Abstract
Background
African Americans have historically had high HDL-C compared to other races and ethnicities.
Objective
We sought to characterize whether there is a cross-sectional association between age and HDL-C in a contemporary community-based study of African Americans.
Methods
Cross-sectional data was modeled by logistic regression for predictors of HDL-C among African-Americans, ages 35–74, participating in the baseline examination of a community-based study of cardiovascular disease in Jackson, MS, during 2000–2004. After excluding persons taking lipid-lowering medications, hormone replacement therapy, oral contraceptives, or thyroid replacement, the analytical data set comprised 2420 persons (1370 women, 1050 men).
Results
HDL-C had a significant positive association with age after controlling for serum triglycerides, sex, waist circumference, percent dietary calories from carbohydrates, alcohol use, and leisure physical activity. Sex was a significant effect modifier of this relationship, whereby the increase in HDL-C with age was steeper for women than for men.
Conclusions
Cross-sectional analysis found a positive association of HDL-C with age while controlling for triglycerides. Careful evaluation of longitudinal data will be needed to confirm whether this is a true effect of aging, or a cohort or survivor effect.
Keywords: high density lipoprotein cholesterol, triglycerides, aging, epidemiology, African Americans, cohort studies
Introduction
African Americans have higher mortality from coronary heart disease (CHD) compared to white or and Hispanic Americans.1 However, until the mid-1980s, African Americans had a lower risk of death from CHD than white Americans of the same sex,2,3 with a favorable risk profile typically characterized by higher levels of HDL-cholesterol (HDL-C) compared to whites.4,5 The historically higher HDL-C among African Americans may have been related to the intensity of occupational physical activity of past occupations of African Americans, particularly among men.6 High protective levels of HDL-C may become less prevalent among younger African Americans who are now working at predominantly sedentary occupations. We sought to determine whether age was associated with HDL-C among a contemporary sample of African Americans.
Cross-sectional and longitudinal studies of the association of HDL-C with age have generally focused on European, white American, or Asian populations.7–15 Cross-sectional studies in these populations have generally noted that HDL-C is stable across age groups,7,8 or slightly increasing with age among women;9 occasional cross-sectional studies have noted an increase with age in both sexes.15 Several longitudinal analyses of HDL-C have shown stable or declining values of HDL-C with age, but these declines were associated with weight gain or an increase in triglycerides;10–13 fewer longitudinal studies have shown an increase with age.14,16 Up to this time, there have been no large studies of HDL-C across age groups among African Americans. The Jackson Heart Study provides an opportunity to investigate the association of age with HDL-C in a large community-based cohort of African Americans.
We sought to characterize the association of serum HDL-C with age among African Americans, ages 35–74, from the Jackson Heart Study (JHS), after controlling for important covariates, including serum triglycerides (TG). HDL-C levels are inversely related to TG levels, as cholesterol ester transferase protein (CETP) exchanges cholesterol esters attached to HDL particles for TG from TG-rich lipoproteins, especially from VLDL.17 Therefore, any description of independent determinants of HDL-C must control for TG.
Methods
The JHS is a longitudinal community-based study of the determinants and trajectory of cardiovascular disease among 5,301 adult African Americans in the Jackson, MS, metropolitan area.18 All participants gave their informed consent to this study, approved by the institutional review boards of Jackson State University, the University of Mississippi Medical Center, and Tougaloo College. At the first clinical examination during 2000–2004, blood pressure and anthropometric measures were obtained and blood samples were drawn after an 8-hour fast. HDL-C was measured in serum following Mg-dextran precipitation of serum (Roche Diagnostics, Indianapolis IN 46250); laboratory coefficient of variation for HDL-C was 2.9%. In an accompanying interview, the participants reported their physical activity, current amedications, usual dietary intake, alcohol and tobacco use, level of educational attainment and usual occupation (if retired, their usual pre-retirement occupation); women reported their menstrual history. The level of physical activity required by the participant's usual occupation was categorized as either sedentary, mostly standing, or strenuous.19
Of the 5,301 participants in the first JHS examination, 4,741 were between the ages of 35–74; sequential exclusions were of participants taking thyroid medication (243), having invalid dietary data (438), missing data on lipid-lowering medications (354), taking lipid-lowering medications (521), women missing menstrual or hormone medications status (27), and women taking oral contraceptives or hormone replacement therapy (496). Additional sequential exclusions were of those participants missing data on serum HDL-C (229), serum TG (1), waist circumference (6), and those with extreme outlier lipid values: TG≥700 (3), LDL≥300 (1), HDL≥130 (2). The resulting analysis set comprised 2420 persons: 1370 women and 1050 men (Table 1). Of the 1370 women, 875 were considered post-menopausal, reporting no menstrual periods during the past 2 years.
Table 1.
Description of analytical population, ages 35–74 Jackson Heart Study Baseline Exam, 2000–2004, n=2420*, mean (S.D.)
| Women n=1370 | Men n=1050 | |
|---|---|---|
| Age | 53.8 (10.7) | 53.4 (10.2) |
| HDL-C, mg/dl | 53.4 (13.6) | 45.8 (12.2) |
| Triglycerides, mg/dl | 95.1 (53.9) | 111.2 (66.8) |
| BMI | 33.0 (7.5) | 29.8 (6.0) |
| Waist, cm | 100.3 (17.0) | 100.7 (14.2) |
| Pct dietary calories as carbohydrates | 51.1 (9.4) | 49.5 (9.0) |
| Physical activity score† | 8.4 (2.5) | 8.8 (2.5) |
| Any alcohol use during past year | 38% | 61% |
| Hypertensive | 56% | 56% |
Excluded participants taking lipid-lowering or thyroid medications, hormone replacement or oral contraceptive.
Composite of 4 domains of physical activity, range 0–20
Data evaluations were conducted for all men and women; women only; men only; and men and women ≥ age 45 wherein women were restricted to those who were post-menopausal. Visual examinations guided decisions on data transformations and functional relationship formulations. Logarithmic (base 2) transformation was performed on TG. Waist circumference, as a measure of body adiposity, was more strongly correlated with HDL-C than body mass index and was offered to regression models. The following independent predictors were examined in each model: age, sex (and menopausal status for women-only model), log2(TG), non-HDL cholesterol, waist circumference, total dietary calories, percent of calories from carbohydrate, and the following dichotomous variables: leisure and household physical activity score above the 90th percentile, sedentary occupation, hypertension (systolic blood pressure>140 or diastolic >90 or taking anti-hypertensive medication), any current alcohol use, any current tobacco use, and non-completion of high school. Because low HDL-C has been associated with uricemia20 and with insulin resistance21,22, these models of HDL-C were also offered terms for the values of serum uric acid and HbA1C.
For each population subgroup examined, unadjusted, fully adjusted, and parsimonious models were examined. We present results for the association of age and HDL-C from the parsimonious model that explained the greatest proportion of the population variation in HDL-C. Models were fit using generalized estimating equations,23 to account for associations arising from the existence of siblings in the study. For each selected final model, randomness of residuals was ascertained for all covariates. SAS v9.2 was used for all analyses (SAS Institute, Cary, NC).
Results
Younger persons were significantly more likely than older persons to report usual occupations classified as sedentary: 56% of ages 35–44 reported sedentary occupations, decreasing to 29% of ages 65–74 (Cochran Cochran-Armitage Trend Test, p<.001).
In all regression models, four covariates explained approximately 90% of the modeled variation in HDL-C: sex, TG, age, and waist circumference. As expected, male sex, TG, and waist circumference were all negatively associated with HDL-C.
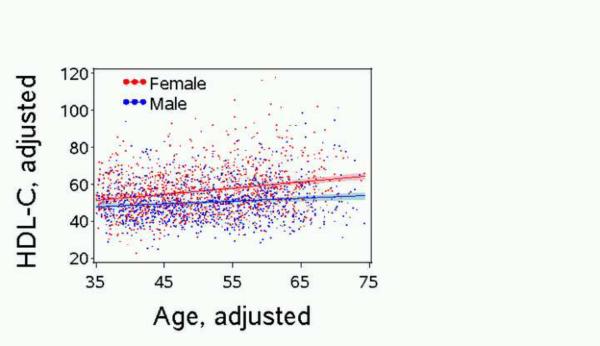
Age accounted for about 10% of this modeled variation and was significantly and positively associated with HDL-C in the final multivariable models. Age was a significant predictor of HDL-C in the model with all persons (Table 2) as well as when the model was restricted to either sex (results not shown) or restricted to men and post-menopausal women of ages 45+ (results not shown). For the model using data from all persons, an interaction term for age-by-sex was also significant, with women showing a faster rate of increase in HDL-C with age than men (0.34 ± .05 mg/dl vs. 0.13 ± .04 mg/dl, per year of age) (Table 2 and Figure 1).
Table 2.
The association of HDL-C, mg/dl, with age and sex Men and women, ages 35–74, Jackson Heart Study Baseline Exam, 2000–2004, n=2420*
| Parameter† | Estimate | 95% Confidence Limits | Pr > |Z| |
|---|---|---|---|
| Intercept | 44.2 | 43.3, 45.1 | <.0001 |
| Female | 7.1 | 6.2, 8.1 | <.0001 |
| Age per year | 0.13 | .06, .20 | 0.0003 |
| Female*Age† | 0.21 | 0.12, 0.30 | <.0001 |
| Triglyceride per doubling, mg/dl | −7.1 | −7.8, −6.4 | <.0001 |
| Waist per cm | −0.15 | −0.18, −0.12 | <.0001 |
| Dietary Carbohydrate per pct dietary calories | −0.10 | −0.15, −0.05 | 0.0001 |
| Alcohol use any vs. none | 2.0 | 1.0–3.0 | <.0001 |
| Leisure physical activity above 90th pctle vs. below | 1.8 | 0.41–3.2 | 0.0109 |
Excluded participants taking lipid-lowering or thyroid medications, female hormone replacement or oral contraceptives. 2283 obs used in regression; 135 obs missing data on physical activity, 2 obs missing data on alcohol use. SAS Proc GenMod, GEE analysis accounted for 2120 sibling clusters.
Continuous covariates were centered at the following values: Age=55 years, Triglycerides=100 mg/dl, Waist=100 cm, Dietary carbohydrates=50% of dietary calories. Thus, the intercept value (44.2 mg/dl) represents the mean estimate of HDL-C for a 55-yr-old man who does not drink alcohol, is not in the upper decile of physical activity, and for whom all other covariates equal the centered values.
Figure 1. Adjusted HDL-C by Adjusted Age*.
Jackson Heart Study Baseline Exam, 2000–2004, n=2420*
*HDL-C and age, each adjusted for waist, log2 triglyceride, percent dietary calories as carbohydrates, alcohol use (Y/N), leisure physical activity above 90th pctle (Y/N)
Alcohol use, leisure physical activity level above sex-specific 90th percentile (both positively associated with HDL-C), and percent of dietary calories from carbohydrate (negatively associated with HDL-C), were significant predictors in the model that used data from all men and women, but these covariates explained little of the modeled variance in HDL-C; sedentary occupation was negatively associated with HDL-C, but its effect did not reach significance.
Upon controlling for the aforementioned covariates, none of these remaining covariates offered to the multivariable models were retained as significantly related to HDL-C: hemoglobin A1C, uric acid, total dietary calories, tobacco use, or non-completion of high school. In the model restricted to women, menopausal status was not a significant predictor of HDL-C, after controlling for TG, waist, and non-HDL cholesterol.
Discussion
In this cross-sectional analysis of data from African American adults, HDL-C was significantly higher in older persons, after controlling for sex, TG, waist circumference, and several other significant but less important predictors of HDL-C. Age was significantly predictive of higher HDL-C, whether using the entire analytical data set of ages 35–74, or restricting the data either to persons age 45+ or to a single sex. We also determined that there was a significant interaction by sex, such that for women there was a greater increase in HDL-C per year of age difference than among men.
In cross-sectional studies of HDL-C in other populations, most have described stable HDL-C levels with age, but TG was not controlled in these analyses.7,8 Heitman, using cross-sectional data from Danish adults, found that HDL-C increased with age in women but not men.9 A recent cross-sectional study in China of 3914 adults found a positive association between age and HDL-C for both sexes: the prevalence of low HDL-C steadily was higher in young adults than in older adults.15
Longitudinal studies evaluating the association of HDL-C with aging have usually found that HDL-C declined or was stable with aging. In these populations, TG also increased with aging, or the changes in TG were not reported.10–13,24,25 In contrast to these findings, the present cross-sectional analysis may reflect an increase with HDL-C that occurs with aging after controlling for TG; because these aforementioned longitudinal analyses of HDL-C did not control for longitudinal changes in TG, it is not surprising that these earlier reports do not comport with our findings.
There have been two notable exceptions whereby longitudinal studies have reported that HDL-C increases with aging. Among Japanese-American men from the Honolulu Heart Study, the HDL-C increased significantly over a 20-year period, wherein increasing HDL-C was associated with decreasing body weight; the longitudinal change in TG was not reported.16 Early longitudinal analysis from mid-1970s to early 1980s of young, white adults in the Framingham Offspring cohort had reported HDL-C declines with aging but with no mention of TG changes;13 however, later longitudinal analysis of serial examinations conducted between 1991–2001of this same cohort, now older, reported a longitudinal rise in mean HDL-C coupled with a decline in TG; the relationship between HDL-C and TG by age was not explicitly assessed.14 The Framingham authors reasoned that these later changes in HDL-C and TG could have be due to change in the U.S. diet from saturated to non-saturated dietary lipids during the 1990s.14
Similar to our own analysis, one other report explicitly controlled for TG in an analysis of the effect of age on HDL-C, using data from two cross-sectional studies, one each from the U.S. and Hong Kong. In univariate analysis of these data, HDL-C appeared to be decreasing with age among Hong Kong women. However, similar to our own results, multivariable analysis that controlled for TG and other covariates found that the odds of low HDL declined with higher age, i.e., that HDL increased with age, in both U.S. and Hong Kong women and in U.S. men; only among Hong Kong men was there was no significant effect of age on HDL-C.26
Although the current report supports other studies that found a positive association of HDL-C with age, especially in women, this finding does not imply that reverse cholesterol transport becomes more effective with age. The contrary situation is more likely: at older ages, HDL particles were less efficient at reverse cholesterol transport; compared to HDL isolated from young subjects, HDL particles from older persons were less able to bring about cholesterol efflux from macrophages, were more susceptible to oxidative damage, and demonstrated impaired activity of paraoxonase, an enzyme that aids in reverse cholesterol transport.27–30
A limitation of the current cross-sectional analysis is that it cannot discern whether the association noted between HDL-C and age is a true effect of aging, a survivor effect, or a cohort effect. Perhaps older persons in this population may carry HDL-C at higher levels of serum TG than younger persons due to epigenetic factors relating to differential experiences between birth cohorts. Younger persons in the current study were more likely than older persons to have reported `usual' occupations that are sedentary, but in our final model, having a sedentary occupation was not a significant predictor of HDL-C after control for leisure physical activity and the other predictive covariates. Many of the older persons in the study have retired from their usual occupation, a factor that may have weakened the relation between occupational physical activity and HDL-C in this analysis. In the JHS population, the younger participants are, on average, heavier than the older participants, reflecting the recent obesity epidemic; but the positive association of age with HDL-C in these models was significant even after controlling for waist circumference and TG. It will be interesting to follow these younger members of the cohort, to learn the trajectory of their HDL-C values with aging, after adjustment for TG and waist measures.
Conclusion
Models of HDL-C using data from a large cross-sectional sample of African Americans found that age was significantly associated with higher HDL-C after controlling for sex, serum triglycerides, waist circumference, dietary carbohydrates, alcohol use, and leisure physical activity. The finding of significant association of age with HDL-C after controlling for triglycerides may indicate an altered relation, with aging, between the HDL particle, cholesterol and TG, a relation that is mediated by CETP and apolipoproteins. Forthcoming analyses will evaluate this relationship in longitudinal data from the Jackson Heart Study, as well as in longitudinal data from cohorts of other races and ethnicities, to establish whether this difference is seen in other populations, and whether the difference appears to be a cohort effect, a survivor effect, or a true effect of aging.
Acknowledgments
The Jackson Heart Study is conducted by the Jackson State University, the University of Mississippi Medical Center, and Tougaloo College, and is supported by National Institutes of Health contracts N01-HC-95170, N01-HC-95171, and N01-HC-95172 from the National Heart, Lung and Blood Institute, and the National Institute for Minority Health and Health Disparities. Anne E. Sumner is supported by the intramural program of the National Institute of Diabetes and Digestive and Kidney Diseases.
Financial support The Jackson Heart Study is conducted by the Jackson State University, the University of Mississippi Medical Center, and Tougaloo College, and is supported by National Institutes of Health contracts N01-HC-95170, N01-HC-95171, and N01-HC-95172 from the National Heart, Lung and Blood Institute and the National Institute for Minority Health and Health Disparities. Anne E. Sumner is supported by the intramural program of the National Institute of Diabetes and Digestive and Kidney Diseases.
Abbreviations
- JHS
Jackson Heart Study
- CHD
coronary heart disease
- HDL-C
HDL-cholesterol
- TG
triglycerides
- CETP
cholesterol ester transfer protein
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest No author has any conflict of interest to disclose.
Reference List
- (1).Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, de SG, Ferguson TB, Ford E, Furie K, Gillespie C, Go A, Greenlund K, Haase N, Hailpern S, Ho PM, Howard V, Kissela B, Kittner S, Lackland D, Lisabeth L, Marelli A, McDermott MM, Meigs J, Mozaffarian D, Mussolino M, Nichol G, Roger VL, Rosamond W, Sacco R, Sorlie P, Roger VL, Thom T, Wasserthiel-Smoller S, Wong ND, Wylie-Rosett J. Heart disease and stroke statistics--2010 update: a report from the American Heart Association. Circulation. 2010;121:e46–e215. doi: 10.1161/CIRCULATIONAHA.109.192667. [DOI] [PubMed] [Google Scholar]
- (2).National Center for Health Statistics [Accessed Dec 30,2010];Coronary Heart Disease in Adults, United States: 1960–1962. 1975 Series 11(10) Available at: http://www.cdc.gov/nchs/data/series/sr_11/sr11_010.pdf. [Google Scholar]
- (3).National Heart Lung and Blood Institute [Accessed Dec 28,2010];Morbidity and Mortality: 2009 Chart Book on Cardiovascular, Lung, and Blood Diseases. 2009 October; Available at: http://www.nhlbi.nih.gov/resources/docs/2009_ChartBook.pdf.
- (4).Tyroler HA, Hames CG, Krishan I, Heyden S, Cooper G, Cassel JC. Black-white differences in serum lipids and lipoproteins in Evans County. Prev Med. 1975;4:541–9. doi: 10.1016/0091-7435(75)90040-7. [DOI] [PubMed] [Google Scholar]
- (5).Morrison JA, deGroot I, Kelly KA, Mellies MJ, Khoury P, Edwards BK, Lewis D, Lewis A, Fiorelli M, Heiss G, Tyroler HA, Glueck CJ. Black-white differences in plasma lipids and lipoproteins in adults: the Cincinnati Lipid Research Clinic population study. Prev Med. 1979;8:34–9. doi: 10.1016/0091-7435(79)90027-6. [DOI] [PubMed] [Google Scholar]
- (6).Crook ED, Clark BL, Bradford ST, Golden K, Calvin R, Taylor HA, Jr., Flack JM. From 1960s Evans County Georgia to present-day Jackson, Mississippi: an exploration of the evolution of cardiovascular disease in African Americans. Am J Med Sci. 2003;325:307–14. doi: 10.1097/00000441-200306000-00002. [DOI] [PubMed] [Google Scholar]
- (7).Wallace RB, Colsher PL. Blood lipid distributions in older persons. Prevalence and correlates of hyperlipidemia. Ann Epidemiol. 1992;2:15–21. doi: 10.1016/1047-2797(92)90032-l. [DOI] [PubMed] [Google Scholar]
- (8).Abbott RD, Garrison RJ, Wilson PW, Epstein FH, Castelli WP, Feinleib M, LaRue C. Joint distribution of lipoprotein cholesterol classes. The Framingham study. Arteriosclerosis. 1983;3:260–72. doi: 10.1161/01.atv.3.3.260. [DOI] [PubMed] [Google Scholar]
- (9).Heitmann BL. The effects of gender and age on associations between blood lipid levels and obesity in Danish men and women aged 35–65 years. J Clin Epidemiol. 1992;45:693–702. doi: 10.1016/0895-4356(92)90046-p. [DOI] [PubMed] [Google Scholar]
- (10).Schubert CM, Rogers NL, Remsberg KE, Sun SS, Chumlea WC, Demerath EW, Czerwinski SA, Towne B, Siervogel RM. Lipids, lipoproteins, lifestyle, adiposity and fat-free mass during middle age: the Fels Longitudinal Study. Int J Obes Relat Metab Disord. 2005;30:251–60. doi: 10.1038/sj.ijo.0803129. [DOI] [PubMed] [Google Scholar]
- (11).Berns MA, de Vries JH, Katan MB. Increase in body fatness as a major determinant of changes in serum total cholesterol and high density lipoprotein cholesterol in young men over a 10-year period. Am J Epidemiol. 1989;130:1109–22. doi: 10.1093/oxfordjournals.aje.a115438. [DOI] [PubMed] [Google Scholar]
- (12).Hubert HB, Eaker ED, Garrison RJ, Castelli WP. Life-style correlates of risk factor change in young adults: an eight-year study of coronary heart disease risk factors in the Framingham offspring. Am J Epidemiol. 1987;125:812–31. doi: 10.1093/oxfordjournals.aje.a114598. [DOI] [PubMed] [Google Scholar]
- (13).Anderson KM, Wilson PW, Garrison RJ, Castelli WP. Longitudinal and secular trends in lipoprotein cholesterol measurements in a general population sample. The Framingham Offspring Study. Atherosclerosis. 1987;68:59–66. doi: 10.1016/0021-9150(87)90094-3. [DOI] [PubMed] [Google Scholar]
- (14).Ingelsson E, Massaro JM, Sutherland P, Jacques PF, Levy D, D'Agostino RB, Vasan RS, Robins SJ. Contemporary Trends in Dyslipidemia in the Framingham Heart Study. Arch Intern Med. 2009;169:279–86. doi: 10.1001/archinternmed.2008.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Zuo H, Shi Z, Hu X, Wu M, Guo Z, Hussain A. Prevalence of metabolic syndrome and factors associated with its components in Chinese adults. Metabolism. 2009;58:1102–8. doi: 10.1016/j.metabol.2009.04.008. [DOI] [PubMed] [Google Scholar]
- (16).Abbott RD, Yano K, Hakim AA, Burchfiel CM, Sharp DS, Rodriguez BL, Curb JD. Changes in total and high-density lipoprotein cholesterol over 10- and 20-year periods (the Honolulu Heart Program) Am J Cardiol. 1998;82:172–8. doi: 10.1016/s0002-9149(98)00310-5. [DOI] [PubMed] [Google Scholar]
- (17).Morton RE, Zilversmit DB. Inter-relationship of lipids transferred by the lipid-transfer protein isolated from human lipoprotein-deficient plasma. J Biol Chem. 1983;258:11751–7. [PubMed] [Google Scholar]
- (18).Taylor HA., Jr. The Jackson Heart Study: an overview. Ethn Dis. 2005;15(S6):1–3. [PubMed] [Google Scholar]
- (19).Dubbert PM, Carithers T, Ainsworth BE, Taylor HA, Jr., Wilson G, Wyatt SB. Physical activity assessment methods in the Jackson Heart Study. Ethn Dis. 2005;15(Suppl 6):56–61. [PubMed] [Google Scholar]
- (20).Kim ES, Kwon HS, Ahn CW, Lim DJ, Shin JA, Lee SH, Cho JH, Yoon KH, Kang MI, Cha BY, Son HY. Serum uric acid level is associated with metabolic syndrome and microalbuminuria in Korean patients with type 2 diabetes mellitus. J Diabetes Complications. 2010 doi: 10.1016/j.jdiacomp.2010.09.004. [DOI] [PubMed] [Google Scholar]
- (21).Manu P, Tsang J, Napolitano BA, Lesser ML, Correll CU. Predictors of insulin resistance in the obese with metabolic syndrome. Eur J Intern Med. 2010;21:409–13. doi: 10.1016/j.ejim.2010.05.015. [DOI] [PubMed] [Google Scholar]
- (22).Brunham LR, Kruit JK, Hayden MR, Verchere CB. Cholesterol in beta-cell dysfunction: the emerging connection between HDL cholesterol and type 2 diabetes. Curr Diab Rep. 2010;10:55–60. doi: 10.1007/s11892-009-0090-x. [DOI] [PubMed] [Google Scholar]
- (23).Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics. 1986;42:121–30. [PubMed] [Google Scholar]
- (24).Ferrara A, Barrett-Connor E, Shan J. Total, LDL, and HDL Cholesterol Decrease With Age in Older Men and Women : The Rancho Bernardo Study 1984–1994. Circulation. 1997;96:37–43. doi: 10.1161/01.cir.96.1.37. [DOI] [PubMed] [Google Scholar]
- (25).Truesdale KP, Stevens J, Cai J. Nine-year changes in cardiovascular disease risk factors with weight maintenance in the Atherosclerosis Risk In Communities cohort. Am J Epidemiol. 2007;165:890–900. doi: 10.1093/aje/kwk072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Cheung BM, Li M, Ong KL, Wat NM, Tam S, Pang RW, Thomas GN, Woo J, Janus ED, Lau CP, Lam TH, Lam KS. High density lipoprotein-cholesterol levels increase with age in American women but not in Hong Kong Chinese women. Clin Endocrinol (Oxf) 2009;70:561–8. doi: 10.1111/j.1365-2265.2008.03361.x. [DOI] [PubMed] [Google Scholar]
- (27).Berrougui H, Isabelle M, Cloutier M, Grenier G, Khalil A. Age-related impairment of HDL-mediated cholesterol efflux. J Lipid Res. 2007;48:328–36. doi: 10.1194/jlr.M600167-JLR200. [DOI] [PubMed] [Google Scholar]
- (28).Rosenblat M, Karry R, Aviram M. Paraoxonase 1 (PON1) is a more potent antioxidant and stimulant of macrophage cholesterol efflux, when present in HDL than in lipoprotein-deficient serum: relevance to diabetes. Atherosclerosis. 2006;187:74–81. doi: 10.1016/j.atherosclerosis.2005.08.026. [DOI] [PubMed] [Google Scholar]
- (29).Jaouad L, de GC, Berrougui H, Cloutier M, Isabelle M, Fulop T, Payette H, Khalil A. Age-related decrease in high-density lipoproteins antioxidant activity is due to an alteration in the PON1's free sulfhydryl groups. Atherosclerosis. 2006;185:191–200. doi: 10.1016/j.atherosclerosis.2005.06.012. [DOI] [PubMed] [Google Scholar]
- (30).Girona J, LaVille AE, Solα R, Motta C, Masana L. HDL derived from the different phases of conjugated diene formation reduces membrane fluidity and contributes to a decrease in free cholesterol efflux from human THP-1 macrophages. Biochimica et Biophysica Acta (BBA) - Molecular and Cell Biology of Lipids. 2003;1633:143–8. doi: 10.1016/s1388-1981(03)00108-2. [DOI] [PubMed] [Google Scholar]