Lower activation of muscle AMPK after resistance exercise training in the metabolic syndrome may be due to higher activation of mammalian target of rapamycin.
Abstract
Context:
Strength training induces muscle remodeling and may improve insulin responsiveness.
Objective:
This study will quantify the impact of resistance training on insulin sensitivity in subjects with the metabolic syndrome and correlate this with activation of intramuscular pathways mediating mitochondrial biogenesis and muscle fiber hypertrophy.
Design:
Ten subjects with the metabolic syndrome (MS) and nine sedentary controls underwent 8 wk of supervised resistance exercise training with pre- and posttraining anthropometric and muscle biochemical assessments.
Setting:
Resistance exercise training took place in a sports laboratory on a college campus.
Main Outcome Measures:
Pre- and posttraining insulin responsiveness was quantified using a euglycemic clamp. Changes in expression of muscle 5-AMP-activated protein kinase (AMPK) and mammalian target of rapamycin (mTOR) pathways were quantified using immunoblots.
Results:
Strength and stamina increased in both groups. Insulin sensitivity increased in controls (steady-state glucose infusion rate = 7.0 ± 2.0 mg/kg · min pretraining training vs. 8.7 ± 3.1 mg/kg · min posttraining; P < 0.01) but did not improve in MS subjects (3.3 ± 1.3 pre vs. 3.1 ± 1.0 post). Muscle glucose transporter 4 increased 67% in controls and 36% in the MS subjects. Control subjects increased muscle phospho-AMPK (43%), peroxisome proliferator-activated receptor γ coactivator 1α (57%), and ATP synthase (60%), more than MS subjects (8, 28, and 21%, respectively). In contrast, muscle phospho-mTOR increased most in the MS group (57 vs. 32%).
Conclusion:
Failure of resistance training to improve insulin responsiveness in MS subjects was coincident with diminished phosphorylation of muscle AMPK, but increased phosphorylation of mTOR, suggesting activation of the mTOR pathway could be involved in inhibition of exercise training-related increases in AMPK and its activation and downstream events.
The prevalences of obesity, the metabolic syndrome (MS), and diabetes have increased globally in the past two decades. Low physical activity levels (1) and low cardiovascular fitness (2) are associated with increased risk of the MS. Exercise is considered one of the cornerstones of diabetes prevention and treatment. Both endurance and strength training ameliorate insulin resistance (3–5) and improve blood sugar control (3, 6). However, the skeletal muscle adaptations and the signaling pathways through which these adaptations occur appear to be specific to the type of exercise performed (7).
Endurance training increases the oxidative capacity of both type 1 (slow twitch, red) and type 2 (fast twitch, white) muscle fibers primarily by increasing oxidative enzyme content through up-regulation of mitochondrial biogenesis (8). These adaptations enhance the efficiency of energy production from fatty acids and glucose. 5-AMP-activated protein kinase (AMPK) is a key energy sensor in most cells and is activated during exercise by an increase in the AMP:AMP ratio (9). Activation of peroxisome proliferator-activated receptor γ coactivator 1α (PGC-1α), a downstream target of AMPK, turns on expression of mitochondrial genes, both in mitochondria and the nucleus (10). Activation of the AMPK signaling pathway is involved in the exercise-related acute translocation of glucose transporter 4 (GLUT4) to the muscle plasma membrane (11) and may be involved in increased GLUT4 expression seen with endurance training (12).
Resistance training results in skeletal muscle hypertrophy, particularly in type 2 fibers, with a concomitant increase in muscular strength (13). Increased protein synthesis occurs through a cell signaling pathway involving mammalian target of rapamycin (mTOR). mTOR integrates intracellular and extracellular signals from growth factors, substrate availability and cellular energy levels to regulate metabolic responses within the cell (14). Upstream of mTOR, growth factors, including insulin and IGF-I, activate a cascade involving phosphatidylinositol 3-kinase, phosphoinositide-dependent kinase 1, and Akt. Akt activation results in phosphorylation of mTOR. mTORC1 in turn phosphorylates 70-kDa S6 protein kinase (S6K1) and 4E binding proteins resulting in increased protein synthesis (14).
The aim of the current study was to evaluate the impact of a resistance training program on insulin responsiveness in subjects with the MS and to quantify the changes in the intramyocyte pathways that mediate changes in insulin action through mitochondrial biogenesis and/or muscle fiber hypertrophy in these subjects.
Subjects and Methods
Subject selection
Nineteen sedentary subjects were recruited to undergo 8 wk of supervised resistance exercise. None of the subjects had performed regular exercise for at least 1 yr. The research protocol and the consent documents were approved by the East Tennessee State University Institutional Review Board. The exercise program was performed at the East Tennessee State University Exercise and Sports Sciences Laboratory supervised by students and faculty from the Department of Kinesiology. Subjects were recruited into two groups: high risk for type 2 diabetes [body mass index (BMI) ≥30 kg/m2; waist circumference ≥40 in. for males or ≥35 in. for females; family history of type 2 diabetes] and low risk for type 2 diabetes (BMI <30 kg/m2; no family history of type 2 diabetes). The 10 subjects at high risk for diabetes qualified for the designation MS, as set forward by the American Diabetes Association and the World Health Organization (15). All 10 had BMI higher than 30 kg/m2 and waist circumference over 40 in. and exhibited insulin resistance in insulin infusion studies. Subjects were instructed to maintain weight during the study period. Muscle biopsies, insulin infusions, blood pressure, blood lipids, body composition, and strength and endurance measurements were performed before and after the full 8-wk training program.
Exercise protocol
Training consisted of large muscle mass free weight exercises (Supplemental Table 1, published on The Endocrine Society's Journals Online web site at http://jcem.endojournals.org). A light familiarization and baseline measurement period was followed by 4 wk of high-volume, low-intensity training (phase I). The intensity of the exercise (based on an estimate of relative repetition maximum) increased approximately 10% each week for the first 3 wk, followed by a 10% drop in intensity from wk 3 to wk 4 to allow for recovery. During phase II (wk 5–8), volume decreased and intensity increased to allow for greater strength gains. Similar to phase I, intensity increased approximately 10% week to week and was decreased by 10% during wk 8 to allow for recovery. All sets (warm-up and target sets) were recorded and calculated as total volume load (sets × repetitions × weight).
Subject assessments
Several anthropometric descriptors were measured at baseline and after 8 wk of resistance training. Body composition was measured by air displacement plethysmography (BodPod, Concord, CA). Waist circumference was measured just above the iliac crest to the nearest 0.1 cm.
Strength testing
Strength was assessed isometrically using a custom-built lifting rack. The lifting rack was set so that the subjects pulled from the mid-thigh pull position used in training. Peak force (PF) was measured on a force plate (Rice Lake Weighing Systems, Rice Lake, WI) with a sampling rate of 1000 Hz. Data were collected and analyzed for PF and rate of force development (RFD) using custom Labview version 8.6 software (National Instruments, Upper Saddle River, NJ).
Endurance testing
Endurance was measured using a Monark Ergomedic 874E cycle ergometer (Monark Exercise AB, Vansbro, Sweden). Expired air was analyzed using a TrueOne 2400 Metabolic Measurement System (ParvoMedics, Sandy, UT). Heart rate, maximal oxygen consumption (VO2max), respiratory exchange ratio, and time to exhaustion were recorded.
Muscle biopsies
Percutaneous needle biopsies of vastus lateralis were performed after an overnight fast and 2 h of quiet recumbency as previously described, using a Bergstrom-Stille 5-mm muscle biopsy needle with suction (16). The second biopsy was performed 24–48 h after the last training session. The sample was divided in half, with one piece frozen immediately in liquid nitrogen for later analysis and the second piece mounted on cork and quickly frozen in a slurry of isopentane cooled in liquid nitrogen. The cork-mounted piece was stored at −80 C and later sectioned on a cryostat (Leica, Wetzlar, Germany) for evaluation of fiber type composition.
Euglycemic-hyperinsulinemic clamp
After a 2-h baseline period, a single infusion of regular insulin was performed at 15 mU/m2 · min for 3 h to achieve a physiological increment in insulin concentration of about 50 μU/ml and a stable glucose infusion rate (GIR) to quantify insulin sensitivity as previously described (17).
Quantification of muscle fiber type composition and fiber size
Fiber composition was determined using methods described by Behan et al. (18). Muscle sections were stained for light microscopy in a two-step method using commercial monoclonal antibodies to fast and slow isoforms of myosin heavy chain. After acetone fixation and incubation with 20% normal rabbit serum, the slow myosin antibody (Sigma clone NOQ7.5.4D; Sigma-Aldrich, St. Louis, MO) was applied, followed by a peroxidase-conjugated rabbit antimouse IgG antibody. The fast myosin antibody (Sigma clone MY-32 alkaline phosphatase conjugate) was then applied (18). Slides were alcohol dehydrated, cleared with xylene, and preserved in synthetic medium. This technique allows discrimination of type 1, type 2a, and type 2b. All sections were coded and then quantified independently by three observers who were unaware of which subject or treatment the image represented.
Preparation of muscle homogenates
A small piece of muscle was removed from the −80 C freezer and slowly thawed on ice. Muscle homogenate was prepared by placing 25–50 mg muscle in 500 μl 0.25 m sucrose, 20 mm HEPES (pH 7.4) containing protease inhibitors (Halt Protease Inhibitor Cocktail Kit; Pierce, Rockford, IL) and homogenized with two 30-sec bursts of a hand-held homogenizer (Pellet Pestle Motor; Kontes, Vineland, NJ).
Mitochondrial markers
A mixture of antibodies to five different mitochondrial components (no. MS604) were purchased from MitoSciences (Eugene, OR). These antibodies were directed against complexes I, II, III, and IV and to ATP synthase subunit α. The principal component used for these analyses was ATP synthase. For these studies, 4–20% gradient gels (Thermo Scientific, Rockford, IL) were loaded with 7.5 μg protein from muscle homogenates. Blots were blocked with 0.25% nonfat dry milk.
Key regulatory factors (AMPK, PGC-1α, mTOR, and S6K1)
Comparison of expression of these proteins were also quantified in immunoblots as previously described (19). Antibodies for AMPK, phospho-AMPKα1, mTOR, phospho-mTOR, and phospho-p70 S6 kinase (2532, 2531, 2972, 2971, and 9205) were purchased from Cell Signaling Technology (Danvers, MA). The antibody for PGC-1α (AB3242) was purchased from Millipore (Billerica, MA). For AMPK and phospho-AMPK, samples containing 5 μg protein from muscle homogenates were applied to 10% polyacrylamide gels. Immunoblots were blocked with 5% nonfat dry milk. Gradient gels (3–8%) (Invitrogen, Carlsbad, CA) were used for mTOR and phospho-mTOR immunoblots. These samples were 10 μg per lane, and blocking was 0.25% nonfat dry milk. PGC-1α immunoblots were from 10% polyacrylamide gels and were blocked with 0.5% nonfat milk. Immunoblots for phospho-S6K1 also used 10% polyacrylamide gels and 0.5% nonfat milk for blocking.
Expression of principal muscle hexose transporters (GLUT4 and GLUT5)
The techniques for quantifying these glucose transporters were described previously (16, 17). Affinity-purified rabbit antibodies against human GLUT5 (GT52-A) were purchased from Alpha Diagnostics (San Antonio, TX). GLUT4 antibodies (AB1049, goat antihuman) were purchased from Chemicon (Temecula, CA).
Statistics
All data are displayed as mean ± sd, except as explicitly indicated. ANOVA was used for data assessment, and for each study group, the paired t test was used for comparing mean levels before and after training. Comparing mean data between the two groups was performed using the independent t test. Effect size correlations were calculated using Cohen's d (20). Relationships between select variables were assessed using a Pearson correlation coefficient. Statistical procedures were performed using SigmaStat version 3.11 from Systat Software (San Jose, CA).
Results
Subject characteristics
The baseline characteristics of the MS subjects were very different from the sedentary control subjects in key variables as listed in Table 1. Body mass and BMI were 30 and 35% higher in MS subjects. Mean fasting insulin concentration was 94% higher in MS subjects. VO2max at baseline was 25% lower in the MS group. Age, blood pressure, and fasting glucose concentration tended to be higher in MS subjects, but these differences were not statistically significant. Additional baseline data are included in subsequent tables. The fasting glucose, insulin, lipids, and blood pressures were determined on the day of the muscle biopsy and euglycemic clamp study before and after the resistance training protocol.
Table 1.
Subject characteristics at baseline
| Controls | MS subjects | |
|---|---|---|
| Number (n) | 9 | 10 |
| Female gender (n) | 5 | 5 |
| Age (yr) | 36.4 ± 12.2 | 45.0 ± 8.6 |
| Body mass (kg) | 69.2 ± 16.2 | 99.5 ± 13.8a |
| BMI (kg/m2) | 24.3 ± 3.6 | 33.7 ± 3.0a |
| Resting systolic blood pressure (mm Hg) | 113 ± 10 | 131 ± 20 |
| Resting diastolic blood pressure (mm Hg) | 77 ± 10 | 82 ± 9 |
| Fasting blood sugar (mm) | 4.9 ± 0.7 | 5.7 ± 0.7 |
| Fasting serum insulin (pmol/liter) | 53 ± 25 | 103 ± 67a |
| VO2max (ml/kg · min) | 31.0 ± 4.5 | 23.3 ± 3.1a |
| IPF (N/kg2/3) | 134 ± 37 | 135 ± 40 |
| RFD (N/sec) | 3180 ± 1320 | 2940 ± 2170 |
Unless indicated otherwise, data are mean ± sd.
Significant difference from controls, P < 0.01.
Anthropometrics, functional capabilities, and volume load
Eight weeks of resistance training had a positive impact on body composition (Table 2). Overall, training had little effect on body mass or fat mass; however, the percent change in body mass was strongly correlated with the percent gain in lean body mass (LBM) in both groups (r = 0.532). Although the increase in LBM was relatively small (d = 0.127 and 0.141), the change was statistically significant in both groups. The decrease in waist circumference was statistically significant (P = 0.022); however, the effect size was moderate in MS and controls (−0.375 and −0.319, respectively). Training tended to decrease body fat percentage in both groups, but the change was statistically significant only in MS (P = 0.010).
Table 2.
The impact of 8 wk resistance training on anthropometrics
| Controls (n = 9) | MS (n = 10) | |
|---|---|---|
| Body Mass (kg) | ||
| Pre | 69.2 ± 6.2 | 99.5 ± 13.8a |
| Post | 69.2 ± 16.1 | 99.3 ± 4.3a |
| d | 0.004 | −0.020 |
| BMI (kg/m2) | ||
| Pre | 24.3 ± 3.6 | 33.7 ± 3.0a |
| Post | 24.5 ± 3.3 | 34.0 ± 3.3a |
| d | 0.057 | 0.099 |
| Waist circumference (cm) | ||
| Pre | 93 ± 11 | 116 ± 6a |
| Post | 90 ± 2 | 114 ± 8a |
| d | −0.319 | −0.375 |
| Fat mass (kg) | ||
| Pre | 19.8 ± 9.3 | 41.4 ± 8.1a |
| Post | 19.5 ± 9.1 | 41.3 ± 8.4a |
| d | −0.024 | −0.006 |
| LBM (kg) | ||
| Pre | 48.4 ± 9.9 | 56.8 ± 9.5a |
| Post | 49.7 ± 9.9c | 58.0 ± 10.1a,b |
| d | 0.141 | 0.127 |
| % Body fat | ||
| Pre | 28.3 ± 9.4 | 42.2 ± 0.4a |
| Post | 27.5 ± 8.4 | 41.7 ± 5.3a,c |
| d | −0.095 | −0.102 |
Data are mean ± sd. d, Cohen's d effect size.
P < 0.01, compared with controls, independent t test.
P < 0.05, compared with baseline, paired t test.
P < 0.01, compared with baseline, paired t test.
Overall, VO2max increased 10% and time to exhaustion increased approximately 35% in both groups. The increase in VO2max was statistically significant in both groups, and both groups achieved relatively large effect sizes (d = 0.819 in MS subjects and 0.535 in controls). The increase in time to exhaustion was also statistically significant (P < 0.001). MS subjects improved in isometric PF (IPF) by 13% and RFD by 28% after training. Controls tended to increase in IPF (7%) and RFD (15%), but the increases were not statistically significant.
Volume load, calculated as sets × repetitions × weight, was tracked for each subject. Average weekly volume load is listed in Table 3. Total volume load was 16% higher in MS subjects than in controls (93,800 ± 32,200 and 80,700 kg ± 27,000 kg, respectively); however, this difference was not statistically significant. IPF measured before training and total volume load were strongly correlated (r = 0.681), indicating that initially stronger subjects were able to handle heavier loads during training. Allometric scaling (divide by weight in kilograms raised to the 2/3 power) adjusts for body size to allow males and females to be similar when combined. Because the MS subjects were much heavier, their unscaled volume load was 16% higher, but when allometrically scaled, the volume load was 9% lower than that of controls.
Table 3.
Average weekly volume load
| Week | Average weekly volume load (kg) |
|
|---|---|---|
| Controls (n = 9) | MS (n = 10) | |
| 1 | 5860 ± 2,570 | 7,010 ± 5,160 |
| 2 | 10,100 ± 3,870 | 11,200 ± 4,980 |
| 3 | 10,300 ± 4,550 | 12,600 ± 5,050 |
| 4 | 11,200 ± 3,910 | 12,800 ± 4,460 |
| 5 | 11,000 ± 3,750 | 13,200 ± 3,740 |
| 6 | 12,200 ± 3,810 | 11,500 ± 3,870 |
| 7 | 13,600 ± 3,870 | 15,000 ± 4,500 |
| 8 | 6,460 ± 1,910 | 7,460 ± 2,090 |
Volume load is an estimate of total work performed and is a product of the sets, repetitions, and load lifted.
There was variability in initial strength among subjects in both groups (sd of allometrically scaled baseline peak power was 28%). To consider whether there was an impact of the differences in workload among subjects, we quantified for each subject the volume load of the initial week, the peak volume load (usually in wk 7), and the total volume load from 8 wk of training sessions. Those data (raw data and allometrically scaled) were compared with changes in strength, LBM, insulin responsiveness, VO2max, and changes in muscle expression of GLUT4, ATP synthase, phospho-AMPK, and phospho-mTOR using the Pearson product moment correlation. We found no statistically significant correlation of the minor subject differences in workload to the differences in response in any of these outcome variables.
Blood lipids, glucose, and insulin
Circulating levels of triglycerides and high-density lipoprotein (HDL) and low-density lipoprotein (LDL) cholesterol were measured at baseline and after training. Triglyceride levels were 114 ± 52 mg/dl in controls and 198 ± 173 mg/dl in MS subjects at baseline. Controls had HDL and LDL cholesterol concentrations of 48 ± 11 and 87 ± 16 mg/dl at baseline, whereas MS subjects had lower HDL cholesterol (40 ± 9 mg/dl) and higher LDL cholesterol (112 ± 41 mg/dl). Triglycerides and total cholesterol tended to be lower in both groups after resistance training, but the differences were not statistically significant and the effect sizes were small (d = −0.158 and −0.148, respectively).
Euglycemic-hyperinsulinemic clamp
Insulin sensitivity was assessed using the euglycemic-hyperinsulinemic clamp technique described in the Subjects and Methods. Fasting insulin was higher in MS subjects both at baseline and after training (103 ± 67 vs. 97 ± 58 pmol/liter). Fasting insulin decreased 22% (53 ± 25 vs. 42 ± 27 pmol/liter) in controls after training (P < 0.05). Fasting blood glucose was higher in MS subjects than controls before and after training.
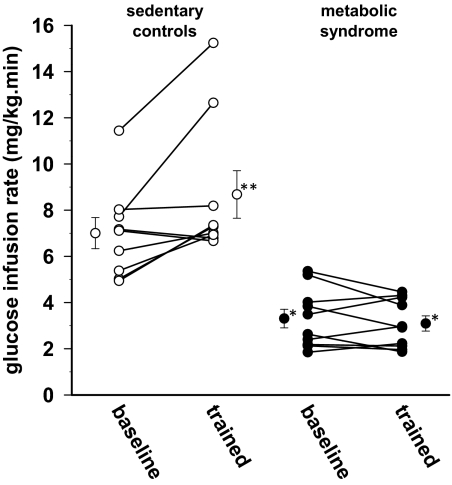
As shown in Fig. 1, MS subjects showed marked insulin resistance at baseline (47% of control baseline, P < 0.01 by independent t test), and training had little effect on GIR (P = 0.37, paired t test). In contrast, controls demonstrated normal insulin sensitivity before training (GIR = 7.0 ± 2.0 mg/kg · min), and GIR increased 25% after resistance training (P = 0.03, paired t test). The increment in insulin concentration achieved by the insulin infusion was similar in both groups before and after training. The insulin concentration increment averaged 52 and 52 μU/ml for MS subjects and controls at baseline and 52 and 49 μU/ml after training, respectively.
Fig. 1.
Changes in insulin responsiveness after 8 wk of resistance exercise training. The data shown here are the individual data and the means and se of the steady-state GIR in the last 30 min of a 3-h insulin infusion at 40 mU/m2 · min. The ANOVA table indicated an important interaction effect between the group factor and training. Because the interaction factor is significant, interpretation of the main effects can be replaced by examination of simple effects of training in each group (which is the paired t test applied to before and after values in controls and MS). The control group shows a significant change in mean level (P = 0.03), and the MS group does not show a statistically significant change in mean level (P = 0.37). *, Significant difference from control means; **, significant difference from mean baseline. The figure shows the control group does have a training effect, whereas the MS group does not. The difference in response to training between the two groups, either absolute or percentage, is significant (P = 0.02, independent-sample t test).
Skeletal muscle fiber composition
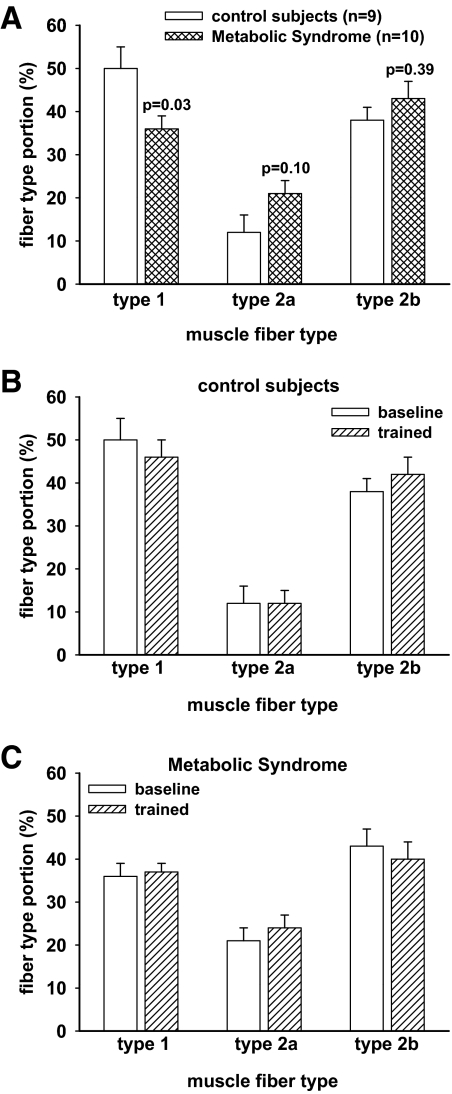
Percutaneous muscle biopsies of the vastus lateralis were performed at baseline and after training, and muscle fiber composition was determined using monoclonal antibodies for fast and slow myosin (18). As shown in Fig. 2, MS subjects had a lower percentage of type 1 muscle fibers than controls at baseline (36.3 ± 10.2 vs. 50.0 ± 17.7%, P = 0.03). Percentage of type 1 fibers was unchanged after training. Training tended to cause a shift in fiber composition from type 2b to type 2a in some MS subjects; however, the effect was small (d = 0.119) and not statistically significant.
Fig. 2.
The effect of resistance training on muscle fiber type composition. Percutaneous needle biopsies of vastus lateralis were obtained before and after 8 wk of supervised resistance training. A, Comparison of the baseline fiber composition in the two groups. MS subjects had 28% less (P = 0.03) type 1 fiber content than the control subjects. B and C, Pre- and posttraining fiber composition for the control subjects and the MS subjects, respectively. Some individuals showed modest changes in fiber composition, but in groups, there was no significant change after training.
Hexose transport proteins
Expression of hexose transport proteins GLUT4 and GLUT5 were also quantified. As shown in Fig. 3A, MS subjects had slightly higher GLUT4 expression than controls before training (2.80 ± 1.41 fmol/10 μg membrane protein, and 2.38 ± 1.11 fmol/10 μg membrane protein, respectively). GLUT4 expression increased significantly after training in both groups (P ≤ 0.05). The percent increase in muscle GLUT4 content was greater in controls than MS subjects (67%, d = 1.634, vs. 36%, d = 0.843, respectively). GLUT5 expression increased significantly in MS subjects (P ≤ 0.05; d = 1.0512) but was unchanged in controls (Fig. 3B).
Fig. 3.
Training-related changes in GLUT4, GLUT5, AMPK, phospho-AMPK, PGC-1α, ATP synthase, total mTOR, and phospho-mTOR. Muscle glucose transporter expression was increased after resistance training. Panels A and B, Examples of typical immunoblots for GLUT4 and GLUT5 as well as bar graphs with the results of image analysis of blots from all of the subjects. In each of the immunoblots displayed, a control subject is on the left and a metabolic subject is shown on the right. The designation A represents the baseline muscle biopsy homogenate, and B indicates the posttraining biopsy sample. Panel C, GLUT4 protein content increased in both groups after training. These data represent the mean expression determined in at least three separate quantitative immunoblots (using chimeric protein standards) of muscle homogenates for each individual (nine controls and 10 MS subjects). Panel B displays the data from GLUT5 immunoblots. GLUT5 expression did not change in the control subjects but increased slightly but significantly in the MS subjects. The P values shown in each panel were calculated as paired t tests. Panels C and D display results of similar analyses of expression of total AMPK and activated phospho-AMPK. Without quantitative protein standards, these data are expressed relative to the control baseline signal intensity. Panel E, Pre- and posttraining data for PGC-1α expression. Panel F, Training-related changes in ATP synthase expression as a direct indicator of mitochondrial enzyme increases. As is displayed, each of these measurements tended to increase after resistance training, but the smaller increases in phospho-AMPK and PGC-1α were not statistically significant for the MS subjects. Panels G and H, Typical immunoblots and summary bar graphs showing the changes that occurred in total mTOR and activated phospho-mTOR expression in response to 8 wk of resistance exercise training. The total mTOR and phospho-mTOR measurements increased significantly in the MS subjects, but the smaller increases in the control group did not achieve statistical significance. In each panel, MS indicates the MS group, and pre and post indicate pretraining and posttraining data.
5-AMP-activated protein kinase
Total and activated AMPK expression were quantified by immunoblot analysis as shown in Fig. 3, C and D. Total AMPK expression increased significantly in both groups, with very strong effect sizes (d = 1.338 in MS subjects, and d = 1.77 in controls). Phospho-AMPK expression increased 50% in controls (P < 0.001; d = 1.924). Phospho-AMPK expression increased 13% in MS subjects; however, the increase was not statistically significant, and the effect size was relatively small (d = 0.368).
Peroxisome proliferator-activated receptor γ coactivator 1α
PGC-1α muscle expression tended to increase in both groups after resistance training (Fig. 3E); however, the increase was only statistically significant in controls (P = 0.029, d =1.181). The increase in PGC-1α expression to resistance training was muted in MS subjects compared with controls (28 and 57%, respectively), and the effect size was moderate in MS subjects (d = 0.542).
ATP synthase
ATP synthase expression, a marker of mitochondrial enzyme activity, increased significantly in both groups in response to training as shown in Fig. 3F. MS subjects tended to have higher ATP synthase expression at baseline than controls (P = 0.112), but training increased ATP synthase expression by 63% in controls compared with 25% in MS subjects.
Mammalian target of rapamycin
Total and activated mTOR, a molecular mediator of protein synthesis, increased significantly in MS subjects in response to training (Fig. 3, G and H). Although the increase in total mTOR expression was not statistically significant in controls, total mTOR expression increased 32%, and the effect size was strong (d = 0.772). Total mTOR expression increased 57% in MS subjects in response to resistance training. Phospho-mTOR expression had a similar response to resistance training. Phospho-mTOR increased 55% in MS subjects (d = 1.49; P < 0.01) and 39% in controls. Again, the effect size was large in controls (d = 0.754), but the increase was not statistically significant.
Discussion
In the present study, 8 wk of supervised resistance training improved several physical and metabolic parameters in healthy, previously sedentary subjects as well as previously sedentary subjects with the MS. The sedentary control subjects improved insulin responsiveness and decreased fasting serum insulin concentrations after resistance training. Despite improvements in strength, endurance, and body composition, MS subjects' insulin resistance was unchanged by 8 wk of resistance training.
The two groups that were studied for this report were very different from each other based on the entry criteria. The MS subjects were obese and had a family history of type 2 diabetes, whereas the sedentary controls were nonobese and had no close family members with diabetes. The baseline data were dramatically different in several of the measured parameters. The MS subjects had higher BMI, higher LBM, higher fat mass and percent body fat, and greater waist circumference. The MS subjects had lower VO2max, nearly double the fasting serum insulin, and less than half the insulin response to a euglycemic insulin infusion.
The lack of improvement in insulin resistance in the present study may have contributions from age, body fat, and baseline strength. Previous studies have suggested that the quantitative adaptations to resistance exercise are reduced with age (21). In the present study, however, older subjects (both controls and MS) showed comparable improvements to younger subjects in strength and endurance. Many studies that suggest resistance training may be effective at improving insulin resistance also demonstrate a decrease in fat mass as a result of training (3, 6). Our study took care to maintain body weight throughout so that the effects observed were primarily due to exercise training and were not confounded by weight loss. In the present study, fat mass did not change in either group.
Homogeneity was not present within either group. In fact, there was overlap in many variables, including GIR, despite no overlap in BMI (dictated by entry criteria). The high variability in the subjects at baseline may have obscured smaller changes that might have been seen if the groups were larger and more homogeneous. Stone and co-workers (22) showed that strength at baseline correlated with response to resistance training in college athletes. Our MS subjects started training slightly stronger (not significant), which likely was related to their higher LBM and the higher proportion of type 2b fibers in their vastus lateralis. When corrected for body mass, though, they were slightly lower in PF development. The percent increase in strength was the same in both groups. Training workload increased each week by about the same increment in all subjects in both groups. No difference was seen in the overall workload, and there was no evidence that the higher responses in insulin responsiveness were the most trained. There was no statistically significant correlation of difference in volume load and GIR response to training.
The lack of improvement in insulin responsiveness may have been related to a low level of baseline fitness from a previous extremely sedentary lifestyle in some subjects. Some of the MS subjects may not have been strong enough at the beginning of the training program to achieve workloads high enough during training to cause significant adaptations in only 8 wk. Higher-volume work increases general fitness and is associated with greater improvements in body composition compared with low-volume training. Some of the subjects may have needed to achieve a higher threshold of strength before they could lift with enough intensity to force adaptation to the higher-volume phase of training. Longer duration and/or higher intensity may be necessary to cause sufficient muscle remodeling in MS subjects, as suggested by Sriwijitkamol et al. (23). However, strength quantified by RFD and by allometrically scaled IPF was essentially the same at baseline in both groups (Table 2). There were seven of the nine control subjects and four of the MS subjects who increased their insulin responsiveness after strength training. Baseline VO2max or RFD data did not identify those who would respond. Surprisingly, the positive responders among the MS subjects tended to have lower VO2max and RFD at baseline, suggesting these parameters of fitness did not correlate with improvement in insulin resistance after strength training.
Lovell and co-workers performed 16 wk of resistance training of older men (24). They found improved strength and improved cardiovascular fitness manifested as increase VO2max, suggesting that in these previously sedentary subjects, the impact of resistance training was mixed (24). There was increased strength and increased endurance. The evidence in our subjects also suggests a mixed benefit. If we had started with recreational athletes (more fit subjects), the impact of resistance training might have been more restricted to increased strength and fiber hypertrophy.
Normal human skeletal muscle contains a mixture of type 1, 2a, and 2b muscle fibers. Each fiber type is suited for different types of physical activity. Type 1 (slow-twitch, red) fibers generally contain more mitochondria and are well suited for oxidative energy production and sustained activity. Although type 1 fibers can provide energy for long periods of time, their relatively slow contraction speed and low force production make them best suited for long-term, low-intensity activity (25). Conversely, type 2b (fast-twitch, white) fibers are best suited for energy production via phosphagens and fast glycolysis. Type 2b fibers have the fastest speed of contraction and highest force production capabilities, making them well suited for short-term, high-intensity activity such as sprints or heavy lifting. Type 2a fibers are an intermediate fiber type with properties of both type 1 and type 2b fibers. Training can cause a shift from type 2b muscle fibers toward type 2a fibers, indicative of the altered energy need of the trained muscles (25). However, even long-term training has not resulted in alteration of the baseline proportion of type 1 and type 2 fibers in humans (26). In view of the divergent functional capabilities of each skeletal muscle fiber type, it is not surprising that many of the adaptations to training are fiber type dependent (27) and may occur through the largely separate cell signaling pathways that predominate in each of the two principal fiber types (7). Our subjects with the MS had a higher proportion of type 2 fibers, which might have made them more adaptable to strength training, but this study did not find a training advantage in this group.
Endurance training is associated with improved efficiency of substrate uptake and use by skeletal muscle. These improvements are brought about by increases in mitochondrial biogenesis, oxidative enzymes, and fatty acid oxidation (28). In addition, endurance training appears to increase glucose uptake into the cell by increasing the expression of GLUT4 in skeletal muscle (29). Many of these adaptations are mediated by AMPK and its downstream targets (30). Resistance training results in increased protein synthesis (31) and may increase GLUT4 expression in skeletal muscle (19, 32). The mTOR signaling pathway appears to be crucial in mediating the fiber hypertrophy response to resistance exercise; however, mTOR's role in improving insulin sensitivity after resistance training has remained elusive.
A bout of exercise will acutely increase AMPK activation, but this increase is transient (33, 34). Endurance exercise training of healthy young men has been shown to increase skeletal muscle total AMPK and activated AMPK (35, 36), but after endurance training, the augmentation induced by an acute exercise bout is less than in untrained subjects (35). An increase in basal activated AMPK does not always occur after exercise training and may be attenuated based on the type of training and the pretraining fitness level of the subjects. Clark and co-workers (37) employed 3 wk of intensified training of well-trained athletes and found despite a shift to increased fat oxidation, that both basal and acute exercise-stimulated AMPK activation did not change. We previously found no change in phospho-AMPK in six very sedentary subjects who underwent 6 wk of stationary cycle training (19). The muscle adaptation responses in the subjects of this earlier study resemble those of the MS group in the current study in that mTOR activation predominated. The current study design was quite different, however, because in this protocol, the duration was longer and the whole-body workload was much higher.
Another way of expressing the impact of the exercise intervention on phosphorylation of AMPK is to adjust the percent increase in phospho-AMPK by the percent increase in the total AMPK, assuming that this expression would better reflect the activity change of the upstream AMPK-kinase (LKB1). Applying this expression to our data by subtracting the percent increase in total AMPK from the increase in phospho-AMPK would result in essentially no increase in adjusted phospho-AMPK in controls (+2%), but the adjusted change in MS subjects would become negative (−22%). Similar adjustment to the phospho-mTOR data to reflect change in the upstream Akt activity would result in a much smaller increase in the MS subject muscle (+7%) and almost no change in the control subjects' adjusted phospho-mTOR (−2%). However, it is clear that the activation of AMPK and/or mTOR will amplify changes in the activity of their upstream kinases and, in this case, is also reflected in downstream effects on PGC-1α and ATP synthase.
Transgenic and knockout mouse studies involving key elements of the AMPK and mTOR pathways have provided some insight into potential mechanisms for the results we observed in these subjects. Chronic activation of AMPK increases muscle mitochondrial content in 5-aminoimidazole-4-carboxamide-1-β-D-ribofuranoside-treated mice (38). A gain-of-function mutation in the γ3 regulatory subunit of AMPK expressed exclusively in mouse type 2 muscle fibers resulted in increased expression of PGC-1α and mitochondria in type 2 fibers without changing the fiber-specific type of myosin heavy chain expression (39). Overexpression of PGC-1α in a transgenic mouse results in a more than 2-fold increase in mitochondria but paradoxically caused muscle insulin resistance, perhaps because of increased intramyocyte lipid (40), whereas a muscle-specific knockout of PGC-1α results in a shift in muscle fiber type from the mitochondria-rich types 1 and 2a to the glycolytic types 2x and 2b (41). These mice exhibited increased inflammatory cytokines and impaired glucose tolerance but normal peripheral insulin sensitivity. Expression of activated Akt1 (a kinase upstream of mTOR) in muscle resulted in mTOR activation and improved metabolic parameters. In this model, Akt1 activation decreased fat pad mass and normalized fasting blood glucose levels, insulin levels, and glucose uptake with a concomitant increase in type 2b muscle fiber size (42). Recently, Selman and co-workers (43) demonstrated increased activation of AMPK in S6K1-null mice and suggested that the beneficial effects of knocking out S6K1, including lower body fat and increased life span, were due to higher AMPK activity in the absence of S6K1 tonic inhibition of LKB1 (AMPK-kinase).
The low-grade chronic inflammation seen in obesity may also impair the activation of AMPK (44). Steinberg and coworkers showed that TNFα suppresses AMPK activity by up-regulation of a phosphatase (protein phosphatase 2C). They further demonstrated that the TNFα-related suppression of AMPK activation was completely abolished in mice lacking both types of TNF receptors showing that the TNFα effect was specific (44). We did not measure TNFα of other inflammatory cytokines in our subjects.
In studies of muscle from lean, obese, and type 2 diabetic subjects, Sriwijitkamol and co-workers (23) found that an acute exercise bout increased phosphorylation of AS160 (a protein thought to be involved in GLUT4 translocation), presumably mediated by AMPK activation. They found that even though obese and diabetic subjects tended to have higher baseline phospho-AMPK in muscle, their increase in the ratio of phospho-AMPK to total AMPK was much less in response to the acute exercise challenges. Biopsies were obtained at times 0, 10, and 40 min of either 50% VO2max or 70% VO2max on a cycle ergometer. The higher-intensity exercise caused increases in phosphorylation of AS160 that paralleled the increased phospho-AMPK in the three groups. The maximum increase in phospho-AMPK was about 40% of that of the lean controls in the obese group and about 30% in the diabetic subjects (23). Their obese subject data on muscle AMPK activation by acute exercise is qualitatively comparable to our 8 wk strength training where total AMPK increased significantly, but the increment in baseline phospho-AMPK was much less. This is consistent with a defect in the activation of AMPK with exercise in obesity/MS (45).
Bandyopadhyay and co-workers (46) measured basal activity and insulin suppression of the activity of muscle AMPK in lean, obese, and type 2 diabetic subjects. They reported basal activity of AMPK and the ratio of phospho-AMPK were decreased in the obese and the diabetic subjects. Insulin suppressed AMPK activity and increased malonyl-coenzyme A levels in lean controls but had no effect in the obese or diabetic groups (46). Unlike their obese subjects, our baseline (untrained) MS AMPK and phospho-AMPK data do not show a difference from controls. Their euglycemic clamp data, however, suggest that dysregulation of AMPK activation is a component of the insulin resistance of obesity and diabetes (46). Further evidence of dysregulation of AMPK activation in obesity was shown by Bruce and co-workers (47). They found that adiponectin stimulation of AMPKα1 and AMPKα2 was diminished and/or delayed in isolated muscle strips from rectus abdominus from obese females who underwent elective hysterectomies (47).
Reports from the HERITAGE Family Study have suggested that the benefits of aerobic exercise training are better correlated with weight loss than to increases in aerobic fitness measurements (48, 49). In this study, 105 of 621 participants were classified as MS. All volunteers were subjected to 20 wk of supervised cycle ergometer training after which the mean VO2max increased by 15 and 18% (male and female) and fat mass decreased by 3–4%. Of 105 MS subjects, 30% improved sufficiently that they no longer met the criteria for the designation of MS (49).
The STRRIDE Study (Studies of a Targeted Risk Reduction Intervention through Defined Exercise) determined the effectiveness of three different exercise training programs in reversing the parameters defining the MS (50). Sixteen to 18 subjects with at least three criteria for the MS were subjected to no intervention, low amount of moderate activity, low amount of vigorous activity, or high amount of vigorous activity for 8 months. The group with low amount of vigorous activity was not different from the no-exercise controls, but the other two groups significantly decreased the number of criteria for the MS (50). Analysis of the actual time spent in organized exercise for each subject suggested that the critical issue was time spent exercising, rather than intensity, because the group with a low amount of vigorous activity averaged less time than the other two groups.
In these studies, sedentary control subjects improved insulin responsiveness, whereas MS subjects did not, despite both groups participating side by side in 8 wk of supervised resistance training. The mechanisms for the lack of equivalent improvement in insulin action in the MS subjects may be related to the intrinsic differences in their untrained muscle. Control subjects had more type 1 fibers in their vastus lateralis, whereas MS subjects had more type 2 fibers, at a level similar to that reported in type 2 diabetes (51, 52).
Increases in GLUT4 and ATP synthase occurred coincident with increased insulin responsiveness in controls; half or less of the increases in GLUT4 and ATP synthase were seen in MS subjects, and GIR increased modestly in only four of 10. Phosphorylation of mTOR was more in the MS group, suggesting mTOR activation is not sufficient to improve insulin responsiveness. We conclude that increases in AMPK activation mitochondrial expression and GLUT4 are either directly involved in improved insulin action or are increased in parallel to a key insulin pathway.
Acknowledgments
We express our appreciation to research nurse Mary Ward, who coordinated the clinical portion of this project, and to Ashley Kavanaugh, Lauren Huskey, Anna Swisher, Chris Plourd, Matt Shifflet, Henry Nowell, Travis Livingston, Brian Hobbs, Trey Maughan, Jacob Wheeler, Jason Eble, Keith Painter, and Guy Hornsby, the students in Kinesiology who acted as training coaches to our subjects.
Funding for these studies came from the National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, DK080488 (to C.A.S.).
Disclosure Summary: A.S.L., S.N., M.A.S., M.E.A.H., M.P.M., M.W.R., M.H.S., and C.A.S. have nothing to declare.
Footnotes
- AMPK
- 5-AMP-activated protein kinase
- GIR
- glucose infusion rate
- GLUT4
- glucose transporter 4
- HDL
- high-density lipoprotein
- IPF
- isometric PF
- LBM
- lean body mass
- LDL
- low-density lipoprotein
- MS
- metabolic syndrome
- mTOR
- mammalian target of rapamycin
- PF
- peak force
- PGC-1α
- peroxisome proliferator-activated receptor γ coactivator 1α
- RFD
- rate of force development
- S6K1
- 70-kDa S6 protein kinase
- VO2max
- maximal oxygen consumption.
References
- 1. Ekelund U, Anderssen SA, Froberg K, Sardinha LB, Andersen LB, Brage S. 2007. Independent associations of physical activity and cardiorespiratory fitness with metabolic risk factors in children: the European youth heart study. Diabetologia 50:1832–1840 [DOI] [PubMed] [Google Scholar]
- 2. LaMonte MJ, Barlow CE, Jurca R, Kampert JB, Church TS, Blair SN. 2005. Cardiorespiratory fitness is inversely associated with the incidence of metabolic syndrome: a prospective study of men and women. Circulation 112:505–512 [DOI] [PubMed] [Google Scholar]
- 3. Cauza E, Hanusch-Enserer U, Strasser B, Ludvik B, Metz-Schimmerl S, Pacini G, Wagner O, Georg P, Prager R, Kostner K, Dunky A, Haber P. 2005. The relative benefits of endurance and strength training on the metabolic factors and muscle function of people with type 2 diabetes mellitus. Arch Phys Med Rehabil 86:1527–1533 [DOI] [PubMed] [Google Scholar]
- 4. Tokmakidis SP, Zois CE, Volaklis KA, Kotsa K, Touvra AM. 2004. The effects of a combined strength and aerobic exercise program on glucose control and insulin action in women with type 2 diabetes. Eur J Appl Physiol 92:437–442 [DOI] [PubMed] [Google Scholar]
- 5. Ishii T, Yamakita T, Sato T, Tanaka S, Fujii S. 1998. Resistance training improves insulin sensitivity in NIDDM subjects without altering maximal oxygen uptake. Diabetes Care 21:1353–1355 [DOI] [PubMed] [Google Scholar]
- 6. Dunstan DW, Daly RM, Owen N, Jolley D, De Courten M, Shaw J, Zimmet P. 2002. High-intensity resistance training improves glycemic control in older patients with type 2 diabetes. Diabetes Care 25:1729–1736 [DOI] [PubMed] [Google Scholar]
- 7. Baar K. 2006. Training for endurance and strength: lessons from cell signaling. Med Sci Sports Exerc 38:1939–1944 [DOI] [PubMed] [Google Scholar]
- 8. Neary JP, Martin TP, Quinney HA. 2003. Effects of taper on endurance cycling capacity and single muscle fiber properties. Med Sci Sports Exerc 35:1875–1881 [DOI] [PubMed] [Google Scholar]
- 9. Carling D. 2005. AMP-activated protein kinase: balancing the scales. Biochimie (Paris) 87:87–91 [DOI] [PubMed] [Google Scholar]
- 10. Hood DA, Irrcher I, Ljubicic V, Joseph AM. 2006. Coordination of metabolic plasticity in skeletal muscle. J Exp Med 209:2265–2275 [DOI] [PubMed] [Google Scholar]
- 11. Thong FSL, Dugani CB, Klip A. 2005. Turning signals on and off: GLUT4 traffic in the insulin-signaling highway. Physiology 20:271–284 [DOI] [PubMed] [Google Scholar]
- 12. Christ-Roberts CY, Pratipanawatr T, Pratipanawatr W, Berria R, Belfort R, Kashyap S, Mandarino LJ. 2004. Exercise training increases glycogen synthase activity and GLUT4 expression but not insulin signaling in overweight nondiabetic and type 2 diabetic subjects. Metabolism 53:1233–1242 [DOI] [PubMed] [Google Scholar]
- 13. Campos GE, Luecke TJ, Wendeln HK, Toma K, Hagerman FC, Murray TF, Ragg KE, Ratamess NA, Kraemer WJ, Staron RS. 2002. Muscular adaptations in response to three different resistance-training regimens: specificity of repetition maximum training zones. Eur J Appl Physiol 88:50–60 [DOI] [PubMed] [Google Scholar]
- 14. Laplante M, Sabatini DM. 2009. mTOR signaling at a glance. J Cell Sci 122(Pt 20):3589–3594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lorenzo C, Okoloise M, Williams K, Stern MP, Haffner SM. 2003. The metabolic syndrome as predictor of type 2 diabetes. Diabetes Care 26:3153–3159 [DOI] [PubMed] [Google Scholar]
- 16. Stuart CA, Yin D, Howell ME, Dykes RJ, Laffan JJ, Ferrando AA. 2006. Hexose transporter mRNAs for GLUT4, GLUT5, and GLUT12 predominate in human muscle. Am J Physiol Endocrinol Metab 291:E1067–E1073 [DOI] [PubMed] [Google Scholar]
- 17. Stuart CA, Howell ME, Zhang Y, Yin D. 2009. Insulin-stimulated translocation of glucose transporter (GLUT) 12 parallels that of GLUT4 in normal muscle. J Clin Endocrinol Metab 94:3535–3542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Behan WM, Cossar DW, Madden HA, McKay IC. 2002. Validation of a simple, rapid, and economical technique for distinguishing type 1 and 2 fibres in fixed and frozen skeletal muscle. J Clin Pathol 55:375–380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Stuart CA, Howell ME, Baker JD, Dykes RJ, Duffourc MM, Ramsey MW, Stone MH. 2010. Cycle training increased GLUT4 and activation of mammalian target of rapamycin in fast twitch muscle fibers. Med Sci Sports Exerc 42:96–106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hedges LV. 1981. Distribution theory for Glass's estimator of effect size and related estimators. J Educ Behav Stat 6:107–128 [Google Scholar]
- 21. Kraemer WJ, Häkkinen K, Newton RU, Nindl BC, Volek JS, McCormick M, Gotshalk LA, Gordon SE, Fleck SJ, Campbell WW, Putukian M, Evans WJ. 1999. Effects of heavy-resistance training on hormonal response patterns in younger vs. older men. J Appl Physiol 87:982–992 [DOI] [PubMed] [Google Scholar]
- 22. Stone MH, Sanborn K, O'Bryant HS, Hartman M, Stone ME, Proulx C, Ward B, Hruby J. 2003. Maximum strength-power-performance relationships in collegiate throwers. J Strength Cond Res 17:739–745 [DOI] [PubMed] [Google Scholar]
- 23. Sriwijitkamol A, Coletta DK, Wajcberg E, Balbontin GB, Reyna SM, Barrientes J, Eagan PA, Jenkinson CP, Cersosimo E, DeFronzo RA, Sakamoto K, Musi N. 2007. Effect of acute exercise on AMPK signaling in skeletal muscle of subjects with type 2 diabetes: a time-course and dose-response study. Diabetes 56:836–848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lovell DI, Cuneo R, Gass GC. 2009. Strength training improves submaximum cardiovascular performance in older men. J Geriatr Phys Ther 32:117–124 [DOI] [PubMed] [Google Scholar]
- 25. Stone MH, Stone ME, Sands WA. 2007. Principles and practice of resistance training. Champaign, IL: Human Kinetics [Google Scholar]
- 26. Coggan AR, Spina RJ, King DS, Rogers MA, Brown M, Nemeth PM, Holloszy JO. 1992. Skeletal muscle adaptations to endurance training in 60- to 70-yr-old men and women. J Appl Physiol 72:1780–1786 [DOI] [PubMed] [Google Scholar]
- 27. Takekura H, Yoshioka T. 1989. Specific mitochondrial responses to running training are induced in each type of rat single muscle fibers. Jpn J Physiol 39:497–509 [DOI] [PubMed] [Google Scholar]
- 28. Hoppeler H, Fluck M. 2003. Plasticity of Skeletal Muscle Mitochondria: Structure and Function. Med Sci Sports Exerc 35:95–104 [DOI] [PubMed] [Google Scholar]
- 29. Houmard JA, Shinebarger MH, Dolan PL, Leggett-Frazier N, Bruner RK, McCammon MR, Israel RG, Dohm GL. 1993. Exercise training increases GLUT-4 protein concentration in previously sedentary middle-aged men. Am J Physiol Endocrinol Metab 264:E896–E901 [DOI] [PubMed] [Google Scholar]
- 30. Winder WW, Taylor EB, Thomson DM. 2006. Role of AMP-activated protein kinase in the molecular adaptation to endurance exercise. Med Sci Sports Exerc 38:1945–1949 [DOI] [PubMed] [Google Scholar]
- 31. MacDougall JD, Gibala MJ, Tarnopolsky MA, MacDonald JR, Interisano SA, Yarasheski KE. 1995. The time course for elevated muscle protein synthesis following heavy resistance exercise. Can J Appl Physiol 20:480–486 [DOI] [PubMed] [Google Scholar]
- 32. Tabata I, Suzuki Y, Fukunaga T, Yokozeki T, Akima H, Funato K. 1999. Resistance training affects GLUT-4 content in skeletal muscle of humans after 19 days of head-down bed rest. J Appl Physiol 86:909–914 [DOI] [PubMed] [Google Scholar]
- 33. Mu J, Brozinick JT, Jr, Valladares O, Bucan M, Birnbaum MJ. 2001. A role for AMP-activated protein kinase in contraction- and hypoxia-regulated glucose transport in skeletal muscle. Mol Cell 7:1085–1094 [DOI] [PubMed] [Google Scholar]
- 34. Wojtaszewski JF, Nielsen P, Hansen BF, Richter EA, Kiens B. 2000. Isoform-specific and exercise intensity-dependent activation of 5′-AMP-activated protein kinase in human skeletal muscle. J Physiol 528(Pt 1):221–226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Nielsen JN, Mustard KJ, Graham DA, Yu H, MacDonald CS, Pilegaard H, Goodyear LJ, Hardie DG, Richter EA, Wojtaszewski JF. 2003. 5′-AMP-activated protein kinase activity and subunit expression in exercise-trained human skeletal muscle. J Appl Physiol 94:631–641 [DOI] [PubMed] [Google Scholar]
- 36. Frøsig C, Jørgensen SB, Hardie DG, Richter EA, Wojtaszewski JF. 2004. 5′-AMP-activated protein kinase activity and protein expression are regulated by endurance training in human skeletal muscle. Am J Physiol Endocrinol Metab 286:E411–E417 [DOI] [PubMed] [Google Scholar]
- 37. Clark SA, Chen ZP, Murphy KT, Aughey RJ, McKenna MJ, Kemp BE, Hawley JA. 2004. Intensified exercise training does not alter AMPK signaling in human skeletal muscle. Am J Physiol Endocrinol Metab 286:E737–E743 [DOI] [PubMed] [Google Scholar]
- 38. Winder WW, Holmes BF, Rubink DS, Jensen EB, Chen M, Holloszy JO. 2000. Activation of AMP-activated protein kinase increases mitochondrial enzymes in skeletal muscle. J Appl Physiol 88:2219–2226 [DOI] [PubMed] [Google Scholar]
- 39. Garcia-Roves PM, Osler ME, Holmström MH, Zierath JR. 2008. Gain-of-function R225Q mutation in AMP-activated protein kinase gamma3 subunit increases mitochondrial biogenesis in glycolytic skeletal muscle. J Biol Chem 283:35724–35734 [DOI] [PubMed] [Google Scholar]
- 40. Choi CS, Befroy DE, Codella R, Kim S, Reznick RM, Hwang YJ, Liu ZX, Lee HY, Distefano A, Samuel VT, Zhang D, Cline GW, Handschin C, Lin J, Petersen KF, Spiegelman BM, Shulman GI. 2008. Paradoxical effects of increased expression of PGC-1α on muscle mitochondrial function and insulin-stimulated muscle glucose metabolism. Proc Natl Acad Sci USA 105:19926–19931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Handschin C, Chin S, Li P, Liu F, Maratos-Flier E, Lebrasseur NK, Yan Z, Spiegelman BM. 2007. Skeletal muscle fiber-type switching, exercise intolerance, and myopathy in PGC-1α muscle-specific knock-out animals. J Biol Chem 282:30014–30021 [DOI] [PubMed] [Google Scholar]
- 42. Izumiya Y, Hopkins T, Morris C, Sato K, Zeng L, Viereck J, Hamilton JA, Ouchi N, LeBrasseur NK, Walsh K. 2008. Fast/glycolytic muscle fiber growth reduces fat mass and improves metabolic parameters in obese mice. Cell Metab 7:159–172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Selman C, Tullet JM, Wieser D, Irvine E, Lingard SJ, Choudhury AI, Claret M, Al-Qassab H, Carmignac D, Ramadani F, Woods A, Robinson IC, Schuster E, Batterham RL, Kozma SC, Thomas G, Carling D, Okkenhaug K, Thornton JM, Partridge L, Gems D, Withers DJ. 2009. Ribosomal protein S6 kinase 1 signaling regulates mammalian life span. Science 326:140–144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Steinberg GR, Michell BJ, van Denderen BJ, Watt MJ, Carey AL, Fam BC, Andrikopoulos S, Proietto J, Görgün CZ, Carling D, Hotamisligil GS, Febbraio MA, Kay TW, Kemp BE. 2006. Tumor necrosis factor α-induced skeletal muscle insulin resistance involves suppression of AMP-kinase signaling. Cell Metab 4:465–474 [DOI] [PubMed] [Google Scholar]
- 45. Hegarty BD, Turner N, Cooney GJ, Kraegen EW. 2009. Insulin resistance and fuel homeostasis: the role of AMP-activated protein kinase. Acta Physiol (Oxf) 196:129–145 [DOI] [PubMed] [Google Scholar]
- 46. Bandyopadhyay GK, Yu JG, Ofrecio J, Olefsky JM. 2006. Increased malonyl-CoA levels in muscle from obese and type 2 diabetic subjects lead to decreased fatty acid oxidation and increased lipogenesis; thiazolidinedione treatment reverses these defects. Diabetes 55:2277–2285 [DOI] [PubMed] [Google Scholar]
- 47. Bruce CR, Mertz VA, Heigenhauser GJ, Dyck DJ. 2005. The stimulatory effect of globular adiponectin on insulin-stimulated glucose uptake and fatty acid oxidation is impaired in skeletal muscle from obese subjects. Diabetes 54:3154–3160 [DOI] [PubMed] [Google Scholar]
- 48. Katzmarzyk PT, Leon AS, Rankinen T, Gagnon J, Skinner JS, Wilmore JH, Rao DC, Bouchard C. 2001. Changes in blood lipids consequent to aerobic exercise training related to changes in body fatness and aerobic fitness. Metabolism 50:841–848 [DOI] [PubMed] [Google Scholar]
- 49. Katzmarzyk PT, Leon AS, Wilmore JH, Skinner JS, Rao DC, Rankinen T, Bouchard C. 2003. Targeting the metabolic syndrome with exercise: evidence from the HERITAGE family study. Med Sci Sports Exerc 35:1703–1709 [DOI] [PubMed] [Google Scholar]
- 50. Johnson JL, Slentz CA, Houmard JA, Samsa GP, Duscha BD, Aiken LB, McCartney JS, Tanner CJ, Kraus WE. 2007. Exercise training amount and intensity effects on metabolic syndrome (from Studies of a Targeted Risk Reduction Intervention through Defined Exercise). Am J Cardiol 100:1759–1766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Kelley DE, Simoneau JA. 1994. Impaired free fatty acid utilization by skeletal muscle in non-insulin-dependent diabetes mellitus. J Clin Invest 94:2349–2356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Stuart CA, Howell ME, Yin D. 2007. Overexpression of GLUT5 in diabetic muscle is reversed by pioglitazone. Diabetes Care 30:925–931 [DOI] [PubMed] [Google Scholar]