Abstract
Platelets contain unspliced heteronuclear IL-1β RNA, which is rapidly spliced and translated upon activation. LPS is a superior agonist for this atypical platelet response, but how LPS induces proinflammatory cytokine production in anucleate cells lacking NF-κB is unknown. Platelets express functional TLR4, and stimulation by LPS induced rapid splicing, translation and secretion of mature IL-1β after caspase-1 processing. LPS stimulated microparticle shedding, and secreted IL-1β was exclusively present in these particles. Microparticles from LPS-stimulated platelets induced VCAM-1 production by cultured human endothelial cells, and blockade of endothelial IL-1β receptor with IL-1 receptor antagonist completely suppressed endothelial activation. Splicing was post-transcriptional as the SR kinase inhibitor TG003 blocked IL-1β RNA production by platelets, but not by monocytes, and was dependent on exogenous CD14 - a property of platelets. We used a combination of small molecule inhibitors, cell-penetrating chimeric peptide inhibitors, and gene-targeted animals to show splicing required MyD88 and TIRAP, and IRAK1/4, AKT and JNK phosphorylation and activation. TRAF6 couples MyD88 to the AKT pathway and, remarkably, a TRAF6 interacting peptide-antennapedia chimera was more effective than LPS in stimulating IL-1β splicing. The TRAF6 chimera did not, however, stimulate microparticle shedding, nor was IL-1β released. We conclude LPS-induced kinase cascades are sufficient to alter cellular responses, that three signals emanate from platelet TLR4, and that AKT and JNK activation are sufficient to initiate post-transcriptional splicing while another event couples microparticle shedding to TLR4 activation. Platelets contribute to the inflammatory response to LPS through production of microparticles that promote endothelial cell activation.
Introduction
Platelet activation plays an important role in a variety of high mortality prothrombotic/proinflammatory disease states, including disseminated intravascular coagulation and acute respiratory distress syndrome (ARDS). Gram-negative sepsis is a leading cause of ARDS, resulting in pulmonary platelet sequestration, elevated pro-inflammatory cytokines, and diffuse alveolar damage (1). Lipopolysaccharide (LPS) of gram-negative bacteria causes rapid thrombocytopenia and platelet sequestration in the lungs and liver (2–4). Despite this, the role of platelets in sepsis is poorly understood. Mice that lack the toll-like receptor 4 (TLR4), the LPS receptor, cannot recognize LPS and are resistant to its pathologic effects (5), and platelet experiments from wild-type mice introduced into TLR4−/− mice show platelets themselves are required for the septic response (6). LPS is not a typical platelet agonist since isolated platelets do not aggregate in its presence (7). In fact, platelets can respond in a variety of ways aside from aggregation, such as bacterial trapping and killing (8), and promoting apoptosis in intraerythrocytic malarial parasites (9). We previously demonstrated LPS is a direct platelet agonist resulting in production and release of pro-inflammatory cytokines (10). Platelets can splice stored intron-containing heteronuclear RNA to produce mature mRNA from which cytokines and other factors are produced (10, 11). Most notably, human platelets splice tissue factor and IL-1β RNA when exposed to thrombin. For these types of responses LPS is more effective than thrombin.
Platelets detect and respond to LPS via TLR4, a trans-membrane member of a family of receptors important in recognizing pathogenic molecules (6, 12, 13). Platelets lack CD14, a lipid-binding chaperone required for TLR4 activation, but plasma contains soluble CD14 in sufficient concentrations to present LPS to platelet TLR4 (14). LPS activated TLR4 recruits either of two downstream signaling complexes that are MyD88-dependant or MyD88-independent. The MyD88-dependant complex recruits and activates the kinases IRAK1 and IRAK4 that, in nucleated cells, promotes IκB degradation and translocation of the transcription factor NF-κB to the nucleus. Although platelets contain NF-κB (15, 16), they lack nuclei and their activation does not include NF-κB driven gene expression. How LPS therefore stimulates a select group of platelet functions is unknown, but likely lies in kinase activation that in nucleated cells are the intermediaries between TLR4 and NF-κB translocation.
Although much is known about MAP kinases in nucleated cells, their role in platelet biology is incompletely understood. Kauskot et al demonstrated that JNK is involved in ADP-dependant collagen-induced platelet aggregation, but not platelet adhesion (17). Studies by Chen et al revealed that oxidized-LDL signaled through CD36 and increased JNK activity via src kinases, contributing to platelet hyperactivity in hyperlipidemia models (18). Akt is a kinase with anti-apoptotic properties in many cell types, but in platelets it is involved in aggregation subsequent to GPVI collagen receptor activation (19, 20). Exceedingly high, non-physiologic amounts of LPS stimulate CD14-independent kinase activation in impure platelet preparations (21), promoting their degranulation. These responses are not seen in response to low amounts of LPS presented by CD14 (10). Whether platelets employ intermediary kinases in their response to LPS when presented in a pathophysiologically relevant way is unknown.
Platelets comprise an essential component of the response to sepsis (4, 22), but what makes platelets distinctive in this cytokine storm evoked by LPS is incompletely described. We hypothesize that one or more of the kinases promoting platelet response to typical agonists would cooperate with kinases found in nucleated cells and together transmit responses from platelet TLR4 in response to LPS. We found LPS does activate a kinase cascade in platelets that is required for stimulated IL-1β production. We also observed that LPS signaling promoted the production of platelet microparticles, and that these were pro-inflammatory by virtue of the caspase-1-dependent IL-1β they express.
Materials and Methods
Cell isolation
Human blood was drawn into acid-citrate-dextrose and centrifuged (200 ×g, 20 min) to obtain platelet-rich plasma in a protocol approved by the Cleveland Clinic IRB. All centrifugations were performed without braking. Platelet-rich plasma was filtered through two layers of 5 μ mesh (BioDesign) to remove nucleated cells and recentrifuged (500 × g, 20 min) in the presence of100 nM prostaglandin E1. The pellet was resuspended in 50 ml PIPES/saline/glucose (5 mM PIPES, 145 mM NaCl, 4 mM KCl, 50 μM Na2HPO4, 1 mM MgCl2, and 5.5 mM glucose) containing100 nM of prostaglandin E1. These cells were centrifuged (500 × g, 20 min), resuspended in AutoMACS sample buffer, 5 μl anti-CD45-, anti-CD15-, anti-CD14- and anti-glycophorin-coated magnetic beads (Miltenyi Biotec) per 109 cells for 25 min with constant rotation before purification in an AutoMACS magnetic separator (Miltenyi Biotec). For some experiments, this negative microbead selection was repeated. Platelets are primarily null for these antigens, but negative selection may exclude small populations that have acquired these markers. This negative selection sorting process resulted in a platelet population containing approximately 1 monocyte per 2×109 platelets based on CD14 mRNA content (10). Light microscopy was used to confirm the cells had a discoidal, unactivated shape. Recovered platelets were centrifuged (500 × g, 20 min) and resuspended in HBSS/A (0.5% human serum albumin in HBSS) at 2 × 108 cells/ml for quantitative reverse transcriptase PCR, and at 8 × 108 platelets/ml for all other uses. Platelet activation was induced for the stated time with 100 ng/ml LPS with addition of 100 ng/ml each of human recombinant CD14 and LPS-binding protein.
Mouse blood was a generous gift from the laboratory of Dr. Clifford Harding (Case Western Reserve University). Briefly, whole blood was obtained via cardiac puncture into acid-citrate-dextrose containing 100 nM of prostaglandin E1 and centrifuged (100 × g, 10 min) to obtain platelet-rich plasma. Reduction of nucleated cells was achieved by gel-filtration of platelet-rich plasma. Platelet yield was determined by cell-counting using a hemocytometer.
HUVEC Cell Culture and VCAM-1 Expression
Human umbilical vein endothelial cells (HUVEC) were kindly provided by Dr. Paul DiCorletto (Cleveland Clinic Foundation, Lerner Research Institute). Briefly, HUVEC were plated overnight in 96-well plates in MCDB-105 media supplemented with 15% fetal bovine serum. The next day, cells were washed twice with PBS (pH 7.4) and preincubated with IL-1 receptor antangonist for 30 minutes. HUVEC were incubated with microparticles for 6 hours. Cells were then washed three times with PBS (pH 7.4) and fixed in 4% paraformaldehyde for 30 min on ice. Cells were subsequently washed and blocked overnight with 5% bovine serum albumin. The day after blocking, cells were incubated with anti-VCAM-1 primary antibody (Santa Cruz Biotechnology) for 2 h at room temperature. After three washes with PBS (pH 7.4), cells were incubated with sheep anti-mouse horseradish peroxidase-conjugated secondary antibody (Biorad) for 1 h at room temperature. 3,3′,5,5′-Tetramethylbenzidine (TMB) substrate was subsequently added to each well and the reaction stopped after 20 min by addition of 1 M HCl. Absorbance was recorded at 450 nm on a 96-well plate reader (Spectramax 384 Plus, Molecular Devices, Sunnyvale, CA).
Microparticle quantification
Human platelet microparticles were counted using known amounts of 3 μm polystyrene latex beads (Sigma) added to FACS tubes just prior analysis. FSC and SSC gates were drawn to include 50,000 3 μm events. Microparticle size was determined using 1 μm beads (Sigma). FACS analysis was performed using settings where the threshold was lowered to 200 and FSC and SSC gates were drawn to include events 1μm in size and smaller.
IL-1β ELISA
Isolated platelets from human donors were incubated for 6 hours at 37° C in the presence or absence of 100 ng/mL of LPS. Cells were lysed and analyzed via ELISA’s detecting mature IL-1β and pro-IL-1β (R&D Systems). We compared resting platelets to treated platelets and then subtracted signal from pro-IL-1β. Ratios were then generated against control platelets, expressing the data as fold-increase of mature IL-1β protein over controls.
RNA isolation and real time RT-PCR
Total RNA from 2.0 × 108 platelets was isolated using RNEasy Mini Kit (Qiagen) and treated with RNase-free DNase (Qiagen). Total RNA was quantitated by NanoDrop and used to normalize PCR samples. Real time reverse transcriptase-PCR primers for: human IL-1β mRNA, sense 5′-AAACCTCTTCGAGGCACAAG-3′ (exon 1), antisense 5′-GTTTAGGGCCATCAGCTTCA-3′ (exon 3); mouse IL-1β: sense 5′-CGAGGCTAATAGGCTCATCT-3′, antisense 5′-GTTTGGAAGCAGCCCTTCAT-3′. Conditions for IL-1β were: reverse transcription (50°C, 30′); PCR (94°C, 15″; 61°C, 30″; 72°C,30″); data collection (80°C, 15″), 40 45 cycles with SYBR Green I in a BioRad MyiQ iCycler. Amplification across intron 2 does not detect unprocessed heteronuclear IL-1β RNA. Results were normalized using real-time PCR data of 18S ribosomal RNA (Ambion). Products were analyzed by melting curve, gel electrophoresis, and sequencing. DNase I treatment did not affect IL-1β mRNA expression, while RNase I treatment and reverse transcriptase removal abolished amplification.
Chemicals and reagents
Chemicals and reagents were purchased from the following sources: sterile filtered HBSS and M199 (Bio Whittaker); sterile tissue culture plates, (Falcon Labware); human serum albumin, (Baxter Healthcare); endotoxin-free PBS, phenol-extracted LPS (Escherichia coli O111:B4) that is free of lipoprotein contamination (List Biological Laboratories); Cdc2-like kinase (CLK-1) inhibitor TG003 (Calbiochem); recombinant soluble CD14, LBP, and IL-1β ELISA kit, (R&D Systems); phospho-JNK, total JNK, phospho-Akt, and total Akt ELISA kits (Cell Signaling). The JNK inhibitor SP600125 and Akt inhibitor VIII were obtained from EMD Biosciensces. The monoclonal3ZD anti-IL-1β antibody, which recognizes both 33-kDa pro-IL-1β and 17-kDa mature IL-1β in Western blot analysis, was a generous gift from the laboratory of Dr. George Dubyak (Case Western Reserve University) and provided by the Biological Resources Branch, National Cancer Institute, Frederick Cancer Research and Development Center (Frederick, MD). Caspase-1 inhibitor (FMK002) was obtained from R&D Systems. Caspase-1 antibody (sc-515) was obtained from Santa Cruz Biotechnologies. Other chemicals were from Sigma-Aldrich or Biomol Research Laboratories. The amino acid sequences of inhibitory peptides (Imgenex) are: MyD88, DRQIKIWFQNRRMKWKKRDVLPGT; TIRAP, DRQIKIWFQNRRMKWKKLQLRDAAPGGAIVS; and Traf6, DRQIKIWFQNRRMKWKKRKIPTEDEY. Underlined amino acids represent antennapedia protein transduction domain.
Expression of data and statistics
Experiments were performed at least three times with cells from different donors, and all assays were performed in triplicate. The standard errors of the mean from all experiments are presented as error bars. Graphing of figures and statistical analyses were generated with Prism4 (GraphPad Software). A value of p < 0.05 was considered statistically significant.
Results
LPS Stimulates Platelet Release of Inflammatory Microparticles Containing IL-1β
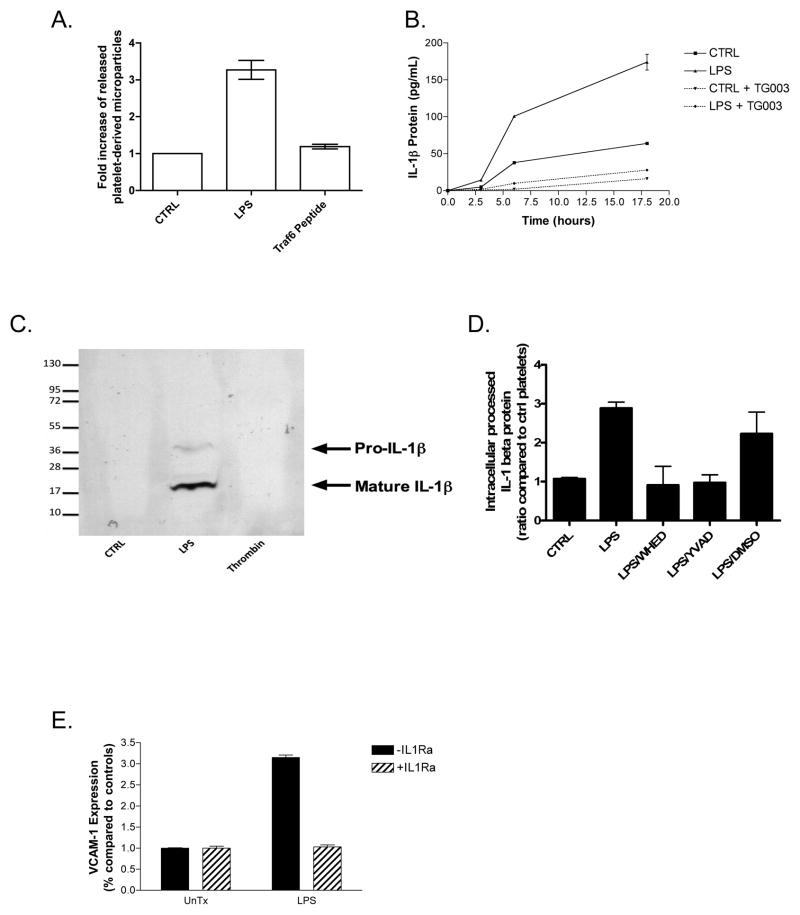
Platelets treated with LPS shed approximately three times as many microparticles as control platelets (Fig. 1A). Release of IL-1β protein from LPS-stimulated human platelets was time-dependant with a five-fold increase 6 hours after stimulation that increased to eight-fold by 18 hours as measured by ELISA (Fig. 1B). Platelets not exposed to LPS also release IL-1β protein during this incubation, although to a lesser extent than stimulated cells. IL-1β protein emanating from LPS-stimulated cells, and those undergoing limited self-activation in the absence of LPS, was completely dependent on splicing of IL-1β heteronuclear RNA to remove introns because the SR kinase inhibitor TG003 that blocks this process (23) abolished IL-1β release. This inhibitor of post-transcriptional RNA processing did not block co-transcriptional IL-1β production in nucleated cells (data not shown). Additionally, the transcriptional inhibitors actinomysin D and α-O-amanatin failed to inhibit this enriched platelet population, but were active against monocyte preparations in concordant experiments (data not shown).
Figure 1.
LPS–stimulated platelets splice IL-1β RNA, process IL-1β protein, and shed IL-1β-laden microparticles, which activate endothelial cells. (A) Platelet microparticles were collected after overnight LPS exposure (100 ng/mL in the presence of recombinant CD14 and LPS-binding protein) or a TRAF6 interacting chimeric peptide before quantitation by flow cytometry. (B) Human platelets (4×108 per condition) were treated with LPS for the stated times in combination with recombinant human CD14 and LPS-binding protein and secreted IL-1β protein was measured by ELISA. (C) Microparticles generated after overnight treatments were resuspended in reducing SDS sample buffer and probed for IL-1β after SDS-PAGE in a western blot. (D) Platelet lysates were probed by ELISA for mature and pro-IL-1β protein after LPS treatment, in the presence or absence of caspase inhibitors. (E) Purified platelet microparticles were added to HUVEC’s for 6 hours before VCAM-1 expression was determined by ELISA. IL-1Ra (150ng/mL) was added 30 minutes prior to microparticle addition. Error bars = +/− 1 SE, N=3 for (A–D) and N=8 for (E).
We collected microparticles from LPS-treated platelets and determined they contained mainly the proteolytically processed form of IL-1β by SDS-PAGE and western blot analysis, while unstimulated platelets and or those treated with thrombin contained undetectable levels of either form of IL-1 due to reduced sensitivity of western blot analysis compared to ELISA data (Fig. 1C vs Fig. 1B). We probed platelet lysates for activated caspase-1 using an antibody recognizing the p10 subunit and found the active 10 kDa fragment in LPS-stimulated cells (data not shown). We also found by ELISA that capsase-1 inhibition by either of two selective inhibitors prevented the formation of mature IL-1β protein (Fig. 1D). Thus, platelets treated with LPS showed significant processing of IL-1β protein, which was absent in control platelets.
We recovered these particles and also found they induced a three-fold increase in VCAM-1 expression in quiescent endothelial cells (Fig. 1E). The inflammatory principle present in the purified microparticles was nearly exclusively particle-bound IL-1β because endothelial cell VCAM-1 expression was ablated by the specific IL-1 receptor antagonist, IL1Ra. VCAM-1 induction was a property of the platelet-derived microparticles themselves, and not from LPS carryover, because only platelets require an exogenous source of CD14, and platelets exposed to LPS without this cofactor did not initiate VCAM-1 expression by HUVEC’s (data not shown). Additionally, HUVEC’s treated with polymixin B to interfere with LPS stimulation responded to LPS-induced microparticles, ruling out LPS carryover as the agent responsible endothelial activation in our experiments. Finally, platelet microparticles generated in response to thrombin stimulation, subsequently incubated with LPS, washed, and then applied to HUVEC’s, failed to stimulate the endothelial cells to produce VCAM-1 (data not shown).
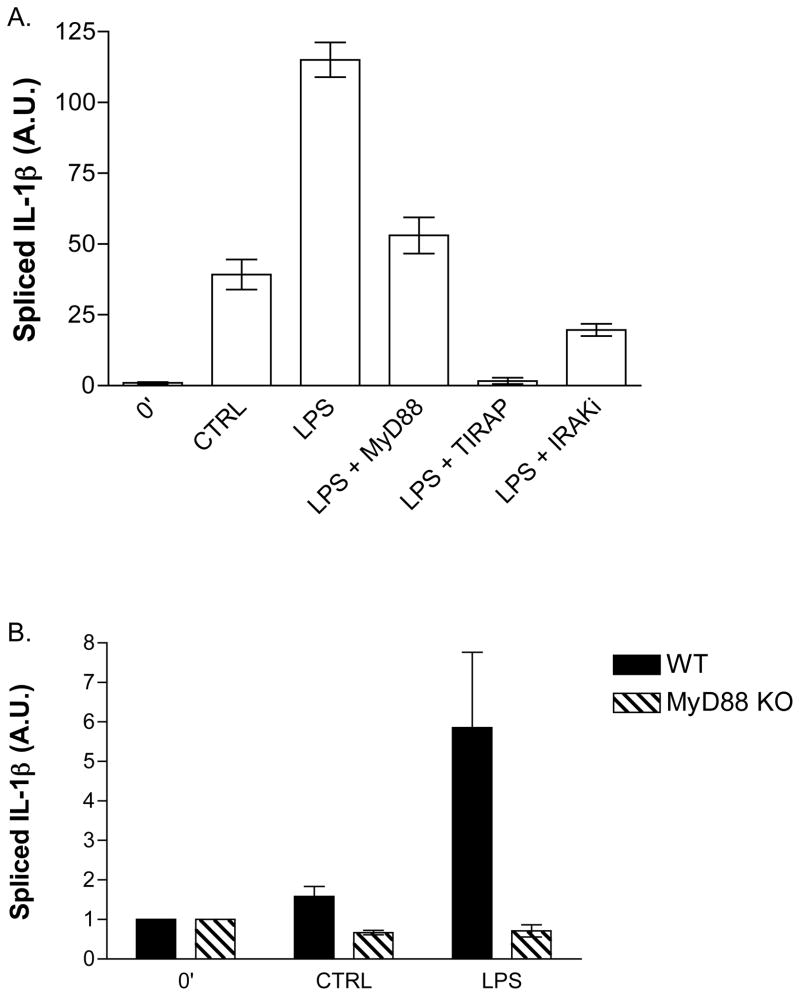
MyD88 and TIRAP Are Involved in LPS-Stimulated IL-1β Production
Resting platelets contain virtually no processed and functional IL-1β mRNA (11). After 3 hours of stimulation by LPS, the level of processed, intronless RNA increased by nearly 100-fold (Fig. 2A). Platelets are sensitive cells and after 3 hours of incubation have undergone mild auto-activation that increased spliced IL-1β RNA by 35-fold. We tested the involvement of MyD88 in the response of platelets to LPS using a chimeric peptide that blocks LPS-stimulated IL-1 production in dendritic cells (24). This peptide consists of an antennapedia sequence that translocates across membranes and a sequence from MyD88 that competitively blocks MyD88 homodimerization. We found that the antennapedia-MyD88 chimeric peptide reduced spliced IL-1β in platelets by nearly half. This reduced level of spliced IL-1β was not significantly different from the content in unstimulated platelets after 3 hours of incubation. A similar competition for binding partners for the MyD88 interacting molecule TIRAP completely abolished LPS (and auto-stimulated, not shown) initiated IL-1β mRNA accumulation. A second TIRAP chimeric peptide [RQIKIWFQNRRMKWKK] (25) also effectively blocked splicing (not shown). We determined whether IRAK activity was required for IL-1β RNA processing using a small molecule inhibitor selective for IRAK 1 and IRAK 4. Inhibition of these kinases dramatically reduced the amount spliced IL-1β in platelets when exposed to LPS, resulting in an 80% reduction to level comparable to control platelets. Loss of LPS-stimulated splicing was not due to cell death as peptide-treated platelets maintained thrombin sensitivity as shown by aggregometry (not shown).
Figure 2.
LPS-induced IL-1β mRNA splicing requires MyD88 and TIRAP. Purified human (A) or mouse (B) platelets were treated with or without LPS, soluble CD14, and LPS-binding protein and for 3 hours at 37°C. RNA was isolated from cell lysates and spliced IL-1β mRNA was measured by real-time quantitative PCR. Peptide-based decoy peptide inhibitors were used at a concentration of 50 μM. IRAK inhibitor was used at a concentration of 2 μM Error bars = +/− SE, N=7 human donors (A). Error bar = +/− 1 SE, N=5 experiments of pooled mice blood from 2–4 mice each (B).
Inhibition of IL-1β mRNA production just to the level of auto-activated cells leaves open the possibility of MyD88-independent processes may contribute to LPS signaling in platelets. MyD88-null mice would provide a definitive answer to this, but we had to first determine whether murine platelets contained unspliced heteronuclear IL-1β RNA, and whether this could be spliced in a stimulated, post-transcriptional manner. We found that murine platelets behaved as human platelets and accumulated spliced RNA in response to LPS. Murine platelets appeared to be less sensitive to stimulation over a prolonged incubation and produced little spliced IL-1β by themselves (Fig. 2B). So, not only is the relative signal after LPS stimulation of murine platelets greater than that of human platelets, there was only a negligible increase in auto-activation levels (i.e., background) to obfuscate the role of MyD88 in splicing. The level of auto-stimulation in MyD88-null platelets was below that of untreated cells, amounting to a reduction by one half when compared to control platelets. IL-1β splicing, at least in murine cells, was completely dependent on MyD88 as platelets from mice lacking this adapter molecule were completely insensitive to LPS exposure (Fig. 2B).
Traf6, Akt, and JNK Promote LPS-induced IL-1β Splicing
LPS couples to JNK phosphorylation in platelets since phosphorylation of residues T183 and Y185 of this kinase increased over two-fold after just 5 minutes of stimulation (Fig 3A). The enhanced level of JNK phosphorylation was prolonged and had diminished by just half 60 minutes after stimulation. LPS also stimulated phosphorylation along the Akt pathway, with a rapid increase in both T308 and S473 phosphorylation (Fig 3B, 3C). In contrast to JNK phosphorylation, phosphorylation of Akt was transient. A modest second wave of S473 phosphorylation appeared after 30 minutes of LPS exposure.
Figure 3.
LPS activates JNK and Akt in human platelets. Platelets were treated with LPS, soluble CD14, and LPS-binding protein before lysates were made at the stated times. A significant increase in JNK (A) and Akt phosphorylation, (B) pTyr308-Akt and (C) pSer473-Akt, was measured by ELISA. Data is expressed as the ratio of phospho-proteins to total kinase protein. Error bars = +/− 1 SE, N=3.
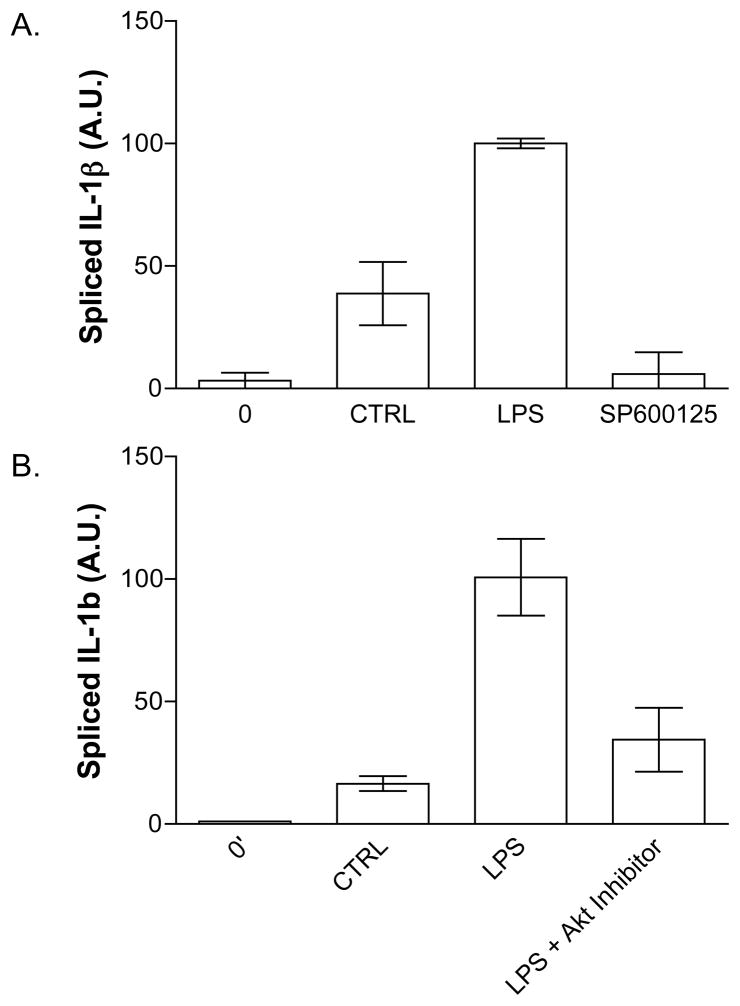
We determined whether Akt or JNK activity was downstream of TLR4 in platelets and required for stimulated IL-1β RNA splicing by using small molecule inhibitors to JNK (Fig. 4A) or Akt (Fig. 4B). Inhibition of either kinase sharply reduced the appearance of mature IL-1β in response to LPS, with the JNK inhibitor SP600125 being almost completely effective.
Figure 4.
Inhibition of JNK Kinase and Akt pathways block IL-1β mRNA splicing in human platelets. Platelets were treated with or without LPS, soluble CD14, and LPS-binding protein for 3 hours and IL-1β splicing determined by real-time quantitative PCR as in Figure 2. The JNK inhibitor, SP600125 (500 nM), inhibits IL-1β splicing (A). An inhibitor of Akt (1.5 uM), also inhibits splicing (B). Error bars = +/− 1 SE, N=3.
Traf6 lies between IRAK1/4 and the Akt and MAP kinase cascades. We used a combination of peptides and pharmacological inhibitors to determine if Traf6 was present and functional in platelets, and whether one or both kinase pathways responded to LPS. To do this, we used a Traf6 decoy peptide derived from CD40-Traf6 interaction. This interaction is defined at atomic resolution (26), and has been shown to functionally interfere with Traf6-mediated signaling (27). We found the Traf6 decoy peptide had a marked effect on the production of spliced IL-1β mRNA, but rather than blocking IL-1β splicing, it exceeded LPS stimulation by five-fold (Fig. 5A). This was not an effect of contaminating LPS in the preparation because, unlike LPS, stimulation by the peptide was CD14-independent. Neither a scrambled Traf6 peptide conjugated to the antennapedia translocation peptide, the antennapedia sequence itself, nor an unconjugated Traf6 sequence induced IL-1β splicing (data not shown).
Figure 5.
TRAF6 peptide activates human platelets in the absence of CD14. Platelets were treated with a chimeric Traf6-antennapedia peptide (5 μM) and lysates were made at stated times. (A) The Traf6 chimeric peptide caused a significant increase in spliced IL-1β mRNA. JNK (B) and Akt (C, D) phosphorylation measured by ELISA. Data is expressed as the ratio of phospho-kinase to total kinase. Error bars = +/− 1 SE, N=7 for Fig. 5A and N=3 for 5B–5E.
The Traf6 decoy peptide is the most effective agent in generating mature IL-1β identified to date, and if this peptide truly affects Traf6 interaction, then the peptide should stimulate Akt and JNK phosphorylation. Indeed, the Traf6 decoy peptide stimulated the accumulation of phospho-JNK over the first 10 minutes of stimulation, and was equally effective at this as LPS (Fig. 5B). Similarly the Traf6 decoy induced a large increase in Akt phosphorylation at residues T308 and S473 (Fig. 5C, 5D). The single notable difference between the Traf6 decoy peptide- and LPS-induced Akt phosphorylation was the delayed response of platelets to the peptide. Inhibitors of JNK, Akt, and CLK1 blocked Traf6-induced IL-1β splicing in human platelets (not shown).
LPS, but not the Traf6 Decoy Peptide, Stimulates Release of IL-1β-expressing Microparticles
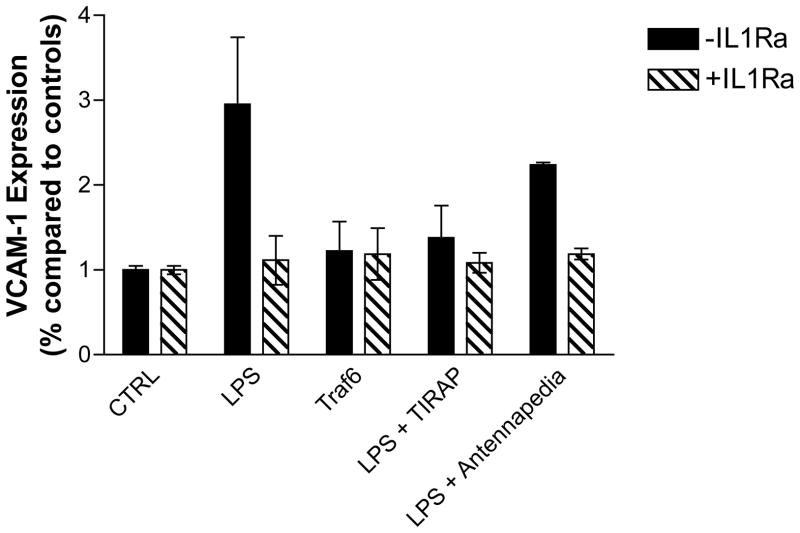
The Traf6-antennapedia chimeric peptide stimulated the Akt and MAP kinase pathways and IL-1β RNA splicing, similar to LPS. To determine if this is sufficient to stimulate the release of IL-1β-expressing microparticles we again treated HUVEC’s with microparticles recovered from platelets treated in various ways before measuring endothelial VCAM-1 expression. Microparticles from LPS-treated platelets caused an increase in VCAM-1 expression that was blocked by the TIRAP peptide and the IL-1 receptor antagonist, but not by the antennapedia control peptide (Fig. 6). In contrast, the Traf6 competing chimera, which was a very effective agonist for IL-1β production, did not produce microparticles (Fig. 1A) able to stimulate HUVEC VCAM-1 production (Fig. 6). We concluded another event below TIRAP but above Traf6 is required for the production of IL-1β-containing microparticles and that IL-1β incorporation into microparticles requires events beyond IL-1β accumulation.
Figure 6.
LPS, but not the Traf6 chimeric peptide, generate IL-1β-containing platelet-derived microparticles. This experiment was performed as in Figure 1, but in the presence or absence of the stated peptides during LPS stimulation. Error bars = +/− 1 SE, N=4
Discussion
In this report we show that human platelets can directly participate in the inflammatory response to endotoxin by activating endothelial cells. Platelets secrete microparticles that contain newly synthesized mature IL-1β, although pro-IL-1β can be detected as well, in response to LPS stimulation. This upregulates endothelial cell VCAM-1 expression that, in turn promotes leukocyte interaction with these cells. This process is dependent on the TLR4 pathway, which leads to JNK and Akt activation in platelets, as in nucleated cells (28, 29). We also show that the compound TG003, which inhibits the splicing kinase CLK1, is an effective inhibitor of LPS-induced IL-1β protein release, indicating that TLR4 activation leads to transcript processing in platelets. In addition to transcript processing, we present data showing that IL-1β protein maturation occurs in a caspase-1-dependent manner. It is notable that the process of activation-dependent splicing, transcription, and transfer of unspliced heteronuclear RNA into platelets as they mature is retained in mice. This indicates that, in contrast to tissue factor RNA (30), heteronuclear RNA for IL-1β can be sorted or differentially processed in both species so that the pro-inflammatory potential, and not the anti-coagulant potential, has been retained over evolution.
Intracellular interference with protein-protein interaction reveals several of the molecular components in the platelet TLR4 pathway leading to IL-1β splicing. First, it is interesting that cells as sensitive as platelets can be exposed to cell-penetrating peptides without becoming sufficiently activated to induce IL-1β splicing, aggregation, or an increase in intracellular calcium (data not shown). We found that a competitive TIRAP peptide inhibitor completely blocked splicing so this interaction is needed for both LPS stimulated and self stimulated IL-1β production, but in contrast, a MyD88 peptide was not equally effective and only reduced splicing to that of control cells. We complemented this approach with peptides by using MyD88−/− mice to find that mice lacking MyD88 were unable splice IL-1β mRNA in response to LPS, so at least in mice that do not undergo self-stimulation, splicing is fully MyD88-dependent.
Unexpectedly, a decoy peptide designed to inhibit Traf6 interaction with its partners, strongly initiated message splicing and kinase activation in platelets. This peptide was marginally effective at blocking message splicing in human monocyte preparations (data not shown), while a CD40-derived peptide failed to block the actions of the Traf6 decoy peptide in human platelets (data not shown). Although our data show the Traf6 decoy peptide is an effective splicing agonist, it failed to elicit IL-1β release from platelets (data not shown) and in fact, failed to stimulate microparticle release beyond the level of control platelets. To date we know of no better agonist that promotes message splicing in human platelets. What this peptide does show is that activation of Akt and MAP kinase signaling is sufficient to initiate heteronuclear RNA splicing, but that another signal, not initiated by the Traf6 peptide, JNK or Akt, is required for particle shedding. This signal is still dependent on MyD88 and TIRAP, but not Traf6 interactions.
Various studies have implicated PI3 kinase in platelet function including aggregation (31, 32) and thrombus formation (33). Akt is a classic downstream target of PI3-Kinase, and several groups have suggested Akt may be an important molecule in platelet function (34–37). Other studies suggest Akt can activate splicing in neurons (38). These facts led us to hypothesize that Akt may modulate mRNA splicing in platelets, and we found that LPS caused a profound increase in phosphorylation of Akt, and that small molecular inhibitors of this kinase block LPS-induced splicing. The timeframe of Akt activation suggests that Akt is a transient second messenger, because while peak Akt activation occurred within minutes, peak splicing and IL-1β protein release occurred well after Akt activation had subsided. The complex interaction of Akt and several platelet mechanisms suggests Akt inhibition may represent a useful adjunct of anti-inflammatory as well as an anti-thrombotic therapy.
Several lines of evidence suggest that TLR4 dependant JNK activation is an important pro-inflammatory pathway (21, 39, 40). Several groups have shown various MAP kinases are activated during platelet stimulation (17, 41, 42). Additionally, Charruyer et al showed that UV-C radiation caused increase ceramide and JNK activation in platelets (43). Because of JNK’s role in inflammation and TLR4 signaling, we hypothesized that LPS-induced mRNA splicing in platelets is dependant on JNK. Like Akt, JNK is highly phosphorylated within minutes of LPS exposure, but then quickly subsides. JNK inhibition effectively blocked this process, indicating this kinase and the Akt pathway separately modulate platelet post-transcriptional splicing. Post-transcriptional splicing, a recently described event (11), has a major role in hematopoietic stem cell maturation (44), and has not been appreciated to require these two kinase cascades.
Zhang et al report that LPS potentiates platelet aggregation via a TLR4-MyD88-cGMP kinase pathway, independent of CD14 presentation of LPS to its receptor (21). The absent role of CD14 may be explained by the extremely high amounts of LPS used in that study, reaching 10 to 100 μg/mL. Although is has been reported that those levels can be achieved in septic patients (45), recent studies suggest most patient plasma samples contain less than 500 pg/mL in the setting of severe sepsis and septic shock (46–48). Additionally, the use of relatively unpurified platelets in the Zhang study may confound results via secretion products of contaminating monocytes and lymphocytes. We eliminate this source of contamination by using two rounds of negative selection based on antibody-coated magnetic beads to CD14, CD15, CD45, and glycophorin leaving behind a very pure population of platelets that are not contaminated with monocytes, which also respond to LPS and make IL-1β. We note that low concentrations of LPS presented by CD14 stimulate signaling events that couple to IL-1β splicing through a kinase cascade above or independent of nitric oxide formation that synergizes with standard agonists to enhance aggregation (21).
Platelet production of IL-1β-enriched microparticles differs from soluble IL-1β release from peripheral blood monocytes in that maximal platelet IL-1β-positive particle release was more rapid, a few hours versus overnight, although ultimately producing less IL-1β. There may also be a qualitative difference between these cellular IL-1β sources in that multiple IL-1β molecules may be expressed by a single platelet-derived microparticle, and these could derive the effective concentration at the IL-1β receptor through avidity. Finally, platelets did not need to be adherent to shed these cytokine-containing microparticles and so all circulating platelets can alter endothelial cell function. We were surprised to find that platelets with robust IL-1β production following exposure to the Traf6 interacting peptide did not shed IL-1β-containing microparticles. These cells, like control platelets, shed some microparticles, but because they lacked IL-1β, we concluded first that an unidentified signal emanating from TLR4 is required for microparticle shedding, and secondly that additional stimulation-dependent events are required for microparticles to acquire IL-1β.
Like IL-1β, caspase-1 is also synthesized as a pro-protein that must be cleaved for activation. Accordingly, we find that platelets contain caspase-1 and activated caspase-1. Platelets activated by thrombin or Platelet-activating Factor have previously been found to contain both pro-IL-1β and processed IL-1β, with the processed form predominating at all times (49).
Finally, through the use of IL-1 receptor antagonist, we found that platelets express functional IL-1 receptors, which couples to IL-1β mRNA splicing like LPS. The slow rate, relative to aggregation, of IL-1β mRNA processing in platelets requires incubation times that are several hours long. We routinely observed “autoactivation” when untreated incubated platelets expressed significantly more processed IL-1β mRNA than freshly isolated resting platelets. This artificially increases background through IL-1β protein-mediated IL-1β RNA splicing via IL-1 receptors, and obfuscates the need for MyD88 in LPS-mediated splicing in human platelets.
These data show that activated, but not necessarily aggregated, platelets can independently influence endothelial activation. Because of the endothelial role in cardiovascular disease, we have provided a new link between platelets and the septic syndrome. Although our experiments used LPS, there are endogenous TLR4 ligands that may play a potential role in mediating aseptic, non-infectious inflammatory states (50, 51). Because platelets lack nuclei, this research represents a useful and simple model for studying post-transcriptional splicing in nucleated cells, such as hematopoietic stem cells, where co-transcriptional processing obfuscates this process.
Acknowledgments
The technical aid of Mark Calabro, and Erin Brady is greatly appreciated. We thank Dr. Clifford Harding and Mr. Daimon Simmons (Department of Pathology, Case Western Reserve University School of Medicine, Cleveland, OH) for the gift of knockout mouse blood, Arundhati Undurti for assistance with VCAM-1 experiments, and Dr. Pavel Shashkin for aid with early experiments. We greatly appreciate the advice and reagents from Dr. George Dubyak (Department of Biophysics and Physiology, Case Western Reserve University School of Medicine, Cleveland, OH). HUVEC’s were kindly provided by Dr. Paul DiCoreletto and his technician Lisa Dechert. We have no commercial conflicts.
Footnotes
This work was supported by Grant 1 P01 HL087018.
References
- 1.Heffner JE, Sahn SA, Repine JE. The role of platelets in the adult respiratory distress syndrome. Culprits or bystanders? Am Rev Respir Dis. 1987;135:482–492. doi: 10.1164/arrd.1987.135.2.482. [DOI] [PubMed] [Google Scholar]
- 2.Davis RB, Meeker WR, McQuarrie D. Immediate effects of intravenous endotoxin on serotonin concentrations and blood platelets. Circ Res. 1960;8:234–239. doi: 10.1161/01.res.8.1.234. [DOI] [PubMed] [Google Scholar]
- 3.Shibazaki M, Kawabata Y, Yokochi T, Nishida A, Takada H, Endo Y. Complement-dependent accumulation and degradation of platelets in the lung and liver induced by injection of lipopolysaccharides. Infect Immun. 1999;67:5186–5191. doi: 10.1128/iai.67.10.5186-5191.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vincent JL, Yagushi A, Pradier O. Platelet function in sepsis. Crit Care Med. 2002;30:S313–317. doi: 10.1097/00003246-200205001-00022. [DOI] [PubMed] [Google Scholar]
- 5.Rumbaut RE, Bellera RV, Randhawa JK, Shrimpton CN, Dasgupta SK, Dong JF, Burns AR. Endotoxin enhances microvascular thrombosis in mouse cremaster venules via a TLR4-dependent, neutrophil-independent mechanism. Am J Physiol Heart Circ Physiol. 2006;290:H1671–1679. doi: 10.1152/ajpheart.00305.2005. [DOI] [PubMed] [Google Scholar]
- 6.Andonegui G, Kerfoot SM, McNagny K, Ebbert KV, Patel KD, Kubes P. Platelets express functional Toll-like receptor-4. Blood. 2005;106:2417–2423. doi: 10.1182/blood-2005-03-0916. [DOI] [PubMed] [Google Scholar]
- 7.Abdelnoor AM, Kassem H, Bikhazi AB, Nowotny A. Effect of gram-negative bacterial lipopolysaccharide-derived polysaccharides, glycolipids, and lipopolysaccharides on rabbit and human platelets in vitro. Immunobiology. 1980;157:145–153. doi: 10.1016/S0171-2985(80)80096-9. [DOI] [PubMed] [Google Scholar]
- 8.Clark SR, Ma AC, Tavener SA, McDonald B, Goodarzi Z, Kelly MM, Patel KD, Chakrabarti S, McAvoy E, Sinclair GD, Keys EM, Allen-Vercoe E, Devinney R, Doig CJ, Green FH, Kubes P. Platelet TLR4 activates neutrophil extracellular traps to ensnare bacteria in septic blood. Nat Med. 2007;13:463–469. doi: 10.1038/nm1565. [DOI] [PubMed] [Google Scholar]
- 9.McMorran BJ, V, Marshall M, de Graaf C, Drysdale KE, Shabbar M, Smyth GK, Corbin JE, Alexander WS, Foote SJ. Platelets kill intraerythrocytic malarial parasites and mediate survival to infection. Science. 2009;323:797–800. doi: 10.1126/science.1166296. [DOI] [PubMed] [Google Scholar]
- 10.Shashkin PN, Brown GT, Ghosh A, Marathe GK, McIntyre TM. Lipopolysaccharide is a direct agonist for platelet RNA splicing. J Immunol. 2008;181:3495–3502. doi: 10.4049/jimmunol.181.5.3495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Denis MM, Tolley ND, Bunting M, Schwertz H, Jiang H, Lindemann S, Yost CC, Rubner FJ, Albertine KH, Swoboda KJ, Fratto CM, Tolley E, Kraiss LW, McIntyre TM, Zimmerman GA, Weyrich AS. Escaping the nuclear confines: signal-dependent pre-mRNA splicing in anucleate platelets. Cell. 2005;122:379–391. doi: 10.1016/j.cell.2005.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aslam R, Speck ER, Kim M, Crow AR, Bang KW, Nestel FP, Ni H, Lazarus AH, Freedman J, Semple JW. Platelet Toll-like receptor expression modulates lipopolysaccharide-induced thrombocytopenia and tumor necrosis factor-alpha production in vivo. Blood. 2006;107:637–641. doi: 10.1182/blood-2005-06-2202. [DOI] [PubMed] [Google Scholar]
- 13.Cognasse F, Hamzeh H, Chavarin P, Acquart S, Genin C, Garraud O. Evidence of Toll-like receptor molecules on human platelets. Immunol Cell Biol. 2005;83:196–198. doi: 10.1111/j.1440-1711.2005.01314.x. [DOI] [PubMed] [Google Scholar]
- 14.Lodrup Carlsen KC, Granum B. Soluble CD14: role in atopic disease and recurrent infections, including otitis media. Curr Allergy Asthma Rep. 2007;7:436–443. doi: 10.1007/s11882-007-0067-2. [DOI] [PubMed] [Google Scholar]
- 15.Liu F, Morris S, Epps J, Carroll R. Demonstration of an activation regulated NF-kappaB/I-kappaBalpha complex in human platelets. Thromb Res. 2002;106:199–203. doi: 10.1016/s0049-3848(02)00130-5. [DOI] [PubMed] [Google Scholar]
- 16.Malaver E, Romaniuk MA, D’Atri LP, Pozner RG, Negrotto S, Benzadon R, Schattner M. NF-kappaB inhibitors impair platelet activation responses. J Thromb Haemost. 2009;7:1333–1343. doi: 10.1111/j.1538-7836.2009.03492.x. [DOI] [PubMed] [Google Scholar]
- 17.Kauskot A, Adam F, Mazharian A, Ajzenberg N, Berrou E, Bonnefoy A, Rosa JP, Hoylaerts MF, Bryckaert M. Involvement of the mitogen-activated protein kinase c-Jun NH2-terminal kinase 1 in thrombus formation. J Biol Chem. 2007;282:31990–31999. doi: 10.1074/jbc.M701596200. [DOI] [PubMed] [Google Scholar]
- 18.Chen K, Febbraio M, Li W, Silverstein RL. A specific CD36-dependent signaling pathway is required for platelet activation by oxidized low-density lipoprotein. Circ Res. 2008;102:1512–1519. doi: 10.1161/CIRCRESAHA.108.172064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barry FA, Gibbins JM. Protein kinase B is regulated in platelets by the collagen receptor glycoprotein VI. J Biol Chem. 2002;277:12874–12878. doi: 10.1074/jbc.M200482200. [DOI] [PubMed] [Google Scholar]
- 20.Kim S, Mangin P, Dangelmaier C, Lillian R, Jackson SP, Daniel JL, Kunapuli SP. Role of phosphoinositide 3-kinase beta in glycoprotein VI-mediated Akt activation in platelets. J Biol Chem. 2009;284:33763–33772. doi: 10.1074/jbc.M109.048553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang B, Ramesh G, Uematsu S, Akira S, Reeves WB. TLR4 signaling mediates inflammation and tissue injury in nephrotoxicity. J Am Soc Nephrol. 2008;19:923–932. doi: 10.1681/ASN.2007090982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kirschenbaum LA, McKevitt D, Rullan M, Reisbeck B, Fujii T, Astiz ME. Importance of platelets and fibrinogen in neutrophil-endothelial cell interactions in septic shock. Crit Care Med. 2004;32:1904–1909. doi: 10.1097/01.ccm.0000139918.80602.57. [DOI] [PubMed] [Google Scholar]
- 23.Muraki M, Ohkawara B, Hosoya T, Onogi H, Koizumi J, Koizumi T, Sumi K, Yomoda J, Murray MV, Kimura H, Furuichi K, Shibuya H, Krainer AR, Suzuki M, Hagiwara M. Manipulation of alternative splicing by a newly developed inhibitor of Clks. J Biol Chem. 2004;279:24246–24254. doi: 10.1074/jbc.M314298200. [DOI] [PubMed] [Google Scholar]
- 24.Uto T, Wang X, Sato K, Haraguchi M, Akagi T, Akashi M, Baba M. Targeting of antigen to dendritic cells with poly(gamma-glutamic acid) nanoparticles induces antigen-specific humoral and cellular immunity. J Immunol. 2007;178:2979–2986. doi: 10.4049/jimmunol.178.5.2979. [DOI] [PubMed] [Google Scholar]
- 25.Horng T, Barton GM, Medzhitov R. TIRAP: an adapter molecule in the Toll signaling pathway. Nat Immunol. 2001;2:835–841. doi: 10.1038/ni0901-835. [DOI] [PubMed] [Google Scholar]
- 26.McWhirter SM, Pullen SS, Holton JM, Crute JJ, Kehry MR, Alber T. Crystallographic analysis of CD40 recognition and signaling by human TRAF2. Proc Natl Acad Sci U S A. 1999;96:8408–8413. doi: 10.1073/pnas.96.15.8408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bharti AC, Takada Y, Shishodia S, Aggarwal BB. Evidence that receptor activator of nuclear factor (NF)-kappaB ligand can suppress cell proliferation and induce apoptosis through activation of a NF-kappaB-independent and TRAF6-dependent mechanism. J Biol Chem. 2004;279:6065–6076. doi: 10.1074/jbc.M308062200. [DOI] [PubMed] [Google Scholar]
- 28.Comalada M, Xaus J, Valledor AF, Lopez-Lopez C, Pennington DJ, Celada A. PKC epsilon is involved in JNK activation that mediates LPS-induced TNF-alpha, which induces apoptosis in macrophages. Am J Physiol Cell Physiol. 2003;285:C1235–1245. doi: 10.1152/ajpcell.00228.2003. [DOI] [PubMed] [Google Scholar]
- 29.Martin M, Rehani K, Jope RS, Michalek SM. Toll-like receptor-mediated cytokine production is differentially regulated by glycogen synthase kinase 3. Nat Immunol. 2005;6:777–784. doi: 10.1038/ni1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pawlinski R, Wang JG, Owens AP, 3rd, Williams J, Antoniak S, Tencati M, Luther T, Rowley JW, Low EN, Weyrich AS, Mackman N. Hematopoietic and non-hematopoietic cell tissue factor activates the coagulation cascade in endotoxemic mice. Blood. 2010;116:806–14. doi: 10.1182/blood-2009-12-259267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cosemans JM, I, Munnix C, Wetzker R, Heller R, Jackson SP, Heemskerk JW. Continuous signaling via PI3K isoforms beta and gamma is required for platelet ADP receptor function in dynamic thrombus stabilization. Blood. 2006;108:3045–3052. doi: 10.1182/blood-2006-03-006338. [DOI] [PubMed] [Google Scholar]
- 32.Trumel C, Payrastre B, Plantavid M, Hechler B, Viala C, Presek P, Martinson EA, Cazenave JP, Chap H, Gachet C. A key role of adenosine diphosphate in the irreversible platelet aggregation induced by the PAR1-activating peptide through the late activation of phosphoinositide 3-kinase. Blood. 1999;94:4156–4165. [PubMed] [Google Scholar]
- 33.Jackson SP, Schoenwaelder SM, Goncalves I, Nesbitt WS, Yap CL, Wright CE, Kenche V, Anderson KE, Dopheide SM, Yuan Y, Sturgeon SA, Prabaharan H, Thompson PE, Smith GD, Shepherd PR, Daniele N, Kulkarni S, Abbott B, Saylik D, Jones C, Lu L, Giuliano S, Hughan SC, Angus JA, Robertson AD, Salem HH. PI 3-kinase p110beta: a new target for antithrombotic therapy. Nat Med. 2005;11:507–514. doi: 10.1038/nm1232. [DOI] [PubMed] [Google Scholar]
- 34.Yin H, Stojanovic A, Hay N, Du X. The role of Akt in the signaling pathway of the glycoprotein Ib-IX induced platelet activation. Blood. 2008;111:658–665. doi: 10.1182/blood-2007-04-085514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kroner C, Eybrechts K, Akkerman JW. Dual regulation of platelet protein kinase B. J Biol Chem. 2000;275:27790–27798. doi: 10.1074/jbc.M000540200. [DOI] [PubMed] [Google Scholar]
- 36.Woulfe D, Jiang H, Morgans A, Monks R, Birnbaum M, Brass LF. Defects in secretion, aggregation, and thrombus formation in platelets from mice lacking Akt2. J Clin Invest. 2004;113:441–450. doi: 10.1172/JCI20267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen J, De S, Damron DS, Chen WS, Hay N, Byzova TV. Impaired platelet responses to thrombin and collagen in AKT-1-deficient mice. Blood. 2004;104:1703–1710. doi: 10.1182/blood-2003-10-3428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jang SW, Liu X, Fu H, Rees H, Yepes M, Levey A, Ye K. Interaction of Akt-phosphorylated SRPK2 with 14-3-3 mediates cell cycle and cell death in neurons. J Biol Chem. 2009;284:24512–24525. doi: 10.1074/jbc.M109.026237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cheng C, Qin Y, Shao X, Wang H, Gao Y, Cheng M, Shen A. Induction of TNF-alpha by LPS in Schwann cell is regulated by MAPK activation signals. Cell Mol Neurobiol. 2007;27:909–921. doi: 10.1007/s10571-007-9215-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rodriguez N, Lang R, Wantia N, Cirl C, Ertl T, Durr S, Wagner H, Miethke T. Induction of iNOS by Chlamydophila pneumoniae requires MyD88-dependent activation of JNK. J Leukoc Biol. 2008;84:1585–1593. doi: 10.1189/jlb.0508304. [DOI] [PubMed] [Google Scholar]
- 41.Flevaris P, Li Z, Zhang G, Zheng Y, Liu J, Du X. Two distinct roles of mitogen-activated protein kinases in platelets and a novel Rac1-MAPK-dependent integrin outside-in retractile signaling pathway. Blood. 2009;113:893–901. doi: 10.1182/blood-2008-05-155978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jackson EC, McNicol A. Cyclic nucleotides inhibit MAP kinase activity in low-dose collagen-stimulated platelets. Thromb Res. 2009;125:147–151. doi: 10.1016/j.thromres.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 43.Charruyer A, Grazide S, Bezombes C, Muller S, Laurent G, Jaffrezou JP. UV-C light induces raft-associated acid sphingomyelinase and JNK activation and translocation independently on a nuclear signal. J Biol Chem. 2005;280:19196–19204. doi: 10.1074/jbc.M412867200. [DOI] [PubMed] [Google Scholar]
- 44.Bowman TV, McCooey AJ, Merchant AA, Ramos CA, Fonseca P, Poindexter A, Bradfute SB, Oliveira DM, Green R, Zheng Y, Jackson KA, Chambers SM, McKinney-Freeman SL, Norwood KG, Darlington G, Gunaratne PH, Steffen D, Goodell MA. Differential mRNA processing in hematopoietic stem cells. Stem Cells. 2006;24:662–670. doi: 10.1634/stemcells.2005-0552. [DOI] [PubMed] [Google Scholar]
- 45.Behre G, Schedel I, Nentwig B, Wormann B, Essink M, Hiddemann W. Endotoxin concentration in neutropenic patients with suspected gram-negative sepsis: correlation with clinical outcome and determination of anti-endotoxin core antibodies during therapy with polyclonal immunoglobulin M-enriched immunoglobulins. Antimicrob Agents Chemother. 1992;36:2139–2146. doi: 10.1128/aac.36.10.2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Opal SM, Scannon PJ, Vincent JL, White M, Carroll SF, Palardy JE, Parejo NA, Pribble JP, Lemke JH. Relationship between plasma levels of lipopolysaccharide (LPS) and LPS-binding protein in patients with severe sepsis and septic shock. J Infect Dis. 1999;180:1584–1589. doi: 10.1086/315093. [DOI] [PubMed] [Google Scholar]
- 47.Ilkka LTJ. Plasma Endotoxin Levels In The Early Phase Of Septic Shock. Intensive Care Med. 1996;22:S9. [Google Scholar]
- 48.Guidet B, Barakett V, Vassal T, Petit JC, Offenstadt G. Endotoxemia and bacteremia in patients with sepsis syndrome in the intensive care unit. Chest. 1994;106:1194–1201. doi: 10.1378/chest.106.4.1194. [DOI] [PubMed] [Google Scholar]
- 49.Lindemann S, Tolley ND, Dixon DA, McIntyre TM, Prescott SM, Zimmerman GA, Weyrich AS. Activated platelets mediate inflammatory signaling by regulated interleukin 1beta synthesis. J Cell Biol. 2001;154:485–490. doi: 10.1083/jcb.200105058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Park JS, Gamboni-Robertson F, He Q, Svetkauskaite D, Kim JY, Strassheim D, Sohn JW, Yamada S, Maruyama I, Banerjee A, Ishizaka A, Abraham E. High mobility group box 1 protein interacts with multiple Toll-like receptors. Am J Physiol Cell Physiol. 2006;290:C917–924. doi: 10.1152/ajpcell.00401.2005. [DOI] [PubMed] [Google Scholar]
- 51.Vogl T, Tenbrock K, Ludwig S, Leukert N, Ehrhardt C, van Zoelen MA, Nacken W, Foell D, van der Poll T, Sorg C, Roth J. Mrp8 and Mrp14 are endogenous activators of Toll-like receptor 4, promoting lethal, endotoxin-induced shock. Nat Med. 2007;13:1042–1049. doi: 10.1038/nm1638. [DOI] [PubMed] [Google Scholar]