Abstract
Cerebellar Purkinje neurons (PNs) possess a well characterized propensity to fuse with bone marrow-derived cells (BMDCs), producing heterokaryons with Purkinje cell identities. This offers the potential to rescue/repair at risk or degenerating PNs in the inherited ataxias, including Spinocerebellar Ataxia 1 (SCA1), by introducing therapeutic factors through BMDCs to potentially halt or reverse disease progression. In this study, we combined gene therapy and a stem cell-based treatment to attempt repair of at-risk PNs through cell-cell fusion in a Sca1154Q/2Q knock-in mouse model. BMDCs enriched for the hematopoietic stem cell (HSC) population were genetically modified using adeno-associated viral vector 7 (AAV7) to carry SCA1 modifier genes and transplanted into irradiated Sca1154Q/2Q mice. Binucleated Purkinje heterokaryons with sex-mismatched donor Y chromosomes were detected and successfully expressed the modifier genes in vivo. Potential effects of the new genome within Purkinje heterokaryons were evaluated using nuclear inclusions (NIs) as a biological marker to reflect possible modifications of the SCA1 disease process. An overall decrease in number of NIs and an increase in the number of surviving PNs were observed in treated Sca1154Q/2Q. Furthermore, Bergmann glia were found to have fusogenic potential with the donor population and reveal another potential route of therapeutic entry into at-risk cells of the SCA1 cerebellum. This study presents a first step towards a proof of principle that combines somatic cellular fusion events with a neuroprotective gene therapy approach for providing potential neuronal protection/repair in a variety of neurodegenerative disorders.
Keywords: Spinocerebellar Ataxia 1, Bone marrow derived cells, Hematopoietic stem cells, Gene therapy, AAV, Stem cell fusion
Introduction
Bone marrow derived cells (BMDCs) have shown evidence of in vivo fusion with somatic cells to give rise to atypical cellular phenotypes in liver, intestine, heart, skeletal muscle and cerebellar Purkinje neurons (Ferrari et al., 1998; Gussoni et al., 1999; Jackson et al., 1999; Petersen et al., 1999; Lagasse et al., 2000; Krause et al., 2001; Orlic et al., 2001; Alvarez-Dolado et al., 2003; Weimann et al, 2003a, 2003b). These findings suggest that cellular fusion is a mechanism that might be exploited to expand the developmental scope of adult stem cells for cellular therapies. Exploring novel therapeutic strategies that could potentially repair or rescue dying neurons, through introduction of new genetic materials via cellular fusion, would be valuable for neuronal populations such as cerebellar Purkinje neurons that are extremely recalcitrant to replacement and yet are vulnerable in many movement disorders.
One neurological disorder with specific Purkinje neuron atrophy that could benefit from this approach is spinocerebellar ataxia 1 (SCA1), a gain of function polyglutamine repeat disease that currently has no effective treatments available (Zoghbi and Orr, 2000; Orr and Zoghbi, 2001, 2007). SCA1 is an autosomal dominant disorder caused by expanded glutamine repeats in the ataxin-1 protein that results in cell death within selective neuronal populations, including cerebellar Purkinje neurons (Zoghbi and Orr, 1995). The underlying cause has not yet been completely elucidated, but evidence indicates protein misfolding, impaired protein clearance (Cummings et al., 1998, 1999; Skinner et al., 2001), as well as altered protein-protein interactions (Tsuda et al., 2005; Lam et al., 2006) are involved. Overexpression of chaperones have shown beneficial effects on the disease progression (Cummings et al., 2001), and other potentially neuroprotective genes also have been identified through a genetic screen using a Drosophila SCA1 model (Fernandez-Funez et al., 2000).
The current study set out to test a concept of using BMDCs as non-invasive delivery vehicles to introduce potentially disease-modifying genes (DnaJB4 and Pcbp3) from the aforementioned screen, into degenerating or at-risk Purkinje neurons of a Sca1 knock-in mouse carrying 154 polyglutamine repeats (Sca1154Q/2Q) (Watase et al, 2002), through cellular fusion. BMDCs were first genetically modified using adeno-associated viruses (AAV) which have shown great potential in gene transfer and therapy (Berns and Giraud., 1996; Muzyczka, 1992). AAV 7 especially has been found to be capable of transducing BMDCs and hematopoietic stem cells (HSCs) efficiently (Maina et al., 2008) and the transduction efficiency is further improved using a self-complementary vector (scAAV) that bypasses the need for viral second-strand DNA synthesis (Wu et al, 2007; Wang et al., 2003). Using this system, we demonstrate here that the transgenes carried by BMDCs are stably expressed within the cerebellum following cell-cell fusion and exert potentially attenuating effects as reflected by a decrease of nuclear inclusions and an increase in the number of surviving Purkinje cells. This is therefore a proof of concept that a combination of cell-cell fusion and gene therapy may be a potential approach for protecting certain at-risk neurons and restoring homeostatic balance within neurodegenerative diseases.
Results
AAV plasmid design and expression of the transgenes in HEK 293T cells
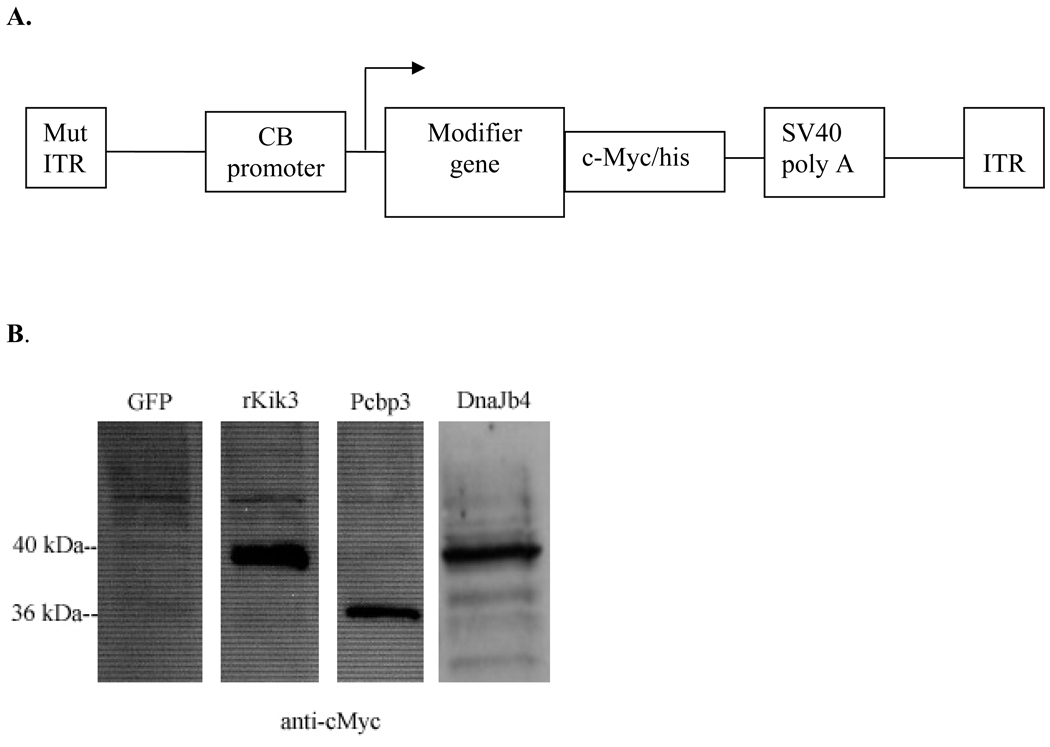
Recent advances show that scAAV7 vectors are capable of transducing Sca-1+, c-kit+, Lin− (SKL) population of bone marrow cells, which have been shown to be HSCs (Krause et al., 2001; Osawa et al., 1996; Spangrude et al., 1988; Muller-Sieburg et al., 1986), with high efficiency (Maina et al., 2008; Han et al., 2008) and therefore suited for carrying SCA1 modifier genes (Fig. 2A). Selection of the transgenes with potential neuroprotective effects was based on previous data published by Fernandez-Funez and colleagues (2000) who showed four genes, via a genetic screen using a SCA1 Drosophila model, with attenuating effects on the course of disease in SCA1. Two of the genes were tested in this study and their murine homologs were determined to be DnaJb4 and Pcbp3, which have functions associated with chaperone activity and mRNA stabilization respectively. Recombinant AAV genomes were constructed through the removal of the AAV coding region and replaced with a modifier gene containing a c-Myc/his reporter sequence for in vivo detection (Fig. 2A). Expression of transgenes was tested through western blotting probed against the c-Myc/his tag (Fig. 2B) and both genes were detected with the correct protein sizes. For initial in vitro detection of viral transduction, whole bone marrow was infected with a scAAV7-GFP vector for easy visualization and approximately 40% of the cells appeared to express GFP. Intracellular c-Myc expression can also be detected through fluorescence activated cell sorting (FACS), but at a much lower percentage of 2–4% as a result of the difficulty in intracellular signal detection and the potential damaging effect of processing cultured bone marrow cells through the cell sorter.
Figure 2. Plasmid design and proviral cassette expression in HEK 293T cells.
A) Both of the double stranded AAV7 viral vectors (scAAV7) contain one copy of a mutated inverted terminal repeat (ITR), the CMV enhancer and chicken β-actin promoter (CB promoter), cDNA of a single modifier gene tethered to a c-Myc/his tag, and the SV40 polyadenylation site. B) Western blot analysis of the proviral cassette expression in vitro probed against the reporter tag, c-Myc/his in 293T cells. The eGFP construct does not contain the c-Myc/his reporter tag and serves as the negative control for unspecific bands. rKiK3 is the original construct from which the c-Myc/his tag was obtained and shows a robust expression of the c-Myc/his sequence tethered to the KiK3 gene. For the Pcbp3 and Dnajb4 constructs, the proteins are expressed at the correct sizes of 36 kDa and 40 kDa respectively.
GFP+ Purkinje neurons are binucleated and possess donor derived Y chromosomes
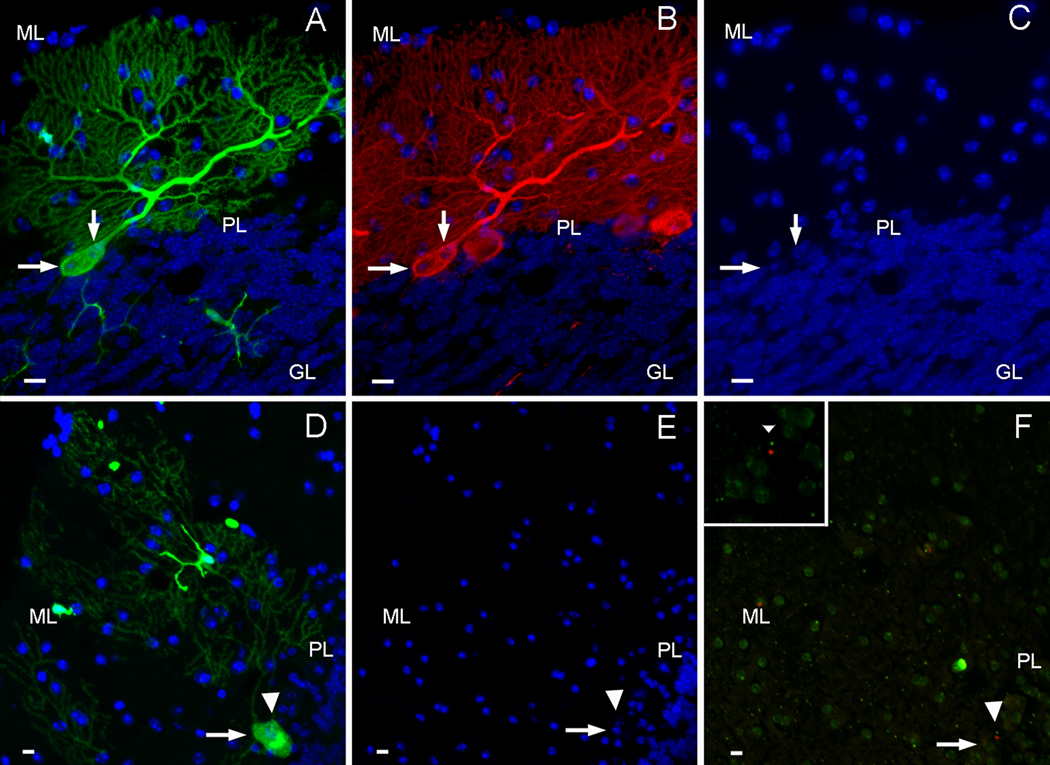
Following isolation of GFP+, SKL HSCs from wildtype male mice, cells were infected with one of the scAAV7 vectors carrying Dnajb4 or Pcbp3. Genetically modified BMDCs/HSCs were immediately injected into heterozygous Sca1154Q/2Q females that had already received whole-body irradiation 48 hrs prior to transplantation (Fig. 1). FACS analysis confirmed that all transplanted mice showed robust peripheral blood reconstitution (60%–100% of GFP+ cells) at the end of all survival periods. GFP+ Purkinje heterokaryons (Fig. 3A) resulting from cell-cell fusion between BMDCs/HSCs and host PNs can be detected in the cerebellum starting around 24 weeks post transplantation, as previously reported (Weimann et al., 2003b; Priller et al., 2001b), and they all expressed the Purkinje neuron region-specific marker Calbindin (Fig. 3B). 12 out of 18 mice receiving BMTs had fused PNs within the cerebellum, and approximately 118 GFP+ Purkinje cells were observed. Cell-cell fusion occurred between BMDCs/HSCs and presumably healthy PNs with full dendritic arbors (Fig. 3A) as well as between seemingly degenerating/atrophied PNs that had smaller cell bodies and thinned, altered dendritic arborizations. GFP+ PNs were further analyzed in serial laser confocal optical sections, and shown to be binucleated (Figs. 3A, B, C, arrows) with two nuclei that were distinctively different in size and morphology as expected from heterotypic cell fusions. To further confirm that the GFP+ PNs resulted from fusion between sex-mismatched donor bone marrow cells and host PNs, FISH analysis was carried out to confirm the presence of a Y chromosome within one of the two nuclei found in the GFP+ Purkinje heterokaryons of female recipient mice. Nuclear patterns, based on DAPI staining before FISH analysis (Fig. 3E; arrow and arrow head), were used to relocate the same two nuclei (Figs. 3D, F; arrow and arrow head) within the fused cell after the GFP fluorescence was abolished during the FISH procedures. Fluorescence-conjugated chromosome probes identified the X chromosomes in green and the Y chromosomes in red (Fig. 3F). In the inset of figure 3F, one of the two nuclei (arrowhead) was clearly shown to contain the cy3-labeled Y chromosome, which indicated that the GFP+ PN was involved in a fusion event with the HSCs and not a product of trans-differentiation. Further more, no GFP− cells were observed to carry Y chromosomes.
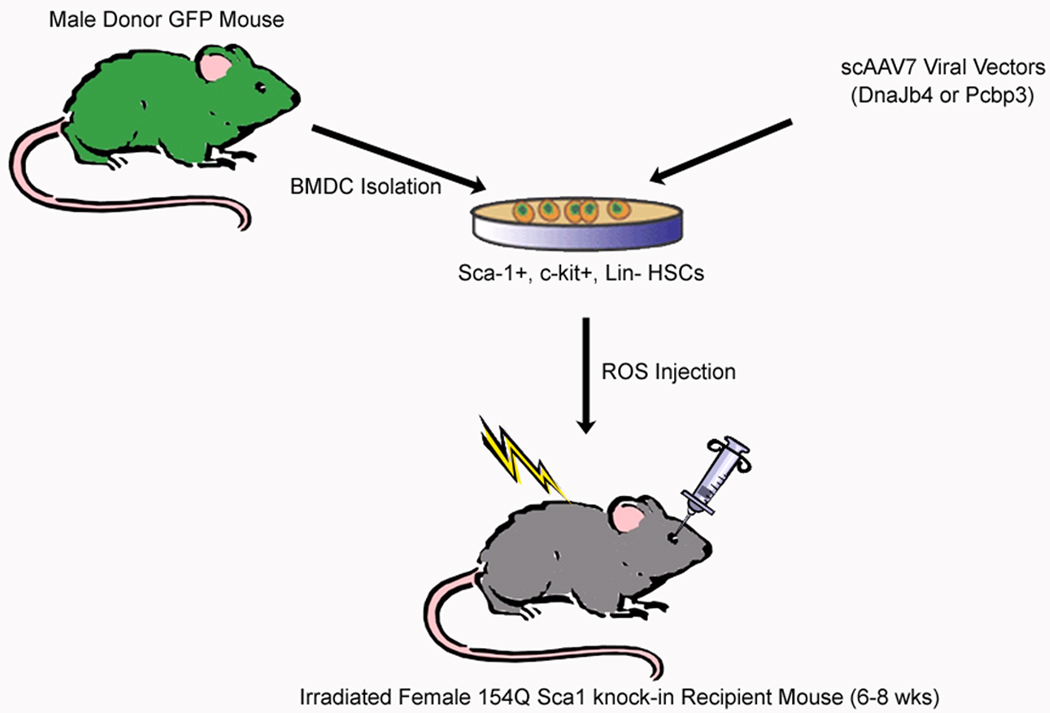
Figure 1. Schematic representation of the experimental paradigm.
GFP+ bone marrow-derived cells (BMDCs), enriched for the hematopoietic stem cell (HSC) population (Sca-1+, c-Kit+, Lin−), are isolated from male GFP transgenic mice through FACS. BMDCs/HSCs are next infected with one of the scAAV7 vectors at 1 × 105 viral particles/cell and transplanted into irradiated female Sca1154Q/2Q recipients through retro-orbital sinus injections.
Figure 3. Fused Purkinje heterokaryons are binucleated and contain Y chromosomes from donor cells.
A) An example of a GFP+ Purkinje neuron (green) derived from BMDCs/HSCs of GFP+ males was observed in female Sca1154Q/2Q recipients. B) The same GFP+ Purkinje neuron also expresses the Purkinje cell marker Calbindin (red). C) DAPI staining (blue) reveals that the fused Purkinje neuron contains two morphologically distinct nuclei of different sizes (arrows). D) Another binucleated GFP+ Purkinje neuron (green with two nuclei pointed to by an arrow and arrowhead) contains one Y chromosome in one of the two nuclei (arrowhead) shown before (E) and after (F) FISH analysis. The cy3-labeled Y chromosome (red, F, inset) is detected amongst FITC-labeled X chromosomes (green) in the female recipient cerebellum. (scale bar=10µm, GL=inner granule layer, PL=Purkinje layer, ML=molecular layer.)
Potentially attenuating effects of cell-cell fusion
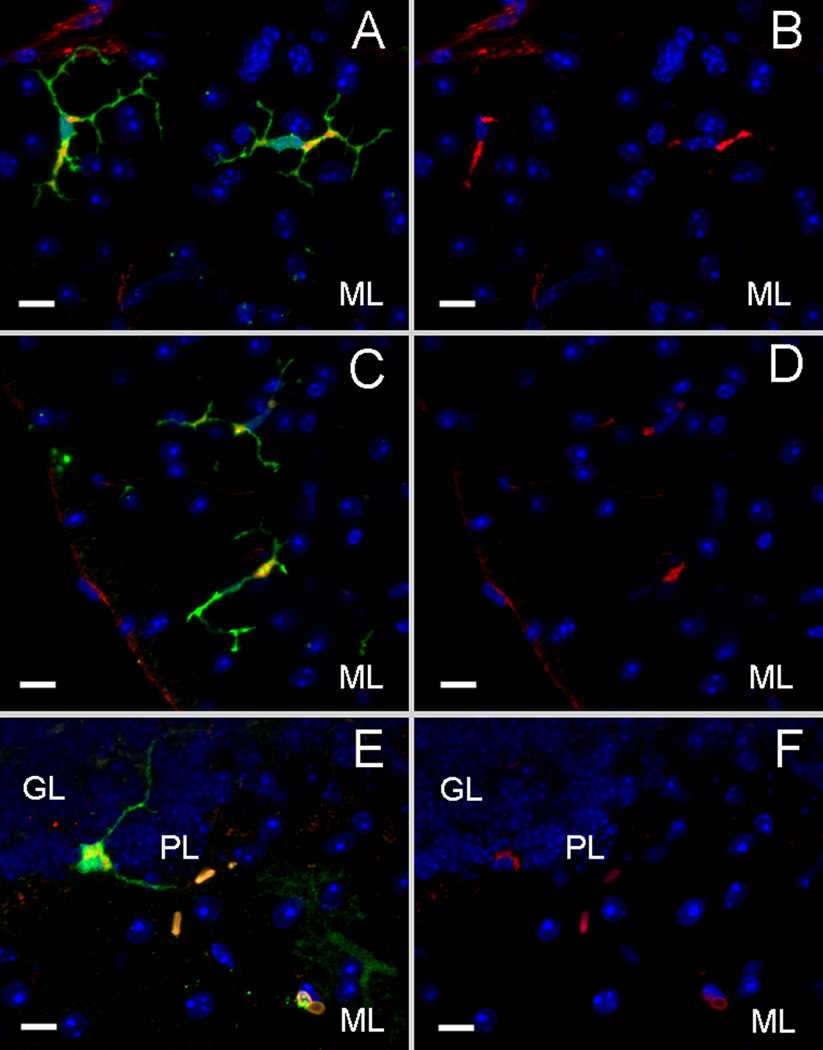
Another prevalent donor derived cell population observed within the recipient cerebella was microglia, with approximately 92% of the GFP+, non-PN cells staining positive for CD11b (Figs. 4A, B). GFP+ microglia were observed in all regions of the brain, including the cortex and the brainstem in addition to the cerebellum. Around 33% of the microglia population also expressed c-Myc (Figs. 4C, D), indicating that the viral genome was stably integrated and expressed within the host environment. C-Myc expressions were found in GFP+ Purkinje heterokaryons as well (Figs. 4E, F) which supported our concept of using bone marrow cells to deliver genes/factors missing within at-risk neuronal populations. In addition to the CNS, c-Myc expressions were also detected in the spleens (data not shown) of the transplanted mice, which further confirmed integration and expression of the viral genomes.
Figure 4. Purkinje heterokaryons and bone marrow-derived microglia express the c-Myc/his reporter tag in vivo.
A) A GFP+ cell type found in Sca1154Q/2Q recipient cerebella has the morphology of a microglial cell (green) and colocalizes with the surface marker CD11b (red) as shown in B. C) 20–30% of the microglia (green) as shown in D) expressed c-Myc (red) in the cytoplasmic regions where both of the modifier genes are localized. E) Fused, GFP+ Purkinje heterokaryons (green) are also immunopositive for c-Myc (red) in F. (scale bar=10µm, GL=inner granule layer, PL=Purkinje layer, ML=molecular layer. DAPI=blue)
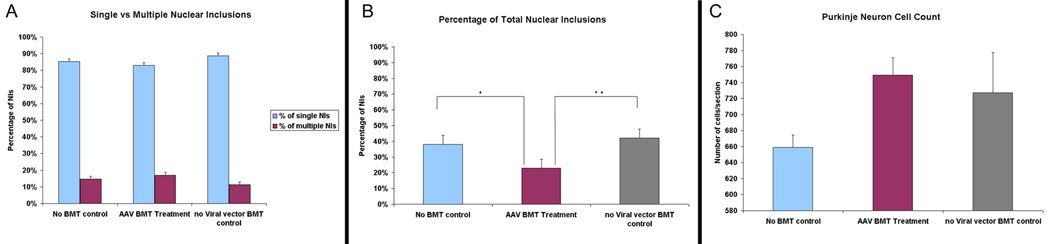
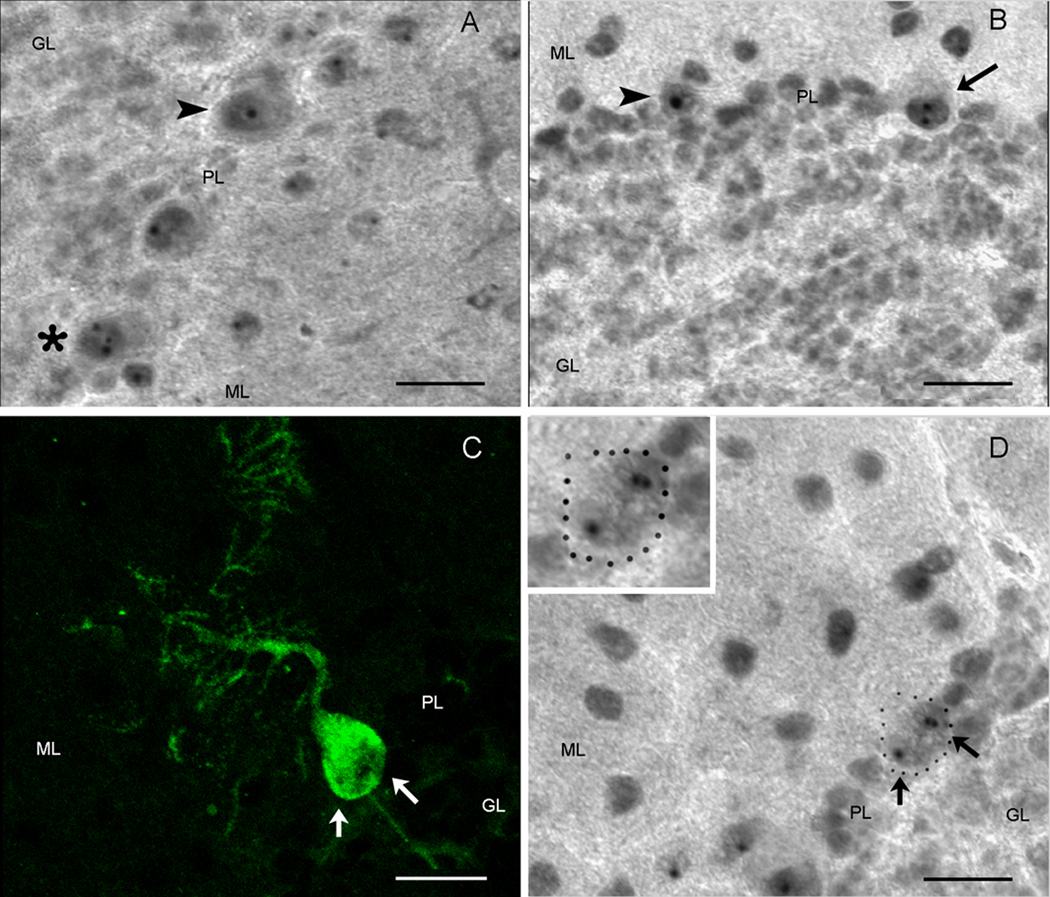
The transgenes used in this study were found through examination of eye phenotypes in a Drosophila model of SCA1 where the pathology was directed to the retina (Fernandez-Funez et al., 2000). In the current murine model, NIs that have been considered to be one of the characteristic neuropathologies of trinucleotide diseases (Servadio et al., 1995) were found in PNs and subsequently used as a marker to reflect any potential impact on the pathological phenotype and/or disease process of SCA1. In an effort to determine any potential beneficial effects seen with the cell-cell fusion or overexpression of the modifier genes, we performed a quantitative analysis on the percentage of the NIs observed as well as the number of survived PNs (Table 1 & Fig. 6). Mice were divided into three groups of 1) AAV-BMT treated group, 2) No viral vector (NVV)-BMT control group, and 3) no-BMT control group. The NIs were immunopositive for ubiquitin and the mutant SCA1 gene product, ataxin-1, and were detected to vary in number within the cell bodies of PNs (Figs. 5A, B) in the current murine model. Specifically, most Purkinje cells contained singular, seemingly larger NIs (Figs. 5A, B; arrow heads) while a smaller number of neurons was observed to harbor two or multiple inclusions that appeared smaller in size through subjective examination (Figs. 5A, asterisk, B, arrow). Similar findings were reported by Skinner and colleagues (1997) in an in vitro Sca1 study where mutant ataxin-1 carrying long glutamine repeats localized within the nuclei of PNs as single, large inclusions, in contrast to the overexpression of wildtype ataxin-1 which accumulated as smaller, multiple inclusions. In the Sca1 Drosophila model, Fernandez-Funez et al. (2000) also described observing altered, more compact nuclear inclusions within the affected cells after treatment with the suppressor genes. We found that the fused GFP+ Purkinje heterokaryons (Fig. 5C) did not contain the singular, dense NIs seen most prevalently in the Sca1 cerebellum, but instead possessed multiple inclusions (Fig. 5D, arrows) which suggested possible modifying effects of the genes overexpressed through the HSCs. However, quantification of the NIs did not show an increase in the multiple NIs in the treatment group when compared to the control groups. In the no-BMT control group, 85.3% of the total NIs counted was single inclusions and 14.7% was multiple inclusions; for the NVV-BMT control group, 88.75% was single inclusions and 11.24% was multiple inclusions; and for the AAV-BMT treatment group, 82.94% was single inclusions and 17.06% was multiple inclusions (Table 1B & Fig. 6A). However, a significant difference was detected between the experimental group and the control groups in the total number of NIs observed. Out of the total number of cells counted, the AAV-BMT group had approximately 23.3% NIs which is significantly lower (p < 0.05) than the 38.78% of NIs found within the no-BMT controls or the 42.18% found in the NVV-BMT control group. (Table 1B & Fig. 6B). For the total number of viable PNs observed, Calbindin+ cells were found to average 659 cells/section in the no-BMT control group, 727 cells/section in the NVV-BMT control as compared to the slightly higher 749 cells/section in the AAV-BMT treatment group (Fig. 6C). The thickness of the molecular layers was also measured to determine if any changes took place since thinning of the Purkinje neuron arborization is another hallmark of SCA1 pathology. Preliminary analysis found that molecular layers of the wt mice were approximately 198.5um thick, the AAV-BMT group was an average of 175.8um, and the NVV-BMT group was 177um which was not significantly different from the experimental AAV-BMT group. Overall, treatment with AAV-BMT did not seem to have beneficial effects on Purkinje neuron dendritic arborization or molecular layer thickness.
Table 1.
A) Average numbers for singular nuclear inclusions (NIs) and multiple NIs, and total number of NIs (includes both singular and multiple NIs) for each experimental group are presented in columns 2, 3, and 4 respectively. Average number of Purkinje neurons immunopositive with Calbindin in the same regions counted for the NIs is presented in the last column.
B) Percentages of singular NIs and multiple NIs are calculated based on the average number of singular and multiple NIs observed out of the total number of cells counted to harbor NIs and presented in columns 2 and 4 respectively. Percentage of overall NIs observed is calculated based on the average number of total Purkinje neurons counted and is presented in column 6. Standard error of means (SEM) for each group is presented in columns 3, 5, and 7. (BMT=bone marrow transplant, AAV/BMT=AAV viral vector with bone marrow transplant, NVV/BMT=no viral vector with bone marrow transplant)
| A. | ||||
|---|---|---|---|---|
| Group: | Average of Singular NIs |
Average of Multiple NIs |
Total NIs | Average of total Purkinje Neurons |
| No BMT | 11500.5 | 1982.5 | 13483 | 34787 |
| AAV-BMT | 9457 | 1945.75 | 11402.75 | 48947.75 |
| NVV-BMT | 10612 | 1344.33 | 11956.33 | 28348.33 |
| B. | ||||||
|---|---|---|---|---|---|---|
| Group: | Percentage of Singular NIs |
SEM | Percentage of Multiple NIs |
SEM | Percentage of Total NIs |
SEM |
| No BMT | 85.30% | 2.26% | 14.70% | 2.11% | 38.78% | 3.82% |
| AAV-BMT | 82.94% | 4.06% | 17.06% | 4.06% | 23.30% | 2.96% |
| NVV-BMT | 88.75% | 1.14% | 11.24% | 2.67% | 42.18% | 3.48% |
Figure 6. Potentially attenuating effects of cell-cell fusion demonstrated through quantitative analysis of percentage of NIs observed and the number of surviving Purkinje neurons found per section.
A) NIs observed were divided into singular NIs and multiple NIs. In the no-BMT control group, 85.32% of the total NIs counted was single inclusions and 14.7% was multiple inclusions, for the NVV-BMT control group, 88.75% was single inclusions and 11.24% was multiple inclusions, and for the AAV-BMT treatment group, 82.94% was single inclusions and 17.06% was multiple inclusions. B) A significant difference was observed between the treatment and control groups in the percentage of total NIs observed. The AAV-BMT group harbored 23% NIs in the total number of Purkinje neurons counted, while the no-BMT control group had 38% NIs and the NVV-BMT control group contained 42%. (asterisks indicate statistical significance with p<0.05) C) Further more, AAV-BMT treated group was also found to have a higher number of viable Purkinje neurons (749 cells/section) than the no-BMT control group (659 cells/section) or the NVV-BMT control group (727 cells/section). (NIs=nuclear inclusions; BMT=bone marrow transplant; NVV=no viral vector)
Figure 5. Nuclear inclusions vary in size and appear as multiple, small aggregates in fused Purkinje heterokaryons.
A) and B) are examples of the ataxin-1+ nuclear inclusions found in the Sca1154Q/2Q mice. Most common are Purkinje neurons with singular large inclusions (arrowheads) but a small number of cells is found to contain two (arrow) or multiple (asterisks) smaller-sized inclusions. C) GFP+ Purkinje heterokaryon are consistently found to contain multiple but smaller ataxin-1+ nuclear inclusions, as shown with an example of a PN dual labeled with GFP in C (green) and with anti-ataxin 1 in D (inset and arrows). (scale bar=20µm, GL=inner granule layer, PL=Purkinje layer, ML=molecular layer)
Bone marrow derived cells can fuse to other cell types within the cerebellum
Previous reports (Alvarez-Dolado et al., 2003; Weimann et al., 2003b; Johansson et al., 2008; Magrassi et al., 2007) found that only PNs and microglia were GFP+, donor-labeled cells located within the CNS following in vivo transplantation. Interestingly, small numbers of other cells were observed within the current paradigm that did not appear to be either PNs or microglia, but instead exhibited morphological characteristics resembling a glial cell type and interneurons within the molecular layer. Another GFP+ cell type that was observed in higher frequency possessed all of the attributes of unipolar protoplasmic astrocytes similar in morphology to Bergmann glial cells which are intimately associated with PNs. These cells had somata located near the cell bodies of PNs, and radial fibers that extended toward the pial surface. In addition, they were immunopositive for glial fibrillary acidic protein (GFAP) as well as S100β, and were found in the correct spatial orientation as Bergmann glia within the cerebellar Purkinje and molecular layers (data not shown). Morphological assessment suggests HSCs may have fused to a glial-like cell and a basket or stellate interneuron in the molecular layer. Conversely, an average of more than ten groups of putative Bergmann glial cells was observed in at least four mice throughout the cerebellum, and thus should not be considered as rare occurrences.
Discussion
Co-culture studies involving stem cells of similar or different lineages showed their propensity to undergo spontaneous cell-cell fusion in vitro (Terada et al., 2002; Ying et al., 2002; Chen et al., 2006; Jessberger et al., 2007) and suggest this mechanism could be used to expand the role of adult stem cells in therapeutic approaches. The current study is a proof of concept that heterotypic cellular fusion can be utilized as a rescue strategy to provide neuroprotective genes/factors for injured or degenerating neurons that currently do not have other therapeutic approaches available. We show that donor-derived PNs are found in the recipient animals approximately six months post BMT, but the number of the fused cells remains low as previously reported (Alvarez-Dolado et al., 2003; Weimann et al., 2003b; Priller et al., 2001b). However, this does not support a notion that fusion events are artifactual rare occurrences (Wagers et al., 2002). A recent study by Johansson et al. (2008) showed that the formation of heterokaryons increased 10–100 fold in chronic inflammatory conditions. Nygren et al. (2008) further demonstrated that fusion could be inhibited through treatment with the anti-inflammatory steroid prednisolone, suggesting that cell fusions are indeed biologically significant events that may serve a protective role following trauma or neurodegenerative disease associated with components of inflammation (Singec and Snyder, 2008). Given these new findings, it is possible that the rate of fusion is slowed down in the current experimental paradigm due to the administration of antibiotics to the experimental mice. Future studies might therefore focus on the search for small molecule compounds that would promote low levels of inflammatory response to increase cell-cell fusion without overwhelming the immune system. Alternative ways to drive cellular fusion include the use of a microfluidic device to pair and fuse cells (Skelley et al., 2009), and introducing chemokines like SDF-1 that increase BMDC homing to the cerebellum (Sano et al., 2005) or other factors (e.g. GM-CSF, Filgrastim, or Neupogen) that might enhance both recruitment and fusion (Sanchez-Ramos et al., 2009; Lee et al., 2009).
We next showed that the expression of neuroprotective genes is detected with approximately 20–30% transduction efficiency as previously reported (Maina et al., 2008). Low transduction efficiency of AAV vectors can be attributed to failure of intracellular trafficking from the cytoplasm into the nucleus and the subsequent degradation by the host proteasome machinery. Phosphorylation by the epidermal growth factor receptor protein tyrosine kinase (EGFR-PTK) at the tyrosine residues of the vector capsid was found to specifically impair the nuclear transport of the vectors (Zhong et al., 2007). Generation of vectors containing point mutations within these tyrosine residues subsequently increased transduction efficiency at lower doses (Zhong et al, 2008) and can therefore be used for future improvements for transgene expressions.
For evaluation of attenuating effects with the current method, ataxin-1+ NIs were examined qualitatively and quantitatively as a biological marker that could reflect any potential modification within the disease process following BMTs. It has been postulated that nuclear aggregations found in many neurodegenerative disorders are derived from an expanded polyglutamine tract that confers toxic gain of function through possible alteration of protein conformation which consequently disrupts normal association with the nuclear matrix or other proteins that accumulate in cell bodies (Cummings et al., 1998; Skinner et al., 1997). It was also shown that overexpression of mutant ataxin-1 in vitro forms large, single inclusions while overexpression of the wildtype ataxin-1 forms multiple inclusions, which was observed in the fused Purkinje heterokaryons in the current in vivo model as well. In addition, mice receiving AAV-BMTs were found to have a lower percentage of NIs as well as an increase in the overall number of surviving PNs in comparison to control NVV-BMT and no-BMT groups; this demonstrates potential beneficial effects resulting from cellular fusion or modifier genes, or both. Further quantitative comparison of the NIs between NVV-BMT control mice and AAV-BMT treated mice, however, shows that mice treated with BMT containing a modifier gene had significantly lower NIs which would suggest that the ameliorating effects are a result of the overexpression of the modifier genes and not merely an effect of BMTs. With each modifier gene being individually expressed in the current study, it might be possible that a synergistic effect may be seen if the genes were co-expressed and will be an important point for future studies. Despite the low number of fused cells, it is worth considering the possibility that through rescuing a few of the PNs may in turn have an overall positive effect on the host environment due to their physical interactions with other PNs and/or other cerebellar cell types. It has been documented that cell-autonomous mutations within one cell type may induce dystrophy of another cell type because of their intimate physical associations (Martí et al., 2001) and the opposite may hold true as well. Another aspect to consider is that bone marrow-derived microglia may be influencing the host milieu by releasing potentially neuroprotective factors. Studies have found that microglial engraftment increased in pathological conditions and were specifically attracted to the site of injury and may be suited as potential delivery vehicles (Priller et al., 2001a). Recent findings in cell therapies involving mesenchymal cells also suggested that the observed therapeutic effects may be attributed in part to trophic factors secreted by the cells themselves (Lee and Park, 2009; Kemp et al., 2010a). Therefore, microglial secretion of neuroprotective/trophic factors may potentially support increased PN survival in an attenuated environment. Alternatively, beneficial effects detected may also be attributed to the immunosuppression nature of the transplantation procedure where reduction of T cells have been implicated to be the cause behind the amelioration of symptoms seen in a Parkinson’s Disease mouse model following BMT (Keshet et al., 2007).
Aside from the successful proof-of-principle demonstration of a system potentially useful for the repair of at-risk PNs, another unexpected observation was the fusion seen with other cerebellar cell types not reported in previous studies (Alvarez-Dolado et al., 2003; Weimann et al, 2003b; Johansson et al., 2008; Magrassi et al., 2007). These included cells exhibiting morphological features similar to molecular layer interneurons, i.e. basket or stellate cells which may be rare “random occurrences” that could be somehow associated with the SCA1 abnormality, and also unipolar astrocytes resembling Bergmann glial cells which were consistently observed in more than one recipient mouse. Bergmann glia have intimate associations with PNs both structurally and functionally (Bellamy, 2006; Yamada et al., 2000), and have been implicated in playing a role in neurodegeneration through impaired glutamate transport in the spinocerebellar ataxias (Custer et al., 2006; Vig et al., 2006). Previous studies have shown that damage within the cerebellum increased the number of fused cells (Magrassi et al., 2007; Bae et al., 2005) and it is possible that selective pressure within the cerebellum, and also potential impairment within the Bergmann glia, play a role in the cell-cell fusion mechanism that is not yet completely understood. Another possibility relates to Bergmann glia being a putative cerebellar stem cell (Alcock et al, 2007; Sottile et al., 2006), and in vitro studies have demonstrated the propensity of different stem cell types to fuse in culture (Terada et al., 2002; Ying et al., 2002; Chen et al., 2006; Jessberger et al., 2007).
Another advantage of the current rescue paradigm is in the delivery method: BMTs have been in clinical use for a long time and are less invasive compared to direct injections into the brain which can cause damage to the parenchyma as well as compromising the blood-brain barrier. Even though whole-body irradiation was used in this experimental paradigm to allow reconstitution of the donor blood cells, previous findings in which parabiosis was used to create chimaerism between two mice showed that peripheral blood reconstitution was above 50% and that irradiation was not necessary to induce fusion (Johansson et al., 2008 in adult mice. In addition, Magrassi and colleagues (2007) showed that mice receiving chemical treatments with Treosulfan and Fludarabine, compared to mice receiving irradiation, achieved similar levels of circulating fluorescent cells and thus showed that irradiation is not a prerequisite for fusion to occur.
Reports of aneuploid Purkinje neurons being found within cerebella from aged rats and mice have been in the literature for quite some time (Del Monte, 2006; Mann et al., 1978; Mares et al., 1971; Lapham, 1968) and it is therefore reasonable to propose that they are the predominant fusogenic-capable CNS cell type able to form heterokaryons with BMDCs/HSCs. Furthermore, these binucleated heterokaryons were shown to be stable and viable in a long term study (Magrassi et al., 2007) and suggest selective cellular fusion offers considerable therapeutic potential in terms of showing promise for rescuing specific cells, especially in the inherited ataxias. We demonstrate here that by combining stem/progenitor cell transplant technologies with viral vector directed gene therapy, a novel therapeutic paradigm may be achieved that could not be easily accomplished using traditional cell replacement or gene therapy alone. With an observable and potentially positive outcome from fusogenic cells carrying neuroprotective genes as reported here, cell-cell fusion warrants further study as an efficacious alternative for rescuing at-risk cellular populations. While we would have liked to see the present combined stem cell fusion-gene therapy approach have a more profound therapeutic effect, an accumulating body of evidence, including studies of other cerebellar mutant mouse models (e.g. Weaver mouse which exhibits profound granule cell loss, versus the Purkinje cell loss studied here) suggests that cues from at-risk neurons may not be enough to support stem cell repair or replacement (Chen et al., 2009). Possibly a result of too much cellular damage and an overall toxic environment, it is clear that complementary methods will need to be developed to enhance stem cell engraftment and repair, either through fusogenic stem cell rescue or widespread cellular replacement. It is encouraging that recent studies (Johansson et al., 2008; Espejel et al., 2009) support a notion that certain experimental interventions, e.g. radiation/inflammation, can increase stem cell fusion within an at-risk cerebellum. Therefore the present study offers encouragement toward this therapeutic, repair regimen since it was first necessary to show a proof of principle whereby potentially therapeutic gene products can be selectively delivered to at-risk populations of fusion-receptive PNs in a strong SCA1 mouse model that is also amenable to tracking disease course and treatment approaches (Oz et al., 2010), and result in albeit so far only modest beneficial phenotypic effects along with potential cellular rescue within a cerebellum that so much resembles the course of human disease in this inherited ataxia.
Materials and Methods
Generation of recombinant scAAV 7 plasmids
DnaJ (Hsp40) homolog, subfamily B, member 4 (DnaJB4) and poly-(rc)-binding protein 3 (Pcbp3) cDNA clones were obtained from Invitrogen. Both were subcloned individually into a dsAAV proviral vector cassette which has been described previously (Wang et al., 2003). A linker containing the multiple cloning sites was also inserted into the vector to facilitate subcloning of the cDNAs. Each of the proviral plasmids contains a cytomegalovirus (CMV) immediate early enhancer and chicken β-actin promoter upstream of a simian virus 40 (SV40) early splice donor/splice acceptor site, a modifier gene (DnaJB4 or Pcbp3), and the SV40 polyadenylation sequence. For reporter activity, c-Myc and polyhistidine (His) tags (c-Myc/his) were also engineered into the C-terminus of the proteins (Fig. 2A).
These double-stranded, self-complementary AAV7 vectors (scAAV7-DnaJb4-c-Myc and scAAV7-pcbp3-c-Myc) were packaged individually using the calcium phosphate precipitation method. Briefly, human embryonic kidney (HEK) 293T cells were plated in 15-cm-diameter plates and cultured until 70–80% confluent. Cells were cotransfected with 15 µg of the proviral plasmid, 45 µg of pAdeno-helper plasmid, and 15 µg of AAV-helper pRC7 plasmid for supply of the rep and cap genes and other necessary helper functions in trans. After 60–72 hr, cells were collected and lysed through three cycles of freeze-thaw treatments. Vectors were purified through the Benzonase treatment, iodixanol step gradient centrifugation, and HiTrap Q HP columns (GE Healthcare Life Sciences). Titers or genome numbers of the viral vectors were determined through DNA slot-blot analysis.
Mice
Donor cells were harvested from 8–12 week-old male transgenic green fluorescence protein (GFP+) mice (strain #C57BL/6-Tg(UBC-GFP)30Scha/J, stock #004353) purchased from the Jackson Laboratory. Recipients were female, 6–8 week-old, Sca1154Q/2Q knock-in mice (Watase et al., 2002; generously provided by the Zoghbi laboratoy, Baylor Medical college/HHMI) and maintained at the University of Florida Animal Care Services.
Murine bone marrow isolation and sorting for Sca1+, c-kit+, Lin− populations
Bone marrow was flushed out of the femurs and tibia of donor GFP+ mice with a 30G needle and a 3cc syringe into Phosphate buffered saline (PBS) and dissociated into single cell suspensions using a 25G needle. Cells were filtered through a nylon filter, triturated with 5ml pipettes, and counted for a final concentration of 2×106 cells/100µl in PBS with 10% Fetal Bovine Serum (FBS). Primary antibodies were added for 30 min at 4°C at 1:200 of APC-conjugated rat CD117/c-kit, and PE-Cy7-conjugated rat Sca-1/Ly-6, 1:10 PE-conjugated Lin cocktail (rat CD4, CD5, CD8a, CD11b, B220, Gr-1, Ter-119, and hamster CD3e) (BD Biosciences). GFP+ and Sca-1+, c-kit+, Lin− cells were sorted into Iscove's Modified Dulbecco's Medium (IMDM, Invitrogen) using a FACS Vantage (BD Biosciences) fluorescence-activated cell (FAC) sorter.
Recombinant AAV7 vector transduction of HSCs and bone marrow transplants
SKL cells were infected with rAAV 7 vectors at a ratio of 1 × 105 viral particles/cell for two hrs at 37°C in serum-free IMDM. Cells were then washed with PBS and used directly for transplantation. 8–12 week-old female Sca1154Q/2Q recipient mice were lethally irradiated 48 hrs prior to transplant with one dose of 950 cGy from a cesium-137 source. 5,000 virally transduced SKL cells, along with 9,000 Sca-1−, c-kit−, Lin+ radio-protective cells, suspended in 150µl total volume of PBS were injected into the right retro-orbital sinus of lethally irradiated mice. Mice were provided with water containing Baytril antibiotics for two weeks post transplant.
Flow cytometric analysis of transgene expression
Peripheral blood was examined at one month post transplantation and at the end of survival period for multilineage and GFP expression analysis. Nucleated cells were separated through ficoll density gradient centrifugation and examined for expression of GFP along with B220 for presence of B lymphocytes, CD11b for macrophages, and CD4 for T lymphocytes by FACS. Data were evaluated using the Cellquest™ software.
Immunohistochemical analysis of BMDCs/HSCs in cerebellum
Treated and control female Sca1154Q/2Q mice were sacrificed between 24 and 32 wk post transplantation and perfused transcardially with 4% paraformaldehyde in 0.1M PBS. Brains were removed, postfixed overnight in perfusate, and transferred to 30% sucrose for another 24 hrs before sectioning in the sagittal plane at a thickness of 14µm using a freezing microtome (Leica). Sections were processed as follows for the different antibodies:
Calbindin and Cd11b: Sections were blocked in PBS supplemented with 0.1% Triton X-100 (PBSt) + 10% FBS for one hour at room temperature (RT) followed by incubation in primary antibodies for GFP (chicken polyclonal 1:1000, Aves Labs) and Calbindin (mouse monoclonal 1:2000, Sigma), or GFP and Cd11b (mouse polyclonal 1:100, BD Biosciences) overnight at 4°C. Sections were then washed in PBSt three times at five-minute intervals before addition of secondary antibodies. Cy3 goat anti-mouse (1:500, Jackson Immunoresearch Labs) and FITC goat anti-chicken (1:1000, Aves Labs) were applied for 2 hours at RT. Sections were again washed in PBS three times at five-minute intervals before coverslipped with Vectashield mounting media containing DAPI (Vector Lab).
Ataxin-1: Procedures for ataxin-1+ nuclear inclusion staining were modified from Skinner et al.28. Briefly, sections were mounted onto plus-charge glass slides, air dried overnight, and microwaved in coplin jars containing 0.01M urea for seven minutes to reach 95–97°C for antigen retrieval. Sections were incubated in heated 0.01M urea for an additional 18 minutes followed by equilibration in PBSt for five minutes. Series of peroxidase blocks (DAKO) and biotin blocks (Vector Lab) ensued and carried out according to the manufacturers’ instructions. Ataxin-1 11NQ (rabbit, 1:1000, Zoghbi lab) was added to the slides for 48 hours at 4°C. Slides were washed in PBSt twice at 10-minute intervals before biotinylated anti-rabbit (goat, 1:150) antibody was added for 30 minutes of incubation at RT. ABC reagent from Vector Labs Vector Elite kit was applied to the sections for another 30 minutes at RT following secondary antibody incubation. A DAB substrate kit (Vector Lab) was prepared and applied to the sections under the light microscope for color development. DAB reactions were stopped in water when punctated NIs were visualized in the Purkinje cell layer. Sections were re-equilibrated in PBS for five minutes before applying the GFP antibody for dual detection of GFP+ PNs and the NIs within the same cells. GFP (chicken polyclonal 1:500, Abcam) was added to the sections and incubated overnight at 4°C. Following three washes, Alexa fluor 488 anti-chicken (donkey, 1:500, Invitrogen) was applied the next day and incubated for 45 min at RT before coverslipped with Vectashield mounting media with DAPI.
c-Myc: Sections were mounted on plus-charged glass slides and air dried overnight. Slides were subjected to high heat retrieval in target retrieval solution (pH9, Dako) for 20 minutes and cooled slowly at RT for an additional 20 minutes. Primary antibodies of c-Myc (rabbit polyclonal 1:100, Santa Cruz Biotechnology) and GFP (chicken polyclonal 1:500, Abcam) were added to the sections for overnight incubation at 4°C. After washing twice in PBSt at 10-minute intervals, Alexa Fluor 594 anti-rabbit (donkey, 1:500, Invitrogen) and Alexa Fluor 488 anti-chicken (donkey, 1:500, Invitrogen) were applied to the sections for 45 minutes in RT before being coverslipped with Vectashield mounting media with DAPI.
Fluorescence in situ Hybridization (FISH) analysis
Parasagittal sections of cerebella were processed for GFP and Calbindin expressions using the standard immunohistochemistry methods described above. The nuclei were counterstained with DAPI and the staining patterns were extensively photodocumented with 10X, 20X, and 40X objectives to locate the exact position of the GFP+ Purkinje neurons before FISH procedures were carried out and the fluorescent signals abolished during the process. Fluorescence conjugated probes specific for mouse X (FITC-conjugated) and Y (cy3-conjugated) chromosomes (Open Biosystems) were used to detect the presence of donor derived Y chromosomes. Briefly, after slides were air dried at RT overnight, they were incubated for 30 min in 0.2N HCl and retrieved in 1M NaSCN in 85°C for 30 minutes. Next, slides were digested in pepsin pre-diluted in prewarmed 0.9% NaCl (pH 2) and hybridized with chromosome probes for 10 minutes at 62°C, followed by further hybridization at 37°C for 48 hours. After hybridization, cells were washed first in 1:1 formamide:2xSSC (Sodium Chloride Sodium Citrate), then 2xSSC, and 4x SSC with 0.1% NP40 at 46°C before re-coverslipped in Vectashield mounting media with DAPI. Locations of the GFP+ PNs were matched in register with nuclei containing fluorescently labeled X and Y chromosomes based on DAPI nuclear patterns and photodocumented with an Olympus IX81-DSU spinning disk confocal microscope.
Quantitative analysis of the nuclear inclusions and total Purkinje cell survival
The number of NIs was counted and compared between three groups containing four age matched Sca1154Q/2Q mice in each group: 1) AAV-BMT (bone marrow transplant) group which received HSCs carrying the viral vector, 2) NVV (non viral vector)-BMT control group that received HSCs only, and 3) no-BMT control group that did not receive any treatment. Every third section of the 14um-thick cerebellar parasagittal sections was counted for the total number of cells containing ataxin-1+ NIs and for the total number of Calbindin+ PNs found within each cerebellum. Percentages of the NIs were calculated based on the number of inclusions found divided by the total number of surviving PNs within the same cerebellar area. A separate quantitative analysis was also carried out for all three groups using different age-matched experimental mice to determine the total number of surviving PNs. Every third section of the14um-thick cerebellar sections was counted for the total number of Calbindin+ PNs observed. A simple student t-test was performed to determine whether there were any statistical significance for the percentages of the NIs as well as for the total number of surviving PNs between the three groups.
Acknowledgements
We are very grateful to Dr. H.Y. Zoghbi and the Zoghbi laboratory for providing Sca1154Q/2Q knock-in mice and the ataxin 1 11NQ antibody, the Srivastava laboratory for providing assistance in the production of viral vectors, M. Jorgensen of the McKnight Brain Institute CTAC facility for immunostaining and FISH analysis on the BMDCs, D. Smith of the McKnight Brain Institute CTAC facility for help with the confocal images, and K. Andrasik, B. Johnson, and S. Suek for excellent technical support including the genotyping of Sca1 mice. This work was supported by NIH/NINDS grants NS37556, NS055165 and the McKnight Brain Institute of the University of Florida.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alcock J, Scotting P, Sottile V. Bergmann glia as putative stem cells of the mature cerebellum. Med Hypotheses. 2007;69:341–345. doi: 10.1016/j.mehy.2007.01.006. [DOI] [PubMed] [Google Scholar]
- Alvarez-Dolado M, Pardal R, Garcia-Verdugo JM, Fike JR, Lee HO. Fusion of bone-marrow-derived cells with Purkinje neurons, cardiomyocytes and hepatocytes. Nature. 2003;425:968–973. doi: 10.1038/nature02069. [DOI] [PubMed] [Google Scholar]
- Bae JS, Furuya S, Shinoda Y, Endo S, Schuchman EH, Hirabayashi Y, Jin HK. Neurodegeneration Augments the Ability of Bone Marrow-Derived Mesenchymal Stem Cells to Fuse with Purkinje Neurons in Niemann-Pick Type C Mice. Hum Gene Ther. 2005;16:1006–1011. doi: 10.1089/hum.2005.16.1006. [DOI] [PubMed] [Google Scholar]
- Bellamy TC. Interactions between Purkinje neurons and Bergmann glia. Cerebellum. 2006;5:116–126. doi: 10.1080/14734220600724569. [DOI] [PubMed] [Google Scholar]
- Berns KI, Giraud C. Biology of adeno-associated virus. Curr Top Microbiol immunol. 1996;218:1–23. doi: 10.1007/978-3-642-80207-2_1. [DOI] [PubMed] [Google Scholar]
- Chen KA, Laywell ED, Marshall G, Walton N, Zheng T, Steindler DA. Fusion of neural stem cells in culture. Exp Neurol. 2006;198:129–135. doi: 10.1016/j.expneurol.2005.11.016. [DOI] [PubMed] [Google Scholar]
- Chen KA, Lanuto D, Zheng T, Steindler DA. Transplantation of embryonic and adult neural stem cells in the granuloprival cerebellum of the weaver mutant mouse. Stem Cells. 2009;27:1625–-1634. doi: 10.1002/stem.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings CJ, Mancini MA, Antalffy B, DeFranco DB, Orr HT, Zoghbi HY. Chaperone suppression of aggregation and altered subcellular proteasome localization imply protein misfolding in SCA1. Nat Genet. 1998;19:148–154. doi: 10.1038/502. [DOI] [PubMed] [Google Scholar]
- Cummings CJ, Reinstein E, Sun Y, Antalffy B, Jiang Y, Ciechanover A, Orr HT, Beaudet AL, Zoghbi HY. Mutation of the E6-AP ubiquitin ligase reduces nuclear inclusion frequency while accelerating polyglutamine-induced pathology inSCA1 mice. Neuron. 1999;24:879–892. doi: 10.1016/s0896-6273(00)81035-1. [DOI] [PubMed] [Google Scholar]
- Cummings CJ, Sun Y, Opal P, Antalffy B, Mestril R, Orr HT, Dillmann WH, Zoghbi HY. Over-expression of inducible HSP70 chaperone suppresses neuropathology and improves motor function in SCA1 mice. Hum Mol Genet. 2001;10:1511–1518. doi: 10.1093/hmg/10.14.1511. [DOI] [PubMed] [Google Scholar]
- Custer SK, Garden GA, Gill N, Rueb U, Libby RT, Schultz C, Guyenet SJ, Deller T, Westrum LE, Sopher BL, La Spada AR. Bergmann glia expression of polyglutamine-expanded ataxin-7 produces neurodegeneration by impairing glutamate transport. Nat Neurosci. 2006;9:1302–1311. doi: 10.1038/nn1750. [DOI] [PubMed] [Google Scholar]
- Del Monte U. The puzzle of ploidy of Purkinje neurons. Cerebellum. 2006;5:23–26. doi: 10.1080/14734220500435894. [DOI] [PubMed] [Google Scholar]
- Espejel S, Romero R, Alvarez-Buylla A. Radiation damage increases Purkinje neuron heterokaryons in neonatal cerebellum. Ann Neurol. 2009;66:100–109. doi: 10.1002/ana.21670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Funez P, Nino-Rosales ML, de Gouyon B, She WC, Luchak JM, Martinez P, Turiegano E, Benito J, Capovilla M, Skinner PJ, McCall A, Canal I, Orr HT, Zoghbi HY, Botas J. Identification of genes that modify ataxin-1-induced neurodegeneration. Nature. 2000;408:101–106. doi: 10.1038/35040584. [DOI] [PubMed] [Google Scholar]
- Ferrari G, Cusella-De Angelis G, Coletta M, Paolucci E, Stornaiuolo A, Cossu G, Mavilio F. Muscle regeneration by bone marrow-derived myogenic progenitors. Science. 1998;279:1528–1530. doi: 10.1126/science.279.5356.1528. [DOI] [PubMed] [Google Scholar]
- Flotte T, Carter B, Conrad C, Guggino W, Reynolds T, Rosenstein B, Taylor G, Walden S, Wetzel lR. A phase I study of an adeno-associated virus-CFTR gene vector in adult CF patients with mild lung disease. Hum Gene Ther. 1996;7:1145–1159. doi: 10.1089/hum.1996.7.9-1145. [DOI] [PubMed] [Google Scholar]
- Flotte TR, Afione SA, Conrad C, McGrath SA, Solow R, Oka H, Zeitlin PL, Guggino WB, Carter BJ. Stable in vivo expression of the cystic fibrosis transmembrane conductance regulator with an adeno-associated virus vector. Proc Natl Acad Sci U S A. 1993;90:10613–10617. doi: 10.1073/pnas.90.22.10613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flotte TR, Brantly ML, Spencer LT, Byrne BJ, Spencer CT, Baker DJ, Humphries M. Phase I trial of intramuscular injection of a recombinant adeno-associated virus alpha 1-antitrypsin (rAAV2-CB-hAAT) gene vector to AAT-deficient adults. Hum Gene Ther. 2004;15:93–128. doi: 10.1089/10430340460732490. [DOI] [PubMed] [Google Scholar]
- Gussoni E, Soneoka Y, Strickland CD, Buzney EA, Khan MK, Flint AF, Kunkel LM, Mulligan RC. Dystrophin expression in the mdx mouse restored by stem cell transplantation. Nature. 1999;401:390–394. doi: 10.1038/43919. [DOI] [PubMed] [Google Scholar]
- Han Z, Zhong L, Maina N, Hu Z, Li X, Chouthai NS, Bischof D, Weigel-Van Aken KA, Slayton WB, Yoder MC, Srivastava A. Stable integration of recombinant adeno-associated virus vector genomes after transduction of murine hematopoietic stem cells. Hum Gene Ther. 2008;19:267–278. doi: 10.1089/hum.2007.161. [DOI] [PubMed] [Google Scholar]
- Jackson KA, Mi T, Goodell MA. Hematopoietic potential of stem cells isolated from murine skeletal muscle. Proc Natl Acad Sci U S A. 1999;96:14482–14486. doi: 10.1073/pnas.96.25.14482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson CB, Youssef S, Koleckar K, Holbrook C, Doyonnas R, Corbel SY, Steinman L, Rossi FM, Blau HM. Extensive fusion of haematopoietic cells with Purkinje neurons in response to chronic inflammation. Nat Cell Biol. 2008;10:575–583. doi: 10.1038/ncb1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay MA, Manno CS, Ragni MV, Larson PJ, Couto LB, McClelland A, Glader B, Chew AJ, Tai SJ, Herzog RW, Arruda V, Johnson F, Scallan C, Skarsgard E, Flake AW, High KA. Evidence for gene transfer and expression of factor IX in haemophilia B patients treated with an AAV vector. Nat Genet. 2000;24:257–261. doi: 10.1038/73464. [DOI] [PubMed] [Google Scholar]
- Kemp K, Hares K, Mallam E, Heesom KJ, Scolding N, Wilkins A. Mesenchymal stem cell-secreted superoxide dismutase promotes cerebellar neuronal survival. J Neurochem. 2010a;114:1569–1580. doi: 10.1111/j.1471-4159.2009.06553.x. [DOI] [PubMed] [Google Scholar]
- Kemp K, Gordon D, Wraith DC, Mallam E, Hartfield E, Uney J, Wilkins A, Scolding N. Fusion between human mesenchymal stem cells and rodent cerebellar Purkinje cells. Neuropathol Appl Neurobiol. 2010b;37:166–178. doi: 10.1111/j.1365-2990.2010.01122.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause DS, Theise ND, Collector MI, Henegariu O, Hwang S, Gardner R, Neutzel S, Sharkis SJ. Multi-organ, multi-lineage engraftment by a single bone marrow-derived stem cell. Cell. 2001;105:369–377. doi: 10.1016/s0092-8674(01)00328-2. [DOI] [PubMed] [Google Scholar]
- Lagasse E, Connors H, Al-Dhalimy M, Reitsma M, Dohse M, Osborne L, Wang X, Finegold M, Weissman IL, Grompe M. Purified hematopoietic stem cells can differentiate into hepatocytes in vivo. Nat Med. 2000;6:1229–1234. doi: 10.1038/81326. [DOI] [PubMed] [Google Scholar]
- Lam YC, Bowman AB, Jafar-Nejad P, Lim J, Richman R, Fryer JD, Hyun ED, Duvick LA, Orr HT, Botas J, Zoghbi HY. ATAXIN-1 interacts with the repressor Capicua in its native complex to cause SCA1 neuropathology. Cell. 2006;127:1335–1347. doi: 10.1016/j.cell.2006.11.038. [DOI] [PubMed] [Google Scholar]
- Lapham LW. Tetraploid DNA content of Purkinje neurons of human cerebellar cortex. Science. 1968;159:310–312. doi: 10.1126/science.159.3812.310. [DOI] [PubMed] [Google Scholar]
- Levine JM, Card JP. Light and electron microscopic localization of a cell surface antigen (NG2) in the rat cerebellum: association with smooth protoplasmic astrocytes. J Neurosci. 1987;7:2711–2720. doi: 10.1523/JNEUROSCI.07-09-02711.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magrassi L, Grimaldi P, Ibatici A, Corselli M, Ciardelli L, Castello S, Podestà M, Frassoni F, Rossi F. Induction and survival of binucleated Purkinje neurons by selective damage and aging. J Neurosci. 2007;27:9885–9892. doi: 10.1523/JNEUROSCI.2539-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maina N, Han Z, Li X, Hu Z, Zhong L, Bischof D, Weigel-Van Aken KA, Slayton WB, Yoder MC, Srivastava A. Recombinant self-complementary adeno-associated virus serotype vector-mediated hematopoietic stem cell transduction and lineage-restricted, long-term transgene expression in a murine serial bone marrow transplantation model. Hum Gene Ther. 2008;19:376–383. doi: 10.1089/hum.2007.143. [DOI] [PubMed] [Google Scholar]
- Mann DM, Yates PO, Barton CM. The DNA content of Purkinje cells in mammals. J Comp Neurol. 1978;180:345–347. doi: 10.1002/cne.901800210. [DOI] [PubMed] [Google Scholar]
- Mares V, Lodin Z, Sácha J. A cytochemical and autoradiographic study of nuclear DNA in mouse Purkinje cells. Brain Res. 1971;53:273–289. doi: 10.1016/0006-8993(73)90214-x. [DOI] [PubMed] [Google Scholar]
- Muller-Sieburg CE, Whitlock CA, Weissman IL. Isolation of two early B lymphocyte progenitors from mouse marrow: a committed pre-pre-B cell and a clonogenic Thy-1-lo hematopoietic stem cell. Cell. 1986;44:653–662. doi: 10.1016/0092-8674(86)90274-6. [DOI] [PubMed] [Google Scholar]
- Muzyczka N. Use of adeno-associated virus as a general transduction vector for mammalian cells. Curr Top Microbiol Immunol. 1992;158:97–129. doi: 10.1007/978-3-642-75608-5_5. [DOI] [PubMed] [Google Scholar]
- Nygren JM, Liuba K, Breitbach M, Stott S, Thorén L, Roell W, Geisen C, Sasse P, Björklund A, Nerlov C, Fleischmann BK, Jovinge S, Jacobsen SE. Myeloid and lymphoid contribution to non-haematopoietic lineages through irradiation-induced heterotypic cell fusion. Nat Cell Biol. 2008;10:584–592. doi: 10.1038/ncb1721. [DOI] [PubMed] [Google Scholar]
- Orlic D, Kajstura J, Chimenti S, Jakoniuk I, Anderson SM, Li B, Pickel J, McKay R, Nadal-Ginard B, Bodine DM, Leri A, Anversa P. Bone marrow cells regenerate infarcted myocardium. Nature. 2001;410:701–705. doi: 10.1038/35070587. [DOI] [PubMed] [Google Scholar]
- Orr HT, Zoghbi HY. SCA1 molecular genetics: a history of a 13 year collaboration against glutamines. Hum Mol Genet. 2001;10:2307–2311. doi: 10.1093/hmg/10.20.2307. [DOI] [PubMed] [Google Scholar]
- Orr HT, Zoghbi HY. Trinucleotide repeat disorders. Annu Rev Neurosci. 2007;30:575–621. doi: 10.1146/annurev.neuro.29.051605.113042. [DOI] [PubMed] [Google Scholar]
- Osawa M, Hanada K, Hamada H, Nakauchi H. Long-term lymphohematopoietic reconstitution by a single CD34-low/negative hematopoietic stem cell. Science. 1996;273:242–245. doi: 10.1126/science.273.5272.242. [DOI] [PubMed] [Google Scholar]
- Oz G, Nelson CD, Koski DM, Henry PG, Marjanska M, Deelchand DK, Shanley R, Eberly LE, Orr HT, Clark HB. Noninvasive detection of presymptomatic and progressive neurodegeneration in a mouse model of spinocerebellar ataxia type 1. J. Neurosci. 2010;30:3831–3838. doi: 10.1523/JNEUROSCI.5612-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen BE, Bowen WC, Patrene KD, Mars WM, Sullivan AK, Murase N, Boggs SS, Greenberger JS, Goff JP. Bone marrow as a potential source of hepatic oval cells. Science. 1999;284:1168–1170. doi: 10.1126/science.284.5417.1168. [DOI] [PubMed] [Google Scholar]
- Priller J, Flügel A, Wehner T, Boentert M, Haas CA, Prinz M, Fernandez-Klett F, Prass K, Bechmann I, de Boer BA, Frotscher M, Kreutzberg GW, Persons DA, Dirnagl U. Targeting gene-modified hematopoietic cells to the central nervous system: use of green fluorescent protein uncovers microglial engraftment. Nat Med. 2001a;7:1356–1361. doi: 10.1038/nm1201-1356. [DOI] [PubMed] [Google Scholar]
- Priller J, Persons DA, Klett FF, Kempermann G, Kreutzberg GW, Dirnagl U. Neogenesis of cerebellar Purkinje neurons from gene-marked bone marrow cells in vivo. J Cell Biol. 2001b;155:733–738. doi: 10.1083/jcb.200105103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Servadio A, Koshy B, Armstrong D, Antalffy B, Orr HT, Zoghbi HY. Expression analysis of the ataxin-1 protein in tissues from normal and spinocerebellar ataxia type 1 individuals. Nat Genet. 1995;10:94–98. doi: 10.1038/ng0595-94. [DOI] [PubMed] [Google Scholar]
- Singec I, Snyder EY. Inflammation as a matchmaker: revisiting cell fusion. Nat Cell Biol. 2008;10:503–505. doi: 10.1038/ncb0508-503. [DOI] [PubMed] [Google Scholar]
- Skelley AM, Kirak O, Suh H, Jaenisch R, Voldman J. Microfluidic control of cell pairing and fusion. Nat Methods. 2009;6:147–152. doi: 10.1038/nmeth.1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner PJ, Koshy BT, Cummings CJ, Klement IA, Helin K, Servadio A, Zoghbi HY, Orr HT. Ataxin-1 with an expanded glutamine tract alters nuclear matrix-associated structures. Nature. 1997;389:971–974. doi: 10.1038/40153. [DOI] [PubMed] [Google Scholar]
- Skinner PJ, Vierra-Green CA, Clark HB, Zoghbi HY, Orr HT. Altered trafficking of membrane proteins in purkinje cells of SCA1 transgenic mice. Am J Pathol. 2001;159:905–913. doi: 10.1016/S0002-9440(10)61766-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder RO, Francis J. Adeno-associated viral vectors for clinical gene transfer studies. Curr Gene Ther. 2005;5:311–321. doi: 10.2174/1566523054065066. [DOI] [PubMed] [Google Scholar]
- Snyder RO, Miao CH, Patijn GA, Spratt SK, Danos O, Nagy D, Gown AM, Winther B, Meuse L, Cohen LK, Thompson AR, Kay MA. Persistent and therapeutic concentrations of human factor IX in mice after hepatic gene transfer of recombinant AAV vectors. Nat Genet. 1997;16:270–276. doi: 10.1038/ng0797-270. [DOI] [PubMed] [Google Scholar]
- Sottile V, Li M, Scotting PJ. Stem cell marker expression in the Bergmann glia population of the adult mouse brain. Brain Res. 2006;1099:8–17. doi: 10.1016/j.brainres.2006.04.127. [DOI] [PubMed] [Google Scholar]
- Spangrude GJ, Heimfeld S, Weissman IL. Purification and characterization of mouse hematopoietic stem cells. Science. 1988;241:58–62. doi: 10.1126/science.2898810. [DOI] [PubMed] [Google Scholar]
- Terada N, Hamazaki T, Oka M, Hoki M, Mastalerz DM, Nakano Y, Meyer EM, Morel L, Petersen BE, Scott EW. Bone marrow cells adopt the phenotype of other cells by spontaneous cell fusion. Nature. 2002;416:542–545. doi: 10.1038/nature730. [DOI] [PubMed] [Google Scholar]
- Tsuda H, Jafar-Nejad H, Patel AJ, Sun Y, Chen HK, Rose MF, Venken KJ, Botas J, Orr HT, Bellen HJ, Zoghbi HY. The AXH domain of Ataxin-1 mediates neurodegeneration through its interaction with Gfi-1/Senseless proteins. Cell. 2005;122:633–644. doi: 10.1016/j.cell.2005.06.012. [DOI] [PubMed] [Google Scholar]
- Vig PJ, Lopez ME, Wei J, D'Souza DR, Subramony S, Henegar J, Fratkin JD. Glial S100B Positive Vacuoles In Purkinje Cells: Earliest Morphological Abnormality In SCA1 Transgenic Mice. J Neurol Sci Turish. 2006;23:166–174. doi: 10.1901/jaba.2006.23-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagers AJ, Sherwood RI, Christensen JL, Weissman IL. Little evidence for developmental plasticity of adult hematopoietic stem cells. Science. 2002;297:2256–2259. doi: 10.1126/science.1074807. [DOI] [PubMed] [Google Scholar]
- Wang Z, Ma HI, Li J, Sun L, Zhang J, Xiao X. Rapid and highly efficient transduction by double-stranded adeno-associated virus vectors in vitro and in vivo. Gene Ther. 2003;10:2105–2111. doi: 10.1038/sj.gt.3302133. [DOI] [PubMed] [Google Scholar]
- Wang X, Willenbring H, Akkari Y, Torimaru Y, Foster M, Al-Dhalimy M, Lagasse E, Finegold M, Olson S, Grompe M. Cell fusion is the principal source of bone-marrow-derived hepatocytes. Nature. 2003;422:897–901. doi: 10.1038/nature01531. [DOI] [PubMed] [Google Scholar]
- Watase K, Weeber EJ, Xu B, Antalffy B, Yuva-Paylor L, Hashimoto K, Kano M, Atkinson R, Sun Y, Armstrong DL, Sweatt JD, Orr HT, Paylor R, Zoghbi HY. A long CAG repeat in the mouse Sca1 locus replicates SCA1 features and reveals the impact of protein solubility on selective neurodegeneration. Neuron. 2002;34:905–919. doi: 10.1016/s0896-6273(02)00733-x. [DOI] [PubMed] [Google Scholar]
- Weimann JM, Charlton CA, Brazelton TR, Hackman RC, Blau HM. Contribution of transplanted bone marrow cells to Purkinje neurons in human adult brains. Proc Natl Acad Sci U S A. 2003a;100:2088–2093. doi: 10.1073/pnas.0337659100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weimann JM, Johansson CB, Trejo A, Blau HM. Stable reprogrammed heterokaryons form spontaneously in Purkinje neurons after bone marrow transplant. Nat Cell Biol. 2003b;5:959–966. doi: 10.1038/ncb1053. [DOI] [PubMed] [Google Scholar]
- Wu J, Zhao W, Zhong L, Han Z, Li B, Ma W, Weigel-Kelley KA, Warrington KH, Srivastava A. Self-complementary recombinant adeno-associated viral vectors: packaging capacity and the role of rep proteins in vector purity. Hum Gene Ther. 2007;18:171–182. doi: 10.1089/hum.2006.088. [DOI] [PubMed] [Google Scholar]
- Xiao X, Li J, Samulski RJ. Efficient long-term gene transfer into muscle tissue of immunocompetent mice by adeno-associated virus vector. J Virol. 1996;70:8098–8108. doi: 10.1128/jvi.70.11.8098-8108.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada K, Fukaya M, Shibata T, Kurihara H, Tanaka K, Inoue Y, Watanabe M. Dynamic transformation of Bergmann glial fibers proceeds in correlation with dendritic outgrowth and synapse formation of cerebellar Purkinje cells. J Comp Neurol. 2000;418:106–120. [PubMed] [Google Scholar]
- Ying QL, Nichols J, Evans EP, Smith AG. Changing potency by spontaneous fusion. Nature. 2002;416:545–548. doi: 10.1038/nature729. [DOI] [PubMed] [Google Scholar]
- Zhong L, Li B, Mah CS, Govindasamy L, Agbandje-McKenna M, Cooper M, Herzog RW, Zolotukhin I, Warrington KH, Jr, Weigel-Van Aken KA, Hobbs JA, Zolotukhin S, Muzyczka N, Srivastava A. Next generation of adeno-associated virus 2 vectors: point mutations in tyrosines lead to high-efficiency transduction at lower doses. Proc Natl Acad Sci U S A. 2008;105:7827–7832. doi: 10.1073/pnas.0802866105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong L, Zhao W, Wu J, Li B, Zolotukhin S, Govindasamy L, Agbandje-McKenna M, Srivastava A. A dual role of EGFR protein tyrosine kinase signaling in ubiquitination of AAV2 capsids and viral second-strand DNA synthesis. Mol Ther. 2007;15:1323–1330. doi: 10.1038/sj.mt.6300170. [DOI] [PubMed] [Google Scholar]
- Zoghbi HY, Orr HT. Spinocerebellar ataxia type 1. Semin Cell Biol. 1995;6:29–35. doi: 10.1016/1043-4682(95)90012-8. [DOI] [PubMed] [Google Scholar]
- Zoghbi HY, Orr HT. Glutamine repeats and neurodegeneration. Annu Rev Neurosci. 2000;23:217–247. doi: 10.1146/annurev.neuro.23.1.217. [DOI] [PubMed] [Google Scholar]