A girl with thyroid dysgenesis had two gene mutations resulting in two defective transcriptional factors important for thyroid development.
Abstract
Context:
Screening of the known candidate genes involved in thyroid organogenesis has revealed mutations in a small subset of patients with congenital hypothyroidism due to thyroid dysgenesis (TD).
Objective:
We studied a girl with TD who had mutations in two transcription factors involved in thyroid development.
Results:
Sequencing analysis of candidate genes involved in thyroid gland development revealed a new paternally inherited heterozygous mutation in the NKX2.5 gene (S265R) and a new maternally inherited heterozygous mutation in the PAX8 promoter region (−456C>T). Both parents and a brother, who was also heterozygous for both mutations, were phenotypically normal. Immunofluorescence microscopy showed a correct nuclear localization of both wild-type (WT) and mutant NKX2.5 proteins. EMSA demonstrated that the mutant NKX2.5 binds to the NKE_2, DIO2, TG, and TPO promoter elements equally well as the WT protein. However, the mutant NKX2.5 protein showed a 30–40% reduced transactivation of the thyroglobulin and the thyroid peroxidase promoters and a dominant-negative effect of the mutant NKX2.5. EMSA studies of the WT and mutant PAX8 promoter sequences incubated with nuclear extracts from PCCL3 cells exhibited a loss of protein binding capacity of the mutant promoter. In addition, the mutant PAX8 promoter showed a significantly reduced transcriptional activation of a luciferase reporter gene in vitro. Thus, this promoter mutation is expected to lead to reduced PAX8 expression.
Conclusions:
We identified new heterozygous mutations in both NKX2.5 and PAX8 genes of a girl with TD. Both defects might contribute to the phenotype.
The majority of cases (80–85%) with congenital hypothyroidism have thyroid dysgenesis (TD), leading to an absent, hypoplastic, or ectopic thyroid gland (1). The molecular mechanisms leading to TD are mostly unknown. Screening of the known candidate genes in individuals with TD have revealed only a few single gene disorders in the thyroid transcription factors 1 and 2 (TTF1 and TTF2); the paired box gene 8 (PAX8) (2–5); the TSH-receptor (TSHR) (6); and recently the NK2 transcription factor related locus 5 (NKX2.5), which not only plays an important role in the development of the heart (7) but is also involved in TD (8). The phenotype in TD varies considerably and mutations, e.g. in the PAX8 gene, can be associated with thyroid dysgenesis or a normal phenotype, even within the same family (9), which might raise the possibility of incomplete penetrance and the presence of genetic or nongenetic modifiers. Those rare cases in which mutations in one of the candidate genes were identified to be the cause of TD were mostly supported by in vitro studies. We here report for the first time a patient with TD with mutations in two transcription factors involved in thyroid development. Whereas one mutation in the PAX8 gene promoter region is reducing PAX8 gene expression, the other mutation leads to a partial loss of NKX2.5 activity.
Case reports
The proposita was born after 35 wk gestation to nonconsanguineous German parents. Weight was 2405 g (25th percentile) and length of 47.5 cm (25th to 50th percentile). Hypothyroidism was diagnosed at neonatal screening (TSH 144 μU/ml) and confirmed 5 d after birth when the serum TSH was still elevated (179 μU/ml, normal <15 μU/ml). At that time her serum T3 was 0.9 nmol/liter (normal 1.2–4) and her serum T4 was 27 nmol/liter (normal 77–205), and l-thyroxine treatment was initiated. With 37.5 μg l-thyroxine daily, she developed normally. At her first visit to Mainz, she was 17 months old and clinically euthyroid. Her physical examination was unremarkable and she had no malformations. An ultrasound of the neck suggested athyreosis. In the follow-up, thyroid function tests were always normal and the l-thyroxine dose was adjusted for age and weight. On an occasion when serum TSH concentration was mildly elevated at 8 mU/liter, her serum thyroglobulin (TG) was very low (5 ng/ml), suggesting that she had no significant thyroid tissue. Currently she is 10 yr old and receiving l-thyroxine 87.5 μg/daily. Her brother is euthyroid and develops normally (see Fig. 1A). He was seen repeatedly in our clinics after we identified the underlying molecular defect in the proposita. Ultrasound confirmed that his thyroid is normal in size and location. The parents, the maternal grandparents, and the paternal grandmother are euthyroid (Fig. 1A). Both children and all investigated family members had no heart malformations or arrhythmias.
Fig. 1.
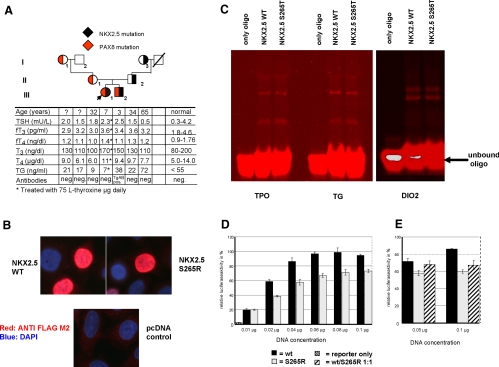
A, Pedigree of the family. The index patient is indicated by an arrow. The transmission of the NKX2.5 mutation is shown by a filled black area within each symbol, whereas the segregation of the PAX8 promoter mutation by a corresponding red area. Results of thyroid function tests are aligned with each symbol. B, Nuclear localization of S265R. HeLa cells were transfected with plasmids expressing the WT NKX2.5 and the mutant NKX2.5 (S265R). The NKX2.5 proteins were visualized by immunofluorescence microscopy as described in Materials and Methods. Both proteins (WT and S265R) are localized in the nucleus. C, EMSA with various NKX2.5 promoter binding sites. Oligonucleotides containing the NKX2.5 binding sequences of the TPO, TG, and DIO2 gene promoters were synthesized and labeled with an infrared dye (IRD700). The in vitro synthesized NKX2.5 proteins were incubated with the IRD700 labeled oligonucleotides and separated on a 4% native polyacrylamide gel and visualized with the Odyssey (Li-Cor, Lincoln, NE). D and E, Functional activity of the mutant NKX2.5 tested using the TG-luciferase reporter gene in HeLa cells. HeLa cells were transiently transfected with 0.25 μg TG-luciferase and various amounts of WT or mutant NKX2.5 expression constructs. At all DNA concentrations tested, the NKX2.5 mutant (gray bars) shows an approximately 30% reduced luciferase activity when compared with the WT (black bars). The empty vector control is marked as a hatched bar (D). When cotransfected in equal amounts (hatched bars), S265R exerts a dominant-negative effect on the WT (E).
Materials and Methods
Details of the genetic and functional analyses are found in the Supplemental Methods, published on The Endocrine Society's Journals Online web site at http://jcem.endojournals.org.
Briefly, after informed consent was obtained from all investigated individuals, the coding sequences of the candidate genes for TD and the promoter sequence of the PAX8 gene were sequenced. For transfection studies, we used expression vectors encoding murine Nkx2.5, wild-type (WT), and mutant S265R to which an N-terminal FLAG tag was added to analyze the nuclear targeting. TG-luc and TPO-luc were used as reporters (10, 11). To study the PAX8 promoter mutation, a 1130-bp fragment containing sequences upstream of the ATG of the PAX8 gene (−602 to +528) was cloned in the pGL3 basic vector upstream of the luciferase gene (PAX8proWT). The PAX8 promoter mutation at position −456 (PAX8proMUT) was introduced using a site-directed mutagenesis kit. HeLa cells were transiently transfected with the FLAG containing NKX2.5 constructs, fixed, permeabilized, and visualized with a monoclonal anti-FLAG and a secondary antibody. For functional analysis of the NKX2.5 mutation, HeLa cells were transiently transfected with the NKX2.5 and the reporter constructs. Forty-eight hours later, the luciferase and the renilla luciferase activities were measured. To analyze the activity of the mutant PAX8 promoter, both PAX8proWT and PAX8proMUT were transiently transfected into HeLa cells and in PCCL3 cells together with the reporter constructs, and luciferase and the renilla luciferase activities were measured 48 h later. For EMSA recombinant mutant and WT NKX2.5 proteins were generated and incubated with the TPO (12), TG (13), DIO2 (14), and NKE_2 (15) oligo sequences, which were labeled with infrared dye. To investigate the protein binding capacity of the WT and the mutant PAX8 promoters, the primers used for mutagenesis of PAX8proWT and PAX8proMUT were labeled with an infrared dye (IRD700; Metabion, Martinsried, Germany) and incubated with proteins extracted from PCCL3 cell nuclei (16). Aliquots of the binding reactions were analyzed by polyacrylamide gel electrophoresis and scanned.
Results
Sequencing analysis of the proposita's genomic DNA revealed a heterozygous missense mutation (c.795A>C, p.S265R) in exon 2 of the NKX2.5 gene. This mutation is located in a highly conserved area of the NKX2.5 gene and was found in one allele of the brother, the father, and the paternal grandmother (Fig. 1A). Furthermore, the proposita had a heterozygous sequence change (C>T) in the promoter area of the PAX8 gene, upstream of the ATG. Her brother, mother, and maternal grandmother were also heterozygous for −456 C>T (Fig. 1A). Both sequence variants were not found in 100 alleles of random samples from individuals of same ethnic background, indicating that they did not represent common polymorphisms.
Because NKX2.5 controls transcription through binding to specific DNA sequences, we first showed that both WT and mutant NXK2.5 proteins were correctly localized into the nucleus (Fig. 1B). EMSA using the NKX2.5 binding sites of the TPO-, TG-, and the DIO2- gene promoter sequences as well as the binding activity to the NKE_2 binding element (Fig. 1C) demonstrate that WT and mutant NKX2.5 proteins bind equally well to all tested promoter sequences. However, S265R showed 30% less induction of the reporter construct when compared with the WT and a dominant-negative effect of the S265R (Fig. 1, D and E).
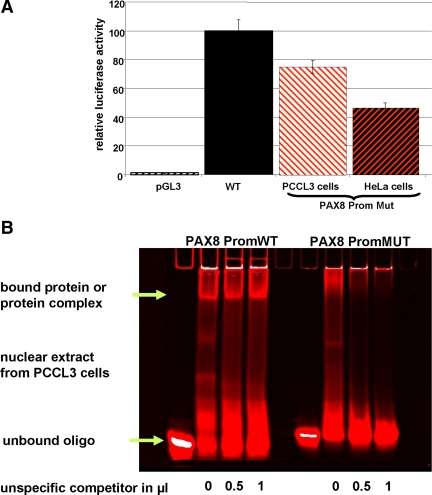
To study the consequence of the PAX8 promoter sequence alteration, both WT (PAX8proWT) and mutant (PAX8proMUT) sequences driving a luciferase reporter gene were transiently transfected into HeLa cells and into PCCL3 cells. Using 1 μg of plasmid DNA, compared with the PAX8proWT construct luciferase expression was 50 and 25% lower in the presence of PAX8proMut in HeLa and PCCL3 cells, respectively (Fig. 2A). In addition, nuclear proteins from PCCL3 cells bound the WT but not the mutant PAX8 promoter sequence (Fig. 2B).
Fig. 2.
A, In vitro effects of the PAX8 promoter mutation. Both PAX8 gene promoter constructs (PAX8proWT and PAX8proMUT) were transiently transfected into HeLa cells and into PCCL3 cells. A, Using 1 μg of plasmid DNA of each promoter construct in both cell lines, a significantly reduced luciferase expression was observed with the PAX8proMut (the red and white hatched bar shows transfection in PCCL3 cells, the red and black hatched bar represents the results in Hela cells) compared with the PAX8proWT (black bar). The empty vector is shown as a white and black hatched bar. B, EMSA with nuclear extracts from PCCL3 with PAX8proWT and PAX8proMUT. Nuclear extracts isolated from PCCL3 cells were incubated with oligonucleotides containing either the WT or the mutant PAX8 promoter sequences. A specific shift with the WT promoter sequence is observed. A specific binding activity is observed only with the WT PAX8 promoter sequence (green arrow).
Discussion
We here report a girl with TD who is heterozygous for two mutations, each encoding a different transcription factor important for the development of the thyroid gland during embryonic life (1). In vitro studies were performed to determine whether the observed gene defects could contribute to the pathogenesis of TD. One of the mutations (S265R) is located within the NKX2.5 gene. Because dominantly inherited mutations in the NKX2.5 gene are known to cause heart conduction defects (17), the involvement of NKX2.5 in TD in humans is still a matter of debate (6). Nkx2.5-deficient mouse embryos have less thyroid precursor cells compared with wild-type animals, which results in hypoplasia of the thyroid bud. The three reported NKX2.5 gene mutations had impaired DNA binding, reduced ability to activate reporter genes driven by thyroid-specific promoters, and showed interference with the wild-type activity (dominant negative effect) (8). Furthermore, as in the case reported herein, the defects were transmitted by an unaffected parent, with no TD and only one of whom had a heart abnormality (8). S265R is located in a highly conserved region of the NKX2.5 gene and is not a common polymorphism. It binds normally to the target sequences of genes regulated by NKX2.5 because the mutation is located outside the homeobox domain of NKX2.5. However, the transcriptional activity of S265R was reduced compared with the WT NKX2.5, and furthermore, S265R exerts a dominant-negative effect. Taken together, our studies show that the mutant S265R has impaired functional activity in vitro. In agreement with the observation by Dentice et al. (8), S265R is likely to have an impact on proper thyroid organogenesis, even though genetically affected family members did not have TD or heart defects.
In addition, we identified a heterozygous mutation in the PAX8 gene promoter. A monoallelic mutation at position −569 in the PAX8 promoter region has been described in four unrelated individuals with thyroid hypoplasia (18), but in vitro functional studies were not reported. Thus, we investigated whether the mutation −456 found in our patient has biological relevance. Indeed, the mutated PAX8 gene promoter sequence showed a reduced ability to activate a reporter gene compared with the WT (Fig. 2A). Furthermore, our EMSA experiments show that in contrast to the normal PAX8 promoter, the altered sequence does not bind nuclear proteins present in PCCL3 cells (Fig. 2B). We do not believe that this band represents NKX2.5 because the latter was unable to activate the WT PAX8 promoter, and NKX2.5 does not bind directly to this PAX8 promoter element in EMSA (data not shown). Taken together, our results indicate that the altered promoter should potentially lead to a reduced PAX8 expression and might therefore contribute to the pathogenesis of TD.
Both NKX2.5 and PAX8 mutations produce a highly variable phenotype. Thus, the absence of TD in members of the family reported herein, despite the presence of a monoallelic PAX8 gene mutation, is not surprising. More surprising was the observation that a younger sibling, also heterozygous for both NKX2.5 and PAX8 promoter mutations and thus carrying the same double hit, had no manifestation of thyroid dysfunction. Whether this phenotypic variability is due to a variable penetrance or expressivity of the mutant gene or whether the genetic background plays a role remains unclear. Haploinsufficiency, monoallelic expression, or imprinting could all have an impact on the phenotype (19).
In summary, our studies show that the herein identified mutations lead to impaired functions of NKX2.5 and PAX8 so that each defect in itself could result in TD. However, their coexistence in the same individual may not always produce TD detectable at birth. A direct interaction of both mutations seems to be unlikely. Interestingly, our EMSA studies show that the PAX8 promoter mutation at position −456 abolishes specific binding of a so-far-unknown protein or protein complex, which could reflect other factors having an impact on the modulation of the phenotype in TD. Further analysis of these factors might help to explain the variable phenotype in the family described here.
Supplementary Material
Acknowledgments
We thank Katja Hilbert and Marita Holl for excellent technical assistance. Furthermore, we thank all family members for their participation in this study and Dr. Paolo Macchia (University of Napoli Federico, Naples, Italy) for providing the murine WT NKX2.5 expression vector. The PAX8 wild-type promoter construct was a gift from Dr. Peter Kopp (Northwestern University, Chicago, IL).
This work was supported in part by National Institutes of Health Grants DK15070, DK020595, DK07011, and RR04999. J.P. was supported by the Fritz-Thyssen-Stiftung (Germany).
Current address for H.G.: Department of Gastroenterology, University of Michigan, Ann Arbor, Michigan 48109.
Disclosure Summary: The authors have nothing to declare.
Footnotes
- NKX2.5
- NK2 transcription factor related locus 5
- PAX8
- paired box gene 8
- TD
- thyroid dysgenesis
- TG
- thyroglobulin
- WT
- wild type.
References
- 1. De Felice M, Di Lauro R. 2004. Thyroid development and its disorders: genetics and molecular mechanisms. Endocr Rev 25:722–746 [DOI] [PubMed] [Google Scholar]
- 2. Montanelli L, Tonacchera M. 2010. Genetics and phenomics of hypothyroidism and thyroid dys- and agenesis due to PAX8 and TTF1 mutations. Mol Cell Endocrinol 322:64–71 [DOI] [PubMed] [Google Scholar]
- 3. Jo W, Ishizu K, Fujieda K, Tajima T. 2010. Congenital hypothyroidism caused by a PAX8 gene mutation manifested as sodium/iodide symporter gene defect. J Thyroid Res 2010:619013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Narumi S, Muroya K, Asakura Y, Adachi M, Hasegawa T. 2010. Transcription factor mutations and congenital hypothyroidism: systematic genetic screening of a population-based cohort of Japanese patients. J Clin Endocrinol Metab 95:1981–1985 [DOI] [PubMed] [Google Scholar]
- 5. Di Palma T, Zampella E, Filippone MG, Macchia PE, Ris-Stalpers C, de Vroede M, Zannini M. 2010. Characterization of a novel loss-of-function mutation of PAX8 associated with congenital hypothyroidism. Clin Endocrinol (Oxf) 73:808–814 [DOI] [PubMed] [Google Scholar]
- 6. Pohlenz J, van Vliet G. 2010. Developmental abnormalities of the thyroid. In: Weiss RE, Refetoff S. eds. Genetic diagnosis of endocrine disease. London: Elsevier; 97–104 [Google Scholar]
- 7. Ouyang P, Saarel E, Bai Y, Luo C, Lv Q, Xu Y, Wang F, Fan C, Younoszai A, Chen Q, Tu X, Wang QK. 2011. A de novo mutation in NKX2.5 associated with atrial septal defects, ventricular noncompaction, syncope and sudden death. Clin Chim Acta 412:170–175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dentice M, Cordeddu V, Rosica A, Ferrara AM, Santarpia L, Salvatore D, Chiovato L, Perri A, Moschini L, Fazzini C, Olivieri A, Costa P, Stoppioni V, Baserga M, De Felice M, Sorcini M, Fenzi G, Di Lauro R, Tartaglia M, Macchia PE. 2006. Missense mutation in the transcription factor NKX2–5: a novel molecular event in the pathogenesis of thyroid dysgenesis. J Clin Endocrinol Metab 91:1428–1433 [DOI] [PubMed] [Google Scholar]
- 9. de Sanctis L, Corrias A, Romagnolo D, Di Palma T, Biava A, Borgarello G, Gianino P, Silvestro L, Zannini M, Dianzani I. 2004. Familial PAX8 small deletion (c. 989_992delACCC) associated with extreme phenotype variability. J Clin Endocrinol Metab 89:5669–5674 [DOI] [PubMed] [Google Scholar]
- 10. Grasberger H, Ringkananont U, Lefrancois P, Abramowicz M, Vassart G, Refetoff S. 2005. Thyroid transcription factor 1 rescues PAX8/p300 synergism impaired by a natural PAX8 paired domain mutation with dominant negative activity. Mol Endocrinol 19:1779–1791 [DOI] [PubMed] [Google Scholar]
- 11. Pohlenz J, Dumitrescu A, Zundel D, Martiné U, Schönberger W, Koo E, Weiss RE, Cohen RN, Kimura S, Refetoff S. 2002. Partial deficiency of thyroid transcription factor 1 produces predominantly neurological defects in humans and mice. J Clin Invest 109:469–473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Francis-Lang H, Price M, Polycarpou-Schwarz M, Di Lauro R. 1992. Cell-type-specific expression of the rat thyroperoxidase promoter indicates common mechanisms for thyroid-specific gene expression. Mol Cell Biol 12:576–588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zannini M, Francis-Lang H, Plachov D, Di Lauro R. 1992. Pax-8, a paired domain-containing protein, binds to a sequence overlapping the recognition site of a homeodomain and activates transcription from two thyroid-specific promoters. Mol Cell Biol 12:4230–4241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dentice M, Morisco C, Vitale M, Rossi G, Fenzi G, Salvatore D. 2003. The different cardiac expression of the type 2 iodothyronine deiodinase gene between human and rat is related to the differential response of the Dio2 genes to Nkx-2.5 and GATA-4 transcription factors. Mol Endocrinol 17:1508–1521 [DOI] [PubMed] [Google Scholar]
- 15. Lee Y, Shioi T, Kasahara H, Jobe SM, Wiese RJ, Markham BE, Izumo S. 1998. The cardiac tissue-restricted homeobox protein Csx/Nkx2.5 physically associates with the zinc finger protein GATA4 and cooperatively activates atrial natriuretic factor gene expression. Mol Cell Biol 18:3120–3129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Schreiber E, Matthias P, Müller MM, Schaffner W. 1989. Rapid detection of octamer binding proteins with ‘mini-extracts,’ prepared from a small number of cells. Nucleic Acids Res 17:6419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Schott JJ, Benson DW, Basson CT, Pease W, Silberbach GM, Moak JP, Maron BJ, Seidman CE, Seidman JG. 1998. Congenital heart disease caused by mutations in the transcription factor NKX2–5. Science 281:108–111 [DOI] [PubMed] [Google Scholar]
- 18. Ramos HE, Nesi-França S, Boldarine VT, Pereira RM, Chiamolera MI, Camacho CP, Graf H, de Lacerda L, Carvalho GA, Maciel RM. 2009. Clinical and molecular analysis of thyroid hypoplasia: a population-based approach in southern Brazil. Thyroid 19:61–68 [DOI] [PubMed] [Google Scholar]
- 19. Vassart G, Dumont JE. 2005. Thyroid dysgenesis: multigenic or epigenetic, or both? Endocrinology 146:5035–5037 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.