We discuss the prevalence and diagnosis of sellar and parasellar masses by pituitary MRI.
Abstract
Context:
Sellar and parasellar masses present with overlapping clinical and radiological features ranging from asymptomatic incidental presentations and hormonal effects to compressive local mass effects. Pituitary masses are diagnosed with increased frequency with magnetic resonance imaging (MRI) advancements and availability, but indications and diagnostic outcomes of MRI screening for sellar lesions are not defined. Although pituitary adenomas are the most frequently encountered sellar mass lesions, other etiologies should be considered in the differential diagnosis of a sellar mass.
Setting:
The study was conducted at a tertiary pituitary center.
Patients:
This study was a retrospective review of 2598 subjects undergoing at least one pituitary MRI scan from 1999 to 2009.
Main Outcome Measure:
Prevalence and diagnosis of specific sellar and parasellar masses as screened by pituitary MRI.
Results:
The most common indications for pituitary imaging, excluding known mass follow-up, were for evaluation of hyperprolactinemia or hypogonadism. A normal pituitary gland was reported in 47% of subjects undergoing pituitary MRI. The most common pituitary adenomas initially identified by MRI included prolactinoma (40%), nonfunctioning adenoma (37%), and GH adenoma (13%). Nonadenomatous sellar masses accounted for 18% of visible lesions, of which the most common were Rathke's cleft cyst (19%), craniopharyngioma (15%), and meningioma (15%). Metastases accounted for 5% of nonpituitary lesions and breast cancer was the most common primary source.
Conclusions:
Half of all pituitary MRI scans performed in a large patient population yielded no visible lesion. Nonadenomatous pituitary lesions should be considered in the diagnosis of sellar masses observed on MRI, and a high clinical suspicion is required to exclude the presence of a nonfunctioning pituitary adenoma.
Pituitary tumors account for up to 15% of all intracranial masses (1), and pituitary adenomas are reported to account for 90% of sellar and parsellar lesions (2, 3). Clinically active pituitary adenomas occur at a prevalence of 1:1064 to 1:1288 to the general population (4, 5). Other sellar lesions include nonneoplastic cystic lesions, germ cell tumors, gliomas, lymphomas, meningiomas, metastatic tumors, vascular lesions, granulomatous and inflammatory lesions, and infections including bacterial abscesses as well as pituitary hyperplasia (2, 3, 6–8). Of the nonadenomatous masses reported in surgical series, Rathke's cleft cyst is the most commonly encountered (23%), metastatic cancer accounts for 12% of cases, and pituitary lymphoma occurred in one of 83 nonpituitary adenomatous masses observed (2). Because only single case reports of rare nonadenomatous lesions are usually published, it is difficult to assess the true prevalence of such lesions.
With advancements in imaging occurring over the past decade as well as the availability of refined endocrine testing techniques, pituitary masses are diagnosed with increased frequency. Increased incidence of pituitary adenomas observed over the second half of an 18-yr study period was due to a 3-fold increased frequency of incidentally discovered pituitary adenomas (9). These pituitary incidentalomas are discovered on computed tomography (CT), or magnetic resonance imaging (MRI) performed for evaluation of unrelated disorders such as head trauma or cancer staging or because of nonspecific symptoms such as headache. MRI scans of clinically normal subjects in the general population have visualized silent pituitary tumors sized 3 mm or greater in diameter in approximately 10% of subjects (10). CT scans of the sellar area in subjects examined for reasons unrelated to pituitary disease revealed sellar lesions sized 3 mm or greater in diameter in 3.7–20% of the subjects (11–13). Pituitary adenomas occur in 1.5–27% of individuals without prior suspected pituitary disease in autopsy series (14). The evaluation and treatment of pituitary incidentalomas are determined by their size and presence of hypersecretory syndromes and/or compressive central symptoms (15, 16). However, further evaluation of these small incidentalomas (<10 mm) may not be cost effective, especially if they are asymptomatic (17).
Specific anatomical landmarks of the sella turcica and the surrounding parasellar region may determine presenting symptoms of masses arising in this region. Nonadenomatous parasellar lesions may present with hypopituitarism, or mass effect with compressive symptoms including headache or visual symptoms, especially when endocrinological evaluation does not identify a hypersecretory syndrome (18). Precise imaging with high contrast and topographic resolution is critical in visualizing this small-volume area to determine both location and specific characteristics of masses, which are important for diagnosis (8, 19, 20).
To date, two surgical case series have reported the prevalence of nonadenomatous sellar and parasellar masses (2, 3). However, the utility of MRI imaging as a diagnostic tool for screening and diagnosis of sellar masses in a large patient population has not been reported. Pituitary MRI identifies sellar tumors and pituitary masses and offers high contrast and multiplanar, thin pituitary cuts enabling evaluation of small soft tissue changes (20). MRI also allows accurate visualization of mass effects on neighboring soft tissues. The objective of this study was to identify the frequency and diagnosis of pituitary masses in an identified patient population undergoing sellar imaging because diagnoses of pituitary-related masses are not all ultimately made by pathological analysis of surgical specimens. We also sought to identify indications for pituitary imaging and the prevalence of incidentalomas, hypopituitarism, and neuroophthalmic symptoms associated with nonadenomatous lesions, as identified by MRI.
Materials and Methods
This study was approved by the Institutional Review Board of the Cedars Sinai Medical Center. Dedicated pituitary MRIs performed at the Cedars Sinai Medical Center from January 1, 1999, to November 12, 2009, were reviewed to identify subjects who had undergone at least one pituitary MRI during this time period. Subjects were excluded from study if the indication for imaging was for purposes of another approved research project. Subjects meeting these criteria were identified, and one reviewer collected data from electronic medical records.
Medical charts were further reviewed for the primary clinical indication that led to a referral for pituitary MRI. In some instances in which indications were nonspecific for pituitary masses, as for evaluation of headache, a concurrent endocrinopathy was either not present or not yet diagnosed. Pituitary MRI scans with findings of questionable lesions of less than 3 mm, without evidence of a concurrent hyperfunctioning endocrinopathy, were considered normal scans. After pituitary MRI scans screened positively for sellar or parasellar masses were identified, the presence of these masses were also sought on prior nonpituitary imaging procedures, if present. Incidentalomas were defined as masses identified on imaging procedures before pituitary MRI for indications unrelated to an endocrinopathy or for visual symptoms consistent with a sellar mass. Endocrinopathies, including hyperfunctioning adenomas, or visual field loss not initially suspected at time of initial presentation was in some cases subsequently diagnosed after further evaluation.
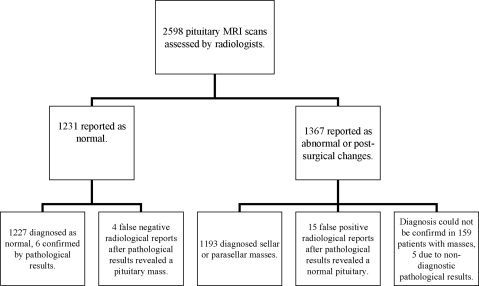
The review of 2598 pituitary MRI records (Fig. 1) revealed a normal pituitary gland reported in 1231 subjects. Within the remaining 1367 subjects in whom a sellar, parasellar mass or postsurgical changes were reported on MRI, a final diagnosis could not be obtained in 159 cases. Reasons for lack of diagnosis included inaccessible outside records (n = 131), serial imaging follow-up (n = 13), diagnosis not pursued by the physician of record (n = 7), nondiagnostic pathologic result (n = 5), and patient lost to follow-up (n = 3). A diagnostic pathological report, considered the gold standard, was available in 435 cases.
Fig. 1.
Screening results of pituitary MRI scans performed in 2598 patients between January 1999 and November 2009. After review and analysis of records, a normal pituitary was diagnosed in 1242 subjects, an abnormal pituitary in 1356 subjects, and 159 masses could not be assessed.
Results
Indications for Imaging
Clinical indications for performing a pituitary MRI are listed in Table 1. The most common indication was for follow-up of a previously identified mass including incidentalomas identified on prior x-ray, CT, or MRI scans. The most common endocrinological indication for performing a pituitary MRI was to evaluate for suspected prolactinoma, followed by evaluation of suspected Cushing's disease and hypogonadism. In 142 cases, a pituitary MRI was ordered for evaluation of neurological symptoms without the presence of a concurrent endocrinopathy. In such instances, headache was the most common indication followed by visual blurriness or loss [second cranial nerve (CN II)], and visual field defects.
Table 1.
Indications for initial pituitary MRI performed in 2598 patients from 1999 to 2009
| Indications | n |
|---|---|
| Follow-up of known massesa | 924 |
| Follow-up of nonpituitary tumors | 4 |
| Suspected mass | |
| Prolactinoma | 455 |
| ACTH adenoma | 349 |
| Nonfunctioning adenoma/mass | 103 |
| GH adenoma | 39 |
| TSH adenoma | 20 |
| Metastases to pituitary | 18 |
| FSH/LH functioning adenoma | 11 |
| Sarcoidosis | 2 |
| Endocrinological work-up | |
| Hypogonadism | 247 |
| Hypopituitarism | 128 |
| GH deficiency | 41 |
| Hypothyroidism | 34 |
| Adrenal insufficiency | 32 |
| Diabetes insipidus | 18 |
| MEN1 syndrome | 17 |
| Postpartum | 8 |
| SIADH | 6 |
| Neurological symptoms | |
| Headache | 75 |
| Visual loss/blurring | 24 |
| Bitemporal hemianopsia | 15 |
| Altered mental status | 5 |
| Syncope | 5 |
| Homonymous hemianopsia | 5 |
| Diplopia | 3 |
| Seizure | 3 |
| Trauma | 3 |
| CVA/TIA | 2 |
| Meningitis | 1 |
| Cranial neuropathy (III, IV, VI) | 1 |
| Total | 2598 |
MEN1, Multiple endocrine neoplasia 1; SIADH, syndrome of inappropriate antidiuretic hormone; CVA, cerebrovacular accident; TIA, transient ischemic attack.
Includes incidentalomas discovered on prior x-rays, CT, or MRI scans and undiagnosed masses.
Evaluation of endocrinopathies resulted in a variable percentage of positive pituitary MRI scans. Screening for acromegaly lead to a finding of a pituitary mass in 69% of pituitary MRIs ordered for this clinical indication. Evaluation of hyperprolactinemia with pituitary MRI resulted in 190 observed masses or 42% of 455 scans. A pituitary abnormality was found in 28% of pituitary MRIs ordered for evaluation of Cushing's disease. Evaluation of panhypopituitarism and hypogonadism resulted in identifying a pituitary lesion in 18 and 16% of pituitary MRIs, respectively.
Pituitary MRI
When comparing pituitary MRI results with the standard of pathological diagnosis, we found pituitary MRI to have a sensitivity and specificity of 99 and 29%, respectively. Four false-negative and 15 false-positive MRI reports were identified. Twenty-one diagnostic pathological reports demonstrated normal pituitary tissue.
Four hundred thirty-five diagnostic pathological reports were available in the electronic medical records. Subjects were excluded from this assessment if pituitary MRI was performed after surgical intervention, which resulted in complete resection of a known mass. When the findings of the first pituitary MRI performed for these patients were analyzed, 382 met such criteria, with 357 true positives, four false negatives, 15 false positives, and six true negatives.
Diagnosis
A pituitary mass was diagnosed in 1197 patients undergoing MRI as shown in Table 2. Of 2598 cases a normal pituitary was diagnosed in 1242 subjects, and 159 pituitary masses could not be diagnosed. Pituitary adenomas accounted for 981 of all diagnosed masses, with most due to prolactinomas and nonfunctioning adenomas. Rare cases of a functioning TSH adenoma, functioning LH/FSH adenoma, and other mixed hormone producing adenomas were also observed. Four hundred thirty-two adenomas were newly diagnosed after pituitary MRI scans. A total of 981 adenomas were identified and were confirmed in 303 cases with a diagnostic pathological report available at this institution (Table 2). Furthermore, 83 macroprolactinomas (>10 mm in diameter) were definitively diagnosed based on concomitant serum prolactin values of greater than 200 ng/ml.
Table 2.
Diagnoses in 2598 patients who underwent at least one pituitary MRI from January 1999 to November 2010
| Diagnoses | Total | Number of masses, [age range], and (average age), at initial pituitary MRIa |
Newly diagnosedb | Pathologyc | |
|---|---|---|---|---|---|
| Males | Females | ||||
| Anterior pituitary tumors | |||||
| Prolactinoma | 395 | 90 [17–81] (46) | 305 [15–89] (36) | 182 | 54 |
| Nonfunctioning adenoma | 364 | 162 [18–85] (54) | 202 [17–87] (47) | 166 | 164 |
| GH adenoma | 127 | 59 [19–84] (49) | 68 [18–82] (45) | 31 | 43 |
| ACTH adenoma | 84 | 18 [19–77] (43) | 66 [10–66] (38) | 47 | 34 |
| GH/prolactin mixed adenoma | 4 | 3 [24–42] (32) | 1 [15] | 1 | 4 |
| Nelson's syndrome | 2 | 0 [24–42] (30) | 2 [44–66] (50) | 1 | 0 |
| Pituitary carcinomad | 2 | 2 [45–75] (60) | 0 | 2 | 2 |
| LH/FSH functioning adenoma | 1 | 1 [59] | 0 | 0 | 1 |
| TSH adenoma | 1 | 0 | 1 [31] | 1 | 1 |
| GH/TSH mixed adenoma | 1 | 0 | 1 [35] | 1 | 0 |
| Cysts | |||||
| Rathke's cleft cyst | 42 | 12 [14–75] (40) | 30 [2–68] (40) | 35 | 25 |
| Craniopharyngioma | 33 | 13 [1–54] (29) | 20 [8–73] (45) | 12 | 20 |
| Arachnoid | 2 | 2 [33–56] (45) | 0 | 0 | 1 |
| Epidermoid | 1 | 0 | 1 [67] | 1 | 1 |
| Pineal cyst | 1 | 1 [11] | 0 | 1 | 0 |
| Nonadenomatous neoplasms | |||||
| Meningioma | 32 | 7 [28–81] (52) | 25 [33–89] (64) | 15 | 13 |
| Chordoma | 3 | 3 [24–69] (43) | 0 | 1 | 3 |
| Pituitary lymphoma | 2 | 1 [48] | 1 [81] | 2 | 2 |
| Chondrosarcoma | 1 | 0 | 1 [24] | 1 | 1 |
| Embryonal rhabdomyosarcoma | 1 | 0 | 1 [10] | 0 | 1 |
| Germinoma | 1 | 1 [12] | 0 | 1 | 1 |
| Granular cell tumor | 1 | 0 | 1 [53] | 0 | 1 |
| Hemangiopericytoma, malignant | 1 | 0 | 1 [77] | 1 | 1 |
| Leiomyosarcoma | 1 | 0 | 1 [36] | 1 | 1 |
| Mucoepidermoid carcinoma | 1 | 0 | 1 [40] | 0 | 1 |
| Pituicytoma | 1 | 1 [38] | 0 | 0 | 1 |
| Xanthogranuloma | 1 | 1 [25] | 0 | 1 | 1 |
| Inflammatory and vasculitidies | |||||
| Lymphocytic hypophysitis | 3 | 1 [45] | 2 [26–34] (30) | 1 | 2 |
| Hypophysitis, unspecified type | 2 | 0 | 2 [37–54] (46) | 0 | 0 |
| Lymphocytic infundibulitis | 1 | 0 | 1 [29] | 0 | 1 |
| Amyloidosis, primary | 1 | 0 | 1 [54] | 1 | 0 |
| Sarcoidosis | 1 | 1 [37] | 0 | 0 | 0 |
| Wegener's granulomatosis | 1 | 1 [44] | 0 | 1 | 1e |
| Infectious | |||||
| Pseudomonas aeruginosa | 1 | 1 [40] | 0 | 1 | 1 |
| Syphilis | 1 | 1 [38] | 0 | 1 | 0 |
| Metastases | |||||
| Breast | 3 | 0 | 3 [32–69] (52) | 3 | 1 |
| CNS lymphoma, to pituitary stalk | 1 | 1 | 0 [72] | 1 | 1 |
| Nasopharyngeal lymphoma | 1 | 0 | 1 [44] | 1 | 1 |
| Liver epitheliod hemangioendothelioma | 1 | 1 [51] | 0 | 0 | 1 |
| Lung, adenocarcinoma | 1 | 1 [71] | 0 | 1 | 0 |
| Pineal germinoma/dysgerminoma | 1 | 1 [18] | 0 | 1 | 1 |
| Plasmacytoma | 1 | 1 [60] | 0 | 1 | 1 |
| Prostate, adenocarcinoma | 1 | 1 [59] | 0 | 1 | 1 |
| Sinusoidal squamous cell carcinoma | 1 | 0 | 1 [70] | 1 | 1 |
| Vascular | |||||
| Apoplexy with masses | 16 | 9 [37–70] (54) | 7 [23–88] (50) | 13 | 9 |
| Carotid aneurysm | 4 | 2 [57–78] (68) | 2 [41–86] (64) | 4 | n/a |
| Hypothalamic cavernous angioma | 1 | 0 | 1 [38] | 1 | 0 |
| Hypothalamic interpeduncular hematoma | 1 | 1 [31] | 0 | 1 | 1 |
| Miscellaneous | |||||
| Empty sella | 21 | 5 [18–48] (36) | 16 [32–62] (47) | 16 | n/a |
| Hyperplasia | 14 | 0 | 14 [14–58] (40) | 10 | 11 |
| Ectopic pituitary gland | 4 | 2 [14 days–25] (12) | 2 [17–67] (42) | 4 | 0 |
| Fibrous dysplasia | 3 | 0 | 3 [26–67] (47) | 1 | 2 |
| Lipoma | 1 | 0 | 1 [23] | 1 | 0 |
| Hypothalamic | |||||
| Astrocytoma | 2 | 0 | 2 [13–37] (25) | 1 | 1 |
| Germinoma | 1 | 1 [26] | 0 | 0 | 0 |
| Hamartoma | 1 | 0 | 1 [10] | 0 | 0 |
| Undiagnosed masses | 159 | 48 [15–93] (48) | 111 [10–89] (43) | n/a | 5f |
| Normal pituitary | 1242 | 436 [2 months–88] (44) | 806 [2–91] (39) | n/a | 21 |
n/a, Not applicable.
Age specified in years, unless otherwise noted. Age range and average noted if applicable;
masses diagnosed after initial pituitary MRI; includes undiagnosed incidentalomas;
number of pathological reports available at this institution to confirm diagnoses;
two cases of pituitary carcinomas initially diagnosed as prolactinomas, which changed at time of biopsy after initial pituitary MRI;
pathology confirmed by serological tests;
five patients with nondiagnostic pathology results.
Two hundred sixteen pituitary masses identified by MRI were not adenomas, accounting for 18% of all observed lesions. One hundred twenty-four of these masses were newly diagnosed after pituitary MRI scans and confirmed with 111 pathological reports. The most common etiology encountered in this group was a pituitary cyst, with Rathke cleft cyst representing 19% of all nonadenomatous lesions. Other commonly observed masses were craniopharyngiomas (15%) and meningiomas (15%).
Eleven cases of pituitary metastases originating from extrapituitary primary cancers were observed. Breast cancer was the most common primary source (n = 3); lung and prostate cancer were also observed. Four cases were due to local tumor spread, including one primary central nervous system (CNS) lymphoma metastasis to the pituitary infundibulum and one nasopharyngeal lymphoma invading the parasellar region without a focal effect on the pituitary gland.
Two other cases of pituitary lymphoma were observed, one diagnosed with a primary diffuse large B cell pituitary lymphoma with lung metastases. Whole-body positron emission tomography/CT demonstrated a suprasellar mass and three pulmonary pleural masses. Biopsies of both the pituitary and pulmonary lesions demonstrated similar pathology. The second patient had stage IV large B cell lymphoma including infiltration of the anterior pituitary without CNS involvement and no primary lymphoma source identified.
Five patients were diagnosed with hypophysitis, three of whom were histologically confirmed as lymphocytic hypophysitis by pathology. Panhypopituitarism was the initial presentation in all five cases, two occurring after pregnancy without a history of significant peripartum bleeding. One patient underwent complete resolution of hypopituitarism without surgical intervention. Pituitary MRI demonstrated large masses (>10 mm in diameter) extending into the suprasellar space with radiographic evidence of chiasmatic compression. Three cases were initially diagnosed as macroadenomas based on MRI characteristics. Hypophysitis was suspected in the other two lesions due to atypical pituitary enhancement, consistent with inflammation. Serial pituitary MRI scans revealed spontaneous pituitary size regression in both cases of hypophysitis treated medically and in the case of lymphocytic hypophysitis diagnosed on surgical biopsy.
Two cases of pituitary infection were observed in immune-compromised patients with HIV infection. One pituitary abscess was due to Pseudomonas aeruginosa. The second patient presented with headache, fevers, and new-onset panhypopituitarism with serology positive for syphilis and extensive work-up negative for other infectious etiologies. MRI demonstrated mild pituitary enlargement and ring enhancement of the gland, mild infundibular thickening, and bony sellar expansion. After penicillin treatment and decreasing titers of serum rapid plasma reagin titers, serial pituitary MRIs demonstrated both decreasing pituitary gland size and differential enhancement consistent with decreasing inflammation.
Other notable lesions observed were pituitary hyperplasia (n = 14) and primary empty sella (n = 21). Five cases of hyperplasia were diffuse without pituitary hyperfunction, and nine others were pathologically proven corticotroph hyperplasias producing ACTH. An ACTH adenoma was initially suspected in all of nine cases with clinical and laboratory evidence of Cushing's disease. Primary empty sella was diagnosed based on MRI findings for evaluation of hypopituitarism, hyperprolactinemia, Cushing's syndrome, incidental findings of nonpituitary MRI scans, or follow-up of a known case.
Neuroophthalmic symptoms and endocrinopathies
Headache (57%) was the most common presenting symptom in patients with nonadenomatous masses identified by MRI, with a significant difference (P < 0.001) between nonfunctioning adenomas (38%) and functioning adenomas (27%) assessed by χ2 test analysis. Furthermore, a significant difference (P < 0.01) was observed for headache prevalence between nonfunctioning and functioning adenomas. Twenty-nine cases of visual field deficits and 25 cases of decreased visual acuity or loss attributed to optic chiasm compression and CN II involvement, respectively, were also observed in nonadenomatous lesions. Invasion of the cavernous sinus leading to cranial nerve involvement was observed in 16 individuals with 11 CN III, six CN IV, and six CN VI palsies. In four individuals, more than one cranial nerve was involved.
Secondary hypogonadism and hyperprolactinemia were the most commonly identified endocrinopathies, both reported in 36% of patients, respectively. Other anterior pituitary dysfunction also occurred frequently with GH deficiency in 24%, central adrenal insufficiency in 21%, and central hypothyroidism in 19% of patients. Although central diabetes insipidus was identified in 11% of nonadenomatous lesions, the occurrence of this endocrinopathy with adenomas was observed far less frequently (0.01%).
Incidental masses
Masses were defined as incidentalomas if they were identified on imaging procedures before pituitary MRI for indications unrelated to an endocrinopathy or for visual symptoms consistent with a sellar mass. Overall, 282 incidental pituitary masses were discovered, with 104 initially discovered by head CT, 176 by brain MRI, and two cases by head or neck x-ray (Table 3).
Table 3.
Diagnoses of 282 incidentalomas discovered by imaging modalitya
| Diagnoses | n |
Diagnoses | n |
||
|---|---|---|---|---|---|
| CT | MRI | CT | MRI | ||
| Anterior pituitary tumors | Metastases | ||||
| Nonfunctioning adenoma | 51 | 66 | CNS lymphoma, to pituitary stalk | 0 | 1 |
| Prolactinoma | 9 | 13 | Liver epitheliod hemangioendothelioma | 1 | 0 |
| GH adenoma | 3 | 5 | Nasopharyngeal lymphoma | 0 | 1 |
| ACTH adenoma | 1 | 3 | Pineal germinoma/dysgerminoma | 0 | 1 |
| GH/prolactin mixed adenoma | 1 | 0 | Plasmacytoma | 1 | 0 |
| GH/TSH mixed adenoma | 1 | 0 | Prostate adenocarcinoma | 0 | 1 |
| TSH adenoma | 0 | 1 | Squamous cell carcinoma of sinus | 1 | 0 |
| Cysts | Infectious | ||||
| Rathke's cleft cyst | 1 | 21 | Pseudomonas aeruginosa | 0 | 1 |
| Craniopharyngioma | 4 | 11 | |||
| Epidermoid | 0 | 1 | Vascular | ||
| Apoplexy with masses | 8 | 6 | |||
| Nonadenomatous neoplasms | Carotid aneurysm | 1 | 0 | ||
| Meningioma | 7 | 11 | Hypothalamic interpeduncular hematoma | 0 | 1 |
| Chordoma | 1 | 2 | |||
| Chondrosarcoma | 0 | 1 | Miscellaneous | ||
| Germinoma | 0 | 1 | Hyperplasia | 4 | 0 |
| Granular cell tumor | 1 | 0 | Empty sella | 0 | 2 |
| Leiomyosarcoma | 1 | 0 | Lipoma | 1 | 0 |
| Mucoepidermoid carcinoma | 0 | 1 | |||
| Xanthogranuloma | 0 | 1 | Undiagnosed masses | 5 | 22 |
| Inflammatory and vasculitidies | Total | 104 | 176 | ||
| Wegener's granulamatosis | 1 | 0 | |||
After screening pituitary MRI scans for sellar and parasellar masses, results were reviewed for identification of these masses on prior nonpituitary imaging procedures.
Not shown are two patients with diagnoses of nonfunctioning adenoma and empty sella identified on head and cervical spine x-rays, respectively.
One hundred eighteen of 282 incidental masses were ultimately identified as nonfunctioning pituitary adenomas. We also observed 37 functioning adenomas and 14 cases of pituitary apoplexy. Indications for imaging leading to incidental discovery of a sellar or parasellar mass are listed in Table 4, with headache (40%) the most common imaging indication. Other less common indications for imaging leading to findings of incidental sellar masses included evaluation for cerebrovascular accidents or transient ischemic attacks (9%), visual loss or blurring (9%), and syncope (7%).
Table 4.
Indications for imaging resulting in discovery of 282 incidentalomas
| Indications | CTa | MRIb | X-ray |
|---|---|---|---|
| Headache | 40 | 72 | |
| CVA/TIA | 10 | 16 | |
| Visual loss/blurring | 5 | 19 | |
| Syncope | 10 | 8 | |
| Altered mental status | 6 | 7 | |
| Diplopia | 6 | 7 | |
| Trauma | 8 | 4 | 1 |
| Dizziness | 3 | 6 | |
| Brain metastases | 3 | 6 | |
| Seizure | 4 | 4 | |
| Cranial nerve palsy | 3 | 5 | |
| Follow-up of nonpituitary brain mass | 1 | 7 | |
| Sinusitis | 2 | 2 | |
| Multiple sclerosis | 0 | 3 | |
| Tinnitus | 1 | 2 | |
| Memory loss | 1 | 1 | |
| Neck pain | 0 | 1 | 1 |
| Mastoiditis | 0 | 1 | |
| SIADH | 0 | 1 |
CVA/TIA, Cerebrovascular accident/transient ischemic attack; SIADH, syndrome of inappropriate antidiuretic hormone.
Indication for imaging was not identified in one case.
Indication for imaging was not identified in two cases.
Discussion
Dedicated pituitary MRI is the preferred diagnostic imaging modality for evaluation of sellar and parasellar tumors, including adenomas. In particular, when functioning adenomas are suspected, a dynamic pituitary MRI, which obtains images within seconds after gadolinium contrast injection, may be more useful because it has higher sensitivity than other imaging modalities for detecting small microadenomas (21). However, small incidental lesions of little or no clinical significance visualized on dynamic pituitary MRI may be misinterpreted as the pathological source of excess hormonal secretion during evaluation of patients for Cushing's disease or acromegaly given lower specificity vs. conventional MRI (21). However, our calculation of the specificity of pituitary MRI is likely limited due to underestimation of true negative values because there are few conditions in which clinicians would obtain pathology results of the pituitary mass when a normal pituitary gland is reported on MRI.
Variability in reported positive MRI results was observed for different clinical indications. Pituitary MRI ordered for the evaluation of patients with suspected acromegaly resulted in a mass reported in 69% of scans, the highest observed among all endocrinopathies. In contrast, 84% of MRI scans ordered for hypogonadism did not reveal a pituitary lesion. These results highlight that pituitary MRI is likely not helpful as a screening tool for patients with hypogonadism. Positive pituitary MRI scans observed with hypogonadotropic hypogonadism were typically observed in cases of severe testosterone deficiency in which total testosterone was less than 100 (normal 250-1000 ng/dl). Given the lack of definitive imaging changes in patients screened for hypogonadism, clinicians should use a higher judgment threshold before ordering pituitary imaging for these patients.
We aimed to identify the prevalence of clinically apparent pituitary masses as screened by MRI scans, and this aim was reflective of the clinical setting because not all pituitary masses are formally diagnosed with histological confirmation. Notably, some etiologies are diagnosed by imaging parameters alone, such as empty sella, or by combination of endocrine values and imaging, as for macroprolactinomas. We observed an 18% incidence of nonadenomatous lesions discovered by MRI, higher than previously reported for surgical series of sellar masses (2, 3). This difference could be attributed to our screening of masses based on imaging parameters, rather than a pathological diagnosis. Other causes for the increased incidence of nonadenomatous masses may be due to our reporting of miscellaneous masses including empty sella syndrome and ectopic pituitary tissue, which are diagnosed only by imaging and would not have been identified in a surgical or pathological series. Furthermore, due to the observational nature of this study, the observed population reflects a selected yet comprehensive group of patients referred for pituitary MRI, rather than the general population as would be encountered in an autopsy series.
Rathke cleft cysts (19%) and craniopharyngiomas (15%) were the most commonly observed nonadenomatous lesions, similar to previous reports. Although both are derived from Rathke's cleft remnants, they can be distinguished on imaging based on magnetic resonance signal appearance and specific characteristics, including homogenous hyperintense T1 signal intensity and midline anterior infundibular displacement in Rathke's cleft cysts, and presence of prominent cystic components and heterogenous hyperintense T1 signal intensity in craniopharyngiomas (22). Furthermore, MRI findings can be instrumental when determining the surgical approach for resection of craniopharyngiomas and likelihood of success in preventing reoccurrences (23).
Previously, large autopsy series have reported pituitary metastases in 1–3.6% of subjects harboring malignant tumors (24–26). Breast and lung cancer are the two most common malignancies that metastasize to the pituitary (27, 28). Breast cancer metastases occur at a high rate, observed in 29% of autopsies of women with disseminated breast cancer (29). Prostate adenocarcinoma may rarely metastasize to the pituitary, accounting for 3% of such cases (27, 28). Metastatic involvement of the pituitary has been reported to account for 12–25% of all nonadenomatous pituitary lesions (2, 3). However, we observed a lower rate (5%), with three cases of breast, one of lung, and one due to prostate cancer. This lower incidence may be due to our screening method and possibly having excluded patients with metastases if they had been imaged with brain MRI or positron emission tomography/CT. Furthermore, most cases of pituitary metastases are clinically silent and diagnosed at autopsy (27).
Although increased incidence of primary CNS or lymphoma has been reported (7, 30, 31), we observed only one primary pituitary lymphoma. This contrasted with the secondary stage IV lymphoma in which a primary source could not be identified, or the third case of a primary CNS lymphoma with pituitary stalk metastasis. These findings confirm that pituitary lymphoma is a rare entity and can be primary or secondary (7, 32).
Infections of the pituitary are rare and potentially life-threatening condition, yet few cases have been reported in literature (33). We observed two cases of infectious disease involving the pituitary gland accounting for 0.2% of all pituitary abnormalities with one due to an abscess caused by P. aeruginosa. Pituitary abscess has been estimated to account for 1% of clinically apparent pituitary disease and isolating an organism from gram stain and/or culture may be difficult with high occurrence of sterile cultures (6). Both cases of pituitary infection observed in this study occurred in immunocompromised patients with prior history of HIV/AIDS. Other risk factors besides an immunocompromised state include presence of preexisting lesions such as an adenoma or Rathke's cleft cyst, previous pituitary surgery, and irradiation of the pituitary (6, 33).
Acquired syphilis of the pituitary is a rare clinical entity, and despite an extensive PubMed literature search, we could not find a reported case in modern literature. However, we identified one case of syphilitic infection of the pituitary in a patient who presented with panhypopituitarism with concurrent systemic and neurosyphilis on serological studies. Pathological destruction of the anterior pituitary by syphilis was first noted in adults as early as 1858, and cases of acquired syphilis involving the pituitary body were reported even before widespread manufacturing of penicillin after World War II (34). Given earlier diagnosis of syphilis with availability of serological tests and accessibility of penicillin, tertiary syphilis has become even less prevalent. The diagnosis of congenital and acquired pituitary syphilis is most often made at autopsy, by identification of interstitial inflammation, gummatas, cystic degeneration, and fibrosis (33). Although a neurosurgical evaluation was not performed on this patient, diagnosis was made with serological studies and pituitary MRI scan consistent with infectious process.
We observed 282 incidentalomas, with nonfunctioning adenoma accounting for the most common incidentally discovered entity. This was not surprising because the most common indication for brain imaging leading to the finding of a pituitary incidentaloma included evaluation for headache, cerebrovascular accident, or transient ischemic attack and decreased visual acuity or visual loss. We found that 23% of pituitary masses were incidentally discovered before pituitary MRI scans. However, because we did not screen CT or MRI scans but rather reviewed them for the presence of incidentalomas after identification of these masses by dedicated pituitary MRI scans, this may not be reflective of the true overall frequency.
Headache is a common clinical indication for imaging leading to discovery of incidental pituitary masses (15) and was commonly observed as leading to a finding of an incidentaloma in this study. Pituitary tumor-related headaches may improve in up to 70% of patients after adenoma resection (35, 36). Furthermore, the presence of headaches does not necessarily correlate with the mass size. Nevertheless, a higher incidence of headache in pregnant women was observed harboring macroprolactinomas vs. microprolactinomas without previous surgical or irradiation therapy (37). Several mechanisms have been proposed for the cause of headaches in patients harboring pituitary masses (38, 39), although these have not been uniformly substantiated (35, 40). Regardless, the higher rate of headache occurrence observed for nonadenomatous lesions vs. both nonfunctioning and functioning adenomas suggests that nonadenomatous lesions are more likely to cause headache (P < 0.001).
Although the diagnosis of the etiology of a sellar or parasellar mass may be confirmed by endocrine, neurological, and radiological techniques, in many cases a pathological specimen is required. For example, as in the leiomyosarcoma and hemangiopericytoma cases observed in this study, a primary neoplasm was diagnosed by pathological specimen evaluation when a nonfunctioning adenoma was initially suspected on radiological imaging. However, as in the case of Wegener's granulomatosis observed in our series, a diagnosis suggested by a pathological result may require confirmation with further serological testing. Overall, given the compelling list of possible diagnoses, when a nonsecreting pituitary mass is observed by MRI, a high clinical suspicion and thorough endocrine and possible pathological assessment is required to exclude the presence of a nonfunctioning pituitary adenoma.
Acknowledgments
We thank Mr. James Mirocha for his help and guidance in the organization and statistical analysis of data.
This work was supported by National Institutes of Health Grant CA 75979.
Disclosure Summary: The authors have nothing to declare.
Footnotes
- CNS
- Central nervous system
- CT
- computed tomography
- MRI
- magnetic resonance imaging.
References
- 1. Terada T, Kovacs K, Stefaneanu L, Horvath E. 1995. Incidence, pathology, and recurrence of pituitary adenomas: study of 647 unselected surgical cases. Endocr Pathol 6:301–310 [DOI] [PubMed] [Google Scholar]
- 2. Freda PU, Wardlaw SL, Post KD. 1996. Unusual causes of sellar/parasellar masses in a large transsphenoidal surgical series. J Clin Endocrinol Metab 81:3455–3459 [DOI] [PubMed] [Google Scholar]
- 3. Valassi E, Biller BM, Klibanski A, Swearingen B. 2010. Clinical features of non-pituitary sellar lesions in a large surgical series. Clin Endocrinol (Oxf) 73:798–807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fernandez A, Karavitaki N, Wass JA. 2010. Prevalence of pituitary adenomas: a community-based, cross-sectional study in Banbury (Oxfordshire, UK). Clin Endocrinol (Oxf) 72:377–382 [DOI] [PubMed] [Google Scholar]
- 5. Daly AF, Rixhon M, Adam C, Dempegioti A, Tichomirowa MA, Beckers A. 2006. High prevalence of pituitary adenomas: a cross-sectional study in the province of Liege, Belgium. J Clin Endocrinol Metab 91:4769–4775 [DOI] [PubMed] [Google Scholar]
- 6. Liu F, Li G, Yao Y, Yang Y, Ma W, Li Y, Chen G, Wang R. 2011. Diagnosis and management of pituitary abscess: experiences from 33 cases. Clin Endocrinol (Oxf) 74:79–88 [DOI] [PubMed] [Google Scholar]
- 7. Giustina A, Gola M, Doga M, Rosei EA. 2001. Clinical review 136: primary lymphoma of the pituitary: an emerging clinical entity. J Clin Endocrinol Metab 86:4567–4575 [DOI] [PubMed] [Google Scholar]
- 8. Kaltsas GA, Evanson J, Chrisoulidou A, Grossman AB. 2008. The diagnosis and management of parasellar tumours of the pituitary. Endocr Relat Cancer 15:885–903 [DOI] [PubMed] [Google Scholar]
- 9. Raappana A, Koivukangas J, Ebeling T, Pirilä T. 2010. Incidence of pituitary adenomas in Northern Finland in 1992–2007. J Clin Endocrinol Metab 95:4268–4275 [DOI] [PubMed] [Google Scholar]
- 10. Hall WA, Luciano MG, Doppman JL, Patronas NJ, Oldfield EH. 1994. Pituitary magnetic resonance imaging in normal human volunteers: occult adenomas in the general population. Ann Intern Med 120:817–20 [DOI] [PubMed] [Google Scholar]
- 11. Chambers EF, Turski PA, LaMasters D, Newton TH. 1982. Regions of low density in the contrast-enhanced pituitary gland: normal and pathologic processes. Radiology 144:109–113 [DOI] [PubMed] [Google Scholar]
- 12. Wolpert SM, Molitch ME, Goldman JA, Wood JB. 1984. Size, shape, and appearance of the normal female pituitary gland. AJR Am J Roentgenol 143:377–381 [DOI] [PubMed] [Google Scholar]
- 13. Peyster RG, Adler LP, Viscarello RR, Hoover ED, Skarzynski J. 1986. CT of the normal pituitary gland. Neuroradiology 28:161–165 [DOI] [PubMed] [Google Scholar]
- 14. Molitch ME. 1997. Pituitary incidentalomas. Endocrinol Metab Clin North Am 26:725–740 [DOI] [PubMed] [Google Scholar]
- 15. Sanno N, Oyama K, Tahara S, Teramoto A, Kato Y. 2003. A survey of pituitary incidentaloma in Japan. Eur J Endocrinol 149:123–127 [DOI] [PubMed] [Google Scholar]
- 16. Serhal D, Weil RJ, Hamrahian AH. 2008. Evaluation and management of pituitary incidentalomas. Cleve Clin J Med 75:793–801 [DOI] [PubMed] [Google Scholar]
- 17. Randall BR, Kraus KL, Simard MF, Couldwell WT. 2010. Cost of evaluation of patients with pituitary incidentaloma. Pituitary 13:383–384 [DOI] [PubMed] [Google Scholar]
- 18. Glezer A, Paraiba DB, Bronstein MD. 2008. Rare sellar lesions. Endocrinol Metab Clin North Am 37:195–211, x [DOI] [PubMed] [Google Scholar]
- 19. Smith JK. 2005. Parasellar tumors: suprasellar and cavernous sinuses. Top Magn Reson Imaging 16:307–315 [DOI] [PubMed] [Google Scholar]
- 20. Rennert J, Doerfler A. 2007. Imaging of sellar and parasellar lesions. Clin Neurol Neurosurg 109:111–124 [DOI] [PubMed] [Google Scholar]
- 21. Tabarin A, Laurent F, Catargi B, Olivier-Puel F, Lescene R, Berge J, Galli FS, Drouillard J, Roger P, Guerin J. 1998. Comparative evaluation of conventional and dynamic magnetic resonance imaging of the pituitary gland for the diagnosis of Cushing's disease. Clin Endocrinol (Oxf) 49:293–300 [DOI] [PubMed] [Google Scholar]
- 22. Donovan JL, Nesbit GM. 1996. Distinction of masses involving the sella and suprasellar space: specificity of imaging features. AJR Am J Roentgenol 167:597–603 [DOI] [PubMed] [Google Scholar]
- 23. Laws ER., Jr 1980. Transsphenoidal microsurgery in the management of craniopharyngioma. J Neurosurg 52:661–666 [DOI] [PubMed] [Google Scholar]
- 24. Abrams HL, Spiro R, Goldstein N. 1950. Metastases in carcinoma: analysis of 1000 autopsied cases. Cancer 3:74–85 [DOI] [PubMed] [Google Scholar]
- 25. Kovacs K. 1973. Metastatic cancer of the pituitary gland. Oncology 27:533–542 [DOI] [PubMed] [Google Scholar]
- 26. Max MB, Deck MD, Rottenberg DA. 1981. Pituitary metastasis: incidence in cancer patients and clinical differentiation from pituitary adenoma. Neurology 31:998–1002 [DOI] [PubMed] [Google Scholar]
- 27. Teears RJ, Silverman EM. 1975. Clinicopathologic review of 88 cases of carcinoma metastatic to the putuitary gland. Cancer 36:216–220 [DOI] [PubMed] [Google Scholar]
- 28. Morita A, Meyer FB, Laws ER., Jr 1998. Symptomatic pituitary metastases. J Neurosurg 89:69–73 [DOI] [PubMed] [Google Scholar]
- 29. Marin F, Kovacs KT, Scheithauer BW, Young WF., Jr 1992. The pituitary gland in patients with breast carcinoma: a histologic and immunocytochemical study of 125 cases. Mayo Clin Proc 67:949–956 [DOI] [PubMed] [Google Scholar]
- 30. Snider WD, Simpson DM, Aronyk KE, Nielsen SL. 1983. Primary lymphoma of the nervous system associated with acquired immune-deficiency syndrome. N Engl J Med 308:45. [DOI] [PubMed] [Google Scholar]
- 31. Eby NL, Grufferman S, Flannelly CM, Schold SC, Jr, Vogel FS, Burger PC. 1988. Increasing incidence of primary brain lymphoma in the US. Cancer 62:2461–2465 [DOI] [PubMed] [Google Scholar]
- 32. Megan Ogilvie C, Payne S, Evanson J, Lister TA, Grossman AB. 2005. Lymphoma metastasizing to the pituitary: an unusual presentation of a treatable disease. Pituitary 8:139–146 [DOI] [PubMed] [Google Scholar]
- 33. Berger SA, Edberg SC, David G. 1986. Infectious disease in the sella turcica. Rev Infect Dis 8:747–755 [DOI] [PubMed] [Google Scholar]
- 34. Oelbaum MH. 1952. Hypopituitarism in male subjects due to syphilis, with a discussion of androgen treatment. Q J Med 21:249–264 [PubMed] [Google Scholar]
- 35. Abe T, Matsumoto K, Kuwazawa J, Toyoda I, Sasaki K. 1998. Headache associated with pituitary adenomas. Headache 38:782–786 [DOI] [PubMed] [Google Scholar]
- 36. Levy MJ, Matharu MS, Meeran K, Powell M, Goadsby PJ. 2005. The clinical characteristics of headache in patients with pituitary tumours. Brain 128(Pt 8):1921–1930 [DOI] [PubMed] [Google Scholar]
- 37. Bronstein MD, Salgado LR, de Castro Musolino NR. 2002. Medical management of pituitary adenomas: the special case of management of the pregnant woman. Pituitary 5:99–107 [DOI] [PubMed] [Google Scholar]
- 38. Forsyth PA, Posner JB. 1993. Headaches in patients with brain tumors: a study of 111 patients. Neurology 43:1678–183 [DOI] [PubMed] [Google Scholar]
- 39. Arafah BM, Prunty D, Ybarra J, Hlavin ML, Selman WR. 2000. The dominant role of increased intrasellar pressure in the pathogenesis of hypopituitarism, hyperprolactinemia, and headaches in patients with pituitary adenomas. J Clin Endocrinol Metab 85:1789–1793 [DOI] [PubMed] [Google Scholar]
- 40. Levy MJ, Jäger HR, Powell M, Matharu MS, Meeran K, Goadsby PJ. 2004. Pituitary volume and headache: size is not everything. Arch Neurol 61:721–725 [DOI] [PubMed] [Google Scholar]