Abstract
The role of bone morphogenetic protein (BMP) signaling in specifying cell fate in the zebrafish tailbud has been well established. In addition to a loss of ventral tissues, such as ventral tailfin and cloaca, some embryos with compromised BMP signaling produce an additional phenotype: a ventrally located secondary tail containing both somitic muscle and notochord. This phenotype has been proposed to reflect a fate-patterning defect due to a change in a hypothesized BMP activity gradient. Here, we show that a defect in morphogenetic movements, not fate patterning, underlies the formation of secondary tails in BMP-inhibited embryos. Our data indicate that BMP signaling is activated in the ventroposterior tailbud to promote cell migration during tailbud protrusion, and that defective migration of these cells in BMP mutants ultimately leads to bifurcation of the caudal notochord. Additionally, we show that non-canonical Wnt signaling is also required for proper tail morphogenesis, possibly by maintaining cohesion of notochord progenitors by regulation of cadherin localization. We propose a model in which BMP and the non-canonical Wnt pathway regulate tail morphogenesis by controlling cell migration and cell adhesion within the tailbud.
Keywords: Zebrafish, BMP, Wnt, Tailbud, Notochord, Morphogenesis
INTRODUCTION
The vertebrate tailbud is a mass of undifferentiated cells that gives rise to posterior tissues of the body during tail development. In zebrafish, the tailbud is derived from cells originating at both the dorsal and ventral margins, which come together at the vegetal pole of the embryo upon completion of epiboly (Kimmel et al., 1995). Ventrally derived cells constitute the posterior portion of the tailbud and are fated to develop into somitic muscle in the tail, whereas the dorsally derived cells constitute the anterior tailbud and form primarily axial tissues of the tail, such as notochord and floor plate (Kanki and Ho, 1997; Myers et al., 2002; Agathon et al., 2003).
Extensive genetic analyses have demonstrated roles for several conserved signaling pathways in the specification, patterning and morphogenesis of the tailbud and its derivatives, including the bone morphogenetic protein (BMP), fibroblast growth factor (FGF), Nodal, and both the Wnt/β-catenin and non-canonical Wnt pathways (Schier and Talbot, 2005). For example, the BMP pathway is required (along with Wnt/β-catenin and Nodal signaling) during gastrulation for the formation of a tail organizer at the ventral margin (Agathon et al., 2003). In embryos completely lacking BMP activity during gastrulation, such as swirl (swr; bmp2b – Zebrafish Information Network) mutants, tail development is essentially nonexistent (Mullins et al., 1996; Kishimoto et al., 1997). During gastrulation, BMP is thought to function as a morphogen, patterning cell fates along the dorsal-ventral (DV) axis, with highest BMP activity inducing the ventral-most fates, including tail mesoderm, intermediate levels specifying lateral fates such as trunk mesoderm, and the absence of BMP activity allowing for the development of dorsal fates such as the notochord (Little and Mullins, 2006).
Independent of its role in fate patterning, BMP signaling also regulates morphogenetic movements during gastrulation, promoting the convergence of lateral mesodermal cells towards the dorsal midline (Myers et al., 2002; von der Hardt et al., 2007). The BMP activity gradient is proposed to negatively regulate cell adhesion, establishing distinct patterns of migratory behavior among mesodermal cells along the DV axis (von der Hardt et al., 2007). Presumably, simultaneous control of cell fate patterning and cell movements by BMP ensures tight coupling of these two processes.
During somitogenesis, BMP acts to pattern cell fates derived from the tailbud. Post-gastrula inhibition of BMP signaling results in embryos showing reduced ventral tailfin and cloaca tissue (Pyati et al., 2005; Pyati et al., 2006; Tucker et al., 2008; Yu et al., 2008). Such embryos show a similar phenotype to that observed in embryos carrying mutations in several conserved components of the BMP pathway: bmp4, mini fin (mfn; also known as tolloid-like 1, tll1 – Zebrafish Information Network), lost-a-fin (laf; also known as alk8 and acvr1l), as well as hypomorphic smad5 alleles (Connors et al., 1999; Mintzer et al., 2001; Kramer et al., 2002; Stickney et al., 2007). It has been suggested that a gradient of BMP signaling specifies distinct cell types in posterior tissues, with the highest levels of BMP activity being required for production of ventral tailfin and cloaca and a lower amount being sufficient for presomitic mesoderm and blood (Stickney et al., 2007).
Cell tracing experiments have shown that the anterior and posterior portions of the tailbud undergo distinct morphogenetic movements during tail outgrowth (Kanki and Ho, 1997). Cells in the anterior (dorsally derived) tailbud continue the convergence and extension (CE) movements observed during gastrulation, with presomitic and somitic mesoderm converging to the midline, driving the posteriorward extension of the embryo. By contrast, posterior (ventrally derived) tailbud cells move laterally away from the midline and subduct beneath the posteriorly migrating anterior tailbud cells.
Some genes that regulate cell movements during gastrulation have also been shown to contribute to proper tail morphogenesis. For example, the non-canonical Wnt pathway, which promotes dorsal convergence independently of BMP during gastrulation (Sepich et al., 2005), is also required for proper tailbud morphogenesis (Marlow et al., 2004). Whether the BMP pathway regulates morphogenesis as well as fate patterning in the tailbud is not known.
A subset of BMP-deficient embryos with defective fate patterning in the tail also produce a curious additional phenotype: a ventrally located secondary tail containing both somitic muscle and notochord (Connors et al., 1999; Pyati et al., 2005; Stickney et al., 2007; Yu et al., 2008). It has been proposed that these secondary tails form as a result of a mis-specification of cell fates due to a change in the postulated gradient of BMP activity in the tail (Stickney et al., 2007). According to this hypothesis, a mild reduction in BMP activity could lead to a shallower slope of the gradient such that it would be insufficient to specify ventral tailfin, but would expose more cells to the intermediate level of activity sufficient for formation of tail mesoderm, leading to an expansion of this tissue.
However, this model does not explain why the excess tail mesoderm would form such a morphologically distinct structure or why the secondary tails observed in BMP-compromised embryos include notochord, which is not hypothesized to be responsive to the BMP gradient. Also, although bmp4 mutant embryos show the loss of ventral tailfin and expansion of blood progenitors and somitic muscle predicted by the gradient model, these embryos do not form secondary tails (Stickney et al., 2007). Furthermore, when expression of a dominant-negative BMP receptor (dnBMPR) is induced at the bud stage, the population of blood progenitors is not expanded, even though ~15% of these embryos form secondary tails (Pyati et al., 2005). Taken together, these observations are inconsistent with the hypothesis that secondary tails form as a consequence of defects in fate patterning.
We show here that a defect in morphogenesis, not fate patterning, underlies formation of secondary tails in BMP mutants, and demonstrate a role for BMP in regulating the migration of presomitic mesodermal cells in the tailbud. We further show that non-canonical Wnt signaling functions in a parallel pathway to prevent secondary tail formation, perhaps by regulating cohesion of caudal notochord cells.
MATERIALS AND METHODS
Zebrafish strains
Mutant lines used were mfntc263a (Connors et al., 1999), knyfr6 (Topczewski et al., 2001), smad5m169 (Kramer et al., 2002), smad5dty40 (Kramer et al., 2002) and snhty68a (Schmid et al., 2000). mfn/+;Tg(flh:EGFP) and kny/+;Tg(flh:EGFP) strains were created by crossing mfn and kny heterozygotes to the Tg(flh:EGFP) transgenic line (Gamse et al., 2003).
In situ hybridization and antibody staining
Whole-mount in situ hybridization was carried out using standard methods (Oxtoby and Jowett, 1993). The following probes were used: col2a (Yan et al., 1995), myoD (Weinberg et al., 1996), neurog1 (Blader et al., 1997), ntl (Schulte-Merker et al., 1992), tbx6 (Hug et al., 1997), gata1 (Detrich et al., 1995), fli1 (Brown et al., 2000) and flh (Talbot et al., 1995). Fluorescent in situ hybridization (FISH) for flh expression was performed using a Fast Red color reaction (Hauptmann and Gerster, 1994), followed by incubation with P-Smad1/5/8 antibody (1:100; Cell Signaling Technology). β-catenin antibody (BD Transduction Laboratories) was applied at 1:400; pan-cadherin antibody (Sigma) was applied at 1:100; Tbx6 antibody (ZIRC) was applied at 1:1000. Appropriate Alexa Fluor (Molecular Probes) secondary antibodies were used.
Morpholino and RNA injection
Morpholino antisense oligonucleotides (dvl1 MO: 5′-ATATGATTTTAGTCTCCGCCATGAG-3′) were purchased from Gene Tools. mfn, dvl2, dvl3 and cdh2 morpholinos have been described previously (Lele et al., 2002; Angers et al., 2006; Jasuja et al., 2006). The standard control morpholino by Gene Tools was used in some experiments. Morpholinos were diluted in Danieau's buffer before injection. mfn MO was injected at a concentration of 1 mg/ml; concentrations of 0.5 mg/ml and 1.5 mg/ml were injected as low and high dose. dvl1, dvl2 and dvl3 MOs were injected at a suboptimal concentration of 1 mg/ml each; a concentration of 1.5 mg/ml was injected as high dose; to fully inhibit dvl2 and dvl3, the concentration was 3 mg/ml. cdh2 MO was injected at a suboptimal concentration of 0.1 mg/ml and a high concentration of 0.25 mg/ml. Synthetic mRNA of membrane-bound RFP (mRFP) was made from pCS2+ constructs with mMessage mMachine kit (Ambion) and injected at a concentration of 25 mg/ml. In all experiments, a volume of 3-5 nl was injected into the yolk of one-cell-stage embryos.
Confocal microscopy
Tg(flh:EGFP), mfn;Tg(flh:EGFP) and kny;Tg(flh:EGFP) embryos were injected with mRFP mRNA to provide contrast. To analyze tail morphogenesis, embryos were mounted at the ten-somite stage in 0.7% low-melt agarose in Ringer's solution. Agarose surrounding the posterior body was removed to ensure the free extension of the tailbud. Images were acquired every 1.5 minutes using a Zeiss LSM 5 Pascal confocal microscope and a 20× lens. Videos were re-aligned using ImageJ.
BMP Inhibition
We treated embryos with dorsomorphin (AMPK Inhibitor, Compound C, Calbiochem) as described with slight modifications (Yu et al., 2008). Dorsomorphin stock solution in DMSO was dissolved in fish water at 60 μM. Embryos were dechorionated and reared in the solution for the duration of the experiment. Control embryos were dechorionated and raised in water containing an equivalent amount of DMSO.
RESULTS
Mesoderm tissues produced in the secondary tail of mini fin
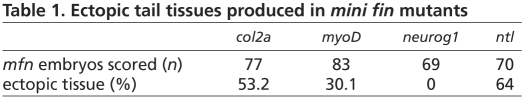
To develop a clearer understanding of the origin of secondary tails in BMP-compromised embryos, we analyzed this process in the mfn mutant, the only mutant reported to consistently produce secondary tails (Connors et al., 1999). To determine which tissues are located in the secondary tail, we examined the expression of the notochord marker col2a (col2a1a – Zebrafish Information Network), muscle marker myoD (myod1 – Zebrafish Information Network), neural marker neurog1 and the notochord/tailbud marker ntl (ntla – Zebrafish Information Network) by in situ hybridization. In mfn embryos at 26 hours post-fertilization (hpf), ectopic col2a (Fig. 1A,B) and myoD (Fig. 1C,D) expression was observed in 53% and 30% of the embryos, respectively (Table 1). Consistent with previous studies, no embryo showed ectopic neurog1 expression (Fig. 1E,F), indicating that secondary tails do not contain neural tissue (Pyati et al., 2005). Ectopic ntl expression (Fig. 1G,H) was detected in 64% of the embryos. In all embryos with a secondary tail, we also observed ectopic expression of two posterior tailbud markers, tbx6 and eve1 (Fig. 1I,J, and data not shown).
Fig. 1.
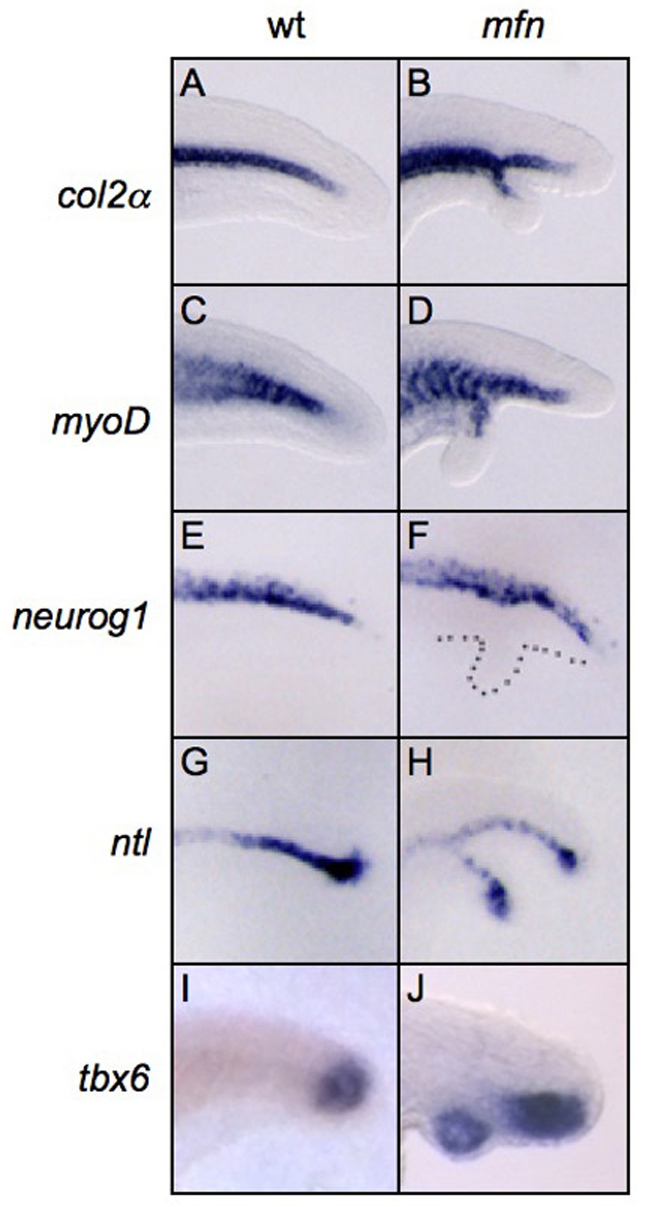
Mesoderm tissues are mis-specified in the secondary tails of mfn. (A-J) Lateral view of expression of col2a (col2α; A,B), myoD (C,D), neurog1 (E,F), ntl (G,H) and tbx6 (I,J) in the posterior tail of wild-type and mfn zebrafish embryos. At 26 hpf, col2a (B), myoD (D) and ntl (H) are ectopically expressed in the secondary tail, but neurog1 is not (F). At 24 hpf, tbx6 (J) is also ectopically expressed in the secondary tail. Embryos in all images are mounted with anterior to the left. Dotted line in F indicates secondary tail.
Table 1.
Ectopic tail tissues produced in mini fin mutants
Secondary tail formation is not accompanied by changed mesoderm fates
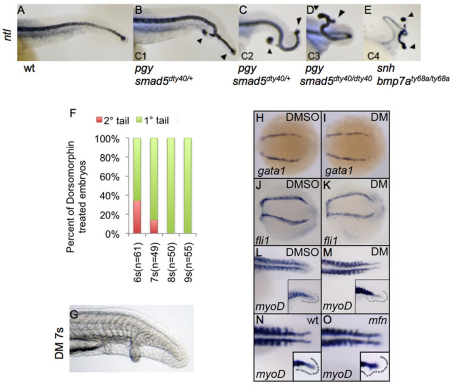
The BMP morphogen model in tail development suggests that secondary tail formation only occurs at a modestly reduced level of BMP signaling in the tailbud, whereas stronger loss of BMP activity results in reduced ventral mesoderm, with no secondary tails formed (Stickney et al., 2007). BMP mutants are variably dorsalized, with the degree of dorsalization typically scored using the dorso-anterior index (DAI), which ranges from very weak (C1) to completely dorsalized (C5). Secondary tail formation is proposed to occur only in embryos with a phenotype intermediate between C1 and C2, which is included in the range of phenotypes typically seen in mfn embryos (Stickney et al., 2007). To test this model, we examined ntl expression in other mutants that reduce BMP signaling: piggytail (smad5 – Zebrafish Information Network) and snailhouse (bmp7a – Zebrafish Information Network). These mutants display a range of dorsalized phenotypes from weak (C1) to severe (C4) (Mullins et al., 1996). In the weak piggytail (smad5m169) allele, we observed that 49% of the C1 homozygotes had a secondary tail (see Table S1 in the supplementary material). In clutches of embryos from an incross of piggytail (smad5dty40) carriers, we observed a range of phenotypes spanning the C1, C2 and C3 classes. The overall penetrance of secondary tail formation in all the mutant embryos was 36% (see Table S1 in the supplementary material). Notably, we observed secondary tail formation in each phenotypic class (Fig. 2A-D). Furthermore, in snailhouse (bmp7ty68) embryos, which are severely dorsalized (C4), 38% formed secondary tails (Fig. 2E and see Table S1 in the supplementary material). Taken together, we observed secondary tails in multiple BMP mutant lines independent of the severity of dorsalization. These results suggest that secondary tail formation does not require BMP signaling to be reduced to a specific level, and it is unlikely to result from expanded mesoderm, as secondary tails can form in embryos with limited tail mesoderm.
Fig. 2.
Secondary tail formation is independent of the role of BMP signaling in fate patterning. (A-E) Lateral view of zebrafish tails in wild-type and dorsalized embryos of indicated genotypes at 24 hpf, after whole-mount in situ for ntl. Primary and secondary tails are marked with arrowheads. C1-C4 refer to the degree of dorsalization using the dorso-anterior index. (F) Distribution of secondary tail phenotype in embryos following dorsomorphin (DM) treatment at the indicated time points. Secondary tails were scored by the presence of ectopic ntl expression. (G) Lateral view of the tail of a live embryo at 24 hpf, after DM treatment at 7 somites (7s). Note the secondary tail with fully developed ventral fin. (H-M) Dorsal view of embryos expressing gata1 (H,I), fli1 (J,K) at the 13-somite stage and myoD (L,M) at 24 hpf in the tail, after treatment with DMSO (H,J,L) and DM (I,K,M) at the 5-somite stage. (N,O) Dorsal view of myoD expression in the tail of wild-type (wt; J) and mfn (K) embryos at 22 hpf. Inset shows the lateral view. Expression of gata1 (I), fli1 (K) and myoD (M,O) is unaffected.
BMP signaling is required during early somitogenesis to inhibit secondary tail formation
To determine the temporal requirements for BMP in this process, we used the BMP antagonist dorsomorphin (DM) (Yu et al., 2008). We treated embryos at progressively later stages, beginning at the 1-somite stage, then scored for the presence of a secondary tail at 24 hpf. Nearly all embryos treated with DM beginning at 6 somites lacked ventral tailfin at 24 hpf, with ~30% also forming a secondary tail (Fig. 2F). Embryos treated with DM after 7 somites showed normal or slightly reduced ventral tailfin with only a few embryos forming a secondary tail (Fig. 2F). In rare cases, such embryos formed a secondary tail even in the presence of a fully formed ventral tail fin (Fig. 2G). This time frame is very similar to that observed in dnBMPR transgenic embryos (Pyati et al., 2005). Our data indicate that the crucial periods for specifying ventral tailfin and for prevention of secondary tail formation overlap significantly. However, the two processes appear to be separable, indicating that secondary tails might be formed in the absence of overt fate patterning defects.
Although bmp4 mutants do not form secondary tails, it has been suggested that the expansion of blood and muscle fates observed in bmp4 embryos could contribute to their formation in other BMP-compromised embryos (Stickney et al., 2007). To investigate whether expansion of blood and somitic mesoderm is associated with secondary tail formation, we examined gata1 (gata1a – Zebrafish Information Network), fli1 (fli1a – Zebrafish Information Network) and myoD expression in DM-treated embryos. For each marker, we treated 60 embryos with DM beginning at the 5-somite stage. We fixed half of these at the 13-somite stage and stained for gata1, fli1 or myoD. To get an estimate of the penetrance of secondary tails in each group of embryos, we stained the other 30 embryos for ntl at 24 hpf. gata1 expression was indistinguishable from wild type in 27/30 embryos (Fig. 2H,I), although three showed weak expansion of gata1 expression at the posterior end (data not shown). Our ntl staining indicated that 11/30 embryos formed a secondary tail in this experiment. Similarly, we observed no expansion of fli1 or myoD in DM-treated embryos (Fig. 2J-M), despite approximately a third (11/30) of the sibling embryos going on to form a secondary tail. As a further test of whether somitic muscle is expanded in embryos producing secondary tails, we stained mfn embryos at 22 hpf with myoD. Whereas myoD expression has been reported to be expanded across the midline of the caudal tail in bmp4 embryos (Stickney et al., 2007), we observed no such expansion in mfn embryos (Fig. 2N,O). Taken together, our data suggest that the role of BMP in preventing secondary tail formation might be independent of its role in cell fate patterning.
Non-canonical Wnt signaling functions with BMP in inhibiting secondary tail formation
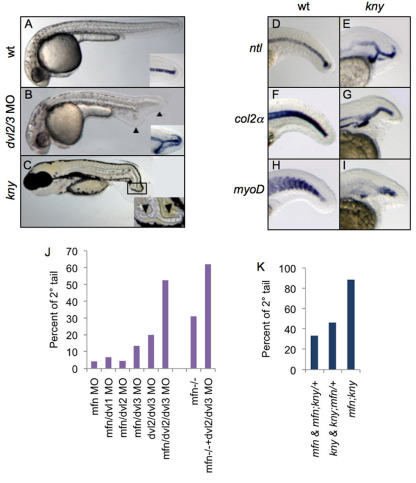
Non-canonical Wnt signaling plays an essential role in governing morphogenesis of the posterior body (Veeman et al., 2003; Seifert and Mlodzik, 2007). ppt (wnt5 – Zebrafish Information Network) and kny (glypican4, gpc4 – Zebrafish Information Network) mutants are defective in components of the non-canonical Wnt pathway and have short tails due to impaired cell movements (Marlow et al., 2004). Strikingly, kny mutants have also been reported to exhibit ectopic eve1 and shh (shha – Zebrafish Information Network) expression domains in the tail (Solnica-Krezel et al., 1996; Marlow et al., 1998), resembling the expression pattern of these markers in secondary tails of dnBMPR embryos (Pyati et al., 2005). Upon examination of later stage (72 hpf) kny embryos, we could observe clear bifurcations of the posterior notochord (Fig. 3C), suggesting that a secondary tail might form in these embryos. We confirmed this by observing ectopic ntl, col2a and myoD expression at 24 hpf (Fig. 3D-I; Table 2).
Fig. 3.
Non-canonical Wnt signaling functions with BMP to inhibit secondary tail formation. (A-C) Lateral view of wild-type (A, 24 hpf), dvl2/3 morphant (B, 24 hpf) and kny mutant (C, 72 hpf) zebrafish. Insets of A and B show ntl expression in the posterior tail. Inset of C shows a close-up of posterior tail. Primary and secondary tails are marked with arrowheads. (D-I) Lateral view of expression of ntl (D,E), col2a (col2α; F,G) and myoD (H,I) in the tail of wild-type and kny embryos fixed at 24 hpf. ntl (E), col2a (G), and myoD (I) exhibit ectopic expression within the tails of kny. (J) Percentages of secondary tail formation in embryos injected with indicated combination of morpholinos (MO; 1 mg/ml each, except for ‘dvl2/dvl3 MO’ condition, where a concentration of 3 mg/ml for each MO was injected). For each column over 100 embryos from three separate experiments were scored. (K) Percentages of secondary tail formation in embryos from incross of mfn/+;kny/+ double mutant carriers. Embryos are categorized by phenotypes. Secondary tails in J and K were scored by ntl expression.
Table 2.
Ectopic tail tissues produced in knypek mutants
We next tested whether this phenotype is unique to kny mutants or is common to other embryos in which non-canonical Wnt signaling is impaired. First, we knocked down three dishevelled homologs, dvl1, dvl2 and dvl3, by morpholino (MO). Knockdown of each dvl gene individually did not result in a secondary tail phenotype (data not shown). However, we found that inhibition of dvl2 and dvl3 simultaneously did give rise to a secondary tail in a subset of embryos (Fig. 3A,B) in addition to the previously reported mild CE defect (Angers et al., 2006).
In contrast to our observations in kny or dvl2/dvl3 MO embryos, we never observed secondary tail formation in embryos in which strabismus (stbm; vangl2 – Zebrafish Information Network), prickle 1a (pk1a), or prickle 2 (pk2) had been inhibited, separately or in combination (see Table S2 in the supplementary material). Lastly, we tested several known non-canonical Wnt ligands (wnt5, wnt11 and wnt11r), knocking them down using morpholinos, singly and in combination, and observed no evidence for a role in secondary tail formation for any of these genes (data not shown).
To investigate whether non-canonical Wnt signaling interacts with BMP signaling in inhibiting secondary tail formation, we tested for synergy between mfn and dvl by injecting suboptimal amounts of morpholinos for each gene and scoring embryos for secondary tail formation (Fig. 3J). Although partial knockdown of mfn showed a very low penetrance of secondary tail formation, we observed moderate enhancement with partial knockdown of mfn and dvl3 simultaneously, and greater enhancement with partial knockdown of mfn, dvl2 and dvl3. Injection of suboptimal amounts of dvl2/dvl3 MO into mfn embryos also caused a robust enhancement (Fig. 3J). Lastly, we scored the penetrance of secondary tails in mfn;kny double mutant embryos. In mfn;kny, strong enhancement of secondary tail formation was observed (88% of double mutant embryos formed a secondary tail, Fig. 3K), approximately what one would expect for a simple additive effect on penetrance of this phenotype. Together, these genetic interactions are consistent with a model in which non-canonical Wnt signaling and BMP signaling function in parallel to inhibit secondary tail formation.
BMP and non-canonical Wnt signaling are required to prevent bifurcation of the caudal notochord during tail outgrowth
During our earlier observations in BMP-deficient embryos, we did not observe a clean bifurcation of the notochord when stained with ntl, but, instead, the normally compact caudal end of the notochord appeared stretched and elongated (data not shown), suggesting the possibility that a defect in notochord morphogenesis could underlie the defect. As the caudal end of the notochord is a source of notochord progenitors, mislocalization of cells from this region could account for the ectopic notochord cells we observe in secondary tails. For simplicity, we will refer to these progenitor cells as caudal notochord (CN) cells.
We first tested whether the secondary tails contained ectopic CN cells by performing in situ hybridization for flh, which is expressed in the CN during tail development. In both mfn and kny embryos at 24 hpf, flh was ectopically expressed in the region of the secondary tail (Fig. 4A-C). We confirmed this result by observing ectopic expression of a second CN marker, fgf4 (see Fig. S1 in the supplementary material).
Fig. 4.
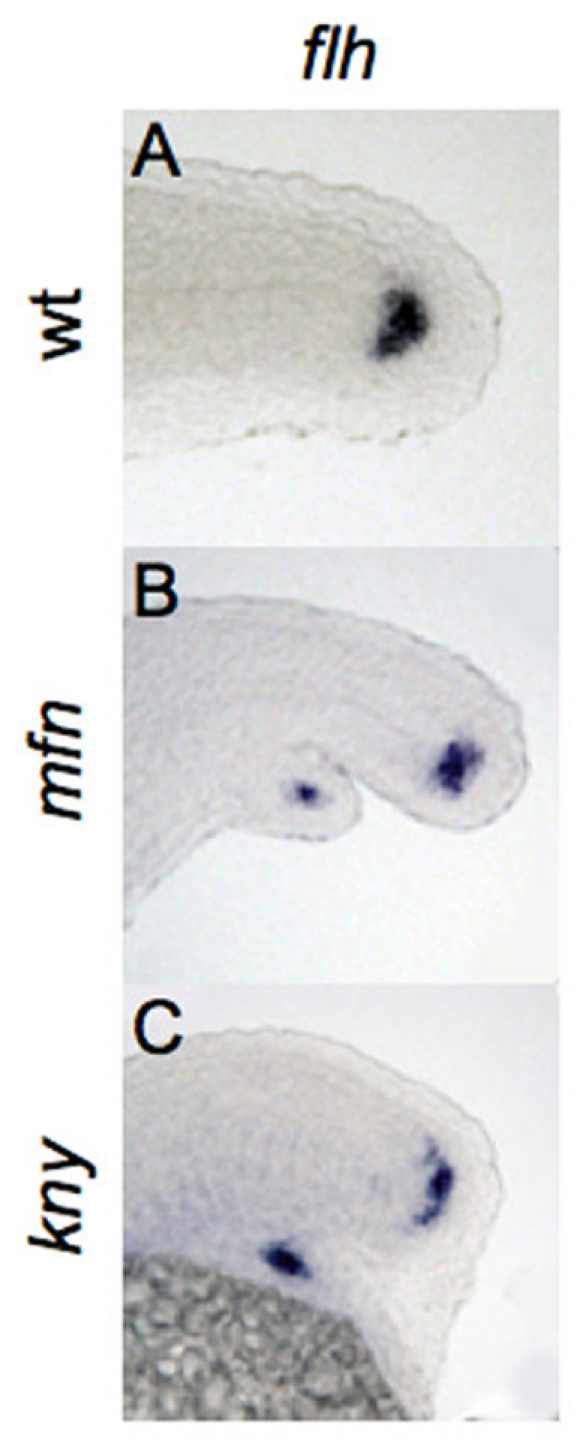
Bifurcation of the caudal end of notochord in BMP and non-canonical Wnt mutants. (A-C) Lateral view of expression of flh, marking the caudal end of notochord in wild-type, mfn and kny zebrafish embryos fixed at 24 hpf. flh is expressed in the secondary tail of mfn (B) and kny (C) mutants.
In order to understand the emergence of the ectopic CN cells, we performed a time-course experiment by fixing mfn, snh and kny embryos hourly during tail outgrowth and using flh expression to determine the onset of the secondary CN domain (see Fig. S2 in the supplementary material). For each mutant, we initially observed a single domain of flh expression in all embryos, but between 18-24 hpf, we observed increasing numbers of embryos with either elongated CN domains or two distinct CN domains (see Fig. S2A-C in the supplementary material). Although these data cannot rule out the possibility that new flh expression is contributing to the ectopic expression that we observe, we favor the interpretation that the CN domain is specified normally in both mfn and kny embryos, but becomes split during tail extension. This suggests that a defect in morphogenetic movements could underlie the secondary tail phenotype, which we confirmed by time-lapse analysis (see below).
N-cadherin is required to maintain tailbud integrity
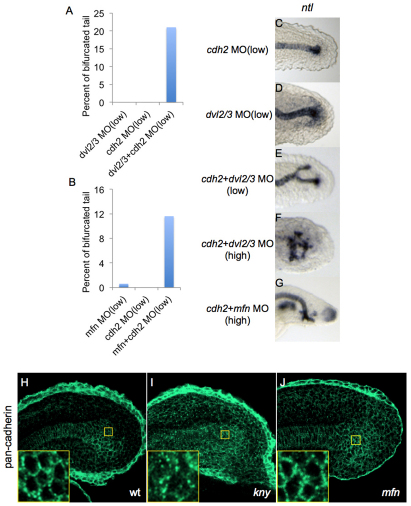
Proper regulation of intercellular adhesion is important for proper cell movements (Halbleib and Nelson, 2006). Both the BMP pathway and non-canonical Wnt signaling have been demonstrated to regulate cell movements during gastrulation, in part by regulation of the localization or activity of cadherins (Ulrich et al., 2005; von der Hardt et al., 2007). As a recent study has shown that a semi-dominant allele of N-cadherin (cdh2) perturbs cell cohesion in the tailbud (Harrington et al., 2007), we asked whether disruption of cell adhesion in the tailbud affects CN morphogenesis. We performed genetic interaction assays between dvl2/3 and cdh2. Co-injection of suboptimal amounts of dvl2/3 MO and cdh2 MO, which did not cause any tail defects when injected separately, induced notochord bifurcations (Fig. 5A,C-E). At higher doses of dvl2/3 MO and cdh2 MO, we observed dramatically scattered notochord cells rather than simple bifurcation (Fig. 5F). Interestingly, in kny embryos, we occasionally observed embryos with triple-branched notochords (see Fig. S3 in the supplementary material), which resemble these severe dvl/cdh2 MO morphants (Fig. 5F). Similarly, cdh2 MO significantly enhanced the secondary tail penetrance in mfn morphants (Fig. 5B). Together, these results indicate that proper regulation of cell adhesion is essential for maintaining the coherence of the CN during tail development, suggesting the possibility that BMP and/or the non-canonical Wnt pathway could act to prevent tail bifurcation by regulating intercellular adhesion in the tailbud.
Fig. 5.
N-cadherin is required to maintain tailbud integrity. (A,B) Percentages of bifurcated tail in zebrafish embryos injected with the indicated combination of morpholinos (MOs). For low concentrations, dvl2/3 (1 mg/ml each), mfn (0.5 mg/ml) and chd2 (0.1 mg/ml) MOs were injected. For each column over 100 embryos from three separate experiments were scored. (C-G) Lateral view of tails of embryos at 24 hpf injected with the indicated combination of morpholinos, after in situ hybridization for ntl. ntl expression in cdh2 and dvl2/3 MO morphants (C,D) is similar to wild type. Note that co-injection of a high concentration of chd2 and dvl2/3 MOs further enhanced the ntl expression from a single fork shaped pattern (E) to a multi-fork pattern (F), but co-injection of high concentration of chd2 and mfn MOs (G) did not result in a similar phenotype. This difference is consistent with the following antibody staining results (see below). For high concentrations, dvl2/3 (1.5 mg/ml each), mfn (1.5 mg/ml) and chd2 (0.25 mg/ml) MOs were injected. (H-J) Representative confocal microscopy images of tailbud in wild-type, kny and mfn embryos stained with pan-cadherin antibody at 21 somites; inset, close-up of the caudal end of notochord. Membrane staining pattern in the caudal end of notochord is shown in the wild type (H) and the mfn (J) mutant, whereas diffused staining pattern is shown in the kny mutant (I).
Non-canonical Wnt signaling is required for proper localization of cadherin
During gastrulation, wnt11 promotes coherence of the anteriorly migrating prechordal plate cells by regulating the localization of E-cadherin (Ulrich et al., 2005). To determine whether cadherin localization is regulated by non-canonical Wnt signaling in the tailbud, we stained embryos with a pan-cadherin antibody. In wild-type embryos, cadherin was localized to the plasma membrane of CN cells (Fig. 5H), whereas in the CN of kny embryos, cadherin was localized more intracellularly (Fig. 5I). In most kny embryos, we also observed a similar mislocalization in more anterior notochord cells also (data not shown). We never observed this aberrant cadherin localization in posterior tailbud cells or presomitic mesoderm cells of kny embryos. We also analyzed the localization of β-catenin in kny embryos, and observed no defects in its normal membrane localization (see Fig. S4 in the supplementary material). Lastly, both cadherin and β-catenin were localized normally in mfn embryos (Fig. 5J and see Fig. S4 in the supplementary material). These results indicate that non-canonical Wnt signaling, but not BMP, is required for proper localization of cadherin both within notochord progenitors and the differentiated notochord.
CN bifurcation occurs during tailbud protrusion
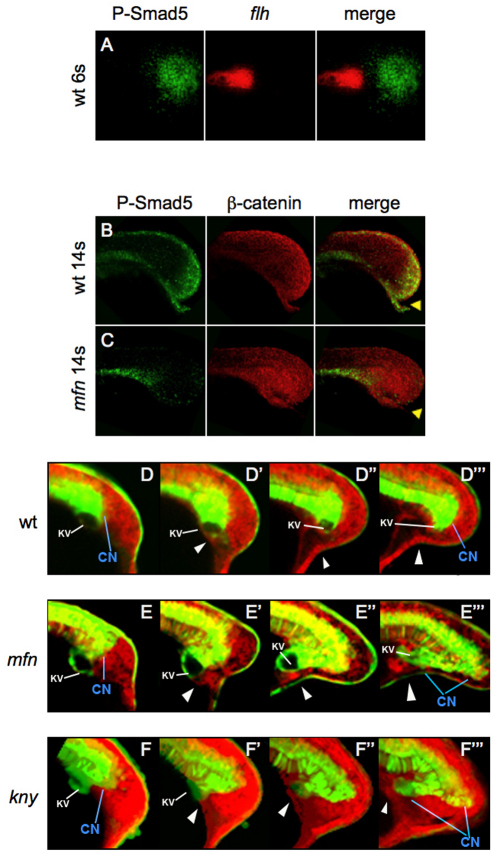
Our temporal studies with dorsomorphin indicate that the 6- to 7-somite stage is a crucial period for BMP signaling, as inhibition of BMP beginning at this stage, but not later, is capable of inducing formation of secondary tails. Known BMP ligands in the tailbud are expressed at this stage in the posterior tailbud, suggesting that the action of BMP could be indirect (Dick et al., 2000; Thisse et al., 2004). To determine more precisely where in the tailbud BMP is active, we performed immunostaining with anti-phosphorylated Smad1/5/8 (P-Smad5) antibody in embryos at the 6-somite stage, and combined this assay with fluorescent in situ hybridization (FISH) using flh probe to visualize the CN. Consistent with a recent study (Esterberg et al., 2008), we observed activation of BMP signaling in the posterior mesoderm of the tailbud, separate from the CN, persisting at least until the 14-somite stage (Fig. 6A,B). By contrast, we observed no activation of BMP signaling in the tailbud of mfn embryos (Fig. 6C), although these embryos showed normal phosphorylation of Smad1/5/8 in the notochord.
Fig. 6.
Defects of the caudal end of notochord in mfn and kny initiate during tailbud protrusion. (A) Dorsal view of flat-mounted tailbud of a 6-somite wild-type zebrafish embryo, after fluorescence in situ hybridization (FISH) for flh (red) and antibody staining for P-Samd5 (green); no colocalization (merge) is seen. (B,C) Lateral view of tails of 14-somite wild-type and mfn embryos exposed to P-Smad5 (green) and β-catenin (red) antibodies. P-Smad5 staining is absent in the posterior tailbud of mfn (C, merge). Arrowheads indicate the ventroposterior cells in the tailbud that are normally positive for P-Smad5 (B, merge). (D-F‴) Confocal time-lapse recording of tailbuds in embryos expressing Tg(flh:EGFP) transgene (green) and mRFP (red) from the 10-somite stage. Shown are wild type (D-D‴) and mfn (E-E‴) and kny (F-F‴) mutants. Note that transgenic EGFP is retained in the caudal notochord, floorplate and periphery of Kupffer's vesicle (KV; marked by the white line). The caudal end of notochord (CN) is indicated by blue line. Arrowheads mark the ventroposterior cells in the tailbud. Embryos were all mounted laterally.
To understand how BMP signaling regulates CN morphogenesis by its activation in the posterior tailbud, we performed confocal time-lapse imaging of the developing tail in Tg(flh:EGFP) and mfn;Tg(flh:EGFP) embryos. During tailbud extension in wild-type embryos, the CN moved along the surface of the yolk cell in close association with Kupffer's vesicle (KV) (Fig. 6D and see Movie 1 in the supplementary material). As the tailbud entered the protrusion phase at the 12-somite stage, the most ventral posterior tailbud cells, coming from a region of high BMP activity (see Fig. 6B), moved anteriorly and began to undercut KV (Fig. 6D′). Progressively, more cells from the ventral side moved in between KV and the yolk, and this was accompanied by notochord extension and yolk constriction (Fig. 6D″). As a result, the CN, together with KV, was lifted away from the surface of the yolk and the tail began to extend off the yolk ball (Fig. 6D‴).
In mfn embryos, after a comparatively normal extension stage (Fig. 6E and see Movie 2 in the supplementary material), the most ventral cells failed to undercut KV. Instead, they moved towards the yolk, often forming an indentation (Fig. 6E′). This had the effect of drawing KV and the CN ventrally towards, or even into, the yolk cell. As tail extension continued, the lengthening notochord eventually caused the CN to adopt an elongated shape (Fig. 6E″). At the end of tailbud protrusion, the stretching of the CN became more severe (Fig. 6E‴). Depending on the embryo, these ectopic CN domains could either be completely ‘left behind’ during tail elongation, or remain connected to the main axis by branching of the notochord.
We also performed time-lapse imaging in kny;Tg(flh:EGFP) embryos (see Movie 3 in the supplementary material). We did not notice any change of CN morphology until at about the 14 somite stage, when the tailbud began to protrude off the yolk (Fig. 6F′). As the most ventral cells in kny undercut KV, we observed cells gradually detaching away from the CN (Fig. 6F″). Over time, the ectopic CN cells formed an elongated cluster (Fig. 6F‴). Our observations suggest that cell movements occur roughly normally in kny embryos, albeit at a reduced rate, and that the CN is unable to maintain coherence during tail extension and protrusion.
BMP regulates migration of posterior mesoderm cells
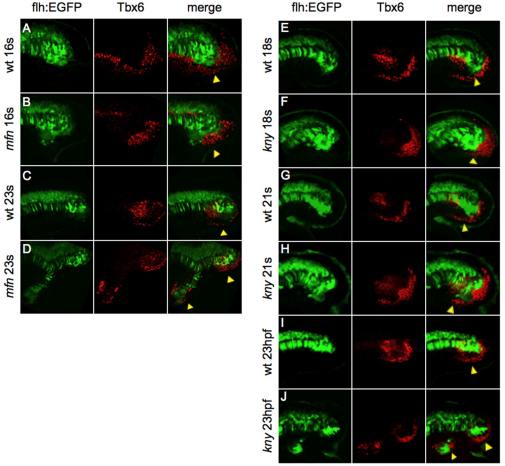
To determine the identity of the cells that undercut KV and the CN, we performed immunostaining with a Tbx6 antibody to label presomitic mesoderm cells in Tg(flh:EGFP) and mfn;Tg(flh:EGFP) embryos at several stages of tail development. In wild type, we observed a band of Tbx6-positive cells located beneath the CN at the 16-somite stage (Fig. 7A), suggesting that those cells migrating beneath the CN are posterior mesodermal cells. Notably, these cells show high levels of P-Smad5 staining (see Fig. S5 in the supplementary material). In mfn embryos, a cluster of Tbx6-positive cells is retained in the tailbud (Fig. 7B). Consistent with our time-lapse video of mfn embryos, at least some of these posterior mesoderm cells eventually moved anteriorly and gradually surrounded the ectopic CN domain (Fig. 7D). Taken together, our data suggest that splitting of the CN upon loss of BMP signaling is likely to be a secondary consequence of the aberrant migration of ventral-posterior presomitic mesoderm cells. The defective movement of these Tbx6-positive cells at least partially accounts for our observation that secondary tails always contain cells expressing markers of presomitic mesoderm (eve1 or tbx6).
Fig. 7.
BMP regulates posterior mesoderm cells of the tailbud to inhibit secondary tail formation. (A-J) All panels show the midline section of tails of Tg(flh:EGFP)-expressing zebrafish embryos (green) at the indicated time points after anti-Tbx6 (red) immunostaining. Tbx6-positive mesoderm cells in mfn embryos (B) shows detention in the posterior tailbud, when compared with wild-type embryos (A) at the same stage. A more wild-type-like movement of posterior mesoderm cells in kny embryos is shown in F and H. Note that eventually ectopic Tbx6-positive mesoderm cells are shown in the secondary tail domain of mfn (D) and kny (J) embryos. Arrowheads mark the ventroposterior mesoderm cells in the taillbud. All tails are flat mounted laterally.
We performed a similar time-course experiment in kny;Tg(flh:EGFP) embryos. Migration of the Tbx6-positive cells is delayed in kny embryos; these cells are retained posteriorly at 18 hpf (Fig. 7F). Only later, at the 21-somite stage, do we observe some Tbx6-positive cells located ventrally beneath the CN (Fig. 7H). At 23 hpf, we observed Tbx6-positive cells colocalized with ectopic CN cells (Fig. 7J), suggesting that these Tbx6 cells were pushed along as the ectopic CN cells drifted out from the main axis. Consistent with our time-lapse video (Fig. 6F″), the undercutting movement of the posterior mesoderm cells still occurs in kny embryos, although with delayed timing.
DISCUSSION
BMP signaling inhibits secondary tail formation
A recently proposed model suggests that a gradient of BMP activity in the tailbud patterns the fates of tail mesoderm, and that within a narrow range of BMP inhibition, ectopic presomitic mesoderm is produced that leads to formation of a secondary tail (Stickney et al., 2007). Two lines of evidence indicate that the gradient model does not adequately explain the formation of secondary tails. First, our observation that secondary tails are produced in several BMP pathway mutants of widely varying strengths indicates that secondary tails are not a consequence of a precise adjustment of a BMP activity gradient within a narrow range. Second, secondary tails are formed in the absence of the fate patterning defects predicted by the BMP gradient model. Thus, our findings do not support the BMP morphogen model as an explanation of secondary tail formation. Rather, we propose that BMP signaling independently regulates morphogenesis and cell fate patterning in the tailbud during early somitogenesis. Our results are consistent with observations of dnBMPR embryos by Pyati and Kimelman, which led them to suggest that mesodermal progenitors were being left behind during tail extension, subsequently forming a secondary tail (Pyati et al., 2005).
During gastrulation, BMP is thought to create a gradient of intercellular adhesion along the DV axis, from low levels on the ventral side, where BMP activity is highest, to high levels dorsally, where BMP activity is low. Migrating mesodermal cells are therefore moving away from regions of high BMP signaling/low adhesion towards regions of low BMP activity/higher adhesion (Myers et al., 2002; von der Hardt et al., 2007). Although we observe a similar directionality (movement of mesodermal cells away from a region of high BMP activity), we do not know if an analogous gradient of adhesion is established in the tailbud by BMP. A key phenotypic distinction between the role of BMP in regulation of cell migration during gastrulation and the defects we observe here is that during gastrulation, dorsalward movement of lateral mesoderm is completely blocked in BMP-compromised embryos, whereas in the tailbud, posterior mesodermal cells still move anteriorly in mfn mutants, but move aberrantly into the yolk cell instead of undercutting KV. Precisely why these cells take this path remains unclear, although one possible explanation is that KV becomes more tightly associated with the yolk cell in BMP-compromised embryos, thereby blocking the movement of the presomitic mesoderm cells. However, in embryos where the dorsal organizer region was surgically extirpated near the end of gastrulation, and also in embryos where formation of KV was blocked by blastula-stage injection into the yolk cell of ntl and spt MOs, we still observed ectopic tbx6-postive cells associated with the yolk extension (Y.Y., unpublished results), indicating that these cells migrate aberrantly even in the absence of KV or the CN.
During gastrulation, BMP is proposed to regulate cell adhesion in a cadherin-dependent manner (von der Hardt et al., 2007). We observed a strong enhancement of the secondary tail phenotype when cdh2 (encoding N-cadherin) was knocked down in conjunction with partial knockdown of BMP. Although this result indicates that regulation of cell adhesion is important for proper morphogenesis of the tailbud, whether BMP functions in this process via regulation of cadherin function is unclear. For example, cdh2 is expressed in the notochord as well as the tailbud (Lele et al., 2002; Harrington et al., 2007), raising the possibility that the synergy we observe is due to separate effects of BMP on the posterior tailbud and N-cadherin on the notochord. Alternatively, cdh2 and BMP might both regulate migration of posterior mesoderm without necessarily functioning in the same pathway. More work, including a detailed examination of morphogenetic movements occurring in the tailbuds of mfn;cdh2 MO embryos, will be required to address these possibilities more definitively.
Somitic muscle formation in secondary tails
Our data indicate that although nearly all secondary tails contain ectopic presomitic mesoderm cells, only a subset of them actually form differentiated somitic muscle. It could be that in their ectopic location, presomitic mesoderm progenitors might not be fully competent to produce somitic muscle or, if they do, they might tend to be incorporated into somites produced by the primary tailbud. When we do observe ectopic myoD cells in the secondary tail, they are invariably part of a group of cells branching off from the main axis (see Fig. 1D).
Interestingly, in other vertebrates, the caudal end of the notochord has been shown to comprise a population of cells, termed the chordoneural hinge (CNH), which has been shown to act as a pool of progenitor cells that can give rise to multiple tissues, including notochord, floor plate and somitic muscle (Charrier et al., 1999; Davis and Kirschner, 2000; Cambray and Wilson, 2002; Cambray and Wilson, 2007). In Xenopus, when the CNH is grafted to host embryos, it is able to produce an ectopic tail (Gont et al., 1993). It is tempting to speculate that the caudal notochord in zebrafish could function similarly, and thus serve as a source for both the ectopic notochord and somitic muscle cells that we observe in secondary tails. However, in zebrafish, there is as yet no evidence that this region possesses the same mix of progenitor cells observed in other vertebrates, so at this time we favor the hypothesis that the ectopic notochord and muscle cells in the secondary tail arise from two distinct populations of cells: the CN and the aberrantly migrating Tbx6-positive cells, respectively.
Non-canonical Wnt signaling and Cadherin localization
To date, although we have observed secondary tails in kny and dvl2/dvl3 MO embryos, the ligand for this putative non-canonical Wnt pathway remains unidentified. Our observation that other genes that regulate CE movements during gastrulation, such as stbm, pk1 and pk2, are not required to prevent secondary tail formation suggests that secondary tails are not formed merely as a by-product of earlier defects in morphogenesis. However, we do observe that inhibition of stbm or pk1 can partially suppress the penetrance of secondary tail formation in kny and dvl2/3 MO embryos (data not shown), suggesting that stbm and pk1 might act antagonistically to kny and dvl in this context. Although such antagonism has been shown between stbm (Van Gogh – FlyBase), pk and frizzled signaling in Drosophila, much work remains to be done in order to clarify the roles of these genes and to identify additional genes that participate in this pathway.
During gastrulation, Wnt11 has been proposed to promote the cycling of E-cadherin between the plasma membrane and endosomes in migrating prechordal plate cells (Ulrich et al., 2005). In the absence of Wnt11, E-cadherin is predominantly localized at the plasma membrane, whereas in Wnt11-overexpressing cells, E-cadherin is observed most often in endosomes. Curiously, in the CN, we observe the opposite effect: cadherin becomes more intracellularly localized in the absence of Wnt signaling, rather than in its presence. The significance of this difference is unclear. One possibility is that prechordal plate cells, which actively migrate across a substrate of other cells and extracellular matrix, need to constantly remodel adhesion complexes in order to both move over their substrate and remain associated with other prechordal plate cells. It is not clear whether CN cells behave in the same way; posteriorward extension of the CN appears to be more passive, driven predominantly by CE movements occurring more anteriorly. Perhaps in this situation, more static localization of cadherin to the plasma membrane is sufficient to maintain coherence of the CN.
A model for the roles of BMP and non-canonical Wnt signaling in tail morphogenesis
Our data suggest that BMP and non-canonical Wnt signaling function independently of each other to ensure proper morphogenesis of the tail (Fig. 8). BMP signaling, acting on ventroposterior mesodermal precursors, promotes the anteriorward migration of these cells, undercutting KV and disrupting the association of the CN with the yolk prior to tail protrusion. When BMP signaling is blocked, aberrant migration of the mesodermal progenitors into the yolk can capture the CN, leading to its bifurcation as tail development proceeds.
Fig. 8.
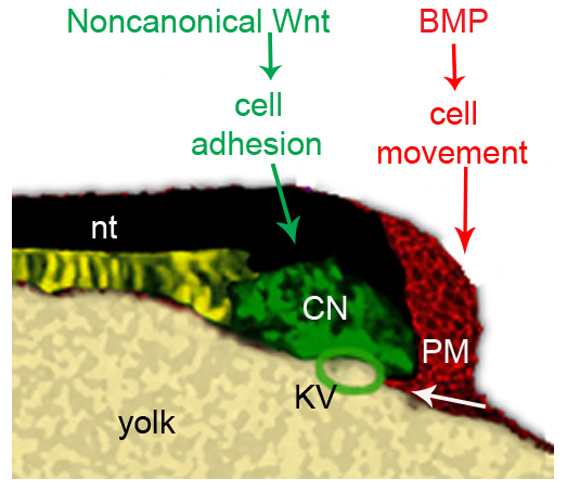
A model for the roles of BMP and non-canonical Wnt signaling in regulating tail development. Depiction of the tailbud of a 10-somite wild-type zebrafish embryo. During tailbud protrusion, BMP signaling in the posterior tailbud plays a crucial role in guiding the ventroposterior mesoderm cells to move beneath the Kupffer's vesicle properly. Non-canonical Wnt signaling promotes intercellular adhesion of cells in the caudal end of notochord, allowing them to maintain cohesion during tail elongation. CNH, the caudal end of notochord; nt: notochord; PM, posterior mesoderm; KV, Kupffer's vesicle.
Non-canonical Wnt signaling promotes the localization of cadherin to the plasma membrane in CN cells, possibly increasing intercellular adhesion. As the CN moves posteriorly during tail extension, it encounters a continuous stream of anteriorly migrating mesodermal progenitors from the posterior tailbud. We hypothesize that the CN might experience some shear stress as the result of moving through this field of cells, and that in the absence of non-canonical Wnt signaling, it is unable to maintain sufficient cohesion, leading to a sloughing off of CN cells. When both pathways are blocked, nearly all embryos form a secondary tail, possibly because a less cohesive CN will nearly always split when ‘caught’ in the yolk. Thus, we propose that these two pathways, which function independently to regulate convergence-extension during gastrulation, work in parallel later in development to govern morphogenesis in distinct regions of the tailbud.
Supplementary Material
Acknowledgements
We thank M. Halpern for kindly sharing the flh:GFP transgenic fish. This work was supported by funding from the Terry C. Johnson Center for Basic Cancer Research and NIH Grant (P20 RR016475) from the INBRE program of the National Center for Research Resources. Deposited in PMC for release after 12 months.
Footnotes
Competing interests statement
The authors declare no competing financial interests.
Supplementary material
Supplementary material for this article is available at http://dev.biologists.org/lookup/suppl/doi:10.1242/dev.058404/-/DC1
References
- Agathon A., Thisse C., Thisse B. (2003). The molecular nature of the zebrafish tail organizer. Nature 424, 448-452. [DOI] [PubMed] [Google Scholar]
- Angers S., Thorpe C. J., Biechele T. L., Goldenberg S. J., Zheng N., MacCoss M. J., Moon R. T. (2006). The KLHL12-Cullin-3 ubiquitin ligase negatively regulates the Wnt-beta-catenin pathway by targeting Dishevelled for degradation. Nat. Cell Biol. 8, 348-357. [DOI] [PubMed] [Google Scholar]
- Blader P., Fischer N., Gradwohl G., Guillemot F., Strahle U. (1997). The activity of neurogenin1 is controlled by local cues in the zebrafish embryo. Development 124, 4557-4569. [DOI] [PubMed] [Google Scholar]
- Brown L. A., Rodaway A. R., Schilling T. F., Jowett T., Ingham P. W., Patient R. K., Sharrocks A. D. (2000). Insights into early vasculogenesis revealed by expression of the ETS-domain transcription factor Fli-1 in wild-type and mutant zebrafish embryos. Mech. Dev. 90, 237-252. [DOI] [PubMed] [Google Scholar]
- Cambray N., Wilson V. (2002). Axial progenitors with extensive potency are localised to the mouse chordoneural hinge. Development 129, 4855-4866. [DOI] [PubMed] [Google Scholar]
- Cambray N., Wilson V. (2007). Two distinct sources for a population of maturing axial progenitors. Development 134, 2829-2840. [DOI] [PubMed] [Google Scholar]
- Charrier J. B., Teillet M. A., Lapointe F., Le Douarin N. M. (1999). Defining subregions of Hensen's node essential for caudalward movement, midline development and cell survival. Development 126, 4771-4783. [DOI] [PubMed] [Google Scholar]
- Connors S. A., Trout J., Ekker M., Mullins M. C. (1999). The role of tolloid/mini fin in dorsoventral pattern formation of the zebrafish embryo. Development 126, 3119-3130. [DOI] [PubMed] [Google Scholar]
- Davis R. L., Kirschner M. W. (2000). The fate of cells in the tailbud of Xenopus laevis. Development 127, 255-267. [DOI] [PubMed] [Google Scholar]
- Detrich H. W., 3rd, Kieran M. W., Chan F. Y., Barone L. M., Yee K., Rundstadler J. A., Pratt S., Ransom D., Zon L. I. (1995). Intraembryonic hematopoietic cell migration during vertebrate development. Proc. Natl. Acad. Sci. USA 92, 10713-10717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick A., Hild M., Bauer H., Imai Y., Maifeld H., Schier A. F., Talbot W. S., Bouwmeester T., Hammerschmidt M. (2000). Essential role of Bmp7 (snailhouse) and its prodomain in dorsoventral patterning of the zebrafish embryo. Development 127, 343-354. [DOI] [PubMed] [Google Scholar]
- Esterberg R., Delalande J. M., Fritz A. (2008). Tailbud-derived Bmp4 drives proliferation and inhibits maturation of zebrafish chordamesoderm. Development 135, 3891-3901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamse J. T., Thisse C., Thisse B., Halpern M. E. (2003). The parapineal mediates left-right asymmetry in the zebrafish diencephalon. Development 130, 1059-1068. [DOI] [PubMed] [Google Scholar]
- Gont L. K., Steinbeisser H., Blumberg B., de Robertis E. M. (1993). Tail formation as a continuation of gastrulation: the multiple cell populations of the Xenopus tailbud derive from the late blastopore lip. Development 119, 991-1004. [DOI] [PubMed] [Google Scholar]
- Halbleib J. M., Nelson W. J. (2006). Cadherins in development: cell adhesion, sorting, and tissue morphogenesis. Genes Dev. 20, 3199-3214. [DOI] [PubMed] [Google Scholar]
- Harrington M. J., Hong E., Fasanmi O., Brewster R. (2007). Cadherin-mediated adhesion regulates posterior body formation. BMC Dev. Biol. 7, 130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauptmann G., Gerster T. (1994). Two-color whole-mount in situ hybridization to vertebrate and Drosophila embryos. Trends Genet. 10, 266. [DOI] [PubMed] [Google Scholar]
- Hug B., Walter V., Grunwald D. J. (1997). tbx6, a Brachyury-related gene expressed by ventral mesendodermal precursors in the zebrafish embryo. Dev. Biol. 183, 61-73. [DOI] [PubMed] [Google Scholar]
- Jasuja R., Voss N., Ge G., Hoffman G. G., Lyman-Gingerich J., Pelegri F., Greenspan D. S. (2006). bmp1 and mini fin are functionally redundant in regulating formation of the zebrafish dorsoventral axis. Mech. Dev. 123, 548-558. [DOI] [PubMed] [Google Scholar]
- Kanki J. P., Ho R. K. (1997). The development of the posterior body in zebrafish. Development 124, 881-893. [DOI] [PubMed] [Google Scholar]
- Kimmel C. B., Ballard W. W., Kimmel S. R., Ullmann B., Schilling T. F. (1995). Stages of embryonic development of the zebrafish. Dev. Dyn. 203, 253-310. [DOI] [PubMed] [Google Scholar]
- Kishimoto Y., Lee K. H., Zon L., Hammerschmidt M., Schulte-Merker S. (1997). The molecular nature of zebrafish swirl: BMP2 function is essential during early dorsoventral patterning. Development 124, 4457-4466. [DOI] [PubMed] [Google Scholar]
- Kramer C., Mayr T., Nowak M., Schumacher J., Runke G., Bauer H., Wagner D. S., Schmid B., Imai Y., Talbot W. S., et al. (2002). Maternally supplied Smad5 is required for ventral specification in zebrafish embryos prior to zygotic Bmp signaling. Dev. Biol. 250, 263-279. [PubMed] [Google Scholar]
- Lele Z., Folchert A., Concha M., Rauch G. J., Geisler R., Rosa F., Wilson S. W., Hammerschmidt M., Bally-Cuif L. (2002). parachute/n-cadherin is required for morphogenesis and maintained integrity of the zebrafish neural tube. Development 129, 3281-3294. [DOI] [PubMed] [Google Scholar]
- Little S. C., Mullins M. C. (2006). Extracellular modulation of BMP activity in patterning the dorsoventral axis. Birth Defects Res. C Embryo Today 78, 224-242. [DOI] [PubMed] [Google Scholar]
- Marlow F., Zwartkruis F., Malicki J., Neuhauss S. C., Abbas L., Weaver M., Driever W., Solnica-Krezel L. (1998). Functional interactions of genes mediating convergent extension, knypek and trilobite, during the partitioning of the eye primordium in zebrafish. Dev. Biol. 203, 382-399. [DOI] [PubMed] [Google Scholar]
- Marlow F., Gonzalez E. M., Yin C., Rojo C., Solnica-Krezel L. (2004). No tail co-operates with non-canonical Wnt signaling to regulate posterior body morphogenesis in zebrafish. Development 131, 203-216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mintzer K. A., Lee M. A., Runke G., Trout J., Whitman M., Mullins M. C. (2001). Lost-a-fin encodes a type I BMP receptor, Alk8, acting maternally and zygotically in dorsoventral pattern formation. Development 128, 859-869. [DOI] [PubMed] [Google Scholar]
- Mullins M. C., Hammerschmidt M., Kane D. A., Odenthal J., Brand M., van Eeden F. J., Furutani-Seiki M., Granato M., Haffter P., Heisenberg C. P., et al. (1996). Genes establishing dorsoventral pattern formation in the zebrafish embryo: the ventral specifying genes. Development 123, 81-93. [DOI] [PubMed] [Google Scholar]
- Myers D. C., Sepich D. S., Solnica-Krezel L. (2002). Bmp activity gradient regulates convergent extension during zebrafish gastrulation. Dev. Biol. 243, 81-98. [DOI] [PubMed] [Google Scholar]
- Oxtoby E., Jowett T. (1993). Cloning of the zebrafish krox-20 gene (krx-20) and its expression during hindbrain development. Nucleic Acids Res. 21, 1087-1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyati U. J., Webb A. E., Kimelman D. (2005). Transgenic zebrafish reveal stage-specific roles for Bmp signaling in ventral and posterior mesoderm development. Development 132, 2333-2343. [DOI] [PubMed] [Google Scholar]
- Pyati U. J., Cooper M. S., Davidson A. J., Nechiporuk A., Kimelman D. (2006). Sustained Bmp signaling is essential for cloaca development in zebrafish. Development 133, 2275-2284. [DOI] [PubMed] [Google Scholar]
- Schier A. F., Talbot W. S. (2005). Molecular genetics of axis formation in zebrafish. Annu. Rev. Genet. 39, 561-613. [DOI] [PubMed] [Google Scholar]
- Schmid B., Furthauer M., Connors S. A., Trout J., Thisse B., Thisse C., Mullins M. C. (2000). Equivalent genetic roles for bmp7/snailhouse and bmp2b/swirl in dorsoventral pattern formation. Development 127, 957-967. [DOI] [PubMed] [Google Scholar]
- Schulte-Merker S., Ho R. K., Herrmann B. G., Nusslein-Volhard C. (1992). The protein product of the zebrafish homologue of the mouse T gene is expressed in nuclei of the germ ring and the notochord of the early embryo. Development 116, 1021-1032. [DOI] [PubMed] [Google Scholar]
- Seifert J. R., Mlodzik M. (2007). Frizzled/PCP signalling: a conserved mechanism regulating cell polarity and directed motility. Nat. Rev. Genet. 8, 126-138. [DOI] [PubMed] [Google Scholar]
- Sepich D. S., Calmelet C., Kiskowski M., Solnica-Krezel L. (2005). Initiation of convergence and extension movements of lateral mesoderm during zebrafish gastrulation. Dev. Dyn. 234, 279-292. [DOI] [PubMed] [Google Scholar]
- Solnica-Krezel L., Stemple D. L., Mountcastle-Shah E., Rangini Z., Neuhauss S. C., Malicki J., Schier A. F., Stainier D. Y., Zwartkruis F., Abdelilah S., et al. (1996). Mutations affecting cell fates and cellular rearrangements during gastrulation in zebrafish. Development 123, 67-80. [DOI] [PubMed] [Google Scholar]
- Stickney H. L., Imai Y., Draper B., Moens C., Talbot W. S. (2007). Zebrafish bmp4 functions during late gastrulation to specify ventroposterior cell fates. Dev. Biol. 310, 71-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talbot W. S., Trevarrow B., Halpern M. E., Melby A. E., Farr G., Postlethwait J. H., Jowett T., Kimmel C. B., Kimelman D. (1995). A homeobox gene essential for zebrafish notochord development. Nature 378, 150-157. [DOI] [PubMed] [Google Scholar]
- Thisse B., Heyer V., Lux A., Alunni V., Degrave A., Seiliez I., Kirchner J., Parkhill J. P., Thisse C. (2004). Spatial and temporal expression of the zebrafish genome by large-scale in situ hybridization screening. Methods Cell Biol. 77, 505-519. [DOI] [PubMed] [Google Scholar]
- Topczewski J., Sepich D. S., Myers D. C., Walker C., Amores A., Lele Z., Hammerschmidt M., Postlethwait J., Solnica-Krezel L. (2001). The zebrafish glypican knypek controls cell polarity during gastrulation movements of convergent extension. Dev. Cell 1, 251-264. [DOI] [PubMed] [Google Scholar]
- Tucker J. A., Mintzer K. A., Mullins M. C. (2008). The BMP signaling gradient patterns dorsoventral tissues in a temporally progressive manner along the anteroposterior axis. Dev. Cell 14, 108-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulrich F., Krieg M., Schotz E. M., Link V., Castanon I., Schnabel V., Taubenberger A., Mueller D., Puech P. H., Heisenberg C. P. (2005). Wnt11 functions in gastrulation by controlling cell cohesion through Rab5c and E-cadherin. Dev. Cell 9, 555-564. [DOI] [PubMed] [Google Scholar]
- Veeman M. T., Axelrod J. D., Moon R. T. (2003). A second canon. Functions and mechanisms of beta-catenin-independent Wnt signaling. Dev. Cell 5, 367-377. [DOI] [PubMed] [Google Scholar]
- von der Hardt S., Bakkers J., Inbal A., Carvalho L., Solnica-Krezel L., Heisenberg C. P., Hammerschmidt M. (2007). The Bmp gradient of the zebrafish gastrula guides migrating lateral cells by regulating cell-cell adhesion. Curr. Biol. 17, 475-487. [DOI] [PubMed] [Google Scholar]
- Weinberg E. S., Allende M. L., Kelly C. S., Abdelhamid A., Murakami T., Andermann P., Doerre O. G., Grunwald D. J., Riggleman B. (1996). Developmental regulation of zebrafish MyoD in wild-type, no tail and spadetail embryos. Development 122, 271-280. [DOI] [PubMed] [Google Scholar]
- Yan Y. L., Hatta K., Riggleman B., Postlethwait J. H. (1995). Expression of a type II collagen gene in the zebrafish embryonic axis. Dev. Dyn. 203, 363-376. [DOI] [PubMed] [Google Scholar]
- Yu P. B., Hong C. C., Sachidanandan C., Babitt J. L., Deng D. Y., Hoyng S. A., Lin H. Y., Bloch K. D., Peterson R. T. (2008). Dorsomorphin inhibits BMP signals required for embryogenesis and iron metabolism. Nat. Chem. Biol. 4, 33-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.