Abstract
Severe sepsis and septic shock are still deadly conditions urging to develop novel therapies. A better understanding of the complex modifications of the immune system of septic patients is needed for the development of innovative immunointerventions. Natural killer (NK) cells are characterized as CD3−NKp46+CD56+ cells that can be cytotoxic and/or produce high amounts of cytokines such as IFN-γ. NK cells are also engaged in crosstalks with other immune cells, such as dendritic cells, macrophages, and neutrophils. During the early stage of septic shock, NK cells may play a key role in the promotion of the systemic inflammation, as suggested in mice models. Alternatively, at a later stage, NK cells-acquired dysfunction could favor nosocomial infections and mortality. Standardized biological tools defining patients' NK cell status during the different stages of sepsis are mandatory to guide potential immuno-interventions. Herein, we review the potential role of NK cells during severe sepsis and septic shock.
1. Introduction
Sepsis is the clinical presentation of a “systemic inflammatory response syndrome” (SIRS) to a severe infection. Most clinical and basic-science research on the immune consequences of severe sepsis conducted during the last decades has focused on the roles of macrophages, neutrophils and conventional T lymphocytes [1]. During recent years, however, it has become increasingly clear that subsets of innate immune cells, such as natural killer (NK) cells, are involved in both protective immunity and immunopathology.
Herein, we review the potential role of NK cells during the different stages of severe sepsis and septic shock.
2. Severe Sepsis: Urgent Needs for “Immunological” Solutions
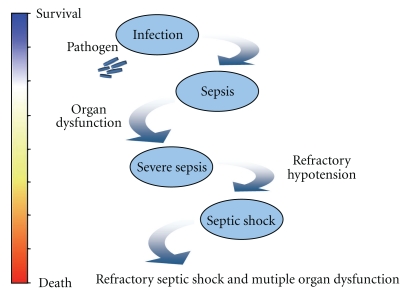
The most threatening infections are referred to as severe sepsis and septic shock [1]. These severe forms of infection, mainly of bacterial origin, represent a major healthcare problem, accounting for thousands of deaths every year worldwide, with more than 200,000 deaths per year just in the United States [2]. Sepsis, severe sepsis, and septic shock are viewed as a continuum that results in increasing mortality (cf. Figure 1) and shares consensual clinical criteria [3]. Mortality is up to 50% in septic shock, and the incidence of sepsis is projected to increase significantly during next years with higher rates of mortality due to more advanced age and/or associated comorbidities (cancer, diabetes, etc.). During the last decades, physicians have made significant progress in the early implementation of symptomatic care through adequate fluid resuscitation, antibiotherapy, and specific organ-support techniques, such as mechanical ventilation and renal-replacement therapy. Unfortunately, these therapeutic strategies have failed to sufficiently reduce mortality in severely septic patients [4, 5]. Moreover, physicians are increasingly concerned about increasing microbial resistance to antibiotics and the slow development of new antimicrobial agents [6]. Thus, there is an urgent need to develop efficacious therapies to treat this deadly disease.
Figure 1.
Continuum from infection to septic shock: the initial response to pathogen is a systemic response, with release of inflammatory mediators and activation of the coagulation cascade, resulting in imbalance between oxygen delivery and oxygen consumption. Ultimately, tissue hypoxia develops and may lead to multiple organ dysfunction and irreversible shock.
Future therapies may emerge from a better understanding of the physiopathology of sepsis [7]. Sepsis, also referred to as SIRS of septic origin, was originally viewed as an exacerbated inflammatory response and a “cytokine storm.” However, most trials that used inhibitors of proinflammatory cytokines or inhibitors of proinflammatory mediators failed to improve patients' outcomes, providing the best proof of the incomplete understanding of its pathogenesis [8, 9]. One of the reasons for the lack of efficacy of anti-inflammatory strategies in patients with sepsis may be because the syndrome changes over time [10].
In its early stages, sepsis is characterized by an increase in inflammatory mediators, but as sepsis persists, there is a shift towards an anti-inflammatory immunosuppressive state. Indeed, a common feature of these patients is the alteration of their immune status, referred to as “compensatory anti-inflammatory response syndrome” (CARS), which is thought to render patients more susceptible to nosocomial infections. It seems that immune dysfunctions that are supposed to play a role in mortality vary between patients who succumb within the first hour after sepsis and those who survive the first critical hours (>80%) but then die later from sepsis-induced multiorgan dysfunction and/or secondary nosocomial infections. It has only been recently that efforts to understand the effect of the inflammatory process on the immune status during septic shock have fully integrated the considerable derangements of both the innate and adaptive immune systems and have better identified the contribution of multiple cellular actors [1, 7].
3. NK Cells: Early Soldiers with Multiple Functions
NK cells are lymphocytes that are classically referred to as part of the innate immunity. NK cells were first described for their ability to kill leukemic cells without prior specific sensitization [11]. They represent a small proportion (4–15%) of blood lymphocytes and do not express a specific receptor for antigens dependent upon RAG-mediated rearrangements [12]. NK cell function is regulated by a multiplicity of activating and inhibitory receptors. Their natural cytotoxicity is largely under the control of natural cytotoxicity receptors, and their antibody-dependent cytotoxicity is linked to the engagement of CD16/FCγ RIIIa [13]. Human NK cells are characterized as CD3− NKp46+CD56+ cells [14]. In humans, blood NK cells can be divided into two major subtypes: CD56bright and CD56dim, corresponding to sequential steps of differentiation [15]. The former subtype represents about 10% of circulating NK cells. These cells express low levels of CD16 and perforin, produce high amounts of cytokines (e.g., interferon gamma or IFN-γ, TNF-α, and granulocyte-macrophage colony-stimulating factor) in response to cytokines such as interleukin (IL)-12 and IL-18, and represent the major fraction of NK cells in lymph nodes. CD56dim NK cells express high levels of CD16, perforin, and killer Ig-like receptors (KIRs). KIRs include inhibitory receptors that recognize MHC class I molecules and dampen NK cell activation. CD56dim NK cells are cytotoxic by granule polarization and exocytosis of various proteins including perforin and granzymes, which mediate target-cell killing and are also cytokine producers.
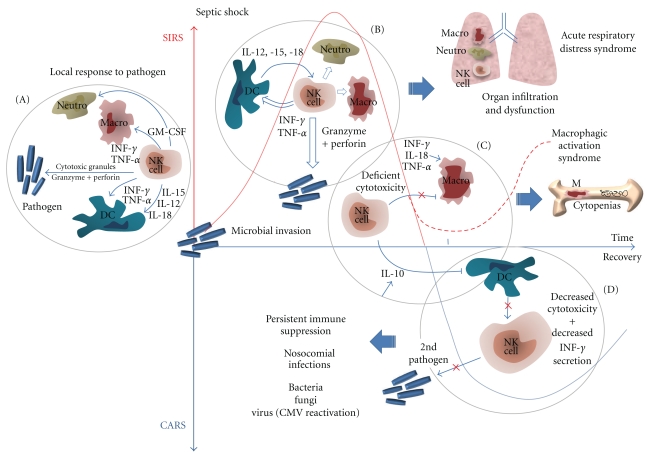
Several lines of evidence suggest that NK cells might be involved in key functions during sepsis. NK cells have a major role in defense against viral infections, in particular herpesvirus [16], influenza viruses [17], or hantavirus [18], by direct cytotoxicity against virus-infected cells and by the early production of cytokines that can control viral replication, such as IFN-γ. NK cells also participate in responses to other types of infections, including those caused by intracellular bacteria, pyogenic bacteria, fungi, and protozoa [19, 20]. As the early and main producers of IFN-γ during sepsis, these cells are equipped with many innate sensors for damage-associated molecular-pattern molecules (DAMPS) and pathogen-associated molecular-pattern molecules (PAMPS) [21]. In addition, if NK cells are found within the blood stream, they are also abundant in some tissues, such as the lungs [22, 23], an organ particularly prone to dysfunction in Intensive Care Unit (ICU) patients. NK cells are also engaged in crosstalks with other immune cells, such as dendritic cells (DCs) [24], monocytes, macrophages [25, 26], and neutrophils [27], which besides being fundamental for NK cell activation in response to most pathogens (by direct contact or cytokine secretion) also participate in the development of the subsequent immune response (Figures 2(A) and 2(B)).
Figure 2.
(A) NK cells initiate a local inflammatory response to pathogens. (B) During SIRS, NK cells amplify the inflammatory response to the spread of the pathogen, which can lead to organ dysfunction. (C) Deficient NK cell cytotoxicity may favor macrophage activation syndrome. (D) During CARS, NK cell global dysfunction may favor nosocomial infections. Note: SIRS and CARS have been separated in time to ease understanding of the figure, but the different stages (B–D) can occur simultaneously. Also, most data shown here are from mouse models and should be further confirmed in septic patients.
4. NK Cells and Severe Sepsis: Lessons and Limits from Murine Models
Most of the current knowledge about the role of NK cells during severe sepsis comes from mouse models. Although NK cell-deficient mice are not reported to present with detectable abnormalities at steady state, all data converge on a detrimental role for NK cells during sepsis. In mice, a challenge with high doses of lipopolysaccharide (LPS) results in a syndrome resembling septic shock in humans, and depletion of NK cells offers protection against LPS-induced shock [28, 29]. Depletion of NK cells by systemic administration of polyclonal antiasialo GM1 or monoclonal anti-NK1.1 antibodies, before the induction of the generalized Schwartzman reaction, leads to a dramatic reduction in mortality and significantly lowers cytokine levels (IFN-γ and TNF-α) following a systemic injection of LPS [28]. The same protective effect against cytokine-induced shock (by administrating IL-12 in combination with IL-2 or IL-15) was observed in mice that underwent depletion of NK cells with antiasialo GM1 antibodies [30].
In addition, there is now increasing evidence of detrimental roles for NK cells in different models of bacterial infections. Depletion of NK cells in SCID mice infected intranasally with Streptococcus pneumoniae resulted in significantly lower bacteremia and inflammatory cytokine production within the lung airways and lung tissue [31]. Improved survival was also observed with NK-cell-depleted mice in a model of septic shock with Streptococcus pneumoniae [32]. In a model of cecal ligation and puncture (CLP), mice treated with anti-asialo-GM1 were protected against CLP-induced mortality compared to IgG-treated controls [32]. During CLP-induced shock, NK cells migrated from blood and spleen to the inflamed peritoneal cavity where they amplified the proinflammatory activities of the myeloid cell populations [33]. NK cells were also involved in the high levels of inflammatory cytokines, lung pathology, and mortality that occur during Escherichia coli peritonitis, as all these parameters were reduced by NK depletion [34].
Altogether, these results suggest that NK cells can promote the inflammatory process occurring during sepsis in vivo, possibly via interactions with macrophages [35, 36], organ infiltration and damage, and the secretion of proinflammatory cytokines, providing a rationale basis to explain how NK-cell depletion increases survival in experimental sepsis. In opposition to this role of amplification of inflammation, recent data show that very early during the course of systemic infections induced by Toxoplasma gondii, Listeria monocytogenes, and Yersinia pestis, IL-12 secreted by DC induces NK cells to produce the broadly immunosuppressive cytokine IL-10, which, in turn, inhibits IL-12 secretion by DC, unveiling an immunosuppressive “regulator” function of NK cells [37]. If documented in humans, NK cells might then contribute to the necessary transition from SIRS to CARS (cf. Figure 2).
Mice are the most commonly used animal models in biomedical research, and rodent studies are an important part of the preclinical studies that determine progression to clinical studies in humans during drug development. However, there are numerous concerns about extrapolation from what is known about mouse to human NK-cell biology during severe sepsis, thus limiting the clinical relevance of the mouse models described above. Because there was no genetic model where NK cells could be selectively deleted, in vivo NK-cell depletion has so far relied on anti-asialo-GM1- or anti-NK1.1-depleting antibodies. Although a depletion of NK cells can be obtained with both antibodies, the selectivity of the depletion depends upon the quantities of antibodies, blurring the interpretation of the results obtained using these methods. More recently, transgenic mice that lack NK cells, but have a normal T/NKT-cell compartment, have been reported [38], but the cause of selective NK-cell ablation in these mice is linked to the expression of the ubiquitous transcription factor, ATF2, which raises the possibility of other defects in the immune system [39]. Moreover, the basic leucine-zipper transcription factor E4BP4 (also called NFIL3) has been proven essential for the generation of the NK-cell lineage and E4BP4-deficient mice specifically lack NK cells [40]. However, E4BP4-deficient mice were also shown to undergo impaired B-cell intrinsic IgE class switching [41]. Finally, taking advantage of the identification of a functional NKp46 promoter, a mouse model of conditional NK cell ablation based on the diphtheria toxin (DT) receptor/DT-based system has been generated [42]. Because DT injection leads to a complete and selective ablation of NK cells in these mice, this model provides a precious tool to explore the role of NK cells in many pathological conditions, including sepsis.
However, even with the availability of selective NK-cell deficient models [42] or of humanized mouse models [43], a constant problem with the murine approach to study sepsis is that rodents are highly resilient to most models of induced inflammation as compared to humans and that results depend on the strain and models of sepsis used (from LPS injection to cecal ligation and puncture). Also, because the supportive care used in humans is not easily transposed into mice [44], these models of septic challenge are not fully relevant to address the particular situation of ICU patients who survive the most severe sepsis and then come to the “immunosuppressive” CARS stage, which is responsible for most deaths.
5. A Role for NK Cells in Human SIRS?
By analogy with the possible use of the NK cell-deficient mouse model, we could consider the study of patients with NK cell-selective deficiency to address the role of NK cells in severe human sepsis. A number of isolated deficiencies of NK cells in humans have been described, but most are complex immunodeficiencies associated with absent or functionally deficient NK cells [45]. Few reports have described patients with isolated NK-cell abnormalities where the main susceptibility is to severe infection with herpesvirus [46]. The paucity of nonambiguous cases of NK-selective deficiencies in humans has hampered the identification of nonredundant NK-cell function. Also, as some SCID-X1 patients with no NK cell reconstitution after allogenic bone-marrow transplantation or gene therapy do not experience severe infections, it has been suggested that NK cells might have redundant anti-infectious functions in humans [47]. However, NK cells have been reported to be key in controlling severe cytomegalovirus (CMV) infection in some patients [48]. In septic shock, as fatality can precede adaptive responses, and in the context of massive and sometimes persistent apoptosis-induced T- and B-cell lymphopenia [49], NK cells may play a crucial and nonredundant role.
Our knowledge of NK cells in severe human sepsis and septic shock could be derived from analyses of NK cells taken directly from patients during the different clinical stages of the disease. However, available data from patients in ICU are scarce and heterogeneous and do not always include evaluation of cell function. Because of all these limitations, these results appear as contradictory. Yet, in patients with severe Gram-negative sepsis, an increased percentage of blood NK cells has been reported, as an improved survival in the patients with high NK counts [50]. Of importance, these patients did not experience septic shock nor were admitted into the ICU. Unfortunately, NK cell effector functions were not monitored in this study. A previous report had observed that NK-cell counts were higher among patients with sepsis of Gram-positive origin than among patients with Gram-negative sepsis [51].
In contrast, previous studies on patients with SIRS [52] and septic shock [53] had reported reduced numbers of NK cells and impaired NK cell in vitro cytotoxicity against K562 tumor cells. However, when NK cell cytotoxicity in patients with severe sepsis or septic shock was assessed in vivo by measuring circulating granzyme A and B levels [54], higher cytotoxicity was found in 50% of septic patients, and these patients had a higher mortality and worse organ function. Altogether, as suggested by a recent prospective study conducted in more than 500 patients with early sepsis, the discrepancies concerning the number and/or function of circulating NK cells are probably due to the heterogeneity of patients in terms of either severity (severe sepsis and/or septic shock) or involvement of pathogens (Gram-negative versus-positive bacteria) [55].
Also, because septic shock is rapidly associated with a dramatic decrease in circulating lymphocytes, the timing of NK-cell analysis might be of particular importance. It is reported that, from their admission into an ICU, the numbers of all lymphocyte subpopulations (including NK cells) of 21 septic-shock patients were diminished, and these alterations remained stable during the first 48 h [56], while no data are available after this short time.
Another caveat in these human studies is that NK cell testing has been obviously limited to peripheral blood. As NK cells can migrate out of the blood into the inflamed tissues, the interpretation of the analysis may be difficult. Indeed, the status of NK cells within tissues might be quite different [57, 58]. Only one study has addressed NK cells at a tissular level in human septic shock. In this paper, Hotchkiss et al. described a profound and progressive, apoptosis-induced loss of B and CD4+ T cells in the spleen and gut-associated lymphoid tissue of adults who had died of sepsis [49]. In contrast, a trend towards an increase of splenic NK cells was observed in septic patients. This result failed to reach statistical significance, most likely as the consequence of the small number of patients.
Thus, with cautious interpretation, due to the mentioned heterogeneity in studied patients and the complete absence of data concerning NK cell cytokine secretion, human studies do not exclude a detrimental role for NK cells in the early stage of septic shock (Figure 1) that was observed in mouse models [49, 54].
6. A Role for NK Cells in Human CARS?
As the vast majority of patients with sepsis survive the initial insult, we should consider not only the initial excessive systemic inflammatory reaction, but also the following sepsis-induced immunosuppressive period and its consequences.
During severe sepsis, some patients can develop secondary hemophagocytic lymphohistiocytic (HLH) syndrome, also termed macrophage activation syndrome (MAS). For intensivists, the features of MAS mainly include nonremitting fever, severe cytopenias, and organ dysfunctions. MAS is characterized by uncontrolled macrophage and Th1-lymphocyte stimulation, with elevated levels of circulating INF-γ, TNF-α, IL-6, and IL-18 [59]. Many clues to the role of NK cells in MAS have been recently discovered. First, a marked decrease in NK cell numbers, as well as a severe decrease in both natural cytotoxicity and ADCC, has been reported in patients with secondary MAS [60]. Instead of being just a consequence of MAS, this defect of NK cell number and function could be part of the pathogenesis. Indeed, the genetic forms of HLH are characterized by an intrinsic defect of NK cell and T-cell cytotoxicity related to the perforin/granzyme release pathway [61], and virally infected perforin KO mice represent a relevant model of MAS [62]. Interactions between human NK cells and macrophages are bidirectional and can result in activation of NK cells or in the regulation of macrophage activity through the killing of activated macrophages by NK cells [63]. Thus, even if NK cells are early and massive sources of INF-γ, and contribute to the initial excessive inflammatory response in severe sepsis, a concomitant defect of their cytotoxic functions could predispose a subset of these septic patients to develop MAS because NK cell dysfunction may contribute to uncontrolled Th1-lymphocyte and macrophage activation (Figure 2(C)).
Reduced NK cell numbers and functions, if persistent, may also contribute to impaired host defenses during CARS (Figure 2(D)). This “compensatory” inhibitory response, which is primarily seen as a regulation for hyperinflammation, can then become deleterious as many immune functions are compromised. These alterations may be directly responsible for the worsening outcome, as they may play a major role in the decreased resistance to nosocomial infections in patients who have survived an initial resuscitation. In humans, this view is actually merely speculative as up to now only a single study has been reported, which includes an evaluation of NK cells in ICU patients with septic shock that was not restricted to the very early stage of shock [64]. In this study, NK cell cytotoxicity was evaluated at different time points from admission, and it was suppressed to <10% in nearly all patients during the complete observation period (up to 14 days). Interestingly, ICU patients presenting with septic shock seem more prone than others to develop reactivation of CMV [65]. These CMV reactivations occur mainly at the late stage of sepsis although the affected patients seem to have sufficient CMV-specific CD4+ or CD8+ T cells [64, 66]. These data suggest that there might be a decrease in NK cell function that favors the progression of the viral infection. Finally, ICU patients with CMV reactivation (up to 30% of all ICU patients) may develop more bacterial/fungal nosocomial infections because of the immunosuppressive properties of CMV [65], but one cannot exclude the possibility that NK cells also participate in protective immunity against nosocomial pathogens [67].
7. NK Cells and Future Therapies for Severe Human Sepsis: Perspectives
Most of the published data on human sepsis do not examine both NK cell numbers and functions and, thus, have incompletely assessed NK cell status. Nowadays, there are rapid and relatively inexpensive methods to assess NK cell functions directly at the patient bedside, using multiparametric functional flow cytometry [68]. Both quantitative and qualitative evaluation of NK status now needs to be broadly performed among ICU patients.
In addition, a single parameter will likely not be sufficient to characterize the complexity of septic patients' immunological status, which rapidly changes over time. Therefore, the introduction of high-throughput technologies represents an emerging solution for the global immunomonitoring of sepsis. DNA microarrays and RNAseq allow genome-wide assessment of changes in mRNA abundance. A first transcriptomic approach could be restricted to NK-specific genes. Strikingly, less than a hundred genes might be sufficient to define the human NK cell-specific signature [69]. One should also look for NK cell-specific combinations of more broadly expressed genes during the different phases of severe sepsis. This global functional approach has been recently performed as a modular approach in different human pathological conditions [70, 71].
As for the potential development of any NK-based immunointervention, new approaches should also allow to better define the complex and dynamic actions of NK cells during the different phases of severe sepsis in humans. At present, targeted NK cell therapies address hematopoietic malignancies using different strategies to enhance NK cell functions and promote their antitumor action [72]. These innovative protocols could be used in the “CARS” period when ICU patients suffer from immunosuppression and nosocomial infection. NK cells stimulation could be achieved by manipulation of NK receptors (i.e., using anti-KIR-specific antibodies that block inhibitory receptors) or by the administration of cytokines as IL-15. The last option has proved successful in murine model of sepsis and pneumonia, where administration of IL-15 could prevent apoptosis, increase the percentage of NK cells that produce IFN-γ, and reverse immune dysfunction [73]. The restoration of INF-γ secretion by NK cells that might be able to migrate and deliver cytokine into the infected tissues at the right time might be more efficient than the direct parenteral administration of INF-γ [74]. Alternatively, in the very early phase of severe sepsis, monoclonal antibodies, targeting, for example, NKp46, could be also designed to downregulate or deplete NK cells and prevent the consequences of uncontrolled inflammation due to their gamma interferon secretion. NK cell depletion should be transient to avoid a supplementary “immunodepression” due to persistent NK depletion during CARS. Also, it might be difficult to use it early enough in patients initiating a septic shock out of the hospital; but instead, inpatients, for example, presenting a postsurgical sepsis, could be carefully screened and receive immunointervention at the earliest phase of sepsis or even at a presymptomatic phase of sepsis, if it can be robustly diagnosed [75].
Translation to its clinical application must carefully take into account the timing of administration of immunotherapeutical agent. Thus, one of the first goals should be first to achieve robust and standardized biological tools that accurately define the patient's immune status, so that physicians can decide who can benefit, and when, from those immunointerventions.
Acknowledgments
The authors thank C. Beziers-Lafosse (CIML) for excellent graphic assistance. E. Vivier is supported by grants from the European Research Council (ERC), Agence Nationale de la Recherche (ANR), and Ligue Nationale contre le Cancer (Equipe labellisée “La Ligue”), and institutional grants from INSERM, CNRS, and Université de la Méditerranée to the CIML. E. Vivier is a scholar from the Institut Universitaire de France. E. Vivier is a cofounder and shareholder of Innate Pharma.
Abbreviations
- CARS:
Compensatory anti-inflammatory response syndrome
- CMV:
Cytomegalovirus
- DCs:
Dendritic cells
- Macro:
Macrophages
- NK:
Natural killer
- Neutro:
Neutrophils
- SIRS:
Systemic inflammatory response syndrome.
References
- 1.Hotchkiss RS, Karl IE. The pathophysiology and treatment of sepsis. New England Journal of Medicine. 2003;348(2):138–150. doi: 10.1056/NEJMra021333. [DOI] [PubMed] [Google Scholar]
- 2.Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Critical Care Medicine. 2001;29(7):1303–1310. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 3.Bone RC, Balk RA, Cerra FB, et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest. 1992;101:1644–1655. doi: 10.1378/chest.101.6.1644. [DOI] [PubMed] [Google Scholar]
- 4.Rivers E, Nguyen B, Havstad S, et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. New England Journal of Medicine. 2001;345(19):1368–1377. doi: 10.1056/NEJMoa010307. [DOI] [PubMed] [Google Scholar]
- 5.Dellinger RP, Levy MM, Carlet JM, et al. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2008. Critical Care Medicine. 2008;36(1):296–327. doi: 10.1097/01.CCM.0000298158.12101.41. [DOI] [PubMed] [Google Scholar]
- 6.Opal SM, Calandra T. Antibiotic usage and resistance: gaining or losing ground on infections in critically ill patients? Journal of the American Medical Association. 2009;302(21):2367–2368. doi: 10.1001/jama.2009.1774. [DOI] [PubMed] [Google Scholar]
- 7.Annane PD, Bellissant PE, Cavaillon JM. Septic shock. Lancet. 2005;365(9453):63–78. doi: 10.1016/S0140-6736(04)17667-8. [DOI] [PubMed] [Google Scholar]
- 8.Fisher Jr. CJ, Agosti JM, Opal SM, et al. Treatment of septic shock with the tumor necrosis factor receptor: Fc fusion protein. New England Journal of Medicine. 1996;334(26):1697–1702. doi: 10.1056/NEJM199606273342603. [DOI] [PubMed] [Google Scholar]
- 9.Opal SM, Fisher Jr. CJ, Dhainaut J-FA, et al. Confirmatory interleukin-1 receptor antagonist trial in severe sepsis: a phase III, randomized, double-blind, placebo-controlled, multicenter trial. Critical Care Medicine. 1997;25(7):1115–1124. doi: 10.1097/00003246-199707000-00010. [DOI] [PubMed] [Google Scholar]
- 10.Bone RC. Immunologic dissonance: a continuing evolution in our understanding of the systemic inflammatory response syndrome (SIRS) and the Multiple Organ Dysfunction Syndrome (MODS) Annals of Internal Medicine. 1996;125(8):680–687. doi: 10.7326/0003-4819-125-8-199610150-00009. [DOI] [PubMed] [Google Scholar]
- 11.Herberman RB, Nunn ME, Lavrin DH. Natural cytotoxic reactivity of mouse lymphoid cells against syngeneic and allogeneic tumors. I. Distribution of reactivity and specificity. International Journal of Cancer. 1975;16(2):216–229. doi: 10.1002/ijc.2910160204. [DOI] [PubMed] [Google Scholar]
- 12.Vivier E, Tomasello E, Baratin M, Walzer T, Ugolini S. Functions of natural killer cells. Nature Immunology. 2008;9(5):503–510. doi: 10.1038/ni1582. [DOI] [PubMed] [Google Scholar]
- 13.Moretta L, Moretta A. Unravelling natural killer cell function: triggering and inhibitory human NK receptors. EMBO Journal. 2004;23(2):255–259. doi: 10.1038/sj.emboj.7600019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vivier E, Raulet DH, Moretta A, et al. Innate or adaptive immunity? The example of natural killer cells. Science. 2011;331(6013):44–49. doi: 10.1126/science.1198687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Romagnani C, Juelke K, Falco M, et al. CD56CD16 killer Ig-like receptor NK cells display longer telomeres and acquire features of CD56 NK cells upon activation. Journal of Immunology. 2007;178(8):4947–4955. doi: 10.4049/jimmunol.178.8.4947. [DOI] [PubMed] [Google Scholar]
- 16.Arase H, Mocarski ES, Campbell AE, Hill AB, Lanier LL. Direct recognition of cytomegalovirus by activating and inhibitory NK cell receptors. Science. 2002;296(5571):1323–1326. doi: 10.1126/science.1070884. [DOI] [PubMed] [Google Scholar]
- 17.Mandelboim O, Lieberman N, Lev M, et al. Recognition of haemagglutinins on virus-infected cells by NKp46 activates lysis by human NK cells. Nature. 2001;409(6823):1055–1060. doi: 10.1038/35059110. [DOI] [PubMed] [Google Scholar]
- 18.Björkström NK, Lindgren T, Stoltz M, et al. Rapid expansion and long-term persistence of elevated NK cell numbers in humans infected with hantavirus. Journal of Experimental Medicine. 2011;208(1):13–21. doi: 10.1084/jem.20100762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tay CH, Szomolanyi-Tsuda E, Welsh RM. Control of infections by NK cells. Current Topics in Microbiology and Immunology. 1998;230:193–220. doi: 10.1007/978-3-642-46859-9_12. [DOI] [PubMed] [Google Scholar]
- 20.Stevenson MM, Riley EM. Innate immunity to malaria. Nature Reviews Immunology. 2004;4(3):169–180. doi: 10.1038/nri1311. [DOI] [PubMed] [Google Scholar]
- 21.Chalifour A, Jeannin P, Gauchat JF, et al. Direct bacterial protein PAMP recognition by human NK cells involves TLRs and triggers α-defensin production. Blood. 2004;104(6):1778–1783. doi: 10.1182/blood-2003-08-2820. [DOI] [PubMed] [Google Scholar]
- 22.Weissler JC, Nicod LP, Lipscomb MF, Toews GB. Natural killer cell function in human lung is compartmentalized. American Review of Respiratory Disease. 1987;135(4 I):941–949. doi: 10.1164/arrd.1987.135.4.941. [DOI] [PubMed] [Google Scholar]
- 23.Grégoire C, Chasson L, Luci C, et al. The trafficking of natural killer cells. Immunological Reviews. 2007;220(1):169–182. doi: 10.1111/j.1600-065X.2007.00563.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Walzer T, Dalod M, Robbins SH, Zitvogel L, Vivier E. Natural-killer cells and dendritic cells: “L’union fait la force”. Blood. 2005;106(7):2252–2258. doi: 10.1182/blood-2005-03-1154. [DOI] [PubMed] [Google Scholar]
- 25.Lapaque N, Walzer T, Méresse S, Vivier E, Trowsdale J. Interactions between human NK cells and macrophages in response to Salmonella infection. Journal of Immunology. 2009;182(7):4339–4348. doi: 10.4049/jimmunol.0803329. [DOI] [PubMed] [Google Scholar]
- 26.Bellora F, Castriconi R, Dondero A, et al. The interaction of human natural killer cells with either unpolarized or polarized macrophages results in different functional outcomes. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(50):21659–21664. doi: 10.1073/pnas.1007654108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Costantini C, Cassatella MA. The defensive alliance between neutrophils and NK cells as a novel arm of innate immunity. Journal of Leukocyte Biology. 2011;89(2):221–233. doi: 10.1189/jlb.0510250. [DOI] [PubMed] [Google Scholar]
- 28.Heremans H, Dillen C, Van Damme J, Billiau A. Essential role for natural killer cells in the lethal lipopolysaccharide-induced Shwartzman-like reaction in mice. European Journal of Immunology. 1994;24(5):1155–1160. doi: 10.1002/eji.1830240522. [DOI] [PubMed] [Google Scholar]
- 29.Emoto M, Miyamoto M, Yoshizawa I, et al. Critical role of NK cells rather than Vα14+NKT cells in lipopolysaccharide-induced lethal shock in mice. Journal of Immunology. 2002;169(3):1426–1432. doi: 10.4049/jimmunol.169.3.1426. [DOI] [PubMed] [Google Scholar]
- 30.Carson WE, Yu H, Dierksheide J, et al. A fatal cytokine-induced systemic inflammatory response reveals a critical role for NK cells. Journal of Immunology. 1999;162(8):4943–4951. [PubMed] [Google Scholar]
- 31.Kerr AR, Kirkham LAS, Kadioglu A, et al. Identification of a detrimental role for NK cells in pneumococcal pneumonia and sepsis in immunocompromised hosts. Microbes and Infection. 2005;7(5-6):845–852. doi: 10.1016/j.micinf.2005.02.011. [DOI] [PubMed] [Google Scholar]
- 32.Sherwood ER, Enoh VT, Murphey ED, Lin CY. Mice depleted of CD8+ T and NK cells are resistant to injury caused by cecal ligation and puncture. Laboratory Investigation. 2004;84(12):1655–1665. doi: 10.1038/labinvest.3700184. [DOI] [PubMed] [Google Scholar]
- 33.Etogo AO, Nunez J, Lin CY, Toliver-Kinsky TE, Sherwood ER. NK but not CD1-restricted NKT cells facilitate systemic inflammation during polymicrobial intra-abdominal sepsis. Journal of Immunology. 2008;180(9):6334–6345. doi: 10.4049/jimmunol.180.9.6334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Badgwell B, Parihar R, Magro C, Dierksheide J, Russo T, Carson WE., III Natural killer cells contribute to the lethality of a murine model of Escherichi coli infection. Surgery. 2002;132(2):205–212. doi: 10.1067/msy.2002.125311. [DOI] [PubMed] [Google Scholar]
- 35.Godshall CJ, Scott MJ, Burch PT, Peyton JC, Cheadle WG. Natural killer cells participate in bacterial clearance during septic peritonitis through interactions with macrophages. Shock (Augusta, Ga.) 2003;19(2):144–149. doi: 10.1097/00024382-200302000-00010. [DOI] [PubMed] [Google Scholar]
- 36.Scott MJ, Hoth JJ, Gardner SA, Peyton JC, Cheadle WG. Natural killer cell activation primes macrophages to clear bacterial infection. American Surgeon. 2003;69(8):679–686. [PubMed] [Google Scholar]
- 37.Perona-Wright G, Mohrs K, Szaba FM, et al. Systemic but not local infections elicit immunosuppressive IL-10 production by natural killer cells. Cell Host and Microbe. 2010;6(6):503–512. doi: 10.1016/j.chom.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim S, Iizuka K, Aguila HL, Weissman IL, Yokoyama WM. In vivo natural killer cell activities revealed by natural killer cell-deficient mice. Proceedings of the National Academy of Sciences of the United States of America. 2000;97(6):2731–2736. doi: 10.1073/pnas.050588297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim S, Song YJ, Higuchi DA, et al. Arrested natural killer cell development associated with transgene insertion into the Atf2 locus. Blood. 2006;107(3):1024–1030. doi: 10.1182/blood-2005-04-1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gascoyne DM, Long E, Veiga-Fernandes H, et al. The basic leucine zipper transcription factor E4BP4 is essential for natural killer cell development. Nature Immunology. 2009;10(10):1118–1124. doi: 10.1038/ni.1787. [DOI] [PubMed] [Google Scholar]
- 41.Kashiwada M, Levy DM, McKeag L, et al. IL-4-induced transcription factor NFIL3/E4BP4 controls IgE class switching. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(2):821–826. doi: 10.1073/pnas.0909235107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Walzer T, Bléry M, Chaix J, et al. Identification, activation, and selective in vivo ablation of mouse NK cells via NKp46. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(9):3384–3389. doi: 10.1073/pnas.0609692104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Unsinger J, McDonough JS, Shultz LD, Ferguson TA, Hotchkiss RS. Sepsis-induced human lymphocyte apoptosis and cytokine production in “humanized” mice. Journal of Leukocyte Biology. 2009;86(2):219–227. doi: 10.1189/jlb.1008615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zanotti-Cavazzoni SL, Guglielmi M, Parrillo JE, Walker T, Dellinger RP, Hollenberg SM. Fluid resuscitation influences cardiovascular performance and mortality in a murine model of sepsis. Intensive Care Medicine. 2009;35(4):748–754. doi: 10.1007/s00134-008-1360-9. [DOI] [PubMed] [Google Scholar]
- 45.Orange JS. Human natural killer cell deficiencies and susceptibility to infection. Microbes and Infection. 2002;4(15):1545–1558. doi: 10.1016/s1286-4579(02)00038-2. [DOI] [PubMed] [Google Scholar]
- 46.Biron CA, Byron KS, Sullivan JL. Severe herpesvirus infections in an adolescent without natural killer cells. New England Journal of Medicine. 1989;320(26):1731–1735. doi: 10.1056/NEJM198906293202605. [DOI] [PubMed] [Google Scholar]
- 47.Fischer A. Human primary immunodeficiency diseases. Immunity. 2007;27(6):835–845. doi: 10.1016/j.immuni.2007.11.012. [DOI] [PubMed] [Google Scholar]
- 48.Kuijpers TW, Baars PA, Dantin C, Van Den Burg M, Van Lier RAW, Roosnek E. Human NK cells can control CMV infection in the absence of T cells. Blood. 2008;112(3):914–915. doi: 10.1182/blood-2008-05-157354. [DOI] [PubMed] [Google Scholar]
- 49.Hotchkiss RS, Tinsley KW, Swanson PE, et al. Sepsis-induced apoptosis causes progressive profound depletion of B and CD4+ T lymphocytes in humans. Journal of Immunology. 2001;166(11):6952–6963. doi: 10.4049/jimmunol.166.11.6952. [DOI] [PubMed] [Google Scholar]
- 50.Giamarellos-Bourboulis EJ, Tsaganos T, Spyridaki E, et al. Early changes of CD4-positive lymphocytes and NK cells in patients with severe Gram-negative sepsis. Critical Care. 2006;10(6, article R166) doi: 10.1186/cc5111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Holub M, Klučková Z, Helcl M, Přihodov J, Rokyta R, Beran O. Lymphocyte subset numbers depend on the bacterial origin of sepsis. Clinical Microbiology and Infection. 2003;9(3):202–211. doi: 10.1046/j.1469-0691.2003.00518.x. [DOI] [PubMed] [Google Scholar]
- 52.Klimpel GR, Herndon DN, Fons M. Defective NK cell activity following thermal injury. Clinical and Experimental Immunology. 1986;66(2):384–392. [PMC free article] [PubMed] [Google Scholar]
- 53.Puente J, Carvajal T, Parra S, et al. In vitro studies of natural killer cell activity in septic shock patients. Response to a challenge with α-interferon and interleukin-2. International Journal of Clinical Pharmacology Therapy and Toxicology. 1993;31(6):271–275. [PubMed] [Google Scholar]
- 54.Zeerleder S, Hack CE, Caliezi C, et al. Activated cytotoxic T cells and NK cells in severe sepsis and septic shock and their role in multiple organ dysfunction. Clinical Immunology. 2005;116(2):158–165. doi: 10.1016/j.clim.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 55.Gogos C, Kotsaki A, Pelekanou A, et al. Early alterations of the innate and adaptive immune statuses in sepsis according to the type of underlying infection. Critical Care. 2010;14, article R96 doi: 10.1186/cc9031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Venet F, Davin F, Guignant C, et al. Early assessment of leukocyte alterations at diagnosis of septic shock. Shock. 2010;34:358–363. doi: 10.1097/SHK.0b013e3181dc0977. [DOI] [PubMed] [Google Scholar]
- 57.Tay CH, Welsh RM. Distinct organ-dependent mechanisms for the control of murine cytomegalovirus infection by natural killer cells. Journal of Virology. 1997;71(1):267–275. doi: 10.1128/jvi.71.1.267-275.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cavaillon JM, Annane D. Compartmentalization of the inflammatory response in sepsis and SIRS. Journal of Endotoxin Research. 2006;12(3):151–170. doi: 10.1179/096805106X102246. [DOI] [PubMed] [Google Scholar]
- 59.Larroche C, Mouthon L. Pathogenesis of hemophagocytic syndrome (HPS) Autoimmunity Reviews. 2004;3(2):69–75. doi: 10.1016/S1568-9972(03)00091-0. [DOI] [PubMed] [Google Scholar]
- 60.Mazodier K, Marin V, Novick D, et al. Severe imbalance of IL-18/IL-18BP in patients with secondary hemophagocytic syndrome. Blood. 2005;106(10):3483–3489. doi: 10.1182/blood-2005-05-1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Stepp SE, Dufourcq-Lagelouse R, Le Deist F, et al. Perforin gene defects in familial hemophagocytic lymphohistiocytosis. Science. 1999;286(5446):1957–1959. doi: 10.1126/science.286.5446.1957. [DOI] [PubMed] [Google Scholar]
- 62.Jordan MB, Hildeman D, Kappler J, Marrack P. An animal model of hemophagocytic lymphohistiocytosis (HLH): CD8+ T cells and interferon gamma are essential for the disorder. Blood. 2004;104(3):735–743. doi: 10.1182/blood-2003-10-3413. [DOI] [PubMed] [Google Scholar]
- 63.Nedvetzki S, Sowinski S, Eagle RA, et al. Reciprocal regulation of human natural killer cells and macrophages associated with distinct immune synapses. Blood. 2007;109(9):3776–3785. doi: 10.1182/blood-2006-10-052977. [DOI] [PubMed] [Google Scholar]
- 64.Von Müller L, Klemm A, Durmus N, et al. Cellular immunity and active human cytomegalovirus infection in patients with septic shock. Journal of Infectious Diseases. 2007;196(9):1288–1295. doi: 10.1086/522429. [DOI] [PubMed] [Google Scholar]
- 65.Chiche L, Forel JM, Roch A, et al. Active cytomegalovirus infection is common in mechanically ventilated medical intensive care unit patients. Critical Care Medicine. 2009;37(6):1850–1857. doi: 10.1097/CCM.0b013e31819ffea6. [DOI] [PubMed] [Google Scholar]
- 66.Chilet M, Aguilar G, Benet I, et al. Virological and immunological features of active cytomegalovirus infection in nonimmunosuppressed patients in a surgical and trauma intensive care unit. Journal of Medical Virology. 2010;82(8):1384–1391. doi: 10.1002/jmv.21825. [DOI] [PubMed] [Google Scholar]
- 67.Wesselkamper SC, Eppert BL, Motz GT, Lau GW, Hassett DJ, Borchers MT. NKG2D is critical for NK cell activation in host defense against Pseudomonas aeruginosa respiratory infection. Journal of Immunology. 2008;181(8):5481–5489. doi: 10.4049/jimmunol.181.8.5481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bryceson YT, Fauriat C, Nunes JM, et al. Functional analysis of human NK cells by flow cytometry. Methods in Molecular Biology. 2010;612:335–352. doi: 10.1007/978-1-60761-362-6_23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Walzer T, Jaeger S, Chaix J, Vivier E. Natural killer cells: from CD3(-)NKp46(+) to post-genomics meta-analyses. Current Opinion in Immunology. 2007;19(3):365–372. doi: 10.1016/j.coi.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 70.Chaussabel D, Quinn C, Shen J, et al. A modular analysis framework for blood genomics studies: application to systemic lupus erythematosus. Immunity. 2008;29(1):150–164. doi: 10.1016/j.immuni.2008.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Berry MPR, Graham CM, McNab FW, et al. An interferon-inducible neutrophil-driven blood transcriptional signature in human tuberculosis. Nature. 2010;466(7309):973–977. doi: 10.1038/nature09247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Terme M, Ullrich E, Delahaye NF, Chaput N, Zitvogel L. Natural killer cell-directed therapies: moving from unexpected results to successful strategies. Nature Immunology. 2008;9(5):486–494. doi: 10.1038/ni1580. [DOI] [PubMed] [Google Scholar]
- 73.Inoue S, Unsinger J, Davis CG, et al. IL-15 prevents apoptosis, reverses innate and adaptive immune dysfunction, and improves survival in sepsis. Journal of Immunology. 2010;184(3):1401–1409. doi: 10.4049/jimmunol.0902307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Döcke WD, Randow F, Syrbe U, et al. Monocyte deactivation in septic patients: restoration by IFN-γ treatment. Nature Medicine. 1997;3(6):678–681. doi: 10.1038/nm0697-678. [DOI] [PubMed] [Google Scholar]
- 75.Lukaszewski RA, Yates AM, Jackson MC, et al. Presymptomatic prediction of sepsis in intensive care unit patients. Clinical and Vaccine Immunology. 2008;15(7):1089–1094. doi: 10.1128/CVI.00486-07. [DOI] [PMC free article] [PubMed] [Google Scholar]