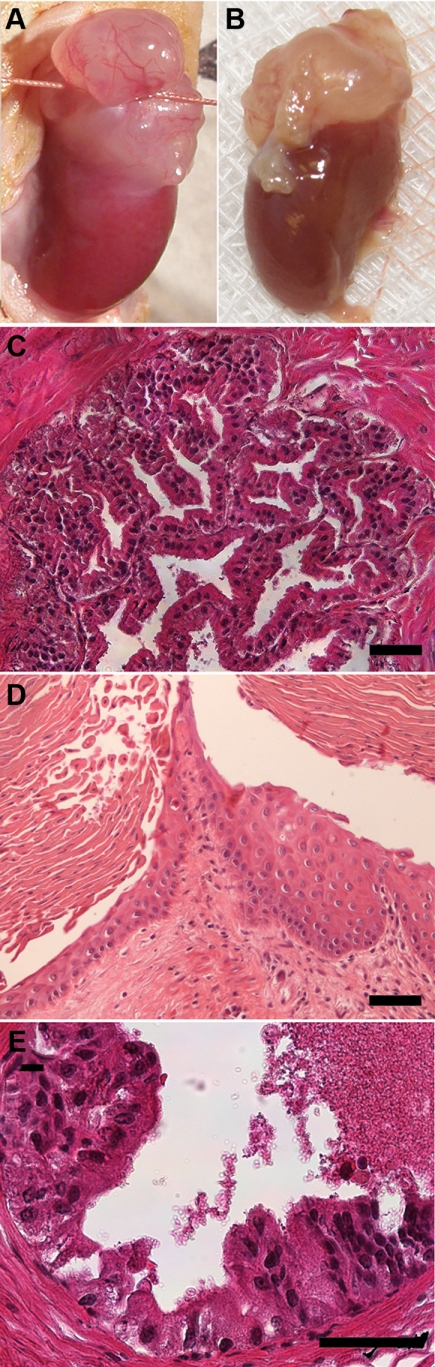
To induce progressive hormonal carcinogenesis in normal human prostate cells, nude mice bearing chimeric prostate grafts were treated with elevated T+E2 over a 1- to 4-month period after the recombinant tissue had fully formed under the renal capsule. In control grafts without steroid pellets for 1–3 months or with T alone for 3 months, the chimeric tissues were histologically normal with no evidence of any prostatic lesion. Subcutaneous pellets of 25 mg of T and 2.5 mg of E2 produced serum steroid levels of 25.4 ± 2.1 ng/ml T and 675 ± 43 pg/ml E2, which is 5- to 9-fold higher than levels measured in untreated male mice as well as human males. A total of 45 grafts were collected at the time of killing after 1–4 months of hormone treatment and histologically examined by a pathologist for evidence of prostate lesions (Table 1). For renal grafts treated with hormones beyond 1 month (n = 35), an aseptic open biopsy was performed at 1, 2, or 3 months after hormone initiation, and the graft was returned to the abdominal cavity for continued growth, thus permitting direct assessment of lesion progression over time (Fig. 6, A and B). One month after initiation of T+E2 treatment, 50% of specimens were graded as normal, whereas 50% contained epithelial hyperplasia and/or squamous metaplasia (SQM) (Fig. 6, C and D). By 2 months of T+E2 treatment and beyond, none of the chimeric prostate grafts were classified as normal, whereas 74% contained SQM, a hallmark lesion of estrogen exposure (Table 1) (46–48). Between 2 and 4 months of treatment with steroids, hyperplasia of the human epithelium, at times atypical, increased from 22% at 2 months to 50% at 3 months and 80% at 4 months with a total incidence of 43% in all examined grafts. HG PIN lesions were first observed in the human epithelium after 2 months of hormone exposure as evidenced by prominent nucleoli (visible at low magnification), nuclear enlargement, hyperchromasia, and overlapping epithelial cells (Fig. 6E). PIN incidence progressively increased over time from 29% at 2 months to 40% at 4 months with an overall HG PIN incidence over 2–4 months of 31%. Several foci of PIN lesions were seen in most grafts when they appeared and were not considered isolated. Importantly, several grafts that contained HG PIN were classified as normal or hyperplastic epithelium in biopsies from prior months (Fig. 6).
Table 1.
Incidence of prostate pathology in human prostate epithelium of chimeric grafts after 1–4 months of T+E2 treatment
| Months | n | Normal | SQM | Hyperplasia | HG PIN | Cancer |
|---|---|---|---|---|---|---|
| 1 | 10 | 50% (5/10) | 30%a (3/10) | 30%a (3/10) | 0% (0/10) | 0% (0/10) |
| 2 | 14 | 0% (0/14) | 93%a (13/14) | 22%a (3/14) | 29% (4/14) | 14% (2/14) |
| 3 | 16 | 0% (0/16) | 69%a (11/16) | 50%a (8/16) | 31% (5/16) | 6% (1/16) |
| 4 | 5 | 0% (0/5) | 40%a (2/5) | 80%a (4/5) | 40%a (2/5) | 20%a (1/5) |
| Cumulative incidence | ||||||
| 2–4 | 35 | 0% (0/35) | 74% (26/35) | 43% (15/35) | 31% (11/35) | 11% (4/35) |
Results show diagnosis of entire graft at final autopsy.
Some specimens contained multiple diagnoses.
Fig. 6.
Graft biopsy after 1 and 2 months of T+E2 exposure revealed SQM and epithelial hyperplasia progressing to HG PIN over time within the same chimeric prostate graft. A, Open biopsy through ligature permitted removal of a portion of the renal graft, which was then returned to the abdominal cavity for continued growth and progression. B, The same graft was removed after 2 months of hormone treatment revealing the continued growth of the chimeric tissue. C, At 1 month of T+E2 exposure, extensive epithelial hyperplasia with infolding of ducts was observed throughout the chimeric graft. D, SQM was frequently observed after 1 month and beyond of T+E2 treatment. E, Histologic examination of the graft shown in B revealed areas of HG PIN with piling and overlapping epithelial cells, nuclear enlargement, hyperchromasia, and prominence of nucleoli. Scale bar, 50 μm.
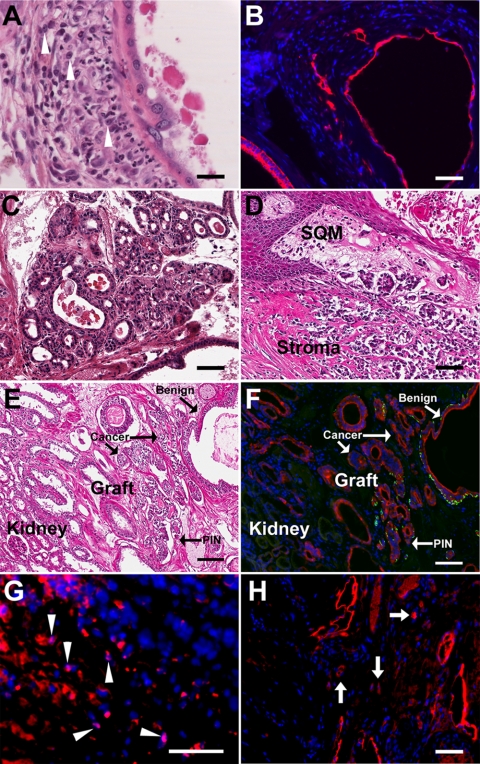
Classification of prostate cancer was assigned to four chimeric grafts collected at 2, 3, and 4 months of T+E2 treatment for an incidence of 11% over the 2- to 4-month period. Two cases at 2 and 3 months were characterized as local microinvasion of neoplastic cells based on nuclear morphology, prominent nucleoli at low magnification, loss of CK14 positive basal cells in neoplastic acini, and presence of CK8/18 positive luminal cells within the stromal region (Fig. 7, A and B). Malignant progression to poorly differentiated adenocarcinoma with invasion to the adjacent kidney paranchyma was observed in two separate chimeric grafts between 2 and 4 months of hormone exposure (Fig. 7, C–H). Earlier biopsies of these grafts had revealed only epithelial hyperplasia or PIN, thus providing evidence of malignant progression over time. Signs of malignancy included irregular small to medium size glandular structures, abortive glandular lumens, back-to-back lumens, infiltrative glands, and loss of the basement membrane and a basal cell layer (Fig. 7, C–E). Immunostaining of the specimens for epithelial-specific CK8/18 and CK14 documented loss of basal cells in the human cancer and kidney invasion of the malignant epithelial cells (Fig. 7F). Immunostaining for human nuclear antigen (Fig. 7G) and PSA (Fig. 7H) in the cancerous regions of the grafts confirmed the human origin of the cancerous cells.
Fig. 7.
Prostate cancer in chimeric prostate renal grafts induced by T+E2 treatment for 2–4 months. A, H&E section of graft exposed to T+E2 for 2 months reveals neoplastic epithelium with enlarged nuclei and prominent nucleoli and their local invasion into the underlying stromal region (cells highlighted with arrowheads). B, CK8/18 immunostained acini shown in A confirms the local invasion of neoplastic cells into the stroma. C and D, Within 2 months of elevated T+E2 exposure, full malignancy was induced in another chimeric renal graft as evidenced by irregular small to medium size glandular structures, abortive glandular lumen, back-to-back lumens, infiltrative glands, and loss of the basement membrane and basal cell layer. Multiple lesions of this type were observed within the graft. E and F, Adjacent sections of a chimeric prostate graft after 4 months of T+E2 treatment with H&E stain (E) and immunofluorescent labeling with CK8/18 (red) and CK14 (green) (F). Staining showed heterogeneous glandular structures with mixture of normal glands, PIN lesions, and carcinoma as well as invasion into kidney. G, Immunostaining with antibody specific to human nuclear antigen (pink nuclei, arrowheads) identifies the human origin of infiltrating epithelial cells (CK8/18+, red) within the stroma. H, Immunolabeling for PSA (red) confirms the human identity of the cancerous ducts and infiltrating cells (arrows) in the grafted tissue. Scale bar, 50 μm.
Discussion
In the present study, we report three major findings that provide an increased understanding of human prostate progenitor cell differentiation and hormone-driven cancer initiation and progression. We have shown for the first time 1) the efficacy of culturing stem/progenitor cells from normal human prostates using a prostasphere assay and the ability to derive normal humanized prostate-like tissues in vivo using a chimeric cell-tissue recombinant renal graft approach; 2) that normal human prostate stem/early progenitor cells express high levels of ER, including ERα, ERβ, and GPR30, and exhibit a proliferative response to 1 nm E2; and 3) that elevated E2 levels in a T-supported milieu can initiate prostate carcinogenesis and promote early progression to adenocarcinoma with an invasive phenotype.
E2 has been classified as a carcinogen by the International Agency for Research on Cancer based on findings in women (49). Although there is accumulating evidence to suggest a central role for estrogens in prostate cancer, direct evidence that estrogens initiate prostate cancer in humans has been elusive. A rising E2:T ratio in aging men (50), association of polymorphisms in estrogen metabolizing genes and urine hydroxy-estrone ratios with higher prostate cancer risk (51, 52), progressive increases in aromatase expression in primary prostate cancers to metastatic prostate cancers (53), and marked alterations in ER expression with cancer progression (3, 5) support the hypothesis that estrogens are involved in the etiology and progression of this disease. Multiple studies over the past several decades using animal models have provided strong evidence of a carcinogenic role for estrogens in the prostate (13, 14, 54, 55), but whether this is directly applicable to the human prostate has not been clarified. To address this issue, we developed an experimental in vivo model using normal human prostate progenitor cells grown as a chimeric prostatic tissue. This novel system permitted the direct assessment of whether E2 was capable of initiating and promoting carcinogenesis in the normal human prostate epithelium. Previous experiments using a tissue recombinant system with BPH-1 cells that are immortalized with large T-antigen and contain chromosomal abnormalities (56) had shown that elevated T+E2 was capable of promoting these preinitiated, yet benign cells into invasive cancers, thus indicating that estrogens can function as a cancer promoter in human prostate epithelium (36, 37). By starting with normal primary PrEC from young, disease-free organ donors, the present study demonstrates for the first time that E2 in a T-supported milieu is sufficient to initiate human PrEC transformation and promote the development of prostatic adenocarcinoma to a locally invasive phenotype. Support that estrogens are the culprit steroid in prostatic hormonal carcinogenesis comes from control grafts without T+E2 or with T implants alone that showed no evidence of prostate pathology after 3 months of growth.
There are a number of possible mechanisms through which estrogens may initiate prostate carcinogenesis and promote progression, and dissection of specific pathways will be directly addressed in future studies using this model. The most obvious mechanism is through direct ER-mediated pathways in prostate epithelium that expresses ERα, ERβ, and GPR30 (3, 57, 58). Augmented signaling through these receptors has been shown to disrupt homeostasis and perturb cell proliferative and apoptotic pathways in a number of estrogen-target tissues and cancers, such as the breast and uterus. Alternatively, because rat UGM cells and some mature rat prostate stromal cells express ERα, but not ERβ (59, 60), it is possible that ERα-mediated signaling in prostatic stroma may contribute to carcinogenesis in the epithelium through paracrine-mediated factors. For example, we have previously shown that early-life estrogens up-regulate stromal Fgf10 expression in rat dorsolateral prostate lobes (61), and paracrine stimulation of murine basal/stem cells by stromal Fgf10 was recently shown to drive adenocarcinoma in a cell recombination model (62). There is also evidence for genotoxic effects of estrogens in several cancers, including the prostate, through formation of catechol estrogens and induction of oxidative stress pathways (63, 64), and it is possible that the elevated estrogen levels used in this study are capable of action through these mechanisms. It deserves mention that increased circulating E2 stimulates the release of pituitary prolactin (PRL) and estrogen effects in prostate dysgenesis, including inflammatory responses may be mediated, in part, through elevation in circulating PRL (65–67). Prostatic, i.e. autocrine, PRL production acting through signal transducer and activator of transcription 5 has been shown to promote human prostate cancer (68) and stimulate basal/stem cell proliferation and prostate carcinogenesis in a novel transgenic murine model (69). Although these data support a role for elevated PRL in prostate carcinogenesis, it is important to note that local PRL production is not under estrogenic regulation.
A critical aspect of the present dataset is the evidence that human prostate stem and early progenitor cells are specific targets for estrogen action in the prostate. The d-7 prostasphere progenitor cells expressed robust levels of estrogen signaling molecules, including ERα, ERβ, and GPR30, that activate common and distinct signaling cascades when liganded by E2. Localization of ERβ in the nucleus, ERα in the nucleus and cytoplasm, and GPR30 in the membrane and cytoplasm provides the progenitor cells with multiple opportunities for responding to estrogens, including rapid membrane-initiated estrogen signaling as well as the classical nuclear transcription factor pathways. That these signaling pathways are functional is supported by their response to 1 nm E2 with increased recruitment of resident stem cells to form prostaspheres as well as increased proliferation producing larger prostaspheres. These findings raise the intriguing possibility that stem and early progenitor cell populations in prostate tissues might be susceptible targets of elevated E2 during the induction of hormonal carcinogenesis. This is particularly appealing in light of the recent evidence that transformation of prostate stem cells is sufficient for prostate cancer initiation in rodent and human models (62, 70). If cancers are seeded by transformed stem cells as the stem cell theory for cancer development posits, an increased number of stem and progenitor cells in response to chronic and/or elevated estrogens would increase prostate cancer risk by the shear presence of more cells available for transformation. Furthermore, it is possible that elevated estrogens, acting through ER signaling pathways in the adult prostate progenitor cells, may directly reprogram or transform these cells, thus rendering them with tumor initiating capacity. Evidence in support of this comes from our studies in rodent models, where developmental estrogen exposures reprogram the prostate, leading to increased basal cell numbers and differentiation defects of the adult epithelium that predispose to dysplasia (71). These ER-dependent responses are mediated through immediate changes in gene expression as well as life-long epigenetic modifications that imprint the developing gland (39, 72, 73).
It is accepted that adult stem cells are involved in normal tissue replenishment, whereas cancer stem cells can support growth and progression of malignancies. Although conventional therapies for prostate cancer eradicate the majority of tumor cells, most patients with advanced prostate cancer progress to androgen-independent, metastatic disease that remains incurable by current treatment strategies. Recent evidence suggests that prostate cancer stem cells, which are AR negative and resistant to androgen/AR-based therapies, may be the underlying cause of disease relapse (74). The therapeutic challenges for prostate cancer may thus involve screening and selecting chemotherapeutic agents that target cancer stem cells. The present findings that human prostate progenitor cells express high levels of ER but no AR and respond to estrogens with increased proliferation sheds a new light on the therapeutic potential for selective ER modulators in the treatment of prostate cancer (75). Although the present studies focused on ER expression in normal prostate progenitor cells, there is a high probability for continued ER expression or perhaps amplified ER levels in diseased prostate stem cells making them likely targets for specific ER antagonists.
The present study documents the efficacy of obtaining prostaspheres containing prostate stem and progenitor cells from normal human prostate specimens that are able to differentiate in vitro as well as reconstitute prostate-like tissue in vivo using the renal graft approach. This approach has previously been used for prostate cancer specimens, benign regions of diseased prostate samples with known field effects (76) as well as from established prostate cancer cell lines (30, 32, 77, 78). That this can be done with relative ease from normal donor prostate specimens provides an opportunity for examining a range of processes during normal human prostate development as well as disease induction and treatment. Although the isolation of prostate stem cells is far more specific using flow cytometry (27, 28, 31), this process results in a relative low cell yield that limits its applications. Witte and co-workers (32), using both approaches simultaneously, have shown that prostate stem cells that express Trop2, CD44, and CD49f markers preferentially exhibited sphere-forming capacity in a 3D culture system. The clear advantage of the latter approach is the expansion of stem cell numbers through self-renewal in culture. Immunolabeling of d-7 prostasphere cells from normal specimens in the present study with multiple stem cell markers revealed that they were committed stem/early progenitor cells that had not begun differentiation into cell lineages. As documented previously in prostate cancer patient samples and cell lines (30, 33, 77, 79), the normal prostaspheres here were capable of cytodifferentiation and functional differentiation in vitro to basal and luminal cell bilayers with lateral outgrowths and lumens. Together, these results indicate that the normal adult prostasphere cells have multipotent differentiation ability. Through the use of the prostate cell-tissue recombinant model, the prostasphere cells from normal human prostate epithelium consistently and reproducibly reconstituted a normal and functional chimeric prostate-like tissue as evidenced by histologic criteria and secretion of PSA. In more than 50 grafts using prostaspheres from multiple organ donors, no instances of prostate pathology of any type have been detected. This model not only affords us the importance of using normal cells of human origin but also allows the in vitro manipulation of stem cells before grafting with resultant impacts observed in vivo using the model presented herein. Although concern has been expressed with regards to the epithelial cell of origin in cross-species recombinants in the murine kidney (80), immunostaining for human nuclear antigen and PSA confirmed the human origin of the prostate epithelium in both the normal as well as malignant tissues in the present studies.
In summary, we document the efficacy of deriving large numbers of prostate stem/early progenitor cells from normal adult prostate glands and using these to generate normal humanized prostate-like tissue in vivo. With these in vitro and in vivo models, we demonstrate that estrogens in an androgen-supported milieu are capable of prostate cancer initiation and progression to adenocarcinoma. Robust expression of ERα, ERβ, and GPR30 in the human prostate progenitor cells and their proliferative response to E2 indicates that prostate stem cells and early progenitor cells may be direct targets for estrogen-induced carcinogenesis. Detailing conversion and progression of normal prostate stem/progenitor cells to cancerous cells in response to steroids will enable a thorough understanding of prostate carcinogenesis, which may lead to a more targeted means of developing future therapeutics.
Supplementary Material
Acknowledgments
We thank Larisa Nonn, Ph.D., for assistance in establishing primary cultures of human PrEC and Lynn Birch with help in manuscript preparation.
This work was supported by National Institute of Environmental Health Sciences Grants RC2-ES018758 and R01-ES-015584. The University of Virginia Center for Research in Reproduction RIA core services were supported by National Institute of Child Health and Human Development Grant U54-HD28934.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AR
- Androgen receptor
- CK
- cytokeratin
- 2D
- two dimensional
- 3D
- three dimensional
- E2
- estradiol-17β
- ER
- estrogen receptor
- GAPDH
- glyceraldehyde-3-phosphate dehydrogenase
- GPR
- G protein-coupled receptor
- H&E
- hematoxylin and eosin
- HG
- high grade
- ICC
- immunocytochemistry
- IHC
- immunohistochemistry
- PIN
- prostate intraepithelial neoplasia
- PR
- progesterone receptor
- PrEC
- prostate epithelial cell
- PrEGM
- Prostate Epithelial Cell Growth Medium
- PRL
- prolactin
- PSA
- prostate-specific antigen
- SQM
- squamous metaplasia
- T
- testosterone
- UGM
- urogenital sinus mesenchyme.
References
- 1. Prins GS. 1997. Developmental estrogenization of the prostate gland. In: Naz RK. ed. Prostate: basic and clinical aspects. Chap 10 Boca Raton, FL:CRC Press; 247–265 [Google Scholar]
- 2. Prins GS, Birch L, Tang WY, Ho SM. 2007. Developmental estrogen exposures predispose to prostate carcinogenesis with aging. Reprod Toxicol 23:374–382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Prins GS, Korach KS. 2008. The role of estrogens and estrogen receptors in normal prostate growth and disease. Steroids 73:233–244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ellem SJ, Risbridger GP. 2007. Treating prostate cancer: a rationale for targeting local oestrogens. Nat Rev 7:621–627 [DOI] [PubMed] [Google Scholar]
- 5. Leung YK, Lam HM, Wu S, Song D, Levin L, Cheng L, Wu CL, Ho SM. 2010. Estrogen receptor β2 and β5 are associated with poor prognosis in prostate cancer, and promote cancer cell migration and invasion. Endocr-Relat Cancer 17:675–689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pienta KJ, Abate-Shen C, Agus DB, Attar RM, Chung LW, Greenberg NM, Hahn WC, Isaacs JT, Navone NM, Peehl DM, Simons JW, Solit DB, Soule HR, VanDyke TA, Weber MJ, Wu L, Vessella RL. 2008. The current state of preclinical prostate cancer animal models. Prostate 68:629–639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. De Marzo AM, Coffey DS, Nelson WG. 1999. New concepts in tissue specificity for prostate cancer and benign prostatic hyperplasia. Urology 53:29–42 [DOI] [PubMed] [Google Scholar]
- 8. Shirai T, Takahashi S, Cui L, Futakuchi M, Kato K, Tamano S, Imaida K. 2000. Experimental prostate carcinogenesis—rodent models. Mutat Res 462:219–226 [DOI] [PubMed] [Google Scholar]
- 9. Gabril MY, Duan W, Wu G, Moussa M, Izawa JI, Panchal CJ, Sakai H, Xuan JW. 2005. A novel knock-in prostate cancer model demonstrates biology similar to that of human prostate cancer and suitable for preclinical studies. Mol Ther 11:348–362 [DOI] [PubMed] [Google Scholar]
- 10. Asamoto M, Hokaiwado N, Cho YM, Takahashi S, Ikeda Y, Imaida K, Shirai T. 2001. Prostate carcinomas developing in transgenic rats with SV40 T antigen expression under probasin promoter control are strictly androgen dependent. Cancer Res 61:4693–4700 [PubMed] [Google Scholar]
- 11. Pollard M, Luckert PH. 1986. Production of autochthonous prostate cancer in Lobund-Wistar rats by treatments with N-nitroso-N-methylurea and testosterone. J Natl Cancer Inst 77:583–587 [PubMed] [Google Scholar]
- 12. Slayter MV, Anzano MA, Kadomatsu K, Smith JM, Sporn MB. 1994. Histogenesis of induced prostate and seminal vesicle carcinoma in Lobund-Wistar rats: a system for histological scoring and grading. Cancer Res 54:1440–1445 [PubMed] [Google Scholar]
- 13. Dunning W. 1963. Prostate cancer in the rat. In: Vollmer E. ed. Biology of the prostae and related tissues. Bethesda, MD: National Cancer Institute; 351–364 [PubMed] [Google Scholar]
- 14. Noble RL. 1980. Production of Nb rat carcinomas of the dorsal prostate and response of estrogen-dependent transplants to sex hormones and tamoxifen. Cancer Res 40:3547–3550 [PubMed] [Google Scholar]
- 15. Zhao H, Nolley R, Chen Z, Peehl DM. 2010. Tissue slice grafts: an in vivo model of human prostate androgen signaling. Am J Pathol 177:229–239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gleave ME, Hsieh JT, Wu HC, von Eschenbach AC, Chung LW. 1992. Serum prostate specific antigen levels in mice bearing human prostate LNCaP tumors are determined by tumor volume and endocrine and growth factors. Cancer Res 52:1598–1605 [PubMed] [Google Scholar]
- 17. Sobel RE, Sadar MD. 2005. Cell lines used in prostate cancer research: a compendium of old and new lines—part 1. J Urol 173:342–359 [DOI] [PubMed] [Google Scholar]
- 18. Corey E, Quinn JE, Buhler KR, Nelson PS, Macoska JA, True LD, Vessella RL. 2003. LuCaP 35: a new model of prostate cancer progression to androgen independence. Prostate 55:239–246 [DOI] [PubMed] [Google Scholar]
- 19. Presnell SC, Petersen B, Heidaran M. 2002. Stem cells in adult tissues. Semin Cell Dev Biol 13:369–376 [DOI] [PubMed] [Google Scholar]
- 20. Beachy PA, Karhadkar SS, Berman DM. 2004. Tissue repair and stem cell renewal in carcinogenesis. Nature 432:324–331 [DOI] [PubMed] [Google Scholar]
- 21. Smith S, Neaves W, Teitelbaum S. 2007. Adult versus embryonic stem cells: treatments. Science 316:1422–1423 [DOI] [PubMed] [Google Scholar]
- 22. Kasper S. 2008. Exploring the origins of the normal prostate and prostate cancer stem cell. Stem Cell Rev 4:193–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Miki J, Rhim J. 2008. Prostate cell cultures as in vitro models for the study of normal stem cells and cancer stem cells. Prostate Cancer P D 11:32–39 [DOI] [PubMed] [Google Scholar]
- 24. Asselin-Labat ML, Vaillant F, Sheridan JM, Pal B, Wu D, Simpson ER, Yasuda H, Smyth GK, Martin TJ, Lindeman GJ, Visvader JE. 2010. Control of mammary stem cell function by steroid hormone signalling. Nature. 465:798–802 [DOI] [PubMed] [Google Scholar]
- 25. Joshi PA, Jackson HW, Beristain AG, Di Grappa MA, Mote PA, Clarke CL, Stingl J, Waterhouse PD, Khokha R. 2010. Progesterone induces adult mammary stem cell expansion. Nature. 465:803–807 [DOI] [PubMed] [Google Scholar]
- 26. Gu G, Yuan J, Wills M, Kasper S. 2007. Prostate cancer cells with stem cell characteristics reconstitute the original human tumor in vivo. Cancer Res 67:4708–4715 [DOI] [PubMed] [Google Scholar]
- 27. Leong KG, Wang BE, Johnson L, Gao WQ. 2008. Generation of a prostate from a single cell. Nature 456:804–808 [DOI] [PubMed] [Google Scholar]
- 28. Vander Griend DJ, Karthaus WL, Dalrymple S, Meeker A, DeMarzo AM, Isaacs JT. 2008. The role of CD133 in normal human prostate stem cells and malignant cancer-initiating cells. Cancer Res 68:9703–9711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Isaacs JT, Coffey DS. 1989. Etiology and disease process of benign prostatic hyperplasia. Prostate Suppl 2:33–50 [DOI] [PubMed] [Google Scholar]
- 30. Lang SH, Stark M, Collins A, Paul AB, Stower MJ, Maitland NJ. 2001. Experimental prostate morphogenesis in response to stroma and three dimensional matrigel culture. Cell Growth Differ 12:631–640 [PubMed] [Google Scholar]
- 31. Goldstein AS, Lawson DA, Cheng D, Sun W, Garraway IP, Witte ON. 2008. Trop2 identifies a subpopulation of murine and human prostate basal cells with stem cell characteristics. Proc Natl Acad Sci USA 105:20882–20887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Garraway IP, Sun W, Tran CP, Perner S, Zhang B, Goldstein AS, Hahm SA, Haider M, Head CS, Reiter RE, Rubin MA, Witte ON. 2010. Human prostate sphere-forming cells represent a subset of basal epithelial cells capable of glandular regeneration in vivo. Prostate 70:491–501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Xin L, Lukacs RU, Lawson DA, Cheng D, Witte ON. 2007. Self-renewal and multilineage differentiation in vitro from murine prostate stem cells. Stem Cells 25:2760–2769 [DOI] [PubMed] [Google Scholar]
- 34. Goldstein AS, Stoyanova T, Witte ON. 2010. Primitive origins of prostate cancer: in vivo evidence for prostate-regenerating cells and prostate-cancer initiating cells. Mol Oncol 4:385–396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Taylor RA, Cowin PA, Cunha GR, Pera M, Trounson AO, Pedersen J, Risbridger GP. 2006. Formation of human prostate tissue from embryonic stem cells. Nat Methods 3:179–181 [DOI] [PubMed] [Google Scholar]
- 36. Hayward SW, Wang Y, Cao M, Hom YK, Zhang B, Grossfeld GD, Sudilovsky D, Cunha GR. 2001. Malignant transformation in a nontumorigenic human prostatic epithelial cell line. Cancer Res 61:8135–8142 [PubMed] [Google Scholar]
- 37. Ricke W, Ishii K, Ricke EA, Simko J, Wang Y, Hayward SW, Cunha GR. 2006. Steroid hormones stimulate human prostate cancer progression and metastasis. Int J Cancer 52 118:2123–2131 [DOI] [PubMed] [Google Scholar]
- 38. Noble RL. 1977. The development of prostatic adenocarcinoma in Nb rats following prolonged sex hormone administration. Cancer Res 37:1929–1933 [PubMed] [Google Scholar]
- 39. Ho SM, Tang WY, Belmonte de Frausto J, Prins GS. 2006. Developmental exposure estradiol and bisphenol A (BPA) increases susceptibility to prostate carcinogenesis and epigenetically regulates phosphodiesterase type 4 variant (PDE4D4) in the rat prostate. Cancer Res 66:5624–5632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lang SH, Smith J, Hyde C, Macintosh C, Stower MJ, Maitland NJ. 2006. Differentiation of prostate epithelial cell cultures by matrigel/stromal cell glandular reconstruction. In Vitro Cell Dev Biol 42:273–280 [DOI] [PubMed] [Google Scholar]
- 41. Lukacs RU, Goldstein AS, Lawson DA, Cheng D, Witte ON. 2010. Isolation, cultivation and characterization of adult murine prostate stem cells. Nat Protoc 5:702–713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Cunha G, Lung B, Reese B. 1980. Glandular epithelial induction by embryonic mesenchyme in adult bladder epithelium of Balb/C mice. J Invest Urol 17:302–304 [PubMed] [Google Scholar]
- 43. Huang L, Pu Y, Hu WY, Birch L, Luccio-Camelo DC, Yamaguchi TP, Prins GS. 2009. The role of Wnt5a in prostate gland development. Dev Biol 358:188–199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Richardson GD, Robson CN, Lang SH, Neal DE, Maitland NJ, Collins AT. 2004. CD133, a novel marker for human prostatic epithelial stem cells. J Cell Sci 117:3539–3545 [DOI] [PubMed] [Google Scholar]
- 45. Draper JS, Pigott C, Thomson JA, Andrews PW. 2002. Surface antigens of human embryonic stem cells: changes upon differentiation in culture. J Anat 200:249–258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Price D. 1936. Normal development of the prostate and seminal vesicles of the rat with a study of experimental postnatal modifications. Am J Anat 60:79–127 [Google Scholar]
- 47. Coffey D, Walsh P. 1990. Clinical and experimental studies of benign prostatic hyperplasia. Urol Clin No Am 17:461–475 [PubMed] [Google Scholar]
- 48. Levine AC, Kirschenbaum A, Droller M, Gabrilove JL. 1991. Effect of the addition of estrogen to medical castration on prostatic size, symptoms, histology and serum prostate specific antigen in 4 men with benign prostatic hypertrophy. J Urology 146:790–793 [DOI] [PubMed] [Google Scholar]
- 49. IARC 1992. Postmenopausal estrogen therapy. IARC Monog 72:399–530 [Google Scholar]
- 50. Vermeulen A, Kaufman JM, Goemaere S, van Pottelberg I. 2002. Estradiol in elderly men. Aging Male 5:98–102 [PubMed] [Google Scholar]
- 51. Cussenot O, Azzouzi AR, Nicolaiew N, Fromont G, Mangin P, Cormier L, Fournier G, Valeri A, Larre S, Thibault F, Giordanella JP, Pouchard M, Zheng Y, Hamdy FC, Cox A, Cancel-Tassin G. 2007. Combination of polymorphisms from genes related to estrogen metabolism and risk of prostate cancers: the hidden face of estrogens. J Clin Oncol 25:3596–3602 [DOI] [PubMed] [Google Scholar]
- 52. Carruba G. 2007. Estrogen and prostate cancer: an eclipsed truth in an androgen-dominated scenario. J Cell Biochem 102:899–911 [DOI] [PubMed] [Google Scholar]
- 53. Montgomery RB, Mostaghel EA, Vessella R, Hess DL, Kalhorn TF, Higano CS, True LD, Nelson PS. 2008. Maintenance of intratumoral androgens in metastatic prostate cancer: a mechanism for castration-resistant tumor growth. Cancer Res 68:4447–4454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Leav I, Merk FB, Kwan PW, Ho SM. 1989. Androgen-supported estrogen-enhanced epithelial proliferation in the prostates of intact Noble rats. Prostate 15:23–40 [DOI] [PubMed] [Google Scholar]
- 55. Bosland MC, Ford H, Horton L. 1995. Induction at high incidence of ductal prostate adenocarcinomas in NBL/Cr and Sprague-Dawley Hsd:SD rats treated with a combination of testosterone and estradiol-17b or diethylstilbestrol. Carcinogenesis 16:1311–1317 [DOI] [PubMed] [Google Scholar]
- 56. Hayward SW, Dahiya R, Cunha GR, Bartek J, Deshpande N, Narayan P. 1995. Establishment and characterization of an immortalized but non-transformed human prostate epithelial cell line: BPH-1. In Vitro Cell Dev Biol Anim. 31:14–24 [DOI] [PubMed] [Google Scholar]
- 57. Chan QK, Lam HM, Ng CF, Lee AY, Chan ES, Ng HK, Ho SM, Lau KM. 2010. Activation of GPR30 inhibits the growth of prostate cancer cells through sustained activation of Erk1/2, c-jun/c-fos-dependent upregulation of p21, and induction of G(2) cell-cycle arrest. Cell Death Differ 17:1511–1523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Mak P, Leav I, Pursell B, Bae D, Yang X, Taglienti CA, Gouvin LM, Sharma VM, Mercurio AM. 2010. ERβ impedes prostate cancer EMT by destabilizing HIF-1α and inhibiting VEGF-mediated snail nuclear localization: implications for Gleason grading. Cancer Cell 17:319–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Prins GS, Birch L. 1997. Neonatal estrogen exposure up-regulates estrogen receptor expression in the developing and adult rat prostate lobes. Endocrinology 138:1801–1809 [DOI] [PubMed] [Google Scholar]
- 60. Prins GS, Marmer M, Woodham C, Chang W, Kuiper G, Gustafsson JA, Birch L. 1998. Estrogen receptor-b messenger ribonucleic acid ontogeny in the prostate of normal and neonatally estrogenized rats. Endocrinology 139:874–883 [DOI] [PubMed] [Google Scholar]
- 61. Huang L, Pu Y, Alam S, Birch L, Prins GS. 2005. The role of Fgf10 signaling in branching morphogenesis and gene expression in the rat prostate gland: lobe-specific supression by neonatal estrogens. Dev Biol 278:396–414 [DOI] [PubMed] [Google Scholar]
- 62. Lawson DA, Zong Y, Memarzadeh S, Xin L, Huang J, Witte ON. 2010. Basal epithelial stem cells are efficient targets for prostate cancer initiation. Proc natl SAcad Sci USA 107:2610–2615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Cavalieri EL, Rogan EG. 2010. Depurinating estrogen–DNA adducts in the etiology and prevention of breast and other human cancers. Future Oncol 6:75–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Tam NN, Leav I, Ho SM. 2007. Sex hormones induce direct epithelial and inflammation-mediated oxidative/nitrosative stress that favors prostatic carcinogenesis in the noble rat. Am J Pathol 171:1334–1341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Lane KE, Leav I, Ziar J, Bridges RS, Rand WM, Ho SM. 1997. Suppression of testosterone and estradiol-17β-induced dysplasia in the dorsolateral prostate of Noble rats by bromocriptine. Carcinogenesis 18:1505–1510 [DOI] [PubMed] [Google Scholar]
- 66. Gilleran JP, Putz O, DeJong M, DeJong S, Birch L, Pu Y, Huang L, Prins GS. 2003. The role of prolactin in the prostatic inflammatory response to neonatal estrogen. Endocrinology 144:2046–2054 [DOI] [PubMed] [Google Scholar]
- 67. Tam NN, Szeto CY, Freudenberg JM, Fullenkamp AN, Medvedovic M, Ho SM. 2010. Research resource: estrogen-driven prolactin-mediated gene-expression networks in hormone-induced prostatic intraepithelial neoplasia. Mol Endocrinol 11:2207–2217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Dagvadorj A, Kirken RA, Leiby B, Karras J, Nevalainen MT. 2008. Transcription factor signal transducer and activator of transcription 5 promotes growth of human prostate cancer cells in vivo. Clin Cancer Res 14:1317–1324 [DOI] [PubMed] [Google Scholar]
- 69. Rouet V, Bogorad RL, Kayser C, Kessal K, Genestie C, Bardier A, Grattan DR, Kelder B, Kopchick JJ, Kelly PA, Goffin V. 2010. Local prolactin is a target to prevent expansion of basal/stem cells in prostate tumors. Proc Natl Acad Sci USA 107:15199–15204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Goldstein AS, Huang J, Guo C, Garraway IP, Witte ON. 2010. Identification of a cell of origin for human prostate cancer. Science 329:568–571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Prins GS, Birch L, Habermann H, Chang WY, Tebeau C, Putz O, Bieberich C. 2001. Influence of neonatal estrogens on rat prostate development. Reprod Fertil Dev 13:241–252 [DOI] [PubMed] [Google Scholar]
- 72. Prins GS, Birch L, Couse JF, Choi I, Katzenellenbogen B, Korach KS. 2001. Estrogen imprinting of the developing prostate gland in mediated through stromal estrogen receptor a: studies with aERKO and bERKO mice. Canc Res 61:6089–6097 [PubMed] [Google Scholar]
- 73. Prins GS, Ho SM. 2010. Early life estrogens and prostate cancer in an animal model. J Dev Origins Health Dis 1:365–370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Maitland NJ, Collins AT. 2008. Prostate cancer stem cells: a new target for therapy. J Clin Oncol 26:2862–2870 [DOI] [PubMed] [Google Scholar]
- 75. Price D, Stein B, Sieber P, Tutrone R, Bailen J, Goluboff E, Burzon D, Bostwick D, Steiner M. 2006. Toremifene for the prevention of prostate cancer in men with high grade prostatic intraepithelial neoplasia: results of a double-blind, placebo controlled, phase IIB clinical trial. J Urol 176:965–970 [DOI] [PubMed] [Google Scholar]
- 76. Nonn L, Ananthanarayanan V, Gann PH. 2009. Evidence for field cancerization of the prostate. Prostate 69:1470–1479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Chu JH, Yu S, Hayward SW, Chan FL. 2009. Development of a three-dimensional culture model of prostatic epithelial cells and its use for the study of epithelial-mesenchymal transition and inhibition of PI3K pathway in prostate cancer. Prostate 69:428–442 [DOI] [PubMed] [Google Scholar]
- 78. Dubrovska A, Kim S, Salamone RJ, Walker JR, Maira SM, García-Echeverría C, Schultz PG, Reddy VA. 2009. The role of PTEN/Akt/PI3K signaling in the maintenance and viability of prostate cancer stem-like cell populations. Proc Natl Acad Sci USA 106:269–273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Bisson I, Prowse DM. 2009. Wnt signaling regulates self-renewal and differentiation of prostate cancer cells with stem cell characteristics. Cell Res 19:683–697 [DOI] [PubMed] [Google Scholar]
- 80. Vander Griend DJ, Konishi Y, De Marzo AM, Isaacs JT, Meeker AK. 2009. Dual-label centromere and telomere FISH identifies human, rat, and mouse cell contribution to multispecies recombinant urogenital sinus xenografts. Prostate 69:1557–1564 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.