A high-fat diet disturbs uteroplacental hemodynamics; maternal obesity exacerbates the placental dysfunction and increases the frequency of stillbirth.
Abstract
Prepregnancy maternal obesity confers an increased risk of stillbirth, but the mechanisms are unknown. Maternal obesity is associated with placental inflammation. We considered that maternal diet may predispose to the increased risk of placental inflammation and stillbirth. We hypothesized that a chronic high-fat diet (HFD) is associated with abnormal uteroplacental circulation and placental inflammation. Here we used a nonhuman primate model to determine the effect of chronic HFD on the uterine and placental hemodynamics, placental histology, and inflammation in a prospective, observational study of 24 Japanese macaques. Overall, there was a statistically significant (38–56%) reduction in uterine volume blood flow from HFD animals, whether they were lean or obese. Consumption of a HFD, independent of obesity, increased placental inflammatory cytokines and the expression of Toll-like receptor 4. We show that HFD consumption by obese mothers with hyperinsulinemia also reduced volume blood flow on the fetal side of the placenta and significantly increased the frequency of both placental infarctions and stillbirth. These results suggest that a HFD, independent of obesity, decreases uterine volume blood flow. Maternal obesity and insulin resistance further exacerbates the placental dysfunction and results in an increased frequency of stillbirth.
The increased prevalence of obesity complicating pregnancy is a direct consequence of the obesity epidemic (1). Recent data from the Centers of Disease Control and Prevention (Atlanta, GA) suggest that one of five women are obese at the start of pregnancy and that the prevalence of obesity in reproductive-age women is 30% (2). Prepregnancy maternal obesity confers an increased risk of fetal growth abnormalities and stillbirth (3, 4). Maternal obesity, defined as a body mass index of 30 kg/m2 or greater, increases the risk of stillbirth by 2- to 5-fold (5–7).
The mechanisms underlying these increased risks are poorly understood but include increased placental inflammation and placental dysfunction as possible contributing factors (6–8). The placenta is the primary organ for nutrient exchange during pregnancy, and abnormal placental development has been associated with virtually every adverse obstetric outcome including abnormalities in fetal growth, preeclampsia, preterm labor, and stillbirth (9–12). In addition, obese gravidae demonstrate impaired endothelial vasodilation when compared with lean counterparts, which might contribute to their increased risk of preeclampsia and placental dysfunction (13).
Recent work from our laboratory using nonhuman primates fed a Western-style diet demonstrated that chronic maternal consumption of a high-fat diet (HFD) resulted in fetal lipotoxicity, as evidenced by increased liver triglycerides and high circulating cytokines (14). Surprisingly, these effects were independent of maternal obesity or insulin resistance. The relative contribution of maternal diet to the obstetric complications associated with obesity in human pregnancies is uncertain. These studies raise the question of whether the consumption of a Western-style diet itself could also impact placenta function and health.
Doppler ultrasound is an established antenatal surveillance method in the clinical management of pregnancies complicated by placental dysfunction (15, 16). Recent advances in ultrasound technology permit the quantitative estimation of uterine artery volume blood flow to assess perfusion and vascular resistance of the maternal side of the placenta (17). This measurement, the calculated uterine artery volume blood flow (cQUta), correlates well with directly measured volume blood flow in the uterine artery (18). The quantitative estimation of blood flow on the fetal side of the placenta, the placental volume blood flow (cQUV) is reduced in pregnancies complicated by intrauterine growth restriction (19, 20).
Our well-characterized nonhuman primate model of excess nutrition provides a unique resource to investigate the impact of a Western-style diet on placental hemodynamics independent of obesity and has the advantage of sharing similar placentation with humans. In the present study, we hypothesized that maternal HFD consumption during pregnancy significantly alters maternal and fetal placental hemodynamics and that these alterations would be associated with placental histological changes and inflammation.
Materials and Methods
Animals
All animal procedures were in accordance with the guidelines of the Institutional Animal Care and Use Committee of the Oregon National Primate Research Center. A total of 24 young adult Japanese macaques were maintained on either a HFD (32% of calories from fat; TAD Primate Diet no. 5L0P; Lab Diet, Richmond, IN) or a control diet (CTR, 14% calories from fat; Primate Diet no. 5000, Lab Diet) for at least 4 yr. The animals were matched for age and had similar reproductive histories. Both diets are sufficient in vitamin, mineral, and protein content for normal growth. Because these animals are socially housed, individual food intake and energy expenditure could not be measured. As previously reported, animals fed the HFD segregated into diet resistant (HFD-R) or diet sensitive (HFD-S) based on body weight and insulin resistance (14). During the breeding season, animals were checked for pregnancies by palpation and confirmed and dated by ultrasound. During the third trimester of pregnancy (as determined by ultrasound), animals underwent an iv glucose tolerance test (GTT) to determine insulin sensitivity. For the experiments involving fetuses, pregnancies were terminated by cesarean section, performed by Oregon National Primate Research Center veterinarians, at gestational day (G) 130 of a 170- to 180-d term gestation. Both groups consisted of primiparous and multiparous pregnant animals. There were no differences in parity between groups. All pregnancies were singleton. There were 59 (26 CTR, 13 HFD-R, 20 HFD-S) total pregnancies during the study period including those pregnancies that were allowed to continue for breeding and juvenile studies. Twenty-four animals underwent the Doppler ultrasound experiments described below and 21 pregnancies were terminated at G130 to collect fetal and placental tissue. The incidence of stillbirth was calculated as the number of stillbirths per group divided by the total number of pregnancies per group.
Maternal insulin sensitivity
We performed iv GTT in the early third trimester (G130) after an overnight fast. Animals were sedated with ketamine (10 mg/kg) and administered a glucose bolus (50% dextrose solution) at a dose of 0.6 g/kg via the saphenous vein. Baseline blood samples were obtained before the infusion, and 1-ml blood samples were taken at 1, 3, 5, 10, 20, 40, and 60 min after infusion via the femoral artery. Glucose was measured immediately using a OneTouch Ultra blood glucose monitor (LifeScan, Milpitas, CA), and the remainder of the blood was kept in heparinized tubes on ice for insulin measurement. After iv GTT, samples were centrifuged, and plasma was stored at −80 C until assayed. Insulin was assayed in plasma by RIA (catalog no. RI-13K; Linco, St. Charles, MO).
RNA isolation and quantitative RT-PCR
Monkey placentas were delivered via cesarean section when fetuses were G130. In ribonuclease-free conditions, membranes were removed from the placenta, and it was weighed and measured. The outer 1 cm of the placental disk was discarded and 1-cm2 pieces were cut from the inner placenta (near the cord) and placed in RNAlater (Ambion, Austin, TX). Tissue was homogenized and RNA isolated using Trizol reagent (Life Technologies Corp., Carlsbad, CA). RNA was then deoxyribonuclease treated (RQ1 Dnase, catalog no. 9PIM610; Promega, Madison, WI) and cleaned up using QIAGEN′s RNeasy minikit (Valencia, CA). Samples were bioanalyzed on a RNA 6000 Nano chip kit (Agilent 2100 Bianalyzer; Agilent Technologies, Inc., Palo Alto, CA) to check for integrity and concentration. Standard RT-PCR was performed using 1 μg of total RNA. Real-time PCR was performed on the ABI 7900HT (Applied Biosystems, Foster City, CA) using the following primer/probe sets specifically designed for the Japanese macaque: monocyte chemoattractant protein (MCP)-1, forward, AGGCTGGCGAGCTATAGAAGAAT, reverse, TCTTGAAGATCACAGCTTCTTTGG, probe, ACCAGCAGCAAGTGT; IL-1β, forward, GACGTCGATGGCCCTAAACA, reverse, CCAGAGGGCAGAGGTCTAGATC, probe, ATGAAGTGCTCCTTCC, for 45 cycles and using β-actin as an internal control. For Toll-like receptor real-time PCR, the Toll-like receptor (TLR) 4 Gene Expression Assay (catalog no. Rh01060206m1; Applied Biosystems) was used.
Ultrasound
Each subject was anesthetized for ultrasound examination. Anesthesia was induced with a 10-mg/kg im injection of ketamine HCl (Ketaved; Bioniche Teoranta, Inverin Co., Galway, Ireland). Once anesthetized, each subject was positioned in dorsal recumbency. Measurements were obtained while subjects breathed room air. The examinations were completed within 30 min. Image-directed pulsed and color Doppler equipment (GE Voluson 730 Expert; Kretztechnik, Zipf, Austria) with a 5- to 9-MHz sector probe was used for ultrasonographic data collection. The sonographer was blinded to treatment group. The lowest high-pass filter level was used (100 Hz), and an angle of 15° or less between the vessel and Doppler beam was deemed acceptable. Blood flow velocity waveforms were obtained from the proximal portion of the uterine artery as previously described (17, 18).
Measurements of the waveforms were done using the software supplied on the ultrasound machine, and the following parameters were obtained: pulsatility index (PI), velocity time integral, and heart rate. The diameter of the uterine artery was measured using power angiography as previously described (17, 18). The cross-sectional area (CSA) of the vessel was calculated as CSA = π (diameter/2)2. cQUta was calculated using the following formula: cQUta= velocity time integral × CSA × heart rate. For the cQUV, the Doppler waveforms were obtained from the straight portion of the intraabdominal umbilical vein as previously described (21). The mean velocity was calculated as 0.5 of the maximum velocity. The inner diameter of the intraabdominal umbilical vein was measured as previously described. cQUV was calculated as: mean velocity × CSA × 60.
Pathology
Representative samples from each placenta were fixed in formalin, paraffin-embedded and histological sections stained for hematoxylin and eosin. Placental histological sections were evaluated by a placental pathologist (T.K.M.) blinded to treatment group. Sections were scored for the presence of infarctions, villous calcification, and accelerated villous maturation characterized by increased architectural arborization, perivillous fibrin, and syncytial knotting (22, 23).
Proteome Profiler Cytokine Array
Relative expression of plasma cytokines was quantified using the Proteome Profiler Human Cytokine Array Panel A array kit (R&D Systems, Minneapolis, MN). The array was performed according to the manufacturer's exact specifications using 250 μl of maternal plasma (n = 4 for controls, n = 4 for HFD) or placental plasma (n = 4 for controls, n = 4 for HFD). For maximum sensitivity, overnight incubation of blots with the plasma was used. Enhanced chemiluminescence incubation was performed for 5 min using the SuperSignal West Femto chemiluminescent kit (Thermo Scientific Pierce, Rockford, IL), and then images were captured and analyzed using FluoroChem FC2 imaging illuminator coupled with AlphaEaseFC software (version 4.0; Alpha Innotech, San Leandro, CA).
Immunohistochemistry
Immunohistochemistry was done as previously described (24).
Briefly, formalin-fixed tissue was paraffin embedded and sliced into 5-μm sections. Tissue was deparaffinized in xylene with rehydration in a series of ethanols. Slides were then treated for antigen retrieval by boiling in citrate buffer [10 mm (pH 6.0)] for 20 min. To block for endogenous peroxidase activity, sections were washed with 2% hydrogen peroxide in absolute methanol for 15 min. Tissue was then blocked with 5% donkey serum in potassium PBS (KPBS) for 30 min. Goat antihuman TLR4 antibody (catalog no. AF2616; R&D Systems; 1:1000 dilution) was incubated with the tissue at 4 C overnight. Sections were washed three times for 5 min in KPBS, and then biotinylated donkey antigoat antibody (catalog no. 705-065-147; Jackson ImmunoResearch Laboratories, West Grove, PA) was added at a 1:600 dilution in 0.4% Triton X-100 KPBS for 1 h. Tissue was washed again three times for 5 min. To form the biotin-avidin complex, the Vectastain Elite ABC solution (catalog no. 101098-314; Vector Laboratories Inc., Burlingame, CA) was incubated with the tissue for 30 min at room temperature. These complexes were then detected using the peroxidase substrate diaminobenzidine kit (catalog no. SK-4100; Vector Laboratories). Sections were counterstained with hematoxylin and coverslipped with Permount (Fisher Chemicals, Springfield, NJ).
Statistics
Data are expressed as mean ± sem. For the maternal and fetal data, differences between the three groups (control, HFD-S, and HFD-R) were tested by one-way ANOVA with Tukey's post hoc analysis. For the ultrasound data, differences between the three groups were tested by one-way ANOVA with Bonferroni post hoc analysis. When comparable changes were observed in the HFD-S and HFD-R, the two groups were combined, and a two-tailed Student's t test was used. To investigate the effects of the maternal diet on gene expression, samples from the HFD and control groups were compared using a Mann-Whitney U test. To investigate the placenta pathological changes comparing the HFD-S or HFD-R groups with controls, a χ2 analysis was used.
Results
These studies used 24 young adult Japanese macaques maintained on a HFD (32% of calories from fat) for at least 4 yr; animals on the CTR diet (14% of calories from fat) were age matched and had similar reproductive histories. As previously reported, animals fed the HFD segregated into the HFD-R or HFD-S groups based on body weight and insulin resistance (14). The HFD-S animals were classified by increased area under the curve (AUC) for insulin secretion at least 2 sd above the mean of controls during iv GTT and had increased body weight and adiposity (Table 1). The cohort of HFD-S animals investigated in this study had a 48% increase in weight, a 4-fold increase in insulin AUC during GTT, a greater than 5-fold increase in leptin levels, and increased fasting insulin levels compared with the control and HFD-R animals (Table 1). As a group, the combined HFD animals (HFD-R and HFD-S) had a significant increase in total triglycerides (Table 1).
Table 1.
Maternal third-trimester weight, insulin, glucose, leptin, and lipids
| Control | HFD-R | HFD-S | |
|---|---|---|---|
| n | 9 | 6 | 9 |
| Weight (kg) | 8.8 ± 0.4 | 10.1 ± 0.6 | 13.0 ± 0.8a |
| Insulin (mU/ml) | 4.9 ± 0.6 | 21.6 ± 5.9 | 51.6 ± 16.5b |
| Glucose (mg/dl) | 44.6 ± 3.7 | 39.0 ± 2.7 | 39.0 ± 2.5 |
| Insulin AUC | 2131 ± 299 | 3612 ± 1015 | 8332 ± 1751c |
| Glucose AUC | 4901 ± 290 | 4125 ± 267 | 5419 ± 462 |
| Leptin (ng/ml) | 14.9 ± 4.8 | 39.3 ± 11.7 | 87.1 ± 6.6d |
| Total TG (mg/ml) | 0.42 ± 0.08 | 0.57 ± 0.07e | 0.59 ± 0.09e |
| True TG (mg/ml) | 0.47 ± 0.08 | 0.61 ± 0.06 | 0.58 ± 0.07 |
| Glycerol (mg/ml) | 0.09 ± 0.01 | 0.10 ± 0.02 | 0.12 ± 0.02 |
Values are mean ± sem. TG, Triglycerides.
P < 0.0003 among control, HFD-R, and HFD-S (ANOVA with Tukey's post hoc analysis);
P < 0.02 among control, HFD-R, and HFD-S (ANOVA with Tukey's post hoc analysis);
P < 0.003 among control, HFD-R, and HFD-S (ANOVA with Tukey's post hoc analysis);
P < 0.0001 among control, HFD-R, and HFD-S (ANOVA with Tukey's post hoc analysis);
P < 0.05 among control vs. combined HFD group (Student's t test).
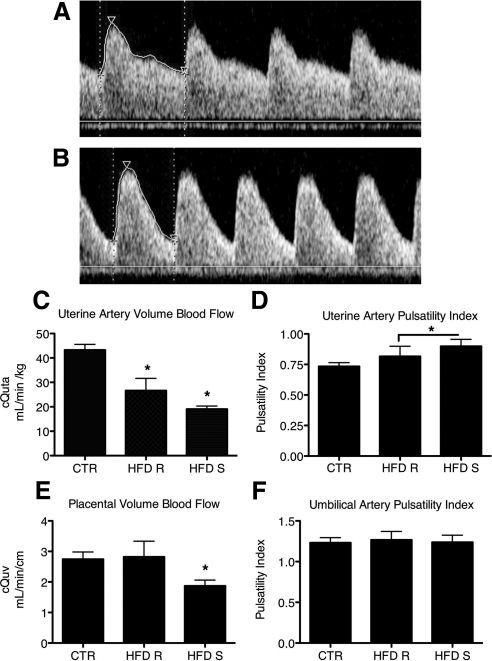
Uterine hemodynamics were examined using Doppler ultrasonography in early third-trimester pregnancies (gestational day 120) from CTR (Fig. 1A), HFD-S (Fig. 1B) and HFD-R animals (not shown). The cQUta, normalized to maternal weight, was significantly decreased in both HFD-R and HFD-S animals (Fig. 1C), with an average 38% reduction in HFD-R and an average 56% reduction in HFD-S animals. The combined HFD group (HFD-R + HFD-S) also had a significant increase in the uterine artery PI (Fig. 1D). In contrast, there was a maternal metabolic phenotype effect on the cQUV. The cQUV was significantly decreased by 32% in only the HFD-S group (Fig. 1E). Furthermore, the cQUV was negatively correlated with maternal weight at the time of GTT (r2 −0.510, P < 0.02) and maternal serum leptin (r2 −0.526, P < 0.02), features that distinguish the HFD-S from the HFD-R animals. The umbilical artery PI was not different in any group (Fig. 1F).
Fig. 1.
Decreased uteroplacental perfusion in animals fed a HFD. Maternal HFD leads to increased uterine artery PI. A, Uterine artery (Uta) PI is 0.74 in a representative control animal. B, The Uta PI is 1.17 in a representative HFD-S animal with a Doppler waveform that demonstrates decreased diastolic flow consistent with increased vascular impedance when compared with A. C, The cQUta normalized to maternal weight was significantly reduced in HFD-R and HFD-S animals when compared with CTR. *, P < 0.05 (one-way ANOVA with Bonferroni's multiple comparison test). D, Uta PI is increased in HFD-S animals when compared with CTR. *, P < 0.05, Student's t test. As a group, HFD (HFD-R + HFD-S) had a significant increase in Uta PI when compared with CTR. *, P < 0.05, Student's t test. E, The cQUV normalized to fetal abdominal circumference was reduced in HFD-S animals when compared with controls. There was no difference in HFD-R animals when compared with controls. *, P < 0.05, one-way ANOVA with Bonferonni's multiple comparison test. F, The umbilical artery (UA) PI was unaffected by diet group. CTR, n = 9; HFD-R, n = 6; HFD-S, n = 9.
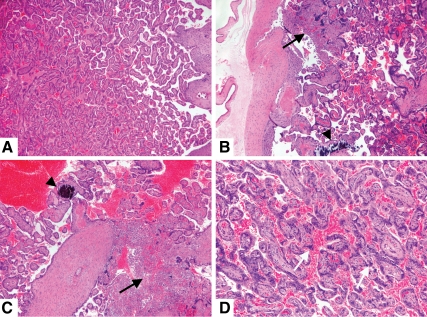
Consistent with the placental dysfunction, the HFD-S placentas displayed evidence of infarctions more often than did controls (62.5 vs. 12.5%, P = 0.04, Fig. 2, B and A). In general, HFD-R placentas displayed minimal evidence of pathology. Neither placental weight nor placental volume was different from control placentas (Table 2). As a group, the HFD animals had a significant increase in placental true triglycerides (Table 2). Hence, the placental histological features of uteroplacental insufficiency are more frequent in the HFD-S placentas, which are functionally characterized by decreased blood flow on both the maternal and fetal sides of the placenta.
Fig. 2.
Placental infarction and chorionic villous calcification in animals fed a HFD. A, Representative histological section from a control animal placenta showing normal term maturation of chorionic villi. B–D, In contrast, sections from animals fed HFD show increased frequency of infarction (bold arrow), calcifications (arrowhead), and syncytial knotting (white arrow). Hematoxylin and eosin-stained sections photographed at magnifications of ×50 (A–C) and ×100 (D).
Table 2.
Placenta weight, volume, and lipids on G130
| Control | HFD-R | HFD-S | |
|---|---|---|---|
| n | 8 | 5 | 8 |
| Weight (g) | 117.2 ± 7.06 | 105.6 ± 8.42 | 102.7 ± 4.26 |
| Primary | 74.29 ± 4.78 | 64.05 ± 8.61 | 67.58 ± 7.84 |
| Accessory | 42.93 ± 3.10 | 51.96 ± 3.35 | 46.81 ± 3.22 |
| Volume (cm3) | 134.3 ± 10.17 | 123.0 ± 22.38 | 113.1 ± 5.06 |
| Primary | 90.77 ± 6.52 | 75.48 ± 15.25 | 71.66 ± 7.91 |
| Accessory | 43.56 ± 6.70 | 59.43 ± 11.80 | 47.40 ± 4.96 |
| Total TG (mg/ml) | 0.13 ± 0.01 | 0.20 ± 0.04 | 0.21 ± 0.05 |
| True TG (mg/ml) | 0.20 ± 0.01 | 0.29 ± 0.04a,b | 0.24 ± 0.02a |
| Glycerol (mg/ml) | 0.04 ± 0.01 | 0.04 ± 0.00 | 0.07 ± 0.03 |
| NEFA (mEq/liter) | 0.15 ± 0.01 | 0.13 ± 0.03 | 0.11 ± 0.03 |
Values are mean ± sem. TG, Triglycerides; NEFA, nonesterified fatty acids.
P < 0.05, combined HFD group vs. controls.
P < 0.05, HFD R vs. control (ANOVA).
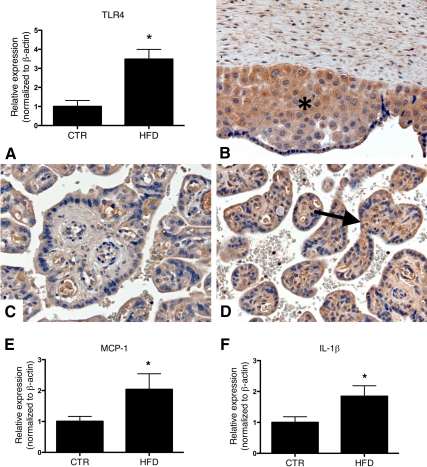
To determine the relative impact of cytokines, we used the Proteome Profiler cytokine panel to screen for plasma cytokine levels in parallel samples of the maternal and placental circulation from control and HFD-S animals. None of the 36 cytokines probed were elevated in maternal plasma (Supplemental Table 1, published on The Endocrine Society's Journals Online web site at http://endo.endojournals.org) as a result of maternal HFD consumption, but 13 of the 36 cytokines were significantly up-regulated in the placentas from HFD-S dams (P < 0.05, Supplemental Table 2). Both IL-1β mRNA and MCP-1 mRNA expression levels were significantly up-regulated in the placentas of HFD animals (combined HFD-S and HFD-R) (P < 0.05, Fig. 3, E and F). TLR4 mRNA levels were significantly up-regulated in the placentas of both HFD-S and HFD-R animals (Fig. 3A). Immunohistochemistry showed TLR4 staining in the extravillous trophoblasts in both control (Fig. 3B) and HFD placentas (data not shown). There was no TLR4 staining in the chorionic villous stroma in control placentas (Fig. 3C). In contrast, TLR4 was expressed in both the chorionic villous stroma and the syncytiotrophoblasts in HFD placentas (Fig. 3D).
Fig. 3.
Maternal HFD leads to an increased expression of TLR4 in the placenta. A, A maternal HFD leads to increased placental mRNA expression of TLR4. B, TLR4 is expressed in the extravillous trophoblast (asterisk) in both control (CTR) and HFD (not shown) placentas. C, Chorionic villous stroma is negative for TLR4 staining in the control placentas. D, TLR4 is expressed by chorionic villous stroma (arrow) and syncytiotrophoblasts in HFD placentas. E, Placenta MCP-1 mRNA expression is increased in HFD animals when compared with CTR. F, Placenta IL-1β mRNA expression is increased in the HFD animals. CTR, n = 5; HFD, n = 8. *, P < 0.05, Mann Whitney U test. The mRNA expression was normalized to β-actin as a housekeeping gene. Chromagen is brown. Sections were counterstained with hematoxylin.
As a group, the HFD fetal offspring did not differ in weight from the control fetal offspring at G130 of a 170- to 180-d term gestation (Table 3). The stillbirth rate was higher in HFD-S animals when compared with controls. In the HFD-S animals, there were seven stillbirths of 20 pregnancies (35%) compared with one stillbirth of a total of 26 control pregnancies (4%), and one stillbirth of a total of 13 HFD-R pregnancies (7%). The fetal deaths in the HFD animals occurred in the second and third trimester of pregnancy and ranged from G67 to G174.
Table 3.
Fetal weight and lipids on G130
| Control | HFD-R | HFD-S | |
|---|---|---|---|
| n | 8 | 5 | 8 |
| Weight (g) | 344.0 ± 8.34 | 333.3 ± 6.47 | 347.5 ± 12.53 |
| Total TG (mg/ml) | 0.17 ± 0.02 | 0.23 ± 0.04 | 0.27 ± 0.06 |
| True TG (mg/ml) | 0.20 ± 0.008 | 0.27 ± 0.04 | 0.22 ± 0.012 |
| Glycerol (mg/ml) | 0.10 ± 0.01 | 0.08 ± 0.006 | 0.11 ± 0.01 |
Values are mean ± sem. TG, Triglycerides.
Discussion
The most notable finding of this study was that chronic maternal HFD consumption led to decreased uterine volume blood flow accompanied by increased placental pathology. These changes were independent of maternal obesity but more severe in pregnancies complicated by maternal obesity and hyperinsulinemia. The physiological consequences of this uteroplacental insufficiency included an increased frequency of placental ischemic changes and fetal deaths in the HFD-S animals. Our data provide intriguing evidence that decreased uterine and placental volume blood flow may contribute to the increased rate of stillbirth in obese gravidae.
Both the HFD-R and HFD-S animals had a significant reduction in the cQUta. These results are consistent with sheep studies of excess nutrition. In a well-characterized adolescent sheep model of acute excess nutrition during pregnancy, overnourished ewes had a uterine flow reduction of 43% at d 88 of gestation compared with controls (25). By late gestation in this sheep model, both uterine and umbilical volume blood flows were reduced; placental mass was reduced by 30–40% (26). The placentas in this adolescent sheep model also demonstrated reduced capillary density, which may explain the decreased QUta (27). The sheep placenta is markedly different in structure from the primate placenta so direct comparisons with this model and humans are not possible (28, 29), but this experimental model suggests a link with diet and uterine and placental volume blood flow. Our studies provide the first evidence of a similar link with diet and primate placental hemodynamics.
We hypothesize that the reduction in the cQUV observed in the HFD-S animals is related to placental ischemic injury. The placental infarcts observed in the HFD-S animals are consistent with this hypothesis. The excess triglycerides from a HFD may have contributed to endothelial dysfunction directly in the placental microcirculation, causing an increased vascular resistance. Maternal lipids could also affect the placental trophoblasts directly by modifying their ability to invade the uterine spiral arteries, as is seen in some preeclamptic pregnancies, thus inhibiting the creation of a low resistance uteroplacental circulation.
The placenta has significant functional reserve to maintain adequate fetal nutrient delivery during pregnancy. Sheep experiments suggest that the pregnant uterus is perfused in sufficient excess to protect the fetus during short-term decreases of less than 50% QUta (30). The umbilical artery (UA) PI reflects the hemodynamic and morphologic events at the level of the placental villi, and increased PI is associated with increased resistance at the level of the placental microcirculation (31). The UA PI is used in clinical practice to follow up pregnancies complicated by intrauterine growth restriction. As the placental resistance increases, there is decreased diastolic flow followed by absent and then reversed end-diastolic flow, which are associated with a significant risk of fetal demise. However, significant increases in the UA PI are estimated to occur only after more than 60% of the placental terminal vascular branches are destroyed (32). Our study did not demonstrate an increase in the UA PI but showed a significant reduction in placental volume blood flow in HFD-S animals. This result is consistent with human data in which it is known that significant reductions in placental volume blood flow occur before the increase in UA PI (33).
The increased placental villous infarcts observed in the HFD-S animals reflect uteroplacental ischemia and likely contribute to the decreased placental volume blood flow in our model. The reduction in placental volume blood flow suggests that obesity with a HFD further compromises the placental circulation by decreasing blood flow on the fetal side of the placenta. This reduction in placental blood flow may exceed the placental functional reserve, thus increasing the susceptibility to stillbirth. The increased frequency of late fetal deaths in the HFD-S animals may reflect a physiologically relevant consequence of the uteroplacental insufficiency. The mechanism for this difference between the HFD animals is uncertain. The cQUV was negatively correlated with both weight and maternal serum leptin, features that distinguish the HFD-S animals from the HFD-R animals.
How could obesity directly affect the placental circulation? The data supporting an increased frequency of adverse obstetric outcomes in obese human gravidae are robust. Prepregnancy obesity, defined as a body mass index of 30 kg/m2 or greater, increases the risk of miscarriage, congenital malformation, preeclampsia, gestational diabetes, fetal growth abnormalities, and stillbirth. Diseases associated with obesity that also increase these risks include diabetes, hypertension, and preeclampsia. However, obese human gravidae display metabolic, inflammatory, and vascular abnormalities, even when there are no associated medical conditions, suggesting that obesity by itself is a contributor to these adverse outcomes (34).
In nongravid humans, obesity is associated with increased production of proinflammatory cytokines (35, 36). Similarly, obesity in pregnancy is associated with an increase in proinflammatory mediators in the placenta (8). Consistent with the reported human data, we report that a HFD is associated with an increase in proinflammatory mediators in the placenta. In our model, the inflammation in the adult female HFD animals is not systemic. We have previously reported increased inflammation in the fetal circulation, liver, and brain and now the placenta (14, 37). This suggests that the end-organ inflammatory response is a local response to the HFD. The increased inflammation in the placenta may contribute to or may be a result of the decreased placental perfusion. TLR4 expression is increased in the placentas complicated by both preeclampsia and maternal obesity (38, 39). The expression of TLR4 in the chorionic villous stroma and syncytiotrophoblasts in the HFD placentas suggests that it may be a mediator of the placental inflammatory response.
Unlike our previous study, we did not find a significant decrease in fetal weights associated with a HFD. In the previously described sheep experiments of acute excess nutrition, the fetal weights of the overnourished ewes were greater in midgestation (d 50 and 90) but were reduced by 20% by the late G130 (27). These sheep experiments suggest that the consequences of decreased uteroplacental perfusion may not be seen until later in gestation when the fetal nutrient demands exceed the placental capabilities.
The strengths of our study include the use of a well-characterized HFD nonhuman primate model whose placentation is structurally very similar to humans (29, 40). Both the placental pathologist and the ultrasonographer were blinded to the treatment group. The main limitation to the study is that uterine and placental blood flow were not measured directly but were estimated by well-validated ultrasonographic measurements. The mechanism to explain the difference in placental hemodynamics between the HFD lean and obese animals is unknown. However, we have identified TLR4 as a potential mediator of the placental inflammatory response to a HFD and obesity. The use of ketamine anesthesia could potentially affect uterine artery volume blood flow. However, iv ketamine infusion in chronically instrumented pregnant ewes did not significantly affect uterine blood flow or fetal hemodynamics (41). These sheep studies confirmed earlier studies on pregnant nonhuman primates that also suggested no significant effects of ketamine on uteroplacental perfusion (42).
In summary, our data suggest that a HFD during pregnancy reduces uteroplacental perfusion and increases placental inflammation. Obesity with a HFD further compromises placental function and leads to decreased blood flow on the fetal side of the placenta and histopathological findings suggestive of placental ischemia and decreased placental perfusion. The physiological consequence of a HFD with obesity is an increase in stillbirths, which recapitulates the human data of increased stillbirth in obese gravidae. This is the first report of placental hemodynamic abnormalities in a primate placenta that are secondary to a HFD and of a more severe phenotype manifesting when obesity compounds the HFD. Additional studies are needed to elucidate the mechanisms that link a HFD to decreased uterine and placental blood flow.
Supplementary Material
Acknowledgments
We acknowledge the technical assistance of Sarah Comstock, Diana Takahashi, and the Division of Animal Resources and Veterinary staff at Oregon National Primate Research Center for their technical assistance with these studies.
This work was supported by National Institutes of Health Grants R24 DK0909640–01 (to A.E.F., K.L.T., K.L.G.), R01 DK-79194 (to K.L.G.), P51 RR00163 (to K.L.G. and Oregon National Primate Research Center), P01 HD34430 (to K.L.T. and K.L.G.), WRHR K12 HD001243-10 (to A.E.F.), and BIRCWH K12 HD043488-06, (to T.K.M.).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AUC
- Area under the curve
- cQUta
- uterine artery volume blood flow
- cQUV
- placental volume blood flow
- CSA
- cross-sectional area
- G
- gestational day
- GTT
- glucose tolerance test
- HFD
- high-fat diet
- KPBS
- potassium PBS
- MCP
- monocyte chemoattractant protein
- PI
- pulsatility index
- TLR
- Toll-like receptor
- UA
- umbilical artery.
References
- 1. Callaway LK, Prins JB, Chang AM, McIntyre HD. 2006. The prevalence and impact of overweight and obesity in an Australian obstetric population. Med J Aust 184:56–59 [DOI] [PubMed] [Google Scholar]
- 2. Kim SY, Dietz PM, England L, Morrow B, Callaghan WM. 2007. Trends in pre-pregnancy obesity in nine states, 1993–2003. Obesity (Silver Spring) 15:986–993 [DOI] [PubMed] [Google Scholar]
- 3. Nohr EA, Vaeth M, Bech BH, Henriksen TB, Cnattingius S, Olsen J. 2007. Maternal obesity and neonatal mortality according to subtypes of preterm birth. Obstet Gynecol 110:1083–1090 [DOI] [PubMed] [Google Scholar]
- 4. Catalano PM, Thomas A, Huston-Presley L, Amini SB. 2003. Increased fetal adiposity: a very sensitive marker of abnormal in utero development. Am J Obstet Gynecol 189:1698–1704 [DOI] [PubMed] [Google Scholar]
- 5. Kristensen J, Vestergaard M, Wisborg K, Kesmodel U, Secher NJ. 2005. Pre-pregnancy weight and the risk of stillbirth and neonatal death. BJOG 112:403–408 [DOI] [PubMed] [Google Scholar]
- 6. Cnattingius S, Bergström R, Lipworth L, Kramer MS. 1998. Prepregnancy weight and the risk of adverse pregnancy outcomes. N Engl J Med 338:147–152 [DOI] [PubMed] [Google Scholar]
- 7. Nohr EA, Bech BH, Davies MJ, Frydenberg M, Henriksen TB, Olsen J. 2005. Prepregnancy obesity and fetal death: a study within the Danish National Birth Cohort. Obstet Gynecol 106:250–259 [DOI] [PubMed] [Google Scholar]
- 8. Challier JC, Basu S, Bintein T, Minium J, Hotmire K, Catalano PM, Hauguel-de Mouzon S. 2008. Obesity in pregnancy stimulates macrophage accumulation and inflammation in the placenta. Placenta 29:274–281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Roberts DJ, Post MD. 2008. The placenta in pre-eclampsia and intrauterine growth restriction. J Clin Pathol 61:1254–1260 [DOI] [PubMed] [Google Scholar]
- 10. Salafia CM, Vogel CA, Bantham KF, Vintzileos AM, Pezzullo J, Silberman L. 1992. Preterm delivery: correlations of fetal growth and placental pathology. Am J Perinatol 9:190–193 [DOI] [PubMed] [Google Scholar]
- 11. Kidron D, Bernheim J, Aviram R. 2009. Placental findings contributing to fetal death, a study of 120 stillbirths between 23 and 40 weeks gestation. Placenta 30:700–704 [DOI] [PubMed] [Google Scholar]
- 12. Amir H, Weintraub A, Aricha-Tamir B, Apel-Sarid L, Holcberg G, Sheiner E. 2009. A piece in the puzzle of intrauterine fetal death: pathological findings in placentas from term and preterm intrauterine fetal death pregnancies. J Matern Fetal Neonatal Med 22:759–764 [DOI] [PubMed] [Google Scholar]
- 13. Stewart FM, Freeman DJ, Ramsay JE, Greer IA, Caslake M, Ferrell WR. 2007. Longitudinal assessment of maternal endothelial function and markers of inflammation and placental function throughout pregnancy in lean and obese mothers. J Clin Endocrinol Metab 92:969–975 [DOI] [PubMed] [Google Scholar]
- 14. McCurdy CE, Bishop JM, Williams SM, Grayson BE, Smith MS, Friedman JE, Grove KL. 2009. Maternal high-fat diet triggers lipotoxicity in the fetal livers of nonhuman primates. J Clin Invest 119:323–335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Trudinger BJ, Cook CM, Giles WB, Connelly A, Thompson RS. 1987. Umbilical artery flow velocity waveforms in high-risk pregnancy. Randomised controlled trial. Lancet 1:188–190 [DOI] [PubMed] [Google Scholar]
- 16. Baschat AA, Gembruch U, Reiss I, Gortner L, Weiner CP, Harman CR. 2000. Relationship between arterial and venous Doppler and perinatal outcome in fetal growth restriction. Ultrasound Obstet Gynecol 16:407–413 [DOI] [PubMed] [Google Scholar]
- 17. Konje JC, Kaufmann P, Bell SC, Taylor DJ. 2001. A longitudinal study of quantitative uterine blood flow with the use of color power angiography in appropriate for gestational age pregnancies. Am J Obstet Gynecol 185:608–613 [DOI] [PubMed] [Google Scholar]
- 18. Acharya G, Sitras V, Erkinaro T, Mäkikallio K, Kavasmaa T, Päkkilä M, Huhta JC, Räsänen J. 2007. Experimental validation of uterine artery volume blood flow measurement by Doppler ultrasonography in pregnant sheep. Ultrasound Obstet Gynecol 29:401–406 [DOI] [PubMed] [Google Scholar]
- 19. Kiserud T, Rasmussen S, Skulstad S. 2000. Blood flow and the degree of shunting through the ductus venosus in the human fetus. Am J Obstet Gynecol 182:147–153 [DOI] [PubMed] [Google Scholar]
- 20. Ferrazzi E, Rigano S, Bozzo M, Bellotti M, Giovannini N, Galan H, Battaglia FC. 2000. Umbilical vein blood flow in growth-restricted fetuses. Ultrasound Obstet Gynecol 16:432–438 [DOI] [PubMed] [Google Scholar]
- 21. Acharya G, Wilsgaard T, Berntsen GK, Maltau JM, Kiserud T. 2005. Doppler-derived umbilical artery absolute velocities and their relationship to fetoplacental volume blood flow: a longitudinal study. Ultrasound Obstet Gynecol 25:444–453 [DOI] [PubMed] [Google Scholar]
- 22. Beebe LA, Cowan LD, Altshuler G. 1996. The epidemiology of placental features: associations with gestational age and neonatal outcome. Obstet Gynecol 87:771–778 [DOI] [PubMed] [Google Scholar]
- 23. Langston C, Kaplan C, Macpherson T, Manci E, Peevy K, Clark B, Murtagh C, Cox S, Glenn G. 1997. Practice guideline for examination of the placenta: developed by the Placental Pathology Practice Guideline Development Task Force of the College of American Pathologists. Arch Pathol Lab Med 121:449–476 [PubMed] [Google Scholar]
- 24. Ma Y, Krikun G, Abrahams VM, Mor G, Guller S. 2007. Cell type-specific expression and function of toll-like receptors 2 and 4 in human placenta: implications in fetal infection. Placenta 28:1024–1031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wallace JM, Milne JS, Matsuzaki M, Aitken RP. 2008. Serial measurement of uterine blood flow from mid to late gestation in growth restricted pregnancies induced by overnourishing adolescent sheep dams. Placenta 29:718–724 [DOI] [PubMed] [Google Scholar]
- 26. Wallace JM, Bourke DA, Aitken RP, Leitch N, Hay WW., Jr 2002. Blood flows and nutrient uptakes in growth-restricted pregnancies induced by overnourishing adolescent sheep. Am J Physiol Regul Integr Comp Physiol 282:R1027–R1036 [DOI] [PubMed] [Google Scholar]
- 27. Redmer DA, Luther JS, Milne JS, Aitken RP, Johnson ML, Borowicz PP, Borowicz MA, Reynolds LP, Wallace JM. 2009. Fetoplacental growth and vascular development in overnourished adolescent sheep at day 50, 90 and 130 of gestation. Reproduction 137:749–757 [DOI] [PubMed] [Google Scholar]
- 28. Reynolds LP, Borowicz PP, Vonnahme KA, Johnson ML, Grazul-Bilska AT, Wallace JM, Caton JS, Redmer DA. 2005. Animal models of placental angiogenesis. Placenta 26:689–708 [DOI] [PubMed] [Google Scholar]
- 29. Carter AM. 2007. Animal models of human placentation—a review. Placenta 28(Suppl A):S41–S47 [DOI] [PubMed] [Google Scholar]
- 30. Skillman CA, Plessinger MA, Woods JR, Clark KE. 1985. Effect of graded reductions in uteroplacental blood flow on the fetal lamb. Am J Physiol 249:H1098–H1105 [DOI] [PubMed] [Google Scholar]
- 31. Acharya G, Erkinaro T, Mäkikallio K, Lappalainen T, Rasanen J. 2004. Relationships among Doppler-derived umbilical artery absolute velocities, cardiac function, and placental volume blood flow and resistance in fetal sheep. Am J Physiol Heart Circ Physiol 286:H1266–H1272 [DOI] [PubMed] [Google Scholar]
- 32. Thompson RS, Trudinger BJ. 1990. Doppler waveform pulsatility index and resistance, pressure and flow in the umbilical placental circulation: an investigation using a mathematical model. Ultrasound Med Biol 16:449–458 [DOI] [PubMed] [Google Scholar]
- 33. Rigano S, Bozzo M, Ferrazzi E, Bellotti M, Battaglia FC, Galan HL. 2001. Early and persistent reduction in umbilical vein blood flow in the growth-restricted fetus: a longitudinal study. Am J Obstet Gynecol 185:834–838 [DOI] [PubMed] [Google Scholar]
- 34. Ramsay JE, Ferrell WR, Crawford L, Wallace AM, Greer IA, Sattar N. 2002. Maternal obesity is associated with dysregulation of metabolic, vascular, and inflammatory pathways. J Clin Endocrinol Metab 87:4231–4237 [DOI] [PubMed] [Google Scholar]
- 35. Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, Sole J, Nichols A, Ross JS, Tartaglia LA, Chen H. 2003. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest 112:1821–1830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr 2003. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest 112:1796–1808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Grayson BE, Levasseur PR, Williams SM, Smith MS, Marks DL, Grove KL. 2010. Changes in melanocortin expression and inflammatory pathways in fetal offspring of nonhuman primates fed a high-fat diet. Endocrinology 151:1622–1632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zhu MJ, Du M, Nathanielsz PW, Ford SP. 2010. Maternal obesity up-regulates inflammatory signaling pathways and enhances cytokine expression in the mid-gestation sheep placenta. Placenta 31:387–391 [DOI] [PubMed] [Google Scholar]
- 39. Kim YM, Romero R, Oh SY, Kim CJ, Kilburn BA, Armant DR, Nien JK, Gomez R, Mazor M, Saito S, Abrahams VM, Mor G. 2005. Toll-like receptor 4: a potential link between “danger signals,” the innate immune system, and preeclampsia? Am J Obstet Gynecol 193:921–927 [DOI] [PubMed] [Google Scholar]
- 40. Blankenship TN, Enders AC. 2003. Modification of uterine vasculature during pregnancy in macaques. Microsc Res Tech 60:390–401 [DOI] [PubMed] [Google Scholar]
- 41. Strümper D, Gogarten W, Durieux ME, Hartleb K, Van Aken H, Marcus MA. 2004. The effects of S+-ketamine and racemic ketamine on uterine blood flow in chronically instrumented pregnant sheep. Anesth Analg 98:497–502 [DOI] [PubMed] [Google Scholar]
- 42. Eng M, Bonica JJ, Akamatsu TJ, Berges PU, Ueland K. 1975. Respiratory depression in newborn monkeys at Caesarean section following ketamine administration. Br J Anaesth 47:917–921 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.