Abstract
Epidemiologic studies have correlated elevated plasma fibrinogen (hyperfibrinogenemia) with risk of cardiovascular disease and arterial and venous thrombosis. However, it is unknown whether hyperfibrinogenemia is merely a biomarker of the proinflammatory disease state or is a causative mechanism in the etiology. We raised plasma fibrinogen levels in mice via intravenous infusion and induced thrombosis by ferric chloride application to the carotid artery (high shear) or saphenous vein (lower shear); hyperfibrinogenemia significantly shortened the time to occlusion in both models. Using immunohistochemistry, turbidity, confocal microscopy, and elastometry of clots produced in cell and tissue factor-initiated models of thrombosis, we show that hyperfibrinogenemia increased thrombus fibrin content, promoted faster fibrin formation, and increased fibrin network density, strength, and stability. Hyperfibrinogenemia also increased thrombus resistance to tenecteplase-induced thrombolysis in vivo. These data indicate that hyperfibrinogenemia directly promotes thrombosis and thrombolysis resistance and does so via enhanced fibrin formation and stability. These findings strongly suggest a causative role for hyperfibrinogenemia in acute thrombosis and have significant implications for thrombolytic therapy. Plasma fibrinogen levels may be used to identify patients at risk for thrombosis and inform thrombolytic administration for treating acute thrombosis/thromboembolism.
Introduction
Elevated plasma fibrinogen is associated with risk of cardiovascular disease and arterial and venous thrombosis.1–9 Several studies have detected dose effects, with increased risk of death or thrombosis in subjects with the highest plasma fibrinogen concentrations.6–9 The Framingham7 and Fragmin During Instability in Coronary Artery Disease8 studies positively correlated fibrinogen levels with risk of cardiovascular disease and incidence of death and/or myocardial infarction, respectively. The Leiden Thrombophilia Study showed that persons with elevated fibrinogen levels (4.0-4.9 vs < 3.0 mg/mL, 130%-160% of normal) have an adjusted odds ratio for venous thrombosis of 1.6, whereas persons with ≥ 5 mg/mL fibrinogen (≥ 170% of normal) have a 4-fold higher thrombotic risk, even after adjusting for C-reactive protein levels.9 These epidemiologic studies suggest that elevated fibrinogen is an independent risk factor for both arterial and venous thrombosis and therefore a potential diagnostic and therapeutic target for predicting and reducing thrombosis.
Importantly, however, epidemiologic studies have not and cannot show a causal relationship between fibrinogen and disease etiology.2,10,11 Fibrinogen levels increase with age, inflammatory processes, hematocrit, hypertension, glucose intolerance, cigarette smoking, and adiposity, and high fibrinogen levels increase plasma viscosity, a demonstrated risk factor for coronary heart disease.5,6,12 These potential confounders have not permitted distinction between fibrinogen's role as a biomarker of inflammation or coincident comorbidity and a direct, causative role in the etiology of cardiovascular disease.
Prior studies using animal models to clarify the role of hyperfibrinogenemia in thrombosis13–17 have been equivocal and controversial. Transgenic mice overexpressing murine fibrinogen (∼ 45% higher than wild-type) demonstrate elevated D-dimer and spontaneous fibrin deposition in the spleen, suggesting that hyperfibrinogenemia is mildly prothrombotic.15 However, these mice demonstrate only marginal shortening of the time to 75% occlusion after 20% ferric chloride (FeCl3) application to the carotid artery, indicating that hyperfibrinogenemia is not important in arterial thrombosis.15 In contrast, rabbits treated with turpentine to elevate fibrinogen before stasis- or mechanical injury-induced venous thrombosis demonstrate a positive correlation between thrombus size, weight, and fibrin content.16 However, because turpentine also increases factor VIII, another thrombosis risk factor, the specific prothrombotic contribution of elevated fibrinogen is difficult to discern. One recent study in which the human γ′-chain of fibrinogen was expressed in transgenic mice suggested that fibrinogen's thrombin-binding properties are antithrombotic,17 further questioning a pathologic mechanism relating hyperfibrinogenemia to disease.
The aim of the current study was to determine whether elevated fibrinogen directly contributes to thrombosis and identify the operant mechanism(s). We used in vivo models to assess fibrinogen's effects on thrombus formation and stability, and cell and tissue factor (TF)-based ex vivo and in vitro methods to identify biochemical and biomechanical mechanisms by which fibrinogen modulates fibrin formation, structure, and function. Our data indicate that hyperfibrinogenemia directly and independently shortened the time to occlusion (TTO) and increased thrombus resistance to thrombolysis. These effects were mediated through enhanced fibrin formation and increased fibrin network density and mechanical and fibrinolytic stability. Together, these findings strongly suggest a causative role for hyperfibrinogenemia in the pathology of thrombosis. Information on plasma fibrinogen levels may be used to identify patients at risk for thrombosis and inform thrombolytic administration for treating arterial and venous thrombosis.
Methods
Proteins and materials
Dulbecco modified Eagle medium with high glucose/2mM l-glutamine, 0.05% trypsin and ethylenediamine tetraacetic acid, and phosphate-buffered saline (10mM phosphate, pH 7.1, 150mM NaCl, phosphate-buffered saline) were from Invitrogen. Thrombin fluorogenic substrate (Z-Gly-Gly-Arg-AMC) and calibrator (α2-macroglobulin/thrombin) were from Diagnostica Stago. Factor Xa chromogenic substrate (Pefachrome FXa) was from Pentapharm. Mouse anti–human TF antibody (HTF-1) was the kind gift of Dr Ronald Bach (University of Minnesota). Tissue-type plasminogen activator (tPA) and goat anti–mouse and anti–rabbit peroxidase-conjugated antibodies were from Calbiochem. Monoclonal anti-fibrin(ogen) antibody (59D8) was the generous gift of Drs Marschall Runge (University of North Carolina [UNC] Department of Medicine) and Charles Esmon (Oklahoma College of Medicine). Biotinylated secondary antibodies were from Vector Laboratories. Target Retrieval Solution was from Dako North America. Nonimmune mouse IgG antibody (MOPC-1), bovine serum albumin (BSA), and adenosine diphosphate were from Sigma-Aldrich. Recombinant human tumor necrosis factor α (TNF-α) was from Millipore. Corn trypsin inhibitor (CTI) and factor X were from Haematologic Technologies. Fibronectin-, plasminogen-, and von Willebrand factor (VWF)–depleted fibrinogen was from Enzyme Research Laboratories. Fibrinogen was further depleted of factor XIII by immunoaffinity chromatography. The AlexaFluor-488 protein labeling kit was from Invitrogen. AlexaFluor-488-labeled fibrinogen (∼ 8 mol fluorophore/mol fibrinogen) was prepared as described.18 Thrombin receptor activation peptide (serine-phenylalanine-leucine-leucine-arginine-asparagine, TRAP) was from Bachem. Collagen was from Chrono-Log. Tenecteplase (TNKase) was the generous gift of Genentech. Contact-inhibited normal pooled plasma (NPP) was prepared from whole blood from 40 healthy subjects (50% female, 68% nonwhite) in a protocol approved by the UNC Institutional Review Board.19 The fibrinogen concentration in human NPP (3 mg/mL) was determined by enzyme-linked immunosorbent assay.
Murine thrombosis and thrombolysis models
Procedures were approved by the UNC Institutional Animal Care and Use Committee. Mice (6- to 8-week-old male C57BL/6, Charles River Laboratories) were anesthetized with 1.5% to 2% isofluorane in 2% oxygen, and the left saphenous vein was exposed under a SZX12 dissecting microscope (Olympus) using a catheter constructed of pulled PE-10 tubing (Braintree Scientific) with a 3.0-mil (0.076-mm diameter) cleaning wire (Hamilton) placed into the lumen as a stylet, as described.20 Human fibrinogen or vehicle (20mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid, pH 7.4, 150mM NaCl [HBS] or HBS/BSA) was administered through the cannula on a per-weight basis (blood volume [milliliters] is 7% of body weight [grams]; plasma is 50% of blood volume) to achieve 135% or 170% of normal (endogenous murine fibrinogen plus infused human fibrinogen) in murine circulation 5 minutes before injury. Final plasma fibrinogen levels were measured as described21 in mice not subject to FeCl3 injury. Nanobead diffusion experiments showed that these fibrinogen concentrations did not alter plasma viscosity (R. Spero, unpublished observation, September 2010).
For carotid artery thrombosis, the right common carotid artery was exposed after midline cervical incision. A Doppler transonic flow probe (Transonic Systems) was applied and connected to a flow meter (model T206; Transonic Systems) supplying a data acquisition system (PowerLab 4/30 model ML866, AD Instruments). The carotid artery was dried and 10% FeCl3 (0.62M FeCl3 on 0.5 × 0.5-mm filter paper) placed on the artery for 3 minutes, removed, and tissues washed 3 times with warm saline. After injury, blood flow was continuously monitored. For saphenous vein thrombosis, the saphenous vein of the right leg was dissected and exposed, 5% FeCl3 (0.31M FeCl3 on 0.5 × 2-mm filter paper) placed on the vein for 3 minutes, removed, and tissues washed 3 times with warm saline. Blood flow was monitored auditorily by Doppler ultrasonic flow probe. In both models, the TTO was the time between FeCl3 administration and lack of flow for 60 consecutive seconds. Experiments were stopped at 45 minutes if no occlusion occurred. Occluded vessels were excised and fixed in 10% formalin.
Thrombolysis was assessed in mice subject to FeCl3 carotid artery thrombosis. After 5 consecutive minutes of blood flow less than 0.1 mL/min, mice were infused with TNKase (0.5-5 mg/kg) through the saphenous vein intravenous catheter while continuously monitoring carotid blood flow.
Hematoxylin and eosin staining and immunohistochemistry
Fixed tissues were dehydrated and paraffin-embedded, and consecutive, 5-μm sections cut and mounted with vectamount (UNC Lineberger Comprehensive Cancer Center Animal Histopathology Core). Slides were stained with hematoxylin and eosin to visualize the thrombus, and imaged with a Retiga 400R camera (Qimaging) linked to an Olympus Bx 61 microscope with a 20× U Plan FL N, 0.5 NA objective lens. A computer equipped with Velocity software (v5.5) was used to operate the system. Images were analyzed with Adobe Photoshop CS (v8.0). For immunohistochemistry, antigen retrieval was performed in Target Retrieval Solution in a 95°C water bath. Slides were stained with anti-fibrin antibody (59D8, 1:1000), which detects both human and mouse fibrin(ogen)15,22 (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article) for 1 hour at room temperature in a humidity-controlled chamber and developed using avidin-biotin complex (Dako North America). Negative controls were stained simultaneously in the absence of primary antibody. Staining intensity of thrombi from at least 4 representative sections per mouse were analyzed by 3 independent, blinded observers on a scale of 0 to 3.
Platelet aggregation
Human platelet-rich plasma (PRP) obtained by centrifugation (150g, 15 minutes) of citrated blood was adjusted to 250 × 103 platelets/mL with autologous platelet-poor plasma (PPP). Aggregation was triggered by TRAP (50 μg/mL, final), collagen (2 μg/mL, final), or adenosine diphosphate (2.5μM, final). Light transmission was recorded on a Chrono-Log Optical Aggregometer, model 470.
Cell culture
Primary human saphenous vein endothelial cells (HSVECs, PromoCell) and human smooth muscle cells (SMCs; Lonza Walkersville) were cultured as directed to 80% to 95% confluence in 5% CO2 at 37°C. Cells were used between passages 3 to 6 to reduce phenotypic drift.
Cellular activity assays
TF activity was measured by chromogenic substrate cleavage on a SpectraMax Plus340 plate reader (Molecular Devices) in the presence and absence of inhibitory anti-TF antibody (HTF-1, 10 μg/mL) or isotype control (MOPC-1, 10 μg/mL), as described.19 Thrombin was measured by calibrated automated thrombography using a Fluoroskan Ascent fluorometer (ThermoLabsystem) as described.19 Thrombin generation was calculated using Thrombinoscope software Version 3.0.0.29 (Thrombinoscope BV). The thrombin generation rate was calculated by dividing peak height by the difference from time to peak to lag time. The endogenous thrombin potential is not reported because thrombin generation curves did not always return to baseline.
Phospholipid vesicles
Phosphatidylcholine (egg), phosphatidylethanolamine (soy), and phosphatidylserine (porcine brain) were from Avanti Polar Lipids. Large unilamellar vesicles (41% phosphatidylcholine/44% phosphatidylethanolamine/15% phosphatidylserine) were made as described.23 Briefly, lipids were combined, dried under nitrogen gas, and resuspended in cyclohexane. Resuspended lipids were lyophilized, resuspended in HBS containing 1mM ethylenediaminetetraacetic acid, and extruded through a 0.2-μm filter 10 times.
Clot formation and lysis by turbidity
For human plasma experiments, recalcified (16mM, final), lipidated (4μM, final) NPP was spiked with fibrinogen to 4.5, 6, or 7.5 mg/mL (150%, 200%, and 250% of normal, respectively, final) and immediately added to washed cell monolayers (67.7% plasma, final). For mouse plasma experiments, recalcified (16mM, final) murine PPP containing 2.4 ± 0.2 mg/mL fibrinogen (100%) was spiked with 2 or 4 mg/mL human fibrinogen to approximately 180% and 270% of normal, respectively, final, diluted 1:3 in HBS, and clotted with TF (Innovin 1:30 000 final). Fibrinolysis assays included tPA (250 μg/mL, final) or TNKase (concentrations indicated) at the reaction start. Clotting and lysis were detected by turbidity at 405 nm in a SpectraMax Plus340 plate reader.18,19
Laser scanning confocal microscopy
Clots were formed over washed cells in Lab-Tek II Chamber #1.5 coverglasses (Nalge Nunc International) with addition of AlexaFluor-488–labeled fibrinogen as described.18,19 Clotting proceeded until a constant final turbidity was reached in separate, parallel reactions. Clots were imaged on a Zeiss LSM5 Pascal laser scanning confocal microscope (Carl Zeiss Inc) linked to a Zeiss Axiovert 200M microscope equipped with a Zeiss 60×/7.4 NA oil immersion plan apo-chromatic lens, as described. The 488nm line of a medium power multi-line argon ion laser was used for excitation and a 505-530-nm band-pass filter for emission. A computer equipped with Carl Zeiss software (v1.5) was used to operate the system. Optical sectioning was achieved by closing the pinhole in the front of the detector to one airy unit. The zoom factor was 1. Thirty optical sections (1024 × 1024 pixels) in 3 randomly chosen locations were collected at 0.36-μm intervals in the z-axis at the cell surface. Image volumes were 146 × 146 × 10 μm. Single images were collected in 15.47 seconds. Optical resolution was ∼0.14 μm in the xy-plane and ∼0.5 μm on the z-axis. The sectioning interval was smaller than the calculated z-axis optical section resolution to achieve Nyquest sampling in z based on the Zeiss software calculation. Images were deconvolved using 3-dimensional deconvolution algorithms in AutoQuant Autodeblur (Version x1.4.1; Media Cybernetics). Fibrin network density was analyzed using ImageJ (1.37V; National Institutes of Health) by placing random grids of 2 pixel crosses on individual slices (121-144 crosses/slice) and counting fibers intersecting the middle of the crosses divided by the total number of crosses, less crosses in the volume occupied by the cells, as described.18,19
Clot viscoelastometry
Human PRP and PPP from blood drawn into 3.2% sodium citrate/18.3 μg/mL CTI was recalcified (16mM, final) and spiked with fibrinogen or BSA in HBS. PRP and PPP had more than 300 × 103 and less than 8 × 103 platelets/mL, respectively. The baseline PRP and PPP fibrinogen concentration was estimated in accord with NPP determinations. PRP was clotted with TF (1pM, final), which corresponds to the TF activity of TNF-α–stimulated HSVECs (TNF-α-HSVEC). PPP was clotted with 1pM TF and 4μM phospholipid. Lysis assays were performed in the presence of tPA (500 ng/mL, final). Clot elastic modulus (CEM) was measured by Hemodyne HAS.24
Statistical methods
For TTO and immunohistochemistry, normal and hyperfibrinogenemic conditions were compared by Mann-Whitney test. For CEM, normal and hyperfibrinogenemic conditions were compared by unpaired Student t tests. For clotting assays, fibrin density, and fibrinolysis parameters, significant differences between groups were identified by one-way analysis of variance and analyzed by Dunnett post-hoc test using 3 mg/mL fibrinogen as the index group (on unstimulated HSVECs, SMCs, or TNF-α-HSVECs as indicated) to limit type I error. Statistical analyses were performed using Kaleidagraph, Version 4.1 (Synergy Software).
Results
Hyperfibrinogenemia shortens the time to vessel occlusion after FeCl3 injury
To determine the contribution of elevated fibrinogen to intravascular thrombus formation, we used 2 murine thrombosis models based on FeCl3 application to the carotid artery (high shear) or saphenous vein (lower shear) after intravenous infusion of human fibrinogen. This infusion-based approach enabled us to precisely control the plasma fibrinogen level during thrombus formation. Control experiments and published studies demonstrate that human fibrinogen is incorporated into murine clots, supports murine platelet aggregation,25 and has appropriate half-life26 in mouse circulation. The endogenous fibrinogen concentration in mice was 2.4 ± 0.2 mg/mL (100%), and levels were raised to 3.2 ± 0.2 or 4.0 ± 0.1 mg/mL, final (135% or 170% of normal, respectively), consistent with levels associated with thrombosis in humans.7,9
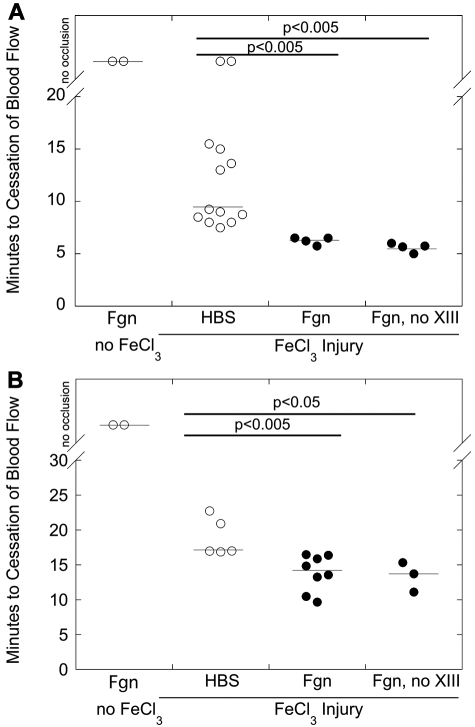
Consistent with transgenic hyperfibrinogenemic mice13,15 and a prior study in which human fibrinogen was injected into BALB/c mice,26 elevated fibrinogen did not trigger spontaneous thrombosis (Figure 1). FeCl3 application to the carotid artery or saphenous vein of HBS-infused mice produced occlusive thrombi in 9.3 and 17 minutes (median values), respectively, confirming the prothrombotic effect of vascular disruption. Infusion of control protein (BSA) did not further shorten the TTO in either vessel (data not shown). Mice infused with fibrinogen to 3.2 ± 0.2 mg/mL (135%) demonstrated a nonsignificant trend to shorter TTO in the carotid artery model (data not shown); we did not test this fibrinogen concentration in the saphenous vein model. Interestingly, compared with control (HBS or BSA infusion), fibrinogen infusion to 4.0 ± 0.1 mg/mL final (170%) before FeCl3 injury significantly (P < .005) shortened the TTO in both carotid artery and saphenous vein models (median, 6.4 and 14.2 minutes, respectively, Figure 1). The shortened TTOs were not the result of factor XIII in the fibrinogen preparation; infusion of factor XIII-depleted fibrinogen also significantly (P < .05) shortened the TTO versus control mice (Figure 1). These data demonstrate a direct contribution of hyperfibrinogenemia to thrombus formation after vascular injury.
Figure 1.
Elevated fibrinogen shortens the time to vessel occlusion after FeCl3 injury. Wild-type C57Bl/6 mice were infused with HBS or fibrinogen (plasminogen-, fibronectin-, and VWF-depleted or plasminogen-, fibronectin-, VWF-, and factor XIII-depleted) to 170% of normal. Thrombosis was induced by FeCl3 application to the carotid artery (A) or saphenous vein (B), and the TTO was determined by flow probe or Doppler, respectively. In vessels that did not occlude, the TTO was recorded as 45 minutes. Each point represents a separate mouse. Lines indicate median values.
Elevated fibrinogen increases the fibrin(ogen) content of thrombi
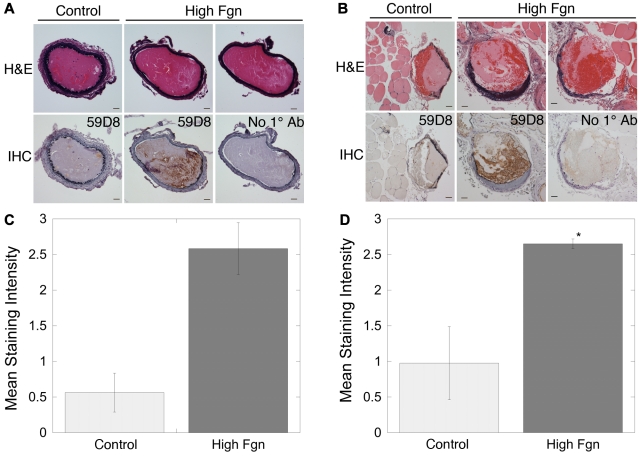
To probe the mechanism for the shortened TTO, we first examined the morphology of thrombi formed in the murine carotid artery and saphenous vein. Hematoxylin and eosin staining showed extensive, occlusive thrombi containing distinct regions of proteinaceous material and erythrocytes in injured vessels from both control and fibrinogen-injected mice (Figure 2A-B). Immunohistochemistry of thrombi in carotid artery and saphenous vein thrombi from control (HBS or BSA-infused) mice demonstrated weak fibrin staining concentrated primarily at the luminal edge of proteinaceous regions. No staining was detected in the absence of primary antibody, confirming that the secondary antibody did not bind mouse tissue nonspecifically (Figure 2A-B). Staining was slightly more intense in thrombi from saphenous vein than carotid artery, consistent with higher fibrin production in lower shear conditions.27 Both carotid artery and saphenous vein thrombi from mice infused with human fibrinogen demonstrated more intense fibrin staining at the periphery of proteinaceous regions and intense, diffuse staining in regions containing erythrocytes (Figure 2). These findings suggest that hyperfibrinogenemia increased thrombus fibrin content in both high and low shear vessels.
Figure 2.
Elevated fibrinogen increases fibrin(ogen) incorporation into thrombi after FeCl3 injury. Representative sections through thrombi after FeCl3 injury to the carotid artery (A) or saphenous vein (B). Hematoxylin and eosin staining shows regions of protein and packed erythrocytes. Immunohistochemistry (IHC) for fibrin (59D8) on corresponding sections shows darker staining for fibrin(ogen) at the thrombi margins and in thrombi from hyperfibrinogenemic (170% fibrinogen) mice. “No 1° Ab” indicates antibody 59D8 was omitted from IHC as a negative control. Staining intensity (on a scale of 0-3) was normalized to assign the value of 3 to the most intensely stained section in each vessel separately. Mean staining intensity from immunohistochemistry images from 3 separate carotid arteries (C) or 4 separate saphenous veins (D) for each condition was determined as described in “Hematoxylin and eosin staining and immunohistochemistry” and compared with control (wild-type) carotid artery (P = .1) and saphenous vein, respectively. *P < .05.
In vitro platelet aggregation is not increased by elevated fibrinogen levels
We then tested whether hyperfibrinogenemia increased platelet aggregation, a process dependent on fibrinogen binding to platelet αIIbβ3. The endogenous fibrinogen concentration of human plasma was 3 mg/mL (100%), and fibrinogen levels were raised with additional human fibrinogen as indicated. As shown in supplemental Figure 2 and supplemental Table 1, elevated fibrinogen did not increase, and even slightly decreased, platelet aggregation induced by TRAP, collagen, or adenosine diphosphate, consistent with a mechanism where high fibrinogen promotes full occupancy of platelet αIIbβ3 and inhibits interplatelet bridging.28 These data suggest that the mechanism by which hyperfibrinogenemia shortened the TTO was not via enhanced platelet aggregation.
Both cellular PCA and elevated fibrinogen increase fibrin formation
We next determined the effect of fibrinogen level on fibrin formation in models of vasculature by incubating cultured cell monolayers with recalcified NPP spiked with fibrinogen. We used unstimulated HSVECs to model unperturbed endothelium and SMCs to model FeCl3-injured vessels. We also used TNF-α–stimulated HSVECs (100 ng/mL TNF-α for 6 hours, TNF-α-HSVECs) to model intact cytokine-stimulated endothelium thought to promote clotting in venous thrombosis (supplemental Figure 3). Whereas quiescent endothelial cells in vivo do not express significant TF, cultured unstimulated endothelial cells exhibit low TF activity.19 However, the net procoagulant activity (PCA) of cultured HSVECs was significantly lower than cultured SMCs or TNF-α-HSVECs (supplemental Figure 3; Table 1).
Table 1.
TF activity and thrombin generation supported by HSVECs, SMCs, and TNF-α-HSVECs
| HSVECs | SMCs | TNF-α-HSVECs* | |
|---|---|---|---|
| TF activity | |||
| Without HTF1, pM/min | 0.073 ± 0.012 | 12.08 ± 0.443† | 3.141 ± 0.459† |
| With HTF1, pM/min | 0.075 ± 0.007 | 0.973 ± 0.021 | 0.066 ± 0.066 |
| Thrombin generation | |||
| Lag time, minutes | 41.9 ± 12.1 | 2.7 ± 0.2† | 6.1 ± 1.7† |
| Rate, nM/min | 11.5 ± 5.0 | 77.1 ± 16.6† | 21.0 ± 1.7† |
| Time to peak, minutes | 48.0 ± 11.6 | 6.8 ± 0.6† | 11.6 ± 1.3† |
| Peak height, nM | 54.1 ± 21.6 | 274.5 ± 48.6† | 113.9 ± 10.9† |
HSVECs treated with 100 ng/mL TNF-α for 6 hours.
P < .05 versus unstimulated HSVECs.
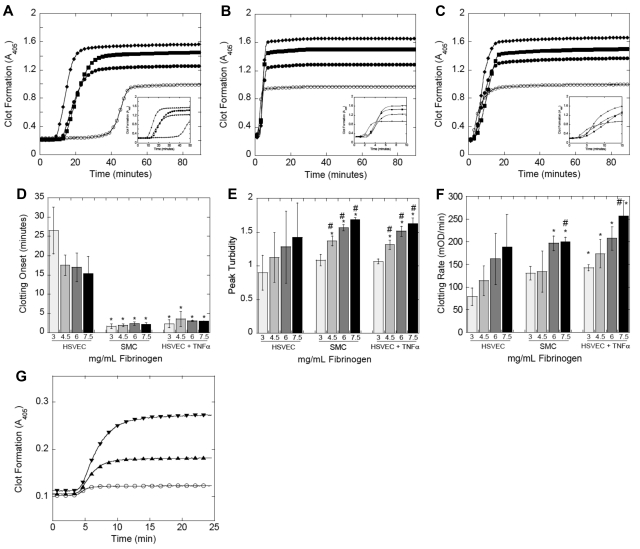
These experiments demonstrated unique effects of cellular PCA and fibrinogen level on fibrin formation (Figure 3A-C). Relative to unstimulated HSVECs, increased PCA of SMCs and TNF-α-HSVECs (supplemental Figure 3; Table 1) significantly (P < .002) shortened the fibrin formation onset (Figure 3D), consistent with the premise that exposure of procoagulant cells to blood triggers clotting. Elevated fibrinogen trended toward a shortened clotting onset in reactions induced by unstimulated HSVECs but did not reach statistical significance because of large variability. In addition, fibrinogen did not further shorten the onset in reactions triggered by the more procoagulant SMCs or TNF-α-HSVECs. Elevated fibrinogen did, however, significantly (P < .001) increase peak turbidity (Figure 3E), indicating increased fibrin(ogen) incorporation into clots. Interestingly, relative to NPP clots produced by unstimulated HSVECs, both cellular PCA (SMCs, P = .08; and TNF-α-HSVECs, P < .02) and hyperfibrinogenemia (P < .001) increased the fibrin formation rate (Figure 3F). We observed a similar increase in the rate and final turbidity of TF-initiated clotting of murine plasma spiked with human fibrinogen (Figure 3G). This increase in the rate and amount of fibrin production is consistent with the shortened TTO observed in the murine thrombosis model (Figure 1).
Figure 3.
Both cellular PCA and elevated fibrinogen promote fibrin formation. (A-F) Recalcified (16mM, final) human NPP spiked with fibrinogen or HBS was added to confluent cell monolayers. Fibrin polymerization was measured by turbidity at 405 nm. (A-C) Polymerization curves representative of 4 independent experiments with human NPP and unstimulated HSVECs (A), SMCs (B), and TNF-α-HSVECs (C). Insets expand the x-axis (time) for each panel. Symbols are as follows: 3 mg/mL (○), 4.5 mg/mL (●), 6 mg/mL (■), and 7.5 mg/mL (♦) fibrinogen, final. (D-F) The onset, final turbidity, and fibrin formation rate (mean ± SD) of all 4 experiments with human NPP, respectively. *P < .05 vs 3 mg/mL fibrinogen on HSVECs. #P < .05 vs 3 mg/mL within each cell type. (G) Recalcified murine PPP was spiked with human fibrinogen or HBS, diluted 1:3 in HBS, and clotting was initiated with TF addition (Innovin 1:30 000 final) and monitored by turbidity at 405 nm. Symbols are as follows: 2.4 mg/mL (○), 4.4 mg/mL (▴), or 6.4 mg/mL (▾) fibrinogen, final. Polymerization curves are from a single experiment representative of 4 independent experiments.
Both cellular PCA and elevated fibrinogen increase fibrin network density
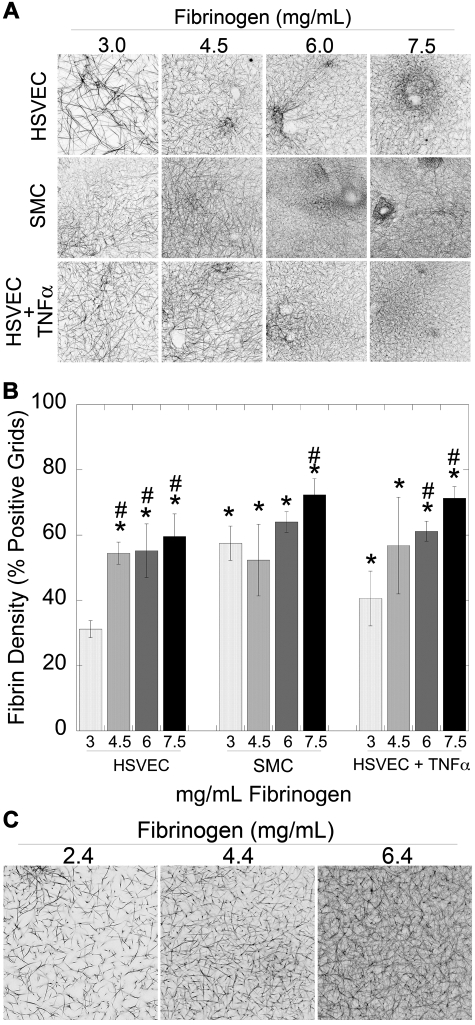
We and others have previously correlated fibrin formation parameters with fibrin network structure.18,19,29,30 To assess the impact of elevated fibrinogen on fibrin structure, we used laser scanning confocal microscopy to examine NPP clots produced by unstimulated HSVECs, SMCs, and TNF-α-HSVECs with increasing fibrinogen concentrations. Clots produced by SMCs and TNF-α-HSVECs were composed of more densely packed fibers (P < .05) than those produced by unstimulated HSVECs (Figure 4), consistent with observations that high thrombin generation promotes formation of dense networks of thin fibers.18,19,29 Notably, fibrin network density also correlated positively and significantly (P < .001) with fibrinogen concentration in both human (Figure 4A-B) and murine (Figure 4C) plasmas, suggesting that elevated fibrinogen levels promote formation of abnormally dense fibrin networks.
Figure 4.
Both cellular PCA and the fibrinogen level modulate fibrin network density. (A-B) Clots were formed by incubating unstimulated HSVECs, SMCs, and TNF-α–stimulated HSVEC monolayers with recalcified human NPP spiked with human fibrinogen or BSA, as indicated, and imaged by laser scanning confocal microscopy as described.18,19 (A) Representative micrographs (146 × 146 μm, xy) show 3-dimensional projections from 10-μm stacks at the cell surface (n ≥ 3). Darker areas represent increased fibrin density. (B) Fibrin network density (mean ± SD) of clots was determined as described in “Laser scanning confocal microscopy.” *P < .05 versus 3 mg/mL fibrinogen on HSVECs. #P < .05 versus 3 mg/mL within each cell type. (C) Clots were formed by addition of TF (1:30 000 Innovin) to recalcified murine PPP spiked with human fibrinogen or HBS.
Elevated fibrinogen increases clot mechanical strength
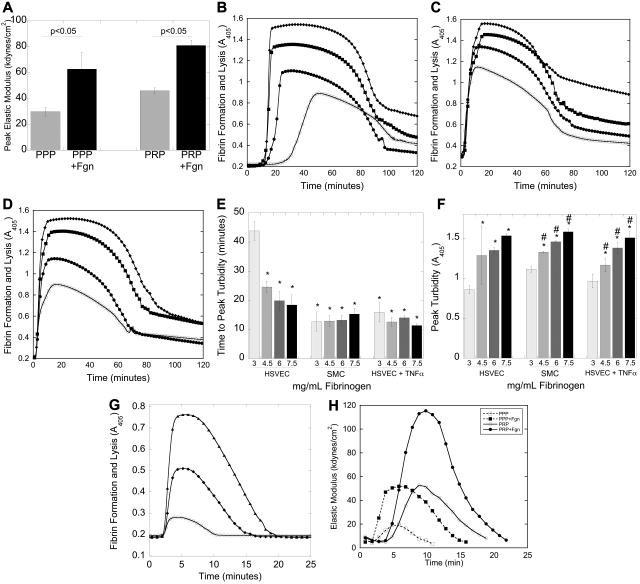
Fibrin network structure determines clot viscoelasticity,24,30 suggesting that elevated fibrinogen alters clot mechanical properties. To assess the impact of fibrinogen level on clot elasticity, we measured the CEM in clots forming in human PRP or PPP spiked with fibrinogen to 6 mg/mL, final (200%) or BSA (control). Because these assays cannot be initiated by cell monolayers, we initiated reactions with 4μM phospholipid and 0 (to model unstimulated endothelium) or 1pM (to model vascular disruption) TF. No CEM developed in the absence of added TF, reflecting the requirement for a procoagulant stimulus (ie, vascular disruption) to initiate clotting, even in hyperfibrinogenemic conditions. Initiation of clotting by TF showed a significant (P < .05) fibrinogen-dependent increase in peak CEM in both PRP and PPP (Figure 5A), suggesting that elevated fibrinogen increases the structural integrity (mechanical strength) of the clot.
Figure 5.
Elevated fibrinogen increases clot stability. (A) Human PRP and PPP prepared from CTI-inhibited whole blood were spiked with fibrinogen (to 6 mg/mL final, 200%) or BSA, recalcified, and clotted with TF (“Clot viscoelastometry”). Bars represent peak CEM (mean ± SD). (B-D) Recalcified human NPP spiked with fibrinogen or control was added to confluent cell monolayers. Fibrin polymerization was initiated in the presence of tPA; clotting and lysis were measured by turbidity at 405 nm. (B-D) Representative turbidity curves with human NPP and unstimulated HSVECs (B), SMCs (C), and TNF-α-HSVECs (D). Symbols are as follows: 3 mg/mL (○), 4.5 mg/mL (●), 6 mg/mL (■), and 7.5 mg/mL (♦) fibrinogen, final. (E) Time to peak turbidity and (F) peak turbidity (mean ± SD, n = 4), respectively. *P < .05 vs 3 mg/mL fibrinogen on HSVECs. #P < .05 vs 3 mg/mL within each cell type. (G) Recalcified murine PPP was spiked with human fibrinogen or HBS to achieve 2.4 mg/mL (○), 4.4 mg/mL (♦), or 6.4 mg/mL (▴) fibrinogen, final, diluted 1:3 in HBS, and clotting was initiated with TF (Innovin 1:30 000 final) and monitored by turbidity. Data are representative polymerization curves (n = 2). (H) Representative elastometry curves (n = 3) of human PRP and PPP prepared from CTI-inhibited whole blood, spiked with human fibrinogen (to 6 mg/mL, final) or BSA, recalcified, and clotted with TF in the presence of tPA (“Clot formation and lysis by turbidity”). The longer initiation phase of PRP clots versus PPP clots reflects the time to platelet activation.31
Elevated fibrinogen increases plasma clot resistance to fibrinolysis
Fibrin network density also determines a clot's resistance to fibrinolysis.18,19,32 To evaluate the effect of elevated fibrinogen on clot resistance to fibrinolysis, we used both turbidimetric and mechanical (elastometry) lysis assays. We first initiated clotting by incubating human NPP spiked with fibrinogen or HBS with HSVECs, SMCs, and TNF-α-HSVECs in the presence of tPA and monitored clotting and lysis by turbidity (Figure 5B-D). Compared with unstimulated HSVECs, SMCs and TNF-α-HSVECs significantly (P < .05) shortened the time to peak turbidity of NPP clots (Figure 5E). In contrast, elevated fibrinogen significantly (P < .05) shortened the time to peak turbidity only on unstimulated HSVECs but increased peak turbidity on all cell types. We observed a similar pattern in mouse PPP spiked with human fibrinogen (Figure 5G). These data indicate that cellular PCA triggers fibrin formation, but fibrinogen concentration dictates fibrin incorporation into the clot and its resistance to lysis.
We then evaluated the effects of elevated fibrinogen on mechanical stability during tPA-mediated lysis of human PRP and PPP clots. Compared with control, addition of fibrinogen to 6 mg/mL, final (200%) increased peak CEM, area under the lysis curve, and half-lysis time (Figure 5H; Table 2). Because fibrinogen produced similar effects in both PRP and PPP, these assays support findings that elevated fibrinogen promotes resistance of clots to fibrinolytic and mechanical disruption by a fibrin-dependent, not platelet-dependent, mechanism.
Table 2.
Elevated fibrinogen increased clot elastic modulus during lysis
| Peak CEM | Area under the curve | Half-lysis | |
|---|---|---|---|
| PPP | 1 | 1 | 1 |
| PPP + fibrinogen | 2.7 ± 0.6* | 3.9 ± 1.5* | 1.3 ± 0.1* |
| PRP | 1 | 1 | 1 |
| PRP + fibrinogen | 2.2 ± 0.6* | 2.6 ± 1.3* | 1.3 ± 0.5 |
Data are presented as fold increase over PPP or PRP with no additional fibrinogen.
P < .05 versus no additional fibrinogen.
Elevated fibrinogen increases resistance to thrombolysis in vivo
Finally, because in vitro experiments suggested that elevated fibrinogen increased clot stability, we assessed thrombolysis in vivo in mice with normal and elevated fibrinogen. Because of the small size of the saphenous vein and its proximity to the saphenous artery, it was not feasible to continuously monitor blood flow during thrombolysis in the saphenous vein. Therefore, thrombolysis was only performed in the carotid artery model. After occlusive thrombus formation, we initiated lysis via bolus infusion of the tPA analog TNKase. TNKase's increased fibrin specificity and enhanced resistance to inhibition by plasminogen activator inhibitor-133 provide a longer plasma half-life and facilitate dosing regimens (single IV bolus vs continuous infusion required for tPA/alteplase). Lysis of murine PPP clots was TNKase-dose-dependent (supplemental Figure 4A). Importantly, human fibrin and murine fibrin are similarly cleaved by murine plasmin,34 and TNKase-mediated lysis was similarly dose-dependent with regard to fibrinogen (human, supplemental Figure 4B; or mouse, supplemental Figure 4C) spiked into murine PPP.
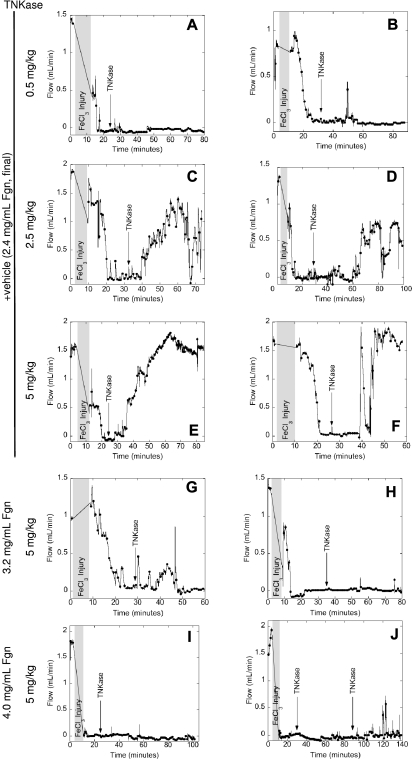
TNKase infusion produced dose-dependent lysis of in vivo thrombi; 0.5, 2.5, and 5 mg/kg TNKase produced no (2 of 2 mice), partial (30%-70% in 2 of 2 mice), or complete (5 of 5 mice) return of flow, respectively, in vehicle-infused mice within 1 hour of infusion (Figure 6A-F). Mice infused to 3.2 ± 0.2 mg/mL final plasma fibrinogen (135%) showed only partial (0%-30%) return of flow in 3 of 3 mice with subsequent reocclusion after 5 mg/kg TNKase infusion (Figure 6G-H). In contrast, mice infused to 4.0 ± 0.1 mg/mL final plasma fibrinogen (170%) did not reacquire flow for up to 120 minutes (3 of 3 mice), even at the highest TNKase dose or with repeat dosing (Figure 6I-J). Together with in vitro lysis data (Figure 5), these in vivo data demonstrate that hyperfibrinogenemia promotes resistance to thrombolysis.
Figure 6.
Hyperfibrinogenemia increases resistance to thrombolysis in vivo. Thrombosis was triggered in the carotid artery of wild-type mice infused with fibrinogen (plasminogen-, fibronectin-, VWF-, and factor XIII-depleted, concentrations indicated in the figure) or vehicle control. After stable occlusion for 5 minutes, mice were infused with TNKase (concentrations indicated in the figure). Blood flow was monitored by flow probe throughout the experiment. Each panel shows data from an individual mouse, representative of at least 2 mice for each condition. Shaded grey area represents the time of FeCl3 treatment plus time to reacquire flow.
Discussion
Although epidemiologic studies have implicated elevated plasma fibrinogen as an independent risk factor for both arterial and venous thrombosis, it remains highly controversial whether fibrinogen is merely a biomarker of a coincident inflammatory state or is also a causative mechanism in the etiology. We addressed this question in vivo by acutely elevating fibrinogen in mice and examining their susceptibility to thrombosis, and used ex vivo and in vitro experiments to elucidate biochemical and biophysical mechanisms by which fibrinogen contributes to thrombus formation and stability. We also used a murine thrombolysis model to test thrombus stability in vivo. Our findings show that elevated fibrinogen levels: (1) specifically and independently shortened the TTO in both high (arterial) and lower (venous) shear injury models, (2) increased thrombus fibrin content, (3) promoted faster fibrin formation, higher network density, and increased clot strength and stability in TF-initiated in vitro models that recapitulate in vivo thrombosis, and (4) increased thrombus resistance to lysis in vivo. Together, these findings show that hyperfibrinogenemia independently promotes thrombosis, identify biochemical and biomechanical mechanisms by which elevated fibrinogen levels contribute to thrombus formation, and demonstrate a critical role for plasma fibrinogen levels in resistance to thrombolytic therapy.
Our study is the first to show elevated fibrinogen significantly and independently shortens the TTO after acute injury. These findings differ somewhat from observations that transgenic hyperfibrinogenemic mice demonstrate a nonsignificant trend to shorter time to 75% occlusion in a FeCl3-carotid model.15 Differences in the 2 studies include the method of elevating fibrinogen levels (infusion vs transgenic expression) and final fibrinogen levels (170% vs 145%).15 In our study, mice infused with lower fibrinogen levels (to 135%) showed a TTO of 9 minutes (median) after carotid artery injury, which was not significantly different from control mice (data not shown). In addition, Kerlin et al15 used mice with elevated murine fibrinogen, whereas the mice in our study had a mixture of human and mouse fibrinogen. Differences between these 2 molecules may make human fibrinogen more prothrombotic. Finally, the higher FeCl3 concentrations (20%) used in the earlier study15 may have dampened differences35 between normal and hyperfibrinogenemic mice. Our findings also differ somewhat from observations that human fibrinogen γ′-chain expression in mice paradoxically decreases thrombus weight after electrolytic femoral injury.17 However, the total fibrinogen levels achieved in human γ′-expressing mice were lower than in controls, so any role of hyperfibrinogenemia was not examined in that study. Our in vivo observations are supported by ex vivo and in vitro cell-based experiments that recapitulate aspects of the murine thrombosis models. Notably, these findings are consistent with studies demonstrating fibrin network density, mechanical stability, and resistance to lysis are positively correlated with fibrinogen concentration in clots triggered by exogenous thrombin24,30,36,37 but extend these findings to a more physiologically relevant model in which clotting was initiated by TF-bearing primary cells. The vascular cells used in the current study model both arterial (atherosclerotic) disease where plaque rupture exposes blood to subendothelial cells (eg, SMCs) and venous disease where clots are thought to arise on intact but inflamed endothelium (eg, TNF-α-HSVECs).19 To our knowledge, these experiments are the first to address the role of elevated fibrinogen in a cellular, TF-based system. Together, these in vivo and in vitro observations link hyperfibrinogenemia to thrombotic disease and suggest specific mechanisms by which elevated fibrinogen is pathogenic.
Our study correlating abnormal fibrin quality with thrombosis in hyperfibrinogenemia informs several patient cohort studies. Of note, both hyperfibrinogenemia and denser, fibrinolysis-resistant clots were observed in plasma from patients with coronary artery disease, uniting observations of elevated fibrinogen, abnormal clot quality, and cardiovascular disease in a single patient cohort.36 Abnormal fibrin network structure and/or function (high mechanical strength and/or increased resistance to lysis) have also been detected in plasma clots from patients with diabetes,38 ischemic stroke,39 pulmonary hypertension,40 myocardial infarction,36,41 venous thromboembolism,42,43 and healthy relatives of patients with premature coronary artery disease.44 Overall, these studies suggest that abnormal fibrin quality is a pathologic mechanism operant not only in hyperfibrinogenemia, but also in other prothrombotic pathologies.
A particularly interesting outcome of our study is the observation that hyperfibrinogenemia did not cause spontaneous thrombosis in vivo. This observation is consistent with reports that injecting human fibrinogen into mice does not cause spontaneous fibrin deposition26 and, importantly, with findings that, although hyperfibrinogenemia is a thrombosis risk factor,1–9 it does not cause thrombosis ipso facto. Virchow proposed that multiple “hits” from abnormalities in plasma composition, vascular cell function, and blood flow are required to trigger thrombosis. This paradigm suggests that an initiating trigger, probably exposure of cellular PCA during plaque rupture or vasculitis, is required to initiate thrombus formation. Indeed, markers of vascular disruption, including circulating leukocyte and endothelial-derived microparticles, are elevated 1.3-fold in venous thromboembolism patients.45 Consistent with this hypothesis, the prognostic importance of elevated fibrinogen levels appears to be independent of, and additive to, myocardial damage (troponin-T levels) in patients with unstable CAD.8 To our knowledge, few diagnostic algorithms simultaneously consider markers of tissue damage and plasma hypercoagulability when assessing thrombosis risk. Of note is one study4 that examined independent and combined effects of elevated fibrinogen and homocysteine (a potential initiator of endothelial damage) on mortality of patients from a high-risk cardiology clinic; elevated levels of both homocysteine and fibrinogen contributed to an increased hazard ratio, consistent with our findings that endothelial dysfunction (TF expression) and hyperfibrinogenemia independently promoted thrombus formation. Reduced blood flow (stasis), the third component of the Virchow triad, is thought to explain differences in arterial and venous thrombosis. The shear rates between arteries and veins differ significantly; in humans, large arteries, such as the carotid, have wall shear rates of 300 to 800 inverse seconds, whereas venous shear rates are in the range of 20 to 200 inverse seconds.46 Interestingly, hyperfibrinogenemia has been correlated with both arterial and venous thrombosis, suggesting that hyperfibrinogenemia contributes to thrombus formation independently of the shear rate. We addressed the role of shear by applying the FeCl3 injury model to both high (artery) and lower (vein) shear vessels. Thrombi from saphenous veins appeared more fibrin-rich than thrombi from carotid arteries (Figure 2), consistent with observations that lower shear promotes fibrin deposition.27 Notably, however, hyperfibrinogenemic mice demonstrated shortened TTOs and increased fibrin content in thrombi in both high and lower shear vessels (Figures 1, 2). These findings do not diminish a role for stasis in thrombosis but rather suggest that the contributions of shear are eclipsed in the setting of hyperfibrinogenemia and potentially other pathologies as well.
Thrombolytic therapy has met limited success. The GUSTO-I trial demonstrated that complete coronary artery perfusion determines 30-day survival after myocardial infarction; however, tPA therapy achieved perfusion in only 54% of patients.47 Similarly, in acute stroke, proximal arterial patency after tPA infusion correlated with positive long-term outcome but was achieved in only 27% of patients.48 Interestingly, although prior studies associated thrombus platelet content with decreased tPA efficacy,49 our study shows that, within a given thrombosis model, increased fibrin content also decreases thrombolytic efficacy. This novel finding suggests that the plasma fibrinogen level present during thrombus formation is an independent predictor not only of thrombotic risk, but also of the potential efficacy of thrombolysis. Of note is the experiment in which we saw no return of flow in the occluded carotid artery despite 2 doses of TNKase (Figure 6J); postmortem dissection revealed diffuse bleeding from microvasculature within the neck and abdominal cavity. Given the risk and devastating consequences of intracerebral hemorrhage with thrombolytic therapy, our data suggest that screening patients for fibrinogen levels may inform risk/benefit analysis before initiating thrombolysis. Confirming a role for fibrinogen level in thrombolysis will require a study of patients who received thrombolytic therapy and for whom both the fibrinogen level and degree of reperfusion are known. Our data provide strong justification for such an investigation.
This study has potential limitations. First, we used human fibrinogen to increase circulating levels in the mouse. However, published studies17,25,26 as well as our data demonstrate that human fibrinogen is stable in murine circulation and incorporated into murine clots. Moreover, elevating either human or mouse fibrinogen in mouse plasma increased peak turbidity and prolonged tPA- or TNKase-mediated fibrinolysis similar to that seen with tPA- or TNKase-mediated lysis of human fibrinogen in human clots (Figure 5; supplemental Figure 4). Second, the FeCl3 model may not fully recapitulate thrombosis/thromboembolism; it will be interesting to examine the effects of hyperfibrinogenemia in other models (stasis- or electrolytic-based) in future studies. Finally, we evaluated the immediate effects of fibrinogen on thrombosis; however, elevated fibrinogen may have additional prothrombotic effects in vivo. Previous studies50 demonstrated fibrin induction of TF in human vascular ECs, suggesting that prolonged exposure of vasculature to hyperfibrinogenemia may feedback on additional cellular mechanisms.
In conclusion, our results show that hyperfibrinogenemia independently promotes thrombus formation and stability via increased fibrin network density and resistance to dissolution. These findings establish hyperfibrinogenemia in the etiology of both arterial and venous thrombosis/thromboembolism and suggest that fibrin is a potential therapeutic target in the management of these pathologies. Furthermore, our study establishes a model for future investigations of plasma hypercoagulability and vascular dysfunction; modulating plasma composition via intravenous procoagulant infusion will allow examination of the specific roles of additional plasma proteins in thrombosis and thrombolysis.
Supplementary Material
Acknowledgments
The authors thank Mr Zach Rotfus, Dr A. Philip Owens III, Ms Laura Gray, and Ms Bethany Walton for their excellent technical assistance, Dr Shrikant Bangdiwala for his consultations regarding statistics, and Dr Robert A. Campbell for his thoughtful contributions.
This work was supported by the National Institutes of Health (R01HL094740, A.S.W.; R21AG031068, F.C.C.), the Gustavus and Louise Pfeiffer Research Foundation (A.S.W.), the National Hemophilia Foundation (A.S.W.), the American Heart Association (10PRE3720011) (K.R.M.), and the UNC-CH Integrative Vascular Biology Program National Institutes of Health T32 HL697668 (J.C.C.).
Footnotes
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: K.R.M. performed experiments, analyzed data, and wrote the manuscript; J.C.C. performed experiments; F.C.C. provided reagents and reviewed the manuscript; and A.S.W. designed and supervised the study, analyzed data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Alisa S. Wolberg, Department of Pathology and Laboratory Medicine, University of North Carolina at Chapel Hill, 815 Brinkhous-Bullitt Bldg, CB #7525, Chapel Hill, NC 27599-7525; e-mail: alisa_wolberg@med.unc.edu.
References
- 1.Lindahl B, Toss H, Siegbahn A, Venge P, Wallentin L. Markers of myocardial damage and inflammation in relation to long-term mortality in unstable coronary artery disease: FRISC Study Group. N Engl J Med. 2000;343(16):1139–1147. doi: 10.1056/NEJM200010193431602. [DOI] [PubMed] [Google Scholar]
- 2.Danesh J, Lewington S, Thompson SG, et al. Plasma fibrinogen level and the risk of major cardiovascular diseases and nonvascular mortality: an individual participant meta-analysis. JAMA. 2005;294(14):1799–1809. doi: 10.1001/jama.294.14.1799. [DOI] [PubMed] [Google Scholar]
- 3.Wilhelmsen L, Svardsudd K, Korsan-Bengtsen K, Larsson B, Welin L, Tibblin G. Fibrinogen as a risk factor for stroke and myocardial infarction. N Engl J Med. 1984;311(8):501–505. doi: 10.1056/NEJM198408233110804. [DOI] [PubMed] [Google Scholar]
- 4.Acevedo M, Pearce GL, Kottke-Marchant K, Sprecher DL. Elevated fibrinogen and homocysteine levels enhance the risk of mortality in patients from a high-risk preventive cardiology clinic. Arterioscler Thromb Vasc Biol. 2002;22(6):1042–1045. doi: 10.1161/01.atv.0000020007.25154.62. [DOI] [PubMed] [Google Scholar]
- 5.van Hylckama Vlieg A, Rosendaal FR. High levels of fibrinogen are associated with the risk of deep venous thrombosis mainly in the elderly. J Thromb Haemost. 2003;1(12):2677–2678. doi: 10.1111/j.1538-7836.2003.0543b.x. [DOI] [PubMed] [Google Scholar]
- 6.Yarnell JW, Baker IA, Sweetnam PM, et al. Fibrinogen, viscosity, and white blood cell count are major risk factors for ischemic heart disease: the Caerphilly and Speedwell collaborative heart disease studies. Circulation. 1991;83(3):836–844. doi: 10.1161/01.cir.83.3.836. [DOI] [PubMed] [Google Scholar]
- 7.Kannel WB, Wolf PA, Castelli WP, D'Agostino RB. Fibrinogen and risk of cardiovascular disease: the Framingham Study. JAMA. 1987;258(9):1183–1186. [PubMed] [Google Scholar]
- 8.Toss H, Lindahl B, Siegbahn A, Wallentin L. Prognostic influence of increased fibrinogen and C-reactive protein levels in unstable coronary artery disease: FRISC Study Group. Circulation. 1997;96(12):4204–4210. doi: 10.1161/01.cir.96.12.4204. [DOI] [PubMed] [Google Scholar]
- 9.Kamphuisen PW, Eikenboom JC, Vos HL, et al. Increased levels of factor VIII and fibrinogen in patients with venous thrombosis are not caused by acute phase reactions. Thromb Haemost. 1999;81(5):680–683. [PubMed] [Google Scholar]
- 10.Meade TW. Fibrinogen measurement to assess the risk of arterial thrombosis in individual patients: yes. J Thromb Haemost. 2005;3(4):632–634. doi: 10.1111/j.1538-7836.2005.01255.x. [DOI] [PubMed] [Google Scholar]
- 11.Lowe GD. Fibrinogen measurement to assess the risk of arterial thrombosis in individual patients: not yet. J Thromb Haemost. 2005;3(4):635–637. doi: 10.1111/j.1538-7836.2005.01256.x. [DOI] [PubMed] [Google Scholar]
- 12.Rokita H, Neta R, Sipe JD. Increased fibrinogen synthesis in mice during the acute phase response: co-operative interaction of interleukin 1, interleukin 6, and interleukin 1 receptor antagonist. Cytokine. 1993;5(5):454–458. doi: 10.1016/1043-4666(93)90035-4. [DOI] [PubMed] [Google Scholar]
- 13.Gulledge AA, Rezaee F, Verheijen JH, Lord ST. A novel transgenic mouse model of hyperfibrinogenemia. Thromb Haemost. 2001;86(2):511–516. [PubMed] [Google Scholar]
- 14.Gulledge AA, McShea C, Schwartz T, Koch G, Lord ST. Effects of hyperfibrinogenemia on vasculature of C57BL/6 mice with and without atherogenic diet. Arterioscler Thromb Vasc Biol. 2003;23(1):130–135. doi: 10.1161/01.atv.0000041037.06509.c2. [DOI] [PubMed] [Google Scholar]
- 15.Kerlin B, Cooley BC, Isermann BH, et al. Cause-effect relation between hyperfibrinogenemia and vascular disease. Blood. 2004;103(5):1728–1734. doi: 10.1182/blood-2003-08-2886. [DOI] [PubMed] [Google Scholar]
- 16.Chooi CC, Gallus AS. Acute phase reaction, fibrinogen level and thrombus size. Thromb Res. 1989;53(5):493–501. doi: 10.1016/0049-3848(89)90204-1. [DOI] [PubMed] [Google Scholar]
- 17.Mosesson MW, Cooley BC, Hernandez I, Diorio JP, Weiler H. Thrombosis risk modification in transgenic mice containing the human fibrinogen thrombin-binding gamma′ chain sequence. J Thromb Haemost. 2009;7(1):102–110. doi: 10.1111/j.1538-7836.2008.03213.x. [DOI] [PubMed] [Google Scholar]
- 18.Campbell RA, Overmyer KA, Bagnell CR, Wolberg AS. Cellular procoagulant activity dictates clot structure and stability as a function of distance from the cell surface. Arterioscler Thromb Vasc Biol. 2008;28(12):2247–2254. doi: 10.1161/ATVBAHA.108.176008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Campbell RA, Overmyer KA, Selzman CH, Sheridan BC, Wolberg AS. Contributions of extravascular and intravascular cells to fibrin network formation, structure, and stability. Blood. 2009;114(23):4886–4896. doi: 10.1182/blood-2009-06-228940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Buyue Y, Whinna HC, Sheehan JP. The heparin-binding exosite of factor IXa is a critical regulator of plasma thrombin generation and venous thrombosis. Blood. 2008;112(8):3234–3241. doi: 10.1182/blood-2008-01-136820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ingram IC. The determination of plasma fibrinogen by the clot-weight method. Biochem J. 1952;51(5):583–585. doi: 10.1042/bj0510583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hui KY, Haber E, Matsueda GR. Monoclonal antibodies to a synthetic fibrin-like peptide bind to human fibrin but not fibrinogen. Science. 1983;222(4628):1129–1132. doi: 10.1126/science.6648524. [DOI] [PubMed] [Google Scholar]
- 23.Hope MJ, Bally MB, Webb G, Cullis PR. Production of large unilamellar vesicles by a rapid extrusion procedure: characterization of size distribution, trapped volume and ability to maintain membrane potential. Biochim Biophys Acta. 1985;812:55–65. doi: 10.1016/0005-2736(85)90521-8. [DOI] [PubMed] [Google Scholar]
- 24.Dempfle CE, Kalsch T, Elmas E, et al. Impact of fibrinogen concentration in severely ill patients on mechanical properties of whole blood clots. Blood Coagul Fibrinolysis. 2008;19(8):765–770. doi: 10.1097/MBC.0b013e32830f1b68. [DOI] [PubMed] [Google Scholar]
- 25.Krystofiak E, Oliver JA. Human fibrinogen supports normal hemostatic function in a mouse platelet system. J Thromb Haemost. 2009;7(suppl 2):PP-MO-039. [Google Scholar]
- 26.Krohn KA, DeNardo SJ, Wheeler DW, DeNardo GL. I-fibrinogen as an oncophilic radiodiagnostic agent: distribution kinetics in tumour-bearing mice. Br J Cancer. 1977;36(2):227–234. doi: 10.1038/bjc.1977.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shen F, Kastrup CJ, Liu Y, Ismagilov RF. Threshold response of initiation of blood coagulation by tissue factor in patterned microfluidic capillaries is controlled by shear rate. Arterioscler Thromb Vasc Biol. 2008;28:2035–2041. doi: 10.1161/ATVBAHA.108.173930. [DOI] [PubMed] [Google Scholar]
- 28.Guy RD, Fogelson AL. Probabilistic modeling of platelet aggregation: effects of activation time and receptor occupancy. J Theor Biol. 2002;219(1):33–53. [PubMed] [Google Scholar]
- 29.Wolberg AS, Monroe DM, Roberts HR, Hoffman M. Elevated prothrombin results in clots with an altered fiber structure: a possible mechanism of the increased thrombotic risk. Blood. 2003;101(8):3008–3013. doi: 10.1182/blood-2002-08-2527. [DOI] [PubMed] [Google Scholar]
- 30.Ryan EA, Mockros LF, Weisel JW, Lorand L. Structural origins of fibrin clot rheology. Biophys J. 1999;77(5):2813–2826. doi: 10.1016/S0006-3495(99)77113-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Machlus KR, Colby EA, Wu JR, Koch GG, Key NS, Wolberg AS. Effects of tissue factor, thrombomodulin and elevated clotting factor levels on thrombin generation in the calibrated automated thrombogram. Thromb Haemost. 2009;102(5):936–944. doi: 10.1160/TH09-03-0180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Collet JP, Park D, Lesty C, et al. Influence of fibrin network conformation and fibrin fiber diameter on fibrinolysis speed: dynamic and structural approaches by confocal microscopy. Arterioscler Thromb Vasc Biol. 2000;20(5):1354–1361. doi: 10.1161/01.atv.20.5.1354. [DOI] [PubMed] [Google Scholar]
- 33.Bennett WF, Paoni NF, Keyt BA, et al. High resolution analysis of functional determinants on human tissue-type plasminogen activator. J Biol Chem. 1991;266(8):5191–5201. [PubMed] [Google Scholar]
- 34.Lijnen HR, van Hoef B, Beelen V, Collen D. Characterization of the murine plasma fibrinolytic system. Eur J Biochem. 1994;224(3):863–871. doi: 10.1111/j.1432-1033.1994.00863.x. [DOI] [PubMed] [Google Scholar]
- 35.Wang X, Xu L. An optimized murine model of ferric chloride-induced arterial thrombosis for thrombosis research. Thromb Res. 2005;115(1):95–100. doi: 10.1016/j.thromres.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 36.Collet JP, Allali Y, Lesty C, et al. Altered fibrin architecture is associated with hypofibrinolysis and premature coronary atherothrombosis. Arterioscler Thromb Vasc Biol. 2006;26(11):2567–2573. doi: 10.1161/01.ATV.0000241589.52950.4c. [DOI] [PubMed] [Google Scholar]
- 37.Kim PY, Stewart RJ, Lipson SM, Nesheim ME. The relative kinetics of clotting and lysis provide a biochemical rationale for the correlation between elevated fibrinogen and cardiovascular disease. J Thromb Haemost. 2007;5(6):1250–1256. doi: 10.1111/j.1538-7836.2007.02426.x. [DOI] [PubMed] [Google Scholar]
- 38.Jorneskog G, Egberg N, Fagrell B, et al. Altered properties of the fibrin gel structure in patients with IDDM. Diabetologia. 1996;39(12):1519–1523. doi: 10.1007/s001250050607. [DOI] [PubMed] [Google Scholar]
- 39.Undas A, Podolec P, Zawilska K, et al. Altered fibrin clot structure/function in patients with cryptogenic ischemic stroke. Stroke. 2009;40(4):1499–1512. doi: 10.1161/STROKEAHA.108.532812. [DOI] [PubMed] [Google Scholar]
- 40.Undas A, Kaczmarek P, Sladek K, et al. Fibrin clot properties are altered in patients with chronic obstructive pulmonary disease: beneficial effects of simvastatin treatment. Thromb Haemost. 2009;102(6):1176–1182. doi: 10.1160/TH09-02-0118. [DOI] [PubMed] [Google Scholar]
- 41.Fatah K, Silveira A, Tornvall P, Karpe F, Blomback M, Hamsten A. Proneness to formation of tight and rigid fibrin gel structures in men with myocardial infarction at a young age. Thromb Haemost. 1996;76(4):535–540. [PubMed] [Google Scholar]
- 42.Undas A, Zawilska K, Ciesla-Dul M, et al. Altered fibrin clot structure/function in patients with idiopathic venous thromboembolism and in their relatives. Blood. 2009;114(19):4272–4278. doi: 10.1182/blood-2009-05-222380. [DOI] [PubMed] [Google Scholar]
- 43.Lisman T, de Groot PG, Meijers JC, Rosendaal FR. Reduced plasma fibrinolytic potential is a risk factor for venous thrombosis. Blood. 2005;105(3):1102–1105. doi: 10.1182/blood-2004-08-3253. [DOI] [PubMed] [Google Scholar]
- 44.Mills JD, Ariens RA, Mansfield MW, Grant PJ. Altered fibrin clot structure in the healthy relatives of patients with premature coronary artery disease. Circulation. 2002;106(15):1938–1942. doi: 10.1161/01.cir.0000033221.73082.06. [DOI] [PubMed] [Google Scholar]
- 45.Rectenwald JE, Myers DD, Jr, Hawley AE, et al. D-dimer, P-selectin, and microparticles: novel markers to predict deep venous thrombosis: a pilot study. Thromb Haemost. 2005;94(6):1312–1317. doi: 10.1160/TH05-06-0426. [DOI] [PubMed] [Google Scholar]
- 46.Kroll MH, Hellums JD, McIntire LV, Schafer AI, Moake JL. Platelets and shear stress. Blood. 1996;88(5):1525–1541. [PubMed] [Google Scholar]
- 47.Simes RJ, Topol EJ, Holmes DR, Jr, et al. Link between the angiographic substudy and mortality outcomes in a large randomized trial of myocardial reperfusion: importance of early and complete infarct artery reperfusion. GUSTO-I Investigators. Circulation. 1995;91(7):1923–1928. doi: 10.1161/01.cir.91.7.1923. [DOI] [PubMed] [Google Scholar]
- 48.Saqqur M, Molina CA, Salam A, et al. Clinical deterioration after intravenous recombinant tissue plasminogen activator treatment: a multicenter transcranial Doppler study. Stroke. 2007;38(1):69–74. doi: 10.1161/01.STR.0000251800.01964.f6. [DOI] [PubMed] [Google Scholar]
- 49.Fay WP, Eitzman DT, Shapiro AD, Madison EL, Ginsburg D. Platelets inhibit fibrinolysis in vitro by both plasminogen activator inhibitor-1-dependent and -independent mechanisms. Blood. 1994;83(2):351–356. [PubMed] [Google Scholar]
- 50.Contrino J, Goralnick S, Qi J, Hair G, Rickles FR, Kreutzer DL. Fibrin induction of tissue factor expression in human vascular endothelial cells. Circulation. 1997;96(2):605–613. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.