Summary:
While plasmacytoid dendritic cells (pDCs), a natural type I interferon (IFN) producing cell type, are regarded as critical for innate immunity to viruses, their role in defense against fungal infections remains unknown. We examined the interactions of pDCs with hyphae of the invasive human fungal pathogen Aspergillus fumigatus. Human pDCs spread over hyphae and inhibited their growth. Antifungal activity was retained in pDC lysates, did not require direct fungal contact, and was partially reversed by zinc. Incubation with hyphae resulted in pDC cytotoxicity, partly due to fungal gliotoxin secretion. Following hyphal stimulation, pDCs released proinflammatory cytokines via a TLR9-independent mechanism. Pulmonary challenge of mice with A. fumigatus resulted in a substantial influx of pDCs into lungs and pDC-depleted mice were hypersusceptible to invasive aspergillosis. These data demonstrate the antifungal activity of pDCs against A. fumigatus and establish their non-redundant role in host defenses against invasive aspergillosis in vivo.
Introduction:
Aspergillus fumigatus has emerged as the most common cause of invasive mold infections. Mortality rates for persons with invasive aspergillosis are high due to the severely immunocompromised status of most inflicted individuals and the relatively weak fungicidal activity of the available therapeutic options (Hohl and Feldmesser, 2007). Exposure to this ubiquitous fungus is frequent and typically occurs by inhalation of airborne conidia. In the suitable host, the inhaled conidia swell and germinate into hyphae, the invasive form of the fungus. Clinical and experimental studies have strongly implicated both innate and adaptive immune responses as being critical for protection against aspergillosis. While neutrophils appear to be of paramount importance, vital contributions of monocytes, macrophages, conventional dendritic cells (DCs) and T cells have been demonstrated (Bozza et al., 2002; Dagenais and Keller, 2009; Hartigan et al., 2009; Hohl et al., 2009; Park et al., 2010). While conidia are efficiently ingested by phagocytes, the large size of the hyphal morphotype generally precludes phagocytosis. However, phagocytes can spread over the hyphae surface and inhibit and kill the fungus via oxidative and non-oxidative mechanisms (Hohl and Feldmesser, 2007; Levitz et al., 1986). Moreover, co-incubation of neutrophils and A. fumigatus results in the formation of neutrophil extracellular traps (NETs) with direct antifungal activity (Bruns et al., 2010).
Plasmacytoid DCs (pDCs), also known as natural type I interferon (IFN) producing cells, rapidly produce copious amounts of type I IFNs upon stimulation with viruses (Colonna et al., 2004). In humans, pDCs comprise 0.2 – 0.8% of the total peripheral blood mononuclear cells (PBMCs) and express the endosomal Toll-like receptors (TLRs) 7 and 9, but not any of the other known TLRs. Upon viral exposure, pDCs initiate protective antiviral responses by secreting up to 1000-fold more type I IFNs than other cell types, predominantly via mechanisms dependent on sensing viral nucleic acids via TLR7 and TLR9 (Lande and Gilliet, 2010; Yang et al., 2005). Activated pDCs link innate to adaptive immunity by secreting cytokines such as IFNα and tumor necrosis factor (TNFα) and by differentiating into mature pDCs with upregulated MHC and co-stimulatory molecules capable of priming naïve T cells (Yu et al., 2010).
pDCs have also been implicated in the pathogenesis of autoimmune diseases and in maintenance of the immunosuppressive environments in neoplasms (Lande and Gilliet, 2010). Recently, certain bacteria were shown to stimulate pDCs (Ang et al., 2010). However, whether pDCs play a role in the detection and responses to fungal pathogens has not been well studied. We and others have shown that purified DNA and RNA from A. fumigatus stimulate pDC cytokine responses (Perruccio et al., 2004; Ramirez-Ortiz et al., 2008). However, it is uncertain whether quantities of fungal DNA and RNA released during the course of a mycotic infection are sufficient to stimulate pDCs. Therefore, in the present investigation, we sought to determine whether pDCs sense live A. fumigatus hyphae and, if so, what are the consequences of the interaction. We found that human pDCs directly inhibit fungal growth via a mechanism that involves A. fumigatus-induced pDC death and the release of antifungal mediators. Moreover, following stimulation with A. fumigatus hyphae, pDCs release IFNα and TNFα via a TLR-independent mechanism. Finally, we found that pDCs are required for effective antifungal defenses in vivo, as mice depleted of pDCs are hypersusceptible to invasive aspergillosis.
Results:
Antimicrobial activity of pDCs against A. fumigatus.
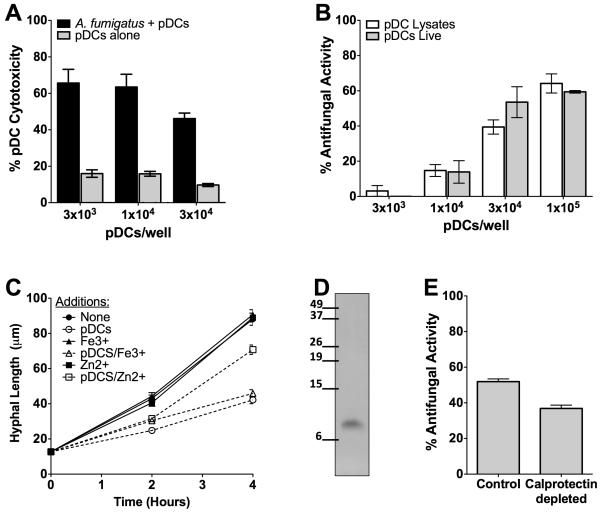
Initial experiments focused on determining whether human blood pDCs had direct antimicrobial activity against the invasive hyphal morphotypes of A. fumigatus. pDCs were incubated with A. fumigatus hyphae for 2 hr at 37°C. Antifungal activity was then measured by the XTT assay. We found that pDCs have potent antifungal activity against A. fumigatus, with nearly 80% antifungal activity observed at the highest ratio (50:1) of pDCs to hyphae tested (Figure 1A). Surprisingly, even at a ratio of 1 pDC to 20 hyphae, over 40% antifungal activity was seen. To confirm the XTT data using an independent assay, we directly measured the hyphal length of A. fumigatus following 2 and 4 hr incubations with pDCs at a 1:2 pDCs to hyphae ratio (Figure 1B). Consistent with the XTT data, hyphal growth was significantly inhibited in the presence of pDCs. Nevertheless, it should be noted that the hyphae did show modest growth suggesting that the pDCs were predominantly fungistatic, rather than fungicidal. pDCs did have fungicidal activity against swollen conidia, and to a lesser extent, resting conidia, as measured by a reduction in CFUs (Supplemental Figure S1A). Anti-hyphal activity increased over time and was greater than or equal to activity seen with other WBC populations (Supplemental Figure S1B and C).
Figure 1. Antimicrobial activity of pDCs against A. fumigatus.
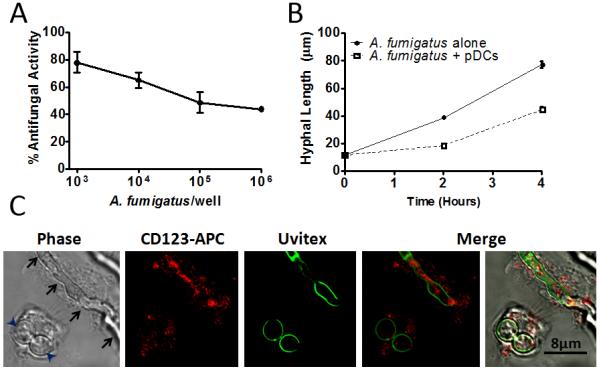
(A) pDCs (5×104) were incubated with the indicated number of A. fumigatus hyphae for 2 hr. Antifungal activity of pDCs was then measured by the XTT assay. Data represent means ± SE from two donors, each tested in duplicate. (B) A. fumigatus hyphae were incubated for the specified times with or without pDCs and hyphal length determined. Data represent means ± SE from two experiments. For each variable, at least 75 hyphal measurements were recorded. p<0.0001, when comparing hyphal lengths in the presence or absence of pDCs at the 2 and 4 hr time points. (C) pDCs (1×105) were incubated with A. fumigatus (1×105) at 37°C for 2 hr. After fixation, pDCs were stained with anti-CD123-efluor650 (red) and A. fumigatus hyphae were stained with Uvitex (green). Samples were analyzed by confocal microscopy. Arrowheads point to two swollen conidia phagocytosed by a pDC. Arrows point to a hypha which is covered by a pDC. The photomicrographs depicted are representative of three independent experiments with similar results.
Confocal microscopy of pDCs incubated with A. fumigatus.
pDCs and A. fumigatus were co-incubated for 2 hr, fixed and observed via confocal microscopy (Figure 1C). Nearly all pDCs were found to be closely associated with fungi. The pDCs spread over hyphae that were too large to be ingested and phagocytosed A. fumigatus swollen conidia.
Effect of A. fumigatus on viability of pDCs.
While observing pDCs by microscopy, we noticed that many of the pDCs incubated with A. fumigatus did not appear healthy, as judged by poor adherence and swollen morphology. Thus, we examined pDC death, as measured by LDH release, following a 2 hr incubation with or without A. fumigatus hyphae (Figure 2A). Remarkably, there was a large increase in death of the pDCs in the presence of A. fumigatus. Cytotoxicity increased over time and was not seen following incubation of hyphae with PBMCs that were depleted of pDCs (Supplemental Figure S2).
Figure 2. A. fumigatus hyphae lyse pDCs but pDC lysates have antifungal activity.
(A) The indicated number of pDCs was incubated with or without 3×104 A. fumigatus hyphae for 2 hr. Supernatants were then collected and cytotoxicity of pDC was assessed by LDH release. Data represent means ± SE of three donors, each studied in triplicate. p< 0.0001 when comparing cytotoxicity with and without hyphae for each concentration of pDCs. (B) A. fumigatus hyphae were incubated with live pDCs or lysates obtained from the indicated number of pDCs. Antifungal activity was measured by the XTT assay. Data represent means ± SE of three donors, each studied in triplicate. No significant differences were observed comparing pDC lysates with live pDCs at any of the cell concentrations studied. (C) A. fumigatus hyphae were incubated with or without pDCs (5×104) in the presence or absence of 10 μM ZnCl2 or 10 μM FeCl3. At the specified times, hyphal length was measured as described in Methods. Solid lines represent A. fumigatus alone whereas dotted lines represent A. fumigatus incubated with pDCs. Data represent means ± SE of two individual experiments. p<0.001 comparing fungal growth in the presence or absence of pDCs. (D) pDCs were hypotonically lysed and 100 μg of total protein were analyzed by Western blot using a monoclonal antibody against human calprotectin. Blot is representative of 3 separate experiments. Lines point to where the indicated molecular size standards (in kD) ran on the gel. (E) The antifungal activity of pDC lysates was determined before and after immunodepletion of calprotectin. Data are from three donors, each studied in triplicate. p<0.001 comparing lysates with and without immunodepletion.
Antifungal activity of pDC lysates.
The finding that pDCs died following incubation with hyphae raised the possibility that dying pDC still had antifungal activity. Thus, we lysed pDCs and compared the activity of pDC lysates with intact pDCs against A. fumigatus hyphae (Figure 2B). We found that the lysates and the live pDCs had comparable antifungal activity.
The contribution of Zn++ and Fe+++ deprivation to the antifungal activity of pDCs.
Zinc- and iron-binding proteins, such as calprotectin and lactoferrin, respectively, are constituents of some phagocytic populations and can exert broad antifungal activity by chelating divalent cations essential for fungal growth (Mambula et al., 2000; Urban et al., 2009). To determine whether nutritional deprivation of zinc or iron contributes to the antifungal activity of the pDCs, we examined whether ZnCl2 or FeCl3 supplementation rescued pDC-mediated growth inhibition of A. fumigatus hyphae (Figure 2C). These experiments were performed by direct microscopic measurement of fungal growth as high ferric concentrations can interfere with the XTT assay (Knight and Dancis, 2006). We found that while addition of exogenous FeCl3 had no effect, 10μM ZnCl2 partially, but significantly, reversed pDC-mediated growth inhibition. In contrast, the non-immune human cell lines, HEK 293 and HeLa, had no antifungal activity in the presence or absence of ZnCl2 (data not shown). While the pDC data suggest a role for calprotectin, the presence of calprotectin in pDCs has not, to our knowledge, been previously documented. Therefore, Western blots probing for calprotectin were performed on pDC lysates. A band of the expected size was found, suggesting that pDCs contain calprotectin (Figure 2D). Moreover, immunodepletion of calprotectin significantly reduced the antifungal activity of pDC lysates (Figure 2E).
Effect of cell contact on pDC cytotoxicity and antifungal activity:
Given our observations that incubation with A. fumigatus results in both pDC death and fungal growth inhibition, we next used Transwells to determine whether contact between the pathogen and the pDCs was required for these events to transpire. pDC death occurred regardless of whether the pDCs were in direct contact with hyphae, although cytotoxicity was greater when contact was allowed (Figure 3A). Similarly, while direct contact promoted antifungal activity, some antifungal activity was still observed in the absence of contact (Figure 3B).
Figure 3. Influence of cell contact on pDC cytotoxicity and antifungal activity.
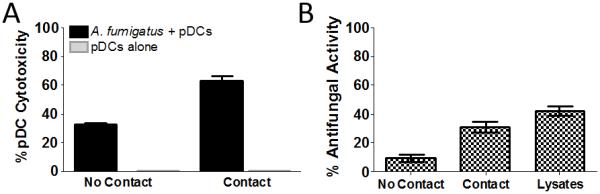
(A) A. fumigatus hyphae (5×104) were grown on the bottom of wells containing a permeable insert (Transwell) with a size exclusion limit of 0.4 μm. pDCs (5×104) were then added either to the top of the insert (No Contact) or below the insert (Contact). After a 2 hr incubation, supernatants were collected and pDC cytotoxicity was measured by LDH assay. p<0.0001, when comparing pDCs in the presence or absence of A. fumigatus for both the “No Contact” and “Contact” groups. (B) As in part A, except antifungal activity was measured by the XTT assay. In addition, the antifungal activity of lysates from 5×104 pDCs was assayed. p< 0.0001, when comparing any two groups except “Contact” and “Lysates”. For parts A and B, data represent means ± SE from four donors, each tested in triplicate.
Role of gliotoxin in the induction of pDC death:
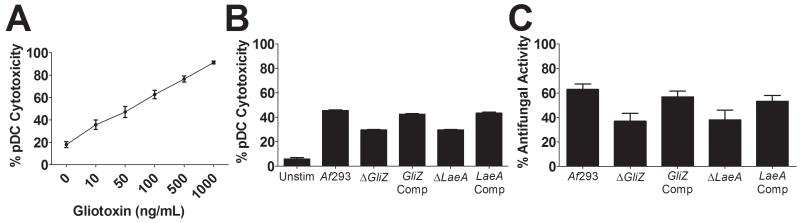
Our findings that pDC death occurs independently of contact with A. fumigatus suggest that secreted factor(s) are responsible for the observed effect. One such candidate is A. fumigatus gliotoxin, which is known to induce apoptosis in many cell types (Bok et al., 2006; Stanzani et al., 2005). Therefore, we incubated pDCs with concentrations of purified gliotoxin within the range found in patients with invasive aspergillosis (Lewis et al., 2005; Spikes et al., 2008; Stanzani et al., 2005). We found that gliotoxin induced pDC death in a dose-dependent manner (Figure 4A). Next, we sought to determine whether hyphae-stimulated pDC death was due to gliotoxin secretion. pDC cytotoxicity was compared following incubation with hyphae from wild-type, gliotoxin-deficient and complemented A. fumigatus strains (see Supplemental Table S1). Our results suggest that pDC death is partially mediated by A. fumigatus gliotoxin, as strains mutated for gliotoxin production induced significantly less pDC death compared with wild type or complemented strains (Figure 4B). Similarly, we observed that the antifungal activity of pDCs against A. fumigatus strains deficient in gliotoxin production was reduced compared with wild-type or complemented strains (Figure 4C). In contrast, as expected, the antifungal activity of pDC lysates was similar against wild-type, gliotoxin-deficient and gliotoxin-complemented A. fumigatus strains (Supplemental Figure S3).
Figure 4. Role of gliotoxin and other A. fumigatus secondary metabolites in the induction of pDC death.
(A) pDCs (5×104) were treated with the indicated concentrations of gliotoxin. After a 2 hr incubation, supernatants were collected and cell mediated cytotoxicity was measured by LDH assay. Data represent means ± SE from four donors, each tested in duplicate. (B, C) pDCs (5×104) were left unstimulated (Unstim) or incubated for 2 hr with hyphae (5×104) from the indicated strain of A. fumigatus (see Supplemental Table 1). pDC cytotoxicity and antifungal activity were analyzed by LDH and XTT assays, respectively. Data represent means ± SE of duplicate experiments from separate donors, each of which was performed in triplicate. p<0.0001, when comparing deletion strains with their complemented counterparts as well as wild-type A. fumigatus (Af293).
Mechanism of pDC death.
In order to gain insights into the mechanism of pDC death, we performed terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assays on pDCs incubated with A. fumigatus hyphae (Figure 5). We found DNA fragmentation in approximately 60% of the pDCs stimulated with hyphae (Figure 5A), suggesting that the pDC death demonstrated in Figure 2 is due to apoptosis or pyroptosis. A similar percentage of TUNEL positive cells were seen following incubation of pDCs with gliotoxin. In contrast, only about 10% of unstimulated pDCs had TUNEL-positive staining.
Figure 5. Mechanism of pDC death.
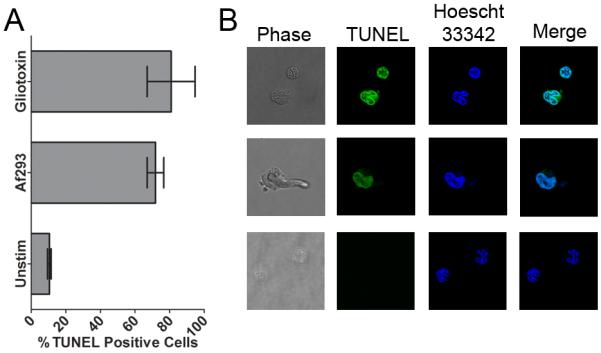
pDCs (1×105) were left unstimulated (Unstim) or stimulated for 2 hr with either A. fumigatus (Af293, 1×105) hyphae or gliotoxin (20 ng/mL). Following incubation, samples were fixed and stained for DNA fragmentation by TUNEL (Alexa Fluor 594) and total DNA (Hoechst 33342). Cells were then examined by confocal microscopy. (A) TUNEL positivity was determined for at least 100 pDCs per group. Data represent means ± SE of two individual experiments performed in duplicate; p<0.01, when comparing unstimulated with A. fumigatus or gliotoxin. (B) Representative confocal microscopy images.
Cytokine release by pDCs stimulated with A. fumigatus hyphae.
Next, we examined whether the interaction of pDCs with hyphae could lead to enhanced immune responses due to cytokine release. pDCs were stimulated with A. fumigatus hyphae for 6 hr following which concentrations of IFNα and TNFα were determined in the supernatants (Figure 6A and B). For comparison, the “flow through” fraction, consisting of the PBMCs which did not adhere to the CD304-coated magnetic beads, was also studied. We found IFNα and TNFα were released from both the pDCs and flow through fractions following stimulation with A. fumigatus hyphae. Human pDCs are TLR9+ but TLR4− (Gilliet et al., 2008; Pietras et al., 2006). Consistent with this observation, the TLR9 ligand, CpG, potently stimulated the pDCs whereas the TLR4 ligand, lipopolysaccharide (LPS), failed to stimulate these cells. As expected, LPS stimulated the flow through fraction (which contains LPS-responsive monocytes) to release IFNα and TNFα. Stimulation of pDCs with hyphae and CpG was additive compared with either stimulus alone (Supplemental Figure S4A).
Figure 6. Cytokine release by pDCs stimulated with A. fumigatus hyphae.
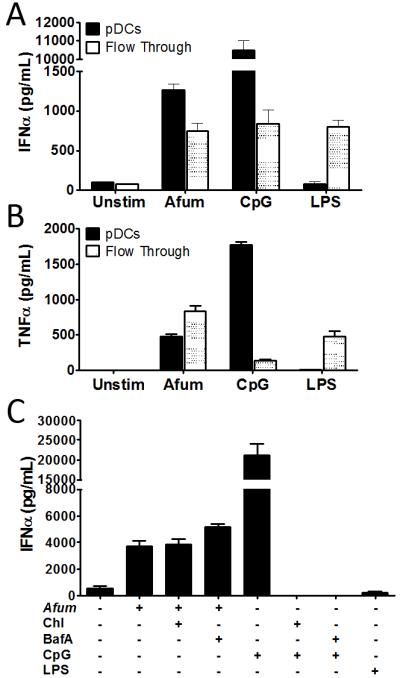
(A) and (B). PBMC were separated into pDC positive (pDCs) and negative (Flow Through) fractions using CD304-coated magnetic beads. The pDCs and flow through cells (5×104/well) were then left unstimulated (Unstim) or stimulated for 6 hr with A. fumigatus hyphae (5×104), CpG (100 ng/mL) or LPS (10 ng/mL). Supernatants were analyzed by ELISA for IFNα (A) and TNFα (B). Data represent means ± SE of cytokine concentrations from two donors, each analyzed in duplicate. p<0.0001 comparing cytokine secretion by unstimulated cells with any stimulus except for unstimulated pDCs compared with LPS-stimulated pDCs. (C) pDCs (5×104) were pretreated for 60 min with 10 μg/mL chloroquine (Chl), 10 μg/mL of bafilomycin A1 (BafA), or left untreated prior to 6 hr stimulation with A. fumigatus hyphae (5×104), CpG (100 ng/mL) or LPS (10 ng/mL). Supernatants were analyzed by ELISA for IFNα. Data represent means ± SE of cytokine concentrations from two donors, each analyzed in triplicate. p<0.0001, when comparing unstimulated pDCs to pDCs incubated with A. fumigatus or CpG. IFNα levels stimulated by A. fumigatus were not significantly affected by treatment with BafA or Chl.
Role of TLR9 in hyphal stimulation of IFNα by pDCs.
We previously demonstrated that purified A. fumigatus DNA stimulates TLR9-dependent cytokine release. Here we examined whether stimulation of IFNα release by intact A. fumigatus hyphae was also mediated by TLR9. As endosomal acidification is essential for TLR9 signaling, we studied the effect of pDC pretreatment with the endosomal acidification inhibitors chloroquine and bafilomycin A1 on IFNα release stimulated by A. fumigatus hyphae (Figure 6C). Inhibiting endosomal acidification did not affect hyphae-induced IFNα release by pDCs, suggesting a TLR9-independent mechanism of stimulation. As expected, chloroquine and bafilomycin A1 abrogated CpG-stimulated IFNα release. We also challenged bone marrow-derived pDC from WT and TLR9 KO mice with A. fumigatus hyphae and found that IL-12p40 release following stimulation was TLR9-independent (Supplemental Figure S4B).
pDCs play a non-redundant role in host defenses against A. fumigatus.
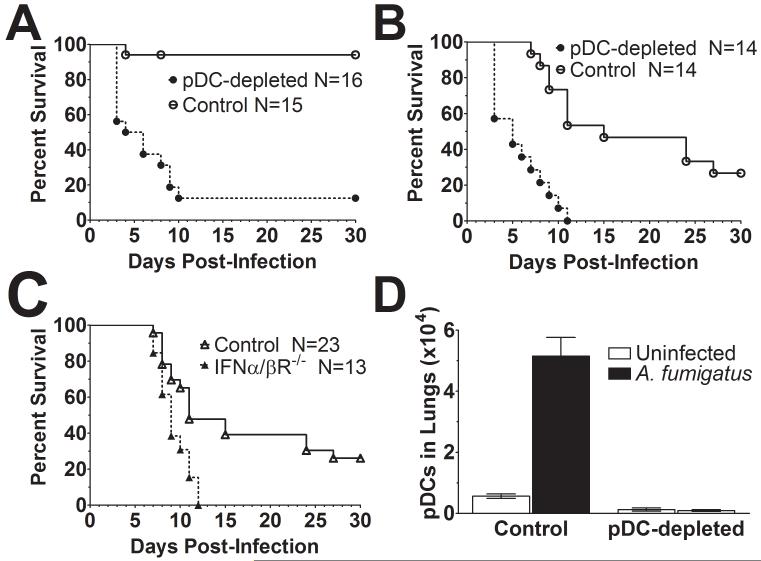
To examine the in vivo contribution of pDCs to host defenses against aspergillosis, mice were treated with either the pDC-depleting mAb, 120G8, or an irrelevant control mAb and then challenged with A. fumigatus. We found that mice depleted of pDCs were dramatically more susceptible to aspergillosis, regardless of whether the challenge was via the intravenous or pulmonary route (Figure 7A and B). As pDCs are the major producer of type I IFNs, we next examined the susceptibility of IFNα/βR−/− mice, which do not respond to type I IFNs, to intravenous challenge with A. fumigatus. We found the KO mice were significantly more susceptible to invasive aspergillosis compared with their wild-type counterparts (Figure 7C).
Figure 7. Contribution of pDCs to defenses against invasive aspergillosis.
Mice were infected with A. fumigatus conidia via pulmonary (A) or intravenous (B and C) challenge and followed 30 days for survival. (A and B) Mice were treated with the antibodies 120G8 (pDC-depleted) or GL113 (Control), as described in Methods, but did not receive any other immunosuppression. (C) Susceptibility of IFNα/βR−/− and wild-type mice to aspergillosis was compared. Data from A, B and C represent combined survival curves of 2 independent experiments, each with similar results. The number (N) of mice per group is indicated in the figure inset. p<0.0001 when comparing survival of pDC depleted mice vs control antibody treated mice. p<0.003 when comparing survival of IFNα/βR−/− mice to WT mice. (D) Wild-type mice were left untreated (Control) or given 120G8 (pDC-depleted). Mice were then either left uninfected or infected via the pulmonary route with A. fumigatus. After 48 hr, the number of pDCs in the lungs was determined. Data represent means +/− SEM of two experiments, each with 3 - 5 mice per group. p<0.0001 comparing the total number of pDC of uninfected or depleted mice to the total number of pDCs of untreated/infected group.
pDCs are recruited into the lungs of A. fumigatus-infected mice.
Mice received a pulmonary challenge with A. fumigatus and 48 hr later the number of pDCs in the lungs was determined. We observed a greater than 6-fold increase in the number of pDCs following challenge with A. fumigatus (Figure 7D). In addition, we confirmed that administration of 120G8 results in profound depletion of pDCs in the lungs. However, depletion of pDCs did not have a significant effect on the number of fungal CFUs in the lungs (Supplemental Figure S5).
Discussion:
Innate responses of phagocytes are thought to be paramount to host defenses against A. fumigatus. Neutrophils, monocytes, macrophages and conventional DC subsets have been shown to recognize and exert antifungal responses that promote clearance of this opportunistic fungus (Bozza et al., 2002; Dagenais and Keller, 2009; Hartigan et al., 2009; Hohl et al., 2009; Park et al., 2010). Here we show that pDCs inhibit the growth of A. fumigatus hyphae, produce cytokines capable of activating and recruiting other immune cells, and are critical to pulmonary and systemic host defenses against invasive aspergillosis.
Hyphae, the tissue invasive form of A. fumigatus, rapidly grow to a size that precludes phagocytosis. However, upon incubation of pDCs with A. fumigatus, we found that within two hr, pDCs had spread over the hyphal surface. While the repertoire of surface receptors on pDCs associated with fungal recognition is not well defined, human pDCs reportedly express dectin-2, but not dectin-1, mannose receptor and DC-SIGN (Graham and Brown, 2009; Meyer-Wentrup et al., 2008). Moreover, human pDCs express some complement and Fc receptors, although we found that hyphal recognition did not require opsonization. Future studies are needed to define receptor(s) and their cognate ligand(s) responsible for recognition of A. fumigatus hyphae by pDCs.
Other cell types, including neutrophils and monocytes, spread over hyphae and cause damage to A. fumigatus by oxidative and non-oxidative mechanisms. Using two independent assays, one that assesses metabolic activity and the other that directly measures hyphal elongation, we demonstrated that human pDCs have antifungal activity against A. fumigatus. However, as opposed to the situation with neutrophils and monocytes, where both growth inhibition and killing of A. fumigatus have been demonstrated, we only found evidence for growth inhibition following incubation of hyphae with pDCs. Thus, hyphal growth proceeded, but at a significantly slower rate, in the presence of pDCs.
In the absence of activating signals, pDCs reportedly undergo spontaneous apoptosis (Grouard et al., 1997; Lepelletier et al., 2010). Interestingly though, after a two hr incubation of pDCs with A. fumigatus hyphae, over half of the pDCs died. Moreover, pDC lysates had antifungal activity against A. fumigatus and some antifungal activity was retained even if the pDCs were separated from the hyphae by a permeable insert. These observations strongly suggest that diffusible, preformed mediators were responsible for the antifungal activity. The essential role of Fe3+ and Zn2+ as fungal growth factors along with the known presence of chelators of these cations in other leukocyte populations (Mambula et al., 2000; Urban et al., 2009), led us to examine whether supplemental Fe3+ or Zn2+ would reverse the pDC-mediated growth inhibition. The observation that ZnCl2 (but not FeCl3) partially restored fungal growth suggests a role for the Zn2+ binding protein, calprotectin (Urban et al., 2009). Neutrophils contain large amounts of cytoplasmic calprotectin. Free and NET-associated calprotectin, released from dying neutrophils, inhibit the growth of C. albicans and A. fumigatus by chelating zinc (Bruns et al., 2010; Lulloff et al., 2004). Although it is unknown whether a process similar to NETosis occurs with pDCs, we do demonstrate that human pDCs contain calprotectin and that immunodepletion of calprotectin reduces the antifungal activity of cell lysates. It is important to emphasize that Zn2+ supplementation only partially restored hyphal growth, suggesting that the antifungal activity of pDCs is probably mediated by more than one pathway.
Two lines of evidence strongly suggest that the high rate of pDC cytotoxicity following incubation with A. fumigatus hyphae is at least partially due to secreted factors released by the fungi. First, pDC cytotoxicity was observed (albeit at a lower level) when the pDCs and hyphae were separated by a Transwell. Second, pDC cytotoxicity was significantly (albeit not completely) reduced following incubation with hyphae from A. fumigatus strains genetically engineered to be deficient in gliotoxin production. Moreover, purified gliotoxin, at concentrations found in the lungs of patients with invasive pulmonary aspergillosis (Lewis et al., 2005; Stanzani et al., 2005), killed pDCs in a dose-dependent manner.
Gliotoxin is a low molecular weight mycotoxin secreted by many fungal species including A. fumigatus (Bok et al., 2006; Kupfahl et al., 2008; Sutton et al., 1994). Induction of apoptosis by gliotoxin has been described in many cell types, including PMNs and monocytes (Stanzani et al., 2005). To dissect the mechanism of pDC death induced by A. fumigatus hyphae, we performed a TUNEL assay. The majority of pDCs incubated with hyphae or purified gliotoxin was TUNEL positive, suggesting that the pDCs are dying by apoptosis. However, recent studies have shown that cells undergoing pyroptosis may also exhibit degradation of DNA and a positive TUNEL reaction (Fink et al., 2008). The finding that pDCs release cytokines when stimulated with hyphae points to their undergoing an inflammatory (rather than an apoptotic) cell death, although it is possible that the fraction of pDCs which remain viable is responsible for the cytokine secretion. Finally, while early apoptotic cells normally preserve their cell membrane integrity, apoptosis can progress to secondary necrosis and membrane leakage (Challa and Chan, 2010), which we speculate contributes to the antifungal activity of the dying pDCs.
pDCs secrete large amounts of type I IFN in response to viral infections and certain DNA and RNA sequences (Cao and Liu, 2007; Pietras et al., 2006), but their role during fungal infections has received little study. While Romani et al. did not find IFNα secretion by pDCs stimulated by A. fumigatus resting conidia (Romani et al., 2004), we found that the tissue invasive hyphal morphotype stimulates pDCs to release IFNα. Furthermore, induction of type I IFN appears to be independent of TLR7 and TLR9 as inhibition of endosomal acidification had no effect on hyphae-stimulated IFNα production. This may not be surprising given that hyphae are extracellular and TLR7 and TLR9 are endosomally localized, although the possibility that hyphal components could eventually end up in lysosomal compartments cannot be excluded. An in vivo role for type I IFNs in invasive aspergillosis was suggested by our finding that compared to wild-type mice, IFNα/βR−/− mice had accelerated mortality after intravenous challenge with A. fumigatus. Similarly, IFNα/βR−/− mice were hypersusceptible to Cryptococcus neoformans and failed to develop protective Th1 cytokine responses (Biondo et al., 2008). Recombinant human IFNα2a and IFNα2b did not have direct antifungal activity in vitro (unpublished studies).
Remarkably, mice treated with the pDC-depleting antibody, 120G8, were dramatically more susceptible to both pulmonary and systemic challenge with A. fumigatus than their control counterparts suggesting that pDCs are critical for defenses against the mold. In addition, following pulmonary infection of wild-type mice with A. fumigatus, a substantial recruitment of pDCs to the lungs was observed. These recruited cells could have antifungal activity, present antigen to T cells and secrete cytokines. While the studies with IFNα/βR−/− implicate a contribution of pDC-derived type I IFNs, further studies are required to determine the exact mechanisms by which pDCs mediate protection. Finally, it should be noted that although widely used to deplete pDCs, the antigen recognized by 120G8 is present, albeit at a reduced density, on plasma cells and may be induced by IFNs (Asselin-Paturel et al., 2003; Blasius et al., 2006).
Thus, our data demonstrate that pDCs play a nonredundant role in host defenses against invasive aspergillosis. The host-pathogen interaction between pDCs and A. fumigatus has unusual, yet seemingly paradoxical, features. The significant antifungal activity of pDCs against A. fumigatus hyphae appears to be dependent, at least in part, on dying pDCs releasing antifungal effector molecules, such as zinc chelators. This process is enhanced by fungal release of cytotoxic molecules, including gliotoxin, which induce apoptosis or pyroptosis of the pDCs. Release of cytokines by fungal stimulated pDCs may serve to recruit and activate other immune cells, thereby boosting innate responses and helping to initiate adaptive immunity. We postulate that pDCs also make critical contributions to defenses against other fungal infections and that immunotherapies that target pDC could prove beneficial in the treatment of invasive mycoses.
Experimental Procedures
Reagents and cell culture.
Reagents were from Sigma-Aldrich unless otherwise stated. DMEM and RPMI-1640 without phenol red were obtained from GIBCO (Invitrogen). pDC media consisted of RPMI-1640 without phenol red supplemented with 100U/ml penicillin, 100U/ml streptomycin, 2mM L-glutamine, 0.5mM HEPES, 1mM sodium pyruvate and 10% autologous serum. CpG 2007 oligonucleotide was synthesized with phosphothiorate linkages by Integrated DNA Technologies. Ultrapure Escherichia coli LPS was purified as described (Huang et al., 2009). All antibodies were from eBiosciences, unless otherwise specified. Monoclonal antibody against human calprotectin was obtained from Santa Cruz Biotechnology.
A. fumigatus strains and culture.
Wild-type A. fumigatus strain 293 was obtained from the Fungal Genetic Stock Center. ΔGliZ, GliZ Comp, ΔLaeA, and LaeA Comp (Bok et al., 2005; Bok and Keller, 2004; Bouhired et al., 2007; Hohl et al., 2005) were generous gifts of Dr. Nancy Keller (University of Wisconsin, Madison). These genetically manipulated strains were on the 293 background (see Supplemental Table 1). There were no differences in growth rates among the different strains as determined using the XTT assay (data not shown). Except as noted, strain 293 was used in the studies.
Cultivation of A. fumigatus, harvesting of conidia and growth into swollen conidia and hyphae was performed as in our previous studies with slight modifications (Mambula et al., 2002). Briefly, fungi were grown on Sabouraud Dextrose Agar slants and conidia were harvested with PBS containing 0.05% Tween 20. The conidia were then vortexed, filtered through a 40-μm nylon mesh washed, counted and stored in water at 4°C for up to a week. To generate hyphae, conidia were incubated at 21°C for 16 hr in pDC medium to swell the conidia, and then an additional 3 hr at 37°C to promote germination.
Isolation of human pDCs and autologous sera.
Human pDCs were isolated as described (Ramirez-Ortiz et al., 2008; Wang et al., 2006). Peripheral blood was collected by venipuncture. A portion was clotted and the autologous serum collected following centrifugation. The remainder of the blood was anticoagulated with heparin, and the peripheral blood mononuclear cells (PBMCs) purified by Ficoll-Hypaque density gradient centrifugation. Human pDCs were positively selected from the PBMC using CD304-coated magnetic beads (Miltenyi). For some experiments, the negatively selected “flow through” cells, consisting of PBMCs depleted of CD304+ cells, were collected too. pDC lysates were generated by hypotonic lysis in sterile distilled water at 37°C for 30 min. Lysis was verified by microscopy. Lysates were immunodepleted of calprotectin by two rounds of sequential incubation with anti-calprotectin antibody and agarose beads coated with Proteins A and G according to the supplied protocol (Santa Cruz Biotech). The high purity of the pDC population was confirmed by analyzing the expression of the pDC markers CD123 and CD303 by flow cytometry (Colonna et al., 2004).
XTT assay.
XTT assay was performed as described (Meshulam et al., 1995). Briefly, A. fumigatus conidia were plated in 96-well half area plates and grown in pDC media to hyphae of 10-20 μm average length. The hyphae were preopsonized by incubation in 10% autologous serum for 30 min followed by washing. Experiments comparing preopsonized and unopsonized hyphae yielded similar results (data not shown). pDCs were then added to the hyphae in a final volume of 100 μl pDC media. For some experiments, where indicated, pDCs lysates were added in lieu of live pDCs or the experiments were conducted in Transwell chambers rather than half area wells. Following a 2 hr incubation, the pDCs were subjected to hypotonic lysis by three gentle washes and a 20 min incubation with sterile distilled water. Supernatants then were removed, taking care not to remove the hyphae. pDC media without serum containing 400 μg/ml of XTT and 50 μg/ml of Coenzyme Q were added and the wells were incubated for 2 hr at 37°C. The OD450 and OD650 were then measured and data expressed as percent antifungal activity according to the formula:
ODAf + pDC is (OD450 – OD650) of wells containing A. fumigatus hyphae with pDCs. ODpDC is (OD450 – OD650) of wells containing pDCs. ODAf is (OD450 – OD650) of wells containing A. fumigatus hyphae alone. ODblank is (OD450 – OD650) of wells containing media alone.
Hyphae Growth Inhibition Assay.
A. fumigatus hyphae were incubated in pDC media at 37°C in 8-well coverslip chambers (Nunc) with or without pDCs. Where indicated, pDC media were supplemented with 10 μM ZnCl2 or 10 μM FeCl3. At the specified times, pDCs were lysed with water and hyphae were fixed with 2% paraformaldehyde. Hyphal length was then measured by microscopy (Nikon Eclipse TE200) using software (SOFTMax Advance, SPOT Imaging Solutions) equipped with a curved and calibrated cursor. At least 10 visual fields containing ≥75 hyphae were scored per group.
Cytotoxicity Assay.
pDCs were incubated with or without preopsonized A. fumigatus or gliotoxin for 2 hr in 150 μl pDC media. Following centrifugation, supernatants were collected and LDH release assayed using a kit (Roche Applied Science). Maximum LDH released was determined by lysis with Triton X-100. All samples were measured in triplicate.
Confocal Microscopy.
Preopsonized A. fumigatus hyphae in 35mm tissue culture slide dishes (MatTek Corporation) were incubated with pDCs in pDC media for 2 hr at 37°C. Cell surface staining was then performed by incubating with the pDC specific antibody CD123-efluor650 (eBiosciences) for 30 min at 0°C. Following three washes with PBS supplemented with 2% FBS, fungal cell walls were stained with the chitin-specific fluorescent dye, 1% Uvitex 2B (Polysciences), by incubation for 20 min at 0°C. Samples were fixed with 2% buffered paraformaldehyde and visualized using a confocal microscope (Leica SP2 AOBS) equipped with a 63X plan apochromatic objective (Zeiss).
Transwell Assay. .
A. fumigatus conidia (5×105 in 100μl) were added to the surface of Transwell 96 well plates containing 0.4 μm pore size polycarbonate membrane inserts (Corning Life Sciences) and germinated to hyphae as described above. pDCs (5×105 in 100μl) were then added either below (allowing direct contact with hyphae) or above (allowing no contact with hyphae) the insert. After 2 hr incubation, pDC cytotoxicity and antifungal activity were assayed as described above.
TUNEL Assay.
TUNEL assay was performed following the manufacturer’s instructions (Molecular Probes). Briefly, pDCs and A. fumigatus hyphae were incubated for 2 hr at 37°C in tissue culture chamber slides and then fixed with 2% paraformaldehyde. pDCs were stained for the surface marker CD123 (CD123-efluor650), washed, permeabilized and stained for fragmented DNA by the Click-it reaction (Alexa Fluor 594). After additional washes, total cellular DNA was stained using Hoechst 33342. Samples were visualized by confocal microscopy, as described above.
Cytokine Secretion.
The indicated concentrations of pDCs or flow through cells were incubated with A. fumigatus hyphae in 96-well flat-bottom plates containing a final volume of 200 μl pDC media supplemented with 10% autologous serum. IFNα and TNFα levels in supernatants were measured by ELISA (Bender MedSystems, Burlingame, CA for human IFNα, eBiosciences for human TNFα and BD Biosciences for murine IL-12p40).
For the inhibition experiments, pDCs were left untreated or incubated for one hr with either 10 μg/ml of bafilomycin A1 or 10 μg/ml of chloroquine. pDCs were then incubated for 6 hr with preopsonized A. fumigatus hyphae, CpG or LPS in a final volume of 200 μl of pDC media without serum. Samples were then assayed for IFNα by ELISA.
Murine models of invasive aspergillosis.
Wild-type C57Bl/6J mice were from Jackson Laboratories. IFNα/βR−/− (also known as IFNARI−/− and IFN-IR−/−) mice, which lack the receptor needed to signal in response to type I IFNs, were from Jon Sprent (The Scripps Research Institute) and backcrossed to the C57Bl/6J background for 14 generations (Kolumam et al., 2005). Mice were 6 to 8 weeks old at the start of the experiments and groups were matched for sex and age. Mice were maintained under microisolation conditions at the University of Massachusetts Medical School under a protocol approved by the Institutional Animal Care and Use Committee.
A. fumigatus conidia were suspended in PBS supplemented with 0.01% Tween20. Pulmonary infection was achieved by anesthetizing mice with isoflurane followed by the oro-tracheal instillation of a total of 5×107 conidia administered in two doses of 50 μL each. For intravenous infections, mice received a tail vein injection of 100 μL of 1×106 A. fumigatus conidia (Bellocchio et al., 2004; Werner et al., 2009). For survival studies, infected mice were monitored at least daily for 30 days. Moribund mice were euthanized.
pDC Influx and CFU Assays.
Mice were infected oro-tracheally, euthanized 48 hr post infection, and the pulmonary arteries perfused with PBS. Lungs were then harvested, minced and incubated with 1 mg/mL of collagenase type IV and 75 μg/mL bovine pancreatic DNAse supplemented with 5% FBS in PBS for 1 hr at 37°C (Wozniak et al., 2006). Following digestion, cells were passed through a 70μm filter, counted and stained for flow cytometry using fluorescently labeled antibodies (eBiosciences) against PDCA-1, CD11c, CD45 and F4/80 (Ang et al., 2010). Labels used were APC, FITC, PE and PercpCy5.5, respectively. Isotype controls were included in each experiment. Samples were analyzed using BD LSRII (Becton Dickinson) and data were analyzed using FlowJo 7.6 version for PC (Tree Star Inc). pDCs were defined as CD45+, F4/80−, CD11cInt, PDCA-1+ cells. The number of pDCs per lung was calculated by multiplying total number of cells per lung by percentage of pDCs.
pDC depletion.
pDC depletion in mice was achieved by intraperitoneal injection of 120G8, a monoclonal rat anti-mouse IgG that depletes pDCs (Asselin-Paturel et al., 2003). Mice were injected with 250 μg of 120G8 one day before infection and then given additional doses of 150 μg every other day for 14 days starting on the day of infection. Control mice received equivalent doses of GL113, a monoclonal rat IgG directed against E. coli β-galactosidase.
Statistical Analysis.
For comparisons of two groups, means ± SE were analyzed by the two-tailed unpaired Student t-test with the Bonferroni correction applied when making multiple comparisons. For comparisons of greater than two groups, significance was determining using the one- or two-way analysis of variance (ANOVA) with the Tukey multiple correction. Kaplan-Meier survival curves were compared with the log rank test. Calculations were performed using a statistical software package (GraphPad Prism 4.02).
Supplementary Material
Acknowledgments:
Dr. Nancy Keller and Lim Fang Yun generously provided the gliotoxin deletion and complemented strains. This work was supported in part by NIH grants RO1 AI066087 and RO1 AI025780 and UMass Medical School.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References.
- Ang DK, Oates CV, Schuelein R, Kelly M, Sansom FM, Bourges D, Boon L, Hertzog PJ, Hartland EL, van Driel IR. Cutting edge: Pulmonary Legionella pneumophila is controlled by plasmacytoid dendritic cells but not type I IFN. J Immunol. 2010;184:5429–5433. doi: 10.4049/jimmunol.1000128. [DOI] [PubMed] [Google Scholar]
- Asselin-Paturel C, Brizard G, Pin JJ, Briere F, Trinchieri G. Mouse strain differences in plasmacytoid dendritic cell frequency and function revealed by a novel monoclonal antibody. J Immunol. 2003;171:6466–6477. doi: 10.4049/jimmunol.171.12.6466. [DOI] [PubMed] [Google Scholar]
- Bellocchio S, Montagnoli C, Bozza S, Gaziano R, Rossi G, Mambula SS, Vecchi A, Mantovani A, Levitz SM, Romani L. The contribution of the Toll-like/IL-1 receptor superfamily to innate and adaptive immunity to fungal pathogens in vivo. J Immunol. 2004;172:3059–3069. doi: 10.4049/jimmunol.172.5.3059. [DOI] [PubMed] [Google Scholar]
- Biondo C, Midiri A, Gambuzza M, Gerace E, Falduto M, Galbo R, Bellantoni A, Beninati C, Teti G, Leanderson T, Mancuso G. IFN-alpha/beta signaling is required for polarization of cytokine responses toward a protective type 1 pattern during experimental cryptococcosis. J Immunol. 2008;181:566–573. doi: 10.4049/jimmunol.181.1.566. [DOI] [PubMed] [Google Scholar]
- Blasius AL, Giurisato E, Cella M, Schreiber RD, Shaw AS, Colonna M. Bone Marrow Stromal Cell Antigen 2 Is a Specific Marker of Type I IFN-Producing Cells in the Naive Mouse, but a Promiscuous Cell Surface Antigen following IFN Stimulation. The Journal of Immunology. 2006;177:3260–3265. doi: 10.4049/jimmunol.177.5.3260. [DOI] [PubMed] [Google Scholar]
- Bok JW, Balajee SA, Marr KA, Andes D, Nielsen KF, Frisvad JC, Keller NP. LaeA, a regulator of morphogenetic fungal virulence factors. Eukaryot Cell. 2005;4:1574–1582. doi: 10.1128/EC.4.9.1574-1582.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bok JW, Chung D, Balajee SA, Marr KA, Andes D, Nielsen KF, Frisvad JC, Kirby KA, Keller NP. GliZ, a transcriptional regulator of gliotoxin biosynthesis, contributes to Aspergillus fumigatus virulence. Infect Immun. 2006;74:6761–6768. doi: 10.1128/IAI.00780-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bok JW, Keller NP. LaeA, a regulator of secondary metabolism in Aspergillus spp. Eukaryot Cell. 2004;3:527–535. doi: 10.1128/EC.3.2.527-535.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouhired S, Weber M, Kempf-Sontag A, Keller NP, Hoffmeister D. Accurate prediction of the Aspergillus nidulans terrequinone gene cluster boundaries using the transcriptional regulator LaeA. Fungal Genet Biol. 2007;44:1134–1145. doi: 10.1016/j.fgb.2006.12.010. [DOI] [PubMed] [Google Scholar]
- Bozza S, Gaziano R, Spreca A, Bacci A, Montagnoli C, di Francesco P, Romani L. Dendritic cells transport conidia and hyphae of Aspergillus fumigatus from the airways to the draining lymph nodes and initiate disparate Th responses to the fungus. J Immunol. 2002;168:1362–1371. doi: 10.4049/jimmunol.168.3.1362. [DOI] [PubMed] [Google Scholar]
- Bruns S, Kniemeyer O, Hasenberg M, Aimanianda V, Nietzsche S, Thywissen A, Jeron A, Latge JP, Brakhage AA, Gunzer M. Production of extracellular traps against Aspergillus fumigatus in vitro and in infected lung tissue is dependent on invading neutrophils and influenced by hydrophobin RodA. PLoS Pathog. 2010;6:e1000873. doi: 10.1371/journal.ppat.1000873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao W, Liu YJ. Innate immune functions of plasmacytoid dendritic cells. Curr Opin Immunol. 2007;19:24–30. doi: 10.1016/j.coi.2006.11.004. [DOI] [PubMed] [Google Scholar]
- Challa S, Chan FK. Going up in flames: necrotic cell injury and inflammatory diseases. Cell Mol Life Sci. 2010;67:3241–3253. doi: 10.1007/s00018-010-0413-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colonna M, Trinchieri G, Liu YJ. Plasmacytoid dendritic cells in immunity. Nat Immunol. 2004;5:1219–1226. doi: 10.1038/ni1141. [DOI] [PubMed] [Google Scholar]
- Dagenais TR, Keller NP. Pathogenesis of Aspergillus fumigatus in Invasive Aspergillosis. Clin Microbiol Rev. 2009;22:447–465. doi: 10.1128/CMR.00055-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink SL, Bergsbaken T, Cookson BT. Anthrax lethal toxin and Salmonella elicit the common cell death pathway of caspase-1-dependent pyroptosis via distinct mechanisms. Proc Natl Acad Sci U S A. 2008;105:4312–4317. doi: 10.1073/pnas.0707370105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilliet M, Cao W, Liu YJ. Plasmacytoid dendritic cells: sensing nucleic acids in viral infection and autoimmune diseases. Nat Rev Immunol. 2008;8:594–606. doi: 10.1038/nri2358. [DOI] [PubMed] [Google Scholar]
- Graham LM, Brown GD. The Dectin-2 family of C-type lectins in immunity and homeostasis. Cytokine. 2009;48:148–155. doi: 10.1016/j.cyto.2009.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grouard G, Rissoan MC, Filgueira L, Durand I, Banchereau J, Liu YJ. The enigmatic plasmacytoid T cells develop into dendritic cells with interleukin (IL)-3 and CD40-ligand. J Exp Med. 1997;185:1101–1111. doi: 10.1084/jem.185.6.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartigan AJ, Westwick J, Jarai G, Hogaboam CM. CCR7 deficiency on dendritic cells enhances fungal clearance in a murine model of pulmonary invasive aspergillosis. J Immunol. 2009;183:5171–5179. doi: 10.4049/jimmunol.0901027. [DOI] [PubMed] [Google Scholar]
- Hohl TM, Feldmesser M. Aspergillus fumigatus: principles of pathogenesis and host defense. Eukaryot Cell. 2007;6:1953–1963. doi: 10.1128/EC.00274-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohl TM, Rivera A, Lipuma L, Gallegos A, Shi C, Mack M, Pamer EG. Inflammatory monocytes facilitate adaptive CD4 T cell responses during respiratory fungal infection. Cell Host Microbe. 2009;6:470–481. doi: 10.1016/j.chom.2009.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohl TM, Van Epps HL, Rivera A, Morgan LA, Chen PL, Feldmesser M, Pamer EG. Aspergillus fumigatus triggers inflammatory responses by stage-specific beta-glucan display. PLoS Pathog. 2005;1:e30. doi: 10.1371/journal.ppat.0010030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H, Ostroff GR, Lee CK, Wang JP, Specht CA, Levitz SM. Distinct patterns of dendritic cell cytokine release stimulated by fungal beta-glucans and toll-like receptor agonists. Infect Immun. 2009;77:1774–1781. doi: 10.1128/IAI.00086-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight SA, Dancis A. Reduction of 2,3-bis(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide inner salt (XTT) is dependent on CaFRE10 ferric reductase for Candida albicans grown in unbuffered media. Microbiology. 2006;152:2301–2308. doi: 10.1099/mic.0.28843-0. [DOI] [PubMed] [Google Scholar]
- Kolumam GA, Thomas S, Thompson LJ, Sprent J, Murali-Krishna K. Type I interferons act directly on CD8 T cells to allow clonal expansion and memory formation in response to viral infection. J Exp Med. 2005;202:637–650. doi: 10.1084/jem.20050821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupfahl C, Michalka A, Lass-Florl C, Fischer G, Haase G, Ruppert T, Geginat G, Hof H. Gliotoxin production by clinical and environmental Aspergillus fumigatus strains. Int J Med Microbiol. 2008;298:319–327. doi: 10.1016/j.ijmm.2007.04.006. [DOI] [PubMed] [Google Scholar]
- Lande R, Gilliet M. Plasmacytoid dendritic cells: key players in the initiation and regulation of immune responses. Ann N Y Acad Sci. 2010;1183:89–103. doi: 10.1111/j.1749-6632.2009.05152.x. [DOI] [PubMed] [Google Scholar]
- Lepelletier Y, Zollinger R, Ghirelli C, Raynaud F, Hadj-Slimane R, Cappuccio A, Hermine O, Liu YJ, Soumelis V. Toll-like receptor control of glucocorticoid-induced apoptosis in human plasmacytoid pre-dendritic cells (pDC) Blood. 2010 doi: 10.1182/blood-2010-05-282913. [DOI] [PubMed] [Google Scholar]
- Levitz SM, Selsted ME, Ganz T, Lehrer RI, Diamond RD. In vitro killing of spores and hyphae of Aspergillus fumigatus and Rhizopus oryzae by rabbit neutrophil cationic peptides and bronchoalveolar macrophages. J Infect Dis. 1986;154:483–489. doi: 10.1093/infdis/154.3.483. [DOI] [PubMed] [Google Scholar]
- Lewis RE, Wiederhold NP, Chi J, Han XY, Komanduri KV, Kontoyiannis DP, Prince RA. Detection of gliotoxin in experimental and human aspergillosis. Infect Immun. 2005;73:635–637. doi: 10.1128/IAI.73.1.635-637.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lulloff SJ, Hahn BL, Sohnle PG. Fungal susceptibility to zinc deprivation. J Lab Clin Med. 2004;144:208–214. doi: 10.1016/j.lab.2004.07.007. [DOI] [PubMed] [Google Scholar]
- Mambula SS, Sau K, Henneke P, Golenbock DT, Levitz SM. Toll-like receptor (TLR) signaling in response to Aspergillus fumigatus. J Biol Chem. 2002;277:39320–39326. doi: 10.1074/jbc.M201683200. [DOI] [PubMed] [Google Scholar]
- Mambula SS, Simons ER, Hastey R, Selsted ME, Levitz SM. Human neutrophil-mediated nonoxidative antifungal activity against Cryptococcus neoformans. Infect Immun. 2000;68:6257–6264. doi: 10.1128/iai.68.11.6257-6264.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meshulam T, Levitz SM, Christin L, Diamond RD. A simplified new assay for assessment of fungal cell damage with the tetrazolium dye, (2,3)-bis-(2-methoxy-4-nitro-5-sulphenyl)-(2H)-tetrazolium-5-carboxanil ide (XTT) J Infect Dis. 1995;172:1153–1156. doi: 10.1093/infdis/172.4.1153. [DOI] [PubMed] [Google Scholar]
- Meyer-Wentrup F, Benitez-Ribas D, Tacken PJ, Punt CJ, Figdor CG, de Vries IJ, Adema GJ. Targeting DCIR on human plasmacytoid dendritic cells results in antigen presentation and inhibits IFN-alpha production. Blood. 2008;111:4245–4253. doi: 10.1182/blood-2007-03-081398. [DOI] [PubMed] [Google Scholar]
- Park SJ, Burdick MD, Brix WK, Stoler MH, Askew DS, Strieter RM, Mehrad B. Neutropenia enhances lung dendritic cell recruitment in response to Aspergillus via a cytokine-to-chemokine amplification loop. J Immunol. 2010;185:6190–6197. doi: 10.4049/jimmunol.1002064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perruccio K, Bozza S, Montagnoli C, Bellocchio S, Aversa F, Martelli M, Bistoni F, Velardi A, Romani L. Prospects for dendritic cell vaccination against fungal infections in hematopoietic transplantation. Blood Cells Mol Dis. 2004;33:248–255. doi: 10.1016/j.bcmd.2004.08.011. [DOI] [PubMed] [Google Scholar]
- Pietras EM, Saha SK, Cheng G. The interferon response to bacterial and viral infections. J Endotoxin Res. 2006;12:246–250. doi: 10.1179/096805106X118799. [DOI] [PubMed] [Google Scholar]
- Ramirez-Ortiz ZG, Specht CA, Wang JP, Lee CK, Bartholomeu DC, Gazzinelli RT, Levitz SM. Toll-like receptor 9-dependent immune activation by unmethylated CpG motifs in Aspergillus fumigatus DNA. Infect Immun. 2008;76:2123–2129. doi: 10.1128/IAI.00047-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romani L, Bistoni F, Gaziano R, Bozza S, Montagnoli C, Perruccio K, Pitzurra L, Bellocchio S, Velardi A, Rasi G, et al. Thymosin alpha 1 activates dendritic cells for antifungal Th1 resistance through toll-like receptor signaling. Blood. 2004;103:4232–4239. doi: 10.1182/blood-2003-11-4036. [DOI] [PubMed] [Google Scholar]
- Spikes S, Xu R, Nguyen CK, Chamilos G, Kontoyiannis DP, Jacobson RH, Ejzykowicz DE, Chiang LY, Filler SG, May GS. Gliotoxin production in Aspergillus fumigatus contributes to host-specific differences in virulence. J Infect Dis. 2008;197:479–486. doi: 10.1086/525044. [DOI] [PubMed] [Google Scholar]
- Stanzani M, Orciuolo E, Lewis R, Kontoyiannis DP, Martins SL, St John LS, Komanduri KV. Aspergillus fumigatus suppresses the human cellular immune response via gliotoxin-mediated apoptosis of monocytes. Blood. 2005;105:2258–2265. doi: 10.1182/blood-2004-09-3421. [DOI] [PubMed] [Google Scholar]
- Sutton P, Newcombe NR, Waring P, Mullbacher A. In vivo immunosuppressive activity of gliotoxin, a metabolite produced by human pathogenic fungi. Infect Immun. 1994;62:1192–1198. doi: 10.1128/iai.62.4.1192-1198.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban CF, Ermert D, Schmid M, Abu-Abed U, Goosmann C, Nacken W, Brinkmann V, Jungblut PR, Zychlinsky A. Neutrophil extracellular traps contain calprotectin, a cytosolic protein complex involved in host defense against Candida albicans. PLoS Pathog. 2009;5:e1000639. doi: 10.1371/journal.ppat.1000639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JP, Liu P, Latz E, Golenbock DT, Finberg RW, Libraty DH. Flavivirus activation of plasmacytoid dendritic cells delineates key elements of TLR7 signaling beyond endosomal recognition. J Immunol. 2006;177:7114–7121. doi: 10.4049/jimmunol.177.10.7114. [DOI] [PubMed] [Google Scholar]
- Werner JL, Metz AE, Horn D, Schoeb TR, Hewitt MM, Schwiebert LM, Faro-Trindade I, Brown GD, Steele C. Requisite role for the dectin-1 beta-glucan receptor in pulmonary defense against Aspergillus fumigatus. J Immunol. 2009;182:4938–4946. doi: 10.4049/jimmunol.0804250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wozniak KL, Vyas JM, Levitz SM. In vivo role of dendritic cells in a murine model of pulmonary cryptococcosis. Infect Immun. 2006;74:3817–3824. doi: 10.1128/IAI.00317-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang GX, Lian ZX, Kikuchi K, Liu YJ, Ansari AA, Ikehara S, Gershwin ME. CD4-plasmacytoid dendritic cells (pDCs) migrate in lymph nodes by CpG inoculation and represent a potent functional subset of pDCs. J Immunol. 2005;174:3197–3203. doi: 10.4049/jimmunol.174.6.3197. [DOI] [PubMed] [Google Scholar]
- Yu CF, Peng WM, Oldenburg J, Hoch J, Bieber T, Limmer A, Hartmann G, Barchet W, Eis-Hubinger AM, Novak N. Human plasmacytoid dendritic cells support Th17 cell effector function in response to TLR7 ligation. J Immunol. 2010;184:1159–1167. doi: 10.4049/jimmunol.0901706. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.