Abstract
Fetal hemoglobin (HbF) is regulated as a multigenic trait. By genome-wide association study, we confirmed that HBS1L-MYB intergenic polymorphisms (HMIP) and BCL11A polymorphisms are highly associated with HbF in Chinese β-thalassemia heterozygotes. In this population, the variance in HbF resulting from the HMIP is 13.5%; that resulting from the BCL11A polymorphism is 6.4%. To identify the functional variant in HMIP, we used 1000 Genomes Project data, single nucleotide polymorphism imputation, comparisons of association results across populations, potential transcription factor binding sites, and analysis of phylogenetic conservation. Based on these studies, a hitherto unreported association between HbF expression and a 3-bp deletion, between 135 460 326 and 135 460 328 bp on chromosome 6q23 was found. This 3-bp deletion is in complete linkage disequilibrium with rs9399137, which is the single nucleotide polymorphism in HMIP most significantly associated with HbF among Chinese, Europeans, and Africans. Chromatin immunoprecipitation assays confirmed erythropoiesis-related transcription factors binding to this region in K562 cells. Based on transient expression of a luciferase reporter plasmid, the DNA fragment encompassing the 3-bp deletion polymorphism has enhancer-like activity that is further augmented by the introduction of the 3-bp deletion. This 3-bp deletion polymorphism is probably the most significant functional motif accounting for HMIP modulation of HbF in all 3 populations.
Introduction
More than 3% of Chinese in Hong Kong are heterozygous carriers of β-thalassemia.1 Homozygotes or compound heterozygotes for β-thalassemia are usually severely ill and require monthly transfusions. Increased production of fetal hemoglobin (HbF; α2γ2) can modulate the disease severity by compensating for the shortfall of β globin caused by the β-thalassemia mutations.
HbF level in adults varies and is regulated as a multigenic trait.2 Three major HbF quantitative trait loci (QTL) have been identified: the C/T single nucleotide polymorphism (SNP, rs7482144) at promoter nucleotide (nt) 158 bp 5′ upstream of HBG2 on chromosome 11p15,3 the HBS1L-MYB intergenic polymorphism (HMIP) on chromosome 6q23,4 and the BCL11A polymorphism on chromosome 2p16.5 They can modulate HbF and disease severity in β-thalassemia,6–9 and sickle cell anemia.10 The relative contributions of these 3 QTLs to HbF regulation appear to differ among populations.5,11 The functional motif for each of these 3 QTLs responsible for their effects on HbF is not known.
In a genome-wide SNP association study (GWAS) among Chinese adult β-thalassemia heterozygotes in Hong Kong, both HMIP and BCL11A polymorphisms are highly associated with HbF level. To identify the functional variant in HMIP, we devised a novel strategy using the 1000 Genomes Project data, SNP imputation, variations between populations, and phylogenetic conservation to identify a hitherto unreported association between HbF expression and a 3-bp deletion polymorphism within the HMIP. Chromatin immunoprecipitation (ChIP) assays revealed binding of erythropoiesis-related transcription factors near the polymorphism. The DNA fragment surrounding the 3-bp deletion polymorphism has enhancer-like activity. These findings indicate that this 3-bp deletion polymorphism is most probably the functional motif accounting for HMIP modulation of HbF.
Methods
Study subjects
Chinese β-thalassemia adult carriers were parents of β-thalassemia major or intermedia patients at the Queen Mary, Prince of Wales, Tuen Mun, Queen Elizabeth, and Princess Margaret Hospitals in Hong Kong. All subjects signed informed consent in accordance with the Declaration of Helsinki. Identifying information was removed from data files prepared for analyses. An additional 300 archived genomic DNA samples from unrelated adult β-thalassemia heterozygotes at the Queen Mary Hospital were also studied. This research was approved by the Institutional Review Boards of the Boston University School of Medicine and each of the 5 hospitals in Hong Kong.12
African American subjects with sickle cell anemia were from the Cooperative Study of Sickle Cell Disease as reported by Solovieff et al.11
Hematology and hemoglobin analyses
Peripheral blood samples anticoagulated with ethylenediaminetetraacetic acid were delivered within 1 day after phlebotomy to the Division of Hematology, Department of Pathology, Queen Mary Hospital for clinical laboratory testing.12 F-cell measurement by flow cytometry based on detection by anti-Hb F antibody was previously described.12
Globin genotyping
Genomic DNA was extracted from peripheral blood leukocytes. β-Thalassemia mutations and XmnI polymorphism in HBG2 promoter were determined as described.12
SNP genotyping and quality control procedures
Genome-wide genotyping was performed on 659 subjects using the Illumina Human 610-Quad BeadChip array according to the Illumina Infinium II Assay protocol. A total of 500 ng of genomic DNA was used per sample. Genotypes were called by the Illumina BeadStudio Genotyping Module using Illumina supplied predetermined clusters for each SNP.
Quality checks on the 582 539 genotyped SNPs completed on 659 subjects were performed to identify SNP call rates < 95%, SNPs with a minor allele frequency (MAF) < 1%, and SNPs not in Hardy-Weinberg equilibrium at a cut-off of P < .001. These procedures identified 17 282 SNPs with call rates below the threshold, 85 091 SNPs with a low MAF, and 5797 SNPs not in Hardy-Weinberg equilibrium. These 108 170 SNPs were excluded from the analysis. In addition, 32 persons with missing data for > 8% of the SNPs and 8 persons with extreme HbF values were excluded. The latter included subjects who were heterozygous for (δβ)0-thalassemia, (Aγδβ)0-thalassemia, or HPFH deletions. For the remaining 619 subjects, the total genotyping rate was 99.2%.
Statistical analysis
HbF level was log-transformed to normalize the distribution. Tests for association between SNPs and HbF level were conducted using linear regression models implemented in PLINK, Version 1.05 (www.pngu.mgh.harvard.edu/purcell/plink). Regression models were adjusted for the presence of the promoter nt −28 A > G or nt −29 A > G β+-thalassemia mutations. Using a Bonferroni correction to adjust for multiple testing, a P value cutoff of 1.05 × 10−7 was set to establish genome-wide significance.
SNP imputation and analyses
SNP imputation within the HMIP region was carried out using the software program MACH, Version 1.013,14 together with the 1000 Genomes Project phased data released in August 2009 (www.1000genomes.org). For SNP imputation in Chinese adult β-thalassemia heterozygotes, a reference panel of 120 haplotypes from unrelated HapMap Chinese/Japanese (CHB/JPT) persons was used. For imputation in African Americans with sickle cell anemia, a merged reference panel of 242 haplotypes from unrelated HapMap white and Yoruban (YRI) persons was used. Only those imputed SNPs with an imputation quality of r2 more than 0.30 and MAF more than 0.01 were included for further analyses. Association analyses were performed using linear additive models of the normalized percentage HbF and the imputed allele dosages. Regression analyses were completed with the Im function available in the R software package Version 2.9.2 (cran.r-project.org). Models for the Chinese adult β-thalassemia heterozygotes were adjusted for the presence of promoter nt −28 and −29 β+-thalassemia mutations.
Amplification refractory mutation system test for the 3-bp deletion
A bidirectional polymerase chain reaction (PCR) test using 2 pairs of allele-specific oligonucleotide primers was designed to detect the TAC 3-bp deletion on chromosome 6q23. The primer pairs were TAC-1 5′-TCACTCTGGACAGCAGATGTTACTAT-3′; and TAC-2, 5′-CTCAGTGATGGTATTTCTGGAGAC-3′ to detect the sequence with intact TAC with a PCR product of 207 bp; TAC-3, 5′-AGCCCGTCCAGACACTCATTGTT-3′; and TAC-4, 5′-GCCCTGATAACATTTTGTGGTTTTCATTTAACAT-3′ to detect the sequence with the 3-bp deletion with a PCR product of 276 bp.
PCR reaction was carried out in 10 μL, containing 50 ng of target DNA; 5 μL of multiplex PCR master mix (QIAGEN), 1 μL of Q solution, 0.5 μL of each primer (125nM TAC-1, 500nM TAC-2, 62.5nM TAC-3, 500nM TAC-4), using GeneAmp PCR System 9700 (Applied Biosystems) at 98°C for 15 minutes, followed by 30 cycles of 98°C for 20 seconds, 58°C for 40 seconds, and 72°C for 40 seconds, and ended with the last cycle of 72°C for 10 minutes. A 5-μL aliquot of the PCR reaction was electrophoresed on 2% agarose gel.
Test for phylogenetic conservation
Nucleotide sequences were examined for conservation with 44 vertebrate species using the Phylop and PhastCon software analyses available through the University of California Santa Cruz (UCSC) Genome Browser tracks.15 The references and documentation for the software used to generate these tracks for the UCSC Genome browser are found at compgen.bscb.cornell.edu/phast.
ChIP assay
The SimpleChIP Enzymatic Chromatin IP Kit with magnetic beads (Cell Signaling Technology) was used according to the manufacturer's protocol. Briefly, K562 cells were treated with formaldehyde (final concentration 1%) for 10 minutes at room temperature. Glycine (final concentration 125mM) was added to quench cross-linking. Cells were washed with ice-cold phosphate-buffered saline, pelleted by centrifugation at 4°C, and were lysed (buffer A) for 10 minutes on ice. Nuclei released were resuspended in ice-cold buffer B and digested with micrococcal nuclease (5 μL, 2000 gel units/μL) for 20 minutes at 37°C. The enzyme reaction was stopped by ethylenediaminetetraacetic acid, and the sheared chromatin was collected by centrifugation for 10 minutes at 4°C. A total of 5 μg of antibodies each against TAL1 (04-123, clone BTL73, Millipore), E47 (554077, clone G127-32, BD Biosciences), GATA-1 (SC-266, clone N1, Santa Cruz Biotechnology), GATA-2 (MAB2046, clone 527530, R&D systems), RUNX1 (ab23980, polyclonal, Abcam), and histone H3 (D2B12, Cell Signaling Technology) as well as normal IgG control were used for immunoprecipitation with 10 μg of sheared chromatin.
After overnight incubation at 4°C, ChIP-grade protein G magnetic beads were added and incubated for 2 hours at 4°C. Protein G magnetic bead pellet was washed with low- and high-salt ChIP buffer. Cross-linking was reversed, and chromatin DNA was eluted with DNA elution buffer. Real-time PCR with Power SYBR Green PCR Master Mix (Applied Biosystems) using primer set, forward (5′-ATTCACTCTGGACAGCAGATGTTA-3′) from chromosome 6, 135 460 304 to 135 460 327 and reverse (5′-CCAGTAAGTGTCTTCTGAGGGAAC-3′) from chromosome 6, 135 460 383 to 135 460 360 was performed. The amount of immunoprecipitated DNA in each sample was determined as a fraction relative to input chromatin in percentages. The assay with histone H3 antibody served as a positive control using SimpleChIP Human RPL30 Exon 3 primer set.
For ChIP assays at a region approximately 1.5 kb centromeric to the 3-bp deletion polymorphism, the primer sets are forward (5′-GTGTCTCACACACTGAGGACACTA-3′) from chromosome 6, 135 458 859 to 135 458 882 and reverse (5′-CTGTAGGCACAGAGATTGAAGAGG-3′) from chromosome 6, 135 458 966 to 135 458 943.
For ChIP assays at a region approximately 2.5 kb telomeric to the 3-bp deletion polymorphism, the primer sets are forward (5′-CTGGAGTACAGTGGTGTGATCTTG-3′) from chromosome 6, 135 462 802 to 135 462 825 and reverse (5′-CACACCTGTAATCCCAGCTACTTG-3′) from chromosome 6, 135 462 903 to 135 462 880.
Cell culture, plasmid constructs, and site-directed mutagenesis
Culture of human erythroleukemia K562 cells and plasmid construct of luciferase reporter driven by 1.4 kb human HBG2 globin gene promoter (pGL3-1383/+49 Gγ/Luc) were previously described.16 The 61-bp wild-type and 3-bp deletion fragments (see Figure 3A) were generated by PCR and cloned into the Kpn I and Spe I sites of the pGL3-1383/+49 Gγ/Luc (upstream of the 1.4 kb HBG2 globin gene promoter): the pGl3-WT-1383/+49 Gγ/Luc and pGL3-Del-1383/+49 Gγ/Luc plasmids. These constructs were sequenced to confirm their nucleotide fidelity. Mutations in the E-box (from CAGATG to TTTTAT), 5′-GATA (from TATC to AAAA), RUNX1 binding (from AACCACA to TTTTTTT), and 3′-GATA (from TATC to AAAA) binding sites of 61 bp WT fragment were introduced into either plasmid using the QuikChange II site-directed mutagenesis kit (Stratagene) according to the manufacturer's protocols. All mutations were verified by nucleotide sequence analysis.
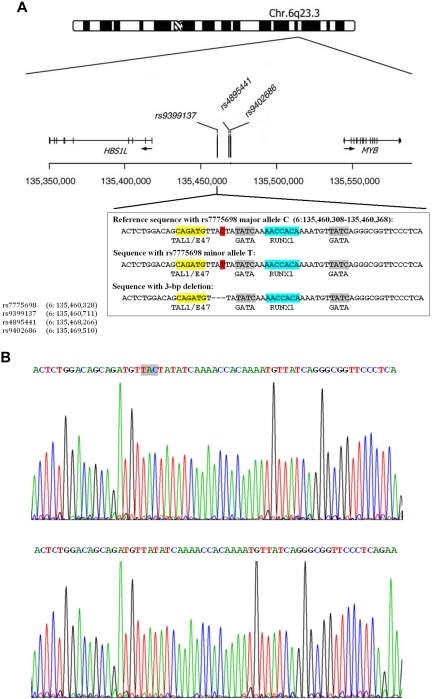
Figure 3.
The 3-bp deletion in the HMP on chromosome 6q23. (A) Upper figure: Chromosome 6q23 HMIP is adapted from Thein et al.2 The 3 SNPs shown represent those with the strongest association signal in different studies: rs9399137,5,10,19 rs4895441,6,10 and rs9402686.19 Below are shown nucleotide sequences between chromosome 6: 135 460 308 to 135 460 368 bp, and transcription factor binding motifs: a, sequences with the rs7775698 major allele C in red; b, sequences with the rs7775698 minor allele T in red; and c, sequences with the 3-bp deletion. Yellow represents sequence that is the TAL1/SCL and E47 binding motif; gray, the GATA binding motif; and blue, the RUNX1 binding motif. The locations of the 4 SNPs on chromosome 6 are shown on the lower left hand corner. (B) Upper figure: Nucleotide sequences surrounding rs7775698 in a Chinese β-thalassemia heterozygote who is homozygous for the rs9399137 major allele, T/T. This person is also homozygous for having intact TAC without deletion. Lower figure: Nucleotide sequences surrounding rs7775698 in a Chinese β-thalassemia heterozygote who is homozygous for the rs9399137 minor allele, C/C. This person is also homozygous for the TAC 3-bp deletion.
Transient transfection and luciferase assays
K562 cells were seeded at 1.0 to 2.0 × 105 cells/well in 24-well tissue culture plates. The transfection and luciferase assay were as previously described,17 except that 0.25 μg of plasmid DNA was used in each transfection.
Results
Genome-wide SNP association study
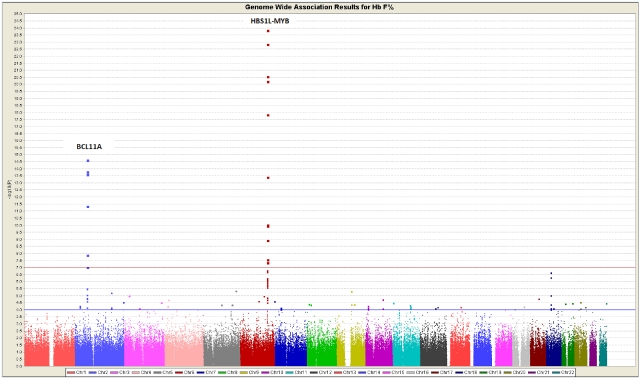
A GWAS for HbF level was performed among 619 unrelated Chinese adult β-thalassemia heterozygotes, and the results are shown in the Manhattan plot (Figure 1). The highly significant SNPs in HMIP and BCL11A are tabulated in supplemental Table 1 (available on the Blood Web site; see the Supplemental Materials link at the top of the online article). The most significant SNP in HMIP was rs9399137 (P = 1.39 × 10−24) and in BCL11A was rs766432 (P = 2.40 × −15).18 SNP rs7482144 was not interrogated by the Illumina Human 610-Quad BeadChip array and was not analyzed in this GWAS. The linkage disequilibrium (LD) plots for the HMIP on chromosome 6q23 and BCL11A region on chromosome 2p16 generated using Haploview Version 3.2 are shown in supplemental Figure 1A and B.
Figure 1.
Manhattan plot showing the GWAS results of 619 unrelated adult β-thalassemia heterozygotes in Hong Kong, using the additive model. The association was with normalized HbF expressed as percentage determined by HPLC.
Effect of BCL11A and HMIP on HbF
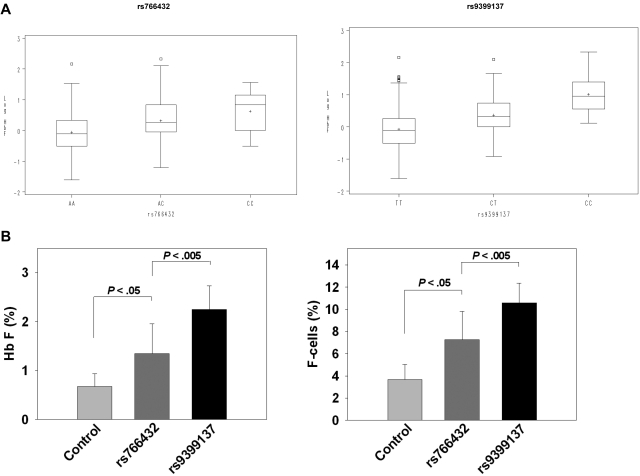
The distribution of log-transformed HbF values according to SNP genotypes for rs766432 in BCL11A and rs9399137 in HMIP are shown in box plots (Figure 2A). For both SNPs, homozygotes for the major allele have the lowest median HbF, whereas homozygotes for the minor allele have the highest median HbF. The variance in HbF level because of rs766432 was 6.4%; that because of rs9399137 was 13.5%. Tests to detect interaction between these 2 SNPs were not significant (P > .05), suggesting that their effects on HbF are independent.
Figure 2.
Effect of BCL11A and HMIP QTL upon HbF and F cells. (A) Box plots showing the complete distribution of HbF in 619 Chinese β-thalassemia heterozygotes, expressed as percentage in log scale on the y-axis. Each rectangle represents the data between the 25th and 75th quartiles, and the bar in each rectangle is the median value for the HbF in log scale. rs766432: AA, AC, and CC represent the SNP genotypes for rs766432 in BCL11A on chromosome 2p16. rs9399137: TT, CT, and CC represent the SNP genotypes for rs9399137 in HMIP on chromosome 6q23. (B) Effect of the 3 major HbF QTLs on HbF and F-cell levels in adult β-thalassemia heterozygotes who had HbF and F cells determined. Data are mean ± SD. Control indicates 80 subjects (age, 48.3 ± 9.0 years) who are homozygous for the major alleles for rs7482144 in HBG2 promoter, rs766432 in BCL11A, and rs9399137 in HMIP. rs766432: 8 subjects (age 46.9 ± 9.6 years) with identical HbF QTL genotypes as those in control, except that they are homozygous for the minor allele for rs766432 in BCL11A. rs9399137: 6 subjects (age 40.3 ± 13.8 years) with identical HbF QTL genotypes as those in control, except that they are homozygous for the minor allele for rs9399137 in HMIP.
Among subjects in whom both HbF and F cells (erythrocytes containing HbF) were determined, 80 were found to be homozygotes for the major alleles of SNPs in all 3 major QTLs (labeled as control in Figure 2B). Heterozygotes for the promoter nt −28 and −29 β+-thalassemia mutations were excluded because these mutations are known to up-regulate HbF and F cells.12 Homozygotes for the minor allele of rs766432 in BCL11A had twice the HbF and F cells compared with control. Homozygotes for the minor allele of rs9399137 in HMIP had even higher HbF and F cells (Figure 2B).
Mining data from the 1000 Genomes Project
To identify the functional variant in HMIP that accounts for its association with HbF expression, we first interrogated the 1000 Genomes Project database (www.1000genomes.org) for all known polymorphisms within HMIP region spanning approximately 126 kb from chromosome 6: 135 417 715 to 135 544 146 bp.4 In the 59 CHB/JPT, 57 CEU, and 56 YRI HapMap samples, which were sequenced for the 1000 Genomes Project, there were 478 SNPs in the CHB/JPT, 323 in the CEU, and 633 in the YRI samples. Of the SNPs found in CHB/JPT and CEU subjects, 235 were present in both populations. Twenty-seven of these are in strong LD (Pearson correlation r2 > 0.54) with rs9399137 (supplemental Table 2).
We searched for insertion or deletion polymorphisms in the HMIP-2 block, approximately 24 kb from chromosome 6: 135 452 921 to 135 477 194,4 in the 1000 Genomes Project database and found a TAC 3-bp deletion between chromosome 6: 135 460 326 and 135 460 328 (Figure 3A) that is in very strong LD with rs9399137 in Chinese, Europeans, and African populations. SNP rs7775698 is located at chromosome 6: 135 460 328 bp, with C/T being its major/minor alleles, respectively. In Chinese and European populations, the T allele as determined by the probe for rs7775698 primarily tags the 3-bp deletion (supplemental Table 3). In African populations, the T allele only very occasionally tags the 3-bp deletion. In contrast, the T allele in African populations primarily tags an ancestral sequence containing rs7775698 (T) allele without the 3-bp deletion (supplemental Table 3). This rs7775698 (T) allele without the 3-bp deletion found in African populations is also the reference allele found in several simian primates, such as chimpanzee, gorilla, orangutan, and Rhesus monkey. The frequency of this ancestral sequence in African populations is comparable with the frequency of the sequence with the 3-bp deletion found in Chinese and European populations.
The rs7775698(T)-rs9399137(C) haplotype that tags the 3-bp deletion is very common in both the CHB and CEU 1000 Genomes Project samples with frequency of 31.5% and 21.4%, respectively. In the YRI population, this 3-bp deletion haplotype is much less common with a frequency of 5.1%. To examine the difference between non-African and African populations, we calculated the r2 between rs7775698 and rs9399137 in the 11 HapMap populations. All the non-African populations had a mean r2 (0.94) much higher than that in African populations (0.17) (supplemental Table 4). The frequency of the rs7775698(T)-rs9399137(C) haplotype is 0.23 in non-African HapMap populations but only 0.05 in African HapMap populations.
The difference in the r2 is the result of the presence of the ancestral sequence containing rs7775698 (T) without the 3-bp deletion that is present in 17% of the African populations but is rarely found in non-African populations (< 0.01). The rs9399137 (C) tags the sequence containing rs7775698 (T) with the 3-bp deletion in the Chinese and European populations examined. As a result of this LD pattern between rs9399137 (C) and the 3-bp deletion, the deletion is as highly significantly associated with HbF levels as the nearby (383 bp) rs9399137 (C) allele (see “Confirmation of the 3-bp deletion in study cohort”).
Confirmation of the 3-bp deletion in study cohort
To determine whether our Chinese study cohort had the 3-bp deletion in LD with rs9399137, we sequenced this region in 36 subjects who were homozygous for either the major allele T (n = 16) or minor allele C (n = 20) at rs9399137. All 16 T/T homozygotes are homozygous for having intact TAC in their nucleotide sequences (Figure 3B upper figure), and their HbF was 0.85% ± 0.70%. All 20 C/C homozygotes are homozygous for the TAC 3-bp deletion (Figure 3B lower figure), and their HbF was 3.24% ± 2.09%.
A PCR-based amplification refractory mutation system test capable of detecting homozygosity, heterozygosity, and wild-type for the 3-bp deletion was designed. This was applied to 335 unrelated Chinese β-thalassemia heterozygotes. There were 17 homozygotes for the 3-bp deletion, 115 heterozygotes, and 203 wild-type. The 3-bp deletion frequency is 0.22. Furthermore, the 3-bp deletion is in complete LD with rs9399137, the SNP found in GWAS to be most significantly associated with HbF. Both polymorphisms were equally associated with HbF with an identical P value of 1.0 × 10−20 among these 335 subjects.
Evidence for the functionality of the TAC 3-bp deletion
1. Comparison of association results between populations.
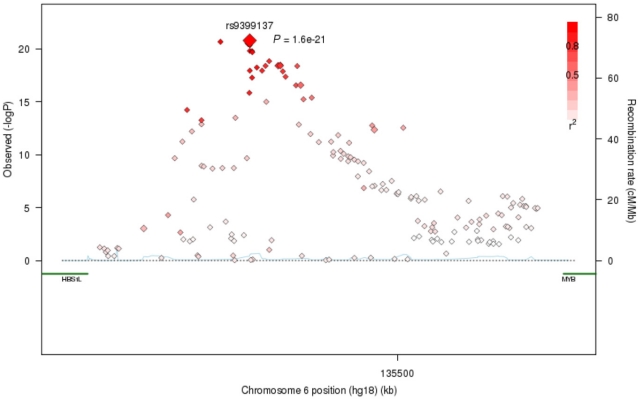
Under the assumption that the functional variant should be the most strongly associated with HbF in all populations, we sought to leverage the cross-population differences in LD patterns and allele frequencies in the region to narrow the field of candidates. We imputed SNPs in our study cohort of 619 Chinese β-thalassemia heterozygotes and in 848 African Americans with sickle cell anemia11 using 1000 Genomes Project haplotype reference panels. Results of the association analyses of the imputed SNPs with HbF in Chinese subjects are shown in Figure 4. The most significant SNP is rs9399137 (P = 7.34 × 10−24). All other SNPs with significant HbF association are also in LD with rs9399137.
Figure 4.
Association of genotyped and imputed SNPs throughout the HMIP region on chromosome 6q23 with HbF levels among 619 adult Chinese β-thalassemia heterozygotes.
A total of 27 genotyped and imputed SNPs were found to be in strong LD with rs9399137 and highly associated with HbF in the Chinese β-thalassemia heterozygotes (supplemental Table 2). Among African Americans, 14 of these SNPs have MAFs greater than 0.17 yet are only weakly associated with HbF, with rs4895440 being the most significant (P = 4.93 × 10−4). One other SNP (chromosome 6q23 135 474 996 bp) is monomorphic in African Americans. Given the weak association results with these SNPs and the fact that these SNPs had significantly high minor allele frequencies to afford sufficient power to detect association with HbF if present, these 15 SNPs were excluded from consideration as probably functional motifs. Two more SNPs (rs9494145 and rs9483788) were also removed from consideration because they were substantially less strongly associated with HbF (P = 1.73 × 10−18 and 4.59 × 10−16, respectively) than rs9399137 (P = 7.34 × 10−24) in the Chinese samples. The remaining 10 SNPs and the 3-bp deletion polymorphism merit further study in the search for the functional variant (supplemental Table 5).
2. Transcription factor binding sites and phylogenetic conservation.
We next assessed the nucleotide sequences near each of the 11 polymorphisms for potential transcription factor binding sites using the transcription factor binding site search program ConSite (www.consite.genereg.net). The transcription factor binding site scores are based on a profile model that uses position specific weight matrices to assign a score to the candidate sequences in conserved regions. The results are presented in supplemental Table 5. The region flanking the 3-bp deletion is of particular importance because it provides a potential platform for the binding of 4 transcriptional factors known to be essential for erythroid cell differentiation and hemopoiesis: TAL1/SCL, E47, GATA, and RUNX1/AML1 (Figure 3A).
The sequences flanking the 3-bp deletion polymorphism are highly conserved among 44 vertebrate species with log odds conserved element scores of 64 and 84 (supplemental Figure 2). Sequences near rs11154792 are conserved with a lesser log odds score of 17. Sequence conservation was not found in regions near the other variants.
3. Transcription factor binding.
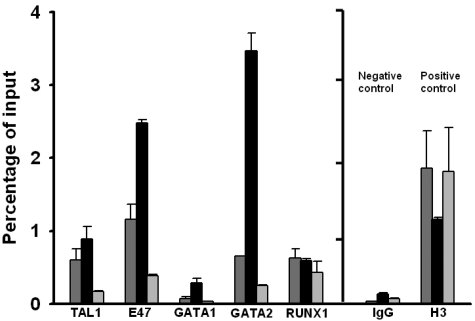
Based on nucleotide sequencing results, K562 cells are heterozygous for the 3-bp deletion polymorphism. ChIP assay using K562 cells revealed that GATA-2 binds to the immediate vicinity of the 3-bp deletion polymorphism, the signal being 5- to 15-fold higher compared with binding at sites 1.5 kb telomeric or 2.5 kb centromeric to the 3-bp deletion polymorphism, respectively (Figure 5; supplemental Figure 3). The signal for GATA-1 binding, if present at all, is much less than GATA-2. These results are consistent with a published report on ChIP-seq experiments on genome-wide GATA binding site occupancy.20 One GATA-2 ChIP-seq peak, but not GATA-1, was located between chromosome 6: 135 460 050 to 135 460 379, which encompasses the 3-bp deletion polymorphism under study at chromosome 6: 135 460 326 to 135 460 328. No GATA ChIP-seq peak is found near the rs9399137 located at chromosome 6: 135 460 711. (See also ENCODE data later in Results.)
Figure 5.
ChIP assay. Relative real-time PCR signal intensities of the ChIP assay, showing significant binding of TAL1, E47, GATA2, and RUNX1 near the 3-bp deletion polymorphism.  represents transcription factor binding signals in the immediate vicinity of the 3-bp deletion polymorphism;
represents transcription factor binding signals in the immediate vicinity of the 3-bp deletion polymorphism;  binding signals at approximately 1.5 kb centromeric to the 3-bp deletion polymorphism; and
binding signals at approximately 1.5 kb centromeric to the 3-bp deletion polymorphism; and  binding signals at approximately 2.5 kb telomeric to the 3-bp deletion polymorphism. IgG indicates negative control using IgG for immunoprecipitation; H3, positive control using antibody against histone H3; and RT-PCR, using exon 3 primer set.
binding signals at approximately 2.5 kb telomeric to the 3-bp deletion polymorphism. IgG indicates negative control using IgG for immunoprecipitation; H3, positive control using antibody against histone H3; and RT-PCR, using exon 3 primer set.
Significant binding of TAL1, E47, and RUNX1 to the DNA region surrounding the 3-bp deletion polymorphism is also shown by ChIP assays in K562 cells (Figure 5). E47 binding is 2- to 7-fold higher compared with binding signal at sites 1.5 kb telomeric or 2.5 kb centromeric to the 3-bp deletion polymorphism. Less difference is observed with the TAL1 binding. No difference is observed with the RUNX1 binding.
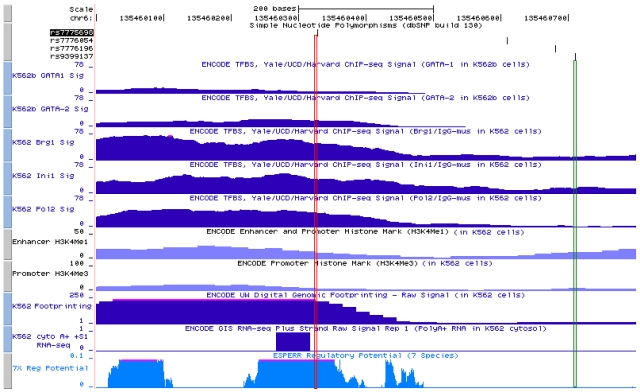
The ENCODE datasets provide a rich resource of experimental results showing potential regulatory regions throughout the genome.21 Figure 6 shows that, within the immediate vicinity of the 3-bp deletion polymorphism in K562 cells, ChIP-seq experiments revealed GATA-2 occupancy, less so for GATA-1, and occupancy of BRG1, INI1 also known as BAF47, and RNA polymerase II. Present also are the digital DNase genomic footprinting and RNA transcripts.22,23 There is a strong signal for ESPERR regulatory potential in this region among several species.24 In contrast, these transcription regulatory markers are either absent or present at a low level in the immediate vicinity of rs9399137 (Figure 6). The ChIP-seq signals for histones H3K4Me1 and H3K4Me3 in the vicinity of the 3-bp deletion polymorphism and rs9399137 region are relatively low, and there appears to be no significant enrichment between these 2 sites.
Figure 6.
ENCODE data display beginning from the top row down of ChIP-seq signals in K562 cells on occupancy of GATA-1, GATA-2, BRG1, Ini1, RNA polymerase 2, and histones H3K4Me1 and H3K4Me3. Also shown is genomic footprinting, RNA transcript22,23 both also in K562 cells and ESFERR regulatory potential,24 among different species. The vertical red lines represent the site of the 3-bp deletion polymorphism (chromosome 6: 135 460 326-135 460 328), which is in complete LD with rs9399137. The green lines represent the site of rs9399137 (chromosome 6: 135 460 711), the SNP that was found in multiple GWASs to have the most significant P value in HbF association. These ENCODE data were generated by the ENCODE consortium and are available on the Genome Browser at UCSC.21 The GATA-1, GATA-2, BRG1, Ini1, and RNA Polymerase II ChIP-Seq data were generated by the laboratories of M. Snyder, M. Gerstein, and S. Weissman at Yale; P.J. Farnham at the University of California-Davis; and K. Struhl at Harvard. The H3K4Me1 and H3K4Me3 data were generated by the Bernstein Laboratory at the Broad Institute. The Digital Genomic Footprinting data were generated by the University of Washington ENCODE group. The GIS RNA-seq data were generated by the Genome Institute of Singapore.
4. Enhancer-like activity.
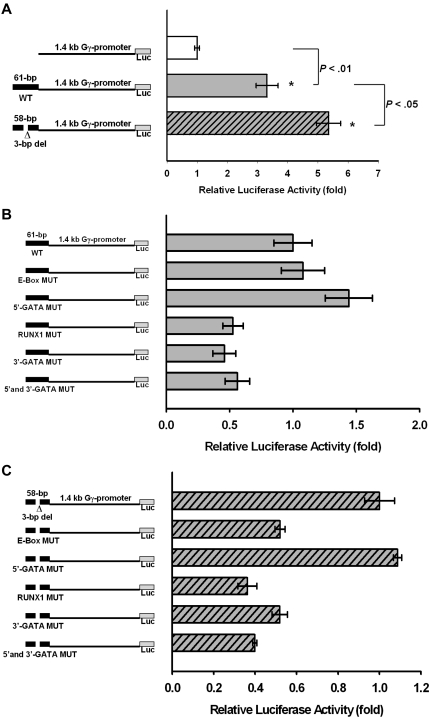
In an initial attempt to demonstrate functionality of the DNA fragment surrounding the 3-bp deletion polymorphism, DNA fragment either without or with the 3-bp deletion (Figure 3A) was ligated to an expression vector consisting of 1.4 kb HBG2 proximal promoter and luciferase reporter gene. These were transiently transfected into K562 cells, and the luciferase activity was measured after 48-hour cell culture.
The 61-bp DNA fragment without the 3-bp deletion enhances the HBG2 promoter transcriptional activity by 3.4-fold, compared with the plasmid without the 61-bp DNA fragment (Figure 7A). The 58-bp DNA fragment with the 3-bp deletion enhances the HBG2 promoter transcriptional activity by 5.4-fold, compared with the plasmid without the 58-bp DNA fragment (Figure 7A).
Figure 7.
Enhancer-like activity of the DNA fragment encompassing the 3-bp deletion. (A) Enhancer-like activity of the 61-bp DNA fragment surrounding the 3-bp deletion polymorphism on HBG2 proximal promoter linked to a luciferase expression vector. The luciferase activity of the plasmid without the 61-bp DNA fragment was normalized to be 1.0. The 61-bp DNA fragment enhances the HBG2 promoter activity to 3.4-fold of the control. The 58-bp DNA fragment with the 3-bp deletion enhances the HBG2 promoter activity to 5.4-fold of the control. (B) Effect of mutation of transcription factor binding sites in the 61-bp DNA fragment on its enhancer-like activity. The luciferase activity of the plasmid with the 61-bp DNA fragment was normalized to be 1.0. Mutation of either RUNX1 or 3′ GATA binding site down-regulates the enhancer-like activity to 50%. Mutation of the E-box or 5′ GATA binding site does not decrease the enhancer-like activity of the DNA fragment. (C) Effect of mutation of transcription factor binding sites in the 58-bp DNA fragment with the 3-bp deletion on its enhancer-like activity. The luciferase activity of the plasmid with the 58-bp DNA fragment was normalized to be 1.0. Mutation of the E-box, RUNX1, or 3′ GATA binding site down-regulates the enhancer-like activity to approximately 50%. Mutation of the 5′ GATA binding site does not decrease the enhancer-like activity of the DNA fragment.
The enhancer-like activity of the 61-bp DNA fragment is dependent on intact RUNX1 and 3′ GATA binding sites. Mutation of either of these 2 binding sites down-regulates the enhancer-like activity of the DNA fragment to approximately half of the intact and unmutated DNA fragment (Figure 7B). Mutation of the E-box or the 5′ GATA binding site does not negatively perturb the enhancer-like activity (Figure 7B). Mutation of both GATA sites results in lower enhancer-like activity, suggesting that the 3′ GATA site rather than the 5′ GATA site has an important role in modulating the enhancer-like activity (Figure 7B). The 3′ GATA site (TGATAA) matches well with the WGATAR sequence found most commonly in the DNA segments occupied by GATA in erythroid cells. On the other hand, the 5′ GATA site (TGATAT) is not as good a match to the consensus sequence.
The enhancer-like activity of the 58-bp DNA fragment with the 3-bp deletion is dependent on intact E- box, RUNX1, and 3′ GATA binding sites. Mutation of any of the 3 binding sites significantly down-regulates the enhancer-like activity of the DNA fragment to approximately half of the intact and unmutated DNA fragment with the 3-bp deletion (Figure 7C).
Discussion
The HbF QTL on chromosome 6q23 was first identified in an Asian Indian family segregating with β-thalassemia and hereditary persistence of fetal hemoglobin.25 This locus is now localized within HMIP.4 HMIP also impacts on erythrocyte, platelet, monocyte numbers, and erythrocyte parameters, such as hematocrit, mean cell volume, mean cell hemoglobin, and mean cell hemoglobin concentration.26–28
HMIP and the BCL11A polymorphism are highly associated with HbF in Chinese adult β-thalassemia carriers.18,29 Although GWASs have robustly identified genes and loci associated with diseases or phenotypes, identification of responsible functional variants has proven difficult. The 1000 Genomes Project (www.1000genomes.org) provides a large number of publicly available genomic sequences from different populations that can be interrogated to identify common and rare variants within haplotype blocks of interest.30,31 Lists of these candidate variants can be further refined based on bioinformatics and variations among populations. Using this strategy, we identified a hitherto unreported association between HbF expression and a 3-bp deletion polymorphism in HMIP. A recent study resequenced 32.8 kb of the HbF QTL from chromosome 6: 135 460 328 to 135 493 110, and did not report this 3-bp deletion, which is located on chromosome 6: 135 460 326 to 135 460 328.32
We first interrogated the 1000 Genomes Project database for polymorphisms within HMIP in high LD with rs9399137. This SNP is most significantly associated with HbF in Chinese β-thalassemia heterozygotes (supplemental Table 1). It tags the HMIP-2 block, which is strongly associated with HbF and F-cell numbers among Europeans.4,5 This SNP is also associated with HbF among sickle cell anemia patients of African descent,10,11,19,33 although less significantly compared with the other 2 populations because of much lower minor allele frequencies. We hypothesized that the functional variant should be in high LD with rs9399137 in all 3 populations.
The differences in HbF association for variants with comparable allele frequencies between Chinese and blacks can be used to filter out unlikely functional variant candidates (supplemental Table 2). As an example, the minor allele frequency of rs7776054 (chromosome 6: 135 460 609) among the African Americans is 0.25. If this SNP were the functional motif, it should have a highly significant HbF association P value, but its P value is only 3.8 × 10−2 (supplemental Table 2). Therefore, this SNP can be eliminated for consideration of being a possible functional SNP, even though it is highly associated with HbF (P = 1.5 × 10−23) in the Chinese population.
We identified 10 SNPs and one 3-bp deletion in strong LD (r2 > 0.54) with rs9399137 in the Chinese β-thalassemia heterozygotes (supplemental Table 4) as potential candidates for the functional motif. The region flanking the 3-bp deletion polymorphism contains possible binding sites for 4 essential erythropoiesis- and hemopoiesis-related transcription factors, TAL1/SCL, E47, GATA, and RUNX1/AML1 (Figure 3A; supplemental Table 5). Furthermore, these sequences are phylogenetically conserved among many vertebrate species (supplemental Figure 2).
TAL1/SCL and E47 are basic helix-loop-helix transcription factors34,35 and bind to E-box. The spatial orientation of the E box (CANNTG or CAGATG) and GATA motif is important for enhancing TAL1/SCL binding affinity.36–38 Nearby is a binding motif for RUNX1/AML1: TG(C/T)GGT(C/T).39 These DNA-binding proteins and their coregulators probably interact and form transcriptional complexes that can modulate target gene expression. An erythroid DNA-binding transcriptional complex composing TAL1/SCL, GATA-1, E47, plus LMO2 and LDB1 is known to regulate erythroid differentiation and gene expression.40,41
HMIP is probably an erythroid distal regulatory region previously shown to possess DNase I hypersensitivity, GATA binding, RNA polymerase II interaction, and strong histone acetylation.42 The DNA fragment surrounding the 3-bp deletion is in the midst of the aforementioned markers. We (Figure 5) and Fujiwara et al20 have shown GATA-2 binding within the immediate vicinity of the 3-bp deletion polymorphism. ChIP assays also revealed binding of TAL1, E47, and RUNX1 to the region surrounding the 3-bp deletion polymorphism in K562 cells. Furthermore, the ENCODE ChIP-seq results show that, in the same region, there is GATA-2 binding, occupancy of BRG1, INI1, RNA polymerase 2, and to a lesser extent histones H3K2Me1 and H3K2Me4, as well as footprinting (Figure 6). The presence of 50-bp RNA transcripts near the 3-bp deletion polymorphism is also suggestive of the presence of an enhancer.22,23
We hypothesize that the region surrounding the 3-bp deletion polymorphism has enhancer-like activity, which is substantiated by results of transfection experiments using the HBG2 1.4-kb proximal promoter linked to a luciferase reporter gene. Without the deletion, the DNA fragment enhances the HBG2 promoter activity by 3.4-fold (Figure 7A). With the 3-bp deletion, the promoter activity enhancement increased to 5.4-fold (Figure 7A). Mutating the various transcription factor binding sites led to down-regulation of the enhancer-like activity (Figure 7B-C), indicating that transcription factor binding is necessary for the enhancer-like activity. These are initial attempts to demonstrate functionality of the DNA fragment surrounding the 3-bp deletion polymorphism. Further investigations are needed to ascertain in vivo biologic function of this DNA fragment in primary human adult erythroid cell cultures with or without the 3-bp deletion, and to identify its target genes for the enhancer-like activity.
The 3-bp deletion polymorphism is located at 42.6 kb upstream of HBS1L and 83.8 kb upstream of MYB, near the erythroid specific DNase hypersensitive site 2 within the HMIP-2 block.42 MYB protein can increase erythroid cell proliferation and inhibit cellular differentiation. Overexpression of MYB, but not HBS1L, in human K562 erythroid cells was reported to decrease HBG expression and supports the hypothesis that MYB modulates HbF production by altering erythropoiesis kinetics.43 Down-regulation of MYB expression by shRNAs in adult erythroid cell cultures was reported to result in increased HBG expression.44 On the other hand, HBS1L, but not MYB, expression was found to correlate with HMIP alleles associated with high HbF in primary human adult erythroid cell cultures.4 HBS1L has 4 guanosine triphosphate-binding motifs,45 but its biologic functions are not understood. It remains undetermined whether HMIP QTL modulates HbF production primarily through MYB, HBSIL, both, or neither. Our transfection experiments carried out in K562 cells showed that the 58-bp DNA fragment with the 3-bp deletion, representing the minor allele of chromosome 6q23 QTL, which is associated with elevated HbF, has increased enhancer-like activity. These observations suggest that the function of this HbF QTL is not necessarily mediated through direct transcriptional regulation of MYB. Near the QTL, there is PDE7B coding for a cyclic adenosine 5′-monophosphate-dependent phosphodiesterase which can effect HBG expression and erythroid cell differentiation through cyclic adenosine 5′-monophosphate pathway.46 Located further telomeric is MAP3K5.47 Histone hyperacetylation by activation of p38 MAP kinase pathway is linked to HBG induction.48,49 The presence of RNA transcripts in the vicinity of the 3-bp deletion polymorphism shown by the ENCODE data (Figure 6) needs to be confirmed and explored further for their possible biologic functions. Additional investigations are needed to fully understand the molecular mechanisms and pathways involved in the modulation of HbF by this QTL.
The 3-bp deletion, when present in people of either Chinese, European, or African descent, changes the normal DNA binding configuration of transcription factors and results in possible changes to the spatial orientation for DNA-protein binding and/or protein-protein interactions,50 which might account for the observed up-regulation of the enhancer-like activity. The consistent agreement of the association results across multiple populations, erythropoiesis-related transcription factor binding, phylogenetic conservation, and enhancer-like activity all suggest that the 3-bp deletion polymorphism is probably the most significant functional variant within HMIP accounting for its modulation of HbF production.
Supplementary Material
Acknowledgments
The authors thank Ms Yvonne Chu and Ms Amanda Mok for their effort in subject recruitment in Hong Kong, Ms Stella Tsang for carrying out clinical laboratory testing, and all study subjects who agreed to participate in this research project.
This investigation was supported by National Institute of Diabetes and Digestive and Kidney Diseases (grant RO1 DK069646; D.H.K.C.) and National Heart, Lung, and Blood Institute (grant RO1 HL068970; M.H.S.).
Footnotes
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: J.J.F. and R.M.S. designed research, analyzed the data, and wrote the manuscript; Z.-y.C., H.-y.L., B.F.C., and C.T.B. undertook molecular testing and SNP genotyping, analyzed data, and edited the manuscript; S.Y.H., C.K.L., A.C.W.L., R.C.H.L., C.K.L., H.L.Y., J.C.C.S., E.S.K.M., L.C.C., and V.C. supervised subject recruitment and clinical laboratory testing, analyzed data, and edited the manuscript; and L.A.F., P.S., C.T.B., M.H.S., and D.H.K.C. conceived of and designed research, analyzed data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: David H. K. Chui, Evans 248, Department of Medicine, Boston University School of Medicine, 72 East Concord St, Boston, MA 02118; e-mail: david.chui@bmc.org.
References
- 1.Lau YL, Chan LC, Chan YY, et al. Prevalence and genotypes of alpha- and beta-thalassemia carriers in Hong Kong: implications for population screening. N Engl J Med. 1997;336(18):1298–1301. doi: 10.1056/NEJM199705013361805. [DOI] [PubMed] [Google Scholar]
- 2.Thein SL, Menzel S, Lathrop M, Garner C. Control of fetal hemoglobin: new insights emerging from genomics and clinical implications. Hum Mol Genet. 2009;18(R 2):R216–R223. doi: 10.1093/hmg/ddp401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gilman JG, Huisman THJ. DNA sequence variation associated with elevated fetal Ggamma globin production. Blood. 1985;66(4):783–787. [PubMed] [Google Scholar]
- 4.Thein SL, Menzel S, Peng X, et al. Intergenic variants of HBS1L-MYB are responsible for a major quantitative trait locus on chromosome 6q23 influencing fetal hemoglobin levels in adults. Proc Natl Acad Sci U S A. 2007;104(27):11346–11351. doi: 10.1073/pnas.0611393104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Menzel S, Garner C, Gut I, et al. A QTL influencing F cell production maps to a gene encoding a zinc-finger protein on chromosome 2p15. Nat Genet. 2007;39(10):1197–1199. doi: 10.1038/ng2108. [DOI] [PubMed] [Google Scholar]
- 6.Uda M, Galanello R, Sanna S, et al. Genome-wide association study shows BCL11A associated with persistent fetal hemoglobin and amelioration of the phenotype of beta-thalassemia. Proc Natl Acad Sci U S A. 2008;105(5):1620–1625. doi: 10.1073/pnas.0711566105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Galanello R, Sanna S, Perseu L, et al. Amelioration of Sardinian beta0 thalassemia by genetic modifiers. Blood. 2009;114(18):3935–3937. doi: 10.1182/blood-2009-04-217901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ma Q, Abel K, Sripichai O, et al. beta-globin gene cluster polymorphisms are strongly associated with severity of HbE/beta0-thalassemia. Clin Genet. 2007;72(6):497–505. doi: 10.1111/j.1399-0004.2007.00897.x. [DOI] [PubMed] [Google Scholar]
- 9.Nuinoon M, Makarasara W, Mushiroda T, et al. A genome-wide association identified the common genetic variants influence disease severity in beta0-thalassemia/hemoglobin E. Hum Genet. 2010;127(3):303–314. doi: 10.1007/s00439-009-0770-2. [DOI] [PubMed] [Google Scholar]
- 10.Lettre G, Sankaran VG, Bezerra MA, et al. DNA polymorphisms at the BCL11A, HBS1L-MYB, and beta-globin loci associate with fetal hemoglobin levels and pain crises in sickle cell disease. Proc Natl Acad Sci U S A. 2008;105(33):11869–11874. doi: 10.1073/pnas.0804799105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Solovieff N, Milton JN, Hartley SW, et al. Fetal hemoglobin in sickle cell anemia: genome-wide association studies suggest a regulatory region in the 5′ olfactory receptor gene cluster. Blood. 2010;115(9):1815–1822. doi: 10.1182/blood-2009-08-239517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gibney GT, Panhuysen CI, So JC, et al. Variation and heritability of Hb F and F cells among beta-thalassemia heterozygotes in Hong Kong. Am J Hematol. 2008;83(6):458–464. doi: 10.1002/ajh.21150. [DOI] [PubMed] [Google Scholar]
- 13.Li Y, Willer CJ, Sanna S, Abecasis GR. Genotype imputation. Annu Rev Genomics Hum Genet. 2009;10:387–406. doi: 10.1146/annurev.genom.9.081307.164242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li Y, Willer CJ, Ding J, Scheet P, Abecasis GR. MaCH: using sequences and genotype data to estimate haplotypes and unobserved genotypes. Genet Epidemiol. 2010;34(8):816–834. doi: 10.1002/gepi.20533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rhead B, Karolchik D, Kuhn RM, et al. The UCSC Genome Browser database: update 2010. Nucleic Acids Res. 2010;38:D613–D619. doi: 10.1093/nar/gkp939. (Database issue) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen Z, Luo HY, Basran RK, et al. A T-to-G transversion at nucleotide −567 upstream of HBG2 in a GATA-1 binding motif is associated with elevated hemoglobin F. Mol Cell Biol. 2008;28(13):4386–4393. doi: 10.1128/MCB.00071-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen Z, Luo HY, Steinberg MH, Chui DHK. BCL11A represses HBG transcription in K562 cells. Blood Cells Mol Dis. 2009;42(2):144–149. doi: 10.1016/j.bcmd.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 18.Sedgewick AE, Timofeev N, Sebastiani P, et al. BCL11A is a major HbF quantitative trait locus in three different populations with beta-hemoglobinopathies. Blood Cells Mol Dis. 2008;41(3):255–258. doi: 10.1016/j.bcmd.2008.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Creary LE, Ulug P, Menzel S, et al. Genetic variation on chromosome 6 influences F cell levels in healthy individuals of African descent and HbF levels in sickle cell patients. PLoS ONE. 2009;4(1):e4218. doi: 10.1371/journal.pone.0004218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fujiwara T, O'Geen H, Keles S, et al. Discovering hematopoietic mechanisms through genome-wide analysis of GATA factor chromatin occupancy. Mol Cell. 2009;36(4):667–681. doi: 10.1016/j.molcel.2009.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rosenbloom KR, Dreszer TR, Pheasant M, et al. ENCODE whole-genome data in the UCSC Genome Browser. Nucleic Acids Res. 2009;38:D620–D625. doi: 10.1093/nar/gkp961. (Database issue) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ren B. Transcription: enhancers make non-coding RNA. Nature. 2010;465(7295):173–174. doi: 10.1038/465173a. [DOI] [PubMed] [Google Scholar]
- 23.Kim TK, Hemberg M, Gray JM, et al. Widespread transcription at neuronal activity-regulated enhancers. Nature. 2010;465(7295):182–187. doi: 10.1038/nature09033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Taylor J, Tyekucheva S, King DC, Hardison RC, Miller W, Chiaromonte F. ESPERR: learning strong and weak signals in genomic sequence alignments to identify functional elements. Genome Res. 2006;16(12):1596–1604. doi: 10.1101/gr.4537706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Garner C, Mitchell J, Hatzis T, Reittie J, Farrall M, Thein SL. Haplotype mapping of a major quantitative-trait locus for fetal hemoglobin production, on chromosome 6q23. Am J Hum Genet. 1998;62(6):1468–1474. doi: 10.1086/301859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Menzel S, Jiang J, Silver N, et al. The HBS1L-MYB intergenic region on chromosome 6q23. 3 influences erythrocyte, platelet, and monocyte counts in humans. Blood. 2007;110(10):3624–3626. doi: 10.1182/blood-2007-05-093419. [DOI] [PubMed] [Google Scholar]
- 27.Soranzo N, Spector TD, Mangino M, et al. A genome-wide meta-analysis identifies 22 loci associated with eight hematological parameters in the HaemGen consortium. Nat Genet. 2009;41(11):1182–1190. doi: 10.1038/ng.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ganesh SK, Zakai NA, van Rooij FJ, et al. Multiple loci influence erythrocyte phenotypes in the CHARGE Consortium. Nat Genet. 2009;41(11):1191–1198. doi: 10.1038/ng.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.So CC, Song YQ, Tsang ST, et al. The HBS1L-MYB intergenic region on chromosome 6q23 is a quantitative trait locus controlling fetal haemoglobin level in carriers of β-thalassaemia. J Med Genet. 2008;45(11):745–751. doi: 10.1136/jmg.2008.060335. [DOI] [PubMed] [Google Scholar]
- 30.Durbin RM, Abecasis GR, Altshuler DL, et al. A map of human genome variation from population-scale sequencing. Nature. 2010;467(7319):1061–1073. doi: 10.1038/nature09534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pennis E. Genomics: 1000 Genomes Project gives new map of genetic diversity. Science. 2010;330(6004):574–575. doi: 10.1126/science.330.6004.574. [DOI] [PubMed] [Google Scholar]
- 32.Galarneau G, Palmer CD, Sankaran VG, Orkin SH, Hirschhorn JN, Lettre G. Fine-mapping at three loci known to affect fetal hemoglobin levels explains additional genetic variation. Nat Genet. 2010;42(12):1049–1051. doi: 10.1038/ng.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Makani J, Menzel S, Nkya S, et al. Genetics of fetal hemoglobin in Tanzanian and British patients with sickle cell anemia. Blood. 2011;117(4):1390–1392. doi: 10.1182/blood-2010-08-302703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Porcher C, Swat W, Rockwell K, Fujiwara Y, Alt FW, Orkin SH. The T cell leukemia oncoprotein SCL/tal-1 is essential for development of all hematopoietic lineages. Cell. 1996;86(1):47–57. doi: 10.1016/s0092-8674(00)80076-8. [DOI] [PubMed] [Google Scholar]
- 35.Kassouf MT, Chagraoui H, Vyas P, Porcher C. Differential use of SCL/TAL-1 DNA-binding domain in developmental hematopoiesis. Blood. 2008;112(4):1056–1067. doi: 10.1182/blood-2007-12-128900. [DOI] [PubMed] [Google Scholar]
- 36.Hsu HL, Huang L, Tsan JT, et al. Preferred sequences for DNA recognition by the TAL1 helix-loop-helix proteins. Mol Cell Biol. 1994;14(2):1256–1265. doi: 10.1128/mcb.14.2.1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cohen-Kaminsky S, Maouche-Chretien L, Vitelli L, et al. Chromatin immunoselection defines a TAL-1 target gene. EMBO J. 1998;17(17):5151–5160. doi: 10.1093/emboj/17.17.5151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cheng Y, Wu W, Ashok Kumar S, et al. Erythroid GATA1 function revealed by genome-wide analysis of transcription factor occupancy, histone modifications, and mRNA expression. Genome Res. 2009;19(12):2172–2184. doi: 10.1101/gr.098921.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cohen MM., Jr Perspectives on RUNX genes: an update. Am J Med Genet A. 2009;149A(12):2628–2646. doi: 10.1002/ajmg.a.33021. [DOI] [PubMed] [Google Scholar]
- 40.Xu Z, Huang S, Chang LS, Agulnick AD, Brandt SJ. Identification of a TAL1 target gene reveals a positive role for the LIM domain-binding protein Ldb1 in erythroid gene expression and differentiation. Mol Cell Biol. 2003;23(21):7585–7599. doi: 10.1128/MCB.23.21.7585-7599.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hansson A, Zetterblad J, van Duren C, Axelson H, Jonsson JI. The Lim-only protein LMO2 acts as a positive regulator of erythroid differentiation. Biochem Biophys Res Commun. 2007;364(3):675–681. doi: 10.1016/j.bbrc.2007.10.071. [DOI] [PubMed] [Google Scholar]
- 42.Wahlberg K, Jiang J, Rooks H, et al. The HBS1L-MYB intergenic interval associated with elevated HbF levels shows characteristics of a distal regulatory region in erythroid cells. Blood. 2009;114(6):1254–1262. doi: 10.1182/blood-2009-03-210146. [DOI] [PubMed] [Google Scholar]
- 43.Jiang J, Best S, Menzel S, et al. cMYB is involved in the regulation of fetal hemoglobin production in adults. Blood. 2006;108(3):1077–1083. doi: 10.1182/blood-2006-01-008912. [DOI] [PubMed] [Google Scholar]
- 44.Sankaran VG, Menne TF, Scepanovic D, et al. MicroRNA-15a and -16–1 act via MYB to elevate fetal hemoglobin expression in human trisomy 13. Proc Natl Acad Sci U S A. 2011;108(4):1519–1524. doi: 10.1073/pnas.1018384108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wallrapp C, Verrier SB, Zhouravleva G, et al. The product of the mammalian orthologue of the Saccharomyces cerevisiae HBS1 gene is phylogenetically related to eukaryotic release factor 3 (eRF3) but does not carry eRF3-like activity. FEBS Lett. 1998;440(3):387–392. doi: 10.1016/s0014-5793(98)01492-6. [DOI] [PubMed] [Google Scholar]
- 46.Kuroyanagi Y, Kaneko Y, Muta K, et al. cAMP differentially regulates gamma-globin gene expression in erythroleukemic cells and primary erythroblasts through c-Myb expression. Biochem Biophys Res Commun. 2006;344(3):1038–1047. doi: 10.1016/j.bbrc.2006.03.203. [DOI] [PubMed] [Google Scholar]
- 47.Wyszynski DF, Baldwin CT, Cleves MA, et al. Polymorphisms near a chromosome 6q QTL area are associated with modulation of fetal hemoglobin levels in sickle cell anemia. Cell Mol Biol (Noisy-le-grand) 2004;50(1):23–33. [PubMed] [Google Scholar]
- 48.Witt O, Monkemeyer S, Ronndahl G, et al. Induction of fetal hemoglobin expression by the histone deacetylase inhibitor apicidin. Blood. 2003;101(5):2001–2007. doi: 10.1182/blood-2002-08-2617. [DOI] [PubMed] [Google Scholar]
- 49.Wei GH, Zhao GW, Song W, et al. Mechanisms of human gamma-globin transcriptional induction by apicidin involves p38 signaling to chromatin. Biochem Biophys Res Commun. 2007;363(4):889–894. doi: 10.1016/j.bbrc.2007.06.191. [DOI] [PubMed] [Google Scholar]
- 50.Wozniak RJ, Keles S, Lugus JJ, et al. Molecular hallmarks of endogenous chromatin complexes containing master regulators of hematopoiesis. Mol Cell Biol. 2008;28(21):6681–6694. doi: 10.1128/MCB.01061-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.