Abstract
IL-15 uses the heterotrimeric receptor IL-2/IL-15Rβ and the γ chain shared with IL-2 and the cytokine-specific IL-15Rα. Although IL-15 shares actions with IL-2 that include activation of natural killer (NK) and CD8 T cells, IL-15 is not associated with capillary leak syndrome, activation-induced cell death, or with a major effect on the number of functional regulatory T cells. To prepare for human trials to determine whether IL-15 is superior to IL-2 in cancer therapy, recombinant human IL-15 (rhIL-15) was produced under current good manufacturing practices. A safety study in rhesus macaques was performed in 4 groups of 6 animals each that received vehicle diluent control or rhIL-15 at 10, 20, or 50 μg/kg/d IV for 12 days. The major toxicity was grade 3/4 transient neutropenia. Bone marrow examinations demonstrated increased marrow cellularity, including cells of the neutrophil series. Furthermore, neutrophils were observed in sinusoids of enlarged livers and spleens, suggesting that IL-15 mediated neutrophil redistribution from the circulation to tissues. The observation that IL-15 administration was associated with increased numbers of circulating NK and CD8 central and effector-memory T cells, in conjunction with efficacy studies in murine tumor models, supports the use of multiple daily infusions of rhIL-15 in patients with metastatic malignancies.
Introduction
Several cytokines, including IFNα and GM-CSF, have been approved for use in cancer immunotherapy.1,2 IL-2 was approved by the US Food and Drug Administration (FDA) to treat metastatic renal cell cancer and malignant melanoma.3 However, IL-2 is not optimal because its administration is associated with capillary leak syndrome, activation-induced cell death (AICD), and a major expansion of CD4+CD25+ regulatory T cell (Treg) numbers.4,5 IL-15 is a 14- to 15-kDa member of the 4-α helix bundle family of cytokines that stimulates T cells and natural killer (NK) cells.6–10 IL-15 uses a heterotrimeric receptor that includes the cytokine-specific IL-15Rα, IL-2/15Rβ subunit shared with IL-2, and the common γ chain (γc) shared with IL-2, IL-4, IL-7, IL-9, and IL-21. Because of shared receptor components, IL-2 and IL-15 share several functions, including stimulation of the proliferation of multiple T-cell subsets, facilitation of synthesis of immunoglobulin by B cells, and persistence and activation of NK and NK T cells.6–10 However, in many adaptive immune responses, IL-2 and IL-15 have distinct and often contrasting roles.6–10 Through its contribution to AICD and its participation in the maintenance of CD4+CD25+ Tregs, IL-2 is involved in the elimination of self-reactive T cells with a role in autoimmune diseases but also in antitumor immune responses. In contrast, IL-15 does not play a major role in the maintenance of Tregs. Furthermore, in IL-15–transgenic mice, there is no expansion of Tregs, and IL-15 inhibits IL-2–mediated AICD.11 Trans presentation of IL-15 by antigen-presenting cells, including dendritic cells, to CD8 T cells contributes to the generation and maintenance of long-lasting, high-avidity, antigen-specific CD8+ memory T cells.12–24 These observations from ex vivo functional studies are supported by analysis of mice with disrupted cytokine or cytokine-receptor genes. IL-2 and IL-2Rα–deficient mice develop marked enlargements of peripheral lymphoid organs that are associated with polyclonal expansions of T- and B-cell populations and the development of autoimmune disorders.25 In contrast, mice deficient in IL-15 or IL-15Rα do not develop lymphoid enlargement, increased serum immunoglobulin concentrations, or autoimmunity.26,27 Instead, these mice have marked reductions in numbers of NK cells, intestinal intraepithelial lymphocytes, and CD8+CD44hi memory-phenotype T cells.26,27
There are considerable implications concerning the distinct functions of IL-2 and IL-15 in terms of their suitability as agents for use in cancer therapy. IL-2 has been approved for use in patients with metastatic renal cell cancer and metastatic malignant melanoma.3 However, as noted above, IL-2 is not optimal. IL-15, with its ability to maintain and activate many subsets of T and NK cells, its inhibition of AICD, its lack of major contributions to the generation of Tregs, and its role in NK- and T-cell functions, may be a better choice than IL-2 in cancer treatment.6–10 Attempts to treat tumor models in mice by administration of IL-15 have proven effective.28–35 Whereas wild-type C57Bl/6 mice died by 40 days after IV injection of MC38 colon carcinoma cells, IL-15 transgenic mice did not develop metastases and survived.28 Furthermore, therapy with IL-15 prolonged survival of mice that received syngeneic CT26, MC38 colon carcinoma cells, or B16 melanoma cells.28,33 Klebanoff et al demonstrated that IL-15 enhanced the in vivo activity of tumor-reactive CD8+ T cells in the T-cell receptor–transgenic mouse (Pmel-1), in which CD8 T cells recognize an epitope derived from the melanoma antigen GP100.31 In other studies, synergy between IL-21 and IL-15 in regulating CD8+ T-cell expansion and function yielded cures of established large B16 melanomas.32 In addition, IL-15 prolonged survival of mice with established TRAMP C2 prostatic cancer. When IL-15 was administered with an anti-CD40 antibody that increased IL-15Rα expression, most of the mice were cured.35
As part of the preclinical evaluation in the present study, recombinant human IL-15 (rhIL-15) was administered to 18 rhesus macaques divided into groups that received 10, 20, and 50 μg/kg/d of rhIL-15, with an additional 6 animals receiving vehicle control daily for 12 days. There were no animal deaths, evidence of autoimmune diseases, or infections. There was a dose-related increase in serum concentrations of IL-2Rα and IL-18 during rhIL-15 administration, providing surrogate markers of IL-15 activity. The only other laboratory abnormality observed was a transient grade 3/4 neutropenia. Bone marrow examination demonstrated a reduction in adipocytes and an increase in marrow cellularity, including cells of the neutrophil series. The only other pathological alteration was an increase in leukocytes, including neutrophils in the sinusoids of enlarged livers and spleens, suggesting that a redistribution of neutrophils from the blood pool to the tissues was the cause of the neutropenia.
Methods
Production of rhIL-15
IL-15 is a 14- to 15-kDa member of the 4-α helix bundle family of cytokines. The Biopharmaceutical Development Program of the National Cancer Institute, in collaboration with the Laboratory of Thomas A. Waldmann of the National Cancer Institute, produced rhIL-15 under current good manufacturing practices (supplemental Methods, available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Design of toxicology protocol
rhIL-15 was evaluated for safety (toxicity), pharmacokinetics, immunogenicity, autoimmunity, and impact on elements of the normal immune system in nonpremedicated animals receiving 1- to 2-minute infusions. Within this toxicology protocol, there were 4 groups of 3 animals each receiving infusions of vehicle diluent or 10, 20, or 50 μg/kg/d of rhIL-15 for 12 days followed within 24 hours by killing and necropsy. An additional 4 groups of 3 animals each received the same dose and dosing schedule but had their necropsy at day 48 after a 36-day recovery period. In the first 4 groups, IL-15 pharmacokinetics were determined with an assay of serum IL-15 concentrations before the infusions and at 10 minutes, 30 minutes, 1 hour, 4 hours, and 24 hours after initial rhIL-15 administration. For the remaining 4 groups, IL-15 pharmacokinetics were determined at the same time periods after the final (12th) dose of rhIL-15. A single additional animal received 200 μg/kg/d in a pilot study. Pharmacokinetics were determined after the initial dose in this animal. All studies were approved by the Animal Care and Use Committee of the National Cancer Institute.
Clinical signs
All monkeys were observed daily for adverse clinical signs and weekly for evaluation of body weight, body temperature, and food consumption for the duration of the study.
Clinical pathology
Blood was drawn from each monkey for clinical pathologic examination 1-7 days before the infusions and then on days 8 and 13 for animals killed on day 13 and on days 8, 13, 15, 22, 29, and 48 for animals killed on day 48. Hematologic, clinical pathology tests, and FACS analyses are outlined in the supplemental Methods.
Autoimmunity assessment
To determine whether the administration of IL-15 leads to the development of autoimmunity, animals were evaluated clinically and the same samples as indicated in the hematology schedule were analyzed for the development of antinuclear antibodies.
Necropsy procedure
Half of the monkeys were euthanized by sodium pentobarbital at day 13 within 24 hours after the last dose of IL-15, and the remaining animals on day 48 after the initial dose of IV IL-15. Select tissues were collected and fixed in 10% neutral-buffered formalin and eyes were preserved in Davidson fixative. Fixed tissues embedded in paraffin blocks were cut approximately 5-μm thick and stained with hematoxylin and eosin. All necropsy tissues were evaluated by John M. Pletcher, DVM (Charles River Laboratories Pathology Associates, Frederick, MD).
Pharmacokinetic procedures
The rhIL-15 concentrations in serum were assessed using a human IL-15–specific ELISA kit from R&D Systems according to the manufacturer's directions. Noncompartmental pharmacokinetic analysis of data was undertaken using the software package WinNonlin, Version 5.0 (Pharsight Corporation). Peak plasma concentration (Cmax) is reported as back-extrapolated values. The area under the plasma concentration-versus-time curve was calculated using the linear trapezoidal method from time 0 (at drug administration) to time of last sample with measurable drug concentration for each individual. The inferred AUC value was calculated by extrapolation by dividing the last measurable drug concentration by the rate constant at the terminal phase. All statistical analyses were carried out using NCSS 2004 software (J. Hintze, Kaysville, UT). P < .05 was considered to be statistically significant in paired t tests.
Serum antibody to test material rhIL-15 in rhesus macaques
All samples were analyzed for the development of antibodies to rhIL-15 using the ELISA method (see supplemental Methods). This procedure achieved a sensitivity of 156 ng antibody/mL, which provided a limit of quantification of 470 ng/mL in rhesus macaque serum analyzed at a 1:3 dilution.
Results
Production of rhIL-15
rhIL-15 was produced using current good manufacturing practices in an E coli expression system (see supplemental Methods). rhIL-15 was generated as a nonglycosylated, single-chain polypeptide of 115 amino acids having a calculated molecular weight of 12 901 Da. An E coli–based fermentation and purification process was developed for the production of clinical grade rhIL-15. DNA sequences for human IL-15 inserted into the pET28 b plasmid were expressed in E coli host BL 21-AI. Inclusion bodies of IL-15 produced in E coli were solubilized, refolded, and orthogonally purified to yield active IL-15. Purification consisted of orthogonal column chromatography and filtration methods that included Superdex-200 chromatography, refolding of denatured rhIL-15, butyl hydrophobic interaction chromatography, Source 15Q chromatography, QXL chromatography, and Superdex-75 chromatography (GE Healthcare) to produce a sterile-filtered pure drug substance formulated in 25mM sodium phosphate and 500mM sodium chloride, pH 7.4.
Pharmacokinetics of rhIL-15 in rhesus macaques
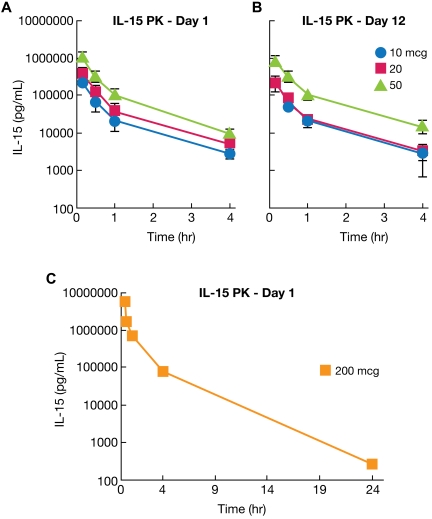
The pharmacokinetics of rhIL-15 in rhesus macaques receiving 12 daily doses of drug was evaluated in 11 animals after the first infusion and in 7 animals after the last (12th) infusion. IL-15 concentrations were determined preinfusion and at 10, 30, and 60 minutes, 4 hours, and 24 hours after rhIL-15 administration. Analysis of pharmacokinetic parameters is provided in Table 1 and data are presented in Figure 1, supplemental Table 1, and supplemental Figure 1. There were no statistical differences in parameter values between animals evaluated at day 1 (first infusion) and day 12 (final infusion) by Student t test. (Table 1, Figure 1A-B, and supplemental Figure 1). Means of various parameters for the day 1 study for different groups were as follows: Cmax for animals receiving 10 μg/kg/d was 475 ± 37 ng/mL, for 20 μg/kg/d, 702 ± 281 ng/mL; and for 50 μg/kg/d, 2085 ± 421 ng/mL. Survival terminal half-time was 1.11 ± 0.25 hours for the 10 μg/kg/d group, 1.10 ± 0.16 hours for the 20 μg/kg/d group, and 0.91 ± 0.11 hours for the 50 μg/kg/d group. IL-15 was not detectable at the 24-hour time point for any animal at any dose with a limit of quantitation of 188 pg/mL. A single additional animal that received a 200 μg/kg/d dose was also examined. In this animal, 270 pg/mL of rhIL-15 was detected at 24 hours, a value only 0.04% of the ∼ 6 × 106 pg/mL present at the 10-minute time point (Figure 1C). Therefore, only in this latter animal was there evidence for a prolonged persistence of rhIL-15.
Table 1.
Data analysis of rhIL-15 pharmacokinetic studies performed in rhesus macaques after the first or last of 12 daily infusions at 10, 20, or 50 μg/kg/d
| Parameter, units | Study day 1 |
Study day 12 |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 10 μg/kg/d |
20 μg/kg/d |
50 μg/kg/d |
10 μg/kg/d |
20 μg/kg/d |
50 μg/kg/d |
|||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| Cmax, ng/mL | 475.4 | 36.7 | 584.9 | 281 | 1994.7 | 421 | 450.7 | 70.9 | 460.9 | 358.9 | 1705.6 | 554.3 |
| AUC0-4 h, ng·h/mL | 147.0 | 36.3 | 260.3 | 91.0 | 719.4 | 149 | 138.9 | 16.2 | 155.6 | 34.2 | 641.9 | 69.1 |
| Terminal slope, h−1 | 0.642 | 0.13 | 0.639 | 0.08 | 0.772 | 0.08 | 0.694 | 0.36 | 0.678 | 0.12 | 0.71 | 0.15 |
| Half-life, h | 1.113 | 0.25 | 1.101 | 0.16 | 0.907 | 0.11 | 1.315 | 0.93 | 1.039 | 0.19 | 2.159 | 1.29 |
| AUCinf, ng·h/mL | 151.4 | 36.4 | 269.6 | 95.6 | 733.5 | 149 | 147.5 | 12.7 | 161.3 | 30.6 | 660.9 | 95.1 |
| AUCinf/dose, ng·h/mL/μg | 15.14 | 3.64 | 13.48 | 4.78 | 14.67 | 2.98 | 14.75 | 1.27 | 12.04 | 7.61 | 13.22 | 1.90 |
| CL, L/h/kg | 0.069 | 0.02 | 0.082 | 0.03 | 0.070 | 0.01 | 0.068 | 0.01 | 0.0832 | 0.04 | 0.076 | 0.01 |
| Vss, L/kg | 0.050 | 0.01 | 0.063 | 0.02 | 0.047 | 0.01 | 0.044 | 0.04 | 0.06 | 0.06 | 0.043 | 0.014 |
AUC0-4 h indicates area under the curve; AUCinf, inferred AUC; CL, clearance; and Vss, volume of distribution at steady state.
Figure 1.
Pharmacokinetics of rhIL-15 assessed in rhesus macaques. (A) rhIL-15 pharmacokinetics were evaluated in rhesus macaques, with one set of animals receiving 12 infusions of 10 (•), 20 (■), and 50 (▴) μg/kg/d of rhIL-15 for 12 days, followed within 24 hours by killing and necropsy. IL-15 pharmacokinetics in this group were evaluated at 10, 30, 60 minutes, 4 hours, and 24 hours after the initial rhIL-15 administration. The mean terminal half-time was 1.11 ± 0.25 hours for the 10 μg/kg/d group, 1.10 ± 0.16 hours for the 20 μg/kg/d group, and 0.91 ± 0.11 hours for the 50 μg/kg/d group. (B) Other groups of animals each received the same doses and dosing schedule but were killed and necropsied at day 48 after a 36-day recovery period. In this set of animals, IL-15 pharmacokinetics were evaluated in the same schedule as in panel A after the 12th dose of rhIL-15. There was no statistical difference in the parameter values between day 1 (first infusion) and day 12 (final infusion) by Student t test, with the exception of the terminal slope of the 50 μg/kg/d animal. (C) A single additional animal received a 200 μg/kg/d dosage. At 24 hours after the IL-15 infusion, there was 270 pg/mL of rhIL-15 retained in this animal, a value only 0.04% of the ∼ 6 × 106 pg/mL at the 10-minute time point. Therefore, only in this latter animal was there any evidence for a prolonged persistence of rhIL-15 in the serum.
Clinical features of animals receiving rhIL-15
Adverse clinical signs in rhesus macaques receiving rhIL-15 included reduced appetite, diarrhea, emesis, and weight loss. Reduced appetite was seen intermittently and primarily during the dosing phase, when physical restraint for dosing may have contributed. There was an 11% weight loss in animals receiving 20 μg/kg/d and 12% in animals dosed with 50 μg/kg/d. Diarrhea (3 of 6 animals) and emesis (2 of 6 animals) that responded well to supportive care occurred during the dosing phase in monkeys receiving 50 μg/kg/d. In addition to the 24 animals in the major toxicology study, a single animal received 200 μg/kg/d of rhIL-15 for 8 doses in a pilot toxicology study. This animal developed decreased appetite, diarrhea, and emesis, and infusions were stopped on day 7. After a rapid recovery, dosing was reinitiated on day 9, but was then terminated because of return of the same clinical abnormalities. None of the animals died during the study.
Analysis of immunogenicity of rhIL-15 in rhesus macaques
The immunogenicity of rhIL-15 in rhesus macaques was analyzed using a specific 2-arm capture ELISA procedure in samples collected at the same time points as indicated for hematology. The sensitivity of the ELISA assay was 156 ng/mL. No antibodies to administered rhIL-15 were detected at any time point.
Results of hematology, chemistry, and coagulation assays in rhesus macaques receiving rhIL-15
The circulating total white blood cells, RBCs, hemoglobin, hematocrit, mean corpuscular volume, platelet count, basophils, neutrophils, lymphocytes, monocytes, and eosinophils were determined before therapy and on days 8 and 13 for animals killed on day 13 and before therapy and on days 8, 13, 15, 22, 29, and 48 for those killed on day 48. The only biologically meaningful clinical chemistry or hematologic abnormality observed was a grade 3/4 reduction in circulating neutrophils observed in 3 of 6 of the animals receiving 20 μg/kg/d of rhIL-15 and in 3 of 6 of the animals receiving 50 μg/kg/d of rhIL-15 (Figure 2 and supplemental Table 2). These reductions were transient, with a return toward normal by 72 hours after cessation of rhIL-15 administration. Serum clinical chemistries were determined on the same schedule as hematology assays. None of the animals manifested serum chemical abnormalities compared with published values for rhesus macaques with the exception of one animal receiving 20 μg/kg/d in a pilot study, in which there was an aminotransferase increase to 178 μL on day 8 that normalized on day 15, a creatinine kinase increase from 187 to 2860 on day 15 that normalized by day 22, and in the animal receiving 200 μg/kg/d, elevated triglycerides and decreased phosphorus. There were consistent increases in soluble IL-2Rα and IL-18 levels on days 2, 8, and 13 during and immediately after the rhIL-15 infusions (in those animals evaluable; Tables 2–3). IL-6 and troponin-T levels remained within the normal range on those days.
Figure 2.
rhIL-15 administration reduces the circulating absolute neutrophil numbers in rhesus macaques. Vehicle diluent or rhIL-15 at 10, 20, and 50 μg/kg/d was administered daily for 12 days to rhesus macaques. The circulating neutrophil numbers were determined before therapy and on days 8 and 13 for animals killed on day 13, and before therapy and on days 8, 13, 15, 22, 29, and 48 for those killed on day 48. Three of 6 animals receiving 20 μg/kg/d and 3 of 6 animals receiving 50 μg/kg/d of rhIL-15 had a grade 3/4 reduction in circulating neutrophil numbers. These reductions were transient, with a return toward normal levels by 72 hours after cessation of rhIL-15 administration.
Table 2.
Soluble IL-2Rα (CD25) levels detected in the serum of rhesus macaques receiving 12 daily infusions of rhIL-15
| Macaque ID | rhIL-15 dose, μg/kg | Soluble IL-2Rα level, pg/mL |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Pre-therapy | Day 2 | Day 8 | Day 13 | Day 15 | Day 22 | Day 29 | Day 48 | ||
| 395 | Vehicle | 356 | ND | 394 | 390 | ND | ND | ND | ND |
| CFIT | Vehicle | 769 | ND | 668 | 770 | ND | ND | ND | ND |
| XDD | Vehicle | 422 | ND | 768 | 481 | ND | ND | ND | ND |
| Mean | 516 | 610 | 547 | ||||||
| SD | 222 | 194 | 198 | ||||||
| XFH | 10 | 681 | 1643 | 2753 | 2792 | ND | ND | ND | ND |
| DA5F | 10 | 1388 | 2663 | 2842 | 3259 | ND | ND | ND | ND |
| CF5F | 10 | 581 | 1861 | 1623 | 1506 | ND | ND | ND | ND |
| 446 | 10 | 388 | ND | 2118 | ND | 1874 | 705 | 716 | 334 |
| XDT | 10 | 488 | ND | 1103 | ND | 777 | 414 | 439 | 415 |
| CJ5P | 10 | 645 | ND | 2336 | ND | 1646 | 844 | 969 | 703 |
| Mean | 695 | 2056 | 2129 | 2519 | 1432 | 654 | 708 | 484 | |
| SD | 356 | 537 | 671 | 908 | 579 | 219 | 265 | 194 | |
| RQ3552 | 20 | 1056 | 1556 | 2194 | 2860 | ND | ND | ND | ND |
| H450 | 20 | 435 | 1234 | 1971 | 2485 | ND | ND | ND | ND |
| CK3D | 20 | 351 | 998 | 1658 | 1917 | ND | ND | ND | ND |
| VCT | 20 | 942 | ND | 3167 | ND | 2033 | 965 | 750 | 839 |
| VVX | 20 | 754 | ND | 3523 | ND | 1971 | 938 | 755 | 590 |
| CK2X | 20 | 774 | ND | 1858 | ND | 1237 | 645 | 683 | 599 |
| Mean | 719 | 1263 | 2395 | 2421 | 1747 | 849 | 729 | 676 | |
| SD | 277 | 280 | 764 | 475 | 443 | 177 | 40 | 141 | |
| CJ64 | 50 | 962 | 2751 | 6726 | 6885 | ND | ND | ND | ND |
| OIE123 | 50 | 512 | 1173 | 3794 | 4885 | ND | ND | ND | ND |
| CF65 | 50 | 1080 | 1843 | 7910 | 6041 | ND | ND | ND | ND |
| XFK | 50 | 349 | ND | 3934 | ND | 1914 | 615 | 458 | 378 |
| CK18 | 50 | 814 | ND | 3805 | ND | 2076 | 892 | 697 | 718 |
| CF4C | 50 | 602 | ND | 4349 | ND | 3052 | 932 | 663 | 632 |
| Mean | 720 | 1922 | 5086 | 5937 | 2347 | 813 | 606 | 576 | |
| SD | 280 | 792 | 1780 | 1004 | 616 | 173 | 129 | 177 | |
ND indicates not determined.
Table 3.
IL-18 levels detected in the serum of rhesus macaques receiving 12 daily infusions of rhIL-15
| Macaque ID | rhIL-15 dose, μg/kg | IL-18 level, pg/mL |
||||
|---|---|---|---|---|---|---|
| Pre-therapy | Day 8 | Day 13 | Day 15 | Day 22 | ||
| 395 | Vehicle | 82 | 66 | ND | ND | ND |
| CFIT | Vehicle | 47 | 56 | ND | ND | ND |
| XDD | Vehicle | 79 | 61 | ND | ND | ND |
| XAF | Vehicle | 189 | 170 | ND | ND | ND |
| XCJ | Vehicle | 131 | 160 | ND | ND | ND |
| CK3C | Vehicle | 132 | 128 | ND | ND | ND |
| Mean | 110 | 107 | ||||
| SD | 51 | 52 | ||||
| XFH | 10 | 192 | 620 | 560 | ND | ND |
| DA5F | 10 | 192 | 389 | 485 | ND | ND |
| CF5F | 10 | 371 | 531 | 532 | ND | ND |
| 446 | 10 | 113 | 373 | 414 | ND | 169 |
| XDT | 10 | 179 | 544 | 547 | ND | 239 |
| CJSP | 10 | 173 | 417 | 507 | ND | 231 |
| Mean | 203 | 479 | 508 | 213 | ||
| SD | 87 | 100 | 53 | 38 | ||
| RQ3552 | 20 | 157 | 339 | 390 | ND | ND |
| H450 | 20 | 134 | 299 | 543 | ND | ND |
| CK3D | 20 | 115 | 448 | 490 | ND | ND |
| VTC | 20 | 132 | 376 | ND | 303 | 154 |
| VVX | 20 | 152 | 688 | 650 | ND | 208 |
| CK2X | 20 | 95 | 422 | 649 | ND | 230 |
| Mean | 131 | 429 | 544 | 197 | ||
| SD | 23 | 138 | 111 | 39 | ||
| CJ64 | 50 | 199 | 703 | 712 | ND | ND |
| OIE123 | 50 | 100 | 508 | 568 | ND | ND |
| CF65 | 50 | 86 | 408 | 615 | ND | ND |
| XFK | 50 | 103 | 320 | ND | 343 | 216 |
| CK18 | 50 | 62 | 388 | 757 | ND | 231 |
| CF4C | 50 | 150 | 596 | 632 | ND | 201 |
| Mean | 117 | 487 | 657 | 216 | ||
| SD | 50 | 143 | 76 | 15 | ||
ND indicates not determined.
Prothrombin time, activated partial thromboplastin time, and fibrinogen levels remained normal throughout the study. Sera were negative for antinuclear antibody reactivity.
A detailed FACS analysis of the impact of rhIL-15 on lymphocyte subsets was published previously.36 A transient lymphocytopenia at day 2 in animals receiving 20 and 50 μg/d preceded T- and NK-cell expansion with a mean lymphocyte count on day 2 of 1036 compared with 1515 before therapy. The absolute number of lymphocytes per microliter rose from the pre-bleed mean of 1810/μL for all animals to 4219/μL on day 13 in animals receiving 10 μg/kg/d, to 3452 in animals receiving 20 μg/kg/d, and to 4614/μL in animals receiving 50 μg/kg/d, respectively. Lymphocyte levels returned to pre–IL-15 administration levels by day 29 (supplemental Table 3). During and immediately after rhIL-15 infusions, there was an increase in the absolute number of NK cells from a baseline of 73 to 180, 171, and 321 on day 8 for animals receiving 10, 20, and 50 μg/kg/d, respectively. NK-cell values returned to normal by day 48. There was a 2-fold increase in the absolute number of CD3 cells, a 1.7-fold increase in CD4 cells, and a 2.5-fold increase in CD8 T cells at the day of rhIL-15 infusion (day 8) and immediately afterward (day 13/15). By day 48, these values returned to normal with the exception that the elevated CD8:CD4 ratio was maintained. In these studies, there was a 4-fold expansion of central memory CD8 T cells and a 6-fold expansion of effector memory CD8 T cells observed during rhIL-15 administration, with a decline in the expanded T- and NK-cell populations in blood soon after discontinuing rhIL-15 administration.36
Necropsies and pathological examinations
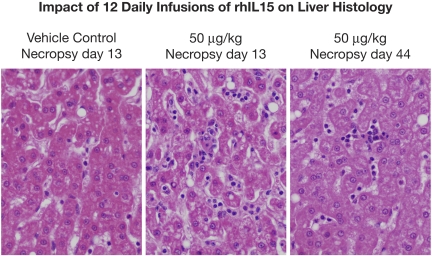
Gross lesions identified at necropsy in animals killed on day 13 (1 day after the last infusion) included the following. Enlarged axillary lymph nodes were seen in the group receiving 50 μg/kg/d. Most histopathological lesions observed were clearly incidental in nature, with the exception of hyperplastic bone marrow and a reduction in the number of adipocytes in animals receiving 20 and 50 μg/kg/d of rhIL-15 (Figure 3). Furthermore, there were dose-related enlargements of livers and spleens with increases comparable to those of control animals of 21%, 26%, and 85%, respectively, for liver size among animals receiving 10, 20, and 50 μg/kg/d and 164%, 79%, and 377% increases, respectively, in the sizes of spleens of animals receiving these same doses (supplemental Table 4). Examination of the livers showed normal hepatocytes but an increase in leukocytes, including lymphocytes and neutrophils within liver sinusoids in 2 of 3 animals receiving 10 μg/kg/d, in 1 of 3 animals receiving 20 μg/kg/d, and in 2 of 3 animals receiving 50 μg/kg/d (Figure 4). Alterations from control values in bone marrow and liver histology returned to or toward normal in the animals examined at day 44 or 48. There was a modest increase in the weight of the lungs at day 13 during IL-15 administration that persisted at day 44. Histological analyses only revealed minimal pulmonary edema in single animals receiving 10 and 50 μg/kg/d, and a mild perivascular lymphocytic and eosinophilic infiltrate in animals receiving 50 μg/kg/d. The single animal receiving 200 μg/kg/d of IL-15 had a moderate thrombosis in a branch of the pulmonary artery. This lesion could have been the result of the presence of a catheter, meaning that it was mechanical in nature.
Figure 3.
rhIL-15 administration is associated with hypercellular marrow and a reduction in adipocytes. Shown is the impact of rhIL-15 on the bone marrow examination of animals receiving vehicle diluent control or rhIL-15 at 50 μg/kg/d for 12 days. Left panel is the vehicle control bone marrow at day 44. Center panel is the bone marrow of animals receiving 50 μg/kg/d for 12 days determined at necropsy at day 13 within 24 hours of the last rhIL-15 administration showing marrow hyperplasia with a reduction in adipocytes. Right panel is the bone marrow examination of rhesus macaques receiving 50 μg/kg/d for 12 days studied at necropsy at day 44, 32 days after the last rhIL-15 administration, showing a return to a normal marrow pattern.
Figure 4.
rhIL-15 administration is associated with an enlarged liver with normal hepatocytes, but with the presence of leukocytes including lymphocytes and neutrophils in the liver sinusoids. Left panel is the liver histology of an animal receiving vehicle control for 12 days with a necropsy on day 13. Center panel is the liver histology of an animal receiving 50 μg/kg/d for 12 days, with necropsy at day 13, showing normal hepatocytes but the presence of leukocytes including lymphocytes and neutrophils in the liver sinusoids. Right panel is the liver histology of an animal receiving 50 μg/kg/d of rhIL-15 for 12 days with necropsy at day 44, showing a return toward a normal pattern.
Review of bone marrow and liver pathology
Because of the granulocytopenia observed after repeated administrations of high-dose rhIL-15, bone marrow aspirates and biopsies and bone marrow and liver tissues at necropsy received special examination. In bone marrow aspirates performed on day 8 and necropsies on day 13 during or immediately after rhIL-15 administration, but not those done on day 44/48, there was a hypercellular marrow with a reduction in adipocytes and a shift to the left (immaturity) in myeloid maturation (Figure 3 center panel). There was a 90%-100% marrow cellularity in 1 animal and 100% in 2 animals receiving 50 μg/kg/d of IL-15 studied at day 13 compared with 50%-80% in those receiving either vehicle or those receiving 50 μg/kg/d at day 44/48. The bone marrow myeloid:erythroid ratios were 3-4:1, 2-3:1, and 2-3:1 in animals receiving IL-15 compared with 3:1, 1-3:1, and 1-3:1 in those receiving vehicle. Bone marrow obtained at day 44/48 returned to normal (Figure 3 right panel). Review of liver pathology examined at necropsy on day 13 confirmed the normal hepatocytes but also the presence of leukocytes, including lymphocytes and neutrophils in liver sinusoids (Figure 4 center panel), which returned to normal in animals examined at day 44/48 (Figure 4 right panel). As noted in the discussion, these findings are most consistent with IL-15–mediated neutrophil migration from the blood pool to tissues.
Discussion
IL-15 has several characteristics, including maintenance and activation of mature NK cells, a positive action on effector and especially memory CD8 T cells, a lack of capillary leak syndrome or support for AICD or a major effect on Tregs, which suggest that it may be superior to IL-2 in the FDA-approved use of IL-2 in the treatment of metastatic malignancy.6–10 Attempts to prevent or treat syngeneic tumors in mice by administration of IL-15 have proven effective.28–35
In the present study, we evaluated the pharmacokinetics of rhIL-15 in rhesus macaques as part of an effort to define a rational dosing strategy. Previously, we described a unique feature of IL-15 in that it forms stable complexes with IL-15Rα on cell surfaces (predominantly activated dendritic cells but also on nonhematopoietic cells of lungs and small intestine) that present IL-15 in trans to neighboring NK and CD8 cells that express IL-2/IL-15Rβ (CD122) and the common γc (CD132) but not the private IL-15Rα.12 Moreover, membrane IL-15/IL-15Rα complexes undergo endosomal internalization but survive lysosomal degradation, allowing the complex to recycle back to the cell surfaces.12 Membrane IL-15–expressing cells give rise to augmented retention of IL-15 in the circulation and tissues. To compare the pharmacokinetics of different γc cytokines, we administered IL-2, IL-7, and IL-15 to mice.37 Serum IL-2 and IL-7 concentrations declined with a terminal half-time of less than 1 hour.37 However, serum concentrations of IL-15 manifested a different kinetic profile. There was a rapid decline during the α phase (first 24 hours), followed by a β phase in which IL-15 levels remained detectable (> 10 pg/mL) after the administration of 5 μg of human IL-15 even 120 hours after injection. By performing IL-15 pharmacokinetic studies in IL-15Rα−/− and IL-15Rα transgenic mice, we demonstrated that prolonged IL-15 retention was mediated by a specific mechanism involving IL-15Rα.
An analysis of pharmacokinetic parameters of rhIL-15 in rhesus macaques in the present study is provided in Table 1, Figure 1, and supplemental Figure 1, and the data are presented in supplemental Table 1 and supplemental Figure 1. The Cmax for the 10, 20, and 50 μg/kg/d groups was 475 ± 37 ng/mL, 702 ± 280 ng/mL, and 2085 ± 421 ng/mL, respectively, in animals evaluated after first infusion. The terminal half-times for 10, 20, and 50 μg/kg/d were 1.11 ± 0.25 hours, 1.10 ± 0.16 hours, and 0.91 ± 0.11 hours, respectively. At the earliest time points, the levels of IL-15 were sufficient to act through the β/γ receptors alone. IL-15 was not detectable at the 24-hour time point for any animal receiving 10, 20, or 50 μg/kg/d with a limit of quantitation of 188 pg/mL at a 1:3 dilution. Failure to find prolonged persistence of rhIL-15 in serum may reflect the fact that rhIL-15 in the present study was produced in E coli and was not glycosylated. However, an alternate likely explanation is that there was only a very modest expression of IL-15Rα on resting antigen-presenting cells in rhesus macaques and, as noted above, prolonged survival of IL-15 requires expression of IL-15Rα and its associated recycling of IL-15.
Using an ELISA procedure sensitive to 156 ng/mL of antibody to rhIL-15, the immunogenicity of rhIL-15 was evaluated in the 18 rhesus macaques that received daily doses of this cytokine for 12 days at 10, 20, and 50 μg/kg per dose. Although E coli produces rhIL-15 that is not glycosylated and could theoretically yield aggregates, and although human IL-15 has 6 amino acid differences from that of rhesus macaques, none of the animals developed antibodies to administered rhIL-15.
In previously reported studies by others in rhesus macaques, doses ranging from 5-25 μg/kg/d of IL-15 were used and did not cause detrimental effects.16,38–40 Mueller et al demonstrated that IL-15 increased effector memory CD8 T cells and NK cells in simian immunodeficiency virus–infected macaques at a dose of 10 μg/kg/d.16 IL-15 administration induced a nearly 3-fold increase in the number of peripheral blood CD8+CD3− NK cells and in CD8 T cells, mainly due to an increase in effector-memory T cells. To evaluate possible toxic effects of IL-15 treatment, animals were monitored with an array of clinical, chemical, and hematologic measures. All tests remained within physiologic limits with the exception of platelet numbers, which increased from 200 × 103/μL of blood to 333 × 103/μL of blood. Necropsy did not reveal any abnormalities. Picker et al demonstrated that a dose of 10-50 μg/kg given twice a week led to increased numbers of NK cells and to an increase in the numbers of Ki67-expressing T cells. Increases in the absolute number of CD4 or CD8 memory T cells were either not observed or were of low amplitude and transient.39
Berger et al40 evaluated the safety and immunologic effects of IL-15 administered subcutaneously twice a week in 7 animals and in 3 rhesus macaques administered daily. Intermittent administration of IL-15 yielded very modest effects on NK and CD8 subset levels but was not associated with cytotoxicity. One of 3 animals receiving daily administrations of IL-15 developed persistently elevated plasma IL-15 levels and transient neutropenia in association with hypocellularity of the bone marrow, which is consistent with a lack of neutrophil production. In a second animal, there was a decline in hemoglobin and the bone marrow showed an infiltration of lymphocytes; however, in a third animal, the neutrophil numbers remained within normal limits. Berger et al concluded that the neutropenia observed in one animal was due to a lack of neutrophil production rather than to accelerated turnover or migration from the blood pool. This is not consistent with our data, as discussed below.
In our study, we evaluated rhIL-15 given IV at 10, 20, and 50 μg/kg/d for 12 consecutive doses. The only clinical abnormalities were diarrhea in 3 of 6 and vomiting in 2 of 6 animals receiving 50 μg/kg/d of rhIL-15 for 12 days. In terms of clinical chemistry analysis, no meaningful abnormalities were observed that might suggest disorders of renal, hepatic, or other organ abnormalities. There were dose-related elevations of serum IL-18 and serum IL-2Rα in all animals receiving rhIL-15. Previously it had been demonstrated by signaling through the IL-2/IL-15Rβ-γ receptor that IL-15 administration was associated with an up-regulation of expression of both RNA encoding IL-2Rα and the receptor protein.41 The assay for IL-2Rα could provide a simple surrogate marker of IL-15 action when rhIL-15 is administered in therapeutic trials.
The only clinically meaningful hematologic abnormality observed was a grade 3/4 neutropenia in certain animals receiving 20 or 50 μg/kg/d of rhIL-15. We have a different perspective on the cause and significance of this neutropenia from the view presented by Berger et al that the neutropenia was due to a lack of neutrophil production.40 For the 9 animals in our study that received rhIL-15 IV daily for 12 days, bone marrow sections obtained at necropsy on day 13 were hypercellular with a reduced number of adipocytes and a large number of cells of the neutrophil series. In no animal did we observe a hypocellular marrow. What we did observe was the appearance of neutrophils and lymphocytes in the sinusoids of livers, an increase of mean liver weight by 85%, and an increase of mean spleen weight by 375% in animals receiving 50 μg/kg/d when observed at day 13 (supplemental Table 4). In animals necropsied at days 44/48, bone marrow cellularity was normal and the sizes of livers and spleens had returned to those observed in animals receiving vehicle control. Thus, in contrast to the conclusions of Berger et al, we suggest that the neutropenia observed during the course of IL-15 administration represents a redistribution of neutrophils from the blood pool to tissues.
Verri et al42 reported that IL-15 mediates antigen-induced neutrophil migration by triggering IL-18 production. Their results demonstrated that IL-15 plays an important role in neutrophil migration during inflammation, triggering a sequential IL-15, IL-18, MIP-2, MIP-1α, TNFα, LTB4 neutrophil migration signaling cascade. To determine whether such a cascade might have played a role in the neutropenia seen after rhIL-15 administration in the present study, we quantitated IL-18. In animals receiving a high dose of rhIL-15 that manifested neutropenia, there was a consistent elevation of serum IL-18 levels on day 8 during the rhIL-15 infusion phase and on day 13 immediately after the infusions (Table 3). The values returned to near preinfusion levels by day 22 in all but the 50 mg/kg/d animals. Thus, our data, in conjunction with those of Verri, support the view that IL-15 mediates transient neutropenia via neutrophil migration from the blood pool to tissues, including the liver and spleen, which is mediated by a migration signaling cascade that is initiated by IL-15 triggering of IL-18 expression.
On the basis of the neutropenia and anemia observed in one animal each during daily IL-15 administration, Berger et al40 suggested that subcutaneous, intermittent (every 3 days) IL-15 administration should be used in therapeutic trials instead of daily administration. In the clinical situation, in which a major expansion of NK and CD8 T cells is desirable, we have a different perspective. Intermittent (every 3 days) administration leads to only exceedingly modest increases in NK and CD8 T-cell levels in mice and rhesus macaques compared with daily administration. In a therapeutic study we performed using the TRAMP-C2 prostatic tumor model in mice, a schedule of 5 μg/mouse of murine IL-15 given twice a week for 3 weeks was much less effective than a dose of 2.5 μg/mouse given 5 times a week for the same time period in terms of maximum levels of circulating NK, CD8, and CD44hiCD8 T cells (M. Zhang and T.A. Waldmann, unpublished observations). In the rhesus macaque studies of Berger et al using subcutaneous administration every 3 days and with the preparation of rhIL-15 used in the present study on the same schedule (M. Sneller, unpublished observations), there was no statistically significant elevation in circulating NK-cell numbers and only a very modest increase in numbers of CD8 central or effector memory cells.40 In contrast to daily IV administration of rhIL-15 at comparable doses used in the present study, there was a much more marked (4-fold) increase in the number of circulating NK cells and NK T cells, a modest expansion of CD4+CD25+Foxp3+ cells without apparent functional activity, and a 4- and 6-fold increase in the numbers of central and effector memory T cells, respectively, during IL-15 administration. After cessation of IL-15 administration, lymphocyte subpopulations in the blood returned to normal with the exception that CD8 cells still outnumbered CD4 cells.36
In summary, administration of rhIL-15 in rhesus macaques was not associated with undesirable features of IL-2, such as capillary leak syndrome or IL-2–mediated checkpoints, including activation-induced cell death and increased numbers of Tregs. Furthermore, given that the only abnormality of daily administration of rhIL-15 in the present study appeared to be a redistribution of neutrophils from the blood pool to tissues, we suggest that our present study supports a phase 1 dose-escalation clinical trial of 12 daily infusions of rhIL-15 in patients with metastatic malignancy. An FDA-approved clinical protocol for a phase 1 trial to evaluate a dose-escalation analysis (3, 7, 10, 15, 20, and 25 μg/kg) of the rhIL-15 preparation evaluated in this toxicology study has been initiated in patients with metastatic malignant melanoma and metastatic renal cell cancer.
Supplementary Material
Acknowledgments
We thank SAIC-Frederick, Inc and the Biopharmaceutical Development Program at the National Cancer Institute–Frederick for providing the rhIL-15 used in this study.
This research was supported by the intramural programs of the National Cancer Institute (Frederick, MD) and the National Institute of Allergy and Infectious Diseases, National Institutes of Health (Bethesda, MD).
Footnotes
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: T.A.W. conceived and supervised the study, analyzed the data, and wrote the paper; E.L., C.K.G., B.R.B., and T.A.F. performed the experiments; J.L.Y., S.P.C., and L.P.P. produced the rhIL-15; J.V.S. and R.P.M. provided veterinary support; W.D.F. analyzed the pharmacokinetic data; J.M.P. performed the pathological analyses; R.J.K. and D.E.K. analyzed the bone marrow and liver pathology; and T.A.W., M.R., H.C.L., M.C.S., and J.M.D. supervised the study.
Conflict-of-interest declaration: The authors declare no competing financial interests.
Correspondence: Thomas A. Waldmann, MD, National Institutes of Health, Bldg 10, Rm 4N115, 10 Center Dr, Bethesda, MD 20892-1374; e-mail: tawald@helix.nih.gov.
References
- 1.Wadler S, Schwartz EL. Antineoplastic activity of the combination of interferon and cytotoxic agents against experimental and human malignancies: a review. Cancer Res. 1990;50(12):3473–3486. [PubMed] [Google Scholar]
- 2.Metcalf D. The colony-stimulating factors and cancer. Nat Rev Cancer. 2010;10(6):425–434. doi: 10.1038/nrc2843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rosenberg SA, Yang JC, Topalian SL, et al. Treatment of 283 consecutive patients with metastatic melanoma or renal cell cancer using high-dose bolus interleukin-2. JAMA. 1994;271(12):907–913. [PubMed] [Google Scholar]
- 4.Lenardo MJ. Fas and the art of lymphocyte maintenance. J Exp Med. 1996;183(3):721–724. doi: 10.1084/jem.183.3.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maloy KJ, Powrie F. Fueling regulation: IL-2 keeps CD4+ Treg cells fit. Nat Immunol. 2005;6(11):1071–1072. doi: 10.1038/ni1105-1071. [DOI] [PubMed] [Google Scholar]
- 6.Waldmann TA, Tagaya Y. The multifaceted regulation of interleukin-15 expression and the role of this cytokine in NK cell differentiation and host response to intracellular pathogens. Annu Rev Immunol. 1999;17:19–49. doi: 10.1146/annurev.immunol.17.1.19. [DOI] [PubMed] [Google Scholar]
- 7.Waldmann TA, Dubois S, Tagaya Y. Contrasting roles of IL-2 and IL-15 in the life and death of lymphocytes: implications for immunotherapy. Immunity. 2001;14(2):105–110. [PubMed] [Google Scholar]
- 8.Waldmann TA. The biology of interleukin-2 and interleukin-15: implications for cancer therapy and vaccine design. Nat Rev Immunol. 2006;6(8):595–601. doi: 10.1038/nri1901. [DOI] [PubMed] [Google Scholar]
- 9.Fehniger TA, Caligiuri MA. Interleukin 15: biology and relevance to human disease. Blood. 2001;97(1):14–32. doi: 10.1182/blood.v97.1.14. [DOI] [PubMed] [Google Scholar]
- 10.Fehniger TA, Cooper MA, Caligiuri MA. Interleukin-2 and interleukin-15: immunotherapy for cancer. Cytokine Growth Factor Rev. 2002;13(2):169–183. doi: 10.1016/s1359-6101(01)00021-1. [DOI] [PubMed] [Google Scholar]
- 11.Marks-Konczalik J, Dubois S, Losi JM, et al. IL-2 induced activation-induced cell death is inhibited in IL-15 transgenic mice. Proc Natl Acad Sci U S A. 2000;97(21):11445–11450. doi: 10.1073/pnas.200363097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dubois S, Mariner J, Waldmann TA, Tagaya Y. IL-15Ralpha recycles and presents IL-15 in trans to neighboring cells. Immunity. 2002;17(5):537–547. doi: 10.1016/s1074-7613(02)00429-6. [DOI] [PubMed] [Google Scholar]
- 13.Oh S, Berzofsky JA, Burke DS, Waldmann TA, Perera LP. Coadministration of HIV vaccine vectors with vaccinia viruses expressing IL-15 but not IL-2 induces long-lasting cellular immunity. Proc Natl Acad Sci U S A. 2003;100(6):3392–3397. doi: 10.1073/pnas.0630592100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oh S, Perera LP, Burke DS, Waldmann TA, Berzofsky JA. IL-15/IL-15Ralpha-mediated avidity maturation of memory CD8+ T cells. Proc Natl Acad Sci U S A. 2004;101(42):15154–15159. doi: 10.1073/pnas.0406649101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kutzler MA, Robinson TM, Chattergoon MA, et al. Coimmunization with an optimized IL-15 plasmid results in enhanced function and longevity of CD8 T cells that are partially independent of CD4 T cell help. J Immunol. 2005;175(1):112–123. doi: 10.4049/jimmunol.175.1.112. [DOI] [PubMed] [Google Scholar]
- 16.Mueller YM, Petrovas C, Bojczuk PM. Interleukin-15 increases effector memory CD8+ T cells and NK cells in simian immunodeficiency virus-infected macaques. J Virol. 2005;79(8):4877–4885. doi: 10.1128/JVI.79.8.4877-4885.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oh S, Perera LP, Terabe M, Ni L, Waldmann TA, Berzofsky JA. IL-15 as a mediator of CD4+ help for CD8+ T cell longevity and avoidance of TRAIL-mediated apoptosis. Proc Natl Acad Sci U S A. 2008;105(13):5201–5206. doi: 10.1073/pnas.0801003105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang X, Sun S, Hwang I, Tough DF, Sprent J. Potent and selective stimulation of memory-phenotype CD8+ T cells in vivo by IL-15. Immunity. 1998;8(5):591–599. doi: 10.1016/s1074-7613(00)80564-6. [DOI] [PubMed] [Google Scholar]
- 19.Lodolce JP, Burkett PR, Boone DL, Chien M, Ma A. T cell-independent interleukin 15Ralpha signals are required for bystander proliferation. J Exp Med. 2001;194(8):1187–1194. doi: 10.1084/jem.194.8.1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Surh CD, Sprent J. Homeostasis of naive and memory T cells. Immunity. 2008;29(6):848–862. doi: 10.1016/j.immuni.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 21.Becker TC, Wherry EJ, Boone D, et al. Interleukin 15 is required for proliferative renewal of virus-specific memory CD8 T cells. J Exp Med. 2002;195(12):1541–1584. doi: 10.1084/jem.20020369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Geginat J, Lanzavecchia A, Sallusto F. Proliferation and differentiation potential of human CD8+ memory T-cell subsets in response to antigen or homeostatic cytokines. Blood. 2003;101(11):4260–4266. doi: 10.1182/blood-2002-11-3577. [DOI] [PubMed] [Google Scholar]
- 23.Schluns KS, Klonowski KD, Lefrancois L. Transregulation of memory CD8 T-cell proliferation by IL-15Ralpha+ bone marrow-derived cells. Blood. 2004;103(3):988–994. doi: 10.1182/blood-2003-08-2814. [DOI] [PubMed] [Google Scholar]
- 24.Ku CC, Murakami M, Sakamato A, Kappler J, Marrack P. Control of homeostasis of CD8+ memory T cells by opposing cytokines. Science. 2000;288(5466):675–678. doi: 10.1126/science.288.5466.675. [DOI] [PubMed] [Google Scholar]
- 25.Sadlack B, Kuhn R, Schorle H, Rajewsky K, Muller W, Horak I. Development and proliferation of lymphocytes in mice deficient for both interleukins-2 and -4. Eur J Immunol. 1994;24(1):281–284. doi: 10.1002/eji.1830240144. [DOI] [PubMed] [Google Scholar]
- 26.Lodolce JP, Boone DL, Chai S, et al. IL-15 receptor maintains lymphoid homeostasis by supporting lymphocyte homing and proliferation. Immunity. 1998;9(5):669–676. doi: 10.1016/s1074-7613(00)80664-0. [DOI] [PubMed] [Google Scholar]
- 27.Kennedy MK, Glaccum M, Brown SN, et al. Reversible defects in natural killer and memory CD8 T cell lineages in interleukin 15-deficient mice. J Exp Med. 2000;191(5):771–780. doi: 10.1084/jem.191.5.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kobayashi H, Dubois S, Sato N, et al. Role of trans-cellular IL-15 presentation in the activation of NK cell-mediated killing, which leads to enhanced tumor immunosurveillance. Blood. 2005;105(2):721–727. doi: 10.1182/blood-2003-12-4187. [DOI] [PubMed] [Google Scholar]
- 29.Munger W, DeJoy SQ, Jeyaseelan R, Sr, et al. Studies evaluating the antitumor activity and toxicity of interleukin-15, a new T cell growth factor: comparison with interleukin-2. Cell Immunol. 1995;165(2):289–293. doi: 10.1006/cimm.1995.1216. [DOI] [PubMed] [Google Scholar]
- 30.Evans R, Fuller JA, Christianson G, Krupke DM, Troutt AB. IL-15 mediates anti-tumor effects after cyclophosphamide injection of tumor-bearing mice and enhances adoptive immunotherapy: the potential role of NK cell subpopulations. Cell Immunol. 1997;179(1):66–73. doi: 10.1006/cimm.1997.1132. [DOI] [PubMed] [Google Scholar]
- 31.Klebanoff CA, Finkelstein SE, Surman DR, et al. IL-15 enhances the in vivo antitumor activity of tumor-reactive CD8+ T cells. Proc Natl Acad Sci U S A. 2004;101(7):1969–1974. doi: 10.1073/pnas.0307298101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zeng R, Spolski R, Finkelstein SE, et al. Synergy of IL-21 and IL-15 in regulating CD8+ T cell expansion and function. J Exp Med. 2005;201(1):139–148. doi: 10.1084/jem.20041057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dubois S, Patel HJ, Zhang M, Waldmann TA, Müller JR. Preassociation of IL-15 with IL-15Ralpha-IgG1-Fc enhances its activity on proliferation of NK and CD8+/CD44high T cells and its antitumor action. J Immunol. 2008;180(4):2099–2106. doi: 10.4049/jimmunol.180.4.2099. [DOI] [PubMed] [Google Scholar]
- 34.Kudo-Saito C, Wansley EK, Gruys ME, Wiltrout R, Schlom J, Hodge JW. Combination therapy of an orthotopic renal cell carcinoma model using intratumoral vector-mediated costimulation and systemic interleukin-2. Clin Cancer Res. 2007;13(6):1936–1946. doi: 10.1158/1078-0432.CCR-06-2398. [DOI] [PubMed] [Google Scholar]
- 35.Zhang M, Yao Z, Dubois S, Ju W, Müller JR, Waldmann TA. Interleukin-15 combined with an anti-CD40 antibody provides enhanced therapeutic efficacy for murine models of colon cancer. Proc Natl Acad Sci U S A. 2009;106(18):7513–7518. doi: 10.1073/pnas.0902637106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lugli E, Goldman CK, Perera LP, et al. Transient and persistent effects of IL-15 on lymphocyte homeostasis in nonhuman primates. Blood. 2010;116(17):3238–3248. doi: 10.1182/blood-2010-03-275438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sato N, Patel HJ, Waldmann TA, Tagaya Y. The IL-15/IL-15Ralpha on cell surfaces enables sustained IL-15 activity and contributes to the long survival of CD8 memory T cells. Proc Natl Acad Sci U S A. 2007;104(2):588–593. doi: 10.1073/pnas.0610115104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Villinger F, Miller R, Mori K, et al. IL-15 is superior to IL-2 in the generation of long-lived antigen specific memory CD4 and CD8 T cells in rhesus macaques. Vaccine. 2004;22(25–26):3510–3521. doi: 10.1016/j.vaccine.2003.07.022. [DOI] [PubMed] [Google Scholar]
- 39.Picker LJ, Reed-Inderbitzin EF, Hagen SI, et al. IL-15 induces CD4+ effector memory T cell production and tissue emigration in nonhuman primates. J Clin Invest. 2006;116(6):1514–1524. doi: 10.1172/JCI27564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Berger C, Berger M, Hackman RC, et al. Safety and immunological effects of IL-15 administration in nonhuman primates. Blood. 2009;114(12):2417–2426. doi: 10.1182/blood-2008-12-189266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Alileche A, Goldman CK, Waldmann TA. Differential effects of IL-2 and IL-15 on expression of IL-2 receptor alpha. Biochem Biophys Res Commun. 2001;285(5):1302–1308. doi: 10.1006/bbrc.2001.5332. [DOI] [PubMed] [Google Scholar]
- 42.Verri WA, Jr, Cunha TM, Ferreira SH, et al. IL-15 mediates antigen-induced neutrophil migration by triggering IL-18 production. Eur J Immunol. 2007;37(12):3373–3380. doi: 10.1002/eji.200737488. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.