Abstract
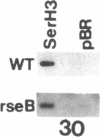
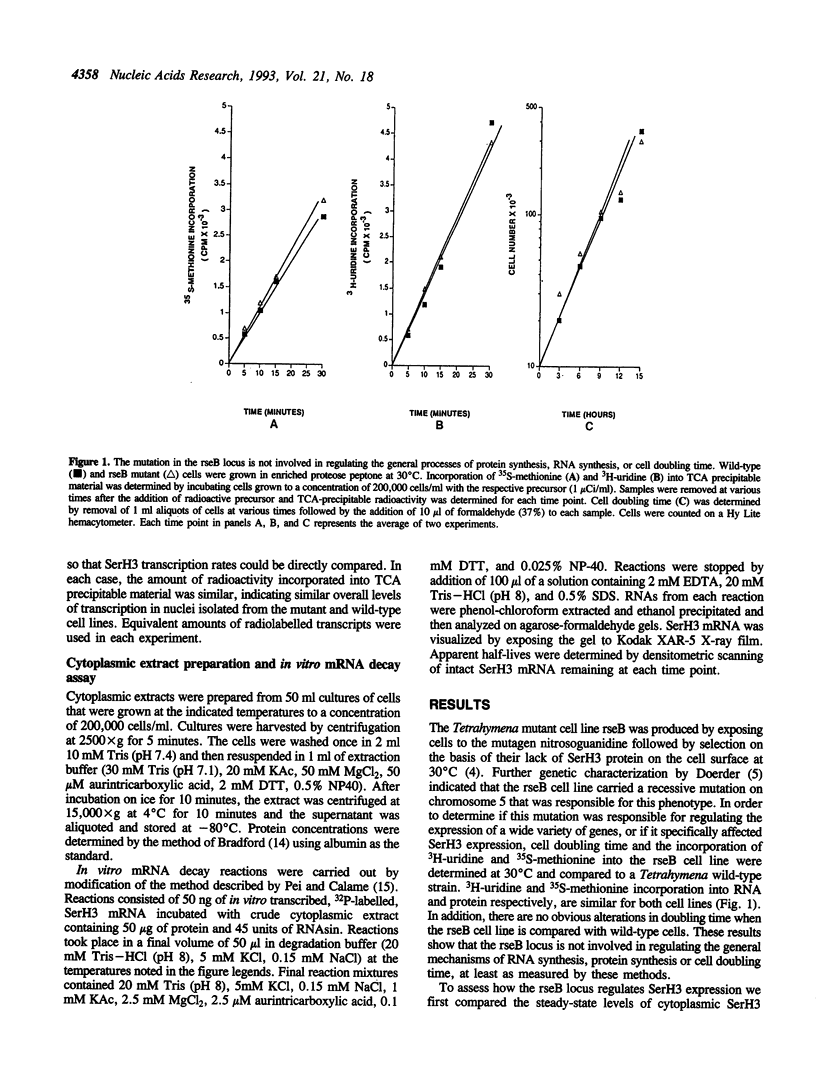
In Tetrahymena thermophila, the expression of a temperature-specific surface protein known as SerH3 is primarily controlled by a temperature-dependent change in the stability of the mRNA that encodes this protein. At 30 degrees C the SerH3 mRNA displays a half-life of 60 minutes while at 40 degrees C the half-life decreases to only 3 minutes. We used a Tetrahymena mutant cell line (rseB) defective in expression of SerH3 at 30 degrees C to explore the mechanisms involved in temperature-dependent mRNA stability. The results of in vitro nuclear run-off assays and Northern and slot blot analysis of cytoplasmic and nuclear RNAs show that the rseB locus encodes a temperature-sensitive product that has no effect on SerH3 gene transcription or the steady-state levels of SerH3 nuclear RNA. However, the product of this locus does have a dramatic effect on cytoplasmic levels of the SerH3 mRNA at 30 degrees C, indicating that SerH3 gene expression is affected post-transcriptionally within the cytoplasm. To explore the possibility that the rseB locus controls SerH3 mRNA stability we developed an in vitro mRNA decay assay. This assay successfully duplicates the differential decay of the SerH3 mRNA observed in wild-type cells grown at different temperatures. The apparent half-life of the SerH3 mRNA in cytoplasmic extracts derived from cells grown at 30 degrees C is approximately 45 minutes while in cytoplasmic extracts derived from cells grown at 40 degrees C it is only 6 minutes. When similar experiments are performed using extracts prepared from the Tetrahymena rseB cell line, we find that the SerH3 mRNA is only stable in extract prepared from cells grown under conditions in which the mRNA accumulates to detectable levels in the cytoplasm. These results indicate that the product of the rseB locus is a trans-acting cytoplasmic factor that exerts its effect on SerH3 gene expression by regulating SerH3 mRNA stability.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atwater J. A., Wisdom R., Verma I. M. Regulated mRNA stability. Annu Rev Genet. 1990;24:519–541. doi: 10.1146/annurev.ge.24.120190.002511. [DOI] [PubMed] [Google Scholar]
- Bannon G. A., Calzone F. J., Bowen J. K., Allis C. D., Gorovsky M. A. Multiple, independently regulated, polyadenylated messages for histone H3 and H4 in Tetrahymena. Nucleic Acids Res. 1983 Jun 25;11(12):3903–3917. doi: 10.1093/nar/11.12.3903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechhofer D. H., Dubnau D. Induced mRNA stability in Bacillus subtilis. Proc Natl Acad Sci U S A. 1987 Jan;84(2):498–502. doi: 10.1073/pnas.84.2.498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein P. L., Herrick D. J., Prokipcak R. D., Ross J. Control of c-myc mRNA half-life in vitro by a protein capable of binding to a coding region stability determinant. Genes Dev. 1992 Apr;6(4):642–654. doi: 10.1101/gad.6.4.642. [DOI] [PubMed] [Google Scholar]
- Bohjanen P. R., Petryniak B., June C. H., Thompson C. B., Lindsten T. An inducible cytoplasmic factor (AU-B) binds selectively to AUUUA multimers in the 3' untranslated region of lymphokine mRNA. Mol Cell Biol. 1991 Jun;11(6):3288–3295. doi: 10.1128/mcb.11.6.3288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Bridges K. R., Cudkowicz A. Effect of iron chelators on the transferrin receptor in K562 cells. J Biol Chem. 1984 Nov 10;259(21):12970–12977. [PubMed] [Google Scholar]
- Calzone F. J., Angerer R. C., Gorovsky M. A. Regulation of protein synthesis in Tetrahymena: isolation and characterization of polysomes by gel filtration and precipitation at pH 5.3. Nucleic Acids Res. 1982 Mar 25;10(6):2145–2161. doi: 10.1093/nar/10.6.2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darnell J. E., Jr Variety in the level of gene control in eukaryotic cells. Nature. 1982 Jun 3;297(5865):365–371. doi: 10.1038/297365a0. [DOI] [PubMed] [Google Scholar]
- Doerder F. P., Berkowitz M. S., Skalican-Crowe J. Isolation and genetic analysis of mutations at the SerH immobilization antigen locus of Tetrahymena thermophila. Genetics. 1985 Oct;111(2):273–286. doi: 10.1093/genetics/111.2.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doerder F. P. Regulatory Serotype Mutations in TETRAHYMENA PYRIFORMIS, Syngen 1. Genetics. 1973 May;74(1):81–106. doi: 10.1093/genetics/74.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreyfuss G. Structure and function of nuclear and cytoplasmic ribonucleoprotein particles. Annu Rev Cell Biol. 1986;2:459–498. doi: 10.1146/annurev.cb.02.110186.002331. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. "A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity". Addendum. Anal Biochem. 1984 Feb;137(1):266–267. doi: 10.1016/0003-2697(84)90381-6. [DOI] [PubMed] [Google Scholar]
- Gorovsky M. A., Yao M. C., Keevert J. B., Pleger G. L. Isolation of micro- and macronuclei of Tetrahymena pyriformis. Methods Cell Biol. 1975;9(0):311–327. doi: 10.1016/s0091-679x(08)60080-1. [DOI] [PubMed] [Google Scholar]
- Kuchka M. R., Goldschmidt-Clermont M., van Dillewijn J., Rochaix J. D. Mutation at the Chlamydomonas nuclear NAC2 locus specifically affects stability of the chloroplast psbD transcript encoding polypeptide D2 of PS II. Cell. 1989 Sep 8;58(5):869–876. doi: 10.1016/0092-8674(89)90939-2. [DOI] [PubMed] [Google Scholar]
- Love H. D., Jr, Allen-Nash A., Zhao Q. A., Bannon G. A. mRNA stability plays a major role in regulating the temperature-specific expression of a Tetrahymena thermophila surface protein. Mol Cell Biol. 1988 Jan;8(1):427–432. doi: 10.1128/mcb.8.1.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malter J. S. Identification of an AUUUA-specific messenger RNA binding protein. Science. 1989 Nov 3;246(4930):664–666. doi: 10.1126/science.2814487. [DOI] [PubMed] [Google Scholar]
- Müllner E. W., Kühn L. C. A stem-loop in the 3' untranslated region mediates iron-dependent regulation of transferrin receptor mRNA stability in the cytoplasm. Cell. 1988 Jun 3;53(5):815–825. doi: 10.1016/0092-8674(88)90098-0. [DOI] [PubMed] [Google Scholar]
- Nilsson G., Belasco J. G., Cohen S. N., von Gabain A. Growth-rate dependent regulation of mRNA stability in Escherichia coli. Nature. 1984 Nov 1;312(5989):75–77. doi: 10.1038/312075a0. [DOI] [PubMed] [Google Scholar]
- Owen D., Kühn L. C. Noncoding 3' sequences of the transferrin receptor gene are required for mRNA regulation by iron. EMBO J. 1987 May;6(5):1287–1293. doi: 10.1002/j.1460-2075.1987.tb02366.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei R., Calame K. Differential stability of c-myc mRNAS in a cell-free system. Mol Cell Biol. 1988 Jul;8(7):2860–2868. doi: 10.1128/mcb.8.7.2860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao K., Harford J. B., Rouault T., McClelland A., Ruddle F. H., Klausner R. D. Transcriptional regulation by iron of the gene for the transferrin receptor. Mol Cell Biol. 1986 Jan;6(1):236–240. doi: 10.1128/mcb.6.1.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saini K. S., Summerhayes I. C., Thomas P. Molecular events regulating messenger RNA stability in eukaryotes. Mol Cell Biochem. 1990 Jul 17;96(1):15–23. doi: 10.1007/BF00228449. [DOI] [PubMed] [Google Scholar]
- Shapiro D. J., Blume J. E., Nielsen D. A. Regulation of messenger RNA stability in eukaryotic cells. Bioessays. 1987 May;6(5):221–226. doi: 10.1002/bies.950060507. [DOI] [PubMed] [Google Scholar]
- Shaw G., Kamen R. A conserved AU sequence from the 3' untranslated region of GM-CSF mRNA mediates selective mRNA degradation. Cell. 1986 Aug 29;46(5):659–667. doi: 10.1016/0092-8674(86)90341-7. [DOI] [PubMed] [Google Scholar]
- Stargell L. A., Karrer K. M., Gorovsky M. A. Transcriptional regulation of gene expression in Tetrahymena thermophila. Nucleic Acids Res. 1990 Nov 25;18(22):6637–6639. doi: 10.1093/nar/18.22.6637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tondravi M. M., Willis R. L., Love H. D., Jr, Bannon G. A. Molecular characterization of SerH3, a Tetrahymena thermophila gene encoding a temperature-regulated surface antigen. Mol Cell Biol. 1990 Nov;10(11):6091–6096. doi: 10.1128/mcb.10.11.6091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vreken P., Buddelmeijer N., Raué H. A. A cell-free extract from yeast cells for studying mRNA turnover. Nucleic Acids Res. 1992 May 25;20(10):2503–2510. doi: 10.1093/nar/20.10.2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu M., Allis C. D., Richman R., Cook R. G., Gorovsky M. A. An intervening sequence in an unusual histone H1 gene of Tetrahymena thermophila. Proc Natl Acad Sci U S A. 1986 Nov;83(22):8674–8678. doi: 10.1073/pnas.83.22.8674. [DOI] [PMC free article] [PubMed] [Google Scholar]