IREα/XBP1a pathway is linked with the insulin-dependent hepatic lipogenesis.
Abstract
The role of the IRE1α-X-box-binding protein 1 (XBP1) pathway in the insulin-mediated hepatic lipogenic program and associated mechanisms were investigated in this study. We observed that phosphorylation of IRE1α (an upstream activator of XBP1) and splicing (activation) of XBP1 were elevated in the liver of the C57BL/6 mice with insulin resistance/hyperinsulinemia induced by high-fat diet. Treatment of nonobese diabetic mice with insulin activated hepatic XBP1. In cultured primary mouse hepatocytes, prolonged exposure to insulin induced IRE1α phosphorylation and XBP1 splicing significantly in the presence of insulin resistance. Overexpression of the activated XBP1 elevated the promoter activities of the sterol regulatory element-binding protein (SREBP)-1c and fatty acid synthase (FAS) genes. Knockdown of either the IRE1α or XBP1 gene by small interfering RNA prevented the insulin-stimulated promoter activities of both SREBP-1 and FAS genes. In investigating the associated mechanisms, we found a direct interaction between XBP1 and SREBP-1 promoter detected by the chromatin immunoprecipitation assays. Furthermore, the XBP1-mediated stimulation of the FAS promoter was eliminated by knocking down the SREBP-1c gene (Srebf1). Finally, we observed that insulin activation of the IRE1α-XBP1 pathway was prevented by inhibition of mammalian target of rapamycin-dependent protein synthesis. In conclusion, our results show that the IRE1α-XBP1-mediated unfolded protein response pathway is an integrated part of the insulin-induced hepatic lipogenic program and functions at an increased basal level in the presence of insulin resistance and hyperinsulinemia. Besides, the insulin-mediated protein synthesis is tightly connected with the insulin-mediated lipogenic program.
Lipogenesis is necessary for fat accumulation. Fat accumulation, in particular ectopic fat accumulation, is a critical/necessary component of insulin resistance (1, 2). Insulin resistance and its associated hyperinsulinemia are either a precursor or key component of numerous modern health problems, such as obesity, metabolic syndrome, type 2 diabetes mellitus, cardiovascular disorders, nonalcoholic fatty liver disease, Alzheimer's disease, asthma, some cancers, and aging (3–11). Although it is well established that fat accumulation in any place requires insulin, there are still significant gaps in understanding the insulin-mediated hepatic lipogenesis.
The IRE1α-X-box-binding protein 1 (XBP1) is one of the three pathways that activate the unfolded protein response (UPR) in the endoplasmic reticulum (ER) (12–14). UPR is required for resolving the overloading of ER due to increased unfolded or misfolded proteins (13, 14). The unresolved ER overloading may lead to the so-called ER stress, which has been shown to be associated with insulin resistance (12, 14). Decreased UPR capacity by genetically knocking down the IRE1α-XBP1 pathway can also lead to ER stress and insulin resistance (12). Importantly, it is known that ER stress is closely connected with lipogenesis (13, 14). The connection between ER stress and lipogenesis is reciprocal. Lipids are known activators of ER stress, i.e. lipid accumulation can activate ER stress, whereas ER stress in turn can promote lipogenesis (14). Knockdown of a key UPR gene, XBP1 gene, cannot only induce ER stress through decreasing the capacity of UPR but also lead to decreased lipogenesis and hypolipidemia (15, 16), suggesting a critical role for XBP1 in lipogenesis. Nevertheless, the role of XBP1 in the insulin-mediated hepatic lipogenesis and associated mechanisms remain to be investigated. In this study, we have studied the role of IRE1α-XBP1 pathway in the insulin-mediated hepatic lipogenesis and associated mechanisms.
Materials and Methods
Antibodies and reagents
Antibody against phospho-IRE1α (catalog no. PA1-16927) was purchased from Thermo Fisher Scientific, Inc. (Waltham, MA). Antibodies against XBP1 (catalog no. sc-7160) and poly(ADP-ribose) polymerase 1 (PARP1) (catalog no. sc-7150) were from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). Antibodies against IRE1α (catalog no. 3294), phospho-phosphatidylinositol 3 kinase (PI3K) p85 (catalog no. 4228), total/phospho-serine-threonine kinase (Akt) (serine 473/308, catalog nos. 9271 and 9275, respectively) were purchased from Cell Signaling Technology (Danvers, MA). Antibody against sterol regulatory element-binding protein (SREBP)-1 (catalog no. ab44153) was from Abcam (Cambridge, MA). The triglyceride (TG) assay kit (catalog no. TR0100), the recombinant human insulin (catalog no. I9278), and antisera against β-actin were from Sigma (St. Louis, MO). Plasma insulin levels were measured with a Linco insulin enzyme-linked immunosorbent assay kit (Linco Research, St. Charles, MO). Protein assay kits were from Bio-Rad (Hercules, CA). Rapamycin was from Calbiochem (catalog no. 553210; Calbiochem, La Jolla, CA). Other materials were all obtained commercially and are of analytical quality.
Animal experiments
Animals were housed under the usual day (12-h daylight) and night (12-h darkness) circadian rhythm and fed ad libitum. The following animal experiments were included. 1) Obese/hyperinsulinemia model. C57BL/6 (B6) mice were fed with either the normal rodent chow diet (ND) or the high-fat diet (HFD) (Research Diets catalog no. D12330: 58.0 kcal % fat, 16.0 kcal % protein, and 26 kcal % carbohydrate) for 8 wk. 2) Insulin-deficient mice with prolonged exposure to insulin. Nonobese diabetic (NOD)/ShiLtj mice were purchased from The Jackson Laboratory (Bar Harbor, ME). The diabetic NOD mice were treated with either Detemir or the vehicle solution (saline, 100 μl) via sc injections once every 12 h for 2 wk. Euglycemia was reached and maintained for at least 2 d in each animal with various amount of Detemir. Application of Detemir was similar to the so-called current conventional treatment of human type 1 diabetes mellitus. All animal studies were approved by the Institutional Animal Care and Use Committee of The Hamner Institutes for Health Sciences and fully complied with the guidelines from the National Institutes of Health.
Cells
Mouse primary hepatocytes were isolated from B6 mice that were fed with ND and cultured in Williams' medium E supplemented with 10% fetal bovine serum as previously described (11–13). Mouse hepatoma 1c1c7 cells were maintained in high glucose DMEM supplemented with 10% fetal bovine serum.
DNA transfection, small interfering RNA (siRNA) and luciferase assay, and lentiviral infection
DNA plasmids were introduced into the indicated cells by Lipofectamine2000 transfection reagent (Invitrogen, Carlsbad, CA). To knock down cognate mRNA expression, the pLentiLox3.7 short hairpin RNA expression system with the U6-RNA promoter was employed as previously described. The sequences used for targeting IRE1α and XBP1 were AAGGCGATGATCTCTGACTTT and GGGATTCATGAATGGCCCTTA, respectively. The siRNA pool to knock down SREBP-1 (catalog no. sc-36558) was purchased from Santa Cruz Biotechnology, Inc. Promoter activity was detected by a luciferase assay system (Promega, Madison, WI) with a Wallac 1420 Multilabel Counter (PerkinElmer Life Sciences, Waltham, MA) and normalized to the protein level.
Immunoblotting
Immunoblotting was performed with standard procedures. In brief, cells were lysed in Triton X-100 lysis buffer [1% Triton X-100, 150 mm NaCl, 10% glycerol, 2 mm EDTA, 20 mm Tris (pH 8.0), 1 mm dithiothreitol, 1 mm sodium orthovanadate, 1 mm phenylmethylsulfonyl fluoride, 2 μg/ml leupeptin, and 10 μg/ml aprotinin]. Cell lysates (15 μg/lane) were resolved in 4–20% Tris/glycine gels (Invitrogen) and transferred to nitrocellulose membranes (Bio-Rad). Target proteins were detected by immunoblotting with primary antibodies as indicated and alkaline phosphatase-conjugated secondary antisera. The fluorescent bands were visualized with a Typhoon 9410 variable mode imager (GE Healthcare, Princeton, NJ) and then quantified by densitometry using ImageQuant 5.2 software (GE Healthcare).
RNA extraction and real-time PCR
Total RNA were extracted from cells or tissues with an RNeasy mini kit (QIAGEN, Valencia, CA) and reverse transcribed into cDNA, which were quantified by TaqMan real-time PCR with specific probes and primers listed in the Supplemental Table 1 (published on The Endocrine Society's Journals Online web site at http://endo.endojournals.org) normalized to levels of β-actin and/or 36B4.
Fractionation of nuclear and cytosolic extracts
Liver tissues and hepatocytes were homogenized and washed twice with the homogenization buffer. The pellet was resuspended in ice-chilled buffer I [0.33 m sucrose, 10 mm HEPES (pH7.4), 1 mm MgCl2, and 0.1% Triton X-100] with protease inhibitors in 5:1 (buffer:pellet). After incubating on ice for 15 min, the cytosolic supernatants were collected by centrifugation at 3000 rpm for 5 min (4 C). A pure preparation of nuclei without the nuclear membrane was resuspended in ice-cold buffer II [0.45 m NaCl and 10 mm HEPES (pH 7.4)] containing protease inhibitors. After incubating the suspension on ice for 15 min, nuclear extract was collected by centrifugation at maximum speed for 5 min at 4 C.
Chromatin immunoprecipitation (ChIP) assays
ChIP assays were performed using the EZ ChIP kit from Upstate (Lake placid, NY). Briefly, SREBP-1 promoter plasmids were introduced into hep1c1c7 hepatocytes by using Lipofectamine2000 transfection reagent (Invitrogen). Cells were cross-linked with 1% formaldehyde and lysed in sodium dodecyl sulfate lysis buffer supplemented with the protease inhibitors. Sheared and precleaned chromatin samples were precipitated with the anti-XBP1 antisera (sc-7160; Santa Cruz Biotechnology, Inc.). Purified antibody-bound protein/DNA complexes were analyzed by PCR with primers amplifying the mouse Srebf1 promoter fragment containing the CCACG-box (forward, 5′-GTCTGAGCAAGGCAGCTTCT-3′ and reverse, 5′-GCCAGGTGCCTGAATA AAGA-3′), generating a 211-bp product. PCR products were visualized in 2% agarose gels. The antiacetyl histone H3 antibody as a positive control and normal rabbit IgG as a negative control were included. Aliquots of chromatin samples without the treatment with antibodies were used as an input control.
Statistical analysis
Data are presented as the mean ± se. Data were compared by Student's t test using GraphPad Prism version 5.0 for Windows (GraphPad, San Diego, CA). Differences at values of P < 0.05 were considered significant.
Results
Hepatic lipogenic program and the IRE1α-XBP1 pathway are both activated in mice with insulin resistance/hyperinsulinemia induced by the HFD
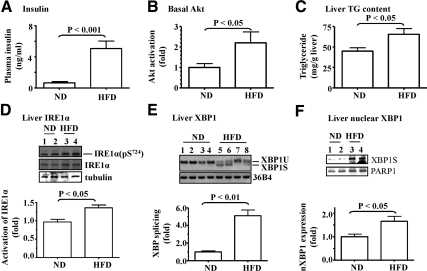
To determine the activity level of the IRE1α-XBP1 pathway in the liver of the mice with elevated hepatic lipogenesis, B6 mice were fed with the HFD for 8 wk, followed by evaluations of plasma level of insulin, basal level of hepatic Akt phosphorylation, hepatic TG content, and activations of IRE1α and XBP1 in the liver. As shown in Fig. 1A, the fasting plasma insulin level was significantly elevated by the HFD. In these same animals, we previously showed that these mice had obvious insulin resistance measured by insulin tolerance test (Supplemental Fig. 1) (17). The basal level of hepatic Akt phosphorylation was increased by the HFD (Fig. 1B), implying that hepatic insulin signaling was continuously activated by the HFD. The hepatic TG content was also increased by the HFD as predicted (Fig. 1C). To examine the status of hepatic lipogenic program, expression levels of key lipogenic genes were evaluated. As shown in Supplemental Fig. 2, transcripts of SREBP-1c, SREBP-2, fatty acid synthase (FAS), and acetyl coenzyme A carboxylases were all increased by the HFD, indicating elevated hepatic lipogenesis.
Fig. 1.
IRE1α-XBP1 pathway was activated in the liver of mice with insulin resistance and hyperinsulinemia induced by the HFD. C57BL/6 (B6) mice (8–10 wk) were fed with ND (n = 8) or HFD (n = 7) for 8 wk. A, Plasma insulin level was evaluated after an overnight fast. Basal Akt activation (B) and TG content (C) in the liver were measured. D, IRE1α phosphorylation at serine 724 in the liver was detected by immunoblotting with the indicated antibodies. E, The spliced form of XBP1 mRNA in the liver was evaluated by RT-PCR. The transcript 36B4 was used as a loading control. F, Nuclear XBP1 (nXBP1) in the liver was estimated with immunoblotting with the anti-XBP1 antibody and normalized to a housekeeping nuclear protein PARP1. Data represent mean ± sem.
Next, activity level of the IRE1α-XBP1 pathway was measured. As shown in Fig. 1D, phosphorylation (activation) level of IRE1α was significantly increased in the liver by the HFD. It is noteworthy that IRE1α protein level appeared to be increased slightly by the HFD. IRE1α is a bifunctional transmembrane kinase with both protein kinase and endoribonuclease activity for processing the splicing (activation) of the XBP1 mRNA (10, 15). The splicing (activation) of the XBP1 mRNA was also significantly elevated in the liver by the HFD (Fig. 1E). Further analysis revealed that the protein level of the spliced XBP1 (XBP1S) was significantly augmented in the nuclear extracts of the liver samples from the mice on the HFD (Fig. 1F). These results show that the IRE1α-XBP1 pathway, together with hepatic lipogenic program, is stimulated the liver of mice with the HFD-induced insulin resistance and hyperinsulinemia.
Hepatic lipogenic program and XBP1 activation are elevated by the chronic exposure to insulin in NOD mice
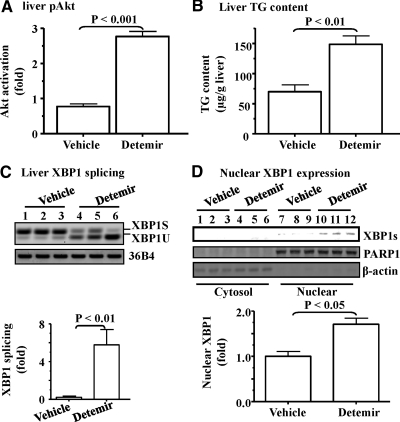
To determine the potential role of hyperinsulinemia in activation of the IRE1α-XBP1 pathway, a long-acting insulin reagent, Detemir, was used to treat the insulin-deficient NOD mice, followed by evaluations of Akt phosphorylation, lipogenic program, and XBP1 activity in the liver. Changes in blood glucose, body weight, consumptions of food and water, and insulin sensitivity measured by insulin tolerance test in these animals have been previously published (18). The animals treated with increasing amount of Detemir had obvious insulin resistance (Supplemental Fig. 3) (18). As shown in Fig. 2, A and B, the basal level of Akt phosphorylation and TG content were both increased significantly by the treatment with Detemir. Transcript levels of several key lipogenic genes (Srebf1, Fasn, and Hmgcr) tended to increase although did not reach a statistical significance (Supplemental Table 2). Protein levels of key lipogenic genes SREBP-1c and FAS were both increased in the liver significantly by Detemir (Supplemental Fig. 4). The spliced mRNA level of XBP1 was significantly elevated by the treatment with Detemir (Fig. 2C). Although level of the cytosolic XBP1 was not altered by Detemir (data not shown), the spliced form of XBP1 at the protein level was significantly increased in the nuclear extracts of the liver samples from the mice treated with Detemir (Fig. 2D). Together, these results indicate that chronic exposure to insulin stimulates the activation (splicing) of XBP1 in the liver.
Fig. 2.
XBP1 was activated in the liver of NOD mice by the chronic exposure to insulin. NOD mice with fasting blood glucose levels at more than 300 mg/dl were treated with either the vehicle solution (saline, n = 6) or Detemir (n = 5) for 2 wk. A and B, Phosphorylation of Akt and TG content in the liver were measured. C, The spliced XBP1 mRNA were evaluated by RT-PCR. The transcript 36B4 was used as a loading control. D, Extracts of cytosole and nuclei were prepared from liver tissue as described in Materials and Methods, and protein levels were determined by immunoblottings with the indicated antibodies. Levels of nuclear XBP1 expression were quantified and normalized to PARP1, a housekeeping nuclear protein (lower panel). Results represent mean ± sem.
XBP1 activation and lipogenic program are both increased by the prolonged exposure to insulin in primary hepatocytes with insulin resistance
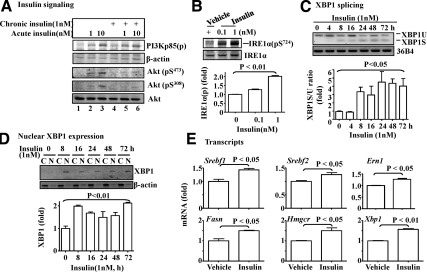
To recapitulate the in vivo findings, isolated mouse primary hepatocytes were treated with insulin for various amount of time, followed by evaluations of insulin signaling, activity of the IRE1α-XBP1 pathway, and transcripts of key lipogenic genes. As shown in Fig. 3A, acute insulin challenge stimulated phosphorylation of both PI3K (p85) and Akt, and the phosphorylation of Akt was blunted by the chronic preexposure to insulin for 24 h, indicating the presence of insulin resistance in the cells exposed to insulin for 24 h.
Fig. 3.
Prolonged exposure to insulin activated splicing (activation) and nuclear translocation of XBP1 in primary hepatocytes. Primary hepatocytes were isolated from B6 mice fed with ND. A, Isolated hepatocytes were cultured in the presence or absence of insulin (1 nm) for 24 h, followed by a thorough wash with warm PBS. Cells were then acutely challenged by using insulin (1 or 10 nm) for 5 min as indicated, and subsequently, phosphorylation levels of insulin signaling components were determined by immunoblotting with the antibodies as noted. B, Isolated hepatocytes were incubated with different amount of insulin for 24 h, followed by measurement of IRE1α phosphorylation at serine 724 by immunoblotting with the indicated antibodies and quantification and normalization to the level of total IRE1α. C and D, Isolated hepatocytes were treated with insulin (1 nm) for various amount of time, followed by evaluations of the spliced XBP1 mRNA by RT-PCR and then quantified and normalized, and nuclear level of XBP1 proteins by immunoblotting and normalized to cytosolic β-actin. The 36B4 transcripts were used as a loading control. C, Cytoplasm; N, nuclear extracts. E, Transcripts of the noted genes were measured by the real-time PCR in the isolated hepatocytes exposed to insulin (1 nm) for 8 h. All results represent mean ± sem of three independent experiments, each in triplicate.
To determine the effect of the prolonged exposure to insulin on the activation of IRE1α-XBP1 pathway, hepatocytes were incubated with insulin for 24 h, followed by measurements of IRE1α phosphorylation. As shown in Fig. 3B, IRE1α phosphorylation was significantly enhanced by the prolonged exposure to insulin. The protein level of IRE1α appeared to be slightly increased by insulin. Next, hepatocytes were incubated with insulin for an increasing amount of time, followed by evaluating levels of the spliced form of XBP1 mRNA (XBP1S) and nuclear XBP1 proteins. As shown in Fig. 3C, level of the spliced form of XBP1 was increased significantly after an exposure to insulin for 8 h and lasted at least 72 h. Similarly, the protein level of the XBP1S in the nuclear extracts was increased by the exposure to insulin for 8 h or longer (Fig. 3D). Together, these results show that the IRE1α-XBP1 pathway was activated by the prolonged exposure to insulin in isolated hepatocytes with insulin resistance.
To determine the level of lipogenic program in cells with insulin resistance, hepatocytes were treated with insulin for 8 h, followed by evaluation of key lipogenic genes. As shown in Fig. 3E, transcript levels of Srebf-1, Fasn, Srebf-2, and Hmgcr were all stimulated by insulin as predicted. It is noteworthy that mRNA levels of both Ern1 (IRE1α gene) and Xbp1 genes were increased by insulin, implying that insulin may promote XBP1 activation by not only IRE1α activation but also expressions of the IRE1α and XBP1 genes.
Overexpression of the spliced (activated) form of XBP1 stimulates transcription of the FAS promoter
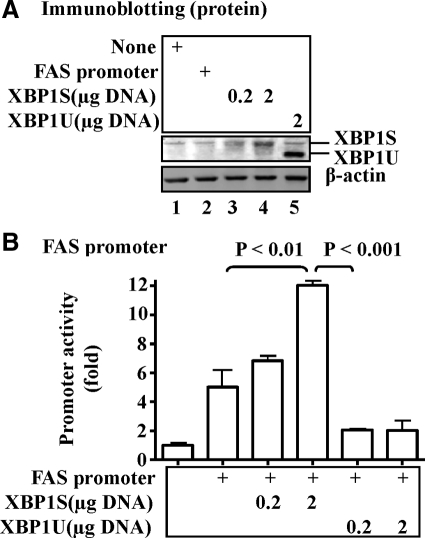
To determine whether or not XBP1 directly participates in lipogenesis, different forms of XBP1 were overexpressed in hepa1c1c7 hepatoma cells, followed by evaluating their effects on activity of the FAS promoter. As shown in Fig. 4A, overexpression of XBP1 was achievable. Note: The molecular weight of spliced XBP1S protein is larger than the unspliced form due an in-frame change in the spliced form (19). Overexpression of the XBP1S indeed significantly stimulated the activity of the FAS promoter in a dose-dependent manner (Fig. 4B). In contrast, overexpression of the unspliced form of XBP1 (XBP1U) did not have a similar effect.
Fig. 4.
Overexpression of XBP1 stimulated promoter activity of the FAS promoter. A, Spliced (XBP1S) or unspliced (XBP1U) was introduced into hepa1c1c7 cells with Lipofectamine2000 for 72 h. Protein levels were determined by immunoblotting with specific antibodies noted. B, The FAS promoter was introduced into hepa1c1c7 cells with XBP1S and XBP1U proteins via transient transfection for 36 h. Promoter activity was measured by luciferase assays and normalized to protein levels. Results represent mean ± sem of three independent experiments, each in triplicate.
Knockdown of the IRE1α gene prevents the insulin-mediated activation of the lipogenic program in hepatoma cells
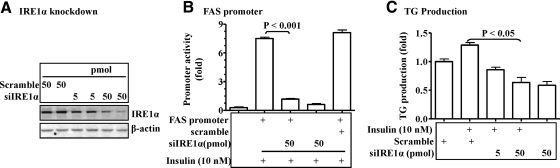
To determine whether or not the IRE1α-XBP1 pathway is required for the insulin-mediated activation of hepatic lipogenic program, the IRE1α gene was silenced by the specific cognate siRNA. As shown in Fig. 5A, the target siRNA was effective in knocking down IRE1α. Knockdown of the IRE1α gene with the specific siRNA prevented the insulin-stimulated activation of the FAS promoter, whereas the control siRNA had no effect (Fig. 5B). Similarly, the insulin-stimulated TG accumulation in hepa1c1c7 cells was prevented by knocking down the IRE1α gene (Fig. 5C). Together, these results indicate that the IRE1α-XBP1 pathway is a key component of the insulin-mediated activation of hepatic lipogenic program.
Fig. 5.
Knockdown of the IRE1α gene with siRNA reduced lipogenic program in hepatocytes. A, The siRNA against the IRE1α gene or the scrambled siRNA were introduced into hepa1c1c7 cells for 48 h followed by evaluating levels of IRE1α proteins by immunoblotting with noted antibodies. B, The FAS promoter was introduced into hepa1c1c7 cells with the noted siRNA via transient transfection for 36 h. Promoter activity was stimulated by insulin (10 nm) for 6 h, measured by luciferase assays, and normalized to the protein level. C, The siRNA against IRE1α were introduced into isolated primary hepatocytes as described in Fig. 3. After 16 h, cells were exposed to insulin (10 nm) for 48 h (fresh insulin was added every 12 h to maintain the concentration of insulin). TG content was measured and normalized to the protein level. Results represent mean ± sem of three independent experiments, each in triplicate.
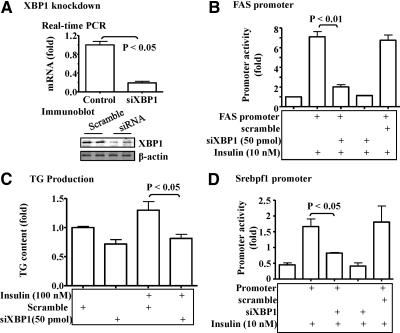
Knockdown of the XBP1 gene prevents the insulin-mediated activation of the lipogenic program in hepatoma cells
To further study the potential role of IRE1α-XBP1 pathway in the insulin-induced activation of hepatic lipogenic program, the XBP1 gene was knocked down with the specific siRNA in hepa1c1c7 hepatoma cells. As shown in Fig. 6A, levels of both mRNA and proteins of XBP1 were efficiently decreased by the target siRNA. Knockdown of the XBP1 gene significantly reduced the insulin-induced activation of the FAS promoter (Fig. 6B). Similarly, knockdown of the XBP1 gene prevented the insulin-induced TG accumulation in hepatoma cells (Fig. 6C).
Fig. 6.
Knockdown of the XBP1 gene reduced lipogenesis in hepatocytes. A, The siRNA against XBP1 were transiently transfected into hepa1c1c7 cells for 48 h. The levels of XBP1 mRNA and proteins were determined by the real-time PCR or immunoblotting as noted. B, The FAS promoter constructs were introduced into hepa1c1c7 cells together with siRNA as noted via transient transfection for 36 h. Promoter activity was stimulated by insulin (10 nm) for 6 h, measured by luciferase assays, and normalized to the protein level. C, The siRNA against XBP1 or scrambled siRNA were introduced into isolated primary hepatocytes for 24 h, followed by stimulation with insulin (10 nm) for 48 h. TG content in the hepatocytes was measured and normalized to the protein level. D, SREBP-1 promoter was introduced into hepa1c1c7 cells together with the siRNA against XBP1 or the scrambled siRNA. After 24 h, cells were exposed to insulin (10 nm) for another 6 h. Activity of the REBP1c promoter was determined by luciferase assays and normalized to the protein level. Results represent mean ± sem of three independent experiments, each in triplicate.
It is well established that the insulin-mediated transcription of the FAS gene is SREBP-1c dependent (20). Therefore, we examined the effect of XBP1 knockdown on expression of the SREBP-1c gene. As predicted, insulin stimulated the activity of the SREBP-1c promoter (Fig. 6D, lane 2). The insulin-induced activation of the SREBP-1c promoter was blocked by knocking down the XBP1 gene with siRNA (Fig. 6D). Together, these results indicate that the IRE1α-XBP1 pathway is a constitutive component of the insulin-mediated activation of hepatic lipogenic program, and a target of the IRE1α-XBP1 pathway is probably the transcription of the SREBP-1c gene (Srebpf1).
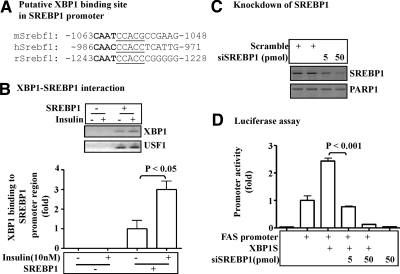
XBP1 interacts with the promoter of the SREBP-1c gene
To further study the role of XBP1 in the insulin-mediated lipogenic program in hepatocytes, we examined whether or not XBP1 interacts with the promoter of the SREBP-1c gene. It is established that XBP1 preferentially binds to the motif containing a CCACG-box (18, 19). Interestingly, and the CCACG-box is highly conserved in the SREBP-1c promoter of mouse, rat, and human (Fig. 7A). The SREBP-1c promoter was transiently introduced into hep1c1c7 cells and stimulated by insulin. Subsequently, ChIP assays were performed with anti-XBP1 antibodies. As shown in Fig. 7B, an element of the Srebf1 promoter was coprecipitated by the anti-XBP1 antibodies, and the precipitation was enhanced by the treatment with insulin. As a control, the anti-upstream transcription factor 1 antibodies also coprecipitated with the Srebf1 promoter, but the precipitation was unaffected by insulin (Fig. 7B). Together, these results show that XBP1 interacts with the SREBP-1 promoter and the interaction is promoted by insulin.
Fig. 7.
SREBP-1 is required for the XBP1-mediated lipogenesis in hepatocytes. A, Consensus sequences of the putative XBP1 binding site in the SREBP-1 gene promoter of different species. The CCACG-box and the CAAT sequence are highlighted. B, SREBP-1 promoter constructs were introduced into hepa1c1c7 cells via transient transfection for 36 h, followed by treatment with insulin (10 nm) for 8 h. Coprecipitates of XBP1 proteins and Srebf1 DNA were evaluated by ChIP assays with antibodies as noted. C, The siRNA against the Srebf1 gene or the scrambled siRNA were transiently transfected into hepa1c1c7 cells for 48 h. Levels of SREBP-1 proteins in the nuclear extracts were determined by immunoblottings. D, The siRNA against the Srebf1 gene or the scrambled siRNA were introduced into hepa1c1c7 cells for 24 h, followed by the second transfections with the constructs encoding either the FAS promoter or XBP1S. After an additional 24 h, promoter activity was measured by luciferase assays and normalized to the protein level. Results represent mean ± sem of three independent experiments, each in triplicate.
SREBP-1c is involved in the IRE1α-XBP1-mediated lipogenic function
To further examine the role of SREBP-1c in the IRE1α-XBP1-mediated lipogenesis, the SREBP-1 was knocked down by siRNA, followed by evaluating the activity of the FAS promoter. As shown in Fig. 7C, the specific siRNA knocked down the Srebpf1 gene efficiently in hepa1c1c7 cells. Overexpression of XBP1S stimulated the FAS promoter activity, but the stimulation was significantly reduced by knocking down the Srebf1 gene (Fig. 7D). Thus, SREBP-1c appears to be involved in the function of the IRE1α-XBP1 pathway in promotion of hepatic lipogenesis.
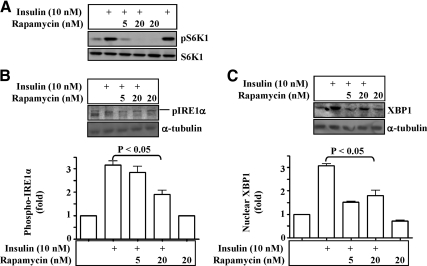
Activation of the IRE1α-XBP1 pathway by the chronic exposure to insulin depends upon mammalian target of rapamycin (mTOR)-dependent protein synthesis
To investigate how chronic exposure to insulin activates the IRE1α-XBP1 pathway, we tested the hypothesis that the chronic exposure to insulin stimulated the UPR through increasing protein synthesis. For this purpose, an inhibitor of protein synthesis, rapamycin (a well-established mTOR inhibitor), was applied in hepa1c1c7 cells. As shown in Fig. 8A, phosphorylation of S6 kinase (S6K) was stimulated by the exposure to insulin for 12 h, but the stimulation was prevented by rapamycin as expected. IRE1α phosphorylation was increased by the prolonged exposure to insulin, but the increase was prevented by rapamycin (Fig. 8B). Similarly, the XBP1S form was elevated by the prolonged exposure to insulin, and the elevation was prevented by rapamycin (Fig. 8B). These results indicate that prolonged exposure to insulin activates the IRE1α-XBP1 pathway through increasing protein syntheses.
Fig. 8.
Activation of the IRE1α-XBP1 pathway by the prolonged exposure to insulin can be saturated by the mTOR inhibitor rapamycin. Hepa1c1c7 cells were treated with insulin (10 nm) in the presence or absence of rapamycin (5 and 20 nm) for 12 h and then lysed in Triton X-100 buffer. Levels of phospho-/total-S6K (A), phospho-IRE1α (B), and nuclear XBP1 (C) were evaluated by immunoblotting with specific antibodies as noted. Results represent mean ± sem of three independent experiments. An arrow indicates target band.
Discussion
Hepatic lipogenesis is necessary for converting the positive energy imbalance caused by overeating and/or lack of physical activity into obesity and its numerous associated major health problems. A fully understanding of hepatic lipogenesis will undoubtedly provide more specific targets for prevention and reversion of obesity and its associated diseases. In this study, we have revealed a constitutive role for the IRE1α-XBP1 UPR pathway in the insulin-mediated hepatic lipogenesis and related mechanisms.
Previously, it has been shown that UPR, including XBP1 splicing, is activated in animals with insulin resistance and hyperinsulinemia induced either by the HFD or genetic defect (ob/ob mice), leading to ER stress (12). However, it was unclear in those studies how XBP1 activation and the associated UPR or ER stress were activated. Here, our results similarly show that ER stress-associated factors, such as IRE1α phosphorylation and XBP1 splicing, are increased in the liver of mice with insulin resistance and hyperinsulinemia induced by the HFD. Moreover, our results show that chronic exposure of NOD mice to insulin induces XBP1 activation. Thus, our results imply that the increased UPR such XBP1 activation in animals with insulin resistance and hyperinsulinemia is mediated by insulin. This implication is strongly supported by our results from cultured hepatocytes, which clearly show that chronic exposure to insulin activates the IRE1α-XBP1 pathway in the presence of insulin resistance.
Previously, it has been shown that activation of XBP1 is involved in the carbohydrate-induced hepatic lipogenesis, and the XBP-mediated lipogenesis is independent of SREBP-1 and carbohydrate responsive element binding protein (15). Here, our results show that the XBP1-mediated hepatic lipogenesis is connected with SREBP-1c. Specifically, our results show that XBP1 interacts with the promoter of the SREBP-1c gene activated by insulin, and insulin-mediated expression of SREBP-1c requires XBP1. Likewise, the XBP1-mediated lipogenesis also requires SREBP-1c. It appears that our results are different from the earlier report. However, the early study might not necessarily exclude the connection between XBP1 and SREBP-1c. In that early study, knockout of the XBP1 gene did not alter the basal expression levels of SREBP-1c and carbohydrate responsive element binding protein but did reduce the expression of SREBP-1c in animals under the challenge with the high-carbohydrate diet (15). Thus, both the early study by others and our current study support a connection between XBP1 and SREBP-1c in regulation of hepatic lipogenesis.
A role for XBP1 has previously been indicated in hepatic lipogenesis induced by the high-carbohydrate diet (15). However, the potential role for XBP1 in the insulin-mediated lipogenesis has not been established. Our results from this study show that silencing of either the IRE1α or XBP1 gene prevents the insulin-induced TG accumulation and transcriptions of FAS and SREBP-1c genes. These results strongly support the notion that the IRE1α and XBP1 pathway plays a constitutive role in the insulin-induced lipogenesis.
It is still unclear how insulin activates the IRE1α and XBP1 pathway. It has been recently shown by two independent groups that subunit of the PI3K (p85), a key component of insulin signaling, can interact with XBP1, and the interaction promotes the nuclear translocation (activation) of XBP1 (21, 22). However, these studies did not address the role of the interaction between p85 and XBP1 in hepatic lipogenesis. Results from our study here show that prolonged exposure to insulin activates the IRE1α and XBP1 pathway probably through the excess protein synthesis, which normally requires mTOR and S6K signaling (23). All newly synthesized and misfolded proteins normally need to be folded or processed by ER. It is conceivable that prolonged exposure insulin promotes syntheses of excess unfolded proteins that demands more ER function and will eventually overload ER, leading to increased UPR, including activation of the IRE1α-XBP1 pathway. Thus, activation of the IRE1α-XBP1 pathway is not only required for resolving the increased ER loading or ER stress caused by the increased production of unfolded proteins but also promoting hepatic lipogenesis. This notion is particularly important in explaining the elevated hepatic lipogenesis and ER stress in subjects with insulin resistance and hyperinsulinemia. We and others have previously shown that the basal level of the Akt-dependent insulin signaling is increased in the presence of insulin resistance and hyperinsulinemia (17, 24). It is easy to imagine how the continuously increased Akt-dependent insulin signaling in these subjects can do in promoting both UPR/ER stress and hepatic lipogenesis.
In summary, results from this study strongly indicate a constitutive role for the IRE1α-XBP1 pathway in the insulin-mediated hepatic lipogenesis, which is necessary for the development of insulin resistance. Furthermore, activity of the IRE1α-XBP1 pathway is elevated by the continuous functioning of insulin signaling at an increased basal level in the presence of insulin resistance and hyperinsulinemia. Because insulin resistance/hyperinsulinemia is closely associated with many major health problems caused by the modern lifestyle characterized by overeating and/or lack of physical activity, our results may provide new clues for prevention and treatment of these major health problems.
Acknowledgments
We thank Dr. Laurie Glimcher (Harvard Medical School, Boston, MA) and Dr. Michael S. Brown (University of Texas Southwestern Medical Center, Dallas, TX) for kindly providing us XBP1S and XBP1U expression plasmids and SREBP-1c promoter construct.
Present address for T.H.: The First Affiliated Hospital of the University of South China, Hengyang, Hunan, People's Republic of China, 421001.
This work was supported by the National Institutes for Health Grant R01DK076039 (to W.C.) and the American Diabetes Association Grant 7-09-BS-27) (to W.C.).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- Akt
- Serine-threonine kinase
- ChIP
- chromatin immunoprecipitation
- ER
- endoplasmic reticulum
- FAS
- fatty acid synthase
- HFD
- high-fat diet
- mTOR
- mammalian target of rapamycin
- ND
- normal rodent chow diet
- NOD
- nonobese diabetic
- PARP1
- poly(ADP-ribose) polymerase 1
- PI3K
- phosphatidylinositol 3 kinase
- siRNA
- small interfering RNA
- S6K
- S6 kinase
- SREBP
- sterol regulatory element-binding protein
- TG
- triglyceride
- UPR
- unfolded protein response
- XBP1
- X-box-binding protein 1
- XBP1S
- spliced XBP1
- XBP1U
- unspliced form of XBP1.
References
- 1. Kim JY, van de Wall E, Laplante M, Azzara A, Trujillo ME, Hofmann SM, Schraw T, Durand JL, Li H, Li G, Jelicks LA, Mehler MF, Hui DY, Deshaies Y, Shulman GI, Schwartz GJ, Scherer PE. 2007. Obesity-associated improvements in metabolic profile through expansion of adipose tissue. J Clin Invest 117:2621–2637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wang MY, Grayburn P, Chen S, Ravazzola M, Orci L, Unger RH. 2008. Adipogenic capacity and the susceptibility to type 2 diabetes and metabolic syndrome. Proc Natl Acad Sci USA 105:6139–6144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Accili D. 2004. Lilly lecture 2003: the struggle for mastery in insulin action: from triumvirate to republic. Diabetes 53:1633–1642 [DOI] [PubMed] [Google Scholar]
- 4. Burns JM, Donnelly JE, Anderson HS, Mayo MS, Spencer-Gardner L, Thomas G, Cronk BB, Haddad Z, Klima D, Hansen D, Brooks WM. 2007. Peripheral insulin and brain structure in early Alzheimer disease. Neurology 69:1094–1104 [DOI] [PubMed] [Google Scholar]
- 5. Cole GM, Frautschy SA. 2007. The role of insulin and neurotrophic factor signaling in brain aging and Alzheimer's disease. Exp Gerontol 42:10–21 [DOI] [PubMed] [Google Scholar]
- 6. Crowell JA, Steele VE, Fay JR. 2007. Targeting the AKT protein kinase for cancer chemoprevention. Mol Cancer Ther 6:2139–2148 [DOI] [PubMed] [Google Scholar]
- 7. Moreira PI, Santos MS, Seiça R, Oliveira CR. 2007. Brain mitochondrial dysfunction as a link between Alzheimer's disease and diabetes. J Neurol Sci 257:206–214 [DOI] [PubMed] [Google Scholar]
- 8. Al-Shawwa BA, Al-Huniti NH, DeMattia L, Gershan W. 2007. Asthma and insulin resistance in morbidly obese children and adolescents. J Asthma 44:469–473 [DOI] [PubMed] [Google Scholar]
- 9. Sterry W, Strober BE, Menter A. 2007. Obesity in psoriasis: the metabolic, clinical and therapeutic implications. Report of an interdisciplinary conference and review. Br J Dermatol 157:649–655 [DOI] [PubMed] [Google Scholar]
- 10. Popa C, Netea MG, van Riel PL, van der Meer JW, Stalenhoef AF. 2007. The role of TNF-α in chronic inflammatory conditions, intermediary metabolism, and cardiovascular risk. J Lipid Res 48:751–762 [DOI] [PubMed] [Google Scholar]
- 11. Okazaki R. 2008. Links between osteoporosis and atherosclerosis; beyond insulin resistance. Clin Calcium 18:638–643 [PubMed] [Google Scholar]
- 12. Ozcan U, Cao Q, Yilmaz E, Lee AH, Iwakoshi NN, Ozdelen E, Tuncman G, Görgün C, Glimcher LH, Hotamisligil GS. 2004. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science 306:457–461 [DOI] [PubMed] [Google Scholar]
- 13. Sriburi R, Jackowski S, Mori K, Brewer JW. 2004. XBP1: a link between the unfolded protein response, lipid biosynthesis, and biogenesis of the endoplasmic reticulum. J Cell Biol 167:35–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Basseri S, Austin RC. 2008. ER stress and lipogenesis: a slippery slope toward hepatic steatosis. Dev Cell 15:795–796 [DOI] [PubMed] [Google Scholar]
- 15. Lee AH, Scapa EF, Cohen DE, Glimcher LH. 2008. Regulation of hepatic lipogenesis by the transcription factor XBP1. Science 320:1492–1496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Glimcher LH, Lee AH. 2009. From sugar to fat: how the transcription factor XBP1 regulates hepatic lipogenesis. Ann NY Acad Sci 1173(Suppl 1):E2–E9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Liu H, Hong T, Wen GB, Han J, Zuo D, Liu Z, Cao W. 2009. Increased basal level of Akt-dependent insulin signaling may be responsible for the development of insulin resistance. Am J Physiol Endocrinol Metab 297:E898–E906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Liu HY, Cao SY, Hong T, Han J, Liu Z, Cao W. 2009. Insulin is a stronger inducer of insulin resistance than hyperglycemia in mice with type 1 diabetes mellitus (T1DM). J Biol Chem 284:27090–27100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yoshida H. 2007. Unconventional splicing of XBP-1 mRNA in the unfolded protein response. Antioxid Redox Signal 9:2323–2333 [DOI] [PubMed] [Google Scholar]
- 20. Eberlé D, Hegarty B, Bossard P, Ferré P, Foufelle F. 2004. SREBP transcription factors: master regulators of lipid homeostasis. Biochimie 86:839–848 [DOI] [PubMed] [Google Scholar]
- 21. Park SW, Zhou Y, Lee J, Lu A, Sun C, Chung J, Ueki K, Ozcan U. 2010. The regulatory subunits of PI3K, p85α and p85β, interact with XBP-1 and increase its nuclear translocation. Nat Med 16:429–437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Winnay JN, Boucher J, Mori MA, Ueki K, Kahn CR. 2010. A regulatory subunit of phosphoinositide 3-kinase increases the nuclear accumulation of X-box-binding protein-1 to modulate the unfolded protein response. Nat Med 16:438–445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jastrzebski K, Hannan KM, Tchoubrieva EB, Hannan RD, Pearson RB. 2007. Coordinate regulation of ribosome biogenesis and function by the ribosomal protein S6 kinase, a key mediator of mTOR function. Growth Factors 25:209–226 [DOI] [PubMed] [Google Scholar]
- 24. Aoyama H, Daitoku H, Fukamizu A. 2006. Nutrient control of phosphorylation and translocation of Foxo1 in C57BL/6 and db/db mice. Int J Mol Med 18:433–439 [PubMed] [Google Scholar]