Estrogen receptor transcriptional activity oscillates in time with a period and amplitude that are tissue-specific and only partially modulated by circulating estrogens.
Abstract
By the use of in vivo imaging, we investigated the dynamics of estrogen receptor (ER) activity in intact, ovariectomized, and hormone-replaced estrogen response element-luciferase reporter mice. The study revealed the existence of a long-paced, noncircadian oscillation of ER transcriptional activity. Among the ER-expressing organs, this oscillation was asynchronous and its amplitude and period were tissue dependent. Ovariectomy affected the amplitude but did not suppress ER oscillations, suggesting the presence of tissue endogenous oscillators. Long-term administration of raloxifene, bazedoxifene, combined estrogens alone or with basedoxifene to ovariectomized estrogen response element-luciferase mice showed that each treatment induced a distinct spatiotemporal profile of ER activity, demonstrating that the phasing of ER activity among tissues may be regulated by the chemical nature and the concentration of circulating estrogen. This points to the possibility of a hierarchical organization of the tissue-specific pacemakers. Conceivably, the rhythm of ER transcriptional activity translates locally into the activation of specific gene networks enabling ER to significantly change its physiological activity according to circulating estrogens. In reproductive and nonreproductive organs this hierarchical regulation may provide ER with the signaling plasticity necessary to drive the complex metabolic changes occurring at each female reproductive status. We propose that the tissue-specific oscillatory activity here described is an important component of ER signaling necessary for the full hormone action including the beneficial effects reported for nonreproductive organs. Thus, this mechanism needs to be taken in due consideration to develop novel, more efficacious, and safer hormone replacement therapies.
The normal aging process in women results in menopause, which is characterized by the cessation of ovarian function and the decrease of sex hormone synthesis. Among the most common effects associated with menopause are vasomotor instability, loss of body mass, and altered lipid profile; in addition, several epidemiological studies indicated that after menopause there is a significant increase of cardiovascular (1, 2), immune (3), skeletal (4, 5), and central nervous system (6, 7) disorders, suggesting a protective action of estrogens also in tissues not directly associated with reproductive functions. The extension of the negative effects after the reduced synthesis of estrogens may be explained by the lack of activation of the two estrogen receptors (ER; ERα and ERβ) because ERs are expressed in most mammalian cells in which, by controlling specific transcription programs, they are deeply involved in the whole-cell metabolism (8).
Attempts to reinstate estrogen beneficial effects with hormone replacement therapies (HRT) did not provide the expected results so far. HRT has been carried out administering 17β-estradiol or conjugated estrogens (CE) to hysterctomized women; in nonhysterectomized women a combined therapy has been applied with progestagens opposing the hyperplastic effects of estrogen therapy in the uterus (9). To avoid estrogen unwanted effects on uterus and mammary gland but retain their beneficial effects in other organs efforts were made to identify ER ligands able to mimic the hormone activity in nonreproductive organs and to antagonize its effects in the uterus and mammary gland. Several such molecules, named selective estrogen receptor modulators (SERM) in virtue of their tissue-selective ER agonist or antagonist properties, were developed and entered clinical practice (10). To date, however, none of the SERM developed appear to be provided with the ideal balance of ER agonist and antagonist activity for an optimal postmenopausal therapy (11). For instance, the SERM used so far tend to be ER antagonists in the central nervous system, thus accentuating menopause vasomotor symptoms (11). To overcome these limitations, a novel HRT concept was proposed, which consists in the combination of natural estrogens with a SERM: in this way the whole spectrum of ER agonists effects can be obtained and the activity in the reproductive organs is blocked. This HRT has been named tissue selective estrogen complex (TSEC) (12, 13).
In all cases, HRTs are carried out by the continuous administration of the hormone aimed at maintaining a constant level of circulating estrogens: this is in contrast with the systemic, periodic fluctuations of ER activity due to the changes of ovarian functions during the menstrual cycle. The impact of continuous stimulation of a receptor system developed to respond to oscillating levels of hormone is unknown; thus, a better understanding of the periodic nature of ER signaling in the whole organism may be an important factor for the generation of more efficacious HRT. The goal of the present study was to investigate the effects of HRTs on the temporal organization of ER transcriptional activity in reproductive and nonreproductive tissues. To this aim, we took advantage of the estrogen response element (ERE)-luciferase (Luc) transgenic mouse characterized by the ubiquitous, ER-regulated expression of the luciferase gene (14). In this animal model, the possibility to measure luciferase activity in vivo by bioluminescence-based imaging provides the opportunity to investigate the ER systemic activity in time (14–17). Here we studied in a model of surgical menopause the extent to which current modalities of HRT, based on estrogens, SERM, and TSEC administration, were able to restore the physiological oscillation occurring in healthy, cycling mice (12, 13).
We show that in intact mice ER activity oscillates at a pace that is similar in each tissue but is not always synchronized on estrogen synthesis in the ovaries. In addition, our results demonstrate that, in each tissue, the continuous administration of natural or synthetic estrogens induces profiles of ER oscillation with an amplitude and frequency that are characteristic of each compound administered. Because of that, upon hormone therapy (HT) the phasing of ER activity among target organs may be significantly disrupted compared with what is observed in healthy, cycling mice. We propose that the decentralized ER oscillatory behavior observed in intact mice might respond to precise physiological needs and that the study of HT effects on ER oscillatory profile might provide a novel mean for the identification of safer and more efficacious HRT.
Materials and Methods
Animal studies
In the present study, we used heterozygous C57BL/6 repTOPERE-Luc (Transgenic Operative Products srl, Lodi, Italy) females 2–3 months of age (18). Mice were housed in individually ventilated plastic cages with hardwood chip bedding and animal house, fed ad libitum with a standard diet (4RF21 standard diet; Mucedola, Settimo Milanese, Italy) and provided with filtered water. The animal room was maintained within a temperature range of 22–25 C, relative humidity of 50 ± 10%, and under an automatic cycle of 12-h light, 12-h dark (lights on at 0700 h).
All animal experimentation was carried out in accordance with the Guide for the Care and Use of Laboratory Animals as adopted and promulgated by the U.S. National institutes of Health and also in accordance with the European Guidelines for Animal Care and Use of Experimental Animals, approved by the Italian Ministry of Research and University and controlled by the panel of experts of the Department of Pharmacological Sciences (University of Milan, Mila, Italy). Mice were ovariectomized 3 wk before the beginning of the study. Animals were assigned to a specific experimental group and treated per os (gavage) for 6 h (acute treatment) or daily for 21 d (chronic treatment) with vehicle, CE (3 mg/kg), bazedoxifen (BZA; 2 and 10 mg/kg), or BZA in association with 3 mg/kg CE (TSEC) and raloxifen (RAL; 2 and 10 mg/kg). All compounds tested (Wyeth, Collegeville, PA) were dissolved in dimethylsulfoxide and subsequently diluted in vehicle (2% Tween 80 and 0.5% carboxymethylcellulose water solution). Controls were treated with vehicle. Compounds were administered always at 0930 h; the in vivo imaging session took place at 1500 h.
In vivo imaging
Mice were injected ip with 80 mg/kg d-luciferin (beetle luciferin potassium salt; Promega, Madison, WI) 15 min before each in vivo imaging session. Previous experiments had demonstrated that this dose and time are sufficient to obtain an uniform biodistribution of the substrate (19, 20). Mice were anesthetized using isofluorane (Isofluorane-Vet; Merial, Lyon, France) and Xenogen XGI-8 gas anesthesia system (Caliper Life Sciences, Hopkinton, MA) and kept under anesthesia during the whole imaging session [gas anesthesia system setting: vaporizer value 2.5%; oxygen flow 1.5 l/min in the induction chamber and 0.25 l/min to the mice during the in vivo imaging in the charge-coupled device (CCD) camera]. Photon emission in mice was measured for 5-min-long periods using a CCD camera (Xenogen IVIS Lumina system; Caliper Life Sciences) consisting of a scientific-grade thermoelectrically cooled CCD camera mounted on a light-tight imaging chamber. Bioluminescence was measured with tiff images of 512 × 512 pixels at 16 bits. Each pixel contained the number of photon counts detected over the exposure period at the resolution of about 0.3 pixels/mm. Instrumental efficiency was measured with appropriate luminescent substrates (Glowell; Lux Biotechnology, Edinburgh, UK).
Automatic analysis of mouse images: segmentation algorithm
Anatomical areas (head, limbs, tail, reproductive area, hepatic area, abdominal area) were segmented in Matlab environment (TOP Transgenic Operative Products srl) using an algorithm previously described (21). The anatomical areas analyzed were: skeletal areas (bone and limbs), reproductive areas (genital and mammary glands), and hepatic and abdominal areas. In each anatomical area, photon emission was defined as the number of photons (p) per second per centimeter squared corrected for instrument efficiency. All the measurements were in the linearity range of the detector (IVIS Lumina; Caliper Life Sciences) and were previously validated by the comparison with the manual analysis carried out using the CCD camera software (Living Image 3.0; Caliper Life Sciences).
Luciferase enzymatic assay
Tissues were homogenized in 200 μl of lysis buffer (100 mm KPO4; 1 mm dithiothreitol; 4 mm EGTA; 4 mm EDTA, pH 7.8) with a 5-mm inox bead in a TissueLyser (QIAGEN GmbH, Hilden, Germany), subjected to one freezing-thawing cycle, and centrifuged for 30 min at 4900 × g at 4 C (Allegra 25R; Beckman Coulter, Brea, CA), and supernatants containing luciferase were collected in ice. In samples containing luciferase, protein concentration was measured by the Bradford assay, following the manufacturer's instructions (Thermo Fisher Scientific Inc., Waltham, MA). Luciferase enzymatic activity was then assayed with a commercial luciferase assay buffer (Promega). Light intensity was measured with a luminometer (Glomax96 microplate luminometer; Promega) and expressed as relative light units over 10 sec per microgram of proteins.
Vaginal smear
Vaginal smears were carried out at 0, 7, 14, and 21 d at 0900 h by vaginal flushing with sterile physiological solution, which was subsequently air dried on glass microscope slides and stained with the May-Grünwald and Giemsa methods (MGG Quick stain kit,; Bio-Optica, Milan, Italy) following the protocol provided by the manufacturer.
Measurement of the phasing of ER oscillatory in different body areas
The synchronic phasing of ER activity was analyzed manually by counting each body area by the total number of peaks of ER activity/mouse during the 21-d-long experiment and then the number of coincident peaks for each couple of body areas. The percentages of coincident/noncoincident peaks obtained from the six to 10 individual animals observed were then averaged.
Real-time PCR
Total liver RNA was extracted with RNeasy minikit (QIAGEN). cDNA was prepared as described (22). RT-PCR experiments were done by Sybr Green and TaqMan technology using the following primers: Fasn (forward, 5′-cctctgatcagtggcctcctc-3′; reverse, 5′-ggattcgggaatacaagtggc-3′); Acly (forward, 5′-gaagctgaccttgctgaaccc-3′; reverse, 5′-ccgtaattcgccagttcattg-3′); Pmvk (forward, 5′-atggggctgtgatacagacag-3′; reverse, 5′-caaagttcccaaagttgtcca-3′); and 36b4 as reference gene (forward, 5′-ggcgacctggaagtccaact-3′; reverse, 5′-ccatcagcaccacagccttc-3′). TaqMan gene expression assays for Foxo1 (Mm00490672_m1), Igf-1 (Mm00439561_m1), and as a reference gene assay 18S rRNA VIC-MGB-PDAR (Applied Biosystems by Life Technologies, Carlsbad, CA). The reaction was carried out according to the manufacturer's protocol using the 7900HT fast real-time PCR system (Applied Biosystems by Life Technologies). Data were analyzed using the 2−ΔΔCt method (23).
Statistical analysis
P values were calculated with one-way or two-way ANOVA followed by Bonferroni post hoc test or t test with GraphPad Prism version 5.02 for Windows (GraphPad Software, San Diego, CA).
Results
ER transcriptional activity is not centrally synchronized
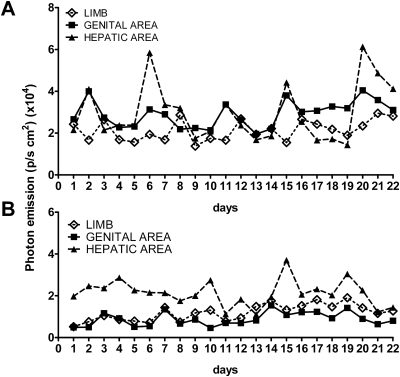
We first investigated the extent to which ER activity oscillated in intact and ovariectomized (ovx) mice. To this aim, we measured luciferase-dependent photon emission in nine intact and nine ovx mice for 21 d. In the cycling mice (Fig. 1A), luciferase activity oscillated with time. The amplitude of the oscillation was different in each body area: for instance, it was more pronounced in hepatic than the genital area or limbs. In most body areas, the frequency of oscillation appeared to be around 4 d (average in all tissues 4.4d); this was expected, considering that in mice the length of estrous cycle is 4–5 d; however, ER oscillations did not occur synchronously in all the body areas (e.g. in the limbs the peak of photon emission was generally retarded by 1 d with respect to the hepatic and genital areas that tended to oscillate in phase). Quite unexpectedly in these animals, ER activity fluctuated after ablation of the ovaries with an oscillation period of about 4 d (Fig. 1B), but the amplitude of the oscillation was lower than in intact mice and the phasing among organs was altered: for instance, photon emission in the hepatic and genital areas was seldom in phase.
Fig. 1.
Profile of photon emission in time in cycling and ovx ERE-Luc mice. Photon emission was measured daily at 1500 h in 6-month-old ERE-Luc mice cycling or ovariectomized 3 wk before the initiation of the study. Each animal group was constituted of nine mice. The figure represents daily photon emission (plotted as photons per second per square centimeter) in head, genital, and hepatic areas of a single mouse representative of the pattern of ER activity in intact (A) and ovx (B) female mice.
These results suggested that, in intact mice, ER activity oscillates with a frequency that is compatible with the estrous cycle; however, 17β-estradiol (E2) cannot be the master regulator of ER oscillatory activity because the oscillations were asynchronous and persisted after ovariectomy.
The measurement of mRNAs encoded by endogenous ER target genes confirms that ER activity is asynchronous in liver and bone of intact mice
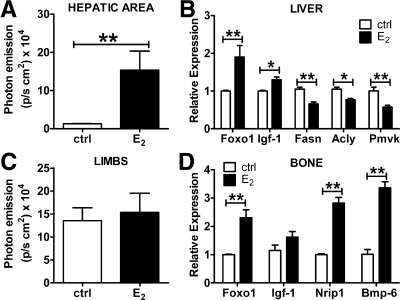
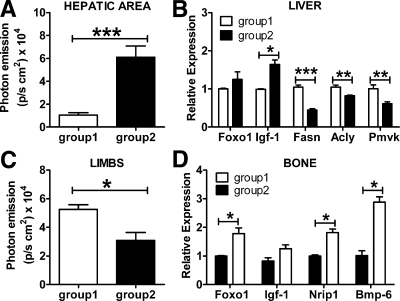
To assess the validity of the observation based on luciferase as an indicator of ER activity, we measured the content of several endogenous genes in bone and liver during the estrous cycle. The target genes were selected on the basis of previous reports (24–26) and of studies carried out in our laboratory (Stell A., personal communication). First, we demonstrated that the genes selected were good indicators of ER activity by measuring their mRNA content in tissue extracts of ovx mice treated with E2 for 6 h at the dose of 10 μg/kg sc. This treatment was sufficient to increase photon emission from 12,800 to 150,000 p/sec · cm2 in liver (Fig. 2A); the effect of acute E2 treatment on luciferase in bone was less visible (Fig. 2C). Real-time PCR analysis carried out with liver extracts showed that after the E2 treatment, Foxo1 and Igf-1 mRNA were significantly increased compared to controls (+140 and +31%, respectively). This is in line with previous publications by our and other laboratories. Conversely mRNAs such as fatty acid synthase (Fasn; −38%), ATP citrate lyase (Acly; −27%), and phosphomevalonate kinase (Pmvk; −43%), known to be encoded by genes repressed by ER, were significantly decreased by the treatment (Fig. 2B). In bone, the mRNAs encoding for Foxo1, Nrip1, and Bmp-6 were significantly increased by E2; in all experimental animals, Igf-1 mRNA content appeared to be higher than in controls, but the increase did not reach a statistically significant value (Fig. 2D). Next, we measured luciferase activity in 15 cycling ERE-Luc mice that were euthanized when luciferase activity was low in liver and high in bone (group 1) or, on the contrary, high in liver and low in bone (group 2) (Fig. 3, A and C). Real-time quantitative PCR analysis demonstrated a full correspondence between the content of the endogenous genes and luciferase activity as shown in Fig. 3, B and D. Indeed, Fig. 3B shows that in liver the genes induced by estrogens were low in group 1 (low liver luciferase) and high in group 2 (high liver luciferase). Conversely, the genes repressed by estrogens were lower in group 2 than in group 1. Similarly in bone (Fig. 3D), in which the genes selected were all positively regulated by E2, we observed that the mRNA content of Foxo1, Nrip1, and Bmp-6 was higher in group 1 than in group 2. These results led us to conclude that luciferase is a reliable indicator of ER transcriptional activity and can be used as a surrogate target gene in our studies on the effect of selected HT.
Fig. 2.
Luciferase activity in selected tissues of ERE-Luc mice and relative endogeneous gene target expression after acute treatment with E2. Photon emission in the hepatic area (A) and in limbs (C) was measured by a 5-min exposure time to a CCD camera (see Materials and Methods) in mice at 6 h after treatment with 10 μg/kg of E2 or vehicle (ctrl). After the imaging session, mice were euthanized and tissues rapidly dissected. B, Foxo1, Igf-1, Fasn, Acly, and Pmvk mRNA content in liver of control and E2-treated mice. D, Foxo1, Igf-1, Nrip1, and Bmp-6 mRNA content in bone of control and E2-treated mice. Columns represent means ± sem of groups of six mice each. *, P < 0.05; **, P < 0.01. P values were calculated with t test.
Fig. 3.
Comparative analysis of luciferase activity mice and endogeneous gene target expression in selected tissues of intact ERE-Luc. Photon emission in the hepatic area (A) and limbs (C) of mice of group 1 (low liver/high bone photon emission) and group 2 (high liver/low bone photon emission). B, Foxo1, Igf-1, Fasn, Acly, and Pmvk mRNA content measured by real-time quantitative PCR in tissue extracts of liver of group 1 and group 2 mice. D, Foxo1, Igf-1, Nrip1, and Bmp-6 mRNA content in bone of mice belonging to group 1 and group 2. Columns represent means ± sem of groups of six mice each. *, P < 0.05; **, P < 0.01; ***, P < 0.001. P values were calculated with t test.
Tissue-specific action of selected HT in short-term treatment (6 h)
The HT selected for the present study were: 1) natural estrogens largely used in HT (Premarin or CE); 2) two SERMs, BZA and RAL; and 3) TSEC (BZA together with CE) (12, 13). The selection of the dosage to be administered with regard to CE, BZA, and RAL was based on previous reports (27). Before starting the long-term study with the different types of HRT currently in use, we demonstrated their ability to regulate the luciferase reporter when administered orally at a dosage previously found to mimic HT in humans (10, 11, 15, 27). For the TSEC treatment, we tested two different concentrations of BZA (2 and 10 mg/kg) to identify the concentration necessary and sufficient to block CE effects when used in the combined therapy.
The study was carried out in ovx ERE-Luc mice at 2–3 months of age; surgery was performed 3 wk before HT. Two weeks after surgery, circulating levels of estradiol were undetectable (not shown) and the uterus weight was significantly decreased (−73% vs. proestrus, the phase of the reproductive cycle during which estrogen circulating levels are highest) (Supplemental Fig. 1A, published on The Endocrine Society's Journals Online web site at http://endo.endojournals.org). Ovarian ablation reduced significantly ER activity on the reporter gene (Supplemental Fig. 2), yet the persistence of luciferase pointed to the existence of factors other than ovarian estrogens able to elicit ER activity.
Six hours after treatment, CE induced a strong transcriptional activity of ER in the hepatic and genital areas (Supplemental Fig. 1, B and C). With other treatments, a trend to increase was observed in several of the areas studied, but none of the changes reached statistical significance. These results were confirmed by a more quantitative study in which luciferase activity was directly measured in tissue extracts (Supplemental Fig. 3): in CE-treated mice, luciferase activity was increased in the uterus (+550%), breast (+230%), liver (+3451%), and bone (+145%). The high, but not the low, concentration of both SERMs caused an increased ER activity in the breast (BZA, +289%; RAL, +286% vs. vehicle); RAL at both dosages was able to increase ER activity in the intestine (+159%). In this short-term study, BZA and RAL did not increase ER activity in bone despite the well-known protective effects of the two compounds against ovx-induced osteoporosis.
This preliminary study established that the sensitivity of the bioluminescence imaging was sufficient to investigate the effects of the treatments.
Long-term −21-d effect of HT with CE, SERM, or TSEC
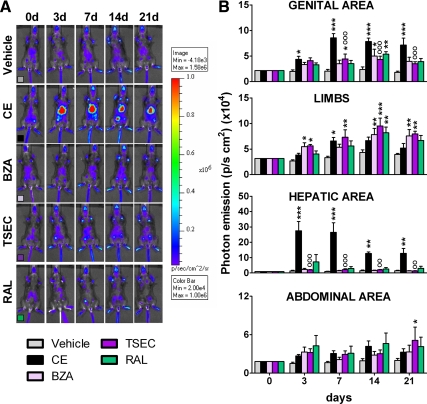
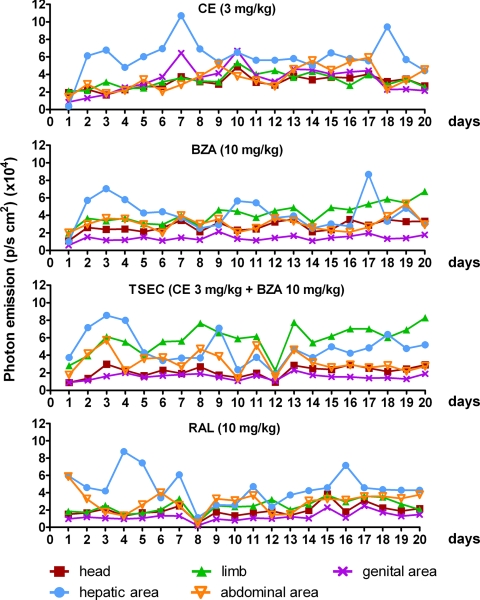
For the long-term study, ovx mice were treated daily by gavage with CE (3 mg/kg); BZA (10 mg/kg); TSEC (CE 3 mg/kg + BZA 10 mg/kg), and RAL (10 mg/kg). Details of the treatments are described in the methodology section. Photon emission at 3, 7, 14, and 21 d of treatment (Fig. 4, A and B) indicated the efficacy of each HT in specific body areas. In line with the short-term treatment, CE significantly increased ER activity in the genital area (up to +318% vs. vehicle at d 7); this effect was blocked by BZA. In this body area, SERM affected ER activity only transiently (d 14). Measurement of the uterus weight in long-term-treated animals further supported the lack of estrogenic effects of BZA, RAL, and TSEC in this organ (Supplemental Fig. 4). In limbs, CE and BZA were able to significantly increase ER activity transiently (CE, +98% at d 7 and BZA, +105% at d 3); although a trend to an increase was present all through out the study, TSEC had a strong effect augmenting ER activity in limbs from d 3 until the end of the treatment (d 3: +111%; d 7: +120%; d 14: +118%; d 21: +101%), and RAL had a delayed effect (d 14: +88%), which lasted to d 21 (+70%). In line with previous reports of estrogens in liver (14), CE had a major effect in the hepatic area that was blocked by BZA; BZA and RAL alone did not affect ER activity in this body region. Interestingly, in the hepatic area, but not in the genital area, the response to CE decreased with time, suggesting a tissue-specific mechanism diminishing ER activity upon prolonged stimulation. We failed to see any significant change in the abdominal area with the exception of TSEC, which had a delayed effect (d 21: +160%). The study of luciferase enzymatic activity in the tissues dissected from the animals at the end of the treatment (Supplemental Fig. 5) fully supported the in vivo data indicating that after 21 d of treatment, ER activity was still elevated in the uterus (+435%) and the liver (+868%) of mice treated with CE and in the bone (+64%) and the intestine (+333%) of those exposed to TSEC.
Fig. 4.
Photon emission of selected areas of ERE-Luc mice undergoing HT. A, Pseudocolor image of photon emission from one representative animal/group at 0, 3, 7, and 21 d of chronic oral treatment with vehicle, CE (3 mg/kg), BZA (10 mg/kg), TSEC (BZA 10 mg/kg + CE 3 mg/kg), and RAL (10 mg/kg). B, Photon emission measured as photons per second per square centimeter from specific body areas. Data represent mean ± sem of three groups of three animals (total nine animals/group). *, P < 0,05; **, P < 0,01; ***, P < 0,001 vs. vehicle; °°, P < 0,01; °°°, P < 0,001 vs. CE. P values were calculated with two-way ANOVA followed by Bonferroni post hoc test.
The observation that during the long-term treatment, photon emission appeared to decrease with time in the hepatic area but not in the genital area and limbs and that in selected areas the changes were transient, led us to further investigate the dynamics of ER activity by measuring photon emission daily.
Dynamics of ER activity during HT
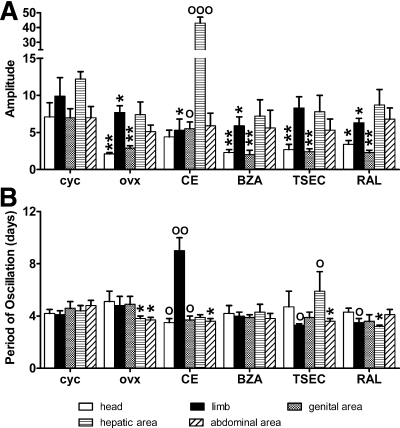
To obtain a more detailed knowledge of ER spatiotemporal activity, we measure photon emission daily in ovx mice treated as before and in intact, cycling mice. Despite the expected variability among individuals, luciferase activity appeared to oscillate in time with an amplitude and a frequency dependent on the type of treatment and the tissue taken in consideration (Fig. 5). The application of Fourier transform for the analysis of the effect of each treatment on ER activity in time showed that ovariectomy reduced the amplitude of the ER oscillations in the bone (head, −70% and limb, −22%, ovx vs. cycling), genital (−59%), and hepatic (−39%) areas but not in the abdominal area. CE treatment increased significantly the amplitude of photon emission in the genital (+90%) and hepatic areas (+481%) but in the bone and abdominal area was not able to restore the amplitude of oscillation observed in intact mice. The effect of BZA, TSEC, and RAL was not significant in any of the areas taken into consideration, even if a trend to increase was observed with TSEC in head (+29% and +62%, respectively) (Fig. 6A). On the other hand, OVX decreased significantly the period of ER oscillation in the hepatic (−14%) and abdominal areas (−23%). Only TSEC was able to reverse this effect in the hepatic area (+55% vs. ovx). In the bone areas, the effect of the treatments varied: CE increased the period in the limb (+88% vs. ovx) but decreased it in the head (−31%), whereas TSEC and RAL decreased the period in the limb (−31 and −27% vs. ovx, respectively) but did not affect the head. Only CE decreased the ER period of oscillation in the genital area (−20% vs. ovx) (Fig. 6B).
Fig. 5.
Profile of photon emission in time in ERE-Luc mice undergoing HT. Photon emission was measured daily in head, limbs, and genital, hepatic, and abdominal areas at 1500 h (6 h after the treatment) using a segmentation algorithm (22). The experiment was done in experimental groups each composed of nine mice. Graphs reproduce data obtained from a single, representative mouse/group.
Fig. 6.
Fourier transform (FT) analysis of the profile of ER activity in time to measure the amplitude and frequency of luciferase oscillation in different body areas of ERE-Luc intact (cyc) and ovx mice and in ovx mice with HT. FT was applied to the data described in Figs. 4 and 5. A, Average amplitude of cycles in each group of nine mice estimated by measuring the degree of displacement from the resting state (calculated as the square root of the 95th percentile of the power spectra). cyc, Cycling mice (or intact mice). B, Period of oscillation estimated by the inverse of the frequencies under the amplitude previously calculated. Bars represent average ± sem of groups of nine mice each. *, P < 0.05; **, P < 0.01 vs. cycling animals; °, P < 0.05; °°, P < 0.01 vs. ovx mice. P values were calculated with one-way ANOVA followed by Bonferroni post hoc test.
Is ER oscillatory activity relevant for the tissue-specific activity of the receptor?
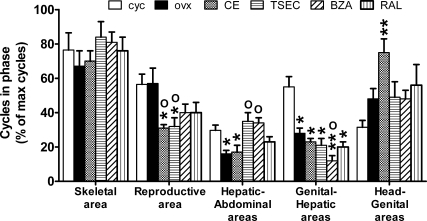
The above results did not point to a straightforward relationship between changes in the amplitude and period of ER transcriptional oscillation and the therapeutic efficacy of the compounds tested. For instance, the decreased amplitude observed in the skeletal tissues with ovx might suggest a relationship between cycle amplitude and ER state of activity. However, in ovx mice, compounds like RAL and BZA, known to protect against ovx-induced bone loss, were unable to rescue the amplitude of oscillation observed in the head and limbs of cycling animals. With respect to the period of oscillation, no difference was observed in the skeletal and genital areas between ovx and intact animals, suggesting that this parameter was not indicative of the efficacy of ER activity in those tissues. These observations suggested that other parameters should be taken into consideration for the identification of the compounds able to reinstate the beneficial effects of the natural estrous cycle. In view of the large number of tissues in which ER is active, we speculated that the beneficial effects of the hormone are due to a harmonic sequence of events in which the relative synchronization of ER activity in the different tissue is critical. We tried to test this hypothesis by counting the number of synchronous cycles in two body areas at the time during the 21 d of the experiment (Fig. 7). We reasoned that during the estrous cycle, the body areas representing functionally related tissues should cycle in synchrony. Indeed, in the skeletal areas (photon emission from head and limb) of cycling mice, the percentage of synchronic cycling was generally quite high (76.5%); also, reproductive (breast and genital area) and genital and hepatic areas were cycling relatively in phase (56 and 55% of the cycles were in phase, respectively). The latter observation had been previously reported (14). In the hepatic and abdominal areas, the percentage of synchrony was significantly lower (29.7%).
Fig. 7.
Effect of HT on the phasing of luciferase oscillation among different body areas of living ERE-Luc mice. The number of synchronous cycles/total cycles in the 21-d observation period was scored analyzing the profile of ER activity of the body areas of each single animal. Data represent the average percentage of synchronic cycling in four clusters of anatomical regions: skeletal area (head and limbs), reproductive area (breast and genital area), hepatic-abdominal areas, reproductive-hepatic areas, and head-genital areas. The experiment was done in experimental groups each composed of nine animals. cyc, Cycling mice (or intact mice).
Ovariectomy had a disruptive effect by decreasing the extent of synchronous phasing in the hepatic and abdominal areas (from 29.7 to 16%) and in the genital and hepatic area (from 55 to 28%). No significant change was observed in the skeletal and reproductive areas, whereas a trend to an increased phasing was measured in the head and genital areas.
Among HT, only TSEC and BZA were able to completely rescue the effect of ovx in the phasing between hepatic and abdominal areas (from 16 to 35 and 34%, respectively).
All HT, including CE, tended to decrease the synchrony of cycling of reproductive areas (from 57% to CE, 31%; TSEC, 32%; BZA, 40%; RAL, 40%). A trend to increase in the synchronic oscillation among skeletal tissues was observed with TSEC (from 67 to 84%), BZA (to 81%), and RAL (to 76%). When we compared head and genital areas, the only significant change was observed with the CE treatment that increased the phasing with respect to cycling mice (+138%).
Discussion
Pulsatility characterizes the secretion of several hormones (e.g. GH, GnRH, insulin), and the maintenance of a specific pattern of secretion is necessary for the hormone full endocrine effects (25–28). This may be valid also for estrogens; indeed, current view predicts that a finely tuned feedback system in the hypothalamic-pituitary-gonadal axis ensures the maintenance of the reproductive cycle regulated by the cyclic synthesis of ovarian sex hormones and the activation of ER transcriptional activity in reproductive as well as nonreproductive organs. The observations reported here argue against such a unicentric model of ER regulation because it is shown that: 1) in intact mice the systemic production of estrogens by the ovaries fails to synchronize ER activity among target organs; 2) after ovariectomy ER maintains a cyclic activity; and 3) each organ regulates autonomously the frequency and the amplitude of ER transcriptional activity in response to the administration of estrogens of different chemical nature or of a combination of estrogenic compounds.
At the present time, the mechanisms underlying the long-paced, rhythmic oscillation of ER activity as well as its physiological function may be only object of speculation. Western blot analysis and RT-PCR carried out on liver and uterus extracts show that during the long-paced ER transcriptional oscillation, the content of the receptor protein and mRNA is unchanged (not shown). Thus, we do not believe that the oscillation described here is a consequence of ER down-regulation.
The observation that the phasing of the oscillation is independent from a central control and is very susceptible to the nature of circulating estrogens may suggest that in each cell the ER activity is controlled autonomously via interlocking transcription/translation feedback loops. This would be in line with what reported for the rhythmic fluctuations of the circadian genes, which appear to be able to oscillate independently from the central oscillator located in the suprachiasmatic nucleus (28–30). Further supporting the hypothesis of cell autonomous regulatory mechanisms are recent studies carried out in isolated cells in which it was demonstrated that, in the constant presence of the ligand, ER activity fluctuates rapidly (in the order of minutes). The mechanisms driving the receptor oscillatory activity include changes in coregulator recruitment (31), assembly of the components of the preinitiation complex (32), and posttranslational regulation of the receptor leading to proteolysis of the ER complex (33). These oscillations are believed to be necessary to poise the receptors for a proper response to hormonal stimulus.
The fluctuations of ER activity described here have a periodicity very similar to the estrous cycle (4.4 d as average in the different tissues of cycling mice) yet are not synchronized on the fluctuations of the sex hormones in the blood stream. Because it is well known that several nonsteroidal stimuli may activate ER transcriptionally (15, 34–38), it is tempting to speculate that endocrine factors other than ovarian estrogen are responsible for the waves of receptor activation observed in the different ER-expressing cells. Supporting this hypothesis is a recent study on mice carrying a liver-selective ablation of ERα (39) in which it was demonstrated that hepatic ER play a major role in the synthesis and secretion of Igf-1, a liver hormone essential for several physiological functions including the maturation of the uterus epithelium and the full execution of the reproductive cycle. Considering the major involvement of ER in reproductive functions, a decentralized control of the activity of these receptors would serve the purpose to grant the reciprocal regulatory feedbacks to ensure that reproduction occurs in the most favorable energetic/metabolic/health conditions and to enable the significant metabolic adaptations associated with changes in the reproductive status (e.g. puberty, pregnancy, lactation). It is conceivable that in each target tissue the significant changes in estrogen synthesis and metabolism reported in different reproductive conditions are instrumental to modulate large transcription programs that trigger the metabolic response necessary for the successful reproduction. Several studies have underlined the relevance of the chemical stimulus triggering ER activity for the selection of the genes to be transcribed (40–43). We also showed by chip-on-chip analysis (data not shown) that in liver, ER associated with very distinct classes of promoters during the different phases of the estrous cycle. On the other hand, the ability of factors other than estrogens to regulate ER activity may be required to prevent pregnancy in case of disease or insufficient nutritional contribution. According to this view and in agreement with the results of the present study, the nature of the estrogenic stimulus may act as a trigger for the differential, rhythmic, and harmonic modulation of ER in the different organs necessary for the activation of the gene programs fulfilling the necessary metabolic program.
It remains to be established which is the hormonal setting that needs to be reestablished in the case of HT in the postmenopause. The study indicated that the use of SERM or a combination of natural hormones and SERM may have a significant effect on the relative phasing and intensity of ER activity in the target organs; this prospects the possibility to reproduce pharmacologically the desired complexity of ER action in the whole organism. What is lacking at the present time is a clear view of the pattern of ER activity that would have the most favorable effects for women's health during aging. In the absence of such knowledge, we believe that the mere analysis of the effects of HRT on a single parameter (e.g. the effect on the period or amplitude of ER activity in different organs) is not sufficient to establish the superiority of a treatment on others. In a recent study, Rando et al. (44) applied an algorithm developed for the comparative analysis of multivariate parameters to the study of the activity of synthetic ER ligands. Most interestingly, the study showed that the application of such an algorithm enables the identification of structurally related compounds by comparing their spatiotemporal effects on the ERE-Luc promoter. In addition, the study showed that the method enables one to measure the ability of each family of compounds to reproduce the state of activation of ER that characterize the intact, cycling mouse. We believe that these methodologies may, at the present time, facilitate the identification of HT to be applied.
Our current hypothesis that the hierarchical ER activation in different tissues is a mechanism set in evolution to enable ER to recognize the changes in the reproductive status and to alert and adapt the entire organism to the novel energetic requirements may help in a better understanding of the metabolic changes occurring after menopause and in devising novel, more efficacious therapeutic interventions.
Supplementary Material
Acknowledgments
We thank Paolo Sparaciari for veterinary assistance and A. Buscemi, C. Roncoroni, E. Galioto, and V. Benedusi for their assistance in the generation of the data and for their comments during the preparation of the manuscript.
This work was supported by European Union Grant STREP EWA LSHM-CT-2005-518245; National Institutes of Health Grant RO1AG027713; and Pfizer Pharmaceutical Co.
Disclosure Summary: S.D.T., A.B., G.R., G.M., and P.C. have nothing to declare. B.K. is employed by Pfizer and has stock/stock options in Pfizer. A.M. received grant support from Wyeth/Pfizer and received consulting fees from Wyeth/Pfizer.
Footnotes
- BZA
- Bazedoxifene
- CCD
- charge-coupled device
- CE
- conjugated estrogen
- E2
- 17β-estradiol
- ER
- estrogen receptor
- ERE
- estrogen response element
- HRT
- hormone replacement therapy
- HT
- hormone therapy
- Luc
- luciferase
- ovx
- ovariectomized
- RAL
- raloxifen
- SERM
- selective estrogen receptor modulator
- TSEC
- tissue selective estrogen complex.
References
- 1. Mendelsohn ME, Karas RH. 1999. The protective effects of estrogen on the cardiovascular system. N Engl J Med 340:1801–1811 [DOI] [PubMed] [Google Scholar]
- 2. Bolego C, Vegeto E, Pinna C, Maggi A, Cignarella A. 2006. Selective agonists of estrogen receptor isoforms: new perspectives for cardiovascular disease. Arterioscler Thromb Vasc Biol 26:2192–2199 [DOI] [PubMed] [Google Scholar]
- 3. Straub RH. 2007. The complex role of estrogens in inflammation. Endocr Rev 28:521–574 [DOI] [PubMed] [Google Scholar]
- 4. Imai Y, Kondoh S, Kouzmenko A, Kato S. 2009. Regulation of bone metabolism by nuclear receptors. Mol Cell Endocrinol 310:3–10 [DOI] [PubMed] [Google Scholar]
- 5. Cauley JA, Robbins J, Chen Z, Cummings SR, Jackson RD, LaCroix AZ, LeBoff M, Lewis CE, McGowan J, Neuner J, Pettinger M, Stefanick ML, Wactawski-Wende J, Watts NB. 2003. Effects of estrogen plus progestin on risk of fracture and bone mineral density: the Women's Health Initiative randomized trial. JAMA 290:1729–1738 [DOI] [PubMed] [Google Scholar]
- 6. Sherwin BB. 2003. Estrogen and cognitive functioning in women. Endocr Rev 24:133–151 [DOI] [PubMed] [Google Scholar]
- 7. Maggi A, Ciana P, Belcredito S, Vegeto E. 2004. Estrogens in the nervous system: mechanisms and nonreproductive functions. Annu Rev Physiol 66:291–313 [DOI] [PubMed] [Google Scholar]
- 8. Dahlman-Wright K, Cavailles V, Fuqua SA, Jordan VC, Katzenellenbogen JA, Korach KS, Maggi A, Muramatsu M, Parker MG, Gustafsson JA. 2006. International Union of Pharmacology. LXIV. Estrogen receptors. Pharmacol Rev 58:773–781 [DOI] [PubMed] [Google Scholar]
- 9. Anderson GL, Limacher M, Assaf AR, Bassford T, Beresford SA, Black H, Bonds D, Brunner R, Brzyski R, Caan B, Chlebowski R, Curb D, Gass M, Hays J, Heiss G, Hendrix S, Howard BV, Hsia J, Hubbell A, Jackson R, Johnson KC, Judd H, Kotchen JM, Kuller L, LaCroix AZ, Lane D, Langer RD, Lasser N, Lewis CE, Manson J, Margolis K, Ockene J, O'Sullivan MJ, Phillips L, Prentice RL, Ritenbaugh C, Robbins J, Rossouw JE, Sarto G, Stefanick ML, Van Horn L, Wactawski-Wende J, Wallace R, Wassertheil-Smoller S. 2004. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women's Health Initiative randomized controlled trial. JAMA 291:1701–1712 [DOI] [PubMed] [Google Scholar]
- 10. Turgeon JL, McDonnell DP, Martin KA, Wise PM. 2004. Hormone therapy: physiological complexity belies therapeutic simplicity. Science 304:1269–1273 [DOI] [PubMed] [Google Scholar]
- 11. Johnson KA. 2006. Editorial: the SERM of my dreams. J Clin Endocrinol Metab 91:3754–3756 [DOI] [PubMed] [Google Scholar]
- 12. Kharode Y, Bodine PV, Miller CP, Lyttle CR, Komm BS. 2008. The pairing of a selective estrogen receptor modulator, bazedoxifene, with conjugated estrogens as a new paradigm for the treatment of menopausal symptoms and osteoporosis prevention. Endocrinology 149:6084–6091 [DOI] [PubMed] [Google Scholar]
- 13. Komm BS. 2008. A new approach to menopausal therapy: the tissue selective estrogen complex. Reprod Sci 15:984–992 [DOI] [PubMed] [Google Scholar]
- 14. Ciana P, Raviscioni M, Mussi P, Vegeto E, Que I, Parker MG, Lowik C, Maggi A. 2003. In vivo imaging of transcriptionally active estrogen receptors. Nat Med 9:82–86 [DOI] [PubMed] [Google Scholar]
- 15. Klotz DM, Hewitt SC, Ciana P, Raviscioni M, Lindzey JK, Foley J, Maggi A, DiAugustine RP, Korach KS. 2002. Requirement of estrogen receptor-α in insulin-like growth factor-1 (IGF-1)-induced uterine responses and in vivo evidence for IGF-1/estrogen receptor cross-talk. J Biol Chem 277:8531–8537 [DOI] [PubMed] [Google Scholar]
- 16. Di Lorenzo D, Villa R, Biasiotto G, Belloli S, Ruggeri G, Albertini A, Apostoli P, Raviscioni M, Ciana P, Maggi A. 2002. Isomer-specific activity of dichlorodyphenyltrichloroethane with estrogen receptor in adult and suckling estrogen reporter mice. Endocrinology 143:4544–4551 [DOI] [PubMed] [Google Scholar]
- 17. Mussi P, Liao L, Park SE, Ciana P, Maggi A, Katzenellenbogen BS, Xu J, O'Malley BW. 2006. Haploinsufficiency of the corepressor of estrogen receptor activity (REA) enhances estrogen receptor function in the mammary gland. Proc Natl Acad Sci USA 103:16716–16721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ciana P, Di Luccio G, Belcredito S, Pollio G, Vegeto E, Tatangelo L, Tiveron C, Maggi A. 2001. Engineering of a mouse for the in vivo profiling of estrogen receptor activity. Mol Endocrinol 15:1104–1113 [DOI] [PubMed] [Google Scholar]
- 19. Biserni A, Giannessi F, Sciarroni AF, Milazzo FM, Maggi A, Ciana P. 2008. In vivo imaging reveals selective peroxisome proliferator activated receptor modulator activity of the synthetic ligand 3-[1-(4-chlorobenzyl)-3-t-butylthio-5-isopropylindol-2-yl]-2,2-dimethylpro panoic acid (MK-886). Mol Pharmacol 73:1434–1443 [DOI] [PubMed] [Google Scholar]
- 20. Rando G, Biserni A, Ciana P, Maggi A. 2010. Profiling of drug action using reporter mice and molecular imaging. Methods Mol Biol 602:79–92 [DOI] [PubMed] [Google Scholar]
- 21. Rando G, Casiraghi E, Arca S, Campadelli P, Maggi A. Automatic segmentation of mouse images. In: Capasso V, et al., Stereology and image analysis. Proc 10th European Congress of ISS, Bologna (Italy), 2009, Esculapio, 60–64 [Google Scholar]
- 22. Ciana P, Biserni A, Tatangelo L, Tiveron C, Sciarroni AF, Ottobrini L, Maggi A. 2007. A novel peroxisome proliferator-activated receptor responsive element-luciferase reporter mouse reveals gender specificity of peroxisome proliferator-activated receptor activity in liver. Mol Endocrinol 21:388–400 [DOI] [PubMed] [Google Scholar]
- 23. Livak KJ, Schmittgen TD. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2[−ΔΔ C(T)] method. Methods 25:402–408 [DOI] [PubMed] [Google Scholar]
- 24. Stossi F, Barnett DH, Frasor J, Komm B, Lyttle CR, Katzenellenbogen BS. 2004. Transcriptional profiling of estrogen-regulated gene expression via estrogen receptor (ER) α or ERβ in human osteosarcoma cells: distinct and common target genes for these receptors. Endocrinology 145:3473–3486 [DOI] [PubMed] [Google Scholar]
- 25. Krum SA, Miranda-Carboni GA, Lupien M, Eeckhoute J, Carroll JS, Brown M. 2008. Unique ERα cistromes control cell type-specific gene regulation. Mol Endocrinol 22:2393–2406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bland R. 2000. Steroid hormone receptor expression and action in bone. Clin Sci (Lond) 98:217–240 [PubMed] [Google Scholar]
- 27. Peano BJ, Crabtree JS, Komm BS, Winneker RC, Harris HA. 2009. Effects of various selective estrogen receptor modulators with or without conjugated estrogens on mouse mammary gland. Endocrinology 150:1897–1903 [DOI] [PubMed] [Google Scholar]
- 28. Vujovic N, Davidson AJ, Menaker M. 2008. Sympathetic input modulates, but does not determine, phase of peripheral circadian oscillators. Am J Physiol Regul Integr Comp Physiol 295:R355–R360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mahoney CE, Brewer D, Costello MK, Brewer JM, Bittman EL. 2010. Lateralization of the central circadian pacemaker output: a test of neural control of peripheral oscillator phase. Am J Physiol Regul Integr Comp Physiol 299:R751–R761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Escobar C, Cailotto C, Angeles-Castellanos M, Delgado RS, Buijs RM. 2009. Peripheral oscillators: the driving force for food-anticipatory activity. Eur J Neurosci 30:1665–1675 [DOI] [PubMed] [Google Scholar]
- 31. Métivier R, Penot G, Hübner MR, Reid G, Brand H, Kos M, Gannon F. 2003. Estrogen receptor-α directs ordered, cyclical, and combinatorial recruitment of cofactors on a natural target promoter. Cell 115:751–763 [DOI] [PubMed] [Google Scholar]
- 32. Stenoien DL, Patel K, Mancini MG, Dutertre M, Smith CL, O'Malley BW, Mancini MA. 2001. FRAP reveals that mobility of oestrogen receptor-α is ligand- and proteasome-dependent. Nat Cell Biol 3:15–23 [DOI] [PubMed] [Google Scholar]
- 33. Lonard DM, Nawaz Z, Smith CL, O'Malley BW. 2000. The 26S proteasome is required for estrogen receptor-α and coactivator turnover and for efficient estrogen receptor-α transactivation. Mol Cell 5:939–948 [DOI] [PubMed] [Google Scholar]
- 34. Ignar-Trowbridge DM, Nelson KG, Bidwell MC, Curtis SW, Washburn TF, McLachlan JA, Korach KS. 1992. Coupling of dual signaling pathways: epidermal growth factor action involves the estrogen receptor. Proc Natl Acad Sci USA 89:4658–4662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kato S, Endoh H, Masuhiro Y, Kitamoto T, Uchiyama S, Sasaki H, Masushige S, Gotoh Y, Nishida E, Kawashima H, Metzger D, Chambon P. 1995. Activation of the estrogen receptor through phosphorylation by mitogen-activated protein kinase. Science 270:1491–1494 [DOI] [PubMed] [Google Scholar]
- 36. Coleman KM, Smith CL. 2001. Intracellular signaling pathways: nongenomic actions of estrogens and ligand-independent activation of estrogen receptors. Front Biosci 6:D1379–D1391 [DOI] [PubMed] [Google Scholar]
- 37. Walters MR, Dutertre M, Smith CL. 2002. SKF-82958 is a subtype-selective estrogen receptor-α (ERα) agonist that induces functional interactions between ERα and AP-1. J Biol Chem 277:1669–1679 [DOI] [PubMed] [Google Scholar]
- 38. Veeneman GH. 2005. Non-steroidal subtype selective estrogens. Curr Med Chem 12:1077–1136 [DOI] [PubMed] [Google Scholar]
- 39. Della Torre S, Rando G, Meda C, Stell A, Chambon P, Krust A, Ibarra C, Magni P, Ciana P, Maggi A. 2011. Amino acid-dependent activation of liver estrogen receptor α integrates metabolic and reproductive functions via IGF-1. Cell Metab 13:205–214 [DOI] [PubMed] [Google Scholar]
- 40. Sismondi P, Biglia N, Ponzone R, Fuso L, Scafoglio C, Cicatiello L, Ravo M, Weisz A, Cimino D, Altobelli G, Friard O, De Bortoli M. 2007. Influence of estrogens and antiestrogens on the expression of selected hormone-responsive genes. Maturitas 57:50–55 [DOI] [PubMed] [Google Scholar]
- 41. Davis AM, Mao J, Naz B, Kohl JA, Rosenfeld CS. 2008. Comparative effects of estradiol, methyl-piperidino-pyrazole, raloxifene, and ICI 182 780 on gene expression in the murine uterus. J Mol Endocrinol 41:205–217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Miki Y, Suzuki T, Nagasaki S, Hata S, Akahira J, Sasano H. 2009. Comparative effects of raloxifene, tamoxifen and estradiol on human osteoblasts in vitro: estrogen receptor dependent or independent pathways of raloxifene. J Steroid Biochem Mol Biol 113:281–289 [DOI] [PubMed] [Google Scholar]
- 43. Chang KC, Wang Y, Bodine PV, Nagpal S, Komm BS. 2010. Gene expression profiling studies of three SERMs and their conjugated estrogen combinations in human breast cancer cells: insights into the unique antagonistic effects of bazedoxifene on conjugated estrogens. J Steroid Biochem Mol Biol 118:117–124 [DOI] [PubMed] [Google Scholar]
- 44. Rando G, Horner D, Biserni A, Ramachandran B, Caruso D, Ciana P, Komm B, Maggi A. 2010. An innovative method to classify SERMs based on the dynamics of estrogen receptor transcriptional activity in living animals. Mol Endocrinol 24:735–744 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.