Abstract
Early-life experience, including maternal care, influences hippocampus-dependent learning and memory throughout life. Handling of pups during postnatal d 2–9 (P2–9) stimulates maternal care and leads to improved memory function and stress-coping. The underlying molecular mechanisms may involve early (by P9) and enduring reduction of hypothalamic corticotropin-releasing factor (CRF) expression and subsequent (by P45) increase in hippocampal glucocorticoid receptor (GR) expression. However, whether hypothalamic CRF levels influence changes in hippocampal GR expression (and memory function), via reduced CRF receptor activation and consequent lower plasma glucocorticoid levels, is unclear. In this study we administered selective antagonist for the type 1 CRF receptor, NBI 30775, to nonhandled rats post hoc from P10–17 and examined hippocampus-dependent learning and memory later (on P50–70), using two independent paradigms, compared with naive and vehicle-treated nonhandled, and naive and antagonist-treated handled rats. Hippocampal GR and hypothalamic CRF mRNA levels and stress-induced plasma corticosterone levels were also examined. Transient, partial selective blockade of CRF1 in nonhandled rats improved memory functions on both the Morris watermaze and object recognition tests to levels significantly better than in naive and vehicle-treated controls and were indistinguishable from those in handled (naive, vehicle-treated, and antagonist-treated) rats. GR mRNA expression was increased in hippocampal CA1 and the dentate gyrus of CRF1-antagonist treated nonhandled rats to levels commensurate with those in handled cohorts. Thus, the extent of CRF1 activation, probably involving changes in hypothalamic CRF levels and release, contributes to the changes in hippocampal GR expression and learning and memory functions.
Genetic factors interact with early-life experience to shape stress responses and learning and memory functions during adulthood (1–4). In the immature rodent, maternal input influences cognitive function and neuroendocrine stress responses during adulthood (5–9). Enhanced maternal care, elicited by brief, daily separation of rat pups from the dam (handling) (6, 7, 10, 11) for the first 1–3 postnatal weeks leads to long-term reduction in stress responses (12) and improved spatial learning and memory (13, 14). The molecular foundations of this long-lasting neuroplasticity may involve enduring reduction of corticotropin-releasing factor (CRF) expression in the hypothalamic paraventricular nucleus (PVN) and increased glucocorticoid receptor (GR) expression in the hippocampus(11, 15).
In exploring the mechanisms by which early-life experience regulates hippocampal GR expression and memory function, we have previously shown that handling from postnatal d 2–9 (P2–9) is sufficient to influence CRF and GR expression long term (11). We also found that hypothalamic CRF mRNA is down-regulated by the end of the handling paradigm on P9 (11). This reprogramming of hypothalamic CRF gene expression is an early change in the molecular cascade initiated by handling, because lower stress-evoked ACTH and corticosterone (CORT) levels are not evident until the third postnatal week. The reduced neuroendocrine stress response, in turn, is followed by elevation of hippocampal GR mRNA levels (by P45) and improved hippocampal-mediated cognitive function (7, 14).
This sequence of changes suggests the possibility that they may be linked. Reduced CRF levels should promote less peptide release in response to stress and thus diminished activation of the major pituitary CRF receptor, CRF1. The resulting reduction in plasma CORT levels should contribute to up-regulation of GR expression (16). An alternative hypothesis suggests that enhanced maternal input influences hippocampal GR expression (and function) independently from changes in CRF expression in the PVN.
To distinguish between these alternatives, we reasoned that if reduced activation of CRF1 by CRF (and consequent lower stress-evoked plasma CORT levels) immediately after the handling paradigm is required for long-lasting up-regulation of GR expression in the hippocampus and improved learning and memory functions, then partially blocking CRF1 receptors in nonhandled rats should recapitulate these changes. We took advantage of the fact that the enduring increase in GR expression in rats handled from P2–9 commences sometime between P23 and P45 (11), supporting the existence of a window of opportunity to intervene, and thus administered a selective CRF1 antagonist (NBI 30775) to nonhandled rats from P10–17. In this study we show that hippocampal GR expression and memory function and hypothalamic CRF levels of nonhandled rats are modified to levels typical of handled rats after this transient, systemic, post hoc administration of CRF1 antagonist.
Materials and Methods
Animals
Timed-pregnant Sprague Dawley rats were housed in a quiet, uncrowded animal facility under a 12-h light, 12-h dark cycle (lights on at 0700 h), with food and water provided ad libitum. Parturition was checked once a day, and the day of birth was considered P0. On P1, litters were mixed (to minimize effects of genetic or prenatal variability) and adjusted to six males and six females. Early-life rearing conditions, either handled or nonhandled, were assigned to each litter. Twelve litters were used. All experimental procedures were approved by university animal care committee and conformed to National Institutes of Health guidelines (http://oacu.od.nih.gov/NIHpolicy).
Experimental design
Sprague Dawley male rats were divided into six experimental groups: handled (H); nonhandled (NH); handled pups that were implanted with an Alzet minipump filled with vehicle (distilled water) on P10 (H+Veh); nonhandled rats that were implanted with vehicle-containing minipump on P10 (NH+Veh); nonhandled rats that were implanted with a pump containing the CRF1-antagonist, NBI 30775 (NH+Antag); and handled rats implanted with NBI 30775-containing pump (H+Antag).
Handling, a brief separation from the dam for 15 min, was carried out daily at 0830 h from P2-9 according to procedures previously described (11, 15); we have found that this duration of the procedure suffices to alter CRF and GR expression long term (11). During this time, nonhandled rats remained undisturbed with the dam. Cages were not changed for any of the experimental conditions from P2-9. On P10, all litters were returned to routine animal facility care (cage changes twice a week) until weaning on P21. On P21, all groups were housed two or three rats per cage.
Administration of the CRF1-antagonist
The selective nonpeptide CRF1 antagonist, NBI 30775 (3-[6-(dimethylamino)-4-methyl-pyrid-3-yl]-2,5-dimethyl-N,N-dipropyl-pyrazolo[2,3-a]-pyrimidin-7-amine, also formerly known as R121919) was a gift from Dr. Dimitri Grigoriadis (Neurocrine Biosciences, San Diego, CA). The compound was dissolved in warm distilled water, and the pH was adjusted. The solution (4 mg/ml) was loaded into a microosmotic Alzet pump (model 1007D, Alzet Corp., Cupertino, CA), calibrated to release 0.5 μl/h (∼2.5 mg/kg·d) (17) over 7 d. Before surgery, two P10 rats (one about to undergo surgery and one nonimplanted control) were removed from the home cage, placed in a separate cage, and kept euthermic on a warming pad (18, 19). Osmotic Alzet minipumps were implanted sc in the nape of the neck using halothane anesthesia (18). Implantation surgery was performed between 0830–1030 h and was completed within 5 min of disturbance. Pups were allowed to recover in the separate cage containing the control pup before being returned to the home cage. CRF1 antagonist was continuously infused from P10–17. The minipumps were removed on the day of weaning, P21 (19). When pumps were removed, pups were treated in the same manner as during implantation surgery.
Morris watermaze test
Hippocampus-dependent learning and memory were tested between P50 and P70 without knowledge of early-life treatment. Spatial memory was tested using a modified Morris watermaze procedure (18, 20). A circular pool (2 m in diameter, 0.6 m deep) was filled with 24 C water made opaque by the addition of powdered milk. A transparent escape platform, 13 cm in diameter, was positioned 2 cm below the surface of the water. Rats were subjected to 2 consecutive days of place training (seven trials per day) to familiarize them with locating and perching on the platform using visual cues in the testing room. During training, the escape platform remained in a fixed location in the center of the northwest quadrant of the pool. At the beginning of each trial, the rat was lowered into the water facing the inside wall of the pool. If the rat did not reach the platform in 60 sec, it was guided to the platform by the experimenter. Rats were kept on the platform for 15 sec and then returned to a holding cage. On the day of testing (d 3), the hidden platform was placed in the southwest quadrant of the pool. Rats were placed in the water at one of three random start positions, which rotated among the remaining quadrants. Rats were subjected to six trials. Latency to reach the escape platform (seconds) was recorded.
Object recognition test
The Morris watermaze involves potentially stressful elements of being forced to swim (18, 20) and may thus be confounded by the differential effects of early-life treatment of the groups' stress responses. Therefore, a second memory test that requires an intact hippocampus and is relatively less stressful than the watermaze was chosen. The 2-d object recognition procedure that tests hippocampus-dependent retrieval function (18, 21) was administered in P50–70 rats. Briefly, adult rats were tested in a 52 × 27 × 21-cm (length × width × height) Plexiglass cage that was lined with opaque paper, leaving the front panel transparent to permit observation by the investigator. The day before sample training, subjects were habituated to the testing cage in a quiet testing room for 20 min. On d 1, each animal was given one sample trial during which it was allowed to explore two objects, randomly placed 6 cm from a wall. Twenty-four hours later, the test trial was administered. During the test trial, the animal was allowed 5 min to explore a duplicate of an object from the sample trial (to avoid olfactory cues) and a novel object. In both sample and test trials, the duration of exploration of each object, defined as sniffing with the animal's nose in direct contact or within 2 cm of the object, was recorded.
Behavioral data analysis
The mean latency in seconds (±se) to reach the Morris watermaze platform per trial was calculated for each group. Significant differences were determined among groups (all groups were included for each analysis) using one-factor ANOVA with repeated measures (trials as the within-subject variable and early-life treatment as the between-subjects variable) followed by Bonferonni's post hoc test (unless otherwise noted). For the object recognition test, significant differences among groups in time spent exploring each object were analyzed using one-factor ANOVA with Bonferonni's post hoc test (unless otherwise noted). In all cases, P < 0.05 was considered significant (PRISM, GraphPad, Inc., San Diego, CA). For descriptive statistics, means and ses were calculated.
Animal/tissue preparation and hormonal assay
Approximately half the rats were killed via decapitation (0800–0900 h), and trunk blood was collected to measure baseline CORT levels. Brains were rapidly removed, immediately frozen on powdered dry ice, and stored at −80 C for mRNA analysis. The remaining animals were subjected to restraint stress. Animals were restrained in an acrylic chamber for 20 min. Thirty minutes after the onset of the restraint stress, trunk blood was collected. Plasma CORT levels were determined using a commercially available RIA kit (ICN Biochemicals, Irvine, CA) as previously described (22). The CORT assay sensitivity was 0.16 μg/dl. Interassay variability, determined by three dilutions of adult rat plasma, averaged 0.5 μg/dl.
In situ hybridization histochemistry (ISH)
Expression of hippocampal GR and PVN CRF mRNA was determined using ISH methods previously described (11, 22, 23). Briefly, 20-μm coronal sections were collected on gelatin-coated slides and stored at −80 C. Sections were thawed, air-dried, and postfixed in 4% paraformaldehyde in phosphate buffer for 20 min. Sections were dehydrated and rehydrated through graded ethanols (3 min each) and exposed to 0.25% acetic anhydride in 0.1 m triethanolamine (pH 8) for 8 min. After graded dehydration (1 min each), sections were incubated with prehybridization buffer (containing 50% formamide, 5× SET (3 m NaCl, 0.05 m EDTA, 0.6 m Tris buffer), 0.2% sodium dodecyl sulfate, 5× Denhardt's solution, 0.5 mg/ml salmon sperm sheared DNA, 250 μg/ μl yeast tRNA, 100 mm dithiothreitol, and 10% dextran sulfate) in a humidified chamber for 1 h (at 55 C for the GR mRNA probe and at 42 C for the CRF mRNA probe). Hippocampal sections were hybridized overnight with 35S-labeled GR-RNA probe [originally from Dr. K. Yamamoto, courtesy of Dr. J. Masters (Pfizer, Inc., Ann Arbor, MI)] in hybridization buffer. Sections underwent a 30-min ribonuclease digestion at 37 C and were washed successively at 62 C in 2× standard saline citrate (SSC) and 1× SSC for 5 min each (1× SSC denotes 0.15 m NaCl and 15 mm trisodium citrate buffer, pH 7.0), in 0.25× SSC for 30 min, and in 0.1× SSC and 0.03× SSC for 1 h each. PVN sections were hybridized with 35S-labeled CRF DNA probe overnight and washed at 55 C in 2× SSC and 1× SSC for 5 min each, then successively in four fresh solutions of 0.3× SSC for 15 min each. All sections were dehydrated in graded ethanols containing 0.3 m ammonium acetate, followed by 100% ethanol, and apposed to film (Kodak BioMax MR film, MR-1; Eastman Kodak, Rochester, NY) for 10–12 d.
Semiquantitative analysis and statistical considerations
Unbiased methods for section sampling and analyses were conducted without knowledge of treatment (18, 22, 23). GR mRNA ISH signal was analyzed on digitized films using the ImageTool software program (University of Texas Health Science Center, San Antonio, TX) as described previously (22). The linear range of ODs was evaluated using 14C standards. The mean ± se of four anatomically matched sections was calculated for each brain. Statistical significance (P < 0.05) among groups was determined using one-factor ANOVA with Bonferonni's post hoc test (PRISM, GraphPad, Inc.).
Results
Hippocampus-mediated learning and memory function is improved in nonhandled rats after early-life CRF1 antagonist treatment
The Morris watermaze paradigm used here involves 2 d of training, followed by the testing day (d 3). As shown in Fig. 1A, on the first trial of d 3, nonhandled rats treated with CRF1 antagonist reached the platform's new location faster than controls [between groups: F(3,40) = 4.51; P < 0.05] and with similar latencies as naive and CRF1 antagonist-treated, handled rats (P > 0.05). The nonhandled group needed the most time to reach the escape platform across trials on d 3 [between groups: F(3,5) = 35.03; P < 0.001]. The performance of nonhandled rats treated with CRF1 antagonist, did not differ from that of both naive and antagonist-treated handled rats. Figure 1B summarizes performance on all 3 d. The time needed for the antagonist-treated nonhandled rats to reach the platform on each day was significantly faster than that for controls [between groups: F(3,2) = 51.19; P < 0.001] and was indistinguishable from that for handled groups (P > 0.05).
Fig. 1.
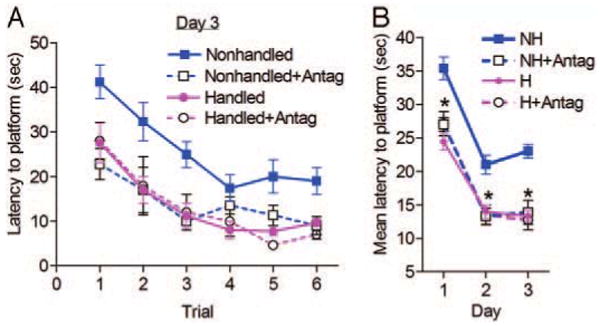
Continuous administration of CRF1 antagonist to nonhandled rats from P10–17 improves spatial memory function in the Morris watermaze. A, Mean time (±se) needed to find the hidden platform on consecutive trials during the test day (d 3; see Materials and Methods). B, Mean time (±se) in seconds required to find the platform considering all trials on each of the training days (d 1 and 2) and on the test day (d 3). NH, Nonhandled rats (n = 15); NH+Antag, CRF1 antagonist-treated nonhandled rats (n = 10); H, handled rats (n = 11); H+Antag, CRF1 antagonist-treated handled rats (n = 8). *, P < 0.05.
As shown in Fig. 2A, all groups learned to find the platform during the 2 d of training, i.e. less time required to reach the platform with subsequent trials [within group d 1: F(3,6) = 54.32; P < 0.001; d 2: F(3,6) = 42.80; P < 0.001], but to a markedly different degree [between groups d 1: F(3,6) = 9.80; P < 0.001; d 2: F(3,6) = 11.63; P < 0.001]. Nonhandled rats treated with CRF1 antagonist escaped faster than controls (P < 0.01), and their performance levels were similar to those of naive and CRF1 antagonist-treated, handled rats (P > 0.05). Vehicle administration did not influence spatial navigation at any point. The mean time (±se) per trial was compared between groups using a paired t test: naive and vehicle-treated nonhandled rats, P = 0.53 (Fig. 2B); naive and vehicle-treated, handled rats, P = 0.28 (Fig. 2B). Therefore, data for naive and vehicle-treated groups were combined.
Fig. 2.
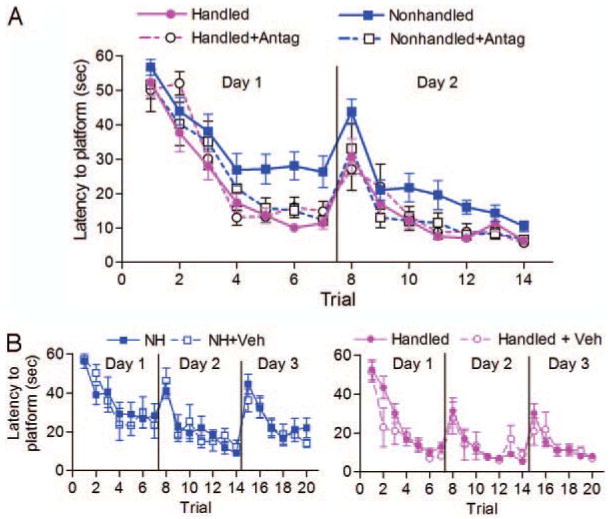
CRF1 antagonist treatment to nonhandled rats from P10–17 facilitates spatial learning during the training phase of the Morris watermaze. Data are given as the mean latency in seconds (±se) per trial required to find the submerged platform. A, Performance of handled (n = 11), handled and CRF1 antagonist-treated (n = 8), nonhandled (n = 15), and nonhandled and CRF1 antagonist-treated (n = 10) rats on d 1 and 2. B, Comparing naive and vehicle-treated subgroups, spatial learning was not different on all 3 d. Left, Nonhandled (n = 9) and vehicle-treated nonhandled (NH+Veh; n = 6); right, handled rats [naive, n = 8; vehicle treated (H+Veh), n = 3].
Hippocampus-dependent retrieval memory was also tested using the object recognition paradigm (18, 21). Objects 1 and 2 were explored for similar amounts of time during the first day of the recognition test (Fig. 3A). The amount of time spent exploring object 1 did not differ between NH and NH+Veh rats (by t test, P = 0.76), and this was also true for object 2 (by t test, P = 0.92; Fig. 3A, upper panel). Therefore, data from naive and vehicle-treated nonhandled groups were combined. Similarly, the exploration time of object 1 (by t test, P = 0.95) and that of object 2 (by t test, P = 0.74) did not differ between H and H+Veh rats (Fig. 3A, middle panel), and these data were consolidated. The lower panel of Fig. 3A shows that on the first day of the test, the time spent exploring objects 1 and 2 did not differ within each group (by t tests: NH, P = 0.98; H, P = 0.73; NH+A, P = 0.73; H+A, P = 0.95). Similarly, the exploration time of each object was not significantly different among groups [by one-factor ANOVA: object 1: F(3,59) = 0.29; P = 0.83; object 2: F (3,59) = 0.24; P = 0.87].
Fig. 3.
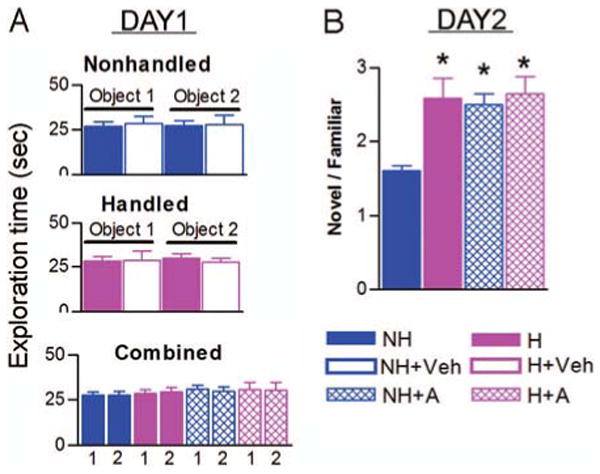
Early-life treatment with CRF1 antagonist improves memory in the relatively stress-free, object recognition test. Data are given as the mean number of seconds (±se) spent exploring each object on d 1 (A) and on d 2 (B), the test day. A, Day 1: upper panel, naive (solid blue bars; n = 9) and vehicle-treated (empty blue bars; n = 6) nonhandled groups; middle panel, naive (solid magenta bars; n = 8) and vehicle-treated (empty magenta bars; n = 3) handled groups. These panels show that object exploration times did not differ between naive and vehicle-treated subgroups. Lower panel, Therefore, the naive and vehicle subgroups were consolidated to compare time spent exploring object 1 (labeled as 1′ for each group) and object 2 (labeled as 2′ for each group). NH, Nonhandled rats; H, handled; NH+A, CRF1 antagonist-treated nonhandled rats (n = 10); H+A, antagonist-treated handled rats (n = 8). Note that on the first day, all groups explored each object for similar amounts of time. B, Day 2, The ratio of time spent exploring the novel object/time spent exploring the familiar object on d 2. A score of 1 indicates that both objects were explored for similar amounts of time. Administration of CRF1 antagonist did not modify the ratio of time spent exploring novel vs. familiar objects in handled rats. However, it significantly increased this ratio in the nonhandled rats. *, P < 0.05, significantly different from the nonhandled group. The values of H, H+A, and NH+A groups are not significantly different.
The second day of the object recognition test examined whether rats remembered the familiar object from the previous day. Indeed, all groups spent more time exploring the novel object and less time exploring the familiar object. However, the ratio of novel/familiar object exploration times differed significantly among groups: NH = 1.6 ± 0.06, H = 2.6 ± 0.27, NH+A = 2.5 ± 0.15; H+A = 2.65 ± 0.23 [one-factor ANOVA: F(3,59) = 8.77; P < 0.001; Fig. 3B]. Total exploration time did not vary among groups on either day of testing [d 1: F(3,59) = 0.33; P = 0.80; d 2: F(3,59) = 1.1; P = 0.37].
CRF1 antagonist increases GR mRNA in nonhandled rats
In accordance with previous findings (11, 15), levels of hippocampal GR mRNA were significantly up-regulated in handled rats compared with levels in nonhandled rats (Fig. 4). In comparison with nonhandled controls (naive and vehicle-treated), CRF1 antagonist treatment increased hippocampal GR mRNA expression in CA1 pyramidal cell layer [F(4,25) = 12.47; P < 0.001] and dentate gyrus granule cells [F(4,25) = 13.45; P < 0.001] to levels indistinguishable from those of naive and vehicle-treated handled animals (Fig. 4A). Administration of vehicle via osmotic pumps influenced neither hippocampal GR expression of nonhandled groups (by t tests: CA1 region, P = 0.71; dentate gyrus, P = 0.83) nor that of handled groups (by t tests: CA1 region, P = 0.66; dentate gyrus, P = 0.24). Treatment-induced augmentation of GR mRNA signal in hippocampus is depicted in bright-field photomicrographs derived from handled, nonhandled, vehicle-treated handled, and CRF1 antagonist-treated nonhandled rats (Fig. 4B).
Fig. 4.
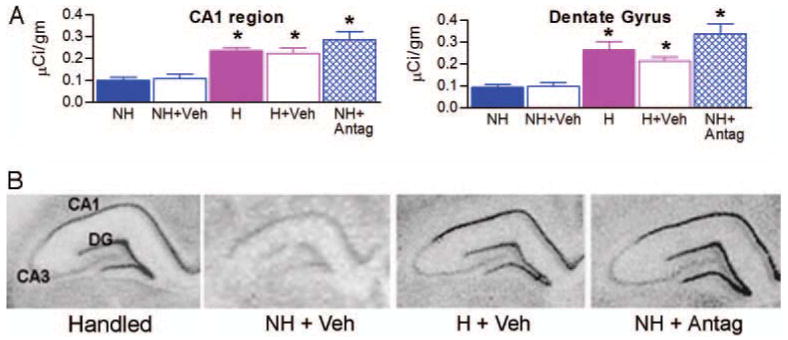
Partially blocking the CRF1 receptor in nonhandled rats during P10–17 reproduces the handling-induced up-regulation of GR mRNA expression in the hippocampus. A and B, Semiquantitative analysis (A) and representative bright-field photomicrographs (B) of coronal hippocampal sections from adult rats subjected to in situ hybridization for GR mRNA. CRF1-antagonist treatment significantly increased GR mRNA levels in both CA1 pyramidal cell layer and dentate gyrus granule cell layer compared with naive and vehicle-treated nonhandled rats. NH, Nonhandled; H, handled rats; NH+Veh, nonhandled and vehicle-treated rats; H+Veh, handled and vehicle-treated rats; NH+Antag, nonhandled and CRF1 antagonist-treated rats. Significant differences among groups were determined using one-factor ANOVA (*, P < 0.05; n = 6 animals/group).
CRF expression in PVN is reduced in CRF1 antagonist-treated nonhandled rats
CRF mRNA levels in PVN of adult rats handled early in life were lower compared with those in nonhandled controls [F(5,19) = 28.69; P < 0.001; Fig. 5]. Administration of CRF1 antagonist significantly down-regulated CRF expression in PVN of nonhandled rats (P < 0.01) to levels that did not differ significantly from those of naive or vehicle-treated handled rats. Expression of CRF mRNA in PVN was not influenced by vehicle administration in either handled or nonhandled groups (P > 0.05).
Fig. 5.
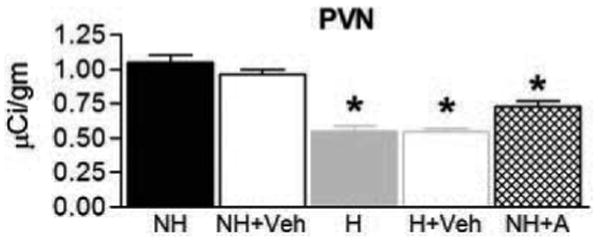
Infusion of CRF1 antagonist down-regulates CRF mRNA levels in PVN in adult nonhandled rats. Semiquantitative analysis depicting the mean (±se). NH, Nonhandled rats (n = 3); NH+Veh, vehicle-treated nonhandled rats (n = 6); H, handled rats (n = 4); H+Veh, vehicle-treated handled rats (n = 3); NH+Antag, nonhandled rats treated with CRF1 antagonist (n = 5). *, Significantly different from NH and NH+Veh groups, P < 0.05. NH+Antag levels were not significantly different from those in the H groups.
Early-life treatment with CRF1 antagonist does not alter adult basal or 30 min poststress plasma CORT levels
Administration of CRF1 antagonist early in life did not influence basal morning CORT levels [F(3,16) = 0.56; P = 0.64; Fig. 6]. A 20-min restraint stress elicited a significant elevation of plasma CORT 30 min after its onset (by t tests per group: NH, P < 0.001; H, P < 0.01; NH+Antag, P < 0.001, H+Antag, P < 0.001). One-factor ANOVA comparing mean (±se) CORT levels among the groups suggested that at 30 min after restraint onset, plasma levels of CORT were similar in CRF1 antagonist-treated handled rats and handled controls (P > 0.05). Levels in both of these groups were significantly lower than those in nonhandled groups [F(3,15) = 30.02; P < 0.001; Fig. 6]. The CRF1 antagonist treatment on P10–17 did not change plasma CORT levels 30 min after stress onset in nonhandled rats (P > 0.05). Adult adrenal weights did not differ among groups [F(3,18) = 0.30; P = 0.82].
Fig. 6.
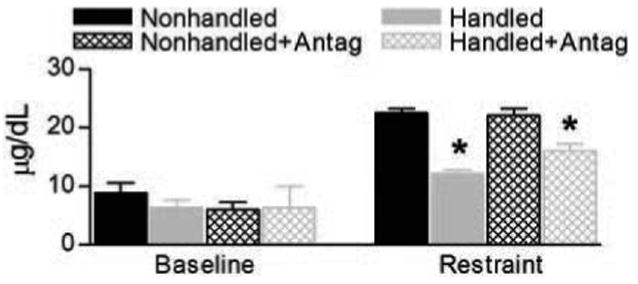
Basal and stress-evoked plasma CORT levels in the experimental groups. Left, Early-life handling and treatment with CRF1 antagonist did not influence basal morning adult plasma CORT levels. Nonhandled, n = 5; handled, n = 7; nonhandled and treated with CRF1 antagonist, n = 5; handled and treated with CRF1 antagonist, n = 4. Right, Thirty minutes after the onset of a 20-min restraint stress, plasma CORT levels in all groups were increased compared with basal levels. However, levels in nonhandled rats (n = 6) were higher than those in the early-life handled group (n = 4). Note that CRF1 antagonist treatment did not influence stress-evoked plasma CORT levels in either nonhandled antagonist-treated (n = 5) or handled antagonist-treated (n = 4) group. *, Significant differences from nonhandled and nonhandled antagonist-treated groups (P < 0.05).
Discussion
The principal findings of the current study are that administration of a selective CRF1-antagonist, NBI 30775, to nonhandled rats from P10–17 1) improves performance on hippocampus-dependent learning and memory tests, 2) increases hippocampal GR mRNA levels and 3) reduces CRF expression in the PVN. Together, these antagonist-evoked changes lead to learning/memory performances and gene expression levels in adult nonhandled rats that resemble those in rats handled early in life.
Adult rats handled early in life perform better in spatial learning tasks, such as the Morris watermaze (14). The current study confirms these findings and shows that CRF1 antagonist treatment to nonhandled rats also facilitates spatial learning and memory. Nonhandled rats treated with CRF1 antagonist required less time to find the escape platform during the 2 training days compared than those in naive and vehicle-treated nonhandled groups. In addition, performance was improved on d 3 of testing, when the hidden platform was placed in a new quadrant in the watermaze, and the ability to apply the previously learned search strategies to find the new platform location using the same spatial cues was tested. On the first trial of d 3, all rats initially swam to the quadrant where the escape platform was located during training. Rats treated with CRF1 antagonist found the hidden platform's new location in less time than nonhandled control groups, with latencies similar to those of handled groups. Because the performance on this series of tests requires an intact hippocampus (20), these findings support a long-lasting influence of early-life handling on hippocampal function and suggest that treatment with NBI 30775 influences hippocampal function of nonhandled rats. (It should be noted that antagonist administration on P10–17 did not influence the learning/memory function of handled rats.)
CRF1 antagonist treatment may improve spatial learning and memory via several potential mechanisms: For example, spatial learning in the watermaze is particularly sensitive to CORT levels, and both excessive or suboptimal amounts may lead to learning and memory impairments (24, 25). As shown here and in previous studies (11, 15), CORT levels after restraint stress are lower in adult rats handled early in life compared with nonhandled cohorts. However, early-life treatment of CRF1 antagonist to nonhandled rats did not reduce plasma CORT levels 30 min after the onset of restraint stress. Because this single time point may not reflect that overall profile of the hormonal stress response of these rats, it is difficult to determine from these data whether alterations in stress-evoked plasma CORT levels account for the improved watermaze performance found in this group.
CORT exerts its effects on hippocampal neurons via activation of receptors, including GR (26). Extensive previous work has demonstrated that GR expression levels are increased in adult rats handled early in life (11, 15). Indeed, the current study finds that CRF1 antagonist treatment increased GR expression in nonhandled rats to levels typical of those in rats handled early in life, supporting the idea that increased GR levels play a key role in regulating the stress responses of handled rats (6, 8, 11); in the presence of similar levels of plasma CORT, higher availability of GR may influence the efficiency of the actions triggered by CORT on hippocampal neurons (27, 28). In this study plasma CORT levels of CRF1 antagonist-treated nonhandled rats 30 min after stress onset were higher than those of handled rats. This may be attributable to the fact that the single time point may not be representative of the complete profile of the hormonal stress response. Future studies should further investigate this point.
It might be argued that the Morris watermaze paradigm involves adverse situations, so that potential subtle changes in the coping abilities of the experimental groups (handled, nonhandled, and antagonist treated) may influence the results. To control for these possibilities, hippocampus-dependent learning and memory were also examined using an independent, comparatively less stressful, object recognition test. Rats were habituated in the testing room and cage for 15 min, 24 h before the first day of testing, to minimize novelty stress. On the second day of testing, memory of the familiar object was significantly improved in the CRF1 antagonist-treated group, i.e. more time was spent exploring the novel object, resembling exploration times of early-life-handled cohorts. These data support the idea that the enhanced memory function of the nonhandled, CRF1 antagonist-treated group is independently regulated from their peripheral CORT responses 30 min after stress.
An alternative mechanism by which antagonist administration post hoc to nonhandled rats might lead to learning and memory improvements is a potential influence on endogenous CRF. In addition to its action on peripheral CRF receptors, when administered as a bolus at the doses used in this study, CRF1 antagonist crosses the blood-brain barrier and partially blocks central CRF1 receptor sites (17). CRF1 is found in many brain regions involved in learning and memory function, e.g. the frontal cortex and amygdala, as well as in the hippocampus (19, 29), the key substrate of the memory functions tested here. Recent research has implicated hippocampal CRF (30) in learning and memory (4, 18, 31). During the time of continuous CRF1 antagonist infusion, from P10-17, the number of CRF-immunoreactive neurons in the CA1 and CA3 hippocampal stratum pyramidal region increases by approximately 300% (from P11–18) (32). In addition, CRF1 receptor levels in hippocampus evolve with age (33). The effects of the endogenous ligand, CRF, on hippocampal CRF1 receptor expression have been demonstrated during the ages studied here (19). It is also conceivable that partially blocking CRF1 receptors from P10–17 may play a role in modulating cognitive function, either directly or via alterations in hippocampal CRF levels. These possibilities merit further investigation.
The mechanisms by which selective CRF1 antagonist treatment led to persisting increases in hippocampal GR expression are not entirely clear. The findings of this study support the postulation that pituitary CRF1 receptors in handled rats are less activated starting on approximately P10, resulting in lower plasma CORT levels in response to intermittent stress during the critical developmental period during the first few weeks of life (11). Because hippocampal GR expression is largely governed by plasma CORT (34), a potential effect of reduced CORT spikes during stress (before ∼P45) might be increased levels of hippocampal GR. Partially blocking CRF1 receptors on P10–17, as in this study in nonhandled rats, may recapitulate the lower plasma CORT levels at this age in handled rats, resulting in enduring increase in hippocampal GR.
Increased levels of hippocampal GR are typically associated with a reduction of hypothalamic CRF levels (13, 15). These latter two changes were found after CRF1 antagonist treatment in the current study. However, the design of the study did not permit us to determine whether the relationship of hippocampal GR and hypothalamic CRF found here is causal.
In summary, the enduring effects of enhanced early-life maternal care in handled rats on hippocampal GR gene expression, CRF mRNA in the PVN, and learning and memory are recapitulated by post hoc partial blockade of CRF1 receptor in nonhandled animals. These results may provide novel molecular targets influencing the long-lasting effects of early-life experience on learning and memory functions (35).
Acknowledgments
We thank Dr. Dimitri Grigoriadis for his kind gift of NBI 30775, and Michele Hinojosa for editorial assistance.
This work was supported by National Institutes of Health Grants 39307, 28912, and 07444.
Abbreviations
- CORT
Corticosterone
- CRF
corticotropin-releasing factor
- GR
glucocorticoid receptor
- ISH
in situ hybridization histochemistry
- P
postnatal day
- PVN
paraventricular nucleus
- SSC
standard saline citrate
References
- 1.Ammerman RT, Cassisi JE, Hersen M, van Hassel VB. Consequences of physical abuse and neglect in children. Clin Psychol Rev. 1986;6:291–310. [Google Scholar]
- 2.Bremner JD, Randall P, Vermetten E, Staib L, Bronen RA, Mazure C, Capelli S, McCarthy G, Innis RB, Charney DS. Magnetic resonance imaging-based measurement of hippocampal volume in posttraumatic stress disorder related to childhood physical and sexual abuse—a preliminary report. Biol Psychiatry. 1997;41:23–32. doi: 10.1016/s0006-3223(96)00162-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sanchez MM, Ladd CO, Plotsky PM. Early adverse experience as a developmental risk factor for later psychopathology: Evidence from rodent and primate models. Dev Psychopathol. 2001;13:419–449. doi: 10.1017/s0954579401003029. [DOI] [PubMed] [Google Scholar]
- 4.Avishai-Eliner S, Brunson KL, Sandman CA, Baram TZ. Stressed out? Or in (utero) Trends Neurosci. 2002;25:518–524. doi: 10.1016/s0166-2236(02)02241-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Walker CD, Scribner KA, Cascio CS, Dallman MF. The pituitary-adrenocortical system of neonatal rats is responsive to stress throughout development in a time-dependent and stressor-specific fashion. Endocrinology. 1991;128:1385–1395. doi: 10.1210/endo-128-3-1385. [DOI] [PubMed] [Google Scholar]
- 6.Liu D, Diorio J, Tannenbaum B, Caldji C, Francis D, Freedman A, Sharma S, Pearson D, Plotsky PM, Meaney MJ. Maternal care, hippocampal glucocorticoid receptors, and hypothalamic-pituitary-adrenal response to stress. Science. 1997;277:1659–1662. doi: 10.1126/science.277.5332.1659. [DOI] [PubMed] [Google Scholar]
- 7.Liu D, Diorio J, Day JC, Francis DD, Meaney MJ. Maternal care, hippocampal synaptogenesis and cognitive development in rats. Nat Neurosci. 2000;3:799–806. doi: 10.1038/77702. [DOI] [PubMed] [Google Scholar]
- 8.Bredy TW, Humpartzoomian RA, Cain DP, Meaney MJ. Partial reversal of the effect of maternal care on cognitive function through environmental enrichment. Neuroscience. 2003;118:571–576. doi: 10.1016/s0306-4522(02)00918-1. [DOI] [PubMed] [Google Scholar]
- 9.Mirescu C, Peters JD, Gould E. Early life experience alters response of adult neurogenesis to stress. Nature Neurosci. 2004;7:841–846. doi: 10.1038/nn1290. [DOI] [PubMed] [Google Scholar]
- 10.Hess JL, Denenberg VH, Zarrow MX, Pfeifer D. Modification of the corticosterone response curve as a function of handling in infancy. Physiol Behav. 1969;4:109–111. [Google Scholar]
- 11.Avishai-Eliner S, Eghbal-Ahmadi M, Tabatchnik E, Brunson KL, Baram TZ. Downregulation of hypothalamic corticotropin-releasing hormone messenger ribonucleic acid precedes early-life hippocampal glucocorticoid receptor-mRNA changes. Endocrinology. 2001;142:89–97. doi: 10.1210/endo.142.1.7917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meerlo P, Horvath KM, Nagy GM, Bohus B, Koolhaas JM. The influence of postnatal handling on adult neuroendocrine and behavioural stress reactivity. J Neuroendocrinol. 1999;11:925–933. doi: 10.1046/j.1365-2826.1999.00409.x. [DOI] [PubMed] [Google Scholar]
- 13.Meaney MJ, Aitken DH, van Berkel C, Bhatnagar S, Sapolsky RM. Effect of neonatal handling on age-related impairments associated with the hippocampus. Science. 1988;239:766–768. doi: 10.1126/science.3340858. [DOI] [PubMed] [Google Scholar]
- 14.Huot RL, Plotsky PM, Lenox RH, McNamara RK. Neonatal maternal separation reduces hippocampal mossy fiber density in adult Long Evans rats. Brain Res. 2002;950:52–63. doi: 10.1016/s0006-8993(02)02985-2. [DOI] [PubMed] [Google Scholar]
- 15.Plotsky PM, Meaney MJ. Early, postnatal experience alters hypothalamic corticotropin-releasing factor (CRF) mRNA, median eminence CRF content and stress-induced release in adult rats. Mol Brain Res. 1993;18:195–200. doi: 10.1016/0169-328x(93)90189-v. [DOI] [PubMed] [Google Scholar]
- 16.De Kloet ER, Oitzl MS, Joels M. Stress and cognition: are corticosteroids good or bad guys? Trends Neurosci. 1999;22:422–426. doi: 10.1016/s0166-2236(99)01438-1. [DOI] [PubMed] [Google Scholar]
- 17.Heinrichs SC, De Souza EB, Schulteis G, Lapsansky JL, Grigoriadis DE. Brain penetrance, receptor occupancy and anti-stress in vivo efficacy of a small molecule corticotropin releasing factor type I receptor selective antagonist. Neuropsychopharmacology. 2002;27:194–202. doi: 10.1016/S0893-133X(02)00299-3. [DOI] [PubMed] [Google Scholar]
- 18.Brunson KL, Eghbal-Ahmadi M, Bender R, Chen Y, Baram TZ. Long-term, progressive hippocampal cell loss and dysfunction induced by early-life administration of corticotropin-releasing hormone reproduce the effects of early-life stress. Proc Natl Acad Sci USA. 2001;98:8856–8861. doi: 10.1073/pnas.151224898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brunson KL, Grigoriadis DR, Lorang MT, Baram TZ. Corticotropin releasing hormone (CRH) downregulates the function of its receptor (CRF1) and induces CRF1 expression in hippocampal and cortical regions of the immature rat. Exp Neurol. 2002;176:75–86. doi: 10.1006/exnr.2002.7937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morris R. Developments of a water-maze procedure for studying spatial learning in the rat. J Neurosci Methods. 1984;11:47–60. doi: 10.1016/0165-0270(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 21.Clark RE, Zola SM, Squire LR. Impaired recognition memory in rats after damage to the hippocampus. J Neurosci. 2000;20:8853–8860. doi: 10.1523/JNEUROSCI.20-23-08853.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eghbal-Ahmadi M, Avishai-Eliner S, Hatalski CG, Baram TZ. Differential regulation of the expression of corticotropin-releasing factor receptor type 2 (CRF2) in the hypothalamus and amygdala of the immature rat by sensory input and food intake. J Neurosci. 1999;19:3982–3991. doi: 10.1523/JNEUROSCI.19-10-03982.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fenoglio KA, Avishai-Eliner S, Chen Y, Baram TZ. Region-specific onset of handling-induced changes in corticotropin releasing hormone and glucocorticoid receptor expression. Endocrinology. 2004;145:121–136. doi: 10.1210/en.2004-0111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oitzl MS, de Kloet RE. Selective corticosteroid antagonist modulates specific aspects of spatial orientation learning. Behav Neurosci. 1992;106:62–71. doi: 10.1037//0735-7044.106.1.62. [DOI] [PubMed] [Google Scholar]
- 25.Oitzl MS, Fluttert M, Sutanto W, de Kloet ER. Continuous blockade of brain glucocorticoid receptors facilitates spatial learning and memory in rats. Eur J Neurosci. 1998;10:3759–3766. doi: 10.1046/j.1460-9568.1998.00381.x. [DOI] [PubMed] [Google Scholar]
- 26.de Kloet RE, Vreugdenhil E, Oitzl MS, Joels M. Brain corticosteroid receptor balance in health and disease. Endocr Rev. 1998;19:269–301. doi: 10.1210/edrv.19.3.0331. [DOI] [PubMed] [Google Scholar]
- 27.Linthorst AC, Flachskamm C, Barden N, Holsboer F, Reul JM. Glucocorticoid receptor impairment alters CNS responses to a psychological stressor: an in vivo microdialysis study in transgenic mice. Eur J Neurosci. 2000;12:283–291. doi: 10.1046/j.1460-9568.2000.00878.x. [DOI] [PubMed] [Google Scholar]
- 28.Cole TJ, Myles K, Purton JF, Brereton PS, Solomon NM, Godfrey DI, Funder JW. GRKO mice express an aberrant dexamethasone-binding glucocorticoid receptor, but are profoundly glucocorticoid resistant. Mol Cell Endocrinol. 2001;173:193–202. doi: 10.1016/s0303-7207(00)00407-x. [DOI] [PubMed] [Google Scholar]
- 29.Chen Y, Brunson KL, Muller MB, Cariaga W, Baram TZ. Immunocytochemical distribution of corticotropin-releasing hormone receptor type-1 (CRF(1))-like immunoreactivity in the mouse brain: light microscopy analysis using an antibody directed against the C-terminus. J Comp Neurol. 2000;420:305–323. doi: 10.1002/(sici)1096-9861(20000508)420:3<305::aid-cne3>3.0.co;2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen Y, Bender RA, Brunson KL, Pomper JK, Grigoriadis DE, Wurst W, Baram TZ. Modulation of dendritic differentiation by corticotropin-releasing factor in the developing hippocampus. Proc Natl Acad Sci USA. 2004;101:15782–15787. doi: 10.1073/pnas.0403975101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Blank T, Nijholt I, Grammatopoulos DK, Randeva HS, Hillhouse EW, Spiess J. Corticotropin releasing factor receptors couple to multiple G-proteins to activate diverse intracellular signaling pathways in mouse hippocampus: role in neuronal excitability and associative learning. J Neurosci. 2003;23:700–707. doi: 10.1523/JNEUROSCI.23-02-00700.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen Y, Bender RA, Frotscher M, Baram TZ. Novel and transient populations of corticotropin-releasing hormone-expressing neurons in developing hippocampus suggest unique functional roles: a quantitative spatiotemporal analysis. J Neurosci. 2001;21:7171–7181. doi: 10.1523/JNEUROSCI.21-18-07171.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Baram TZ, Hatalski CG. Neuropeptide-mediated excitability: a key triggering mechanism for seizure generation in the developing brain. Trends Neurosci. 1998;21:471–476. doi: 10.1016/s0166-2236(98)01275-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Herman JP, Spencer R. Regulation of hippocampal glucocorticoid receptor gene transcription and protein expression in vivo. J Neurosci. 1998;18:7462–7473. doi: 10.1523/JNEUROSCI.18-18-07462.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kaufman J, Charney D. Effects of early stress on brain structure and function: implications for understanding the relationship between child maltreatment and depression. Dev Psychopathol. 2001;13:451–471. doi: 10.1017/s0954579401003030. [DOI] [PubMed] [Google Scholar]


