Abstract
Extrapulmonary poorly differentiated neuroendocrine carcinomas can originate in the gastrointestinal tract, bladder, cervix, and prostate. These high-grade malignancies are characterized by aggressive histological features (high mitotic rate, extensive necrosis, and nuclear atypia) and a poor clinical prognosis. They are infrequently associated with secretory hormonal syndromes (such as the carcinoid syndrome) and rarely express somatostatin receptors.
Most poorly differentiated neuroendocrine carcinomas are locally advanced or metastatic at presentation. First-line systemic chemotherapy with a platinum agent (cisplatin or carboplatin) and etoposide is recommended for most patients with metastatic-stage disease; however, response durations are often short. Sequential or concurrent chemoradiation is recommended for patients with loco-regional disease. In patients with localized tumors undergoing surgical resection, adjuvant treatment (chemotherapy with or without radiation) is warranted in most cases.
Keywords: neuroendocrine carcinomas, neuroendocrine tumors, poorly differentiated, high grade
The terms poorly differentiated and high-grade neuroendocrine carcinoma (NECA) are used synonymously and encompass a variety of histological entities including small-cell carcinoma of the lung, large-cell high-grade NECA of the lung, extrapulmonary small-cell carcinoma, extrapulmonary large-cell NECA, and high-grade NECA with mixed features (combining large- and small-cell features).1 In addition to markers of neuroendocrine differentiation, poorly differentiated (PD) NECAs are characterized by a high mitotic rate (defined as >10 mitotic figures per 10 high-power fields in the lung and variously as >10 or 20 mitoses per 10 high-power fields in the gastrointestinal (GI) tract and other extrapulmonary sites) and extensive necrosis (Fig. 1).2–4 In fact, most carcinomas in this family exhibit substantially more mitoses than these thresholds, typically in the range of 40 to 70 mitoses per 10 high-power fields. If performed, the Ki-67 proliferation index is high (>20% by definition and usually 50%–90%). Up to 40% of PD NECAs contain elements of non-NECAs, usually adenocarcinomas or squamous cell carcinomas. When both components constitute more than 30% of the neoplasm, the diagnosis is “combined NECA,” with the morphological characteristics of both components to be specified. Although this review focuses on the management of extrapulmonary PD NECAs, many of the recommendations are extrapolated from research on small-cell lung cancer.
FIGURE 1.
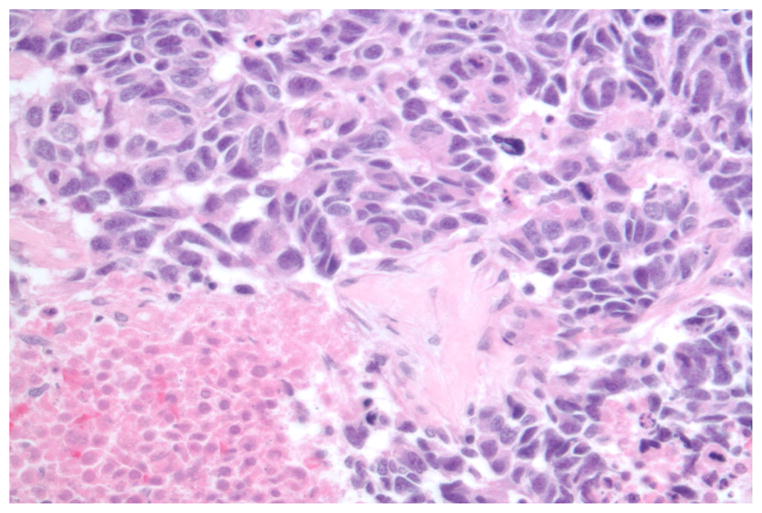
Poorly differentiated neuroendocrine carcinoma exhibiting high mitotic rate, necrosis, and pleomorphism. Photograph courtesy of Dr. Domenico Coppola, H. Lee Moffitt Cancer Center.
EPIDEMIOLOGY
Extrapulmonary PD NECAs are exceedingly rare. They can originate anywhere in the GI tract, with small-cell histological preponderance in the squamous GI tract (esophagus and anus).1 In the jejunum and ileum, PD NECAs represent only 1% of neuroendocrine tumors.5 Extrapulmonary small-cell carcinomas also occur in the bladder (0.3%–1% of all cases), cervix (1% of all cases), and prostate (2% of all cases).6 Prognosis is poor, with median survival durations in patients with localized, regional, and distant disease of 34, 14, and 5 months, respectively.7
IMAGING
Owing to the relative paucity of somatostatin receptor expression in PD NECAs, somatostatin-receptor scintigraphy (Octreoscan) is rarely useful as an imaging modality. Computed tomographic scans, magnetic resonance imaging scans, or [18F]-fluorodeoxyglucose positron emission tomographic scans may be used for baseline staging and for monitoring response to treatment.
MANAGEMENT OF METASTATIC (EXTENSIVE) DISEASE
Based on the established role of cisplatin and etoposide in metastatic small-cell lung cancer, this combination has been investigated in metastatic NECAs of the GI tract. The first such study explored infusional cisplatin and etoposide among 45 patients with metastatic NECAs, of whom 18 had PD tumors. A response rate of 67% was reported in patients with PD NECAs, with response duration of 8 months and a median survival of 19 months.8 A subsequent study of 53 patients with PD NECAs of the GI tract treated with a bolus regimen of cisplatin and etoposide reported a response rate of 42% with response duration of 9 months and a median survival of 15 months.9 Based on these studies, the combination of cisplatin and etoposide is recommended as first-line therapy for metastatic PD NECAs. Alternative regimens substituting carboplatin for cisplatin10 or irinotecan for etoposide11 have been validated in metastatic small-cell lung cancer and are therefore thought to be acceptable options for management of extrapulmonary PD NECAs. The optimal duration of chemotherapy has not been clearly defined, and it remains unclear whether treatment beyond 4 cycles is associated with a survival benefit.
There are no studies of salvage chemotherapy in extra-pulmonary PD NECAs. Second-line chemotherapy regimens in refractory or relapsed small-cell lung cancer are typically associated with modest response rates of 0% to 20%. One study of oral topotecan versus supportive care in relapsed small-cell lung cancer demonstrated a 3-month improvement in median survival.12 Based on this data, topotecan can be recommended as a salvage option for relapsed PD NECAs. Alternatively, retreatment with a platinum and etoposide or irinotecan regimen can be considered in patients who relapse more than 3 to 6 months after termination of first-line chemotherapy. Other agents with similar reported activity in small-cell lung cancer include paclitaxel, docetaxel, vinorelbine, and gemcitabine.6
MANAGEMENT OF LOCO-REGIONAL DISEASE (LIMITED DISEASE)
Poorly differentiated NECAs are characterized by a high proclivity for metastatic dissemination even in patients with clinically localized tumors. This principle is validated by retrospective studies confirming that surgery alone is rarely curative.13,14 Based on the treatment paradigm for limited-stage small-cell lung cancer, a course of definitive chemotherapy (cisplatin or carboplatin and etoposide for 4–6 cycles) and radiation can be considered in many patients with loco-regional extrapulmonary PD NECAs, particularly when surgical resection is difficult. Clinical trials in small-cell lung cancer suggest that concurrent chemoradiation is more efficacious than sequential treatment but at the expense of increased toxicity.15 The optimal sequencing of chemotherapy with radiation in extrapulmonary PD NECA is unknown. Likewise, the benefit of surgery among patients who have completed a course of chemoradiation is uncertain.
Whereas there are no studies examining adjuvant postoperative treatment in PD NECAs, their aggressive behavior warrants consideration of adjuvant therapy in most cases. Chemotherapy (4–6 cycles of cisplatin or carboplatin and etoposide) is recommended. Sequential radiation can also be considered in cases where the risk of local recurrence is thought to be higher than average (eg, carcinomas of the rectum or cervix).
The incidence of brain micrometastases in small-cell lung cancer is high, necessitating prophylactic cranial irradiation for patients with successfully treated limited-stage disease.16 Data on extrapulmonary PD NECAs suggest a lower frequency of central nervous system metastases.17 Consequently, routine prophylactic cranial irradiation cannot be recommended in this population but may be considered among patients with PD NECAs of the head and neck or unknown primary site.
References
- 1.Shia J, Tang LH, Weiser MR, et al. Is nonsmall cell type high-grade neuroendocrine carcinoma of the tubular gastrointestinal tract a distinct disease entity? Am J Surg Pathol. 2008;32(5):719–731. doi: 10.1097/PAS.0b013e318159371c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kloppel G, Heitz PU, Capella C, et al. Pathology and nomenclature of human gastrointestinal neuroendocrine (carcinoid) tumors and related lesions. World J Surg. 1996;20(2):132–141. doi: 10.1007/s002689900021. [DOI] [PubMed] [Google Scholar]
- 3.Capella C, Heitz PU, Hofler H, et al. Revised classification of neuroendocrine tumours of the lung, pancreas and gut. Virchows Arch. 1995;425(6):547–560. doi: 10.1007/BF00199342. [DOI] [PubMed] [Google Scholar]
- 4.Strosberg J, Nasir A, Coppola D, et al. Correlation between grade and prognosis in metastatic gastroenteropancreatic neuroendocrine tumors. Hum Pathol. 2009;40(9):1262–1268. doi: 10.1016/j.humpath.2009.01.010. [DOI] [PubMed] [Google Scholar]
- 5.Surveillance, Epidemiology, and End Results (SEER) Program (www.seer.cancer.gov) SEER*Stat Database: SEER 17 Regs Nov 2006 sub (1973–2004), (ed released April 2006), National Cancer Institute, DCCPS, Surveillance Research Program, Cancer Statistic Branch. 2006.
- 6.Walenkamp AM, Sonke GS, Sleijfer DT. Clinical and therapeutic aspects of extrapulmonary small cell carcinoma. Cancer Treat Rev. 2009;35(3):228–236. doi: 10.1016/j.ctrv.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 7.Yao JC, Hassan M, Phan A, et al. One hundred years after “carcinoid”: epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol. 2008;26(18):3063–3072. doi: 10.1200/JCO.2007.15.4377. [DOI] [PubMed] [Google Scholar]
- 8.Moertel CG, Kvols LK, O’Connell MJ, et al. Treatment of neuroendocrine carcinomas with combined etoposide and cisplatin. Evidence of major therapeutic activity in the anaplastic variants of these neoplasms. Cancer. 1991;68(2):227–232. doi: 10.1002/1097-0142(19910715)68:2<227::aid-cncr2820680202>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 9.Mitry E, Baudin E, Ducreux M, et al. Treatment of poorly differentiated neuroendocrine tumours with etoposide and cisplatin. Br J Cancer. 1999;81(8):1351–1355. doi: 10.1038/sj.bjc.6690325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lassen U, Kristjansen PE, Osterlind K, et al. Superiority of cisplatin or carboplatin in combination with teniposide and vincristine in the induction chemotherapy of small-cell lung cancer. A randomized trial with 5 years follow up. Ann Oncol. 1996;7(4):365–371. doi: 10.1093/oxfordjournals.annonc.a010603. [DOI] [PubMed] [Google Scholar]
- 11.Hanna N, Bunn PA, Jr, Langer C, et al. Randomized phase III trial comparing irinotecan/cisplatin with etoposide/cisplatin in patients with previously untreated extensive-stage disease small-cell lung cancer. J Clin Oncol. 2006;24(13):2038–2043. doi: 10.1200/JCO.2005.04.8595. [DOI] [PubMed] [Google Scholar]
- 12.O’Brien ME, Ciuleanu TE, Tsekov H, et al. Phase III trial comparing supportive care alone with supportive care with oral topotecan in patients with relapsed small-cell lung cancer. J Clin Oncol. 2006;24(34):5441–5447. doi: 10.1200/JCO.2006.06.5821. [DOI] [PubMed] [Google Scholar]
- 13.Casas F, Ferrer F, Farrus B, et al. Primary small cell carcinoma of the esophagus: a review of the literature with emphasis on therapy and prognosis. Cancer. 1997;80(8):1366–1372. [PubMed] [Google Scholar]
- 14.Brenner B, Shah MA, Gonen M, et al. Small-cell carcinoma of the gastrointestinal tract: a retrospective study of 64 cases. Br J Cancer. 2004;90(9):1720–1726. doi: 10.1038/sj.bjc.6601758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Takada M, Fukuoka M, Kawahara M, et al. Phase III study of concurrent versus sequential thoracic radiotherapy in combination with cisplatin and etoposide for limited-stage small-cell lung cancer: results of the Japan Clinical Oncology Group Study 9104. J Clin Oncol. 2002;20(14):3054–3060. doi: 10.1200/JCO.2002.12.071. [DOI] [PubMed] [Google Scholar]
- 16.Meert AP, Paesmans M, Berghmans T, et al. Prophylactic cranial irradiation in small cell lung cancer: a systematic review of the literature with meta-analysis. BMC Cancer. 2001;1:5. doi: 10.1186/1471-2407-1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cicin I, Karagol H, Uzunoglu S, et al. Extrapulmonary small-cell carcinoma compared with small-cell lung carcinoma: a retrospective single-center study. Cancer. 2007;110(5):1068–1076. doi: 10.1002/cncr.22887. [DOI] [PubMed] [Google Scholar]


