Abstract
Objective
The adipocyte-secreted hormone adiponectin exerts important cardioprotective and anti-diabetic effects. Little is known about its effect on vascular smooth muscle cells (VSMC), key cells in restenosis, hypertension, and atherosclerosis.
Methods and Results
Using human coronary artery VSMC, we report that recombinant adiponectin in the HMW or trimeric, but not globular forms induces VSMC differentiation through a mechanism similar to the classic feedback signaling employed by rapamycin, a drug known to effectively inhibit restenosis on drug-eluting stents (DES). Using a combination of pharmacologic agents, siRNA, and overexpression approaches, we demonstrate that adiponectin activates 5′ AMP-activated protein kinase (AMPKα2), leading to inhibition of mammalian target of rapamycin complex 1 (mTORC1) and S6K1. This in turn stabilizes IRS-1, driving Akt2 -mediated inhibition of FoxO4 and subsequent contractile protein induction. While adiponectin and rapamycin have similarly beneficial effects on VSMC phenotype in both cell and organ culture, a direct comparison of the effects of rapamycin versus adiponectin on endothelial cells (EC) revealed distinct differences: rapamycin inhibited, while adiponectin maintained, Akt phosphorylation. Importantly, Akt activity preserves endothelial function.
Conclusions
Adiponectin promotes VSMC differentiation and preserves EC Akt signaling, suggesting that targeting the adiponectin pathway may have advantages over rapamycin in developing new DES therapeutics.
Keywords: Adiponectin, VSMC, differentiation, mTOR, rapamycin, AMPK, Akt2, FoxO4
Introduction
Restenosis is a frequent complication of vascular interventions including bypass grafts, angioplasty, and stenting. Endothelial injury and VSMC phenotypic modulation contribute to this intimal hyperplastic response1. Rapamycin-eluting stents dramatically reduce the incidence of coronary restenosis, but have recently been associated with late-stent thrombosis, a potentially fatal complication2,3. While the underlying mechanisms are still emerging, rapamycin inhibition of re-endothelialization may contribute to late-stent thrombosis4.
Recent studies revealed that low adiponectin levels can predict late in-stent restenosis5 and increase cardiovascular disease risk6. Adiponectin is a 30 kDa hormone produced by white adipose tissue in inverse proportion to fat mass7. Downstream signaling through its known receptors, AdipoR1, AdipoR28 and T-Cadherin9, remains poorly understood. Adiponectin mediates multiple cardioprotective effects, as adiponectin knockout mice exhibit increased neointimal formation and thrombus formation post-injury10,11, higher blood pressure12, and impaired recovery from hind-limb ischemia13. They also develop increased myocardial infarct (MI) size14, increased cardiac hypertrophy with pressure overload15, and exacerbated left ventricular dilation and dysfunction following MI16. Adiponectin also exerts anti-inflammatory and anti-diabetic effects17. While several studies have documented beneficial adiponectin signaling in endothelial cells13,18–20, few have assessed the effects of the hormone on VSMC.
VSMC in mature vessels retain remarkable phenotypic plasticity. VSMC dedifferentiation contributes to intimal hyperplasia, atherosclerosis and hypertension21. Normal mature VSMC are differentiated, quiescent and contractile, while injured VSMC exhibit a proliferative, dedifferentiated, synthetic phenotype. Differentiation markers include contractile proteins smooth muscle myosin heavy chain (SM-MHC), SM α-actin, calponin, and h-caldesmon. Dedifferentiated VSMC lose these markers and upregulate extracellular matrix synthesis21.
We previously showed that rapamycin promotes VSMC differentiation via inhibition of mTORC1 and its effector S6 kinase1(S6K1)22,23. Herein, we test the hypothesis that adiponectin promotes VSMC differentiation through AMPK-mediated inhibition of mTORC1. As late stent thrombosis may be associated with mTORC1 inhibition2,3, we also conduct a direct comparison of the effects of adiponectin versus rapamycin in human endothelial cells.
Materials and Methods
All cell culture experiments employ human coronary artery smooth muscle cells purchased from Cascade Biologics (Portland, OR). Transfections and western blot analysis were performed using standard methods as previously published22–24. Please see detailed Materials and Methods in supplemental data.
Results
Adiponectin induces VSMC differentiation
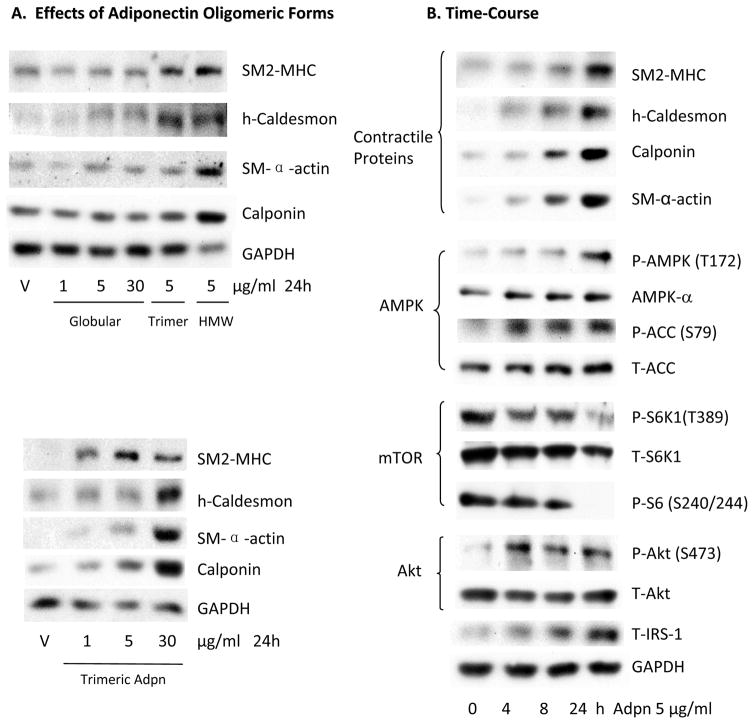
To determine the effects of recombinant human adiponectin on VSMC phenotypic modulation, we employed a human coronary artery VSMC culture model. These cells display a synthetic, proliferative phenotype similar to VSMC in intimal hyperplastic lesions. We have previously reported that rapamycin or prostacyclin analogs can induce VSMC differentiation in this system22–24. Adiponectin exists in multiple oligomeric forms, including trimeric, hexameric, high molecular weight (HMW), and truncated globular forms in vivo25. We examined the effects of different oligomeric preparations on VSMC differentiation. Treatment with a preparation of full length adiponectin enriched in HMW oligomers (12–18mer, ~360–540 kDa) or with trimeric adiponectin (~90 kDa) induced expression of contractile protein markers of VSMC differentiation, including SM-MHC (SM2 isoform), h-caldesmon, calponin, and SM α-actin (Figure 1A). Trimeric adiponectin was slightly less potent than the HMW preparation. Conversely, the truncated globular form did not efficiently induce contractile protein expression (Figure 1A). We next determined that the HMW-enriched adiponectin promotes VSMC differentiation at concentrations (1–10 μg/ml) (Supplemental Figure IA) within the physiologic range of serum adiponectin levels in healthy people (2 to 17 μg/ml)7. We therefore employed 5μg/ml HMW-enriched adiponectin in all subsequent experiments.
Figure 1.
Adiponectin induces VSMC differentiation marker expression and modulates the mTORC1 signaling pathway. A, Human coronary artery VSMC were treated with recombinant human globular, trimeric or HMW-enriched adiponectin preparations as indicated and tested for 24 h prior to western blotting analysis with the primary antibodies indicated. B, VSMC were treated with 5 μg/ml HMW-enriched adiponectin for the indicated time points and harvested for western analysis as indicated.
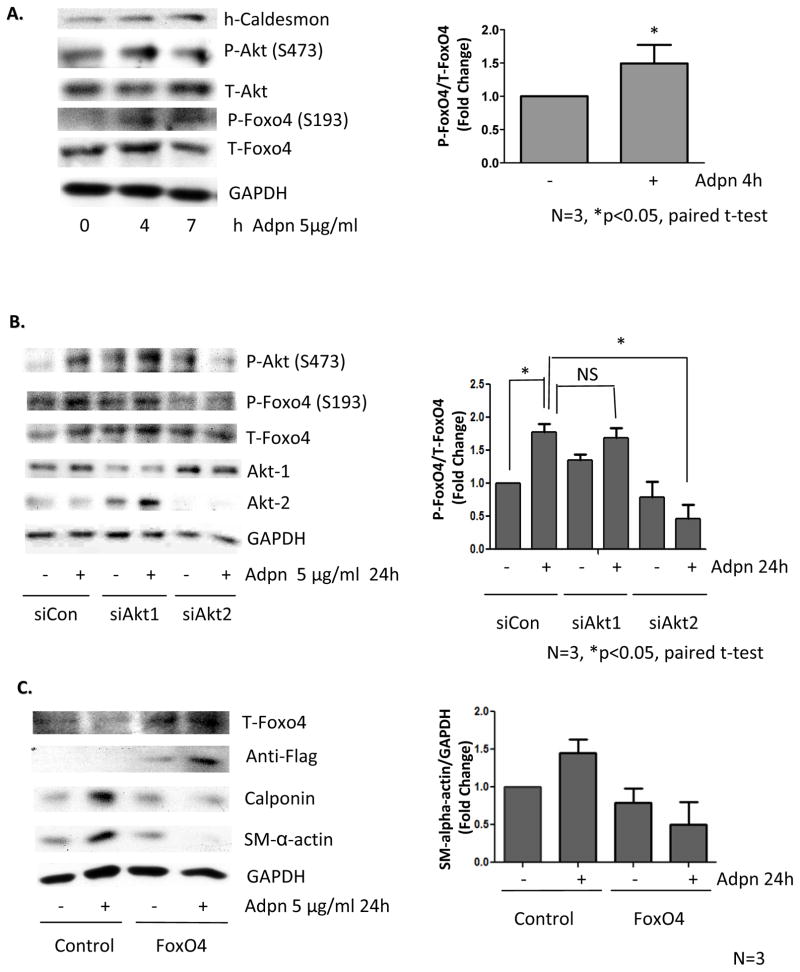
Treating VSMC with adiponectin induced VSMC-specific differentiation marker expression in a time-dependent manner (Figure 1B, Supplemental Figure IB). Adiponectin also activated AMPK over time, as measured by increased AMPK-α subunit phosphorylation (Thr172) and confirmed bythe increased phosphorylation of the AMPK substrate acetyl-CoA carboxylase (ACC) (Ser79) (Figure 1B). Adiponectin concomitantly inhibited mTORC1 signaling as evidenced by the reduced phosphorylation of the mTORC1 substrate S6K1 (Thr389), as well as reduced phosphorylation of the S6K1 substrate ribosomal S6 (Ser240/244) (Figure 1B). We have previously shown that rapamycin inhibition of mTORC1 induces VSMC differentiation through relief of the S6K1-mediated feedback inhibition of IRS-1, leading to Akt activation23. S6K1-mediated serine phosphorylation of IRS-1 promotes its ubiquitination and degradation, attenuating signal transduction to PI3K and Akt23. Like rapamycin, adiponectin also stabilized IRS-1 expression and induced Akt phosphorylation (Ser473) (Figure 1B).
AMPK is necessary for adiponectin-induced differentiation
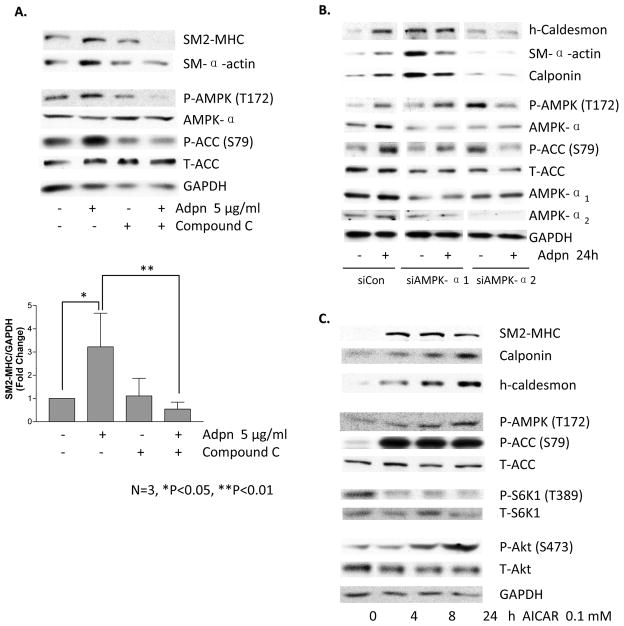
AMPK has been implicated as a key effector of adiponectin signaling in multiple other cell types18,25,26. To determine whether adiponectin induces VSMC differentiation via AMPK, we pretreated VSMC with compound C, a specific inhibitor of AMPK, prior to adiponectin treatment. Compound C inhibited adiponectin-induced AMPK and ACC phosphorylation, as well as contractile protein expression (Figure 2A, Supplemental Figure II). We confirmed this result using siRNA to knockdown the AMPK catalytic subunit isoforms α1 or α2. Notably, only AMPKα2 appears to be required for adiponectin-induced differentiation (Figure 2B). An increase in contractile proteins was noted upon AMPKα1 knockdown, suggesting it may potentially inhibit contractile protein expression. However, treating VSMC with AICAR (5-Aminoimidazole-4-carboxamide-1-b-riboside), a pharmacologic activator of AMPK (which is not known to exert an AMPKα isoform-specific effect), inhibited mTORC1, activated Akt and induced contractile protein expression in a time-dependent manner (Figure 2C). These data indicate that AMPKα2 activation is necessary for adiponectin-induced VSMC differentiation and that AMPK activation is sufficient to induce differentiation.
Figure 2.
Adiponectin-induced VSMC differentiation requires AMPK. A, VSMC were pretreated with compound C (20 μM) for 30 min, followed by treatment with 5 μg/ml adiponectin for 24h and western blotting with the indicated antibodies. Bar graphs represent densitometric quantification (mean fold induction plus standard error of the mean) of 3 separate experiments. p-values (Newman-Keuls multiple comparison post hoc tests) are indicated above the bars. B, VSMC were transfected with siControl, siAMPKα1 or siAMPKα2 for 24h, then treated with vehicle or 5 μg/ml adiponectin for 24h and analyzed by western blotting. Data are representative of two experiments. C. VSMC were treated with 0.1 mM AICAR for the indicated time points and harvested for western blotting analysis as above. Data are representative of 3 separate experiments.
Adiponectin induces differentiation via mTORC1/S6K1 inhibition
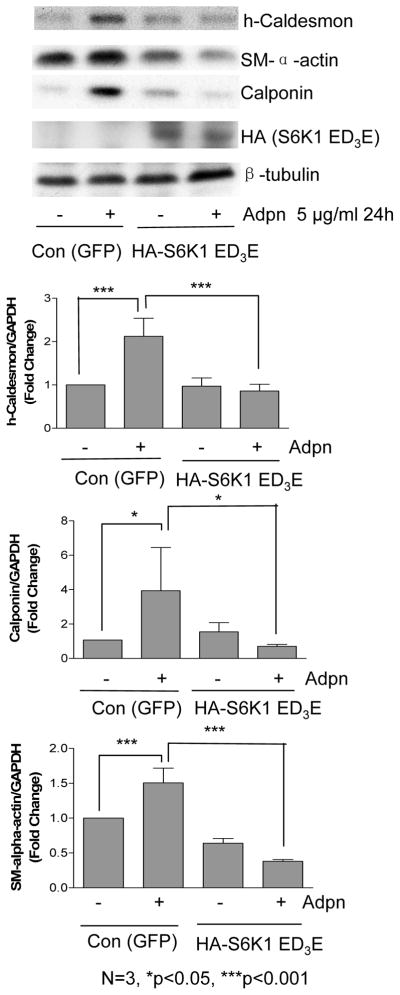
AMPK regulates cellular energy homeostasis by repressing energy-consuming processes, including protein synthesis, while simultaneously enhancing energy-producing processes27. Through phosphorylation of the TSC1/2 complex, AMPK inhibits mTORC1 activity and protein synthesis in skeletal muscle27. Since we previously reported that mTORC1 inhibition with rapamycin induces VSMC differentiation22,23, we next determined whether mTORC1 pathway inhibition is required for adiponectin-induced differentiation. We infected VSMC with an adenovirus encoding GFP alone (control) or GFP and an HA-tagged rapamycin-resistant constitutively active S6K1 mutant (S6K1-ED3E). This mutant is sufficient to block rapamycin-induced VSMC differentiation, IRS-1 stabilization, and Akt activation22. This mutant S6K1 also inhibited adiponectin-induced VSMC differentiation (Figure 3), suggesting that adiponectin inhibition of S6K1 is required for this response. Replicate experiments using S6K1 plasmid verified these findings (data not shown).
Figure 3.
Adiponectin induces VSMC differentiation via S6K1 inhibition. VSMC were infected with control (GFP) or rapamycin-resistant mutant S6K1 adenovirus overnight, and then treated with vehicle or 5 μg/ml adiponectin for 24h prior to western analysis. Bar graphs were prepared from 3 separate experiments as in Fig. 2A. p-values (Newman-Keuls multiple comparison post hoc tests) are indicated above the bars.
Akt-2 inhibition of FoxO4 is required for adiponectin-induced differentiation
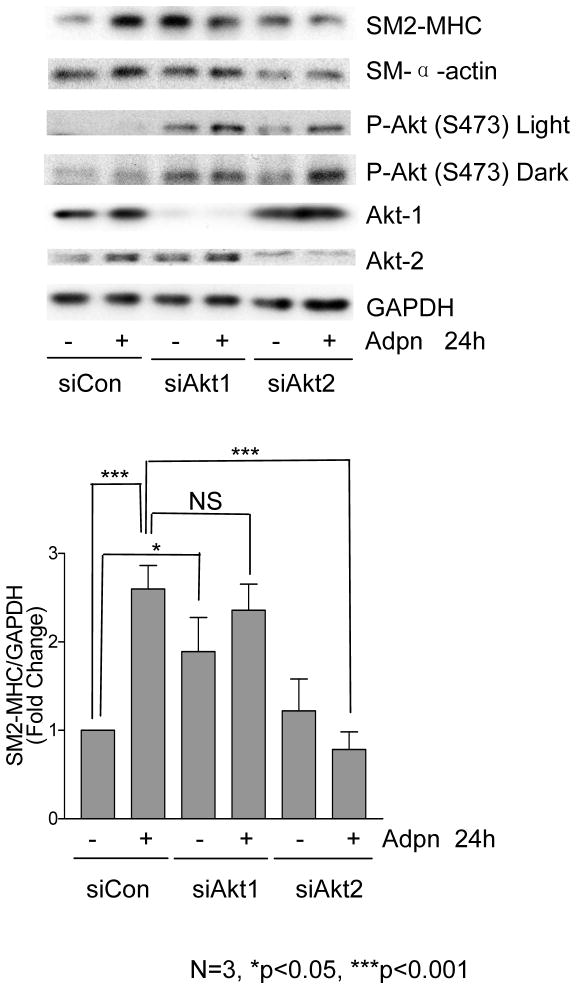
Increasing evidence reveals that Akt1 and Akt2 differentially regulate cell migration and metabolism28. We reported that rapamycin specifically activates Akt2, and that Akt2, but not Akt1, is required for rapamycin-induced VSMC differentiation23. Similarly, siRNA knockdown of Akt2, but not Akt1, inhibited adiponectin-induced contractile protein expression (Figure 4). Interestingly, adiponectin increased Akt phosphorylation (Ser473) whether Akt1 or Akt2 was knocked down, suggesting that, unlike rapamycin, adiponectin may activate both Akt isoforms (Figure 4). In other experiments, we were able to detect a modest but selective activation of Akt2 using an isoforms-specific immunoprecipitation approach (Supplemental Figure IIIA). We previously found that only Akt2 activity is sufficient to induce contractile protein expression23. Adiponectin may activate both Akt1 and Akt2, but it appears that the actions ofAkt2 predominate given the net adiponectin effect on differentiation. Our siRNA data suggest that Akt1 may inhibit contractile protein expression, as Akt1 knockdown increases baseline MHC expression (Figure 4). We report that overexpression of Akt1 does not inhibit basal levels of contractile proteins, but does inhibit their induction by adiponectin or rapamycin (Supplemental Figure IIIB). Finally, we note a compensatory increase in Akt2 expression and phosphorylated Akt2 when Akt1 is knocked down, suggesting another potential mechanism for opposing actions of these Akt isoforms (See Figures 4, 5B, and Supplemental Figure IIIC).
Figure 4.
Adiponectin induces differentiation via Akt2. VSMC were transfected with siControl, siAkt-1 or siAkt-2 for 24 h, then treated with vehicle or 5 μg/ml adiponectin for 24h prior to western analysis. Arepresentative experiment is shown. Bar graphs were prepared from 3 separate experiments as in Fig. 2A.
Figure 5.
Adiponectin induces FoxO4 phosphorylation via Akt2. A, VSMC were treated with 5 μg/ml adiponectin for the indicated time points and harvested for western analysis. B, VSMC were transfected, treated, and analyzed as in Fig. 4. C, VSMC were transfected with plasmid encoding GFP or Flag-Foxo4 for 24h, then treated with vehicle or 5 μg/ml adiponectin for 24h and harvested for western analysis. For all panels, bar graphs were prepared from 3 independent experiments.
FoxO proteins are known substrates of Akt. FoxO4, in particular, has been shown to associate with and inhibit the activity of myocardin, a critical transcriptional coactivator in VSMC differentiation29,30. Akt phosphorylation of FoxO4 inactivates this transcription factor by promoting its nuclear exclusion31. We report that FoxO4 is phosphorylated (Fig 5A) and translocated to the cytosol (Supplemental Fig IV) after adiponectin treatment in a time-dependent manner. Adiponectin-induced FoxO4 phosphorylation was also Akt2-dependent (Figure 5B). Notably, overexpression of FoxO4 prevented adiponectin-induced VSMC differentiation (Fig 5C).
Adiponectin and rapamycin similarly promote VSMC differentiation, but have distinct effects on endothelial cells
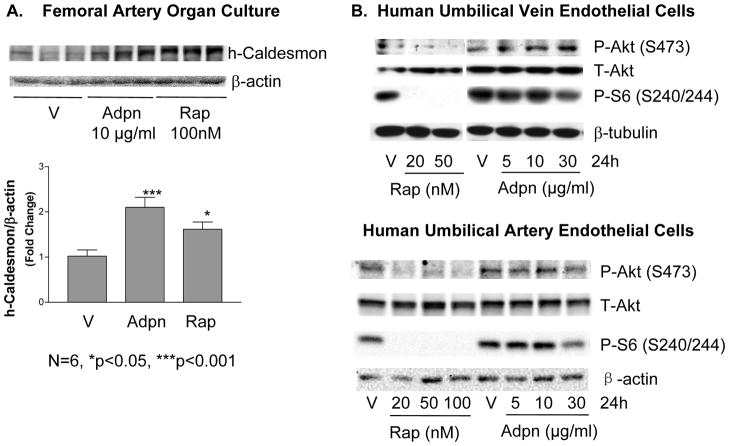
As both adiponectin and rapamycin promote VSMC differentiation via mTORC1 inhibition, we conducted experiments to compare their effects on VSMC and endothelial cells (EC) in parallel. Adiponectin and rapamycin induced SM2-MHC expression to a similar extent and with similar (although non-identical) kinetics in VSMC (Supplemental Figure VA). Notably, both adiponectin and rapamycin also prevented culture-induced contractile protein downregulation in intact vessel segments in an organ culture model (Figure 6A). We find that rapamycin also induces FoxO4 phosphorylation and translocation in a time- and dose-dependent manner (Supplemental Figures VB-C).
Figure 6.
Adiponectin and rapamycin have similar beneficial effects on vessel phenotype, but differ in adverse effects on endothelial cells. A, Freshly isolated pig femoral arteries were placed in organ culture for 3 days then treated with 100nM rapamycin or 10 μg/ml adiponectin for 4 days before harvesting for western analysis. Data from N=6 were quantitated with statistical analysis as in Fig. 2A. B, HUVEC (top panels) or HUAEC (bottom panels) were treated with the indicated concentrations of rapamycin and adiponectin for 24h and harvested for western analysis. HUAEC blot is representative of N=3.
Importantly, the effects of adiponectin and rapamycin differed when directly compared in human EC. Rapamycin significantly inhibited Akt phosphorylation and completely inhibited mTORC1 in human umbilical artery or vein endothelial cells (HUAEC or HUVEC). Notably, adiponectin did not inhibit Akt in HUAEC and slightly activated Akt in HUVEC. In both EC types, adiponectin only modestly inhibited mTORC1 (Figure 6B). Since Akt/mTOR signaling is cytoprotective and promotes EC survival32,33, the divergent effect of rapamycin and adiponectin in endothelial cells suggest that the adiponectin pathway may be a preferable route to promote VSMC differentiation while preventing EC injury.
Discussion
We report the novel findings that 1) HMW or trimeric adiponectin induces VSMC differentiation via mTORC1 inhibition; 2) Adiponectin transduces this signal via AMPKα2, and Akt2 suppression of FoxO4; 3) While adiponectin and rapamycin similarly promoteVSMC differentiation, only adiponectin promotes protective signaling in EC. Our study suggests that maintenance of VSMC phenotype may be an additional cardioprotective effect of endogenous adiponectin. In uncovering these roles of adiponectin, we have identified signaling mechanisms that underlie its actions.
Adiponectin induces VSMC differentiation via AMPK/mTORC1
We report that adiponectin promotes differentiation in human VSMC via AMPK-mediated mTORC1 inhibition (see model in Supplemental Figure VI). AMPK has emerged as a critical mediator of adiponectin’s beneficial effects on metabolism and insulin sensitivity in other tissues25, and on the stimulation of angiogenesis18. We found that AMPK activation with AICAR was sufficient to inhibit mTORC1 and induce VSMC differentiation. Notably, we believe we are the first to specifically implicate the AMPKα2 isoform as a key adiponectineffector. In contrast to other studies of adiponectin signaling in VSMC, ours is the first using multiple methods to manipulate endogenous levels of AMPK, as opposed to adenoviral overexpression34. Our siRNA data interestingly suggest that AMPKα1 may oppose contractile protein expression, as contractile proteins basally increase after AMPKα1 knockdown. AMPKα2 may be the predominant effector of adiponectin induced VSMC differentiation, and maytherefore be a potential therapeutic target for future stent drug development.
Of the two mTOR cellular complexes, only mTORC1 is inhibited by AMPK. mTORC1 and its substrate S6K1 participate in a well-documented feedback loop35 in which growth factor and nutrient signaling limits the activity of the PI3K/Akt pathway: S6K1 phosphorylates IRS-1 on serine residues that promote its ubiquitination and degradation. This pathway modulates cell growth and insulin sensitivity, and its dysregulation is linked to cancer, diabetes, and obesity27. We now report that adiponectin- and rapamycin23-induced VSMC differentiation similarly require S6K1 inhibition. Interestingly, adiponectin inhibits mTORC1 to a lesser degree than rapamycin in both VSMC and EC, perhaps because adiponectin inhibits mTORC1 indirectly via AMPK: AMPK phosphorylates TSC2, which, in turn, inhibits Rheb, a requisite mTORC1 activator. In contrast, rapamycin/FKBP12 directly binds and inhibits mTORC136. Our work suggests that adiponectin may provide an endogenous hormonal signal that limits mTORC1 activity in helping to maintain a healthy VSMC phenotype.
We find that adiponectin, like rapamycin, requires Akt2 activation to promote VSMC differentiation. We now report that FoxO4 is an Akt2 substrate and a critical effector of adiponectin in VSMC, as FoxO4 overexpression inhibits adiponectin-induced differentiation. As FoxO4 is known to interact with and inhibit the activity of myocardin29, and inhibits intimal hyperplasia30, this provides a likely mechanism for transcriptional regulation of contractile protein expression. Unlike rapamycin, our data suggest that adiponectin may also activate Akt1, but Akt1 does not appear to be required for regulation of FoxO4 or differentiation in this model. Our data suggest that Akt1 opposes contractile protein expression, and we will pursue the mechanism in future studies. It will be interesting to determine whether adiponectin might inhibit Akt3, as this isoform has been suggested to promote VSMC proliferation37. We also note potential feedback regulation between Akt isoforms, as Akt1 knockdown increases both total and phospho-Akt2. However, as this Akt2 upregulation did not significantly increase FoxO4 phosphorylation, we hypothesize that there may be Akt2 substrates in addition to FoxO4 that also contribute to contractile protein regulation.
Although adiponectin was previously shown to inhibit VSMC proliferation and migration by directly binding platelet-derived growth factor-BB and inhibiting growth factor-stimulated ERK signaling38, our observations in low serum conditions support a role for intracellular signaling mechanisms in adiponectin-induced differentiation. We found that adiponectin also efficientlyinduces VSMC differentiation in 10% serum conditions in cell (data not shown) and organ culture. Adiponectin is also known to inhibit VSMC and EC apoptosis via AMPK19,39. In vivo, it is likely that adiponectin exerts pleiotropic effects on VSMC as well as EC that contribute to its anti-restenotic effects10,11,34.
Divergent effects of adiponectin in VSMC and EC
Despite the success of rapamycin in combating coronary artery restenosis, recent concerns have been raised regarding sudden death from late stent thrombosis1,3,40. The cause of the late stent thrombosis is not yet clearly understood, but is likely mediated by incomplete re-endothelialization40, and diabetes confers a greater risk2. Rapamycin and other stent drugs have been shown to inhibit re-endothelialization1,2,4, which may contribute to this pathogenesis. A fundamental understanding of rapamycin’s actions on both VSMC and endothelial cells will be required to address this problem.
An ideal stent agent would inhibit VSMC phenotypic modulation while promoting re-endothelialization. We report that the natural hormone adiponectin may fulfill this dual role. Our data also demonstrate in a careful direct comparison that rapamycin inhibits Akt/mTORC1 in human EC, while adiponectin preserves Akt/mTORC1 activity. Adiponectin-induced Akt signaling has been shown to be pro-angiogenic in EC18. Akt promotes eNOS phosphorylation and activity, which is essential for EC migration, and has anti-apoptotic effects in EC13,18. Adiponectin additionallybenefits endothelial function bypromoting EC migration18, inhibiting EC apoptosis19 and inflammatory activation41, inducing endothelial nitric oxide (NO) production20 and decreasing reactive oxygen species production42, suggesting a global EC protective function. Moreover, adiponectin inhibits thrombus formation11, while rapamycin promotes platelet aggregation43. We found that, in contrast to rapamycin, adiponectin only modestly inhibited mTORC1 activity in EC. Notably, mTORC1 inhibition is anti-angiogenic and inhibits EC migration44. The ability of adiponectin to mimic the beneficial effects of rapamycin in VSMC while sparing cytoprotective Akt/mTORC1 signaling in EC suggests that the adiponectin pathway may be a desirable alternative for future DES therapeutic development. As a whole, the adiponectin pathway presents exceptional potential for future anti-restenotic therapy through its unique combination of anti-inflammatory effects, local anti-diabetic properties, and overall promotion of arterial health.
The reasons for the opposing effects of rapamycin on Akt in VSMC versus EC are not yet known, but indirect inhibition of mTORC2 suggests a potential mechanism (see Supplemental Figure VIB). When the mTOR protein resides in mTORcomplex1 with raptor and other accessory proteins, this complexis directly sensitive to rapamycin and regulates processes including protein synthesis. A more recently identified complex, mTORC2, containing mTOR, rictor and other proteins, is required for the phosphorylation of Akt at Ser473, as well as of other kinases including SGK and PKCα36. The kinase activity of mTORC2 is itself insensitive to rapamycin, but mTORC2 assembly can be inhibited due to sequestration of newly synthesized mTOR protein in mTORC1/rapamycin-inhibited complexes. This “chronic” rapamycin inhibition of mTORC2 shows dramatic cell type-specificity and has been observed in EC45. Given the long half-life of rapamycin, this effect can be substantial in some cell types, and could potentially underlie rapamycin inhibition of Akt in EC. Adiponectin does not inhibit mTORC1 activity to the complete extent that rapamycin does in VSMC or EC. Furthermore, rapamycin inhibits mTORC1 by directlybinding the complex, while adiponectin inhibition mediated by AMPK occurs through a distcint, TSC-mediated mechanism. It is therefore likely that adiponectin inhibition of mTORC1 does not result in inhibition of mTORC2. Furthermore, we propose that adiponectin may act through as yet undefined signals to activate Akt in EC. Finally, while active in VSMC, the IGF/Insulin-dependent feedback activation of Akt by rapamycin does not occur in all cell types, perhaps due to cell-type specific patterns of phosphorylation and expression of IRS family proteins.
Remaining challenges
The physical properties of adiponectin, including its short half-life, rapid turnover, and multiple oligomeric forms25, limit its current utility as a DES agent. Understanding the adiponectin-activated pathways in human cells that drive stent pathology is critical for identification of novel targets for development of improved therapeutics. Our data provide important signaling insights as well as identifying the HMW and trimeric, but not globular forms of adiponectin as potent inducers of VSMC differentiation. This is in contrast to skeletal muscle, where globular adiponectin enhanced insulin sensitivity26. Interestingly, Kobayashi et al noted that only the HMW form activated AMPK and inhibited apoptosis in EC, and that it was primarily this HMW form that increased upon weight loss in obese patients19. Whether there are specific receptors and functions for each oligomeric form of adiponectin remains a subject of intense investigation. A small molecule adiponectin receptor agonist may prove to be an ideal agent for DES.
In summary, we report that adiponectin, through AMPK inhibition of mTORC1 and feedback activation of Akt2 and FoxO4 inhibition, promotes VSMC differentiation, in a manner similar to the stent drug rapamycin. In contrast to rapamycin, adiponectin preserves endothelial cell Akt/mTORC1 activity. This work suggests that adiponectin pathway-based therapeutics may have the potential to avoid pro-thrombotic adverse effects associated with rapamycin while maintaining anti-restenotic efficacy.
Supplementary Material
Acknowledgments
a) We thank Drs. Kenneth Walsh, Noriyuki Ouchi, Lee Witters, and Kristina Fetalvero for reagents and helpful discussion. b) This work was supported by NIH NHLBI (HL091013 to KAM) and FAMRI Clinical Innovator Award to KAM.
Footnotes
The authors report no conflicts to disclose.
References
- 1.Inoue T, Node K. Molecular basis of restenosis and novel issues of drug-eluting stents. Circ J. 2009;73:615–621. doi: 10.1253/circj.cj-09-0059. [DOI] [PubMed] [Google Scholar]
- 2.Agostoni P, Van den BF. Drug eluting stents in patients with diabetes. BMJ. 2008;337:a1359. doi: 10.1136/bmj.a1359. [DOI] [PubMed] [Google Scholar]
- 3.Maisel WH. Unanswered questions--drug-eluting stents and the risk of late thrombosis. N Engl J Med. 2007;356:981–984. doi: 10.1056/NEJMp068305. [DOI] [PubMed] [Google Scholar]
- 4.Joner M, Nakazawa G, Finn AV, Quee SC, Coleman L, Acampado E, Wilson PS, Skorija K, Cheng Q, Xu X, Gold HK, Kolodgie FD, Virmani R. Endothelial cell recovery between comparator polymer-based drug-eluting stents. J Am Coll Cardiol. 2008;52:333–342. doi: 10.1016/j.jacc.2008.04.030. [DOI] [PubMed] [Google Scholar]
- 5.Kitta Y, Takano H, Nakamura T, Kodama Y, Umetani K, Fujioka D, Saito Y, Kawabata K, Obata JE, Mende A, Kobayashi T, Kugiyama K. Low adiponectin levels predict late in-stent restenosis after bare metal stenting in native coronary arteries. Int J Cardiol. 2008;131:78–82. doi: 10.1016/j.ijcard.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 6.Han SH, Sakuma I, Shin EK, Koh KK. Antiatherosclerotic and anti-insulin resistance effects of adiponectin: basic and clinical studies. Prog Cardiovasc Dis. 2009;52:126–140. doi: 10.1016/j.pcad.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 7.Arita Y, Kihara S, Ouchi N, Takahashi M, Maeda K, Miyagawa J, Hotta K, Shimomura I, Nakamura T, Miyaoka K, Kuriyama H, Nishida M, Yamashita S, Okubo K, Matsubara K, Muraguchi M, Ohmoto Y, Funahashi T, Matsuzawa Y. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem Biophys Res Commun. 1999;257:79–83. doi: 10.1006/bbrc.1999.0255. [DOI] [PubMed] [Google Scholar]
- 8.Yamauchi T, Kamon J, Ito Y, Tsuchida A, Yokomizo T, Kita S, Sugiyama T, Miyagishi M, Hara K, Tsunoda M, Murakami K, Ohteki T, Uchida S, Takekawa S, Waki H, Tsuno NH, Shibata Y, Terauchi Y, Froguel P, Tobe K, Koyasu S, Taira K, Kitamura T, Shimizu T, Nagai R, Kadowaki T. Cloning of adiponectin receptors that mediate antidiabetic metabolic effects. Nature. 2003;423:762–769. doi: 10.1038/nature01705. [DOI] [PubMed] [Google Scholar]
- 9.Hug C, Wang J, Ahmad NS, Bogan JS, Tsao TS, Lodish HF. T-cadherin is a receptor for hexameric and high-molecular-weight forms of Acrp30/adiponectin. Proc Natl Acad Sci U S A. 2004;101:10308–10313. doi: 10.1073/pnas.0403382101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Matsuda M, Shimomura I, Sata M, Arita Y, Nishida M, Maeda N, Kumada M, Okamoto Y, Nagaretani H, Nishizawa H, Kishida K, Komuro R, Ouchi N, Kihara S, Nagai R, Funahashi T, Matsuzawa Y. Role of adiponectin in preventing vascular stenosis. The missing link of adipo-vascular axis. J Biol Chem. 2002;277:37487–37491. doi: 10.1074/jbc.M206083200. [DOI] [PubMed] [Google Scholar]
- 11.Kato H, Kashiwagi H, Shiraga M, Tadokoro S, Kamae T, Ujiie H, Honda S, Miyata S, Ijiri Y, Yamamoto J, Maeda N, Funahashi T, Kurata Y, Shimomura I, Tomiyama Y, Kanakura Y. Adiponectin acts as an endogenous antithrombotic factor. Arterioscler Thromb Vasc Biol. 2006;26:224–230. doi: 10.1161/01.ATV.0000194076.84568.81. [DOI] [PubMed] [Google Scholar]
- 12.Ohashi K, Kihara S, Ouchi N, Kumada M, Fujita K, Hiuge A, Hibuse T, Ryo M, Nishizawa H, Maeda N, Maeda K, Shibata R, Walsh K, Funahashi T, Shimomura I. Adiponectin replenishment ameliorates obesity-related hypertension. Hypertension. 2006;47:1108–1116. doi: 10.1161/01.HYP.0000222368.43759.a1. [DOI] [PubMed] [Google Scholar]
- 13.Shibata R, Ouchi N, Kihara S, Sato K, Funahashi T, Walsh K. Adiponectin stimulates angiogenesis in response to tissue ischemia through stimulation of AMP-activated protein kinase signaling. J Biol Chem. 2004;279:28670–28674. doi: 10.1074/jbc.M402558200. [DOI] [PubMed] [Google Scholar]
- 14.Shibata R, Sato K, Pimentel DR, Takemura Y, Kihara S, Ohashi K, Funahashi T, Ouchi N, Walsh K. Adiponectin protects against myocardial ischemia-reperfusion injury through. Nat Med. 2005;11:1096–1103. doi: 10.1038/nm1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shibata R, Ouchi N, Ito M, Kihara S, Shiojima I, Pimentel DR, Kumada M, Sato K, Schiekofer S, Ohashi K, Funahashi T, Colucci WS, Walsh K. Adiponectin-mediated modulation of hypertrophic signals in the heart. Nat Med. 2004;10:1384–1389. doi: 10.1038/nm1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shibata R, Izumiya Y, Sato K, Papanicolaou K, Kihara S, Colucci WS, Sam F, Ouchi N, Walsh K. Adiponectin protects against the development of systolic dysfunction following myocardial infarction. J Mol Cell Cardiol. 2007;42:1065–1074. doi: 10.1016/j.yjmcc.2007.03.808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ouchi N, Walsh K. Adiponectin as an anti-inflammatory factor. Clin Chim Acta. 2007;380:24–30. doi: 10.1016/j.cca.2007.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ouchi N, Kobayashi H, Kihara S, Kumada M, Sato K, Inoue T, Funahashi T, Walsh K. Adiponectin stimulates angiogenesis by promoting cross-talk between AMP-activated protein kinase and Akt signaling in endothelial cells. J Biol Chem. 2004;279:1304–1309. doi: 10.1074/jbc.M310389200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kobayashi H, Ouchi N, Kihara S, Walsh K, Kumada M, Abe Y, Funahashi T, Matsuzawa Y. Selective suppression of endothelial cell apoptosis by the high molecular weight form of adiponectin. Circ Res. 2004;94:e27–e31. doi: 10.1161/01.RES.0000119921.86460.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kondo M, Shibata R, Miura R, Shimano M, Kondo K, Li P, Ohashi T, Kihara S, Maeda N, Walsh K, Ouchi N, Murohara T. Caloric restriction stimulates revascularization in response to ischemia via adiponectin-mediated activation of endothelial nitric-oxide synthase. J Biol Chem. 2009;284:1718–1724. doi: 10.1074/jbc.M805301200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Owens GK, Kumar MS, Wamhoff BR. Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiol Rev. 2004;84:767–801. doi: 10.1152/physrev.00041.2003. [DOI] [PubMed] [Google Scholar]
- 22.Martin KA, Rzucidlo EM, Merenick BL, Fingar DC, Brown DJ, Wagner RJ, Powell RJ. The mTOR/p70 S6K1 pathway regulates vascular smooth muscle cell differentiation. Am J Physiol Cell Physiol. 2004;286:C507–C517. doi: 10.1152/ajpcell.00201.2003. [DOI] [PubMed] [Google Scholar]
- 23.Martin KA, Merenick BL, Ding M, Fetalvero KM, Rzucidlo EM, Kozul CD, Brown DJ, Chiu HY, Shyu M, Drapeau BL, Wagner RJ, Powell RJ. Rapamycin promotes vascular smooth muscle cell differentiation through insulin receptor substrate-1/phosphatidylinositol 3-kinase/Akt2 feedback signaling. J Biol Chem. 2007;282:36112–36120. doi: 10.1074/jbc.M703914200. [DOI] [PubMed] [Google Scholar]
- 24.Fetalvero KM, Shyu M, Nomikos AP, Chiu YF, Wagner RJ, Powell RJ, Hwa J, Martin KA. The prostacyclin receptor induces human vascular smooth muscle cell differentiation via the protein kinase A pathway. Am J Physiol Heart Circ Physiol. 2006;290:H1337–H1346. doi: 10.1152/ajpheart.00936.2005. [DOI] [PubMed] [Google Scholar]
- 25.Shetty S, Kusminski CM, Scherer PE. Adiponectin in health and disease: evaluation of adiponectin-targeted drug development strategies. Trends Pharmacol Sci. 2009;30:234–239. doi: 10.1016/j.tips.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 26.Tomas E, Tsao TS, Saha AK, Murrey HE, Zhang CC, Itani SI, Lodish HF, Ruderman NB. Enhanced muscle fat oxidation and glucose transport by ACRP30 globular domain: acetyl-CoA carboxylase inhibition and AMP-activated protein kinase activation. Proc Natl Acad Sci U S A. 2002;99:16309–16313. doi: 10.1073/pnas.222657499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shaw RJ. LKB1 and AMP-activated protein kinase control of mTOR signalling and growth. Acta Physiol (Oxf) 2009;196:65–80. doi: 10.1111/j.1748-1716.2009.01972.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gonzalez E, McGraw TE. The Akt kinases: isoform specificity in metabolism and cancer. Cell Cycle. 2009;8:2502–2508. doi: 10.4161/cc.8.16.9335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu ZP, Wang Z, Yanagisawa H, Olson EN. Phenotypic modulation of smooth muscle cells through interaction of Foxo4 and myocardin. Dev Cell. 2005;9:261–270. doi: 10.1016/j.devcel.2005.05.017. [DOI] [PubMed] [Google Scholar]
- 30.Li H, Liang J, Castrillon DH, DePinho RA, Olson EN, Liu ZP. FoxO4 regulates tumor necrosis factor alpha-directed smooth muscle cell migration by activating matrix metalloproteinase 9 gene transcription. Mol Cell Biol. 2007;27:2676–2686. doi: 10.1128/MCB.01748-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Takaishi H, Konishi H, Matsuzaki H, Ono Y, Shirai Y, Saito N, Kitamura T, Ogawa W, Kasuga M, Kikkawa U, Nishizuka Y. Regulation of nuclear translocation of forkhead transcription factor AFX by protein kinase B. Proc Natl Acad Sci U S A. 1999;96:11836–11841. doi: 10.1073/pnas.96.21.11836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fujio Y, Walsh K. Akt mediates cytoprotection of endothelial cells by vascular endothelial growth factor in an anchorage-dependent manner. J Biol Chem. 1999;274:16349–16354. doi: 10.1074/jbc.274.23.16349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kureishi Y, Luo Z, Shiojima I, Bialik A, Fulton D, Lefer DJ, Sessa WC, Walsh K. The HMG-CoA reductase inhibitor simvastatin activates the protein kinase Akt and promotes angiogenesis in normocholesterolemic animals. Nat Med. 2000;6:1004–1010. doi: 10.1038/79510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Takaoka M, Nagata D, Kihara S, Shimomura I, Kimura Y, Tabata Y, Saito Y, Nagai R, Sata M. Periadventitial adipose tissue plays a critical role in vascular remodeling. Circ Res. 2009;105:906–911. doi: 10.1161/CIRCRESAHA.109.199653. [DOI] [PubMed] [Google Scholar]
- 35.Harrington LS, Findlay GM, Lamb RF. Restraining PI3K: mTOR signalling goes back to the membrane. Trends Biochem Sci. 2005;30:35–42. doi: 10.1016/j.tibs.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 36.Ma XM, Blenis J. Molecular mechanisms of mTOR-mediated translational control. Nat Rev Mol Cell Biol. 2009;10:307–318. doi: 10.1038/nrm2672. [DOI] [PubMed] [Google Scholar]
- 37.Sandirasegarane L, Kester M. Enhanced stimulation of Akt-3/protein kinase B-gamma in human aortic smooth muscle cells. Biochem Biophys Res Commun. 2001;283:158–163. doi: 10.1006/bbrc.2001.4739. [DOI] [PubMed] [Google Scholar]
- 38.Arita Y, Kihara S, Ouchi N, Maeda K, Kuriyama H, Okamoto Y, Kumada M, Hotta K, Nishida M, Takahashi M, Nakamura T, Shimomura I, Muraguchi M, Ohmoto Y, Funahashi T, Matsuzawa Y. Adipocyte-derived plasma protein adiponectin acts as a platelet-derived growth factor-BB-binding protein and regulates growth factor-induced common postreceptor signal in vascular smooth muscle cell. Circulation. 2002;105:2893–2898. doi: 10.1161/01.cir.0000018622.84402.ff. [DOI] [PubMed] [Google Scholar]
- 39.Son BK, Akishita M, Iijima K, Kozaki K, Maemura K, Eto M, Ouchi Y. Adiponectin antagonizes stimulatory effect of tumor necrosis factor-alpha on vascular smooth muscle cell calcification: regulation of growth arrest-specific gene 6-mediated survival pathwayby adenosine 5′-monophosphate-activated protein kinase. Endocrinology. 2008;149:1646–1653. doi: 10.1210/en.2007-1021. [DOI] [PubMed] [Google Scholar]
- 40.Finn AV, Joner M, Nakazawa G, Kolodgie F, Newell J, John MC, Gold HK, Virmani R. Pathological correlates of late drug-eluting stent thrombosis: strut coverage as a marker of endothelialization. Circulation. 2007;115:2435–2441. doi: 10.1161/CIRCULATIONAHA.107.693739. [DOI] [PubMed] [Google Scholar]
- 41.Kobashi C, Urakaze M, Kishida M, Kibayashi E, Kobayashi H, Kihara S, Funahashi T, Takata M, Temaru R, Sato A, Yamazaki K, Nakamura N, Kobayashi M. Adiponectin inhibits endothelial synthesis of interleukin-8. Circ Res. 2005;97:1245–1252. doi: 10.1161/01.RES.0000194328.57164.36. [DOI] [PubMed] [Google Scholar]
- 42.Ouedraogo R, Wu X, Xu SQ, Fuchsel L, Motoshima H, Mahadev K, Hough K, Scalia R, Goldstein BJ. Adiponectin suppression of high-glucose-induced reactive oxygen species in vascular endothelial cells: evidence for involvement of a cAMP signaling pathway. Diabetes. 2006;55:1840–1846. doi: 10.2337/db05-1174. [DOI] [PubMed] [Google Scholar]
- 43.Chatterjee S, Pandey A. Drug eluting stents: friend or foe? A review of cellular mechanisms behind the effects of Paclitaxel and sirolimus eluting stents. Curr Drug Metab. 2008;9:554–566. doi: 10.2174/138920008784892119. [DOI] [PubMed] [Google Scholar]
- 44.Guba M, von Breitenbuch P, Steinbauer M, Koehl G, Flegel S, Hornung M, Bruns CJ, Zuelke C, Farkas S, Anthuber M, Jauch KW, Geissler EK. Rapamycin inhibits primary and metastatic tumor growth by antiangiogenesis: involvement of vascular endothelial growth factor. Nat Med. 2002;8:128–135. doi: 10.1038/nm0202-128. [DOI] [PubMed] [Google Scholar]
- 45.Sarbassov DD, Ali SM, Sengupta S, Sheen JH, Hsu PP, Bagley AF, Markhard AL, Sabatini DM. Prolonged rapamycin treatment inhibits mTORC2 assembly and Akt/PKB. Mol Cell. 2006;22:159–168. doi: 10.1016/j.molcel.2006.03.029. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.