Abstract
Over the last few decades, concepts regarding the presence of hormonal and molecular responses to stress during the first postnatal weeks in the rat and the role of the neuropeptide corticotropin releasing hormone (CRH) in these processes, have been evolving. CRH has been shown to contribute critically to molecular and neuroendocrine responses to stress during development. In turn the expression of this neuropeptide in both hypothalamus and amygdala is differentially modulated by single and recurrent stress, and is determined also by the type of stress (eg, psychological or physiological). A likely transcriptional regulatory factor for modulating CRH gene expression, the cAMP responsive element binding protein CREB, is phosphorylated (activated) in the developing hypothalamus within seconds of stress onset, preceding the transcription of the CRH gene and initiating the activation of stress-induced cellular and neuroendocrine cascades. Finally, early life stress may permanently modify the hypothalamic pituitary adrenal axis and the response to further stressful stimuli, and recent data suggest that CRH may play an integral role in the mechanisms of these long-term changes.
Keywords: corticotropin releasing hormone, CRF, CRH, stress, neonatal, rat, amygdala, paraventricular nucleus, hypothalamus, neuropeptide, glucocorticoids, corticosterone
Introduction
Stress early in life can result in profound permanent changes to both peripheral and central stress circuits, which, in the human, can manifest in cognitive and behavioral disorders later in life.1–4 However, the mechanisms underlying these long-term effects of early life stress remain unclear. In order to gain insight into the full impact of early-life stress on the developing central nervous system, understanding of the basic processes activated by a stressful stimulus, ie, the hormonal, molecular and behavioral stress responses, is required.5
The developmental period between the third and fifteenth postnatal days has been characterized by attenuated hormonal responses and altered gene regulation in response to stress as compared to the adult situation.6–10 This stress hyporesponsiveness during development appears to be stressor-specific, since the hypothalamic pituitary adrenal (HPA) axis is fully capable of responding to stimuli that may be considered stressful to a neonatal rat (eg, cold or saline injection).8,11,12 Although fully operational during development, the magnitude of the hormonal response to stress increases with developmental age.11,13,14
This review focuses on studies highlighting the molecular chain of events triggered by stressful signals early in life, with an emphasis on the role of CRH, the key central nervous system transducer of stressful stimuli.15 Regulation of CRH gene expression during development in response to various stress paradigms, as well as possible factors modulating this regulation, will be described. In addition to the acute molecular and hormonal responses to stress, the long-term effects of stress on the developing nervous system and the role of CRH in mediating these changes in stress circuitry will be discussed.
Neuronal circuitry and CRH-mediated transduction of the responses to stress in the adult and immature brain
The neuroendocrine hypothalamic-pituitary adrenal axis
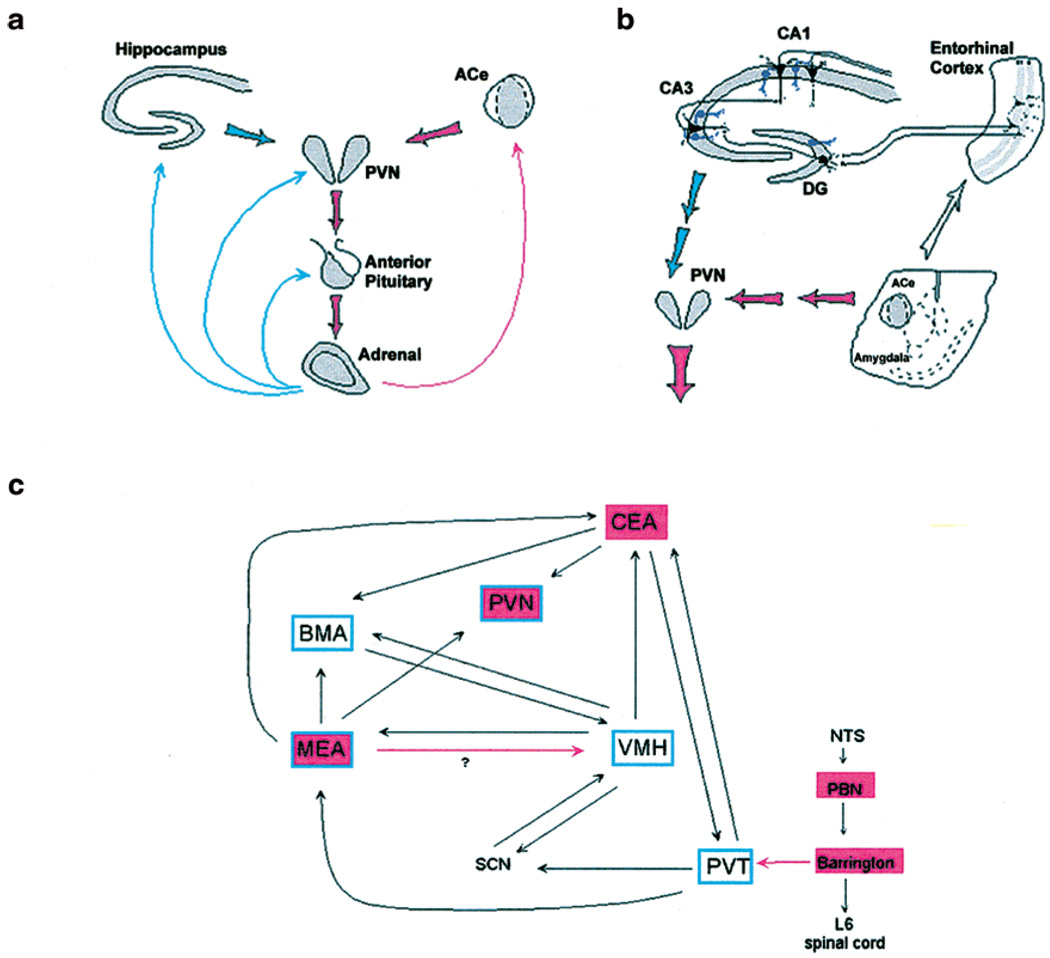
The neuronal networks comprising the stress response in developing and mature rodents and primates include several major circuits. The first is the neuroendocrine circuit, the HPA axis (Figure 1a). It has been well established that stress-induced activation of this circuit is dependent on secretion of the hypothalamic hormone CRH.8,15 Within seconds of exposure to stress, CRH, located in peptidergic neurons in the hypothalamic paraventricular nucleus (PVN), is secreted from nerve terminals to influence rapid secretion of adrenocorticotrophic hormone (ACTH) from the corticotrophs of the anterior pituitary. Subsequent to its secretion, ACTH travels through the bloodstream and acts on the adrenal glands to release glucocorticoids. In the immature rat, this stress-induced elevation of glucocorticoids can be blocked with CRH antisera, further substantiating the role of CRH in mediating this hormonal response.8 During development, proper function of both the activation and the ‘shut-off’ mechanisms of this HPA loop is critical, to permit handling of acute stress, but also to allow normal growth and maturational processes which may be adversely influenced by high levels of glucocorticoids.1,6 Indeed, functional regulation of the HPA loop and the responses to stress is provided in the immature rat by a number of acute or chronic sensory inputs, particularly those related to maternally-derived cues. These influence the nature and magnitude of the neuroendocrine response to stress both acutely and throughout the entire life of the individual.1,2,16,17
Figure 1.
The neuroendocrine (a), limbic (b), and brainstem (c) inter-related, stress-activated corticotropin releasing hormone (CRH) loops. (a) Stress-conveying signals rapidly activate immediate early genes in CRH-expressing neurons of the central nucleus of the amygdala (ACe). Rapid CRH release in the ACe is is thought to activate CRH expressing neurons in the hypothalamic paraventricular nucleus (PVN) to secrete CRH into the hypothalamo-pituitary portal system, inducing ACTH and glucocorticoid secretion from the pituitary and adrenal, respectively. In response to stress, CRH expression is also activated rapidly in these neurons. Glucocorticoids exert a negative feedback on PVN (directly and via hippocampus), yet activate CRH gene expression in the amygdala, potentially promoting further CRH release in this region. (b) Stressors involving ‘psychological’ or multi-modal elements activate the limbic circuit. This consists of ACe, which conveys information both to the hypothalamus and to the hippocampal formation via pathways that likely do not utilize CRH as a neurotransmitter. Within the hippocampus, CRH-expressing GABAergic interneurons (in purple) in the principal cell layers of the hippocampal CA1, CA3 and the dentate gyrus (DG) are positioned to control information flow in the major, tri-synaptic hippocampal pathway. (c) Sensory information regarding physical, somatic and visceral elements of stress is conveyed from sensory organs via a neuroanatomically defined brainstem pathway. Within this general circuit, a chemically defined loop, utilizing CRH (or a similar ligand) as a neurotransmitter which activates the CRF2 receptor may be considered. This afferent pathway contributes to the integration of stress signals, resulting in behavioral and neuroendocrine responses. For panels (a) and (b), red and blue arrows denote established or putative potentiating and inhibitory actions, respectively. Thick arrows do not imply monosynaptic connections. For panel (c), blue frames indicate CRF2 mRNA expression. Red shading over a region indicates the presence of CRH-expressing neurons. Red arrows denote established CRH-containing pathways. CEA = ACe-bed nucleus of the stria terminalis continuum; NTS = nucleus of the solitary tract; PBN = parabrachial nucleus; SCN = suprachiasmatic hypothalamic nucleus; PVT = thalamic paraventricular nucleus; VMH = ventromedial hypothalamic nucleus; MEA and BMA = medial and basomedial amygdalaoid nucleus, respectively. Panels (a) and (b) reprinted from Baram and Hatalski79 with permission from Elsevier Science. Panel (c) reproduced from Eghbal-Ahmadi et al.46 Copyright 1999 by the Society for Neuroscience.
In view of the above, it is not surprising that CRH expression in this HPA circuit is under stringent regulation via multiple feedback loops (Figure 1a). For example, following stress-induced CRH secretion, a ‘compensatory’ increase in CRH mRNA expression in PVN has been observed in the mature18 as well as the developing rat starting on the second week of life.8 Evidence suggests that glucocorticoids suppress this compensatory enhancement of CRH mRNA expression in PVN, to shut down the stress response and return the individual to homeostasis.19–21 The mechanism of this glucocorticoid-induced down-regulation of CRH levels primarily involves activation of glucocorticoid receptors in PVN itself in both mature20 and immature rat.21 In addition, glucocorticoids can bind their cognate receptors in hippocampus to activate indirect pathways leading to suppression of hypothalamic CRH mRNA expression.22–24
The limbic neuroendocrine and behavioral circuit
A second major stress-transducing circuit consists of limbic pathways that are more sensitive to stressors involving higher-order sensory processing (Figure 1b).25–27 These pathways are preferentially activated by stimuli that require assembly and processing of signals from multiple modalities (as opposed to stressors that are an immediate physiological threat). Substantial information, based on lesion studies and analyses of immediate early gene expression following specific stressors28,29 is available about the limbic circuits activated during stress. Thus, the central nucleus of the amygdala (ACe) is a key region in regulating the central response to stress: ablation or stimulation of the ACe attenuate or mimic, respectively, behavioral stress responses, as well as the effects of stress on CRH release from PVN.28,30
However, the precise nature of the effector neurotransmitters/neuromodulators involved in the limbic stress circuit is still not fully understood. Here, too, CRH is a likely contributor to the activation of these pathways in response to stress.31 Indeed, CRH-expressing neurons and CRH receptors are found in select amygdala nuclei, other limbic relay centers, and all hippocampal fields.32–39 Furthermore, administration of receptor-blockers of CRH into the key regions in this limbic circuit, such as ACe40 blocks stress-induced responses. In addition, CRH-containing cells constitute a significant neuronal population in ACe, and send efferent projections to the bed nucleus of the stria terminalis, which then projects to CRH-expressing cells in PVN.34 Thus, CRH-expressing pathways originating in ACe may be responsible for mediating facilitatory stress-related input to the hypothalamus, resulting in CRH release from PVN and activation of the HPA axis.41
As in hypothalamus, stress can modulate CRH expression in ACe of mature and developing rat. Interestingly, certain early life stresses can also modulate CRH expression in hippocampus,27 suggesting involvement of this region in the limbic-neuroendocrine stress circuit of the immature organism (see below).
Brainstem pathways convey physiological stressful signals to effector regions
Physiological stressful stimuli such as dehydration, hypothermia and pain are initially received and processed through brainstem circuits (Figure 1c; see Watts for a review42). The nucleus of the solitary tract (NTS) in the medulla is a key component of these circuits. Diverse types of somatosensory, humoral, and metabolic information are detected peripherally and conveyed to NTS, which can then evoke an autonomic response in addition to relaying information through a number of pathways to the hypothalamus and/or telencephalic structures including the bed nucleus of the stria terminalis, amygdala and hippocampus. The neurotransmitters involved in these brainstem pathways include particularly norepinephrine and serotonin.43–45
Recent studies utilizing the age-specific stress paradigm of maternal separation have yielded important evidence supporting the role of CRH, acting via the CRF2 receptor, in transducing and integrating somatosensory, gustatory, and visceral signals received in brainstem nuclei, such as the NTS and Barrington’s nucleus, with the neuroendocrine stress response (Figure 1c).46 CRH is highly expressed in neurons residing in Barrington’s nucleus,47 as well as in ACe. The CRF2 receptor is expressed in target neurons of these CRH-expressing cells, such as neurons located in the thalamic paraventricular nucleus (PVT), receiving efferents from Barrington’s nucleus, as well as in amygdaloid and hypothalamic nuclei which are targets of ACe cells (Figure 1c). Thus, a neurochemical circuit utilizing CRH (or a similar ligand) as a neurotransmitter which acts on CRF2 to activate postsynaptic cellular processes can be defined. This circuit conveys stress-related somatovisceral signals to the medial-(MEA) and basomedial-(BMA) amygdala nuclei which are then relayed to ‘defensive’ medial hypothalamic centers48 and to ACe for further integration. ACe, the central integrator for autonomic regulation, also receives visceral input from the NTS via the CRH-containing parabrachial nucleus32,49 and modulates central components of the HPA axis (see above), further influenced by input from PVT.50 Thus, this proposed circuit provides a basis for propagating physiological stress-related information from brainstem low-level integrators to limbic and hypothalamic centers.
Regulation of stress-related information in this circuit may conceivably be achieved by modulation of either ligand levels (ie, via regulation of CRH expression or secretion), or by alteration of receptor abundance. Indeed, at least during development,46 absence of specific elements of maternal sensory input leads to selective down-regulation of the robust expression of CRF2 receptors in certain amygdala nuclei, such as BMA (but not in MEA), as well as in ventromedial nucleus of the hypothalamus (VMH)46,51–53 which is reciprocally connected to these regions.46 This is particularly interesting because, as noted above, maternal deprivation has resulted in variable effects on the expression of CRH itself in the hypothalamus (see Table 1).
Table 1.
Stress-induced regulation of the corticotropin releasing hormone (CRH) and its receptors in the immature rat
| Stressor | CRH mRNA expression in PVN |
CRH mRNA expression in ACe |
CRH receptors |
|---|---|---|---|
| Single acute | ⇑ (Yi and Baram, 1994)8 (cold) | ⇒ (Hatalski et al, 1998)26 (cold) | ⇒ CRF1 mRNA (Brunson et al, unpublished) |
| ⇑ (Hatalski et al, 1998)26 (cold) | |||
| ⇑ (Dent et al, 2000)12 (saline) | |||
| Recurrent acute | ⇒ (Hatalski et al, 1998)26 (cold) | ⇑ (Hatalski et al, 1998)26 (cold) | ⇒ CRF1 mRNA (Hatalski et al, unpublished) |
| Maternal separation (24 h) | ⇒ (Avishai-Eliner et al, 1995)86 | no data | ⇓ CRF2 mRNA in VMH (Eghbal-Ahmadi et al, 1999)46 |
| ⇓ (Smith et al, 1997; van Oers et al, 1998)83,85 | ⇒ pituitary CRH receptors (Pihoker et al, 1993)95 |
⇑ upregulation; ⇒ no change; ⇓ downregulation.
Differential effects of single and recurrent stress on hypothalamic and amygdala CRH expression in the developing rat
The response to stress in both developing and adult brain involves complex processes that provide a basis for reacting quickly to environmental challenges that pose immediate physiological threats, while permitting adaptation to non-threatening environmental changes that are part of daily experience. Thus, stress circuitry has evolved unique mechanisms for managing diverse types of acute and chronic stressors. In the developing animal, the HPA axis is selectively activated by specific acute stressors.16,54 In contrast, in the adult, acute stress activates neuroendocrine responses almost universally, and selectivity in the regulation of hypothalamic CRH expression is mostly noted during repeated and chronic stress.55 Thus, in the mature rat, repeated variable- or immobilization-stress has been shown to increase CRH mRNA expression in PVN,55–57 whereas chronic inflammatory stress has been shown to decrease CRH mRNA levels in this nucleus.58 A potential basis for the varying effects of specific recurrent or chronic stressors on CRH synthesis may derive from the qualitative differences of each stressor (eg, physical and psychological components).59
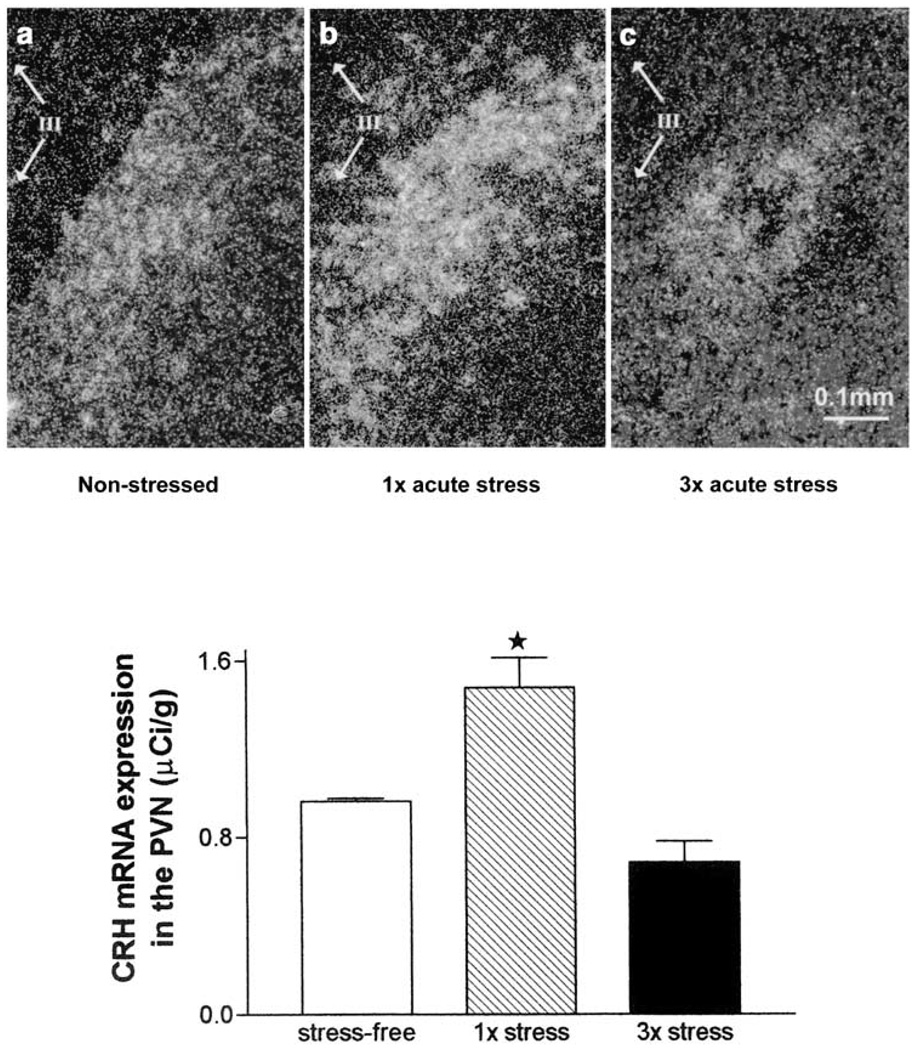
In the immature rat, the effect of a single acute stress on CRH mRNA expression in PVN differs from that of recurrent stress. For example, cold, a potent stressor to the neonatal rat that is considered to comprise both physiological and psychological elements, enhances PVN CRH mRNA levels within 4 h (Figure 2),8,26 consistent with ‘compensatory’ upregulation of CRH gene transcription after enhanced secretion of the peptide from hypothalamic nerve terminals, as found in the mature rodent.18 However, in contrast to the situation in the adult where repeated stress further augmented hypothalamic CRH levels, recurrent cold stress in the immature (10-day-old) rat resulted in PVN CRH expression levels that resembled those of unstressed controls (Figure 2). The mechanisms for this age-specific effect of recurrent stress are not fully resolved. Negative feedback via glucocorticoids,60 released upon the first stress, should suppress CRH expression (see above). It may well be that this feedback is more robust in the infant rat compared with the adult.17,61
Figure 2.
Relative abundance of corticotropin releasing hormone (CRH) mRNA in the hypothalamic paraventricular nucleus (PVN) of 9–10 day old rats under stress-free conditions (a) or after one (b) or three episodes (c) of cold stress. Animals were killed 4 h later. In situ hybridization was performed to detect CRH mRNA in coronal brain sections. Dark-field photomicrographs in panels (a)–(c) demonstrate CRH mRNA hybridization signal, which was quantified as shown in the bar graph. Single exposure to cold stress (1×) resulted in a significant increase in CRH mRNA in PVN (*P < 0.01) compared with stress-free controls. Repeated exposure to cold stress (3 ×) resulted in a nonsignificant trend toward decreased expression of CRH mRNA in PVN (P = 0.09). Each bar represents the mean with standard errors of 16–21 samples from 4–5 brains per group. III in photomicrographs represents the third ventricle. Modified and reproduced from Hatalski et al26 with permission from Blackwell Science Ltd.
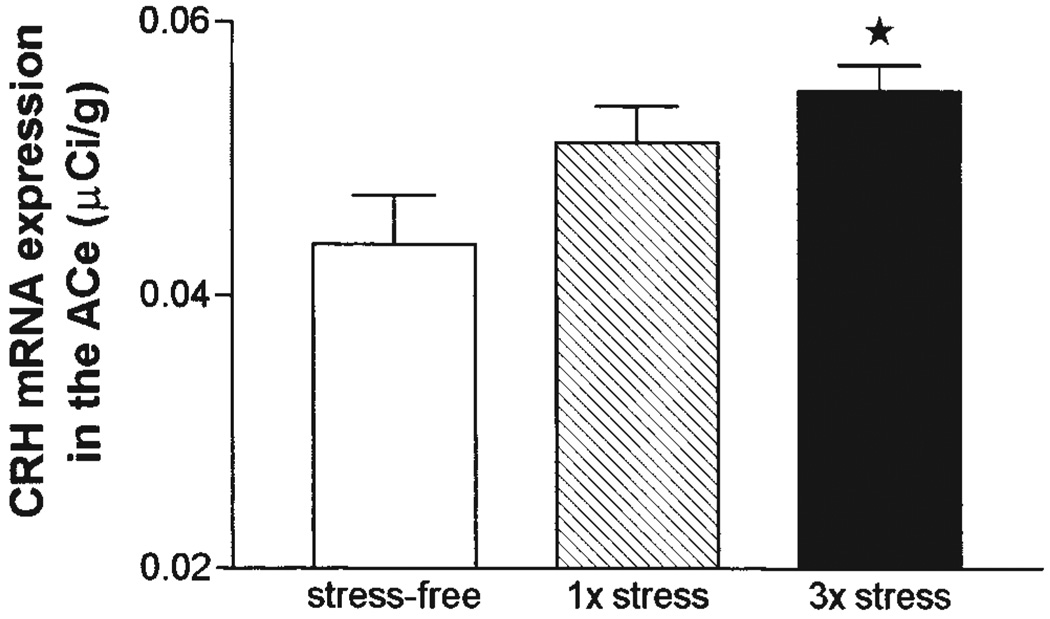
What are the mechanisms, at the circuit, cellular and molecular levels, by which a single acute stress augments CRH expression in the hypothalamic PVN of the immature rat? Facilitatory input from ACe has been implicated in both mature62 and developing rats.63,64 Immediate early gene analyses in adult and developing rat have shown that ACe neurons are rapidly activated by stressful stimuli.29,65 In addition, acute stress in the adult results in CRH release as well as upregulation of CRH mRNA expression in ACe. This upregulation is thought to be mediated by stress-induced glucocorticoids,66–68 and a facilitatory action of glucocorticoids on CRH expression has also been shown in the 10-day-old rat.69 These data suggest that a single acute stress activates CRH-expressing neurons in ACe, leading to a facilitatory input into CRH-expressing PVN neurons and resulting in enhanced CRH expression in this latter region. Indeed, in addition to production of immediate early genes, a single exposure to acute (cold) stress resulted in a trend toward increased expression of CRH mRNA in ACe of the 10-day-old rat,26 and repeated stress resulted in a highly significant upregulation of CRH mRNA in this nucleus (Figure 3).
Figure 3.
Relative abundance of corticotropin releasing hormone (CRH) mRNA in the central nucleus of the amygdala (ACe) of 9–10 day old rats following a single or repeated exposure to cold stress. Hybridization signal for CRH mRNA was analyzed in the ACe of rats killed under stress-free (SF) conditions or subjected once (1×) or three times (3×) to cold stress 4 h prior to killing. Repeated stress resulted in a significant increase in CRH mRNA expression in ACe (*P < 0.01) compared with SF controls. Each bar represents the average with standard error of the mean of 17–30 samples from 4–6 brains per group. Modified and reproduced from Hatalski et al26 with permission from Blackwell Science Ltd.
Taken together, these data suggest the following scenario at the circuit level: a first acute stress elevates plasma glucocorticoids which promotes CRH expression in ACe.66,67,69 This effect is more apparent following repeated stress and cumulatively more glucocorticoid production. Concurrently, upon repeated stress, enhanced glucocorticoid levels may act via local and/or hippocampal receptors to suppress CRH mRNA in the PVN of the more glucocorticoid-sensitive immature rat. This differential regulation of CRH expression in PVN and ACe by glucocorticoids can be biologically beneficial, because it provides a basis for adaptation while maintaining an organism’s ability to respond to stress in the presence of elevated glucocorticoids.62,63
Stress upregulates transcription of the CRH gene via CREB phosphorylation
At the molecular/cellular level, what are the mechanisms by which stress alters expression of the CRH gene in the immature rat? In most mammalian systems, the repertoire of cellular gene expression is typically determined by complex interactions of trans-acting transcriptional regulatory factors with specific DNA sequences. Thus, the ability to rapidly and precisely alter the expression of individual genes in response to environmental stimuli is believed to result from modulation of the interactions of these transcription factors with their corresponding DNA-regulatory elements.70,71 The CRH gene promoter contains a cyclic AMP response element (CRE), that is bound by a complex of trans-activating factors including the CRE binding protein (CREB). This interaction has been shown to be involved in CRH transcriptional regulation.72–74 In order to activate transcription of the CRH gene, CREB must be activated (phosphorylated), permitting its binding to the CRH promoter.75,76
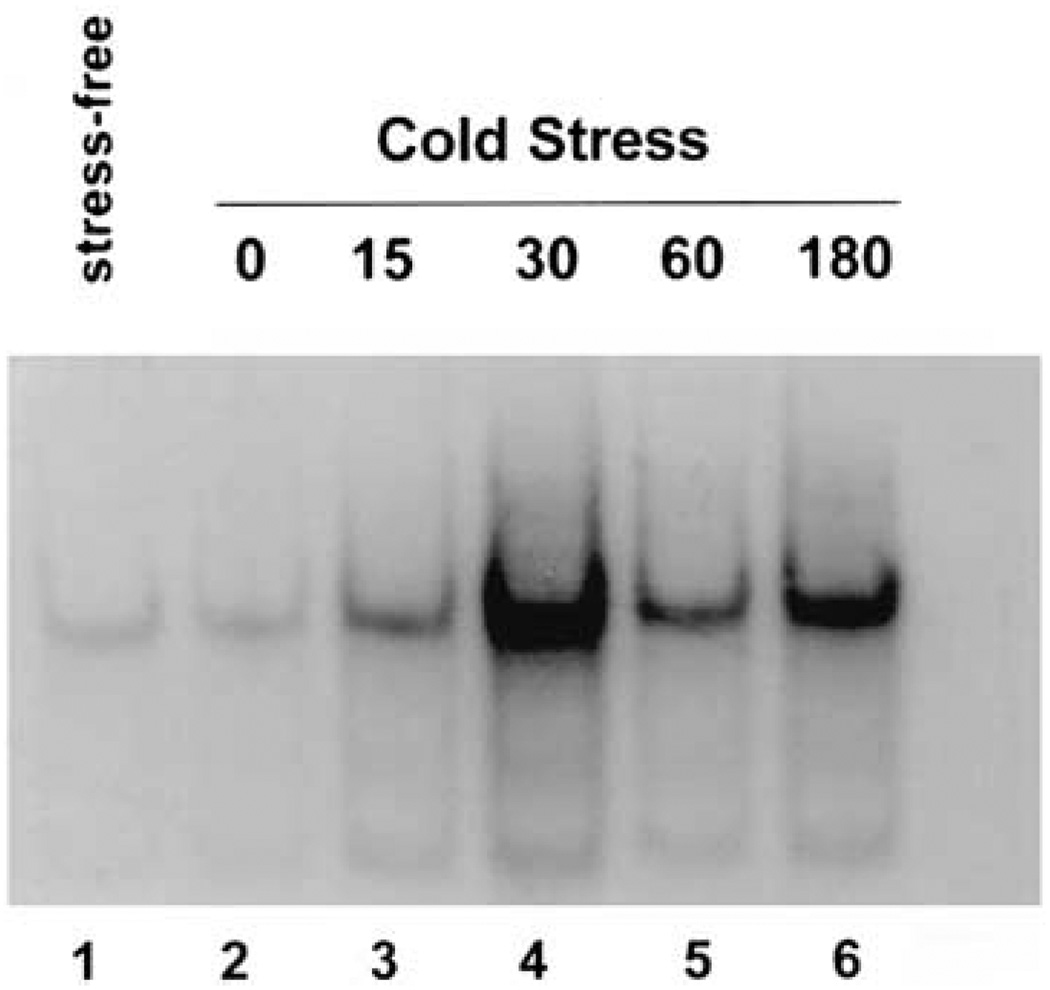
Recent studies77 have yielded evidence that CRE-binding activity is present in the developing rat hypothalamus. Furthermore, CREB is likely to be the principal contributor to this CRE-binding activity since DNA binding is essentially blocked by an antiserum directed against CREB-1. In addition, gel shift and western blot methods have demonstrated that acute cold stress increased the levels of phosphorylated CREB (pCREB), as well as of CRE-related DNA binding capacity in anterior hypothalamic extracts of developing rat (Figure 4). Since stress did not result in an increase in levels of total CREB, it is likely that stress acts via posttranslational mechanisms to activate existing CREB and increase CRE-binding capacity.77 These data are further supported by immunocytochemical analyses showing that acute cold stress leads to phosphorylation of CREB in CRH-expressing medial parvocellular cell groups of PVN within two minutes, preceding the rapid activation of CRH gene transcription as measured by probes for the CRH heteronuclear RNA, the unedited RNA species.78,79 The results are consistent with pCREB induced transcription of the CRH gene in the developing rat, supporting the existence of this stress-induced mechanism which functions in adult PVN80 already during the second postnatal week.
Figure 4.
Increased CRE-binding activity in anterior hypothalamus of developing rat is induced by a single acute stress. Gel shift analysis of DNA containing the CRE consensus sequence in rat anterior hypothalamic extracts (10 µg of protein). The sample in lane 1 was derived from a rat under stress-free conditions. For the other samples rats were killed at 0 min (lane 2), 15 min (lane 3), 30 min (lane 4), 60 min (lane 5), or 180 min (lane 6) after termination of an acute cold stress. A progressive increase in the CRE-binding capacity of the extracts is evident, peaking at the 30 min time point (lane 4). Modified and reproduced with permission from Hatalski and Baram.77
Selectivity and maternal influence on the stress response during early postnatal life
As discussed in the paragraphs above, information regarding acute age-appropriate stress may be conveyed through afferent pathways to hypothalamic PVN, and activate an efferent stress response arm consisting of both release of CRH and upregulation of the expression of this gene. However, whereas the presence of an intact and functional molecular stress response to select stressors throughout the first two postnatal weeks has been established,8,11,12 attenuated hormonal responses to a large number of stressors have been described in the immature rat, leading to the term ‘stress-hyporesponsive’ period. More recent studies have begun to elucidate the complexity of the immature stress response (see Levine for a recent review54), including the molecules and neuronal networks involved in the central regulation of these processes, the time frame of cellular and hormonal responses12 and the age-dependent sensitivities to hormonal and neurotransmitter influences at the pituitary and adrenal levels.81,82
Importantly, these studies have begun to shed light on the profound effects of maternal input, including both sensory stimulation and feeding, on the nature, magnitude and time course of the neuroendocrine responses to stress. The paradigm of maternal separation, combined with selective re-introduction of defined elements of maternal input, has been highly useful to tease out the precise nature of maternally-derived stimuli.46,54,81–84 These studies demonstrated that maternal presence, providing sensory and nutritional input, influenced components of the HPA axis both at rest46,83 and in response to super-imposed stress.12 Further, the direction (hypo- or hyper-activity) and level of modulatory changes were dependent on the timing and length of maternal absence, and of the specific maternal signals that were eliminated.46 Much remained to be studied about the effects of maternal input on the developing HPA axis. For example, maternal separation has been reported to reduce PVN CRH mRNA levels,83 or to not alter the levels of this transcript.86 Thus, while maternal input clearly influences the stress-response early in life, the precise changes and the underlying mechanisms require further investigation.
Long-term consequences of early life stress
Stress early in life can alter gene expression within the brain, leading to permanent modification of the HPA axis.24,87 Later in life, these changes in the HPA axis result in abnormal molecular and hormonal responses to further stressful stimuli.3,24,88 The mechanisms for these long-term effects of early-life stress are not well understood, but it is reasonable to assume that neonatal stressful signals would generate these effects via the activation of the molecular signaling processes that are ‘normally’ induced by stressful challenges in the immature central nervous system.
As discussed above, the propagation and integration of stress-signals and responses in the hypothalamic and limbic stress-circuit of the immature rat involve alterations in the release and synthesis of CRH. Indeed, recent studies have found significant long-term changes in the expression of CRH in regions critical for the regulation of the HPA tone and sensitivity to further stressors. Thus, rearing immature rats without any handling, considered mildly stressful, has led to enhancement of CRH expression in hypothalamus,2 starting already on postnatal day 9.24 In addition, CRH levels in limbic regions that regulate HPA function, eg, the hippocampus23 have been found to be permanently upregulated after a stress-like manipulation during the second postnatal week. Administration of the stress mediator CRH directly into the CNS, reproducing the enhanced endogenous peptide levels found with stress,68 led to dramatic and sustained increase in mRNA expression of CRH in hippocampus.89 The expected effects of these enhanced CRH levels are further compounded by a concomitant increase in the CRH receptor CRF1.89 These enhanced hippocampal levels of CRH as well as of the CRF1 receptor persisted for at least 12 months, and may have significant implications for the human situation. Increased brain and cerebrospinal fluid levels of CRH have been found in individuals with affective disorders, particularly depression.90–93 Importantly, depression in the human may be a consequence of early life stress such as maternal neglect or child abuse.3,88 Thus, early life stress may lead to chronic, sustained alteration in the response to subsequent stressors (eg, in coping) via mechanisms which include permanent alteration of CRH expression in limbic regions that are involved in the regulation of the HPA. Further, CRH at multiple hypothalamic/limbic and perhaps brainstem sites may contribute to the mechanisms of neuroplasticity induced by early life stressful stimuli, to alter the neuroendocrine as well as the behavioral/affective stress responses permanently.
Summary
Stress during a ‘neuroplastic’ developmental period can alter the function of the HPA axis later in life. This has been demonstrated in rodents, non-human primates, and has been well described in humans who were neglected and abused as children.2,4,94 Over the past decade, a great deal of progress has been made in the understanding of the roles of limbic and brainstem pathways in regulating the stress response. Central facilitatory and inhibitory inputs to the hypothalamic core of the HPA axis are crucial in regulating the response to stress both during development and adulthood. The characterization of CRH in the early ‘80s essentially revolutionized the investigation and the understanding of central mechanisms involved in regulating the acute and long-term effects of stress during development. CRH, in addition to acting as a neuroendocrine releasing factor for ACTH, plays an important role as a neuromodulator in brain regions involved in anxiety, mood and learning/memory. The long-term deleterious effects observed after early life stress may involve sustained alteration of CRH expression in these key limbic regions, resulting in dysfunction of the behavioral, molecular and neuroendocrine aspects of the responses to stress throughout life.
Acknowledgements
Research in the authors’ laboratory was supported by NIH grants NS28912 and NS39307.
References
- 1.Levine S. A further study of infantile handling and adult avoidance learning. J Personality. 1957;25:70–80. doi: 10.1111/j.1467-6494.1956.tb01289.x. [DOI] [PubMed] [Google Scholar]
- 2.Plotsky PM, Meaney MJ. Early, postnatal experience alters hypothalamic corticotropin-releasing factor (CRF) mRNA, median eminence CRF content and stress-induced release in adult rats. Mol Brain Res. 1993;18:195–200. doi: 10.1016/0169-328x(93)90189-v. [DOI] [PubMed] [Google Scholar]
- 3.Heim C, Owens MJ, Plotsky PM, Nemeroff CB. Endocrine factors in the pathopysiology of mental disorders. Psychopharmacol Bull. 1997;33:185–192. [PubMed] [Google Scholar]
- 4.Sánchez MM, Hearn EF, Do D, Rilling JK, Herndon JG. Differential rearing affects corpus callosum size and cognitive function of rhesus monkeys. Brain Res. 1998;812:38–49. doi: 10.1016/s0006-8993(98)00857-9. [DOI] [PubMed] [Google Scholar]
- 5.Gold PW, Wong ML, Chrousos GP, Licinio J. Stress system abnormalities in melancholic and atypical depression: molecular, pathophysiological, and therapeutic implications. Mol Psychiatry. 1996;1:257–264. [PubMed] [Google Scholar]
- 6.Levine S. The pituitary-adrenal system and the developing brain. Prog Brain Res. 1970;32:79–85. doi: 10.1016/S0079-6123(08)61521-6. [DOI] [PubMed] [Google Scholar]
- 7.Schoenfeld NM, Leathem JH, Rabii J. Maturation of adrenal stress responsiveness in the rat. Neuroendocrinology. 1980;31:101–105. doi: 10.1159/000123058. [DOI] [PubMed] [Google Scholar]
- 8.Yi SJ, Baram TZ. Corticotropin-releasing hormone mediates the response to cold stress in the neonatal rat without compensatory enhancement of the peptide’s gene expression. Endocrinology. 1994;135:2364–2368. doi: 10.1210/endo.135.6.7988418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Muret L, Priou A, Oliver C, Grino M. Stimulation of adrenocorticotropin secretion by insulin-induced hypoglycemia in the developing rat involves arginine vasopressin but not corticotropin-releasing factor. Endocrinology. 1992;130:2725–2732. doi: 10.1210/endo.130.5.1315256. [DOI] [PubMed] [Google Scholar]
- 10.Vazquez DM. Stress and the developing limbic-hypothalamic-pituitary-adrenal axis. Psychoneuroendocrinology. 1998;23:663–700. doi: 10.1016/s0306-4530(98)00029-8. [DOI] [PubMed] [Google Scholar]
- 11.Walker C-D, Scribner K, Cascio CS, Dallman MF. The pituitary-adrenocortical system of neonatal rats is responsive to stress throughout development in a time-dependent and stressor-specific fashion. Endocrinology. 1991;128:1385–1395. doi: 10.1210/endo-128-3-1385. [DOI] [PubMed] [Google Scholar]
- 12.Dent GW, Smith MA, Levine S. Rapid induction of corticotropin-releasing hormone gene transcription in the paraventricular nucleus of the developing rat. Endocrinology. 2000;141:1593–1598. doi: 10.1210/endo.141.5.7455. [DOI] [PubMed] [Google Scholar]
- 13.Walker CD, Perrin M, Vale W, Rivier C. Ontogeny of the stress response in the rat: role of the pituitary and the hypothalamus. Endocrinology. 1986;118:1445–1451. doi: 10.1210/endo-118-4-1445. [DOI] [PubMed] [Google Scholar]
- 14.Baram TZ, Yi S, Avishai-Eliner S, Schultz L. Development neurobiology of the stress response: multilevel regulation of corticotropin-releasing hormone function. Ann NY Acad Sci. 1997;814:252–265. doi: 10.1111/j.1749-6632.1997.tb46161.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vale W, Spiess J, Rivier C, Rivier J. Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotropin and beta-endorphin. Science. 1981;213:1394–1397. doi: 10.1126/science.6267699. [DOI] [PubMed] [Google Scholar]
- 16.Levine S. The ontogeny of the hypothalamic-pituitary-adrenal axis. The influence of maternal factors. Ann NY Acad Sci. 1994;746:275–288. doi: 10.1111/j.1749-6632.1994.tb39245.x. [DOI] [PubMed] [Google Scholar]
- 17.Oates M, Woodside B, Walker CD. Chronic leptin administration in developing rats reduces stress responsiveness partly through changes in maternal behavior. Hormones Behav. 2000;37:366–376. doi: 10.1006/hbeh.2000.1578. [DOI] [PubMed] [Google Scholar]
- 18.Lightman SL, Young WS. Influence of steroids on the hypothalamic corticotropin-releasing factor and preproenkephalin mRNA responses to stress. Proc Nat Acad Sci. 1989;86:4306–4310. doi: 10.1073/pnas.86.11.4306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Young WS, III, Mezey E, Siegel RE. Quantitative in situ hybridization histochemistry reveals increased levels of corticotropin-releasing factor mRNA after adrenalectomy in rats. Neurosci Lett. 1986;70:198–203. doi: 10.1016/0304-3940(86)90463-5. [DOI] [PubMed] [Google Scholar]
- 20.Swanson LW, Simmons DM. Differential steroid hormone and neural influences on peptide mRNA levels in CRH cells of the paraventricular nucleus: a hybridization histochemical study in the rat. J Comp Neurol. 1989;285:413–435. doi: 10.1002/cne.902850402. [DOI] [PubMed] [Google Scholar]
- 21.Yi SJ, Masters JN, Baram TZ. Effects of a specific glucocorticoid receptor antagonist on corticotropin releasing hormone gene expression in the paraventricular nucleus of the neonatal rat. Dev Brain Res. 1993;73:253–259. doi: 10.1016/0165-3806(93)90145-z. [DOI] [PubMed] [Google Scholar]
- 22.Feldman S, Conforti N. Feedback effects of dexamethasone on adrenocortical responses of rats with fornix transection. Hormone Res. 1976;7:56–59. doi: 10.1159/000178709. [DOI] [PubMed] [Google Scholar]
- 23.Herman JP, Schafer MK, Young EA, Thompson R, Douglass J, Akil H, et al. Evidence for hippocampal regulation of neuroendocrine neurons of the hypothalamo-pituitary-adrenocortical axis. J Neurosci. 1989;9:3072–3082. doi: 10.1523/JNEUROSCI.09-09-03072.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Avishai-Eliner S, Eghbal-Ahmadi M, Tabachnik E, Brunson KL, Baram TZ. Down-regulation of hypothalamic corticotropin releasing hormone-mRNA precedes early-life experience-induced changes in hippocampal glucocorticoid receptor-mRNA. Endocrinology. 2001;142:89–97. doi: 10.1210/endo.142.1.7917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Herman JP, Cullinan WE. Neurocircuitry of stress: central control of the hypothalamo-pituitary-adrenocortical axis. Trends Neurosci. 1997;20:78–84. doi: 10.1016/s0166-2236(96)10069-2. [DOI] [PubMed] [Google Scholar]
- 26.Hatalski CG, Guirguis C, Baram TZ. Corticotropin releasing factor mRNA expression in the hypothalamic paraventricular nucleus and the central nucleus of the amygdala is modulated by repeated acute stress in the immature rat. J Neuroendocrinol. 1998;10:663–669. doi: 10.1046/j.1365-2826.1998.00246.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hatalski CG, Brunson KL, Tantayanubutr B, Chen Y, Baram TZ. Neuronal activity and stress differentially regulate hippocampal and hypothalamic corticotropin releasing hormone expression in the immature rat. Neuroscience. 2000;101:571–580. doi: 10.1016/s0306-4522(00)00386-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Beaulieu S, Pelletier G, Vaudry H, Barden N. Influence of the central nucleus of the amygdala on the content of corticotropin-releasing factor in the median eminence. Neuroendocrinology. 1989;49:255–261. doi: 10.1159/000125125. [DOI] [PubMed] [Google Scholar]
- 29.Honkaniemi J, Kononen J, Kainu T, Pyykonen I, Pelto-Huikko M. Induction of multiple immediate early genes in rat hypothalamic paraventricular nucleus after stress. Mol Brain Res. 1994;25:234–241. doi: 10.1016/0169-328x(94)90158-9. [DOI] [PubMed] [Google Scholar]
- 30.Tannahill LA, Sheward WJ, Robinson IC, Fink G. Corticotropin-releasing factor-41, vasopressin and oxytocin release into hypophysial portal blood in the rat: effects of electrical stimulation of the hypothalamus, amygdala and hippocampus. J Endocrinol. 1991;129:99–107. doi: 10.1677/joe.0.1290099. [DOI] [PubMed] [Google Scholar]
- 31.Koob GF, Bloom FE. Corticotropin-releasing factor and behavior. Fed Proc. 1985;44:259–263. [PubMed] [Google Scholar]
- 32.Swanson LW, Sawchenko PE, Rivier J, Vale WW. Organization of ovine corticotropin releasing factor immunoreactive cells and fibers in the rat brain: an immunohistochemical study. Neuroendocrinology. 1983;36:165–186. doi: 10.1159/000123454. [DOI] [PubMed] [Google Scholar]
- 33.De Souza EB, Insel TR, Perrin MH, Rivier J, Vale WW, Kuhar MJ. Corticotropin-releasing factor receptors are widely distributed within the rat CNS: an autoradiographic study. J Neurosci. 1985;5:3189–3203. doi: 10.1523/JNEUROSCI.05-12-03189.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gray TS, Bingaman EW. The amygdala: corticotropin-releasing factor, steroids, and stress. Crit Rev Neurobiol. 1996;10:155–168. doi: 10.1615/critrevneurobiol.v10.i2.10. [DOI] [PubMed] [Google Scholar]
- 35.Avishai-Eliner S, Yi SJ, Baram TZ. Developmental profile of messenger RNA for the corticotropin-releasing hormone receptor in the limbic system. Dev Brain Res. 1996;91:159–163. doi: 10.1016/0165-3806(95)00158-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yan XX, Toth Z, Schultz L, Ribak CE, Baram TZ. Corticotropin releasing hormone (CRH)-containing neurons in the hippocampal formation: morphological and neurochemical characterization. Hippocampus. 1998;8:231–243. doi: 10.1002/(SICI)1098-1063(1998)8:3<231::AID-HIPO6>3.0.CO;2-M. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Eghbal-Ahmadi M, Hatalski CG, Lovenberg TW, Avishai-Eliner S, Chalmers DT, Baram TZ. The developmental profile of the corticotropin releasing hormone receptor (CRF2) in rat brain predicts distinct age-specific functions. Dev Brain Res. 1998;107:81–90. doi: 10.1016/s0165-3806(98)00002-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen Y, Brunson K, Cariaga W, Baram TZ. Immunocytochemical distribution of corticotropin-releasing hormone receptor type-1 (CRF1)-like immunoreactivity in the mouse brain: light microscopy analysis using an antibody directed against the C-terminus. J Comp Neurol. 2000;420:305–323. doi: 10.1002/(sici)1096-9861(20000508)420:3<305::aid-cne3>3.0.co;2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen Y, Bender RA, Frotscher M, Baram TZ. Novel and transient populations of corticotropin-releasing hormone-expressing neurons in developing hippocampus suggest unique functional roles: a quantitative spatiotemporal analysis. 2001 doi: 10.1523/JNEUROSCI.21-18-07171.2001. (under revision) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Swiergel AH, Takahashi LK, Kalin NH. Attenuation of stress-induced behavior by antagonism of corticotropin-releasing factor receptors in the central amygdala in the rat. Brain Res. 1993;623:229–234. doi: 10.1016/0006-8993(93)91432-r. [DOI] [PubMed] [Google Scholar]
- 41.Gray TS. Amygdaloid CRF pathways. Ann NY Acad Sci. 1993;697:53–60. doi: 10.1111/j.1749-6632.1993.tb49922.x. [DOI] [PubMed] [Google Scholar]
- 42.Watts AG. Understanding the neural control of ingestive behaviors: helping to separate cause from effect with dehydration-associated anorexia. Hormones and Behavior. 2000;37:261–283. doi: 10.1006/hbeh.2000.1581. [DOI] [PubMed] [Google Scholar]
- 43.Fallon JH, Koziell DA, Moore RY. Catecholamine innervation of the basal forebrain. II. Amygdala, suprarhinal cortex and entorhinal cortex. J Comp Neurol. 1978;180:509–532. doi: 10.1002/cne.901800308. [DOI] [PubMed] [Google Scholar]
- 44.Zardetto-Smith AM, Gray TS. Organization of peptidergic and catecholaminergic efferents from the nucleus of the solitary tract to the rat amygdala. Brain Res Bull. 1990;25:875–887. doi: 10.1016/0361-9230(90)90183-z. [DOI] [PubMed] [Google Scholar]
- 45.Howe PR. Blood pressure control by neurotransmitters in the medulla oblongata and spinal cord. J Autonomic Nervous System. 1985;12:95–115. doi: 10.1016/0165-1838(85)90054-2. [DOI] [PubMed] [Google Scholar]
- 46.Eghbal-Ahmadi M, Avishai-Eliner S, Hatalski CG, Baram TZ. Differential regulation of the expression of corticotropin-releasing factor receptor type 2 (CRF2) in hypothalamus and amygdala of the immature rat by sensory input and food intake. J Neurosci. 1999;19:3982–3991. doi: 10.1523/JNEUROSCI.19-10-03982.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sawchenko PE, Imaki T, Potter E, Kovács K, Imaki J, Vale W. The functional neuroanatomy of corticotropin-releasing factor. Ciba Found Sym. 1993;172:5–29. doi: 10.1002/9780470514368.ch2. [DOI] [PubMed] [Google Scholar]
- 48.Swanson LW, Petrovich GD. What is the amygdala? Trends Neurosci. 1998;21:323–331. doi: 10.1016/s0166-2236(98)01265-x. [DOI] [PubMed] [Google Scholar]
- 49.Jia HG, Rao ZR, Shi JW. An indirect projection from the nucleus of the solitary tract to the central nucleus in the rat: a light and electron microscopic study. Brain Res. 1994;663:181–190. doi: 10.1016/0006-8993(94)91262-9. [DOI] [PubMed] [Google Scholar]
- 50.Bhatnagar S, Dallman MF. Neuroanatomical basis for facilitation of hypothalamic-pituitary-adrenal responses to a novel stressor after chronic stress. Neurosci. 1998;84:1025–1039. doi: 10.1016/s0306-4522(97)00577-0. [DOI] [PubMed] [Google Scholar]
- 51.Lovenberg TW, Chalmers DT, Liu C, De Souza EB. CRF2α and CRF2β receptor mRNAs are differentially distributed between the rat central nervous system and peripheral tissues. Endocrinology. 1995;136:4139–4142. doi: 10.1210/endo.136.9.7544278. [DOI] [PubMed] [Google Scholar]
- 52.Richard D, Rivest R, Naimi N, Timofeeva E, Rivest S. Expression of corticotropin-releasing factor and its receptors in the brain of lean and obese Zucker rats. Endocrinology. 1996;137:4786–4795. doi: 10.1210/endo.137.11.8895348. [DOI] [PubMed] [Google Scholar]
- 53.Eghbal-Ahmadi M, Hatalski CG, Avishai-Eliner S, Baram TZ. Corticotropin releasing factor receptor type II (CRF2) messenger ribonucleic acid levels in the hypothalamic ventromedial nucleus of the infant rat are reduced by maternal deprivation. Endocrinology. 1997;138:5048–5051. doi: 10.1210/endo.138.11.5647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Levine S. Influence of psychological variables on the activity of the hypothalamic-pituitary-adrenal axis. Euro J Pharmacol. 2000;405:149–160. doi: 10.1016/s0014-2999(00)00548-3. [DOI] [PubMed] [Google Scholar]
- 55.Ma X-M, Lightman SL, Aguilera G. Vasopressin and corticotropin-releasing hormone gene responses to novel stress in rats adapted to repeated restraint. Endocrinology. 1999;140:3623–3632. doi: 10.1210/endo.140.8.6943. [DOI] [PubMed] [Google Scholar]
- 56.Herman JP, Adams D, Prewitt C. Regulatory changes in neuroendocrine stress—integrative circuitry produced by a variable stress paradigm. Neuroendocrinology. 1995;61:180–190. doi: 10.1159/000126839. [DOI] [PubMed] [Google Scholar]
- 57.Makino S, Smith MA, Gold PW. Increased expression of corticotropin-releasing hormone and vasopressin messenger ribonucleic acid (mRNA) in the hypothalamic paraventricular nucleus during repeated stress: association with reduction in glucocorticoid receptor mRNA levels. Endocrinology. 1995;136:3299–3309. doi: 10.1210/endo.136.8.7628364. [DOI] [PubMed] [Google Scholar]
- 58.Harbuz MS, Rees RG, Eckland D, Jessop DS, Brewerton D, Lightman SL. Paradoxical responses of hypothalamic corticotropin-releasing factor (CRF) messenger ribonucleic acid (mRNA) and CRF-41 peptide and adenohypophysial proopiomelanocortin mRNA during chronic inflammatory stress. Endocrinology. 1992;130:1394–1400. doi: 10.1210/endo.130.3.1537299. [DOI] [PubMed] [Google Scholar]
- 59.Watts AG. The impact of physiological stimuli on the expression of corticotropin-releasing hormone (CRH) and other neuropeptide genes. Front Neuroendocrinol. 1996;17:281–326. doi: 10.1006/frne.1996.0008. [DOI] [PubMed] [Google Scholar]
- 60.Dallman MF, Akana SF, Scribner KA, Bradbury MJ, Walker CD, Strack AM, et al. Stress, feedback and facilitation in the hypothalamo-pituitary-adrenal axis. Neuroendocrinol. 1992;4:517–526. doi: 10.1111/j.1365-2826.1992.tb00200.x. [DOI] [PubMed] [Google Scholar]
- 61.Walker CD, Sapolsky RM, Meaney MJ, Vale WW, Rivier CL. Increased pituitary sensitivity to glucocorticoid feedback during the stress nonresponsive period in the neonatal rat. Endocrinology. 1986;119:1816–1821. doi: 10.1210/endo-119-4-1816. [DOI] [PubMed] [Google Scholar]
- 62.Akana SF, Dallman MF. Chronic cold in adrenalectomized, corticosterone (B)-treated rats: facilitated corticotropin responses to acute restraint emerge as B increases. Endocrinology. 1997;138:3249–3258. doi: 10.1210/endo.138.8.5291. [DOI] [PubMed] [Google Scholar]
- 63.Walker C-D, Dallman MF. Neonatal facilitation of stress-induced adrenocorticotropin secretion by prior stress: evidence for increased central drive to the pituitary. Endocrinology. 1993;132:1101–1107. doi: 10.1210/endo.132.3.8382596. [DOI] [PubMed] [Google Scholar]
- 64.Dallman MF. Moments in time—the neonatal rat hypothalamopituitary-adrenal axis. Endocrinology. 2000;141:1590–1592. doi: 10.1210/endo.141.5.7527. [DOI] [PubMed] [Google Scholar]
- 65.Dubé C, Brunson KL, Nehlig A, Baram TZ. Corticotropin releasing hormone activates specific neuronal circuits, as indicated by c-fos expression and glucose metabolism. J Cereb Blood Flow Metab. 2000;20:1414–1424. doi: 10.1097/00004647-200010000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kalin NH, Takahashi LK, Chen F-L. Restraint stress increases corticotropin-releasing hormone mRNA content in the amygdala and paraventricular nucleus. Brain Res. 1994;656:182–186. doi: 10.1016/0006-8993(94)91382-x. [DOI] [PubMed] [Google Scholar]
- 67.Makino S, Gold PW, Schulkin J. Corticosterone effects on corticotropin-releasing hormone mRNA in the central nucleus of the amygdala and the parvocellular region of the paraventricular nucleus of the hypothalamus. Brain Res. 1994;640:105–112. doi: 10.1016/0006-8993(94)91862-7. [DOI] [PubMed] [Google Scholar]
- 68.Merali Z, McIntosh J, Kent P, Michaud D, Anisman H. Aversive and appetitive events evoke the release of corticotropin-releasing hormone a bombesin-like peptide at the central nucleus of the amygdala. J Neurosci. 1998;18:4758–4766. doi: 10.1523/JNEUROSCI.18-12-04758.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Brunson KL, Khan N, Eghbal-Ahmadi M, Baram TZ. ACTH acts directly on amygdala neurons to down-regulate corticotropin releasing hormone gene expression. Ann Neurol. 2001;49:304–312. [PMC free article] [PubMed] [Google Scholar]
- 70.Brindle PK, Montminy MR. The CREB family of transcription activators. Curr Opin Genet Dev. 1992;2:199–204. doi: 10.1016/s0959-437x(05)80274-6. [DOI] [PubMed] [Google Scholar]
- 71.Lee KA, Masson N. Transcriptional regulation by CREB and its relatives. Biochim Biophys Acta. 1993;117:221–233. doi: 10.1016/0167-4781(93)90191-f. [DOI] [PubMed] [Google Scholar]
- 72.Seasholtz AF, Thompson RC, Douglass JO. Identification of a cyclic adenosine monophosphate-responsive element in the rat corticotropin-releasing hormone gene. Mol Endocrinol. 1988;2:1311–1319. doi: 10.1210/mend-2-12-1311. [DOI] [PubMed] [Google Scholar]
- 73.Guardiola-Diaz HM, Boswell C, Seasholtz AF. The cAMP-responsive element in the corticotropin-releasing hormone gene mediates transcriptional regulation by depolarization. J Biol Chem. 1994;269:14784–14791. [PubMed] [Google Scholar]
- 74.Itoi K, Horiba N, Tozawa F, Sakai Y, Abe K, Demura H, et al. Major role of 3′,5′-cyclic adenosine monophosphate-dependent protein kinase A pathway in corticotropin-releasing factor gene expression in the rat hypothalamus in vivo. Endocrinology. 1996;137:2389–2396. doi: 10.1210/endo.137.6.8641191. [DOI] [PubMed] [Google Scholar]
- 75.Kovács KJ, Sawchenko PE. Regulation of stress-induced transcriptional changes in the hypothalamic neurosecretory neurons. J Mol Neurosci. 1996;7:125–133. doi: 10.1007/BF02736792. [DOI] [PubMed] [Google Scholar]
- 76.Légrádi G, Holzer D, Kapcala LP, Lechan RM. Glucocorticoids inhibit stress-induced phosphorylation of CREB in corticotropin-releasing hormone neurons of the hypothalamic paraventricular nucleus. Neuroendocrinology. 1997;66:86–97. doi: 10.1159/000127224. [DOI] [PubMed] [Google Scholar]
- 77.Hatalski CG, Baram TZ. Stress-induced transcriptional regulation in the developing rat brain involves increased cyclic adenosine 3′,5′-monophosphate-regulatory element binding activity. Mol Endocrinol. 1997;11:2016–2024. doi: 10.1210/mend.11.13.0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hatalski CG, Avishai-Eliner S, Eghbal-Ahmadi M, Baram TZ. Induction of corticotropin releasing factor heteronuclear messenger RNA by acute cold stress in the developing rat hypothalamus. 79th Endocrine Soc Proc Abs. 1997;79:516. [Google Scholar]
- 79.Baram TZ, Hatalski CG. Neuropeptide-mediated excitability: a key triggering mechanism for seizure generation in the developing brain. Trends Neurosci. 1998;21:471–476. doi: 10.1016/s0166-2236(98)01275-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kovács KJ, Sawchenko PE. Sequence of stress-induced alterations in indices of synaptic and transcriptional activation in parvocellular neurosecretory neurons. J Neurosci. 1996;16:262–273. doi: 10.1523/JNEUROSCI.16-01-00262.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Stanton ME, Gutierrez YR, Levine S. Maternal deprivation potentiates pituitary-adrenal stress responses in infant rats. Behav Neurosci. 1988;102:692–700. doi: 10.1037//0735-7044.102.5.692. [DOI] [PubMed] [Google Scholar]
- 82.Suchecki D, Rosenfeld P, Levine S. Maternal regulation of the hypothalamic-pituitary-adrenal axis in the infant rat: the roles of feeding and stroking. Dev Brain Res. 1993;75:185–192. doi: 10.1016/0165-3806(93)90022-3. [DOI] [PubMed] [Google Scholar]
- 83.van Oers HJJ, de Kloet ER, Whelan T, Levine S. Maternal deprivation effect on the infant’s neural stress markers is reversed by tactile stimulation and feeding but not by suppressing corticosterone. J Neursosci. 1998;18:10171–10179. doi: 10.1523/JNEUROSCI.18-23-10171.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Avishai-Eliner S, Hatalski CG, Tabachnik E, Eghbal-Ahmadi M, Baram TZ. Differential regulation of glucocorticoid receptor messenger RNA by maternal deprivation in immature rat hypothalamus and limbic regions. Dev Brain Res. 1999;114:265–268. doi: 10.1016/s0165-3806(99)00031-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Smith MA, Kim S-Y, van Oers HJJ, Levine S. Maternal deprivation and stress induce immediate early genes in the infant rat brain. Endocrinology. 1997;138:4622–4628. doi: 10.1210/endo.138.11.5529. [DOI] [PubMed] [Google Scholar]
- 86.Avishai-Eliner S, Yi SJ, Newth CJ, Baram TZ. Effects of maternal and sibling deprivation on basal and stress-induced hypothalamic-pituitary-adrenal components in the infant rat. Neurosci Lett. 1995;192:49–52. doi: 10.1016/0304-3940(95)11606-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sapolsky RM, Meaney MJ. Maturation of the adrenocortical stress response: neuroendocrine control mechanisms and the stress hyporesponsive period. Brain Res. 1986;396:64–76. doi: 10.1016/s0006-8993(86)80190-1. [DOI] [PubMed] [Google Scholar]
- 88.Coplan JD, Andrews MW, Rosenblum LA, Owens MJ, Friedman S, Gorman JM, et al. Persistent elevations of cerebrospinal fluid concentrations of corticotropin-releasing factor in adult nonhuman primates exposed to early-life stressors: implications for the pathophysiology of mood and anxiety disorders. Proc Nat Acad Sci. 1996;93:1619–1623. doi: 10.1073/pnas.93.4.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Brunson KL, Eghbal-Ahmadi M, Bender R, Chen Y, Baram TZ. Long-term, progressive hippocampal cell-loss and dysfunction induced by early-life administration of corticotropin releasing hormone reproduce the effects of early-life stress. Proc Natl Acad Sci USA. 2001 doi: 10.1073/pnas.151224898. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Nemeroff CB. The role of corticotropin-releasing factor in the pathogenesis of major depression. Pharmacopsychiatry. 1988;21:76–82. doi: 10.1055/s-2007-1014652. [DOI] [PubMed] [Google Scholar]
- 91.Raadsheer FC, Hoogendijk WJ, Stam FC, Tilders FJ, Swaab DF. Increased numbers of corticotropin-releasing hormone expressing neurons in the hypothalamic paraventricular nucleus of depressed patients. Neuroendocrinology. 1994;60:436–444. doi: 10.1159/000126778. [DOI] [PubMed] [Google Scholar]
- 92.Raadsheer FC, van Heerikhuize JJ, Lucassen PJ, Tilders FJ, Swaab DF. Corticotropin-releasing hormone mRNA levels in the paraventricular nucleus patients with Alzheimer’s disease and depression. Am J Psychiatry. 1995;152:1372–1376. doi: 10.1176/ajp.152.9.1372. [DOI] [PubMed] [Google Scholar]
- 93.Bremner JD, Licinio J, Darnell A, Krystal JH, Owens MJ, Southwick SM, et al. Elevated CSF corticotropin-releasing factor concentrations in posttraumatic stress disorder. Am J Psychiatry. 1997;154:624–629. doi: 10.1176/ajp.154.5.624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Trickett PK, McBride-Chang C. The developmental impact of different forms of child abuse and neglect. Dev Rev. 1995;15:311–337. [Google Scholar]
- 95.Pihoker C, Owens MJ, Kuhn CM, Schanberg SM, Nemeroff CB. Maternal separation in neonatal rats elicits activation of the hypothalamic-pituitary-adrenocortical axis: a putative role for corticotropin-releasing factor. Psychoneuroendocrinology. 1993;18:485–493. doi: 10.1016/0306-4530(93)90042-j. [DOI] [PubMed] [Google Scholar]