Abstract
The interaction between mobility and HIV risk is well recognized, but what happens to those same individuals, once infected, as they transition to living with HIV? Does mobility affect their transition into HIV care? If so, do mobile and non-mobile populations achieve similar success with HIV treatment?
The definition of mobility has changed over the centuries to encompass a complex phenotype including permanent migration, frequent travel, circular migration, and distance from HIV treatment centers. The heterogeneity of these definitions leads to discordant findings. Investigations show that mobility has an impact on HIV risk, but fewer data exist on the impact of geographic mobility on HIV care and treatment.
This review will examine existing data on the impact of geographic mobility on access to and maintenance in HIV care and on adherence to antiretroviral therapy. It will also expand the concept of mobility to include data on the impact of the distance from residence to clinic on HIV care and treatment adherence.
Our conclusions are that the existing literature is limited by varying definitions of mobility and the inherent oversimplification necessary to apply a “mobility measure” in a statistical analysis. The impact of mobility on HIV treatment outcomes deserves further exploration to both define the phenomenon and target interventions to these at-risk populations.
Keywords: Human immunodeficiency virus, HIV, mobile population, anti-HIVtreatment
Geographic mobility is known to increase risk for HIV infection and is increasingly recognized as a potential barrier to HIV care and treatment.1, 2 This article will review selected studies relevant to HIV care and treatment for geographically mobile populations. It begins with an overview of how mobile or migrant populations have been identified over the past two centuries, discusses the current scope of geographic mobility in the modern world, and then examines selected literature on mobile populations and HIV care. The literature review is divided into three sections reflecting different aspects of the HIV care-mobility relationship: 1) the effect of geographic mobility on HIV diagnosis and access to HIV care, 2) the impact of mobility on adherence to antiretroviral treatment and maintenance in HIV care, and 3) the increasingly recognized barrier that travel to HIV treatment centers poses for many people living with HIV, particularly in rural or resource-limited settings. We will highlight the strengths and limitations to the current body of literature and provide insight into the complexity of the relationship between HIV care and geographic mobility.
WHAT IS A “MOBILE” POPULATION? A BRIEF AND INCOMPLETE HISTORY OF MIGRATION THEORY
E.G. Ravenstein’s 1885 paper, “The Laws of Migration” represents a starting point for migration theory in the English literature. By comparing census data from the United Kingdom collected in 1871 to 1881, Ravenstein proposed seven “laws.” The paper maintains that economic motivations drive all migration, but is limited by the lack of detail provided by the census data examined. Nevertheless, it highlights some of the issues that make mobility difficult to define today, including variations in patterns of mobility: local migrants (within a city), short-journey migrants (to a bordering county), migration by stages, long-journey migrants, and temporary migrants. Ravenstein asserted that the majority of migrants only travel a short distance, and noted differences in migration patterns by gender. He observed that women migrate more frequently than men but stay within the country, whereas men are more likely to leave their birth kingdom.3 He later expanded on his work to include data from over twenty countries that supported his earlier laws.4
Almost a century later, Everett S. Lee took up Ravenstein’s mantle and defined mobility broadly as “a permanent or semi-permanent change of residence,” intentionally avoiding limitations based upon the distance of or motivation for the move, or the need to cross international borders. He excluded temporary moves, repeated travel to a single destination, and the movements of migrants who have no long-term residence. Lee defined four important factors that affect migration: those associated with the area of origin, those associated with the area of destination, intervening obstacles, and personal factors.5 From Ravenstein’s laws and Lee’s framework come the frequently discussed “push” and “pull” factors thought to govern the act of migration. “Push” factors are those that lead people to leave unfavorable conditions in one place, and “pull” factors are favorable conditions that attract migrants to a destination.
Zelinsky then added a temporal element to migration theory, asserting that, over time and with modernization, personal mobility increases. He also included “circulation” as “short-term, repetitive, or cyclical” movements that represent mobility but without the declared intention of a lasting change in residence.6 This concept has evolved into what many call “circular migration,” a pattern of recurrent migration to and from a specific destination.7, 8 Zelinsky also noted that distance is not necessarily a constant and that a functional approach to space, to account for the time or cost of travel, may be more accurate.6
In the following decades, both Massey and colleagues and Kearney offered up reviews of migration theory.9, 10 Both emphasize the different, and often divergent, conclusions that can be drawn based on individual migration theories and the need for an empiric approach. Kearney also describes the difficulty in finding the appropriate unit of analysis for the study of migration, and promotes a combined “articulation theory” which uses the household as a unit of analysis but examines its interactions with both capitalist and non-capitalist modes of production.10
The work above, along with that of many others, provides a theoretical framework to guide the examination of mobility as a complex behavioral, social, political, and economic phenomenon. It becomes clear that there are multiple axes of mobility, some of which are summarized in Table 1. Considering this overwhelming complexity, the lack of a unifying definition for a “mobile” population or individual is unsurprising. For the purposes of this review, we will use the United Nations (UN) definition, which defines mobile people broadly as those “who move from one place to another temporarily, seasonally, or permanently for a host of voluntary and/or involuntary reasons.” Migrants, by extension, are mobile people who “take up residence or who remain for an extended stay in a foreign country.”1 The UN also specifically recognizes that refugees and asylum seekers may be an important subgroup with regards to risk for HIV/AIDS and other health-related issues.11
Table 1.
Axes which can be used to define and measure mobile populations. References are for migration theory literature discussing each potential division of “mobility”.
CURRENT ESTIMATES OF THE PREVALENCE OF GEOGRAPHIC MOBILITY
International Migration
The UN Department of Economic and Social Affairs estimates the number of international migrants, defined as the number of people living in a country or area other than that in which they were born, to be 214 million in 2010.12
International migration is not evenly distributed; approximately 10% of the population of more developed regions are international migrants, whereas 1.3% of the population of less developed regions are international migrants.12 Data presented in the 2009 Human Development Report from the United Nations Development Programme (UNDP) shows that, among international migrants, approximately one third moved from a developing country to a developed country; the remainder moved within developing countries or within developed countries. The UNDP report argues that geographic mobility leads to global gains in human development.13
In the United States (U.S.), the percentage of the total population represented by international migrants has risen from 9.1% in 1990 to 13.5% in 2010, and there are an estimated 42.8 million international migrants.12 Estimates of how many of these migrants are undocumented/unauthorized vary widely, but a recent report from the Pew Hispanic Research Center shows that the annual flow of unauthorized immigrants has decreased since mid-decade, and the total number of unauthorized immigrants was estimated at 11.1 million in March, 2009.14
Internal Migration
Not surprisingly, the scale of internal migration within a country is much greater than international migration. The UNDP estimates that globally, almost 740 million people are internal migrants, over three times the number of international migrants.13
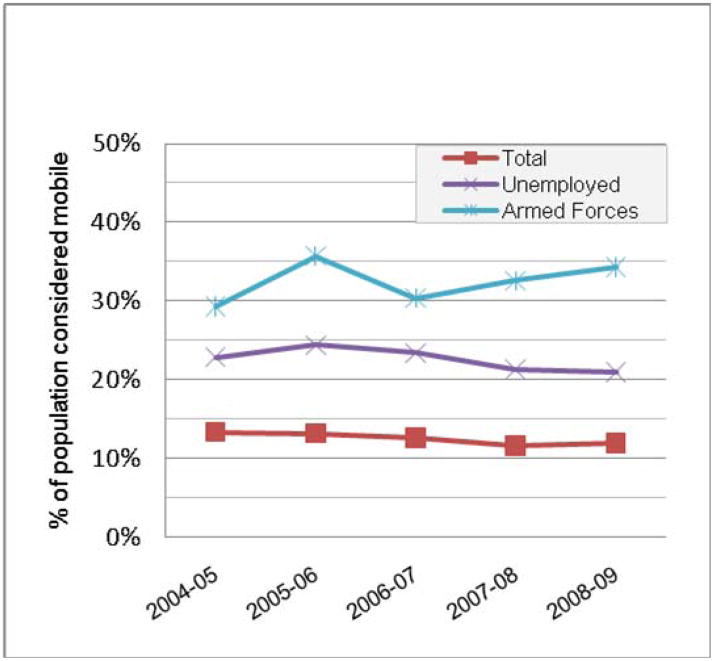
Mobility within the U.S. is estimated by the Current Population Survey, a monthly survey of approximately 50,000 households conducted by the Bureau of the Census for the Bureau of Labor Statistics. In the most recent report, 12% of the U.S. population reported living at a different residence in March, 2009 than their residence one year prior. However, rates of mobility by this measure vary dramatically by employment status (Figure 1) and race or ethnicity (Figure 2). People of color, particularly those self-identifying as Hispanic or black, are 1.5 to 2 times more mobile than the general population. Of note, the above estimates are not estimates of mobile populations by the UN definition,1 as the Current Population survey does not capture temporary or seasonal mobility. In general, estimates of mobility that encompass the entire population covered by the UN definition are rare, and thus, estimates of global “mobility”, as opposed to migration, do not exist.
Figure 1.
Mobility estimates by employment status from the U.S. Current Population Survey, 2004-2009
Figure 2.
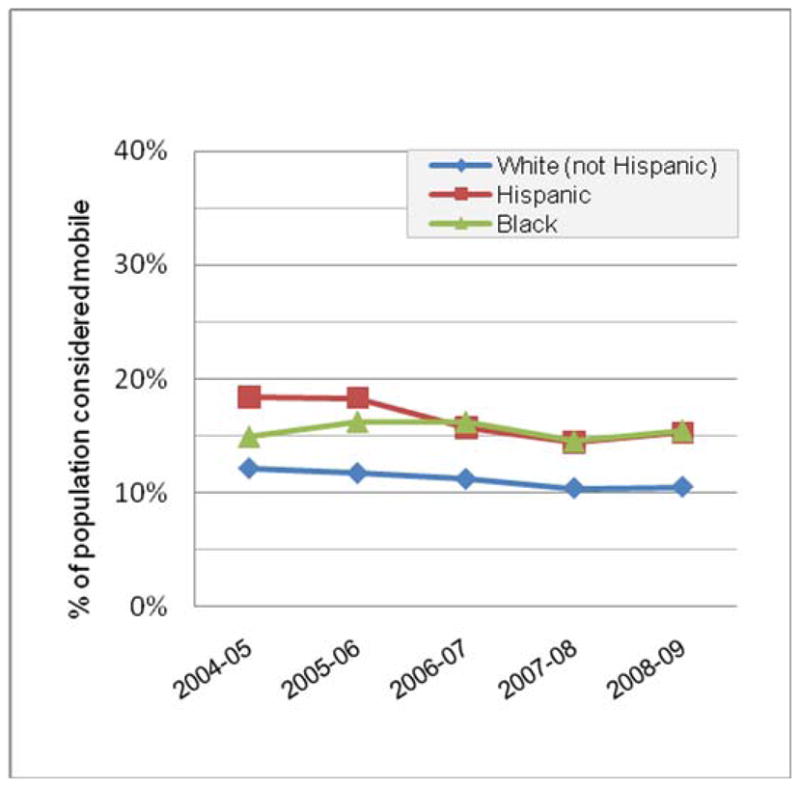
Mobility estimates by race/ethnicity from the U.S. Current Population Survey, 2004–2009
GEOGRAPHIC MOBILITY, ACCESS TO, AND MAINTENANCE IN HIV CARE
Early in the course of the HIV epidemic, governments, public health workers, and researchers argued that migration contributed to the spread of the HIV epidemic.15–19 The link between mobility and risk for HIV infection is well established,18, 20–26 and the strengths and limitations of these data were recently reviewed by Deane and colleagues.27 In 2001, the UN Programme on HIV/AIDS (UNAIDS) attempted to move the debate away from attempts to detain the HIV epidemic by preventing migration. UNAIDS argued that human mobility is highly prevalent, beneficial under many circumstances, and protected by the 1998 International Guidelines on HIV/AIDS and Human Rights.28 The 2001 report highlighted the obstacles that geographically mobile populations face in accessing HIV care once infected.1 A review of the existing literature reveals that the interaction between mobility and access to HIV care can be beneficial or detrimental.
Potential Negative Impact of Mobility on Access to HIV Care
In the short term, migrants may be at risk for delays in HIV diagnosis and entry into HIV care because of lack of medical insurance, language barriers, and fear of deportation if undocumented.
Investigators in Northern California found that immigrants were more likely than U.S.-born patients to be diagnosed with lower CD4+ cell counts, a measure of severe immunodeficiency, and opportunistic infections. These findings imply that immigrants were receiving their HIV diagnosis at a more advanced disease state than non-immigrants. In interviews conducted within the same study, lack of knowledge regarding HIV risk, social stigma surrounding HIV testing and diagnosis, and the need for secrecy were all found to contribute to immigrants’ delay in seeking HIV diagnosis and care.29 A published review of the role of international migration on the Japanese epidemic cites several studies with similar findings showing that immigrants to Japan are diagnosed and seek HIV treatment at more advanced disease stages than non-immigrants.30 As multiple studies link delays in initiation of HIV care and treatment to increased mortality,31–33 mobile populations’ delay in HIV diagnosis and care-seeking could impact their overall survival.
Moves towards Access to HIV Care or Caregivers
Once diagnosed, people living with HIV may move to seek support from caregivers or family members, access better HIV care, or escape stigma.
Two studies, one in Thailand and one in South Africa, examined changes in residence after HIV diagnosis and their relationship to mortality prior to widespread availability of ART in these regions. The South African study found that HIV+ people were more likely to move shortly prior to death, and concluded that people living with HIV were returning home due to their need for end-of-life care.34 The Thai study conducted a similar analysis, but also collected data from patients’ families and key informants which supported the conclusion that people with advanced HIV disease were seeking family support and care.35 Though both these studies linked moves to mortality, moves prior to death may be less necessary in the future as access to ART increases and mortality from HIV declines.
Two investigations using data from the U.S. HIV Cost and Services Utilization Study found that people living with HIV were more likely to change permanent residence than those without HIV, and two times more likely to change state of residence. The results of this survey differed from the South African and Thai studies in that most respondents indicated they were not moving back home. The most common reasons for moving included the desire to be near caregivers, to be in a community with shared needs and interests, to obtain care from a physician specializing in HIV care, and to avoid discrimination.36, 37 This type of mobility, in search of improved care or more favorable circumstances to receive care, could positively impact HIV treatment outcomes by expanding social support and access to services.
Investigators in British Columbia documented an increase in permanent migration of people living with HIV to Vancouver when antiretroviral therapy became available there in the early 1990s. The migration was attributed to desire for the increased access to specialized health care services in the urban center, particularly HIV care and support services. They documented that those with access to HIV care at the time of their diagnosis were less likely to move after diagnosis.38, 39 The same group went on to examine determinants of geographic mobility in a population-based HIV/AIDS drug treatment program, and found that permanent migration, defined as a change in postal code, was low: 3% of people in the cohort changed residence over the 27-month observation period. Mobility was associated with living in a smaller community, being heterosexual, acquiring HIV through intravenous drug use, and the absence of AIDS at the time of HIV diagnosis.40
Finally, two surveys of care utilization in the United Kingdom showed that migrants and asylum seekers were more likely than non-migrants to access HIV support services, but not clinical HIV care.41, 42 Bringing together the above findings, mobile populations, particularly international migrants, may be at risk for delays in HIV diagnosis or entry into HIV care.34, 35 Once diagnosed, people living with HIV may become mobile, particularly within the same country, in order to access better HIV care, receive needed support from caregivers, or escape stigma.36–40
GEOGRAPHIC MOBILITY AND ADHERENCE TO ANTIRETROVIRAL THERAPY
Mobility also has a potential impact on adherence to antiretroviral treatment for HIV. In populations with access to antiretroviral medication, poor adherence to antiretroviral therapy (ART) remains a primary barrier to treatment success, and a strong predictor of disease progression and mortality.43–45 The dominant hypothesis found in the literature is that mobility could negatively impact antiretroviral adherence, leading to poor treatment outcomes.
This adverse impact on adherence could be caused by mobility-induced interruptions in medication supply, increased difficulty in taking medications in settings with less privacy because of fears of disclosure of HIV status, disruptions in daily schedule, conflicting demands on the mobile individual’s time, and loss of social support if this support was found at home.30, 46–48
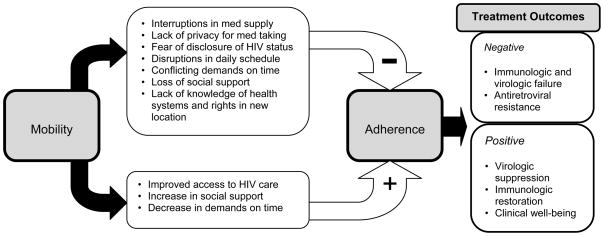
An alternate hypothesis is that mobility might have a positive impact on adherence to ART if people living with HIV were moving into locations where HIV care or caregivers were more readily available or social supports were improved. It is important to note that different types of mobility may have differential impacts on adherence. Figure 3 presents a theoretical framework for the varying impacts of mobility on adherence and, therefore, on the outcomes of antiretroviral therapy, and the following sections review current literature regarding mobility and antiretroviral treatment outcomes. To account for the diversity of definitions of mobility in the literature, and the likelihood that different patterns of mobility will have differential effects on HIV treatment outcomes, the literature review is divided by mobility patterns.
Figure 3.
Theoretical framework for the interaction between geographic mobility and HIV treatment outcomes.
Interactions between Permanent Migration and Adherence to Anti-retroviral Therapy
The Canadian investigators cited above also determined the impact of migration on antiretroviral adherence in British Columbia using the cumulative number of changes of residential address as a time-dependent measure of mobility, and pharmacy refill data to measure adherence. This study showed that individuals who migrated three or more times were 1.79 times more likely to be classified as non-adherent (95%CI: 1.44, 2.21; nonadherence defined as <95% ART coverage by pharmacy refill data), when compared to those with no migration. Similar associations were seen for those individuals moving once or twice during the observation period.49 This study raises additional questions as it did not allow for determination of reasons for non-adherence. Were medications not refilled because of difficulty transferring prescriptions, difficulty accessing care in a new location, or other mobility-induced barriers? Or was the mobility a symptom of some other barrier to adherence such as substance abuse or mental illness?
In a qualitative study on barriers to antiretroviral adherence among people living with HIV in Botswana, Weiser and colleagues interviewed 109 patients and 60 providers. They found that, in addition to financial constraints, forgetfulness, and running out of medications, 13% of patients identified travel or migration as a reason for missed doses of medications. Fifty-four percent of patient participants reported frequent travel or migration, and many patients had to travel great distances for HIV treatment.47
A cross-sectional study of predictors of loss to follow-up among 34,835 patients in the French Hospital Database on HIV, a network of 62 French University Hospitals, found that migrants were at increased risk for loss to follow-up.50 This finding is complicated by the use of a surrogate marker for migrant status, those responding positively to a question about “stays outside France for more than six months since 1978,” which also asks the respondent to specify location of stay. This variable has been shown to provide an approximation of migrant status in other studies.51 In this study, among patients diagnosed within the year, 13% reported a stay in sub-Saharan Africa of over 6 months, and 7% in other foreign non-European Union countries. Compared with those who did not report a stay outside of France, patients reporting stays in sub-Saharan Africa had an odds ratio for loss to follow up of 1.3 (95% CI: 1.0, 1.7) and for those reporting stays in other EU member states the OR was 2.6 (95% CI: 1.4, 4.8).50 Though these findings imply that migrants have higher degrees of loss to follow up, the lack of data regarding when the migration took place in relationship to the loss to follow up makes it difficult to determine whether permanent migration or circular migration was the issue.
Impact of Circular Migration on Adherence to Anti-retroviral Therapy
Few data exist on the impact of circular migration, defined by Zelinsky originally as “short-term, repetitive, or cyclical” movements that represent mobility but without the declared intention of a lasting change in residence,6 on HIV treatment outcomes. Many HIV+ individuals travel regularly for work or family obligations, while keeping their permanent residence constant. Circular migration is frequently implicated in the spread of HIV, and has been cited as a challenge in addressing at-risk populations,8, 52–54 but the effect of circular migration on adherence to antiretroviral therapy is not well described.
It may be that individuals in the French Hospital Database on HIV study described above fit the definition of circular migrants, as a subset clearly maintained residence in France while occasionally spending over 6 months in a different country, but the data did not permit close examination of migration patterns. Similarly, the Weiser study combined migration and frequent travel, so the impact of circular migration alone is more difficult to assess.
Sellier and colleagues conducted a cross-sectional observational study of antiretroviral adherence in 61 HIV-infected people from sub-Saharan Africa living in Paris who had returned from travel to their country of origin within the last 12 months. Self-reported adherence decreased significantly during the participants’ travel, with 26% frequently missing doses in Paris compared to 49% frequently missing doses in sub-Saharan Africa (p=0.015). Some of the common reasons for missed doses while traveling were being busy, fear of social stigma, and lack of a confidential place to store medications. The authors report that the duration of the visit and knowledge of HIV infection status among destination household members also appeared to affect adherence.46
AN ADDITIONAL ASPECT OF MOBILITY – DISTANCE FROM CLINIC
Though not included in traditional concepts of “mobility”, travel to access medical care can become a significant source of mobility for people living with HIV. Some data demonstrate that difficulty finding money for or time for transportation to and from clinic is a barrier to effective ART. Particularly where dedicated HIV care clinics are scarce, people living with HIV may travel hours or even days to receive medical care.
The cost of this mobility, both in terms of payment for transportation and in hours which could be dedicated to other tasks, is usually borne by the HIV+ patient and their family. Studies in Africa have shown that distance from clinic and difficulty paying for transportation is linked to poor ART adherence and loss to follow up.47, 55–57
Several qualitative studies have shown that people living with HIV in Uganda cite distance from clinic and the transportation costs of travel to clinic as a reason for poor ART adherence.55, 56 Another investigation, also from Uganda, showed that though the rate of perfect adherence to ART decreased as distance from clinic increased, the adherence rates for those living <20km and >20km from clinic did not differ.58
Distance from clinic has also been implicated as a reason for loss to follow up or delay in seeing HIV care. In an analysis of predictors of loss to follow up in a large program (n=46,400) in Western Kenya, those who spent over an hour traveling to clinic did not have higher rates of loss to follow up than those spending less than an hour.59 In a Ugandan study, the most common reasons cited by patients for not returning to clinic were: lack of transportation (50%) and distance to clinic (42%). Sixty-three percent of those who were “lost to follow up” were alive, and, of those interviewed, 83% of the survivors had transferred care to a new clinic, implying that the barrier of distance was being overcome by transfer of care to a closer treatment center.60 In Cameroon, retention in care was shown to be associated with having good access to care, defined as living within 40km of the clinic site or within 80km if living on a main road.57
In non-resource-limited settings, the impact of distance from clinic may differ. In Kansas City, distance between residence and clinic was not found to be associated with missed clinic visits,61 and travel to a clinic in the Southern United States was not found to be associated with delay in HIV care initiation in one study.62 In two separate analyses of distance traveled to services in England, where 80% of people living with HIV reside within 5 km of an HIV care center, people from more affluent areas were more likely to travel for HIV care than those from deprived areas, implying that travel for care was a choice.63, 64
CONCLUSION
This review highlights the strengths and limitations of the existing literature on mobility and HIV. Many studies have documented the increased risk for HIV infection incurred by mobile populations, and migrants/mobile populations are now listed among the UN’s vulnerable or “most-at-risk” populations. Fewer studies have assessed the impact of mobility on HIV+ populations, but those that have show that mobility may have both positive and negative effects on HIV care. Geographic mobility, particularly international migration, may lead to delays in HIV diagnosis or care-seeking behavior, adversely impacting HIV treatment outcomes. Mobility may also interfere with adherence to life-saving antiretroviral therapy. However, it may also lead to greater access to HIV care, as some individuals move towards HIV services and support systems, or away from stigma.
However, the existing literature is limited by varying definitions of mobility and the inherent oversimplification necessary to apply a quantitative measure of mobility. The various axes of mobility described in Table 1 are not considered in most of the investigations, and the complexity of the mobility “phenotype” may inhibit comparison across studies. This is exemplified by the lumping of migration and travel to clinic,47 and the lack of exploration of individual-level drivers of mobility and its impact on ART adherence in most studies to date.46, 49, 50 Despite these limitations, the investigations reviewed above shed light on the positive and negative impacts of mobility on HIV care and demonstrate that further investigations are essential to define the phenomenon and target interventions to these at-risk populations.
Acknowledgments
This work was supported in part by a grant from the National Institute for Allergy and Infectious Diseases (NIAID) K23AI081538 [B.S.T.]
The authors would like to gratefully acknowledge the participants in the Dominican HIV Cohort “Estudio SeR” who have generously given their time and support to examining HIV treatment outcomes in the Dominican Republic, and the outstanding team of care providers at the Instituto Dermatológico y Cirugía de Piel “Dr. Huberto Bogaert Diaz” (Dr. Lina Jose, Dr. Carmen Javier, Nurse Primitiva Rodriguez, Technician Ramona Franco, Counselor Sandra Guerrero, and Maireini Guerrera) and Profamilia Clínica Evangelina Rodriguez (Dr. Geraldine Mir, Nurse Eliza del Rosario, and Technician Margarita De Los Santos).
References
- 1.UNAIDS. Population Mobility and AIDS: UNAIDS Technical Update. Geneva: UNAIDS; Feb, 2001. [Google Scholar]
- 2.UNAIDS. Report on the Global AIDS Epidemic. Geneva: UNAIDS; 2010. [Google Scholar]
- 3.Ravenstein EG. The Laws of Migration. Journal of the Statistical Society of London. 1885;48(2):167–235. [Google Scholar]
- 4.Ravenstein EG. The Laws of Migration. Journal of the Statistical Society of London. 1889;52:241–301. [Google Scholar]
- 5.Lee ES. A Theory of Migration. Demography. 1966;3(1):47–57. [Google Scholar]
- 6.Zelinsky W. The Hypothesis of the Mobility Transition. Geographical Review. 1971 April;61(2):219–249. doi: 10.1111/gere.12310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rannveig Agunias D, Newland K. Circular Migration and Development: Trends, Policy Routes, and Ways Forward. Washington D.C: Migration Policy Institute; Apr, 2007. [Google Scholar]
- 8.Deren S, Shedlin M, Decena CU, Mino M. Research challenges to the study of HIV/AIDS among migrant and immigrant Hispanic populations in the United States. J Urban Health. 2005 Jun;82(2 Suppl 3):iii13–25. doi: 10.1093/jurban/jti060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Massey DS, Arango J, Hugo G, Kouaouci A, Pellegrino A, Taylor JE. Theories of international migration: A review and appraisal. Population and Development Review. 1993;19(3):431–466. [Google Scholar]
- 10.Kearney M. From the invisible hand to visible feet: Anthropological studies of migration and development. Annual Review of Anthropology. 1986;15:331–361. doi: 10.1146/annurev.an.15.100186.001555. [DOI] [PubMed] [Google Scholar]
- 11.UNAIDS. Refugees and AIDS: UNAIDS Point of View. Geneva: UNAIDS Best Practice Coalition; 1997. [Google Scholar]
- 12.United Nations, Department of Economic and Social Affairs, Population Division. Trends in International Migrant Stock: The 2008 Revision. 2009. (United Nations database, POP/DB/MIG/Stock/Rev.2008) [Google Scholar]
- 13.United Nations Development Programme. Human Development Report. New York: United Nations; 2009. Overcoming barriers: Human mobility and development. [Google Scholar]
- 14.Passel JS, Cohn DV. US Unauthorized Immigration Flows Are Down Sharply Since Mid-Decade. Washington D.C: Pew Hispanic Center; Sep 1, 2010. [Google Scholar]
- 15.Jochelson K, Mothibeli M, Leger JP. Human immunodeficiency virus and migrant labor in South Africa. Int J Health Serv. 1991;21(1):157–173. doi: 10.2190/11UE-L88J-46HN-HR0K. [DOI] [PubMed] [Google Scholar]
- 16.Barongo LR, Borgdorff MW, Mosha FF, et al. The epidemiology of HIV-1 infection in urban areas, roadside settlements and rural villages in Mwanza Region, Tanzania. AIDS. 1992 Dec;6(12):1521–1528. doi: 10.1097/00002030-199212000-00016. [DOI] [PubMed] [Google Scholar]
- 17.Quinn TC. Population migration and the spread of types 1 and 2 human immunodeficiency viruses. Proc Natl Acad Sci U S A. 1994 Mar 29;91(7):2407–2414. doi: 10.1073/pnas.91.7.2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abdool Karim Q, Abdool Karim SS, Singh B, Short R, Ngxongo S. Seroprevalence of HIV infection in rural South Africa. AIDS. 1992 Dec;6(12):1535–1539. doi: 10.1097/00002030-199212000-00018. [DOI] [PubMed] [Google Scholar]
- 19.Decosas J, Kane F, Anarfi JK, Sodji KD, Wagner HU. Migration and AIDS. Lancet. 1995 Sep 23;346(8978):826–828. doi: 10.1016/s0140-6736(95)91631-8. [DOI] [PubMed] [Google Scholar]
- 20.Lurie MN, Williams BG, Zuma K, et al. The impact of migration on HIV-1 transmission in South Africa: a study of migrant and nonmigrant men and their partners. Sexually Transmitted Diseases. 2003 Feb;30(2):149–156. doi: 10.1097/00007435-200302000-00011. [DOI] [PubMed] [Google Scholar]
- 21.Brewer TH, Hasbun J, Ryan CA, et al. Migration, ethnicity and environment: HIV risk factors for women on the sugar cane plantations of the Dominican Republic. AIDS. 1998 Oct 1;12(14):1879–1887. doi: 10.1097/00002030-199814000-00020. [DOI] [PubMed] [Google Scholar]
- 22.Poudel KC, Okumura J, Sherchand JB, Jimba M, Murakami I, Wakai S. Mumbai disease in far western Nepal: HIV infection and syphilis among male migrant-returnees and non-migrants. Trop Med Int Health. 2003 Oct;8(10):933–939. doi: 10.1046/j.1365-3156.2003.01110.x. [DOI] [PubMed] [Google Scholar]
- 23.Lagarde E, Schim van der Loeff M, Enel C, et al. Mobility and the spread of human immunodeficiency virus into rural areas of West Africa. Int J Epidemiol. 2003 Oct;32(5):744–752. doi: 10.1093/ije/dyg111. [DOI] [PubMed] [Google Scholar]
- 24.Lydie N, Robinson NJ, Ferry B, Akam E, De Loenzien M, Abega S. Mobility, sexual behavior, and HIV infection in an urban population in Cameroon. J Acquir Immune Defic Syndr. 2004 Jan 1;35(1):67–74. doi: 10.1097/00126334-200401010-00010. [DOI] [PubMed] [Google Scholar]
- 25.Khan MR, Patnaik P, Brown L, Nagot N, Salouka S, Weir SS. Mobility and HIV-related sexual behavior in Burkina Faso. AIDS and Behavior. 2008 Mar;12(2):202–212. doi: 10.1007/s10461-007-9314-8. [DOI] [PubMed] [Google Scholar]
- 26.Rees D, Murray J, Nelson G, Sonnenberg P. Oscillating migration and the epidemics of silicosis, tuberculosis, and HIV infection in South African gold miners. American Journal of Industrial Medicine. 2010;53(4):398–404. doi: 10.1002/ajim.20716. [DOI] [PubMed] [Google Scholar]
- 27.Deane KD, Parkhurst JO, Johnston D. Linking migration, mobility and HIV. Trop Med Int Health. 2010 Dec;15(12):1458–1463. doi: 10.1111/j.1365-3156.2010.02647.x. [DOI] [PubMed] [Google Scholar]
- 28.United Nations High Commissioner for Human Rights, Joint United Nations Programme on HIV/AIDS. HIV/AIDS and Human Rights: International Guidelines. Geneva: 1998. [Google Scholar]
- 29.Levy V, Prentiss D, Balmas G, et al. Factors in the delayed HIV presentation of immigrants in Northern California: implications for voluntary counseling and testing programs. Journal of immigrant and minority health/Center for Minority Public Health. 2007 Jan;9(1):49–54. doi: 10.1007/s10903-006-9015-9. [DOI] [PubMed] [Google Scholar]
- 30.Komatsu R, Sawada T. The role of international migration in infectious diseases: the HIV epidemic and its trends in Japan. Int J Health Serv. 2007;37(4):745–759. doi: 10.2190/HS.37.4.j. [DOI] [PubMed] [Google Scholar]
- 31.Nash D, Katyal M, Brinkhof MW, et al. Long-term immunologic response to antiretroviral therapy in low-income countries: a collaborative analysis of prospective studies. AIDS. 2008 Nov 12;22(17):2291–2302. doi: 10.1097/QAD.0b013e3283121ca9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kitahata MM, Gange SJ, Abraham AG, et al. Effect of early versus deferred antiretroviral therapy for HIV on survival. The New England Journal of Medicine. 2009 Apr 30;360(18):1815–1826. doi: 10.1056/NEJMoa0807252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sobrino-Vegas P, Garcia-San Miguel L, Caro-Murillo AM, et al. Delayed diagnosis of HIV infection in a multicenter cohort: prevalence, risk factors, response to HAART and impact on mortality. Curr HIV Res. 2009 Mar;7(2):224–230. doi: 10.2174/157016209787581535. [DOI] [PubMed] [Google Scholar]
- 34.Clark SJ, Collinson MA, Kahn K, Drullinger K, Tollman SM. Returning home to die: circular labour migration and mortality in South Africa. Scandinavian Journal of Public Health. 2007 Aug;69:35–44. doi: 10.1080/14034950701355619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Knodel J, VanLandingham M. Return migration in the context of parental assistance in the AIDS epidemic: the Thai experience. Soc Sci Med. 2003 Jul;57(2):327–342. doi: 10.1016/s0277-9536(02)00361-1. [DOI] [PubMed] [Google Scholar]
- 36.Berk ML, Schur CL, Dunbar JL, Bozzette S, Shapiro M. Short report: migration among persons living with HIV. Soc Sci Med. 2003 Sep;57(6):1091–1097. doi: 10.1016/s0277-9536(02)00487-2. [DOI] [PubMed] [Google Scholar]
- 37.London AS, Wilmoth JM, Fleishman JA. Moving for care: findings from the US HIV Cost and Services Utilization Study. AIDS Care. 2004 Oct;16(7):858–875. doi: 10.1080/09540120412331290149. [DOI] [PubMed] [Google Scholar]
- 38.Hogg RS, Schechter MT, Schilder A, et al. Access to health care and geographic mobility of HIV/AIDS patients. AIDS Patient Care. 1995 Dec;9(6):297–302. doi: 10.1089/apc.1995.9.297. [DOI] [PubMed] [Google Scholar]
- 39.Hogg RS, Whitehead J, Ricketts M, et al. Patterns of geographic mobility of persons with AIDS in Canada from time of AIDS index diagnosis to death. Clin Invest Med. 1997 Apr;20(2):77–83. [PubMed] [Google Scholar]
- 40.Wood E, Yip B, Gataric N, et al. Determinants of geographic mobility among participants in a population-based HIV/AIDS drug treatment program. Health & Place. 2000 Mar;6(1):33–40. doi: 10.1016/s1353-8292(99)00028-3. [DOI] [PubMed] [Google Scholar]
- 41.Madden HC, Phillips-Howard PA, Hargreaves SC, et al. AIDS Care. Jan 31, 2011. Access to HIV community services by vulnerable populations: evidence from an enhanced HIV/AIDS surveillance system; pp. 1–8. [DOI] [PubMed] [Google Scholar]
- 42.Cook PA, Downing J, Rimmer P, Syed Q, Bellis MA. Treatment and care of HIV positive asylum seekers. J Epidemiol Community Health. 2006 Oct;60(10):836–838. doi: 10.1136/jech.2005.044776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bangsberg DR, Perry S, Charlebois ED, et al. Non-adherence to highly active antiretroviral therapy predicts progression to AIDS. AIDS. 2001 Jun 15;15(9):1181–1183. doi: 10.1097/00002030-200106150-00015. [DOI] [PubMed] [Google Scholar]
- 44.Hogg RS, Heath K, Bangsberg D, et al. Intermittent use of triple-combination therapy is predictive of mortality at baseline and after 1 year of follow-up. AIDS. 2002 May 3;16(7):1051–1058. doi: 10.1097/00002030-200205030-00012. [DOI] [PubMed] [Google Scholar]
- 45.Mannheimer S, Friedland G, Matts J, Child C, Chesney M. The consistency of adherence to antiretroviral therapy predicts biologic outcomes for human immunodeficiency virus-infected persons in clinical trials. Clin Infect Dis. 2002 Apr 15;34(8):1115–1121. doi: 10.1086/339074. [DOI] [PubMed] [Google Scholar]
- 46.Sellier P, Clevenbergh P, Ljubicic L, et al. Comparative evaluation of adherence to antiretroviral therapy in sub-Saharan African native HIV-infected patients in France and Africa. Clin Infect Dis. 2006 Sep 1;43(5):654–657. doi: 10.1086/506436. [DOI] [PubMed] [Google Scholar]
- 47.Weiser S, Wolfe W, Bangsberg D, et al. Barriers to antiretroviral adherence for patients living with HIV infection and AIDS in Botswana. J Acquir Immune Defic Syndr. 2003 Nov 1;34(3):281–288. doi: 10.1097/00126334-200311010-00004. [DOI] [PubMed] [Google Scholar]
- 48.Magis-Rodriguez C, Gayet C, Negroni M, et al. Migration and AIDS in Mexico: an overview based on recent evidence. J Acquir Immune Defic Syndr. 2004 Nov 1;37( Suppl 4):S215–226. doi: 10.1097/01.qai.0000141252.16099.af. [DOI] [PubMed] [Google Scholar]
- 49.Lima V, Fernandes K, Rachlis B, Druyts E, Montaner J, Hogg R. Migration adversely affects antiretroviral adherence in a population-based cohort of HIV/AIDS patients. Soc Sci Med. 2009 Mar;68(6):1044–1049. doi: 10.1016/j.socscimed.2008.12.043. [DOI] [PubMed] [Google Scholar]
- 50.Lanoy E, Mary-Krause M, Tattevin P, et al. Predictors identified for losses to follow-up among HIV-seropositive patients. Journal of Clinical Epidemiology. 2006 Aug;59(8):829–835. doi: 10.1016/j.jclinepi.2005.11.024. [DOI] [PubMed] [Google Scholar]
- 51.Simon G. Les migracions internationales. INED Populat Soc. 2002;382:1–4. [Google Scholar]
- 52.Lurie MN, Harrison A, Wilkinson D, Abdool Karim SS. Circular migration and sexual networking in rural KwaZulu/Natal: implications for the spread of HIV and other sexually transmitted diseases. Health Transition Review. 1997;7(Supplement 3):17–27. [Google Scholar]
- 53.Williams BG, Gouws E. The epidemiology of human immunodeficiency virus in South Africa. Philosophical Transactions of the Royal Society of London. 2001 Jul 29;356(1411):1077–1086. doi: 10.1098/rstb.2001.0896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ferreira-Pinto JRR, Shedlin M. Mexican men, female sex workers, and HIV/AIDS at the U.S.-Mexico Border. In: Mishra S, Conner R, Magaña J, editors. AIDS Crossing Borders: The Spread of HIV Among Migrant Latinos. Boulder: Westview Press; 1996. pp. 113–136. [Google Scholar]
- 55.Tuller DM, Bangsberg DR, Senkungu J, Ware NC, Emenyonu N, Weiser SD. Transportation costs impede sustained adherence and access to HAART in a clinic population in southwestern Uganda: a qualitative study. AIDS and Behavior. 2010 Aug;14(4):778–784. doi: 10.1007/s10461-009-9533-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Olupot-Olupot P, Katawera A, Cooper C, Small W, Anema A, Mills E. Adherence to antiretroviral therapy among a conflict-affected population in Northeastern Uganda: a qualitative study. AIDS. 2008 Sep 12;22(14):1882–1884. doi: 10.1097/QAD.0b013e3283112ba6. [DOI] [PubMed] [Google Scholar]
- 57.Mosoko JJ, Akam W, Weidle P, et al. Survival and adherence to ART in an era of decreasing drug cost in Limbe, Cameroon. 14th Conference on Retroviruses and Opportunistic Infections, Abstract 536 Vol Abstract #536; Los Angeles. 2007. [Google Scholar]
- 58.Boyarinova G. Antiretroviral HIV Therapy at TASO Clinic in Mulago Hospital in Kampala, Uganda: Medical, Cultural and Ethical Factors Influencing ART. Journal of Nursing, Allied Health & Health Education. 2007;1(1):1–12. [Google Scholar]
- 59.Ochieng V, Ochieng D, Sidle J, et al. Gender and loss-to follow-up (LTFU) from a large HIV treatment program in Western Kenya. Paper presented at: XVII International AIDS Conference; 3–8 August, 2008; Mexico City. [Google Scholar]
- 60.Geng EH, Bangsberg DR, Musinguzi N, et al. Understanding reasons for and outcomes of patients lost to follow-up in antiretroviral therapy programs in Africa through a sampling-based approach. J Acquir Immune Defic Syndr. 2010 Mar 1;53(3):405–411. doi: 10.1097/QAI.0b013e3181b843f0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rao K, Gantt T, McField Dean S, Griffin R, Bamberger D. Non-Engagement in HIV Care. Paper presented at: Infectious Diseases Society of America 48th Annual Meeting; October 23, 2010; Vancouver. [Google Scholar]
- 62.Krawczyk CS, Funkhouser E, Kilby JM, Kaslow RA, Bey AK, Vermund SH. Factors associated with delayed initiation of HIV medical care among infected persons attending a southern HIV/AIDS clinic. South Med J. 2006 May;99(5):472–481. doi: 10.1097/01.smj.0000215639.59563.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Huntington S, Chadborn T, Rice B, Brown A, Delpech V. Travel for HIV care in England: a choice or a necessity? HIV Med. 2010 Nov 22; doi: 10.1111/j.1468-1293.2010.00891.x. [DOI] [PubMed] [Google Scholar]
- 64.Cook PA, Downing J, Wheater CP, et al. Influence of socio-demographic factors on distances travelled to access HIV services: enhanced surveillance of HIV patients in north west England. BMC Public Health. 2009;9:78. doi: 10.1186/1471-2458-9-78. [DOI] [PMC free article] [PubMed] [Google Scholar]