Abstract
Waardenburg syndromes are characterized by pigmentation and autosensory hearing defects, and mutations in genes encoding transcription factors that control neural crest specification and differentiation are often associated with Waardenburg and related disorders. For example, mutations in SOX10 result in a severe form of Waardenburg syndrome, Type IV, also known as Waardenburg-Hirschsprung disease, characterized by pigmentation and other neural crest defects, including defective innervation of the gut. SOX10 controls neural crest development through interactions with other transcription factors. The MADS box transcription factor MEF2C is an important regulator of brain, skeleton, lymphocyte and cardiovascular development and is required in the neural crest for craniofacial development. Here, we establish a novel role for MEF2C in melanocyte development. Inactivation of Mef2c in the neural crest of mice results in reduced expression of melanocyte genes during development and a significant loss of pigmentation at birth due to defective differentiation and reduced abundance of melanocytes. We identify a transcriptional enhancer of Mef2c that directs expression to the neural crest and its derivatives, including melanocytes, in transgenic mouse embryos. This novel Mef2c neural crest enhancer contains three functional SOX binding sites and a single essential MEF2 site. We demonstrate that Mef2c is a direct transcriptional target of SOX10 and MEF2 via this evolutionarily conserved enhancer. Furthermore, we show that SOX10 and MEF2C physically interact and function cooperatively to activate the Mef2c gene in a feed-forward transcriptional circuit, suggesting that MEF2C might serve as a potentiator of the transcriptional pathways affected in Waardenburg syndromes.
Keywords: MEF2, MEF2C, SOX10, Melanocyte, Neural crest, Pigment, Mouse
INTRODUCTION
Neural crest cells are multipotent progenitor cells that originate from the dorsal neural tube at the border of the neural plate and the lateral epidermis to give rise to various tissues, including the skeletal elements of the face and head, dorsal root and sympathetic ganglia, the glial cells of the peripheral and enteric nervous systems, and melanocytes (Baker and Bronner-Fraser, 1997; Knecht and Bronner-Fraser, 2002; Le Douarin et al., 2004; Selleck et al., 1993; Trainor, 2005). Neural crest-derived melanocytes originate as non-pigmented precursors called melanoblasts, which migrate along characteristic pathways to various destinations, such as the dermis and epidermis, the inner ear and the choroids of the eye (Crane and Trainor, 2006; Dupin and Le Douarin, 2003; Sauka-Spengler and Bronner-Fraser, 2006; Steel and Barkway, 1989). The development of mature pigment-producing melanocytes from their neural crest precursors is a complex process involving melanoblast specification, proliferation, survival, migration and differentiation. Several transcription factors are known to be involved in melanocyte development, and mutations in the genes encoding these factors have been linked to pigment disorders and other neurocristopathies (Baxter et al., 2004; Mollaaghababa and Pavan, 2003; Pingault et al., 2010; Spritz et al., 2003).
SOX10 is an HMG box transcription factor that is a well-established regulator of neural crest development (Hong and Saint-Jeannet, 2005; Mollaaghababa and Pavan, 2003). Loss-of-function mutations in human SOX10 result in haploinsufficiency and are associated with Waardenburg-Hirschsprung disease, a neurocristopathy that involves hypopigmentation, deafness and aganglionic colon (Baxter et al., 2004; Inoue et al., 2004; Parisi and Kapur, 2000; Pingault et al., 1998; Pingault et al., 2010; Spritz et al., 2003). Similarly, Sox10 heterozygous mice also exhibit hypopigmentation, as well as distal bowel aganglionosis, and, therefore, these mice serve as an excellent animal model of Waardenburg-Hirschsprung disease (Herbarth et al., 1998; Lane and Liu, 1984; Southard-Smith et al., 1999; Southard-Smith et al., 1998). Sox10-null mice completely lack pigmentation and fail to develop a functional peripheral nervous system, suggesting a role for SOX10 in the specification of neural crest cells into melanocytes and glia in the peripheral and enteric nervous systems (Britsch et al., 2001; Herbarth et al., 1998; Potterf et al., 2001; Southard-Smith et al., 1998).
The MADS domain transcription factor MEF2C is important in the development of several lineages, including cardiac and skeletal muscle, hematopoietic cells, the craniofacial and axial skeletons, and the central nervous system (Black and Cripps, 2010; Potthoff and Olson, 2007). Inactivation of Mef2c in the germline of mice results in embryonic lethality by embryonic day (E)10 due to severe defects in cardiac and vascular development (Bi et al., 1999; Lin et al., 1998; Lin et al., 1997). In the neural crest, Mef2c expression can be detected as early as E8.5 in the mouse in the region adjacent to the neural folds (Edmondson et al., 1994; Verzi et al., 2007). Inactivation of Mef2c specifically in the neural crest using a conditional knockout approach in mice results in lethality at birth due to airway obstruction and defective craniofacial development (Verzi et al., 2007).
In the present study, we have identified a novel role for MEF2C in neural crest-derived melanocyte development. Inactivation of Mef2c in the neural crest using Wnt1-Cre transgenic mice results in reduced expression of melanocyte genes during development and a significant reduction in the number of melanocytes at birth. We have also identified a highly conserved transcriptional enhancer from the Mef2c locus that directs expression to the neural crest and its derivatives, including the craniofacial mesenchyme, the peripheral and enteric nervous systems, and melanocytes. We demonstrate that Mef2c is a direct transcriptional target of SOX10 in developing melanocytes and peripheral nervous system via this evolutionarily conserved transcriptional enhancer. Finally, we show that MEF2C physically interacts with SOX10 and, together, these proteins cooperatively activate transcription. Thus, we propose that Mef2c has a role in melanocyte development as a transcriptional target and partner for SOX10.
MATERIALS AND METHODS
Cloning and mutagenesis
The 7039-bp Mef2c-F1 enhancer fragment was amplified by PCR from the mouse Mef2c locus using the primers F1-F: 5′-AGTGGGAAGCATAAGGCCCGGGAACTCTGAT-3′ and F1-R: 5′-ATGGTACCGTGTATGGTGGTCCCGGGAATGT-3′. The resulting PCR product was digested and cloned into the XmaI sites in plasmid hsp68-lacZ (Kothary et al., 1989) to create plasmid Mef2c-F1-lacZ. The 300-bp conserved region of Mef2c-F1 was generated as an XmaI-HindIII subfragment and was cloned into plasmid hsp68-lacZ to create plasmid Mef2c-F1[3-3.3]-lacZ for transgenic analyses or into plasmid pTK-β-gal to create plasmid Mef2c[3-3.3]-F1-TK-lacZ for transfection analyses. Mutations of cis-regulatory elements within Mef2c-F1 were generated using the gene SOEing method (Horton, 1997) and were confirmed by sequencing on both strands.
Generation and analysis of transgenic mice
Transgenic mice were generated by oocyte microinjection as described previously (Dodou et al., 2003; Hogan et al., 1994). Sox10Dom mice were purchased from the Jackson Laboratories. Wnt1-Cre, Mef2c+/− and Mef2cflox/flox mice have been described previously (Danielian et al., 1998; Lin et al., 1997; Vong et al., 2005). All experiments with animals complied with federal and institutional guidelines and were reviewed and approved by the University of California, San Francisco Institutional Animal Care and Use Committee.
X-gal staining, whole-mount in situ hybridization, and immunofluorescence
β-galactosidase (β-gal) expression in lacZ transgenic embryos or tissues was detected by X-gal staining, as described previously (Dodou et al., 2003). Whole mount in situ hybridization was performed according to standard methods using digoxigenin-labeled antisense probes as described previously (Rojas et al., 2005). Dct (Image Clone ID 30539879), Mitf (Image Clone ID. 40047440), Pmel17 (Image Clone ID 30541702) and Sox10 probes were all designed by cloning the full-length cDNA into pBlueScript. Immunofluorescence labeling of cryosections was performed as described previously (Rojas et al., 2009) using the following primary antibodies at 1:100 dilution in PBS with 3% BSA and 0.1% Triton X-100: anti-SOX10 (R&D AF2864); anti-MEF2C (C-17) (Santa Cruz, sc-13268); anti-DCT (alpha-PEP8).
Electrophoretic mobility shift assay (EMSA)
EMSAs were performed as described previously (Dodou et al., 2003). The sequences of the control SOX10 and MEF2 binding sites have been described previously (Dodou et al., 2003; Peirano and Wegner, 2000). The sense-strand sequences of the Mef2c-F1 oligonucleotides used for EMSA were: Mef2c SOX-1, 5′-GAATGCACTGACTACAAAGTGCATCCTGAAG-3′; Mef2c mutSOX-1, 5′-GAATGCACTGACCCGCGGTTGCATCCTGAAG-3′; Mef2c SOX-2, 5′-GAATAGCTCTATAACAAAGTAACTACAGAGT-3′; Mef2c mutSOX-2, 5′-GAATAGCTCTATCCGCCGTTAACTACAGAGT-3′; Mef2c SOX-3, 5′-GGCCATTTAGCTCACAATGAAGGTCTGTGTT-3′; Mef2c mutSOX-3, 5′-GGCCATTTAGCTCGAGGGTAAGGTCTGTGTT-3′; Mef2c MEF2, 5′-GGGCTCCAACTATTTATAGAACTGAGTA-3′; and Mef2c mutMEF2, 5′-GGGCTCCAACTATCTGTAGAACTGAGTA-3′.
Chromatin immunoprecipitation (ChIP)
B16F10 mouse melanoma cells were transfected in nearly confluent 10-cm plates with 6 μg of pRK5-Sox10[Flag] using Lipofectamine LTX reagent (Invitrogen), according to the manufacturer's protocol. Twenty-four hours post-transfection, ChIP was performed using the ChIP Assay Kit (Upstate/Millipore) according to the manufacturer's instructions, using anti-FLAG antibody (clone M2, Sigma) and Protein G-PLUS agarose beads (Santa Cruz). Immunoprecipitated fragments and input samples were subjected to PCR using the following primers: Mef2c-F1-A, 5′-GTCCTGGAGTCTTGCACAG-3′ and Mef2c-F1-B, 5′-CAGCCCAAGCTTCCGTATGG-3′ to amplify the region of Mef2c-F1 containing the SOX binding sites; or primers VEGFR2-F1, 5′-ATCATGTGACAGCAAGACCG-3′ and VEGFR2-R1, 5′-TCTTGGTATGTTGGGTCACTC-3′, designed to amplify a region of the Vegfr2 promoter not expected to be bound by SOX10.
Transactivation assays
Transfections were performed in C3H10T1/2 cells in 6-cm plates using either Superfect (Qiagen) or Fugene 6 (Roche) according to the manufacturer's instructions. Each transfection contained 1 μg of reporter and 1 μg of expression plasmid. In transfections with two expression plasmids, the amount of each expression plasmid was adjusted to 0.5 μg each. In transfections without an expression construct, the parent expression plasmid was added to keep the total amount of DNA in each transfection constant at 2 μg. Cells were cultured for 48 hours after transfection, then harvested and assayed by using the Luminescent β-gal Detection System (Clontech). SOX10 and MEF2C-VP16 were each expressed from the pRK5 mammalian expression vector (BD Pharmingen).
DOPA staining and electron microscopy
Staining of 3,4-dihydroxy-l-phenylalanine (DOPA) and electron microscopy (EM) on skin samples isolated from neonatal pups from a region of the back located between the shoulders were performed as previously described (Nguyen and Wei, 2007). For EM analyses, DOPA-stained back skin was fixed in modified Karnovsky's fixative (2% paraformaldehyde, 2% glutaraldehyde and 0.06% CaCl2 in 100 mM cacodylate buffer, pH 7.3). The tissue was then washed twice in 100 mM cacodylate buffer, fixed in 1.5% potassium ferrocyanide and 2% osmium tetraoxide for 2 hours, rinsed in water, dehydrated through a series of ethanol washes, and embedded in Epon resin overnight. Epon blocks were sectioned and mounted onto formvar-coated grids. Grids were stained in 10% uranyl acetate in 50% methanol and viewed on a Zeiss 10A electron microscope at 60 kV. Melanosome density per unit area of melanocyte cytoplasm was determined by measuring the cytoplasmic area with NIH ImageJ software and counting DOPA-stained melanosomes within the cytoplasmic area.
Glutathione S-transferase (GST) pull-down and co-immunoprecipitation assays for protein-protein interaction
GST pull-down assays were performed as described previously (Chen et al., 2000). The GST-MEF2C expression plasmid was generated by inserting the entire coding sequence of MEF2C and an in-frame C-terminal FLAG epitope (Khiem et al., 2008) in-frame into the PGEX-2T expression vector (Pharmacia). SOX10 full length and SOX10 DNA binding domain truncation proteins were generated from pCITE-Sox10[FL] and pCITE-Sox10[DBD], respectively. pCITE-Sox10[DBD] contains a 235-amino acid N-terminal fragment of SOX10 that includes the DNA binding domain cloned in-frame into pCITE. The SOX10 full length and DBD proteins were synthesized and radiolabeled with [35S]-methionine in vitro using the TNT Quick Coupled Transcription/Translation System (Promega), according to the manufacturer's directions.
Co-immunoprecipitation was performed in lysates from co-transfected B16F10 mouse melanoma cells. Nearly confluent 10-cm plates were co-transfected with 3 μg each of pRK5-MEF2C-FLAG (Khiem et al., 2008) and pRK5-Sox10[HA] using Lipofectamine LTX reagent according to the manufacturer's protocol. Cells were grown for 30 hours after transfection and then harvested in NP-40 lysis buffer (50 mM Tris-HCl, 150 mM NaCl, 1% NP-40, pH 7.4) containing protease inhibitors for 30 minutes at 4°C with gentle agitation followed by centrifugation to remove insoluble material. Lysates were then incubated overnight at 4°C with 3 μg of mouse anti-MEF-2 antibody (sc-17785, Santa Cruz) or mouse IgG1 antibody (ab18447, Abcam) as an isotype control. Samples were then incubated for 2.5 hours at 4°C with 100 μl of 50% Protein G Sepharose bead in NP-40 lysis buffer. Bound beads were washed 4 times with NP-40 lysis buffer and eluted in SDS sample buffer containing 100 mM DTT and boiled for 4 minutes before analysis by SDS-PAGE. Co-immunoprecipitation was determined by western blot with rabbit anti-HA antibody (sc-805, Santa Cruz) at a 1:200 dilution.
RESULTS
Identification of a Mef2c enhancer that directs expression to several developing neural crest lineages, including melanocytes
Although Mef2c function is required in the neural crest (Verzi et al., 2007), its expression in that lineage is not well defined. This is likely to be due to the strong expression of Mef2c in developing cardiac, skeletal muscle and vascular lineages, making the relatively weaker, but essential, expression of Mef2c in the neural crest difficult to visualize by in situ hybridization or immunohistochemistry (Black and Cripps, 2010; Edmondson et al., 1994). It has been observed previously that the expression of Mef2c during mouse embryogenesis is controlled by several discrete modular enhancers that each direct expression to a subset of the complete endogenous expression domain of Mef2c (Black and Cripps, 2010). Therefore, we reasoned that isolation of Mef2c enhancers with activity restricted to the neural crest might be used to determine the detailed expression of Mef2c within the neural crest without obfuscation from stronger expression in other lineages proximal to or overlying neural crest-derived tissues. Towards that goal, we identified a highly conserved enhancer that exhibited transcriptional activity in the developing neural crest and its derivatives (Fig. 1).
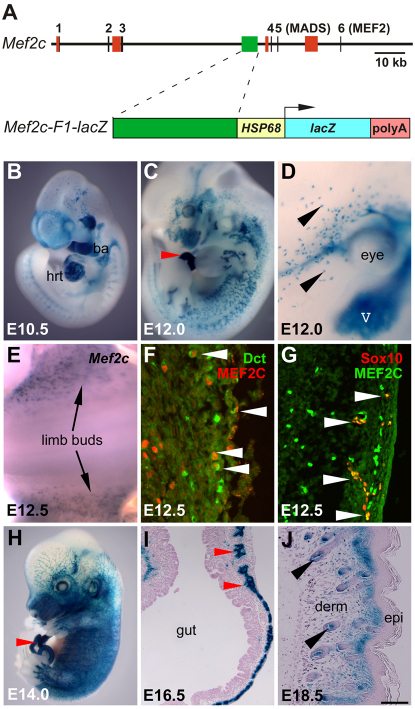
Fig. 1.
Identification of Mef2c-F1, a neural crest enhancer from Mef2c. (A) The top line represents the 5′ end of the mouse Mef2c locus. Vertical black lines depict Mef2c exons. Colored rectangles represent known enhancers. A schematic of the Mef2c-F1-lacZ transgene is shown below. (B-J) Whole-mount (B-E,H) or transverse sections (F,G,I,J) of X-gal-stained Mef2c-F1-lacZ transgenic (B-D,H-J) or wild-type embryos examined by in situ hybridization for Mef2c (E) or by immunofluorescence using antibodies against MEF2C (red in F, green in G), DCT (green in F) and SOX10 (red in G). Black or white arrowheads mark developing melanocytes. Red arrowheads mark peripheral nerves in the gut. V, fifth cranial nerve; ba, branchial arch; derm, dermis; epi, epidermis; hrt, heart. Scale bar: 100 μm.
Mef2c-F1 enhancer activity in the neural crest was detected as early as E8.5 in the neural fold mesenchyme (data not shown). Between E9.0 and E9.5, β-gal was strongly expressed in craniofacial mesenchyme in a pattern similar to endogenous Mef2c at this stage (Verzi et al., 2007) (data not shown). Mef2c-F1 enhancer activity in the craniofacial mesenchyme began to decline by E10.5, but activity became more pronounced in the developing cranial nerves (Fig. 1B). β-Gal activity was also evident in cranial and sympathetic ganglia at E10.5 (Fig. 1B). In addition, Mef2c-F1-lacZ expression was detected in the developing heart (Fig. 1B), but the bona fide regulation of Mef2c in the heart via this enhancer is not a focus of the present study. By E12, β-gal expression in the craniofacial mesenchyme was no longer observed. However, activity in the cranial nerves, sympathetic chain ganglia, peripheral nerves and nerve roots, and enteric ganglia was evident (Fig. 1C). At E14, Mef2c enhancer activity continued throughout the developing peripheral and enteric nervous systems (Fig. 1H). Sections through the developing gut at E16.5 revealed that β-gal expression directed by the Mef2c-F1-lacZ enhancer was within the myenteric plexus (Fig. 1I).
Mef2c-F1-lacZ expression was also evident in the developing melanocyte lineage, beginning at ~E10.5 (Fig. 1B) and continuing throughout embryonic development (Fig. 1J). At E12, robust β-gal activity was apparent in single cells in the peri-ocular region of the embryo (Fig. 1D). This pattern of enhancer activity was consistent with melanocyte expression of the transgene. In perinatal skin, transgene expression was observed in follicular melanocytes and in the melanocytes that occupy the dermal-epidermal border (Fig. 1J). To determine whether the endogenous Mef2c gene was expressed in melanocytes, we examined Mef2c expression by in situ hybridization. As noted above, Mef2c is expressed broadly in many tissues, including the vasculature, somites and nerves (Black and Cripps, 2010; Dodou et al., 2004; Edmondson et al., 1994), making endogenous expression in melanocytes difficult to observe. However, Mef2c expression was evident at E12.5 in the dorsal region of the embryo between the hind limbs in a pattern highly reminiscent of melanocytes (Fig. 1E). To confirm that expression of MEF2C was in melanocytes, we compared the expression of MEF2C protein and the melanocyte marker dopachrome tautomerase (DCT) in the dermis at E12.5 by immunofluorescence (Fig. 1F). Numerous cells co-expressed MEF2C, which was confined to the nucleus, and DCT, which was present in the cytoplasm, confirming MEF2C expression in melanocytes. We also compared expression of MEF2C with SOX10 expression in the dermis at E12.5 and found that all SOX10-expressing cells in the nascent skin also expressed MEF2C (Fig. 1G), although, as expected, many MEF2C-expressing cells did not express SOX10. These results indicate that SOX10 and MEF2C are co-expressed in vivo in a subset of cells in the dermis.
Mice deficient in Mef2c exhibit pigmentation defects at birth
Because of the strong expression of Mef2c and robust activation of the Mef2c-F1 enhancer in melanocytes and in the peripheral and enteric nervous systems, we examined those lineages in detail in Mef2c neural crest knockout (NC KO) neonates to determine whether MEF2C might have requirements in those neural crest-derived lineages. Although Mef2c NC KO mice die at birth of craniofacial defects (Verzi et al., 2007), they exhibited no obvious defects in peripheral or enteric innervation at birth (data not shown). As an initial analysis of melanocyte development, we stained epidermis and dermis taken from the anterior region of the back from Mef2c NC KO and control neonates by incubation with DOPA, which detects pigmented cells, and examined the stained tissue by light microscopy (Fig. 2). We observed a significant reduction in the number of melanocytes in epidermis (Fig. 2, compare panels A and B) and dermis (Fig. 2, compare panels C and D) in Mef2c NC KO mice compared with littermate controls. Within the dermis, we observed a strong reduction in the number of both follicular and interfollicular melanocytes (Fig. 2C,D). Indeed, the number of DOPA-stained cells per field showed that the number of melanocytes in the epidermis was reduced by 87% in Mef2c NC KO neonates compared with littermate controls (Fig. 2E). These observations demonstrate that MEF2C is required for establishing or maintaining the proper number of melanocytes in vivo.
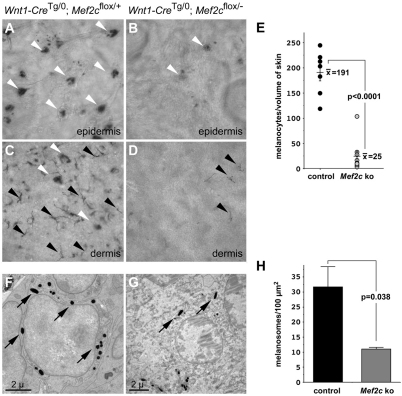
Fig. 2.
Mef2c is required for melanocyte development in mice. (A-D) Whole-mount DOPA-stained epidermis (A,B) or dermis (C,D) from Wnt1-CreTg/0; Mef2cflox/+ control (A,C) and Wnt1-CreTg/0; Mef2cflox/− neural crest knockout (NC KO; B,D) neonatal mice showed many fewer stained follicular (white arrowheads) and interfollicular (black arrowheads) melanocytes in Mef2c NC KO skin compared with controls. (E) Quantification of DOPA-stained melanocytes from Wnt1-CreTg/0; Mef2cflox/+ control and Wnt1-CreTg/0; Mef2cflox/− NC KO epidermis. Filled circles represent individual control mice (191±16 DOPA-stained melanocytes per sample, n=7) and open circles represent individual Mef2c NC KO mice (25±10 DOPA-stained melanocytes per sample, n=9), P<0.0001. (F,G) Electron micrographs of DOPA-stained melanocytes from Wnt1-CreTg/0; Mef2cflox/+ control mice (F) and Wnt1-CreTg/0; Mef2cflox/− NC KO mice (G) showed that Mef2c NC KO mice had fewer melanosomes per melanocyte (black arrows). (H) Quantification of melanosomes in individual DOPA-stained control and Mef2c NC KO neonates indicated the mean number of melanosomes in 100 μm2 for control skin was 31.7±6.8; n=3 mice. The mean number of melanosomes in 100 μm2 within stained melanocytes in Mef2c NC KO neonates was 11.0±0.6; n=3 mice (P=0.038). An average of six randomly selected melanocytes from each neonatal mouse was scored. Data are expressed as the mean number of melanosomes in 100 μm2 + s.e.m.
Analysis of neonatal skin by electron microscopy revealed that the few remaining individual melanocytes in the dermis of Mef2c NC KO mice had fewer melanosomes than did the melanocytes present in littermate controls (Fig. 2, compare panels F and G). Quantification indicated a 65% reduction in the number of electron-dense melanosomes in dermal melanocytes from Mef2c NC KO neonates compared with their littermate controls (Fig. 2H). These data suggest that, in addition to being required for the proper number of melanocytes in the skin, MEF2C function is also required in the neural crest or in neural crest-derived cells for melanocyte function or differentiation in vivo.
Mef2c is required for proper developmental expression of melanocyte marker genes
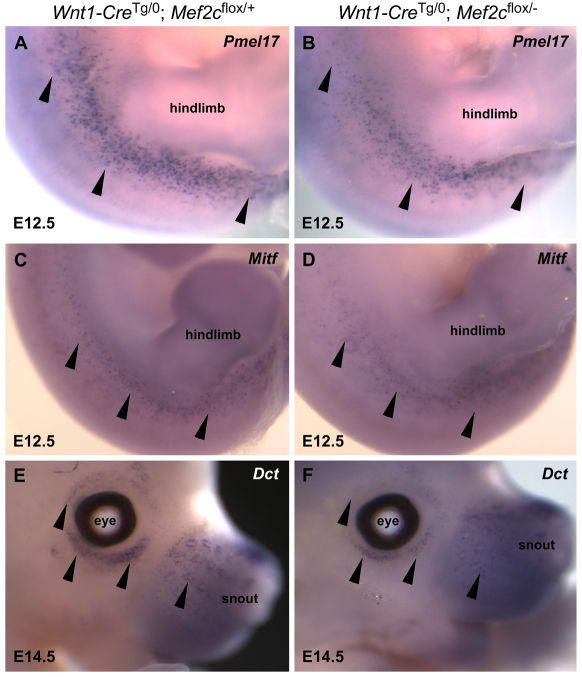
We also examined the expression of well-established markers of melanocyte differentiation and function in Mef2c NC KO and control embryos (Fig. 3). Interestingly, Mitf, Dct and Pmel17 all had reduced expression in Mef2c NC KO embryos compared with their littermate controls (Fig. 3). At E12.5, Pmel17 expression was reduced, including in the trunk region around the hind limbs (Fig. 3, compare panels A and B). Similarly, Mitf expression was reduced in the same region at E12.5 in Mef2c NC KO embryos (Fig. 3, compare panels C and D). Reduced expression of melanocyte markers was apparent in multiple regions of the embryo and over several days of development in Mef2c NC KO embryos. For example, the expression of the melanocyte differentiation marker Dct was clearly reduced in the region around the eyes in Mef2c NC KO embryos at E14.5 (Fig. 3, compare panels E and F). Quantification of Dct-labeled melanocytes in the interlimb region at E12.5 showed that Mef2c NC KO embryos had on average only 69% as many labeled cells as control embryos (356±98 labeled cells/embryo, n=4 embryos for Mef2c NC KO versus 514±133 labeled cells/embryo, n=4 embryos for control, P=0.0027 by Student's t-test).
Fig. 3.
Mef2c is required for normal expression of melanocyte marker genes during development. (A-F) Whole-mount in situ hybridization for Pmel17 (A,B), Mitf (C,D) and Dct (E,F) transcripts from Wnt1-CreTg/0; Mef2cflox/− conditional knockout embryos (B,D,F) showed reduced expression of each of the melanocyte markers during embryonic development compared with expression in Wnt1-CreTg/0; Mef2cflox/+ control embryos (A,C,E). Black arrowheads point to melanocytes in the trunk region at E12.5 and in the sub- and supra-optic region at E14.5.
A small, evolutionarily conserved region that is necessary and sufficient for Mef2c-F1 neural crest enhancer activity in vivo contains conserved MEF2- and SOX-binding sites
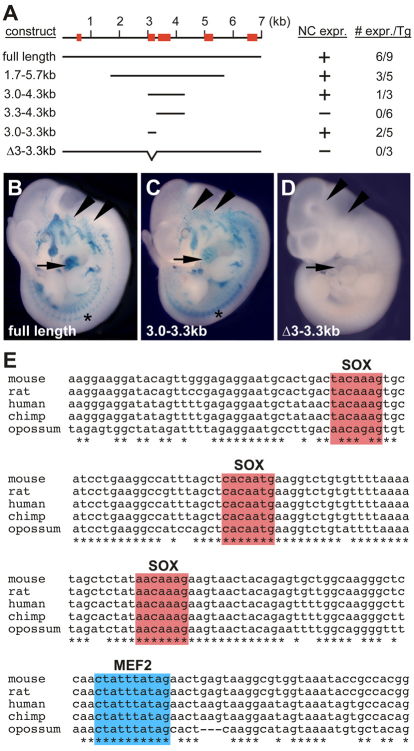
To place Mef2c into a pathway for neural crest and melanocyte development, we next examined the Mef2c-F1 neural crest enhancer for essential cis-regulatory elements by generating a series of deletions within the 7-kb conserved fragment (Fig. 4A). These analyses identified a 300-bp region (3-3.3 kb) that was sufficient for Mef2c-F1 enhancer function in vivo and directed β-gal expression in the same pattern as the full length Mef2c-F1 fragment at E11.5 (Fig. 4, compare panels B and C). This 300-bp region was also required for enhancer function in the context of the full-length 7-kb Mef2c-F1 fragment, as deletion of this region completely abolished enhancer activity in transgenic embryos at E11.5 (Fig. 4D).
Fig. 4.
A minimal fragment of the Mef2c-F1 neural crest enhancer is necessary and sufficient for enhancer activity and contains consensus binding sites for MEF2 and SOX10. (A) Schematic of the Mef2c-F1 enhancer region and the deletion constructs tested for enhancer activity in transgenic mice. Red rectangles depict regions of high conservation between the marsupial opossum and the mouse genomic sequences. The column on the left lists the regions of the Mef2c-F1 fragment tested in deletion analyses. The columns on the right denote the activity and the fraction of independently derived transgenic (Tg) embryos that displayed activity in neural crest (NC) derivatives. (B-D) Representative transgenic embryos for the indicated constructs. Asterisks denote transgene expression in neural crest-derivatives in the trunk. Arrows denote the location of the heart. Arrowheads indicate regions of melanocyte progenitors. (E) ClustalW analysis of Mef2c gene sequences in the core region of the 300-bp necessary and sufficient region of the Mef2c-F1 enhancer. Asterisks denote conserved nucleotides.
We examined the highly conserved, crucial 300-bp region from Mef2c-F1 for candidate cis-regulatory sequences that might be involved in neural crest expression. These analyses revealed three putative SOX binding sites and a single candidate MEF2 binding site (Fig. 4E). The presence of the conserved SOX elements in the Mef2c-F1 enhancer further suggested the possibility that a SOX transcription factor might be a direct upstream activator of Mef2c.
Previous studies have shown that Sox10-null embryos have a nearly complete lack of melanocyte marker expression as early as E11 (Kapur, 1999; Potterf et al., 2001; Southard-Smith et al., 1998). The observations that Mef2c and Sox10 are each required for proper melanocyte differentiation and share common downstream targets in melanocytes, including Dct, Mitf and Pmel17, and that the two factors are co-expressed in melanocytes in the dermis (Fig. 1G) is consistent with the idea that the two transcription factors might function in a common pathway in the neural crest and its derivatives. Based on the observation that the Mef2c NC KO pigmentation phenotype was similar, but substantially less severe, than the pigmentation phenotype in Sox10-null embryos, we hypothesized that Mef2c might function downstream of Sox10 during trunk neural crest development. Moreover, we found that the expression of Sox10 was unaffected in the peripheral nervous system in Mef2c NC KO embryos when compared with control embryos (data not shown), which is consistent with the notion that Sox10 might function upstream of Mef2c in the trunk neural crest and its derivatives.
Mef2c-F1 is bound and activated by SOX10 and MEF2C
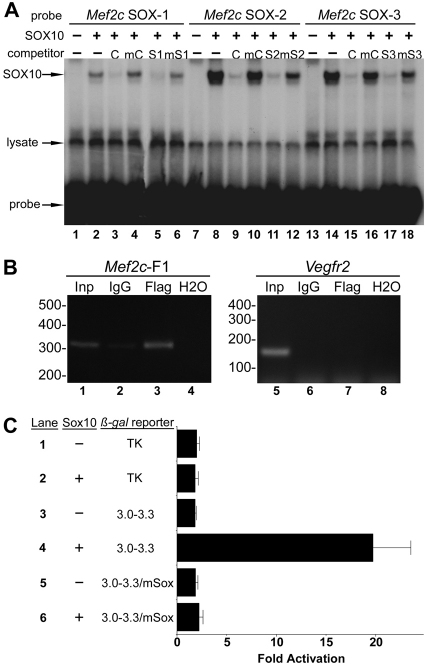
To determine whether the putative SOX elements in the Mef2c-F1 enhancer might represent bona fide cis-acting elements, each was tested for SOX10 binding in vitro by EMSA (Fig. 5A). Indeed, SOX10 bound robustly and specifically to each of the putative SOX sites from the Mef2c-F1 neural crest enhancer (Fig. 5A, lanes 2, 8 and 14). We also examined the ability of SOX10 to bind to the Mef2c-F1 SOX binding sites in the endogenous Mef2c locus by ChIP in vivo in B16F10 mouse melanoma cells transfected with FLAG epitope-tagged SOX10 (Fig. 5B). Importantly, the DNA fragment encompassing the SOX sites in the endogenous Mef2c-F1 enhancer was precipitated and amplified by the anti-FLAG antibody (Fig. 5B, lane 3) but not by the isotype control IgG antibody (Fig. 5B, lane 2). Furthermore, precipitation with anti-FLAG antibody did not result in PCR amplification of a bona fide enhancer from the Vegfr2 (Kdr – Mouse Genome Informatics) gene (De Val et al., 2008), which was used as a non-specific enhancer target (Fig. 5B, lane 7). These results indicate that SOX10 binds to the SOX sites in the Mef2c-F1 enhancer in vitro and in vivo in a relevant cell line.
Fig. 5.
SOX10 binds and activates the Mef2c-F1 enhancer in a SOX site-dependent manner. (A) In vitro-translated SOX10 was incubated with the following Mef2c-F1 radiolabeled probes: SOX-1 (lanes 1-6), SOX-2 (lanes 7-12) and SOX-3 (lanes 13-18). SOX10 efficiently retarded the mobility of each of the sites (lanes 2, 8, 14). Binding was competed by excess unlabeled control probe (C; lanes 3, 9, 15) but not by a mutant version (mC; lanes 4, 10, 16). Binding was also competed by excess unlabeled self probe (S1, lane 5; S2, lane 11; S3, lane 17) but not by excess mutant versions (mS1, lane 6; mS2, lane 12; mS3, lane 18). Unprogrammed reticulocyte lysates are shown in lanes 1, 7 and 13. Lysate-derived, non-specific bands and the free, unbound probe are indicated. (B) Sox10[Flag]-transfected B16F10 mouse melanoma cells were subjected to ChIP to detect SOX10 bound to the endogenous Mef2c-F1 enhancer using an anti-FLAG antibody. Following ChIP, the region of the Mef2c gene surrounding the SOX sites in the Mef2c-F1 enhancer (lanes 1-4), or a region of the Vegfr2 enhancer (lanes 5-8) as a non-specific control, was amplified by PCR and analyzed by agarose gel electrophoresis. Lanes 1 and 5: amplification prior to immunoprecipitation; lanes 2 and 6: amplification following non-specific isotype-matched IgG ChIP; lanes 3 and 7: amplification following ChIP with anti-FLAG to detect SOX10-bound DNA; lanes 5 and 8: amplification without added template (H20). Sizes in bp are shown. (C) Co-transfection of the Mef2c[3-3.3]-F1-TK-lacZ reporter plasmid with pRK5-Sox10 expression plasmid (+) or with pRK5 (−) resulted in strong activation in a SOX10-dependent manner (lanes 3, 4). Mutation of all three SOX sites abolished the ability of SOX10 to transactivate the Mef2c-F1 enhancer (lane 6). SOX10 did not transactivate the parental TK-lacZ reporter (lane 2). Data are expressed as the mean fold activation + s.e.m. from three independent transfections and analyses.
To determine whether the binding of SOX10 to the Mef2c-F1 enhancer was functionally relevant, we tested the ability of SOX10 to trans-activate the Mef2c-F1 enhancer (Fig. 5C). Co-transfection of a SOX10 expression plasmid with the 300-bp Mef2c reporter plasmid Mef2c[3-3.3]-F1-TK-lacZ resulted in robust activation of the reporter gene (Fig. 5C, lane 4). This activation was dependent upon the presence of the SOX binding sites, as mutation of these elements in the Mef2c enhancer eliminated SOX10-dependent activation (Fig. 5C, lane 6). Taken together with the EMSA results shown in Fig. 5A and the ChIP results shown in Fig. 5B, these trans-activation studies strongly support the notion that the SOX binding sites in the Mef2c-F1 enhancer represent bona fide SOX response elements and that Mef2c is a direct transcriptional target of SOX10.
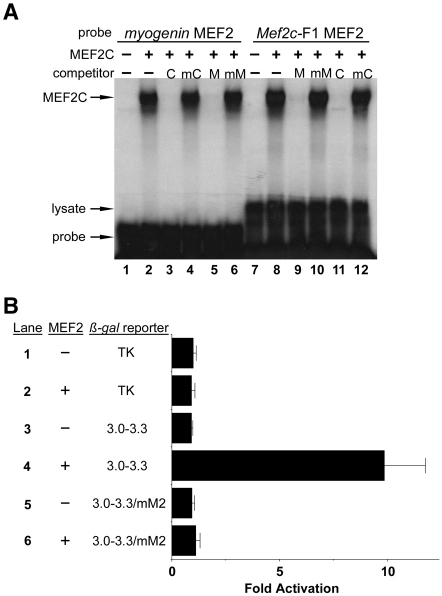
We also examined the MEF2 site in the Mef2c-F1 neural crest enhancer for binding by MEF2C in EMSA (Fig. 6A). MEF2C bound robustly to the bona fide MEF2 element from the myogenin promoter (Dodou et al., 2003) and to the MEF2 site from Mef2c-F1 (Fig. 6A, lanes 2 and 8). The MEF2 element in the Mef2c-F1 enhancer was also responsive to MEF2-dependent activation in C3H10T1/2 cells (Fig. 6B). Co-transfection of a MEF2C-VP16 expression plasmid with the 300-bp Mef2c-F1 enhancer plasmid Mef2c[3-3.3]-F1-TK-lacZ resulted in significant trans-activation (Fig. 6B, lane 4). The activation of the Mef2c-F1 enhancer by MEF2C was dependent on the MEF2 site in the enhancer as mutation of the site completely abolished activation (Fig. 6B, lane 6). Together, the results of Fig. 6A,B supports the notion that the MEF2 element in the Mef2c-F1 enhancer represents a bona fide binding site, and suggest that Mef2c is subject to auto-regulation by itself or cross-regulation by other members of the MEF2 family in developing neural crest lineages.
Fig. 6.
MEF2C binds the MEF2 site in the Mef2c-F1 enhancer. (A) In vitro translated MEF2C was incubated with radiolabeled probes corresponding to a consensus bona fide MEF2 site from the myogenin promoter (lanes 1-6) or from the Mef2c-F1 enhancer (lanes 7-12). MEF2C bound the Mef2c-F1 MEF2 (lane 8) and myogenin control MEF2 (lane 2) sites with similar affinity. Excess unlabeled Mef2c-F1 MEF2 site (M; lanes 5, 9) and myogenin control (C; lanes 3, 11) probes each competed efficiently for binding. Mutant versions of the control probe (mC) or the Mef2c-F1 probe (mM) failed to compete for binding to either labeled probe (lanes 4, 6, 10, 12). Unprogrammed reticulocyte lysate was used in EMSA with each probe (lanes 1, 7) and no specific binding was observed. Lysate-derived, non-specific binding and the free, unbound probe are indicated. (B) Co-transfection of the Mef2c[3-3.3]-F1-TK-lacZ reporter plasmid with a MEF2C-VP16 activator plasmid (+) or with pRK5 (−) demonstrates that MEF2 can activate the Mef2c-F1 enhancer (lanes 3, 4). Mutation of the MEF2 site in Mef2c-F1 completely abolished activation by MEF2C-VP16 (lane 6). No activation of the parental minimal promoter-containing reporter plasmid TK-lacZ by MEF2C-VP16 was observed (lane 2). Data are expressed as the mean fold activation + s.e.m. from three independent transfections and analyses.
MEF2C and SOX10 physically interact and cooperatively activate transcription
Based on the observation that MEF2C and SOX10 each trans-activated the Mef2c-F1 enhancer (Fig. 5C and Fig. 6B), we tested whether the two could cooperatively activate the enhancer (Fig. 7A). Indeed, when SOX10 and MEF2C-VP16 expression plasmids were co-transfected with the 300-bp Mef2c-F1 reporter plasmid, the two transcription factors synergistically activated the enhancer (Fig. 7A, lane 7). The strong transcriptional synergy by MEF2C and SOX10 suggested that the two transcription factors might physically associate. We tested this possibility in GST pull-down assays in vitro and co-immunoprecipitation assays in vivo (Fig. 7B,C). In control reactions, neither SOX10 nor an N-terminal fragment of SOX10, which includes the HMG DNA-binding domain, interacted with GST alone (Fig. 7B, lanes 2 and 4). However, full length SOX10 and the HMG domain of SOX10 each specifically interacted with GST-MEF2C fusion protein (Fig. 7B, lanes 1 and 3). Interaction of GST-MEF2C with the N-terminal portion of SOX10 was more robust than the interaction with full length SOX10 protein (Fig. 7B, compare lanes 1 and 3), which is consistent with the interaction that has been observed previously between another SOX protein, SOX18, and MEF2C (Hosking et al., 2001). SOX10 and MEF2C also interacted in vivo (Fig. 7C). B16F10 mouse melanoma cells were transfected with MEF2C and HA-tagged SOX10 expression constructs and were harvested and subjected to immunoprecipitation with anti-MEF2C antibody. Following immunoprecipitation, cells were subjected to western blot analyses with anti-HA to detect co-precipitated SOX10. HA-SOX10 was specifically detected only in the presence of anti-MEF2C antibody (Fig. 7C, lane 4) and not by isotype-matched IgG (Fig. 7C, lane 3) or by beads alone (Fig. 7C, lane 2), indicating that SOX10 was specifically precipitated by MEF2C co-expression in B16F10 cells. Taken together, these results show that MEF2C and SOX10 cooperatively activate the Mef2c-F1 neural crest enhancer, and suggest that this transcriptional synergy might be dependent on a physical association between the two transcription factors.
Fig. 7.
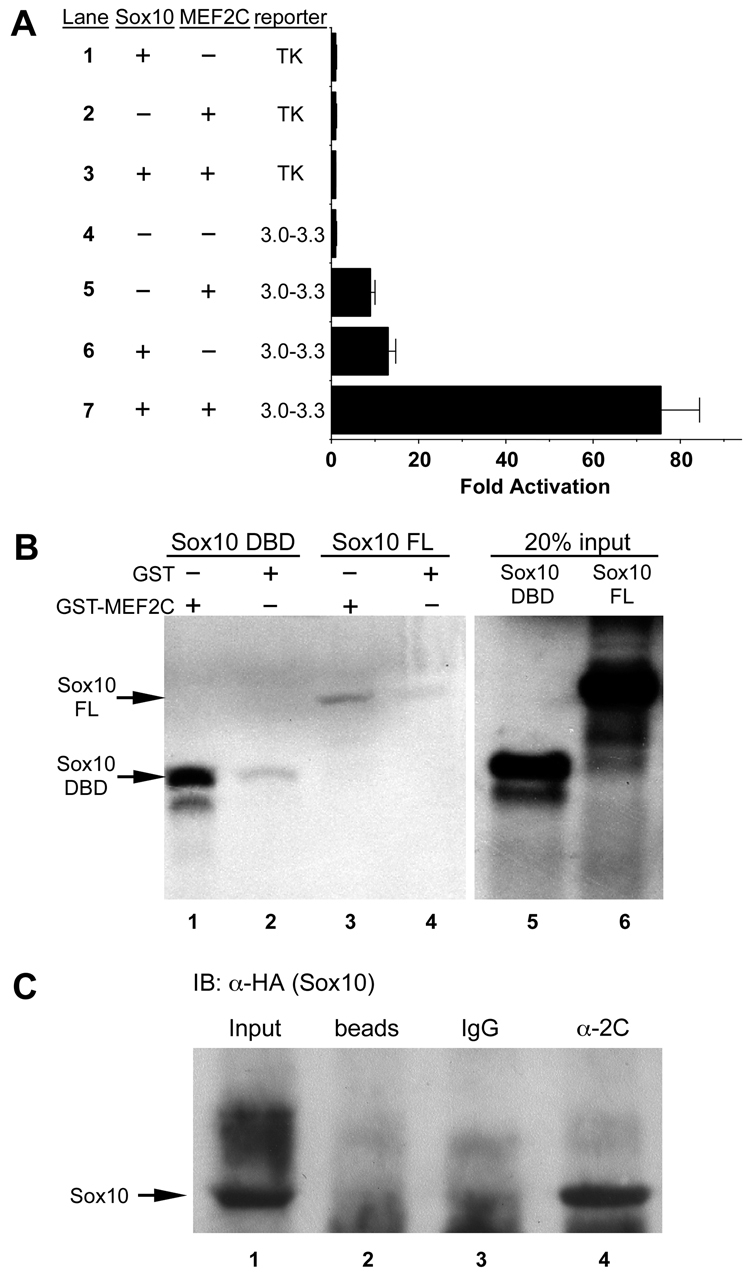
MEF2C and SOX10 physically interact and synergistically activate the Mef2c-F1 enhancer. (A) Co-transfection of expression plasmids for MEF2C-VP16 and SOX10 with the Mef2c[3-3.3]-F1-TK-lacZ reporter plasmid resulted in synergistic activation of the Mef2c-F1 enhancer (lane 7) compared with transfection of the reporter with MEF2C-VP16 (lane 5) or SOX10 (lane 6) alone. No activation of the reporter with empty expression vectors or of the parental TK-lacZ reporter was observed (lanes 1-4). Data are expressed as the mean fold activation + s.e.m. from three independent transfections and analyses. (B) Agarose conjugated to either GST-MEF2C (lanes 1, 3) or GST alone (lanes 2, 4) were used as bait in pull-down assays with radiolabeled DNA binding domain (DBD) of SOX10 (lanes 1, 2) or with radiolabeled full length (FL) SOX10 (lanes 3, 4). Twenty percent of the input amount of radiolabeled SOX10 DBD and SOX10 FL are included in lanes 5 and 6, respectively. (C) MEF2C and SOX10 interact in vivo in B16F10 melanoma cells. MEF2C- and SOX10[HA]-transfected cells were subjected to immunoprecipitation with anti-MEF2C followed by western blot analysis with anti-HA (SOX10). SOX10 was only detected following precipitation with anti-MEF2C (α-2C, lane 4) but not with isotype-matched IgG (lane 3) or with beads alone (lane 2). Lane 1 contains input sample only for western blot (no prior immunoprecipitation) as a positive control for SOX10[HA] detection.
Mef2c is a direct transcriptional target of SOX10 in developing neural crest lineages
To test the requirement for the MEF2 and SOX cis-regulatory elements in the Mef2c-F1 enhancer in vivo, the MEF2 site or all three SOX sites were mutated in the context of the full-length 7-kb Mef2c-F1 enhancer, which was then tested for activity in transgenic embryos (Fig. 8). Mutation of the MEF2 site in the enhancer had only a partial effect on the activity of the enhancer in the nascent neural crest at E8.5 (Fig. 8, compare panels A and B, arrowheads) but resulted in a nearly complete loss of transgene expression by E11.5 (Fig. 8, compare panels C and D). These observations suggest that the initial activation of Mef2c-F1 does not require the MEF2 site but that maintenance of Mef2c expression in the neural crest via the Mef2c-F1 enhancer requires auto- and cross-regulation by MEF2C and other MEF2 factors.
Fig. 8.
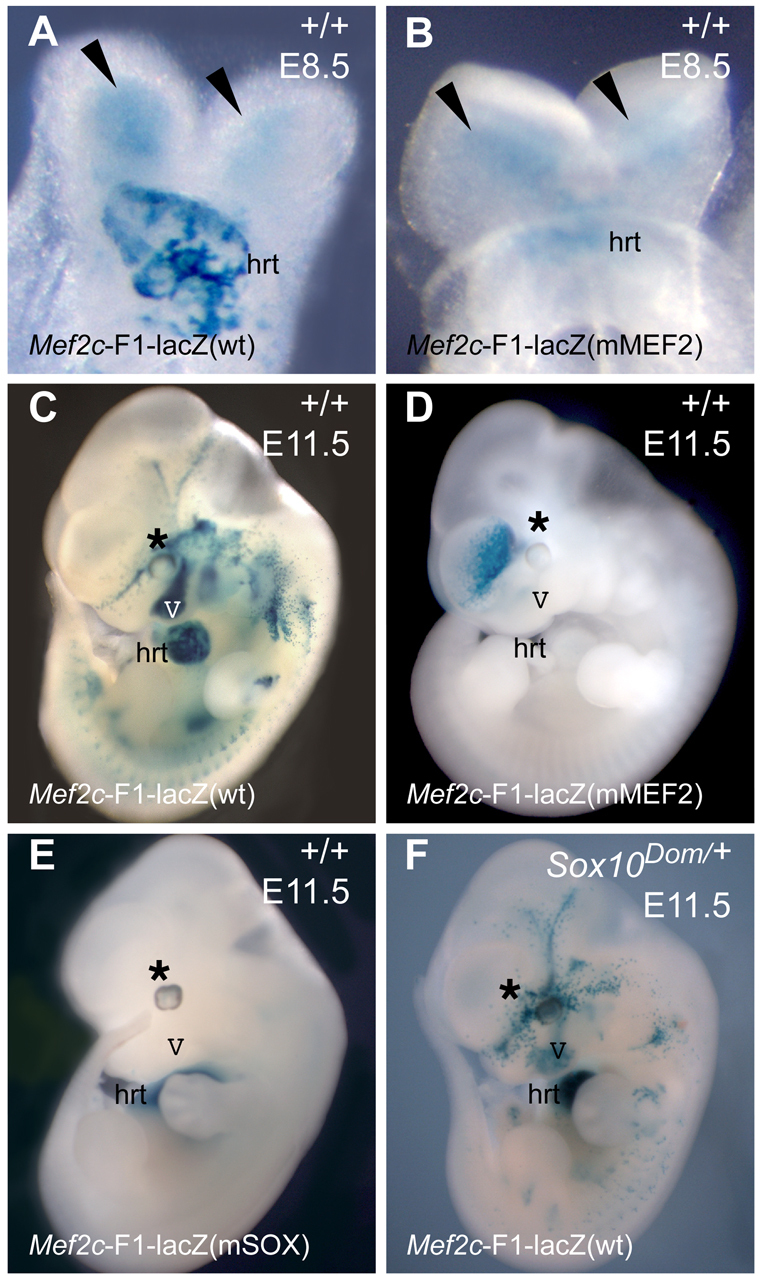
The Mef2c-F1 neural crest enhancer is regulated by MEF2 and SOX10 in vivo. (A,C) The wild-type Mef2c-F1 transgene in an otherwise wild-type background (+/+) directs expression to the nascent neural crest adjacent to the neural folds at E8.5 (A) and in neural crest derivatives, including melanocytes, at E11.5 (C). The same pattern was observed in six out of nine independent transgenic lines examined at both E8.5 and E11.5. (B,D) Mutation of the MEF2 site in Mef2c-F1 completely abolished transgene expression in the neural crest and its derivatives at E11.5 (D) but not at E8.5 (B). Note that the expression in the forebrain region in D was not reproducible and represents ectopic transgene expression. Similar activity of the MEF2 mutant transgene was observed in each of three independent transgenic lines examined at E8.5 and E11.5 and an additional three F0 transient transgenic embryos examined at E11.5 only. (E) Mutation of all three SOX sites in the Mef2c-F1 enhancer abolished transgene expression in neural crest derivatives, but not in the heart (hrt), in each of five independent mSOX transgenic lines examined. (F) Crossing the wild-type Mef2c-F1-lacZ transgene into a Sox10 heterozygous (Sox10Dom/+) background resulted in a diminution of transgene expression in neural crest derivatives at E11.5 but did not affect transgene expression in the heart. Arrowheads indicate neural crest cells. Asterisks indicate melanocytes in the supraocular region at E11.5. hrt, heart; V, fifth cranial nerve.
Mutation of the three SOX sites in the Mef2c-F1 enhancer also abolished expression in all neural crest-derived lineages at all stages of embryonic development, including at E11.5 (Fig. 8, compare panels C and E). Interestingly, the SOX sites were not required for Mef2c-F1 enhancer activity in the heart at E11.5 (Fig. 8E), demonstrating the specificity of those sites for neural crest-specific activity of the enhancer in vivo. To test specifically the role of SOX10 as a possible upstream activator of Mef2c via the Mef2c-F1 enhancer, we crossed mice harboring the wild-type Mef2c-F1-lacZ transgene into a heterozygous Sox10-mutant background (Sox10Dom/+). Mef2c-F1 enhancer activity was significantly reduced in all neural crest lineages when one functional copy of Sox10 was missing (Fig. 8, compare panels C and F), further supporting a possible role for SOX10 specifically as an upstream regulator of Mef2c in the neural crest.
DISCUSSION
A role for MEF2C in melanocyte development
MEF2C is a well-known regulator of gene expression in skeletal and cardiac muscle, the central nervous and immune systems, and the axial and craniofacial skeletons (Black and Cripps, 2010; Potthoff and Olson, 2007). Here, we show that loss of Mef2c in the neural crest results in a profound deficit in pigmentation due to a reduction in the number of melanocytes in the skin and impairment of melanocyte differentiation and function. Interestingly, in other lineages in which MEF2C function is required, such as in cardiomyocytes and endothelial cells, initial lineage specification occurs, but the differentiated phenotype fails to stabilize and eventually development of the heart and vasculature collapses in the absence of MEF2C (Bi et al., 1999; Lin et al., 1998; Lin et al., 1997). The phenotype that we observed here is similar in that markers of the melanocyte lineage, including Mitf, Dct and Pmel17 are reduced but still clearly present in Mef2c NC KO fetuses (Fig. 3). However, by birth, pigmentation was much more severely impaired (Fig. 2). We considered the possibility that the observed dramatic loss of pigmentation might be caused by loss of melanocytes due to apoptosis or impaired proliferation between E13.5 and birth, but we observed no differences in TUNEL staining or in BrdU incorporation from E11.5 to E18.5 (data not shown). It is possible that proliferation or apoptosis were affected but below the level of detection of our assays. However, we favor the hypothesis that MEF2C function is required to maintain the differentiation phenotype in melanocytes. The observation that MEF2C function was required for appropriate melanosome number (Fig. 2F-H) supports this notion.
MEF2C and SOX10 function as a positively reinforcing transcriptional circuit
Feed-forward transcriptional regulation is a common theme in developmental gene regulation, in which this positive-acting mechanism helps to reinforce developmental decisions to differentiate (Amin et al., 2010; Penn et al., 2004; Sandmann et al., 2007; Tapscott, 2005; Verzi et al., 2007). MEF2 transcription factors are known to participate in this type of self-reinforcing transcriptional circuitry in other systems, in which they often function to potentiate the activity of other transcription factors in a feed-forward fashion (Black and Cripps, 2010). Here, we identified MEF2C as a direct transcriptional target of SOX proteins and demonstrate that it can function as a partner of SOX10. The function of SOX10 in different neural crest-derived lineages is spatially and temporally modulated by its interaction with other transcriptional factors, including PAX3, MITF, KROX-20 (EGR2 – Mouse Genome Informatics) and SP1, and the functional interaction of these transcriptional partners with SOX10 is often facilitated by direct physical association (Jiao et al., 2004; Kuhlbrodt et al., 1998; Lang and Epstein, 2003; Ludwig et al., 2004; Melnikova et al., 2000). Consistent with this cofactor-based role for SOX10 in neural crest development, we observed a direct physical association between SOX10 and MEF2C (Fig. 7). We also observed co-expression of MEF2C and SOX10 in the same cells during development (Fig. 1G), further supporting the notion that SOX10 might function as a bona fide regulator and cofactor of MEF2C. However, we cannot rule out the possibility that other SOX factors, such as SOX9, might also be involved in Mef2c regulation during neural crest development. Based on the direct regulation of Mef2c by the novel Mef2c-F1 enhancer identified here, the synergistic activation of transcription by MEF2C and SOX10, and the direct physical interaction and co-expression of the two factors, we propose a model in which SOX10 cooperates with MEF2 to activate Mef2c expression in melanocytes in a feed-forward transcriptional pathway.
MEF2C as a candidate gene involved in neural crest disorders
Mutations in human MEF2C are strongly associated with neuronal disorders, including seizures, mental retardation and epilepsy (Le Meur et al., 2010; Novara et al., 2010; Nowakowska et al., 2010), demonstrating the important role for MEF2C in the development of neuronal lineages in humans. Mutations in human SOX10 often cause a severe form of Waardenburg syndrome (Type IV, also known as Waardenburg-Hirschsprung disease), which involves aganglionic colon and severe pigmentation defects (Parisi and Kapur, 2000; Pingault et al., 1998; Pingault et al., 2010). In addition to SOX10, mutations in genes encoding other transcription factors known to function as SOX10 cofactors or to function in a common pathway with SOX10, also cause various forms of Waardenburg syndromes, including PAX3, MITF and SNAI2 (Pingault et al., 2010). Interestingly, only 50% of human patients with classic symptoms of Waardenburg-Hirschsprung disease have mutations in known disease loci, and even in cases with known genetic causes, the severity or the symptoms are often highly variable (Parisi and Kapur, 2000; Pingault et al., 1998; Pingault et al., 2010; Southard-Smith et al., 1999). Distinct mutations in human SOX10 are associated with different severity of disease, depending on the effect of the various mutations on SOX10 protein function (Inoue et al., 2004). It has also been postulated that some of the variability observed in Waardenburg-Hirschsprung patients with SOX10 mutations is due to modifier genes, which are largely hypothetical, because few modifiers of SOX10 have been defined in humans (Parisi and Kapur, 2000; Pingault et al., 2010). In mice, however, several modifiers of Sox10 function have been identified, including genes encoding endothelin signaling components and the related SOX protein SOX8 (Maka et al., 2005; Stanchina et al., 2006). It is important to note that neither heterozygous Mef2c mice nor Mef2c neural crest conditional knockout mice exhibit aganglionosis of the bowel (Lin et al., 1997; Verzi et al., 2007), a hallmark of Waardenburg-Hirschsprung disease, and we did not observe any exaggeration of the Sox10Dom/+ phenotype when crossed into a Mef2c heterozygous background (data not shown). However, we do show here that loss of Mef2c function in the neural crest results in hypopigmentation at birth, which is characteristic of many forms of Waardenburg syndromes; therefore, the MEF2C gene should be considered for a direct or modifier role in congenital neural crest disorders in humans.
Acknowledgements
The authors thank Ivy Hsieh and Jillian Jarrett for excellent technical assistance. This work was supported by Grant #6-FY08-311 from the March of Dimes and R01 DE019118 from the NIDCR to B.L.B. and by grants from the ACS and the Melanoma Research Alliance to M.L.W. Deposited in PMC for release after 12 months.
Footnotes
Competing interests statement
The authors declare no competing financial interests.
References
- Amin N. M., Shi H., Liu J. (2010). The FoxF/FoxC factor LET-381 directly regulates both cell fate specification and cell differentiation in C. elegans mesoderm development. Development 137, 1451-1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker C. V., Bronner-Fraser M. (1997). The origins of the neural crest. Part I: embryonic induction. Mech. Dev. 69, 3-11. [DOI] [PubMed] [Google Scholar]
- Baxter L. L., Hou L., Loftus S. K., Pavan W. J. (2004). Spotlight on spotted mice: a review of white spotting mouse mutants and associated human pigmentation disorders. Pigment Cell Res. 17, 215-224. [DOI] [PubMed] [Google Scholar]
- Bi W., Drake C. J., Schwarz J. J. (1999). The transcription factor MEF2C-null mouse exhibits complex vascular malformations and reduced cardiac expression of angiopoietin 1 and VEGF. Dev. Biol. 211, 255-267. [DOI] [PubMed] [Google Scholar]
- Black B. L., Cripps R. M. (2010). Myocyte enhancer factor 2 transcription factors in heart development and disease. In Heart Development and Regeneration, Vol. 2 (ed. Rosenthal N., Harvey R. P.), pp. 673-699. Oxford: Academic Press. [Google Scholar]
- Britsch S., Goerich D. E., Riethmacher D., Peirano R. I., Rossner M., Nave K. A., Birchmeier C., Wegner M. (2001). The transcription factor Sox10 is a key regulator of peripheral glial development. Genes Dev. 15, 66-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen N., Baudino T., MacDonald P. N., Green M., Kelley W. L., Burnett J. W., Buller R. M. (2000). Selective inhibition of nuclear steroid receptor function by a protein from a human tumorigenic poxvirus. Virology 274, 17-25. [DOI] [PubMed] [Google Scholar]
- Crane J. F., Trainor P. A. (2006). Neural crest stem and progenitor cells. Annu. Rev. Cell Dev. Biol. 22, 267-286. [DOI] [PubMed] [Google Scholar]
- Danielian P. S., Muccino D., Rowitch D. H., Michael S. K., McMahon A. P. (1998). Modification of gene activity in mouse embryos in utero by a tamoxifen-inducible form of Cre recombinase. Curr. Biol. 8, 1323-1326. [DOI] [PubMed] [Google Scholar]
- De Val S., Chi N. C., Meadows S. M., Minovitsky S., Anderson J. P., Harris I. S., Ehlers M. L., Agarwal P., Visel A., Xu S. M., et al. (2008). Combinatorial regulation of endothelial transcription by Ets and Forkhead transcription factors. Cell 135, 1053-1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodou E., Xu S. M., Black B. L. (2003). mef2c is activated directly by myogenic basic helix-loop-helix proteins during skeletal muscle development in vivo. Mech. Dev. 120, 1021-1032. [DOI] [PubMed] [Google Scholar]
- Dodou E., Verzi M. P., Anderson J. P., Xu S. M., Black B. L. (2004). Mef2c is a direct transcriptional target of ISL1 and GATA factors in the anterior heart field during mouse embryonic development. Development 131, 3931-3942. [DOI] [PubMed] [Google Scholar]
- Dupin E., Le Douarin N. M. (2003). Development of melanocyte precursors from the vertebrate neural crest. Oncogene 22, 3016-3023. [DOI] [PubMed] [Google Scholar]
- Edmondson D. G., Lyons G. E., Martin J. F., Olson E. N. (1994). Mef2 gene expression marks the cardiac and skeletal muscle lineages during mouse embryogenesis. Development 120, 1251-1263. [DOI] [PubMed] [Google Scholar]
- Herbarth B., Pingault V., Bondurand N., Kuhlbrodt K., Hermans-Borgmeyer I., Puliti A., Lemort N., Goossens M., Wegner M. (1998). Mutation of the Sry-related Sox10 gene in Dominant megacolon, a mouse model for human Hirschsprung disease. Proc. Natl. Acad. Sci. USA 95, 5161-5165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan B., Beddington R., Costantini F., Lacy E. (1994). Manipulating the Mouse Embryo. Plainview, NY: Cold Spring Harbor Laboratory Press. [Google Scholar]
- Hong C. S., Saint-Jeannet J. P. (2005). Sox proteins and neural crest development. Semin. Cell Dev. Biol. 16, 694-703. [DOI] [PubMed] [Google Scholar]
- Horton R. M. (1997). In vitro recombination and mutagenesis of DNA: SOEing together tailor-made genes. In PCR Cloning Protocols, Vol. 67 (ed. White B. A.), pp. 141-149. Totowa, NJ: Humana Press. [DOI] [PubMed] [Google Scholar]
- Hosking B. M., Wang S. C., Chen S. L., Penning S., Koopman P., Muscat G. E. (2001). SOX18 directly interacts with MEF2C in endothelial cells. Biochem. Biophys. Res. Commun. 287, 493-500. [DOI] [PubMed] [Google Scholar]
- Inoue K., Khajavi M., Ohyama T., Hirabayashi S., Wilson J., Reggin J. D., Mancias P., Butler I. J., Wilkinson M. F., Wegner M., et al. (2004). Molecular mechanism for distinct neurological phenotypes conveyed by allelic truncating mutations. Nat. Genet. 36, 361-369. [DOI] [PubMed] [Google Scholar]
- Jiao Z., Mollaaghababa R., Pavan W. J., Antonellis A., Green E. D., Hornyak T. J. (2004). Direct interaction of Sox10 with the promoter of murine Dopachrome Tautomerase (Dct) and synergistic activation of Dct expression with Mitf. Pigment Cell Res. 17, 352-362. [DOI] [PubMed] [Google Scholar]
- Kapur R. P. (1999). Early death of neural crest cells is responsible for total enteric aganglionosis in Sox10(Dom)/Sox10(Dom) mouse embryos. Pediatr. Dev. Pathol. 2, 559-569. [DOI] [PubMed] [Google Scholar]
- Khiem D., Cyster J. G., Schwarz J. J., Black B. L. (2008). A p38 MAPK-MEF2C pathway regulates B-cell proliferation. Proc. Natl. Acad. Sci. USA 105, 17067-17072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knecht A. K., Bronner-Fraser M. (2002). Induction of the neural crest: a multigene process. Nat. Rev. Genet. 3, 453-461. [DOI] [PubMed] [Google Scholar]
- Kothary R., Clapoff S., Darling S., Perry M. D., Moran L. A., Rossant J. (1989). Inducible expression of an hsp68-lacZ hybrid gene in transgenic mice. Development 105, 707-714. [DOI] [PubMed] [Google Scholar]
- Kuhlbrodt K., Herbarth B., Sock E., Hermans-Borgmeyer I., Wegner M. (1998). Sox10, a novel transcriptional modulator in glial cells. J. Neurosci. 18, 237-250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane P. W., Liu H. M. (1984). Association of megacolon with a new dominant spotting gene (Dom) in the mouse. J. Hered. 75, 435-439. [DOI] [PubMed] [Google Scholar]
- Lang D., Epstein J. A. (2003). Sox10 and Pax3 physically interact to mediate activation of a conserved c-RET enhancer. Hum. Mol. Genet. 12, 937-945. [DOI] [PubMed] [Google Scholar]
- Le Douarin N. M., Creuzet S., Couly G., Dupin E. (2004). Neural crest cell plasticity and its limits. Development 131, 4637-4650. [DOI] [PubMed] [Google Scholar]
- Le Meur N., Holder-Espinasse M., Jaillard S., Goldenberg A., Joriot S., Amati-Bonneau P., Guichet A., Barth M., Charollais A., Journel H., et al. (2010). MEF2C haploinsufficiency caused by either microdeletion of the 5q14.3 region or mutation is responsible for severe mental retardation with stereotypic movements, epilepsy and/or cerebral malformations. J. Med. Genet. 47, 22-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Q., Schwarz J., Bucana C., Olson E. N. (1997). Control of mouse cardiac morphogenesis and myogenesis by transcription factor MEF2C. Science 276, 1404-1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Q., Lu J., Yanagisawa H., Webb R., Lyons G. E., Richardson J. A., Olson E. N. (1998). Requirement of the MADS-box transcription factor MEF2C for vascular development. Development 125, 4565-4574. [DOI] [PubMed] [Google Scholar]
- Ludwig A., Rehberg S., Wegner M. (2004). Melanocyte-specific expression of dopachrome tautomerase is dependent on synergistic gene activation by the Sox10 and Mitf transcription factors. FEBS Lett. 556, 236-244. [DOI] [PubMed] [Google Scholar]
- Maka M., Stolt C. C., Wegner M. (2005). Identification of Sox8 as a modifier gene in a mouse model of Hirschsprung disease reveals underlying molecular defect. Dev. Biol. 277, 155-169. [DOI] [PubMed] [Google Scholar]
- Melnikova I. N., Lin H. R., Blanchette A. R., Gardner P. D. (2000). Synergistic transcriptional activation by Sox10 and Sp1 family members. Neuropharmacology 39, 2615-2623. [DOI] [PubMed] [Google Scholar]
- Mollaaghababa R., Pavan W. J. (2003). The importance of having your SOX on: role of SOX10 in the development of neural crest-derived melanocytes and glia. Oncogene 22, 3024-3034. [DOI] [PubMed] [Google Scholar]
- Nguyen T., Wei M. L. (2007). Hermansky-Pudlak HPS1/pale ear gene regulates epidermal and dermal melanocyte development. J. Invest. Dermatol. 127, 421-428. [DOI] [PubMed] [Google Scholar]
- Novara F., Beri S., Giorda R., Ortibus E., Nageshappa S., Darra F., Bernardina B. D., Zuffardi O., Van Esch H. (2010). Refining the phenotype associated with MEF2C haploinsufficiency. Clin. Genet. 78, 471-477. [DOI] [PubMed] [Google Scholar]
- Nowakowska B. A., Obersztyn E., Szymanska K., Bekiesinska-Figatowska M., Xia Z., Ricks C. B., Bocian E., Stockton D. W., Szczaluba K., Nawara M., et al. (2010). Severe mental retardation, seizures, and hypotonia due to deletions of MEF2C. Am. J. Med. Genet. B Neuropsychiatr. Genet. 153B, 1042-1051. [DOI] [PubMed] [Google Scholar]
- Parisi M. A., Kapur R. P. (2000). Genetics of Hirschsprung disease. Curr. Opin. Pediatr. 12, 610-617. [DOI] [PubMed] [Google Scholar]
- Peirano R. I., Wegner M. (2000). The glial transcription factor Sox10 binds to DNA both as monomer and dimer with different functional consequences. Nucleic Acids Res. 28, 3047-3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penn B. H., Bergstrom D. A., Dilworth F. J., Bengal E., Tapscott S. J. (2004). A MyoD-generated feed-forward circuit temporally patterns gene expression during skeletal muscle differentiation. Genes Dev. 18, 2348-2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pingault V., Bondurand N., Kuhlbrodt K., Goerich D. E., Prehu M. O., Puliti A., Herbarth B., Hermans-Borgmeyer I., Legius E., Matthijs G., et al. (1998). SOX10 mutations in patients with Waardenburg-Hirschsprung disease. Nat. Genet. 18, 171-173. [DOI] [PubMed] [Google Scholar]
- Pingault V., Ente D., Dastot-Le Moal F., Goossens M., Marlin S., Bondurand N. (2010). Review and update of mutations causing Waardenburg syndrome. Hum. Mutat. 31, 391-406. [DOI] [PubMed] [Google Scholar]
- Potterf S. B., Mollaaghababa R., Hou L., Southard-Smith E. M., Hornyak T. J., Arnheiter H., Pavan W. J. (2001). Analysis of SOX10 function in neural crest-derived melanocyte development: SOX10-dependent transcriptional control of dopachrome tautomerase. Dev. Biol. 237, 245-257. [DOI] [PubMed] [Google Scholar]
- Potthoff M. J., Olson E. N. (2007). MEF2: a central regulator of diverse developmental programs. Development 134, 4131-4140. [DOI] [PubMed] [Google Scholar]
- Rojas A., De Val S., Heidt A. B., Xu S. M., Bristow J., Black B. L. (2005). Gata4 expression in lateral mesoderm is downstream of BMP4 and is activated directly by Forkhead and GATA transcription factors through a distal enhancer element. Development 132, 3405-3417. [DOI] [PubMed] [Google Scholar]
- Rojas A., Schachterle W., Xu S. M., Black B. L. (2009). An endoderm-specific transcriptional enhancer from the mouse Gata4 gene requires GATA and homeodomain protein-binding sites for function in vivo. Dev. Dyn. 238, 2588-2598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandmann T., Girardot C., Brehme M., Tongprasit W., Stolc V., Furlong E. E. (2007). A core transcriptional network for early mesoderm development in Drosophila melanogaster. Genes Dev. 21, 436-449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauka-Spengler T., Bronner-Fraser M. (2006). Development and evolution of the migratory neural crest: a gene regulatory perspective. Curr. Opin. Genet. Dev. 16, 360-366. [DOI] [PubMed] [Google Scholar]
- Selleck M. A., Scherson T. Y., Bronner-Fraser M. (1993). Origins of neural crest cell diversity. Dev. Biol. 159, 1-11. [DOI] [PubMed] [Google Scholar]
- Southard-Smith E. M., Kos L., Pavan W. J. (1998). Sox10 mutation disrupts neural crest development in Dom Hirschsprung mouse model. Nat. Genet. 18, 60-64. [DOI] [PubMed] [Google Scholar]
- Southard-Smith E. M., Angrist M., Ellison J. S., Agarwala R., Baxevanis A. D., Chakravarti A., Pavan W. J. (1999). The Sox10(Dom) mouse: modeling the genetic variation of Waardenburg-Shah (WS4) syndrome. Genome Res. 9, 215-225. [PubMed] [Google Scholar]
- Spritz R. A., Chiang P. W., Oiso N., Alkhateeb A. (2003). Human and mouse disorders of pigmentation. Curr. Opin. Genet. Dev. 13, 284-289. [DOI] [PubMed] [Google Scholar]
- Stanchina L., Baral V., Robert F., Pingault V., Lemort N., Pachnis V., Goossens M., Bondurand N. (2006). Interactions between Sox10, Edn3 and Ednrb during enteric nervous system and melanocyte development. Dev. Biol. 295, 232-249. [DOI] [PubMed] [Google Scholar]
- Steel K. P., Barkway C. (1989). Another role for melanocytes: their importance for normal stria vascularis development in the mammalian inner ear. Development 107, 453-463. [DOI] [PubMed] [Google Scholar]
- Tapscott S. J. (2005). The circuitry of a master switch: Myod and the regulation of skeletal muscle gene transcription. Development 132, 2685-2695. [DOI] [PubMed] [Google Scholar]
- Trainor P. A. (2005). Specification of neural crest cell formation and migration in mouse embryos. Semin. Cell Dev. Biol. 16, 683-693. [DOI] [PubMed] [Google Scholar]
- Verzi M. P., Agarwal P., Brown C., McCulley D. J., Schwarz J. J., Black B. L. (2007). The transcription factor MEF2C is required for craniofacial development. Dev. Cell 12, 645-652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vong L. H., Ragusa M. J., Schwarz J. J. (2005). Generation of conditional Mef2cloxP/loxP mice for temporal- and tissue-specific analyses. Genesis 43, 43-48. [DOI] [PubMed] [Google Scholar]