A low dose insulin infusion in type 2 diabetics significantly reduced amyloid precursor protein expression demonstrating the acute effect of insulin.
Abstract
Objective:
Our objective was to determine whether peripheral blood mononuclear cells express amyloid precursor protein (APP) and other mediators involved in the pathogenesis of Alzheimer's disease and whether their expression is suppressed by insulin.
Research Design and Methods:
Ten obese type 2 diabetic patients were infused with insulin (2 U/h with 100 ml 5% dextrose/h) for 4 h. Patients were also infused with 5% dextrose/h or normal physiological saline for 4 h, respectively, on two other days as controls. Blood samples were obtained at 0, 2, 4, and 6 h.
Results:
Insulin infusion significantly suppressed the expression of APP, presenilin-1, presenilin-2, and glycogen synthase kinase-3β in peripheral blood mononuclear cells. Dextrose and saline infusions did not alter these indices. Insulin infusion also caused significant parallel reductions in nuclear factor-κB binding activity and plasma concentrations of serum amyloid A and intercellular adhesion molecule-1.
Conclusions:
A low dose infusion of insulin suppresses APP, presenilin-1, presenilin-2, and glycogen synthase kinase-3β, key proteins involved in the pathogenesis of Alzheimer's disease, in parallel with exerting its other antiinflammatory effects.
Derived from amyloid precursor protein (APP), β-amyloid (Aβ) is the main component of the plaques that in the brain are the hallmark of Alzheimer's disease (AD) (1). The other key pathological feature of AD is the neurofibrillary tangle formed by the τ-protein, which needs to be hyperphosphorylated to form these tangles (2). This phosphorylation is mediated by the enzyme glycogen synthase kinase-3β (GSK-3β) (3). Because these processes are related to inflammatory and oxidative stress-related changes in the brain, it is likely that conditions associated with increased oxidative stress and inflammation are associated with an increased incidence of AD. Indeed, obesity and type 2 diabetes mellitus (T2DM), known to be associated with chronic low-grade inflammation and oxidative stress, have a significantly increased prevalence of AD (4). Both states are also associated with insulin resistance.
Several other factors are also involved in the deposition of the amyloid plaques. They include the enzymes β- and γ-secretase, which convert APP into Aβ, and the enzyme neprilysin, which breaks down Aβ (2, 5–8). Presenilin-1 (PS1) and PS2 are two subunits of γ-secretase. Thus, the assessment of any novel agent aimed at treating or preventing AD would necessitate the investigation of the effects of the agent on these mediators.
Recent work has shown that insulin reduces the expression of Aβ in neuronal cultures in vitro and that Aβ reduces the expression of the insulin receptor in neurites in these cultures (9). Aβ also induces oxidative stress in neurons, which is inhibited by insulin (9), consistent with the previously described reactive oxygen species' suppressive effect on peripheral blood mononuclear cells (MNC) (10). Interestingly, it has also been shown that Aβ induces an increase in nuclear factor-κB (NFκB) binding activity and triggers inflammation (11). A recent study also demonstrated that intranasal inhalation of insulin improves cognitive function in patients with AD (12). Because intranasal administration leads to direct access of insulin into the brain along the olfactory nerves, insulin is able to exert a selective cerebral or neuronal action without its systemic effects on glycemic levels. Furthermore, there are data demonstrating that the expression of insulin, IGF-I and IGF-II receptors, insulin receptor substrate-1, phosphatidylinositol 3-kinase, and AkT kinase is diminished, whereas that of GSK-3β is increased in the brains of patients with AD (13). This study also showed that the expression of insulin, IGF-I, and IGF-II is diminished in these brains. Indeed, on this basis, the authors wondered whether AD could be termed type 3 diabetes.
To explain the increase of AD in insulin-resistant proinflammatory states and the beneficial effects of insulin in AD patients in preliminary studies, it is important that its effects on these factors be investigated. Because our previous work has shown that iv-infused insulin exerts a potent and rapid antiinflammatory effect as manifested by its effect on peripheral blood MNC (10, 14–16), we hypothesized that the MNC expresses APP and τ-protein and that insulin suppresses their expression. In addition, we also hypothesized that insulin suppresses the expression of the γ-secretase subunits PS1 and PS2 and GSK-3β.
Subjects and Methods
Subjects
Twenty-four obese patients with T2DM participated in this study. They were on stable oral antidiabetic medications. All patients were on metformin (1–2 g/d), and 14 patients were on sulfonylureas (glyburide or glipizide 5–10 mg/d). None of the subjects was on insulin or thiazolidinedione therapy or taking any antioxidant or nonsteroidal antiinflammatory drugs. Subjects were divided into three groups of similar baseline characteristics and medications. Over 80% of patients were on stable doses of statins, angiotensin-converting enzyme inhibitors or angiotensin receptor blockers and aspirin. After an overnight fast, 10 subjects [five females, age 48 ± 9 yr, with body mass index (BMI) of 39.2 ± 6.5 kg/m2 and glycosylated hemoglobin (HbA1c) of 7.0 ± 0.8%] were infused with insulin (2 U/h) with 5% glucose and 20 mEq potassium chloride for 4 h followed by 2 h of observation and washout. Blood glucose level was maintained within a target range of 80–130 mg/dl for the entire group within 15 mg/dl from the baseline for each patient. Blood glucose was measured every 15 min. Another eight (four females, age 46 ± 8 yr, with BMI of 38.6 ± 7.2 kg/m2 and HbA1c of 7.3 ± 0.9%) and six (four females, age 42 ± 8 yr, with BMI of 37 ± 6.7 kg/m2 and HbA1c of 7.5 ± 1.1%) subjects were infused with either 5% glucose or normal saline alone, respectively, at a rate of 100 ml/h for 4 h and served as controls. Blood samples were collected at baseline and at 2, 4, and 6 h after the start of the infusion. The protocol was approved by the Human Research Committee of the State University of New York at Buffalo. An informed consent was signed by all subjects.
MNC isolation
Blood samples were collected in Na-EDTA and carefully layered on Lympholyte medium (Cedarlane Laboratories, Hornby, Ontario, Canada). Samples were centrifuged and two bands separated out at the top of the red blood cell pellet. The MNC band was harvested and washed twice with Hanks' balanced salt solution. This method provides yields greater than 95% MNC preparation.
Quantification of APP, PS1, PS2, τ, and GSK-3β expression
The mRNA expression of APP, PS1, PS2, GSK-3β, and τ was measured in MNC by RT-PCR. Total RNA was isolated using commercially available RNAqueous-4PCR Kit (Ambion, Austin, TX). Real-time RT-PCR was performed using Stratagene (La Jolla, CA) Mx3000P QPCR System, Sybr green master mix (QIAGEN, Valencia, CA), and gene-specific primers (Life Technologies, Rockville, MD). All values were normalized to the expression of a group of housekeeping genes including actin, ubiquitin C, and cyclophilin A.
Western blotting
MNC total cell lysates were prepared and electrophoresis and immunoblotting were carried out as described before (10). Monoclonal antibody against APP (Abcam, Cambridge, MA) and actin (Santa Cruz Biotechnology, Santa Cruz, CA) were used, and all values were corrected for loading to actin.
Plasma measurements
Glucose concentrations were measured in plasma by YSI (Yellow Springs, OH) 2300 STAT Plus glucose analyzer. ELISA was used to measure plasma concentrations of insulin (Diagnostic Systems Laboratories Inc., Webster, TX), soluble intercellular adhesion molecule (sICAM) (R&D Systems, Minneapolis, MN), and SAA (Invitrogen, Carlsbad, CA) with coefficients of variation of 3–5 and 4.5–7%, respectively.
Statistical analysis
Statistical analysis was conducted using SigmaStat software (SPSS Inc., Chicago, IL). All data are represented as mean ± se. Changes from baseline were calculated and statistical analysis was carried out using one-way repeated-measures ANOVA (RMANOVA) with Holm-Sidak post hoc test. Two-factor RMANOVA followed by Dunnett's post hoc test was used for multiple comparisons between different treatments.
Results
Insulin and glucose concentrations after insulin infusion
Plasma insulin concentration increased from 20.9 ± 10.9 to 50.5 ± 22.4 μU/ml (P < 0.001) during the insulin infusion, whereas it fell slightly in the dextrose group from 27.6 ± 5.6 to 22.9 ± 6.5 μU/ml at 4 h [not significant (NS)] and in the normal saline group from 20.6 ± 5.5 to 17.9 ± 4.7 μU/ml at 4 h (NS). The mean blood glucose concentrations changed from 122 ± 15 at baseline to 111 ± 10 mg/dl at 4 h (NS) after insulin infusion, and 133 ± 14 mg/dl at baseline to 125 ± 12 mg/dl at 4 h (NS) after dextrose infusion. Blood glucose concentration did not change in the saline group. Blood glucose at baseline and at 4 h was not significantly different between the three groups. No patient experienced hypoglycemia.
Effect of insulin infusion on the expression of AD mediators in MNC
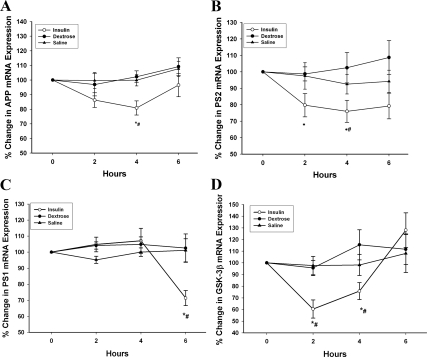
The mRNA expression of APP fell significantly by 19 ± 5% at 4 h after insulin infusion (Fig. 1A, P < 0.05). Insulin infusion also suppressed the mRNA expression of PS2 (subunit of γ-secretase) by 24 ± 9% at 4 h (Fig. 1B, P < 0.05). PS1 expression was suppressed at 6 h by 28 ± 6% (Fig. 1C, P < 0.05), whereas there was no change in its expression at 4 h. There was no significant detectable expression of τ in MNC. However, the infusion of insulin significantly suppressed the expression of GSK-3β by 40 ± 7% at 2 h (Fig. 1D, P < 0.05). There was also a concomitant fall in APP-1 protein levels in MNC by 17 ± 7% at 4 h after insulin infusion (Fig. 2, P < 0.05). There was no significant change in the expression of these indices after the infusion of glucose or saline alone. The expression of PS1, PS2, and GSK-3β was too low to be detected by Western blots.
Fig. 1.
Change in the mRNA expression of APP-1 (A) PS2 (B), PS1 (C), and GSK-3β (D) in MNC after 2 U/h insulin/dextrose infusion (insulin), dextrose alone (dextrose), or saline alone (saline) in obese T2DM for 4 h. Data are presented as mean ± se. *, P < 0.05 by one-way RMANOVA (compared with baseline); #, P < 0.05 by two-way RMANOVA (compared with control groups).
Fig. 2.
Representative Western blot (A) and percent change (B) in APP-1 protein levels by Western blotting from total cell lysates from MNC after 2 U/h insulin/dextrose infusion (insulin), dextrose alone (dextrose), or saline alone (saline) in obese T2DM for 4 h. Data are presented as mean ± se. *, P < 0.05 by one-way RMANOVA (compared with baseline); #, P < 0.05 by two-way RMANOVA (compared with control groups).
Effect of insulin infusion on other inflammatory mediators
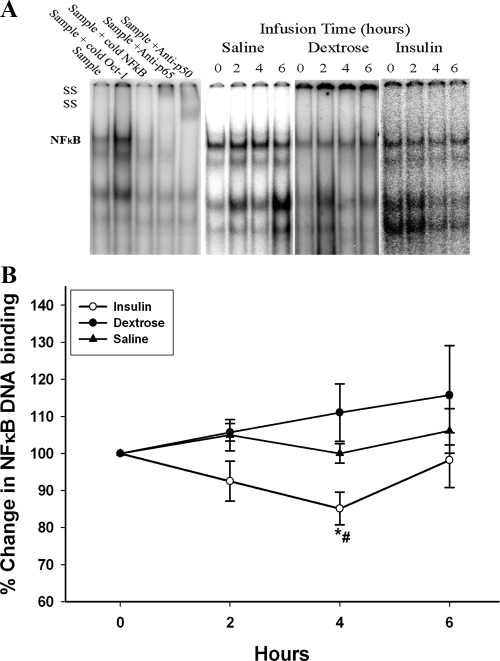
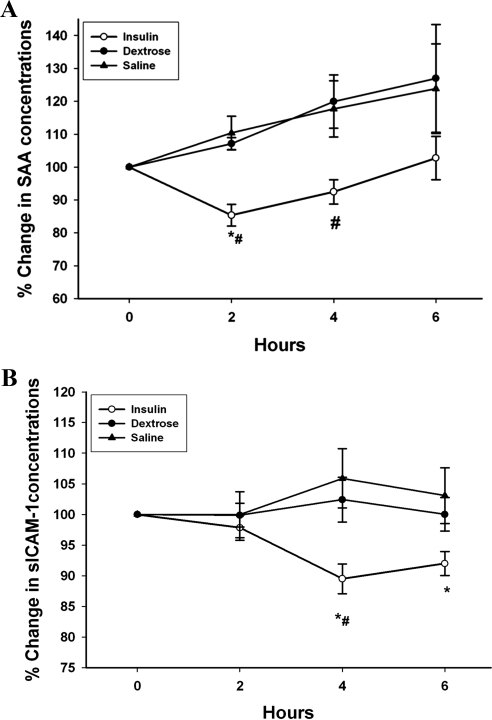
Insulin infusion significantly suppressed the DNA binding of NFκB by 14 ± 5% at 4 h, whereas glucose or saline infusion caused no significant change in NFκB activity (Fig. 3, P < 0.05). After insulin infusion, there was a significant decrease in plasma concentrations of SAA from 9.6 ± 1.6 to 8.2 ± 1.3 μg/ml (by 15 ± 3%) and sICAM from 265 ± 21 to 241 ± 19 ng/ml (by 11 ± 2%) (Fig. 4, P < 0.05). There was no significant change in plasma levels of these inflammatory mediators after dextrose or saline infusions.
Fig. 3.
Change in NFκB DNA binding activity by EMSA to a consensus NFκB binding site in nuclear extracts from MNC after 2 U/h insulin/dextrose infusion (insulin), dextrose alone (dextrose), or saline alone (saline) in obese T2DM for 4 h. Data are presented as mean ± se. *, P < 0.05 by one-way RMANOVA (compared with baseline); #, P < 0.05 by two-way RMANOVA (compared with control groups).
Fig. 4.
Change in plasma concentrations of SAA (A) and sICAM-1 (B) after 2 U/h insulin/dextrose infusion (insulin), dextrose alone (dextrose), or saline alone (saline) in obese T2DM for 4 h. Data are presented as mean ± se. *, P < 0.05 by one-way RMANOVA (compared with baseline); #, P < 0.05 by two-way RMANOVA (compared with control groups).
Discussion
Our data show for the first time that MNC express APP, PS1, PS2, and GSK-3β, which are some of the key proteins involved in the pathogenesis of AD. APP is the precursor of Aβ, whereas PS1 and PS2 are components of γ-secretase, the enzyme that cleaves APP at the cytoplasmic end. We did not observe a significant expression of τ in MNC. On the other hand, GSK-3β, the enzyme responsible for hyperphosphorylating τ was expressed by the MNC. Thus, MNC can be used for investigating the effect of potential drugs that may affect the key proteins related to AD. Although APP expression has previously been shown in monocytic THP-1 cell lines in vitro (17), it has never been shown in peripheral blood MNC.
Our data also demonstrate for the first time that the low-dose insulin infusion suppressed the mRNA expression of APP, PS1, PS2, and GSK-3β. Although APP was suppressed significantly at 4 h, PS2 and GSK-3β were suppressed significantly at 2 h and continued to be suppressed thereafter. On the other hand, the suppression of PS1 was observed only at 6 h, 2 h after the cessation of the insulin infusion. Clearly, PS1 takes longer to suppress, and therefore, future studies would need to be conducted for prolonged periods of time. The known suppression of C-reactive protein by insulin also takes longer periods of infusion (18, 19). The expression of APP was also suppressed significantly at 4 h. This effect appeared to linger at 6 h but was not significant at that time.
Insulin has previously been known to reduce the enzymatic activity of GSK-3β. However, our observation that its expression is reduced significantly in the human in vivo is novel. This observation is also relevant to the fact that the expression of GSK-3β is increased in the brains of patients with AD (13). Because there was no significant expression of τ-protein in MNC, we shall have to investigate an effect of insulin on τ expression in some other tissue in which it is expressed.
It is noteworthy that insulin can potentially inhibit the formation of Aβ in two ways, through the suppression of APP expression and the suppression of the two components of γ-secretase, PS1 and PS2. In addition, insulin would inhibit the hyperphosphorylation of τ by suppressing the expression of GSK-3β.
The suppression of APP after the infusion of insulin occurred in parallel with the suppression of other indices of inflammation as reflected in decreased intranuclear NFκB binding and decreased plasma concentrations of ICAM-1 and SAA. These effects were consistent with the previously demonstrated antiinflammatory effects of insulin including the suppression of chemokines, toll-like receptors, and reactive oxygen species generation (10, 15, 16). Because the pharmacodynamics of these effects on APP and other inflammatory indices were similar, and the other antiinflammatory effects did not precede APP suppression, it is likely that insulin has a direct cellular effect on APP, PS2, PS1, and GSK-3β expression in parallel with its other antiinflammatory actions. If indeed this effect of insulin is a systemic one and occurs in the brain, insulin could be a potential therapeutic agent in the treatment of AD. However, future larger studies are needed to confirm our data and also to confirm the potential benefits to cognitive function demonstrated by the pilot study by Craft's group (12) using intranasal insulin. It is noteworthy the magnitude of NFκB suppression in these patients was less than that described by us previously in obese patients. It is possible that obese patients with type 2 diabetes have a greater level of inflammation and insulin resistance.
Consistent with the above effects, insulin has recently been shown to suppress the expression of Aβ by neurites in culture, whereas Aβ reduces the expression of insulin receptor in neurites (9). A recent report shows that metformin induces an increase in the expression of APP in vitro. However, the incubation of metformin with insulin showed a synergistic effect of metformin with the suppressive effect of insulin (20). This interesting relationship between the key pathogenic peptide of AD and insulin is clearly of interest and points to a potential therapeutic role for insulin in the treatment of this disease. It is also conceptually consistent with insulin resistance being a predisposing factor for AD. In a recent study, intranasal administration of insulin was shown to improve cognitive function in patients with AD (12). In contrast to insulin, glucose is known to induce oxidative stress and inflammation (21). Hyperglycemia in diabetics has recently been shown to be associated with impaired cognitive ability when compared with those with normal glycemic levels (22).
Recent work has revealed another interesting relationship between Aβ and diabetes. Crossbreeding of an APP transgenic mouse and the ob/ob mouse leads to a phenotype with extremely severe insulin resistance and T2DM (23). It is, therefore, possible that Aβ may play other important pathogenic roles including an increase in insulin resistance and diabetogenicity. Thus, our observations on the suppression of APP, PS1, PS2, and GSK-3β by insulin may have implications beyond those for AD. Furthermore, it has been shown that Aβ is capable of inducing increased NFκB binding and to induce molecular changes of inflammation (11, 24). This observation suggests that the role of Aβ in inflammatory processes may potentially be systemic, beyond its currently recognized cerebral actions. By implication, therefore, the suppressive effect of insulin on NFκB binding would systemically be inhibitory of the potential proinflammatory effect of Aβ.
Recent work has demonstrated that APP acts as a proinflammatory mediator in endothelial cells, THP-1 monocytic cell lines, and microglia. Its distribution in the cell membrane is close to β-integrins, and thus, its activation by elements of extracellular matrix induces cytokine generation (25) and increases the adhesion characteristics of these cells (26). It is also expressed in vascular tissue. On the basis of the above evidence, APP may exert its proinflammatory effects in various organs including the brain, independently of its product, Aβ (27). Thus, the suppression of APP by insulin may exert a further systemic antiinflammatory effect.
The obvious weakness of this paper is the absence of data from neurons. However, because that is not possible in humans in vivo, a surrogate cellular model is important. Our data provide that. Further exploration using adipose tissue may be another alternative because that tissue expresses a range of AD-related proteins. Another shortcoming of our data is the lack of protein expression for PS1, PS2, and GSK-3β due to our inability to obtain Western blots. Future studies will have to investigate this aspect with more refined techniques.
In conclusion, APP and γ-secretase complex proteins PS1 and PS2 are expressed in the MNC; a low-dose insulin infusion promptly reduces the expression of APP, PS1, and PS2, in parallel with the reduction in NFκB binding and the expression of several proinflammatory mediators. Insulin also suppresses GSK-3β expression, the enzyme responsible for the hyperphosphorylation of τ. Clearly, insulin has the potential of being developed as a therapeutic agent for AD for which no satisfactory treatment is currently available.
Acknowledgments
P.D. is supported by grants from the National Institutes of Health (R01 DK069805 and RO1 DK075877) and the American Diabetes Association (708CR13) and by grants from Merck, Amylin, and Abbott Pharmaceuticals. S.D. is supported by a grant from the American Diabetes Association.
Participation and Contribution: P.D. was responsible for the hypothesis, planning, analysis of data, and writing; I.M. for laboratory analytical work; H.G. for planning, supervision and coordination of laboratory work, statistical analysis, and writing; C.L.S. for laboratory analysis; S.Dh. for planning, supervision of infusions, and writing; S.Da. for the original idea and hypothesis; A.M. for analysis of data and writing; and A.C. for planning, supervision of recruitment, and infusions.
Disclosure Summary: I.M., H.G., C.L.S., S.Dh., S.Da., A.M., and A.C. have nothing to disclose.
Footnotes
- Aβ
- β-Amyloid
- AD
- Alzheimer's disease
- APP
- amyloid precursor protein
- BMI
- body mass index
- GSK-3β
- glycogen synthase kinase-3β
- HbA1c
- glycosylated hemoglobin
- MNC
- mononuclear cell
- NFκB
- nuclear factor-κB
- NS
- not significant
- PS1
- presenilin-1
- RMANOVA
- repeated-measures ANOVA
- sICAM
- soluble intercellular adhesion molecule
- T2DM
- type 2 diabetes mellitus.
References
- 1. Selkoe DJ. 2001. Alzheimer's disease: genes, proteins, and therapy. Physiol Rev 81:741–766 [DOI] [PubMed] [Google Scholar]
- 2. Selkoe DJ. 1999. Translating cell biology into therapeutic advances in Alzheimer's disease. Nature 399:A23–A31 [DOI] [PubMed] [Google Scholar]
- 3. Hernández F, Gómez de Barreda E, Fuster-Matanzo A, Lucas JJ, Avila J. 2010. GSK3: A possible link between beta amyloid peptide and tau protein. Exp Neurol 223:322–325 [DOI] [PubMed] [Google Scholar]
- 4. Arvanitakis Z, Wilson RS, Bienias JL, Evans DA, Bennett DA. 2004. Diabetes mellitus and risk of Alzheimer disease and decline in cognitive function. Arch Neurol 61:661–666 [DOI] [PubMed] [Google Scholar]
- 5. Haass C, Selkoe DJ. 1993. Cellular processing of β-amyloid precursor protein and the genesis of amyloid β-peptide. Cell 75:1039–1042 [DOI] [PubMed] [Google Scholar]
- 6. Chow VW, Mattson MP, Wong PC, Gleichmann M. 2010. An overview of APP processing enzymes and products. Neuromolecular Med 12:1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Thinakaran G, Koo EH. 2008. Amyloid precursor protein trafficking, processing, and function. J Biol Chem 283:29615–29619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Miners JS, Baig S, Palmer J, Palmer LE, Kehoe PG, Love S. 2008. Aβ-degrading enzymes in Alzheimer's disease. Brain Pathol 18:240–252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. De Felice FG, Vieira MN, Bomfim TR, Decker H, Velasco PT, Lambert MP, Viola KL, Zhao WQ, Ferreira ST, Klein WL. 2009. Protection of synapses against Alzheimer's-linked toxins: insulin signaling prevents the pathogenic binding of Aβ oligomers. Proc Natl Acad Sci USA 106:1971–1976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dandona P, Aljada A, Mohanty P, Ghanim H, Hamouda W, Assian E, Ahmad S. 2001. Insulin inhibits intranuclear nuclear factor κB and stimulates IκB in mononuclear cells in obese subjects: evidence for an anti-inflammatory effect? J Clin Endocrinol Metab 86:3257–3265 [DOI] [PubMed] [Google Scholar]
- 11. Combs CK, Karlo JC, Kao SC, Landreth GE. 2001. β-Amyloid stimulation of microglia and monocytes results in TNFα-dependent expression of inducible nitric oxide synthase and neuronal apoptosis. J Neurosci 21:1179–1188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Reger MA, Watson GS, Green PS, Baker LD, Cholerton B, Fishel MA, Plymate SR, Cherrier MM, Schellenberg GD, Frey WH, 2nd, Craft S. 2008. Intranasal insulin administration dose-dependently modulates verbal memory and plasma amyloid-β in memory-impaired older adults. J Alzheimers Dis 13:323–331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Steen E, Terry BM, Rivera EJ, Cannon JL, Neely TR, Tavares R, Xu XJ, Wands JR, de la Monte SM. 2005. Impaired insulin and insulin-like growth factor expression and signaling mechanisms in Alzheimer's disease: is this type 3 diabetes? J Alzheimers Dis 7:63–80 [DOI] [PubMed] [Google Scholar]
- 14. Dandona P, Aljada A, Mohanty P, Ghanim H, Bandyopadhyay A, Chaudhuri A. 2003. Insulin suppresses plasma concentration of vascular endothelial growth factor and matrix metalloproteinase-9. Diabetes Care 26:3310–3314 [DOI] [PubMed] [Google Scholar]
- 15. Ghanim H, Mohanty P, Deopurkar R, Sia CL, Korzeniewski K, Abuaysheh S, Chaudhuri A, Dandona P. 2008. Acute modulation of toll-like receptors by insulin. Diabetes Care 31:1827–1831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ghanim H, Korzeniewski K, Sia CL, Abuaysheh S, Lohano T, Chaudhuri A, Dandona P. 2010. Suppressive effect of insulin infusion on chemokines and chemokine receptors. Diabetes Care 33:1103–1108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sondag CM, Combs CK. 2010. Adhesion of monocytes to type I collagen stimulates an APP-dependent proinflammatory signaling response and release of Aβ1–40. J Neuroinflammation 7:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chaudhuri A, Janicke D, Wilson MF, Tripathy D, Garg R, Bandyopadhyay A, Calieri J, Hoffmeyer D, Syed T, Ghanim H, Aljada A, Dandona P. 2004. Anti-inflammatory and profibrinolytic effect of insulin in acute ST-segment-elevation myocardial infarction. Circulation 109:849–854 [DOI] [PubMed] [Google Scholar]
- 19. Visser L, Zuurbier CJ, Hoek FJ, Opmeer BC, de Jonge E, de Mol BA, van Wezel HB. 2005. Glucose, insulin and potassium applied as perioperative hyperinsulinaemic normoglycaemic clamp: effects on inflammatory response during coronary artery surgery. Br J Anaesth 95:448–457 [DOI] [PubMed] [Google Scholar]
- 20. Chen Y, Zhou K, Wang R, Liu Y, Kwak YD, Ma T, Thompson RC, Zhao Y, Smith L, Gasparini L, Luo Z, Xu H, Liao FF. 2009. Antidiabetic drug metformin (GlucophageR) increases biogenesis of Alzheimer's amyloid peptides via up-regulating BACE1 transcription. Proc Natl Acad Sci USA 106:3907–3912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mohanty P, Hamouda W, Garg R, Aljada A, Ghanim H, Dandona P. 2000. Glucose challenge stimulates reactive oxygen species (ROS) generation by leucocytes. J Clin Endocrinol Metab 85:2970–2973 [DOI] [PubMed] [Google Scholar]
- 22. Saczynski JS, Jónsdóttir MK, Garcia ME, Jonsson PV, Peila R, Eiriksdottir G, Olafsdottir E, Harris TB, Gudnason V, Launer LJ. 2008. Cognitive impairment: an increasingly important complication of type 2 diabetes. Am J Epidemiol 168:1132–1139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Takeda S, Sato N, Uchio-Yamada K, Sawada K, Kunieda T, Takeuchi D, Kurinami H, Shinohara M, Rakugi H, Morishita R. 2010. Diabetes-accelerated memory dysfunction via cerebrovascular inflammation and Aβ deposition in an Alzheimer mouse model with diabetes. Proc Natl Acad Sci USA 107:7036–7041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Paris D, Patel N, Quadros A, Linan M, Bakshi P, Ait-Ghezala G, Mullan M. 2007. Inhibition of Aβ production by NF-κB inhibitors. Neurosci Lett 415:11–16 [DOI] [PubMed] [Google Scholar]
- 25. Sondag CM, Combs CK. 2006. Amyloid precursor protein cross-linking stimulates β amyloid production and pro-inflammatory cytokine release in monocytic lineage cells. J Neurochem 97:449–461 [DOI] [PubMed] [Google Scholar]
- 26. Susan AA, Colin KC. 2010. Amyloid precursor protein mediates monocyte adhesion in AD tissue and apoE−/− mice. Neurobiol Aging 31:1854–1866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Simón AM, Schiapparelli L, Salazar-Colocho P, Cuadrado-Tejedor M, Escribano L, López de Maturana R, Del Río J, Pérez-Mediavilla A, Frechilla D. 2009. Overexpression of wild-type human APP in mice causes cognitive deficits and pathological features unrelated to Aβ levels. Neurobiol Dis 33:369–378 [DOI] [PubMed] [Google Scholar]